Silencing an ATP-Dependent Caseinolytic Protease Proteolytic Subunit Gene Enhances the Resistance of Rice to Nilaparvata lugens
Abstract
1. Introduction
2. Results
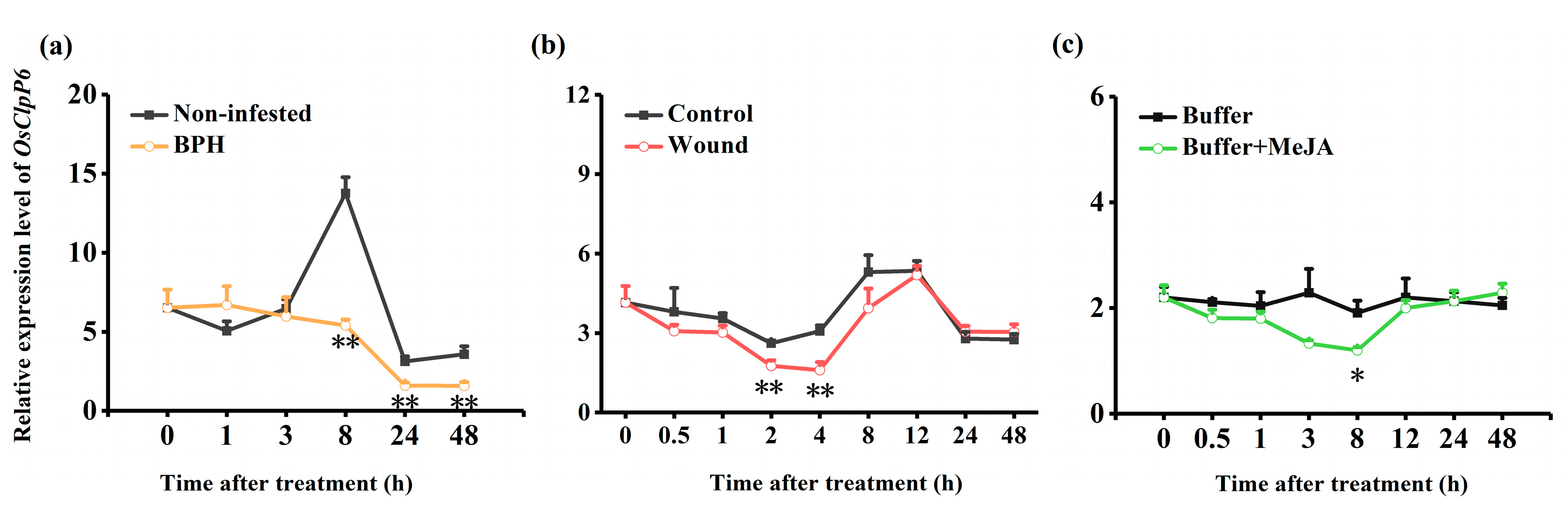
2.1. BPH Infestation, Wounding, and MeJA Treatment Suppress the Expression of OsClpP6
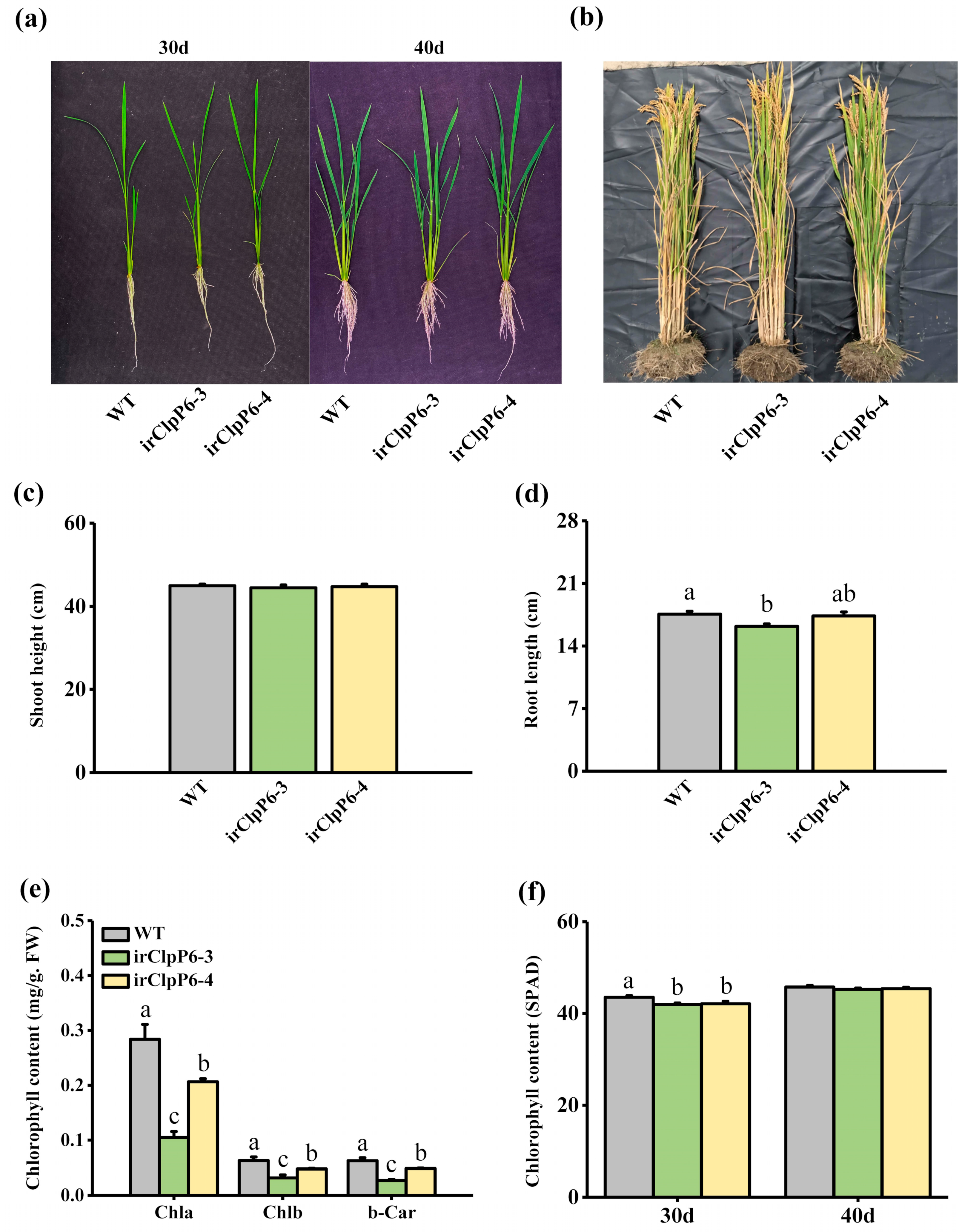
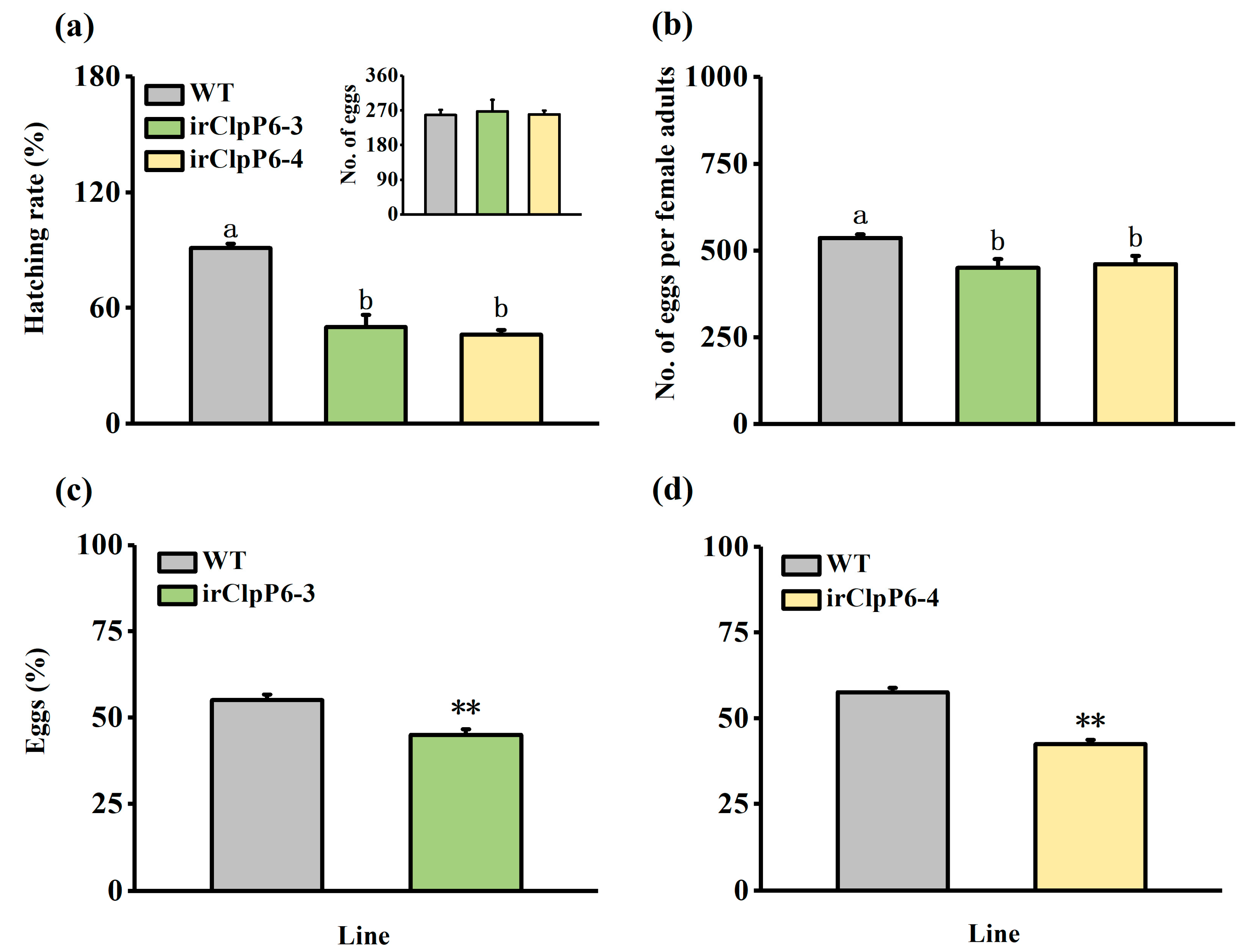
2.2. Silencing OsClpP6 Enhances the Resistance of Rice to BPH
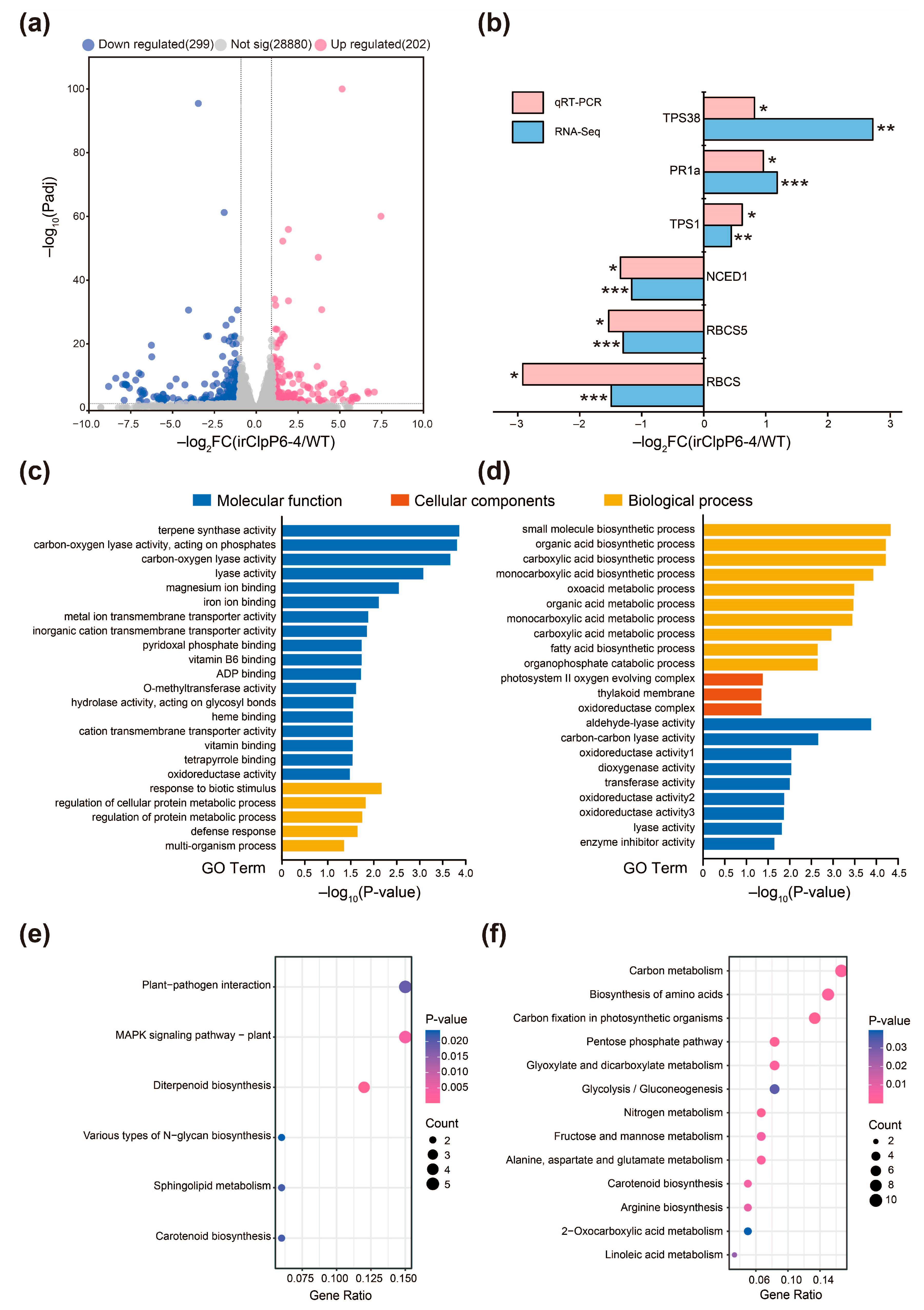
2.3. OsClpP6 Negatively Regulates the Expression of Defense-Related Genes in Rice in Response to BPH Infestation
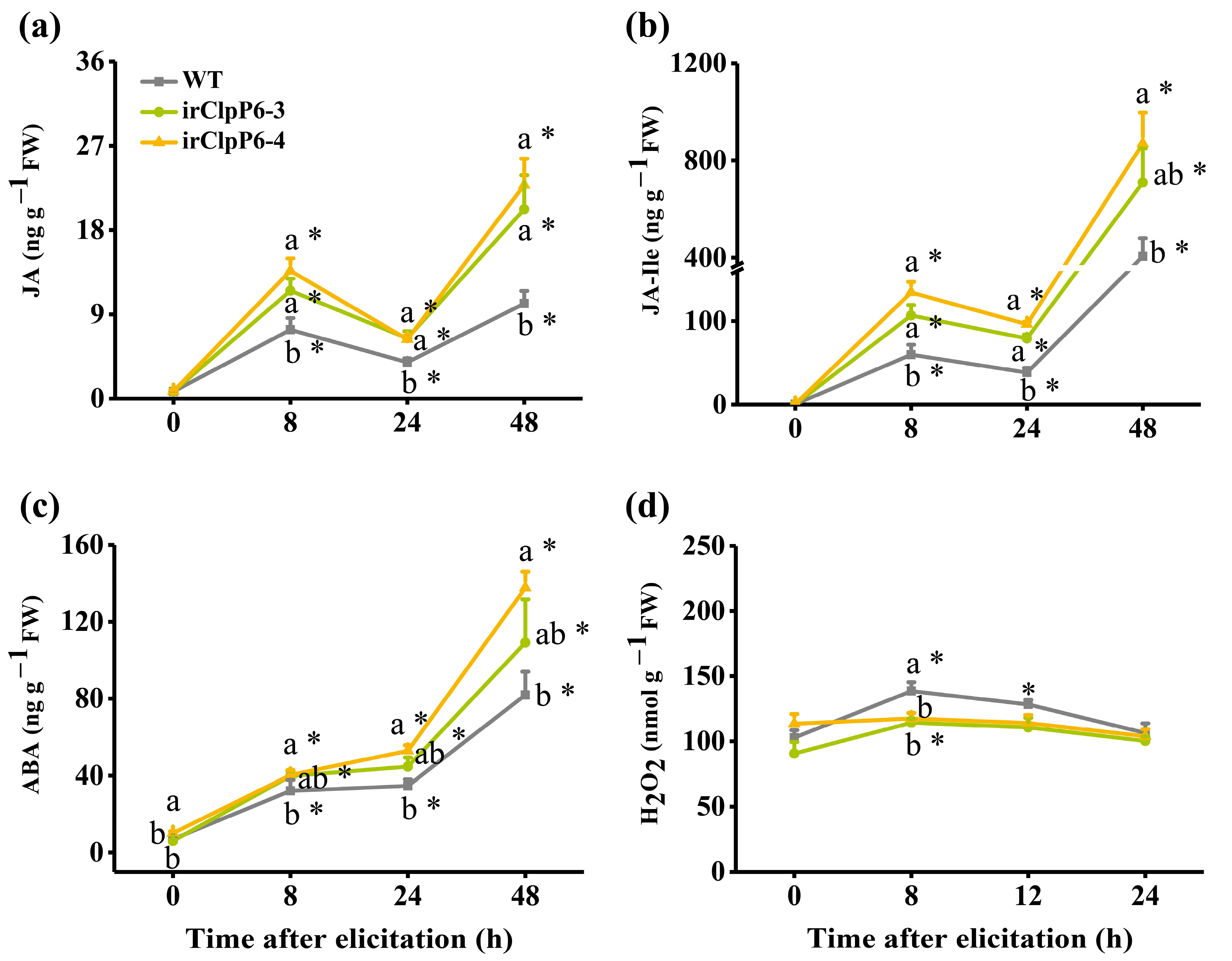
2.4. Silencing OsClpP6 Enhances the Level of BPH-Induced JA, JA-Ile, and ABA but Reduced the Level of H2O2
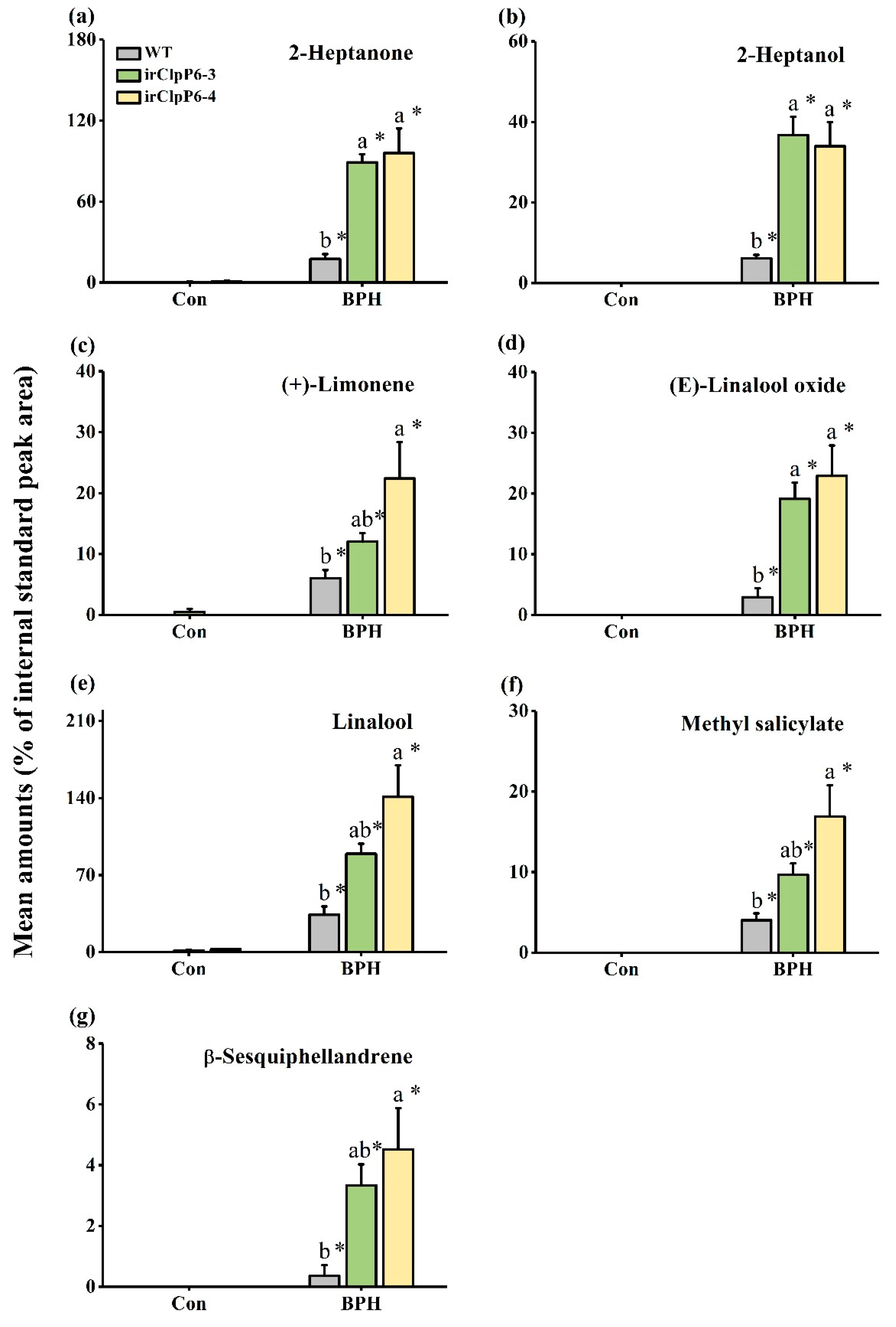
2.5. OsClpP6 Negatively Modulates the Production of BPH-Induced VOCs in Rice
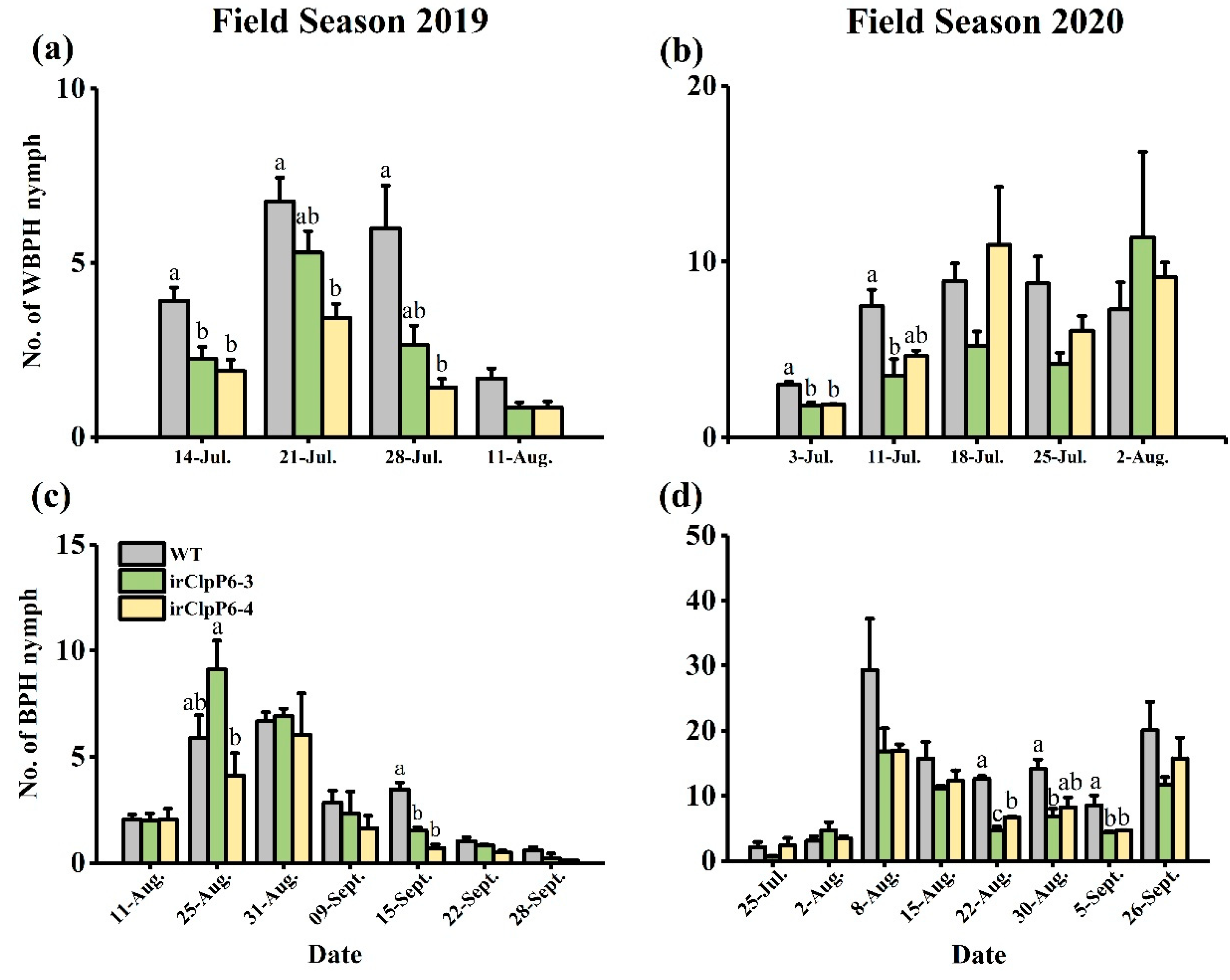
2.6. Silencing OsClpP6 Decreases the Population Densities of BPH and WBPH in the Field
3. Discussion
4. Materials and Methods
4.1. Plants and Insects
4.2. Isolation cDNA of OsClpP6
4.3. Plant Treatments
4.4. Generation and Characterization of Transgenic Plants
4.5. RNA Extraction and qRT-PCR Analysis
4.6. Determination of Rice Growth and Development Parameters
4.7. Measurement of JA, JA-Ile, SA, ABA, and H2O2 Content
4.8. Collection, Isolation, and Identification of the VOCs
4.9. BPH Bioassay
4.10. Field Experiments
4.11. RNA-Seq and Transcriptome Analysis
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erb, M.; Reymond, P. Molecular interactions between plants and insect herbivores. Annu. Rev. Plant Biol. 2019, 70, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Fernandez de Bobadilla, M.; Vitiello, A.; Erb, M.; Poelman, E.H. Plant defense strategies against attack by multiple herbivores. Trends Plant Sci. 2022, 27, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Sozen, C.; Schenk, S.T.; Boudsocq, M.; Chardin, C.; Almeida-Trapp, M.; Krapp, A.; Hirt, H.; Mithofer, A.; Colcombet, J. Wounding and insect feeding trigger two independent MAPK pathways with distinct regulation and kinetics. Plant Cell 2020, 32, 1988–2003. [Google Scholar] [CrossRef] [PubMed]
- Monte, I. Jasmonates and salicylic acid: Evolution of defense hormones in land plants. Curr. Opin. Plant Biol. 2023, 76, 102470. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, Y.T.; Liu, Y.X.; Hao, G.F.; Yang, X.Q. Molecular interaction network of plant-herbivorous insects. Adv. Agron. 2023, 3, 74–82. [Google Scholar] [CrossRef]
- Mamaeva, A.; Taliansky, M.; Filippova, A.; Love, A.J.; Golub, N.; Fesenko, I. The role of chloroplast protein remodeling in stress responses and shaping of the plant peptidome. New Phytol. 2020, 227, 1326–1334. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Protective roles of cytosolic and plastidal proteasomes on abiotic stress and pathogen invasion. Plants 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Montandon, C.; Dougan, D.A.; van Wijk, K.J. N-degron specificity of chloroplast ClpS1 in plants. FEBS Lett. 2019, 593, 962–970. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; D’Andrea, L.; Pulido, P. Control of plastidial metabolism by the Clp protease complex. J. Exp. Bot. 2019, 70, 2049–2058. [Google Scholar] [CrossRef]
- Sjögren, L.L.E.; Clarke, A.K. Assembly of the chloroplast ATP-dependent Clp protease in Arabidopsis is regulated by the ClpT accessory proteins. Plant Cell 2011, 23, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; van Wijk, K.J. Organization, function and substrates of the essential Clp protease system in plastids. Biochim. Biophys. Acta 2015, 1847, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Flores-Pérez, Ú.; Bédard, J.; Tanabe, N.; Lymperopoulos, P.; Clarke, A.K.; Jarvis, P. Functional analysis of the Hsp93/ClpC chaperone at the chloroplast envelope. Plant Physiol. 2015, 170, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Asakura, Y.; Friso, G.; Kim, J.; Oh, S.-H.; Rutschow, H.; Ponnala, L.; van Wijk, K.J. ClpS1 is a conserved substrate selector for the chloroplast Clp protease system in Arabidopsis. Plant Cell 2013, 25, 2276–2301. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Co-Suppression of NbClpC1 and NbClpC2, encoding Clp protease chaperons, elicits significant changes in the metabolic profile of Nicotiana benthamiana. Plants 2020, 9, 259. [Google Scholar] [CrossRef]
- Nishimura, K.; Apitz, J.; Friso, G.; Kim, J.; Ponnala, L.; Grimm, B.; van Wijk, K.J. Discovery of a unique Clp component, ClpF, in chloroplasts: A proposed binary ClpF-ClpS1 adaptor complex functions in substrate recognition and delivery. Plant Cell 2015, 27, 2677–2691. [Google Scholar] [CrossRef]
- Apitz, J.; Nishimura, K.; Schmied, J.; Wolf, A.; Hedtke, B.; van Wijk, K.J.; Grimm, B. Posttranslational control of ALA synthesis includes GluTR degradation by Clp protease and stabilization by GluTR-binding protein. Plant Physiol. 2016, 170, 2040–2051. [Google Scholar] [CrossRef]
- Pulido, P.; Llamas, E.; Llorente, B.; Ventura, S.; Wright, L.P.; Rodríguez-Concepción, M. Specific Hsp100 chaperones determine the fate of the first enzyme of the plastidial isoprenoid pathway for either refolding or degradation by the stromal Clp protease in Arabidopsis. PLoS Genet. 2016, 12, e1005824. [Google Scholar] [CrossRef]
- Llamas, E.; Pulido, P.; Rodriguez-Concepcion, M. Interference with plastome gene expression and Clp protease activity in Arabidopsis triggers a chloroplast unfolded protein response to restore protein homeostasis. PLoS Genet. 2017, 13, e1007022. [Google Scholar] [CrossRef]
- Bouchnak, I.; van Wijk, K.J. Structure, function, and substrates of Clp AAA+ protease systems in cyanobacteria, plastids, and apicoplasts: A comparative analysis. J. Biol. Chem. 2021, 296, 100338. [Google Scholar] [CrossRef]
- Wu, H.; Ji, Y.; Du, J.; Kong, D.; Liang, H.; Ling, H.-Q. ClpC1, an ATP-dependent Clp protease in plastids, is involved in iron homeostasis in Arabidopsis leaves. Ann. Bot. 2010, 105, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Tiller, N.; Diez, M.; Karcher, D.; Tillich, M.; Schottler, M.A.; Bock, R. Generation and characterization of a collection of knock-down lines for the chloroplast Clp protease complex in tobacco. J. Exp. Bot. 2017, 68, 2199–2218. [Google Scholar] [CrossRef]
- D’Andrea, L.; Simon-Moya, M.; Llorente, B.; Llamas, E.; Marro, M.; Loza-Alvarez, P.; Li, L.; Rodriguez-Concepcion, M. Interference with Clp protease impairs carotenoid accumulation during tomato fruit ripening. J. Exp. Bot. 2018, 69, 1557–1568. [Google Scholar] [CrossRef]
- Dong, H.; Fei, G.L.; Wu, C.Y.; Wu, F.Q.; Sun, Y.Y.; Chen, M.J.; Ren, Y.L.; Zhou, K.N.; Cheng, Z.J.; Wang, J.L.; et al. A rice virescent-yellow leaf mutant reveals new insights into the role and assembly of plastid caseinolytic protease in higher plants. Plant Physiol. 2013, 162, 1867–1880. [Google Scholar] [CrossRef]
- Li, W.; Wu, C.; Hu, G.C.; Xing, L.; Qian, W.J.; Si, H.M.; Sun, Z.X.; Wang, X.C.; Fu, Y.P.; Liu, W.Z. Characterization and fine mapping of a novel rice narrow leaf mutant nal9. J. Integr. Plant Biol. 2013, 55, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Islam, M.A.; Cai, K.Y.; Tian, S.X.; Liu, Y.; Kang, Z.S.; Guo, J. TaClpS1, negatively regulates wheat resistance against Puccinia striiformis f. sp. tritici. BMC Plant Biol. 2020, 20, 555. [Google Scholar] [CrossRef] [PubMed]
- Heong, K.L.; Cheng, J.A.; Escalada, M.M. Rice Planthoppers; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Hu, L.F.; Ye, M.; Li, R.; Lou, Y.G. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef]
- Zeng, J.M.; Zhang, T.F.; Huangfu, J.Y.; Li, R.; Lou, Y.G. Both allene oxide synthases genes are involved in the biosynthesis of herbivore-induced jasmonic acid and herbivore resistance in rice. Plants 2021, 10, 442. [Google Scholar] [CrossRef]
- Zhou, G.X.; Qi, J.F.; Ren, N.; Cheng, J.A.; Erb, M.; Mao, B.Z.; Lou, Y.G. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.J.; Zu, H.Y.; Zeng, X.; Baldwin, I.T.; Lou, Y.G.; Li, R. Molecular dissection of rice phytohormone signaling involved in resistance to a piercing-sucking herbivore. New Phytol. 2021, 230, 1639–1652. [Google Scholar] [CrossRef]
- Guo, H.M.; Li, H.C.; Zhou, S.R.; Xue, H.W.; Miao, X.X. Cis-12-oxo-phytodienoic acid stimulates rice defense response to a piercing-sucking insect. Mol. Plant. 2014, 7, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.G.; Du, M.H.; Turlings, T.C.; Cheng, J.A.; Shan, W.F. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J. Chem. Ecol. 2005, 31, 1985–2002. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Hu, X.Y.; Su, S.L.; Liu, Q.S.; Jiao, Y.Y.; Peng, Y.F.; Li, Y.H.; Turlings, T.C. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. eLife 2020, 9, e55421. [Google Scholar] [CrossRef]
- Yang, L.; Li, A.; Zhang, W.L. Current understanding of the molecular players involved in resistance to rice planthoppers. Pest Manag. Sci. 2019, 75, 2566–2574. [Google Scholar]
- Ding, X.; Huang, X.; Sun, L.; Wu, J.; Liu, J. Influence of abscisic acid-biosynthesis inhibitor fluridone on the feeding behavior and fecundity of Nilaparvata lugens. Insects 2019, 10, 57. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.D.; Fang, X.; Chen, X.Y.; Mao, Y.B. Plant specialized metabolism regulated by jasmonate signaling. Plant Cell Physiol. 2019, 60, 2638–2647. [Google Scholar] [CrossRef]
- Luo, C.; Qiu, J.F.; Zhang, Y.; Li, M.Y.; Liu, P. Jasmonates coordinate secondary with primary metabolism. Metabolites 2023, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Dobrogojski, J.; Adamiec, M.; Luciński, R. The chloroplast genome: A review. Acta Physiol. Plant 2020, 42, 98. [Google Scholar] [CrossRef]
- Sjogren, L.L.; Stanne, T.M.; Zheng, B.; Sutinen, S.; Clarke, A.K. Structural and functional insights into the chloroplast ATP-dependent Clp protease in Arabidopsis. Plant Cell 2006, 18, 2635–2649. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.X.; Yang, Y.R.; Cao, P.H.; Cheng, H.; Zhou, H.Y. Plastid caseinolytic protease OsClpR1 regulates chloroplast development and chloroplast RNA editing in rice. Rice 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Maliga, P. The plastid clpP1 protease gene is essential for plant development. Nature 2003, 425, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Zhou, X.J.; Yuan, H.; Álvarez, D.; Sun, T.H.; Schlossarek, D.; Yang, Y.; Shen, G.X.; Zhang, H.; Rodriguez-Concepcion, M. Clp protease and OR directly control the proteostasis of phytoene synthase, the crucial enzyme for carotenoid biosynthesis in Arabidopsis. Mol. Plant. 2018, 11, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Hou, Y.X.; Qiu, J.H.; Wang, H.M.; Wang, S.; Tang, L.Q.; Tong, X.H.; Zhang, J. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice (Oryza sativa). New Phytol. 2020, 228, 1336–1353. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.F.; Zhou, G.X.; Yang, L.; Erb, M.; Lu, Y.; Sun, X.; Cheng, J.A.; Lou, Y.G. The chloroplast-localized phospholipases Da4 and a5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol. 2011, 157, 1987–1999. [Google Scholar] [CrossRef]
- Liu, J.L.; Du, H.T.; Ding, X.; Zhou, Y.D.; Xie, P.F.; Wu, J.C. Mechanisms of callose deposition in rice regulated by exogenous abscisic acid and its involvement in rice resistance to Nilaparvata lugens Stål (Hemiptera: Delphacidae). Pest Manage. Sci. 2017, 73, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, Y.; Portaluri, V.; Woodcock, C.; Pickett, J.A.; Wang, S.S.; Zhou, J.J. Chemical identity and functional characterization of semiochemicals that promote the interactions between rice plant and rice major pest Nilaparvata lugens. J. Agric. Food Chem. 2021, 69, 4635–4644. [Google Scholar] [CrossRef]
- Cock, J.; Yoshida, S.; Forno, D.A. Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Manila, Philippines, 1976. [Google Scholar]
- Ling, Q.H.; Huang, W.H.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lu, J.; Robert, C.A.; Riemann, M.; Cosme, M.; Mene-Saffrane, L.; Massana, J.; Stout, M.J.; Lou, Y.G.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Ye, M.; Kuai, P.; Chen, L.; Lou, Y. Silencing an ATP-Dependent Caseinolytic Protease Proteolytic Subunit Gene Enhances the Resistance of Rice to Nilaparvata lugens. Int. J. Mol. Sci. 2024, 25, 3699. https://doi.org/10.3390/ijms25073699
Chen S, Ye M, Kuai P, Chen L, Lou Y. Silencing an ATP-Dependent Caseinolytic Protease Proteolytic Subunit Gene Enhances the Resistance of Rice to Nilaparvata lugens. International Journal of Molecular Sciences. 2024; 25(7):3699. https://doi.org/10.3390/ijms25073699
Chicago/Turabian StyleChen, Shuting, Miaofen Ye, Peng Kuai, Lin Chen, and Yonggen Lou. 2024. "Silencing an ATP-Dependent Caseinolytic Protease Proteolytic Subunit Gene Enhances the Resistance of Rice to Nilaparvata lugens" International Journal of Molecular Sciences 25, no. 7: 3699. https://doi.org/10.3390/ijms25073699
APA StyleChen, S., Ye, M., Kuai, P., Chen, L., & Lou, Y. (2024). Silencing an ATP-Dependent Caseinolytic Protease Proteolytic Subunit Gene Enhances the Resistance of Rice to Nilaparvata lugens. International Journal of Molecular Sciences, 25(7), 3699. https://doi.org/10.3390/ijms25073699






