Fisetin Alleviates Inflammation and Oxidative Stress in Deep Vein Thrombosis via MAPK and NRF2 Signaling Pathway
Abstract
1. Introduction
2. Results
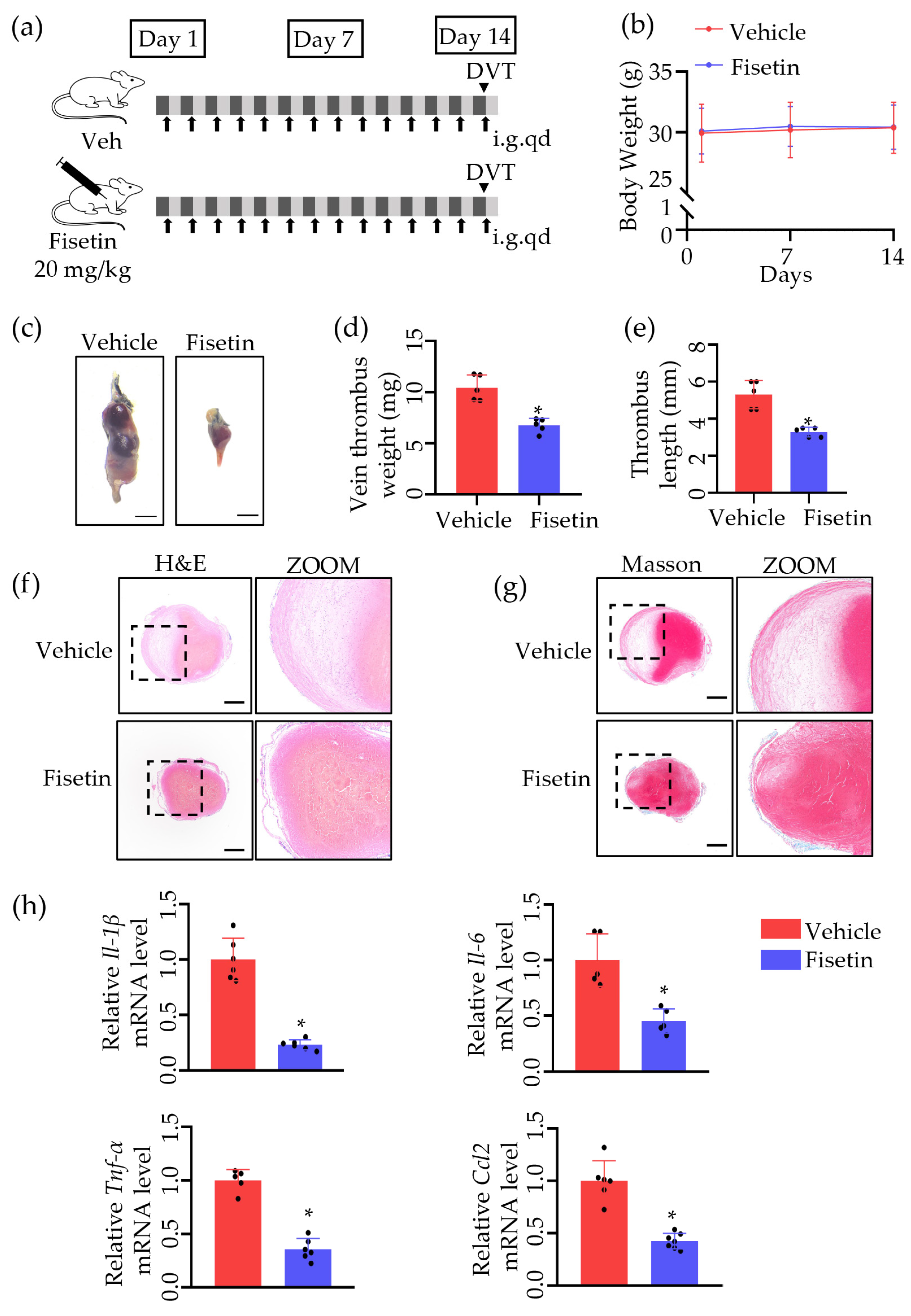
2.1. Fisetin Protected against Venous Thrombosis following IVC Ligation
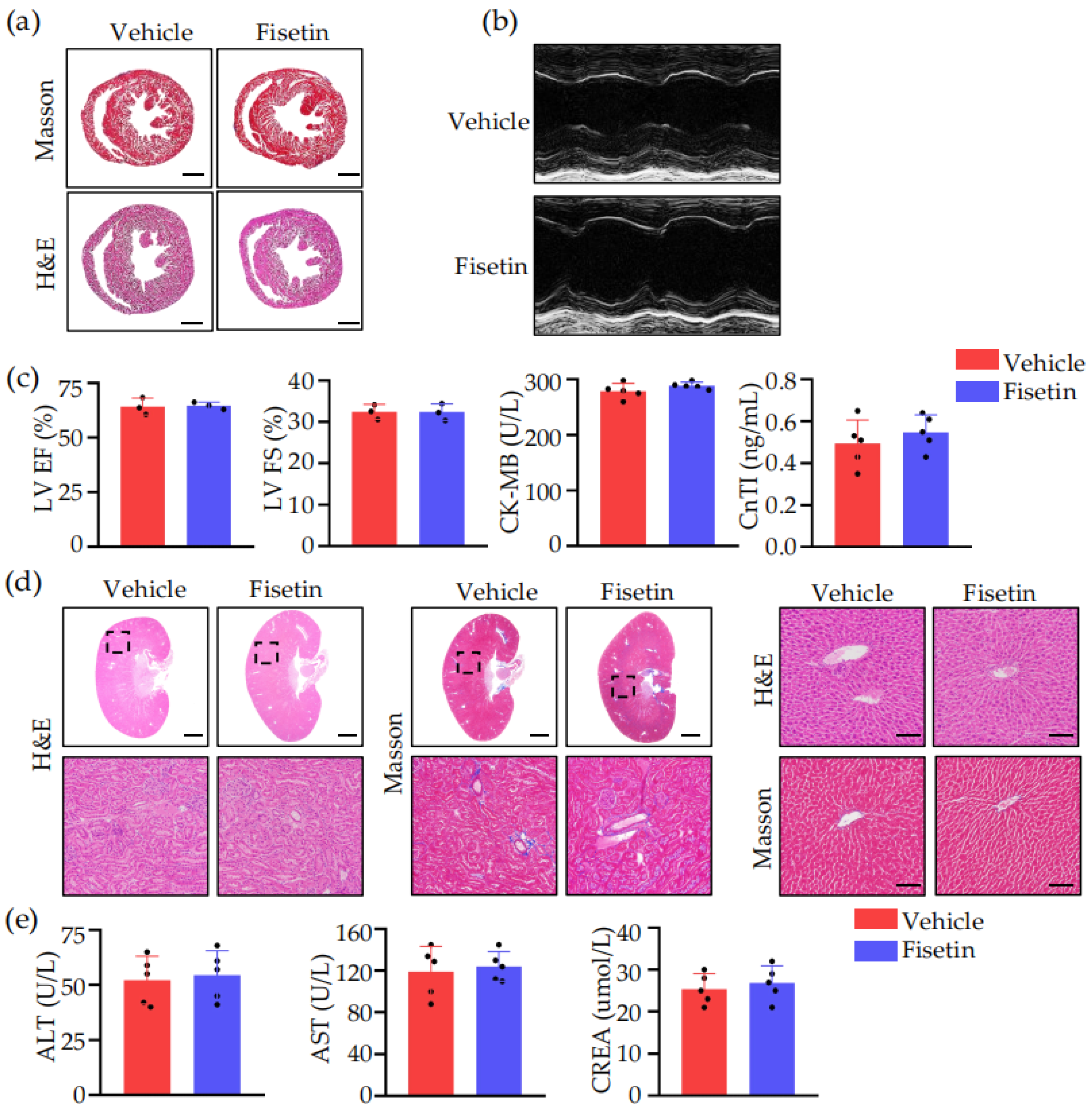
2.2. Fisetin Prevented DVT without Heart, Liver, and Kidney Toxicity
2.3. Fisetin Played a Role in Anti-Inflammation and Leukocytes Adhesion Inhibition in DVT Mice Model
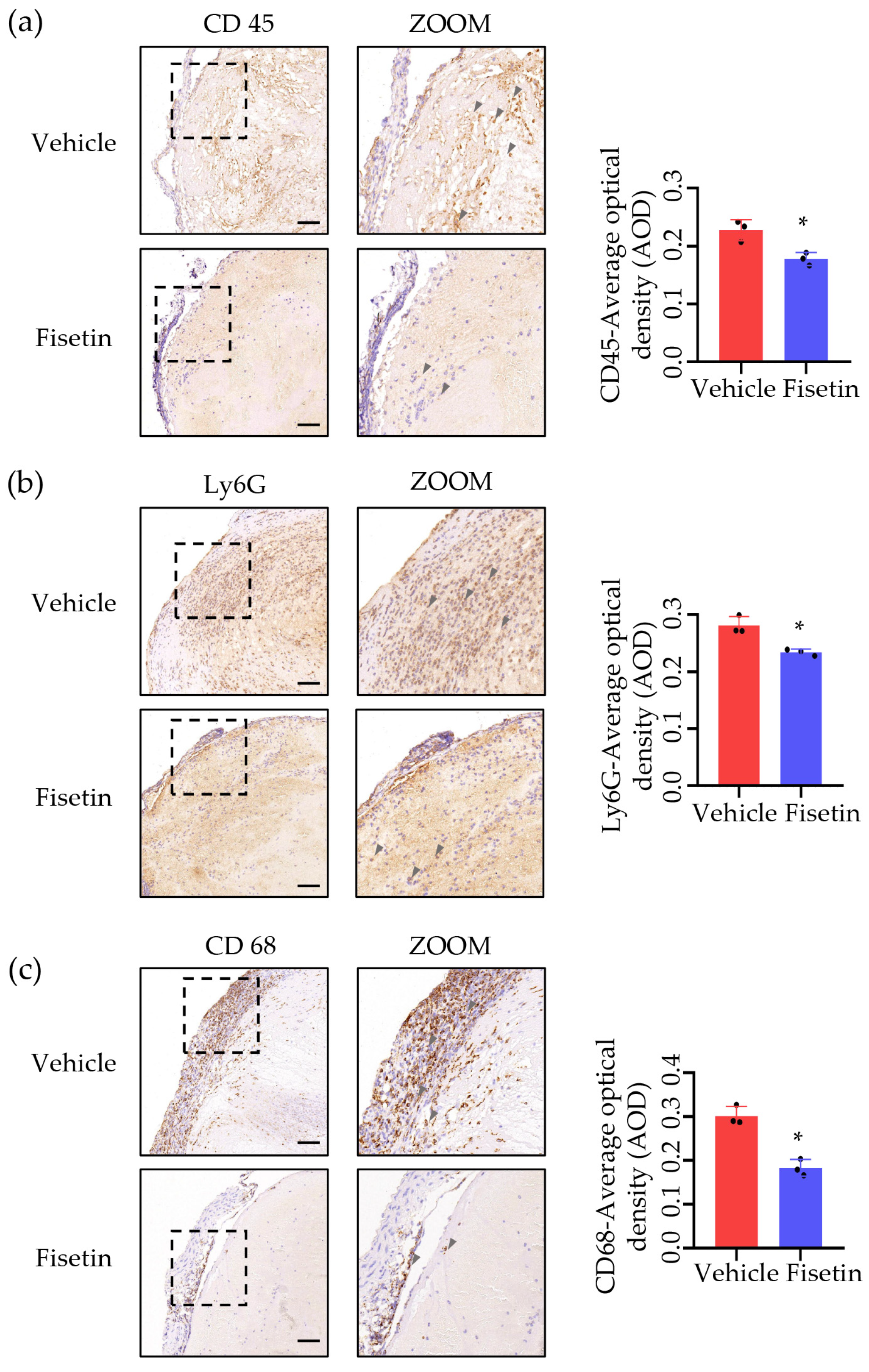
2.4. Fisetin Suppressed Leukocytes Accumulation in DVT Mice Model
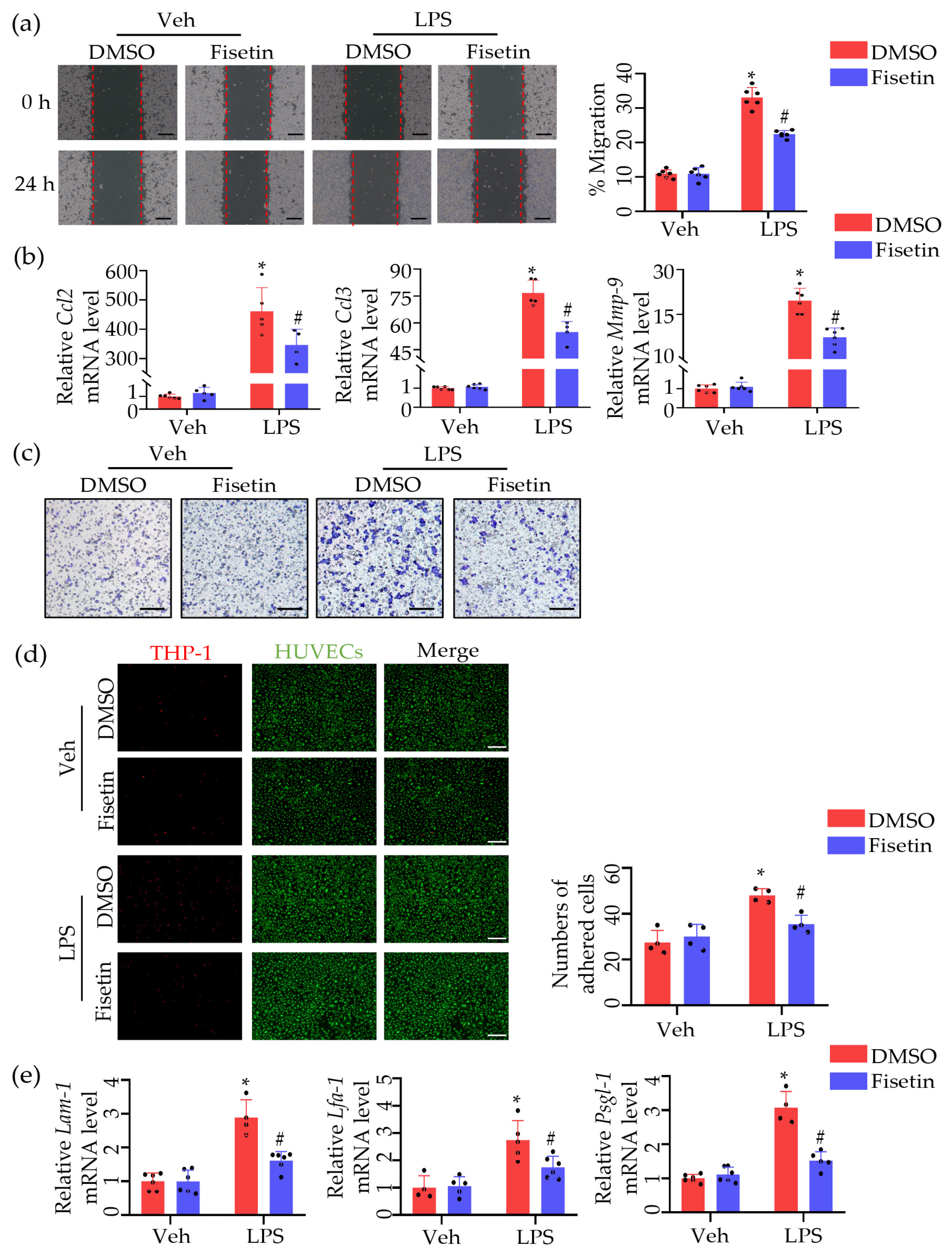
2.5. Fisetin Attenuated Leukocytes Migration and Adhesion
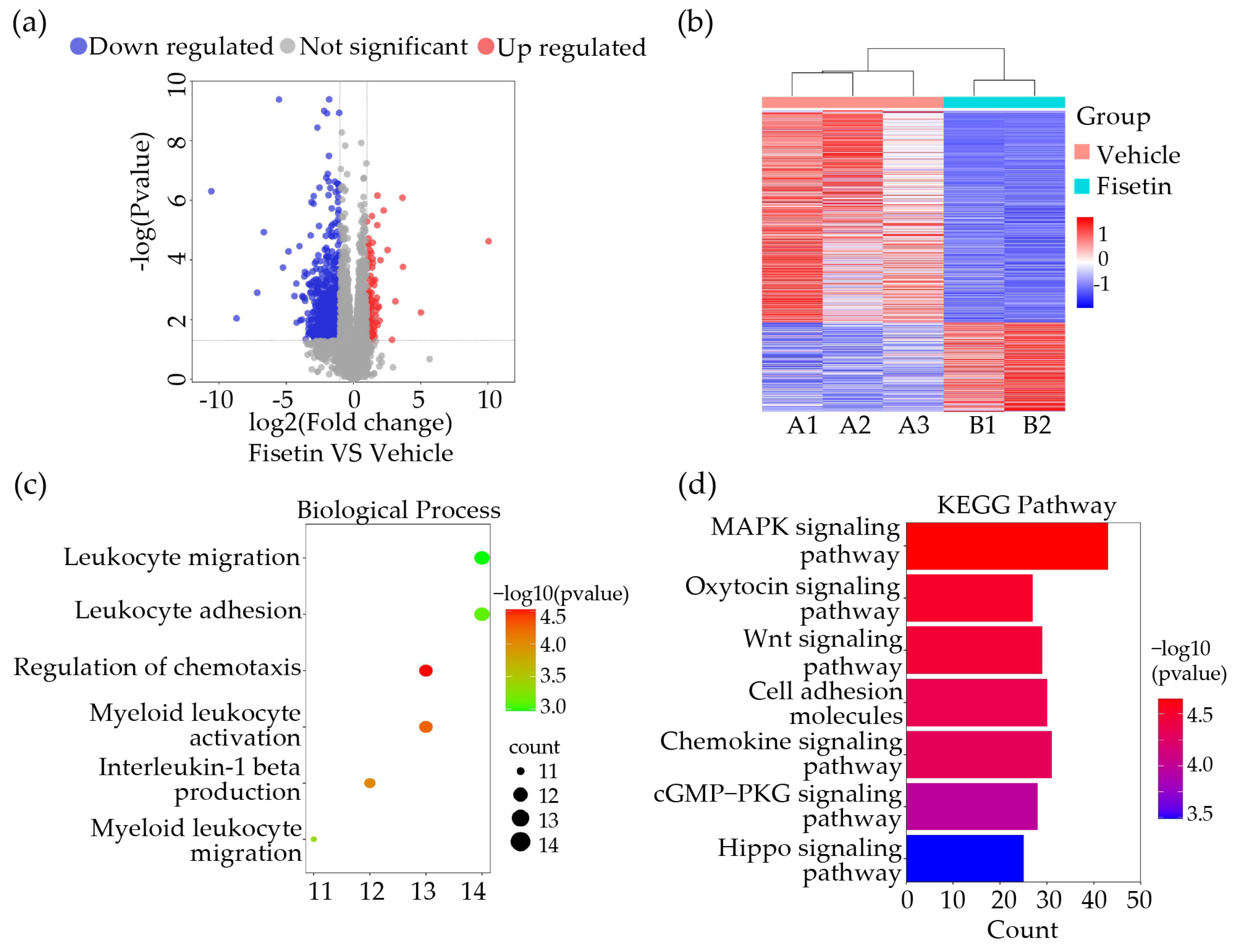
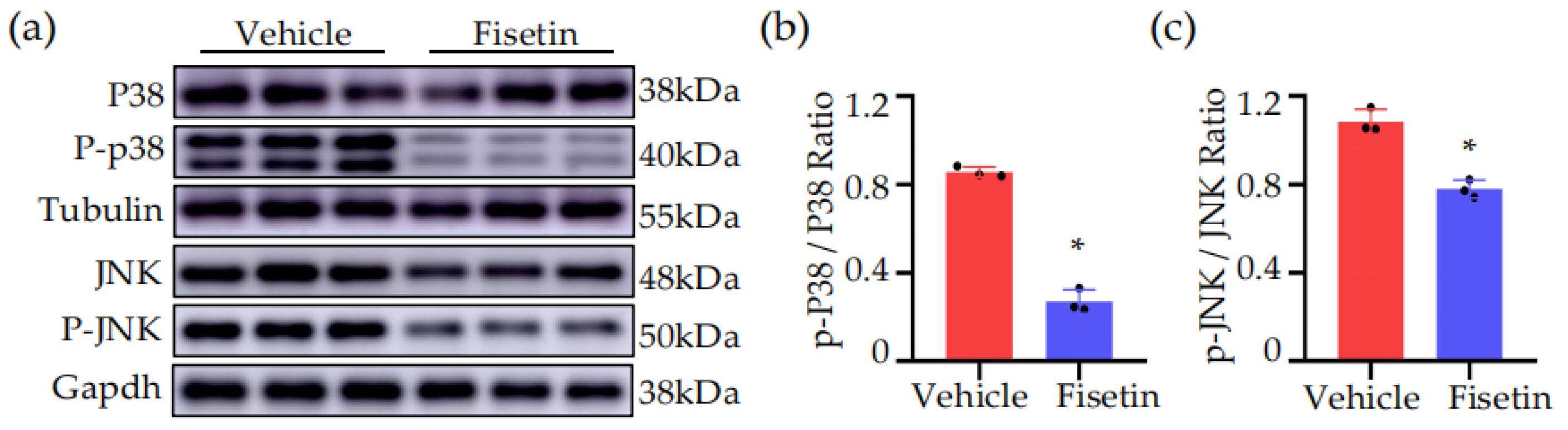
2.6. Fisetin Inhibited MAPK Signaling Pathway in DVT Mice Model
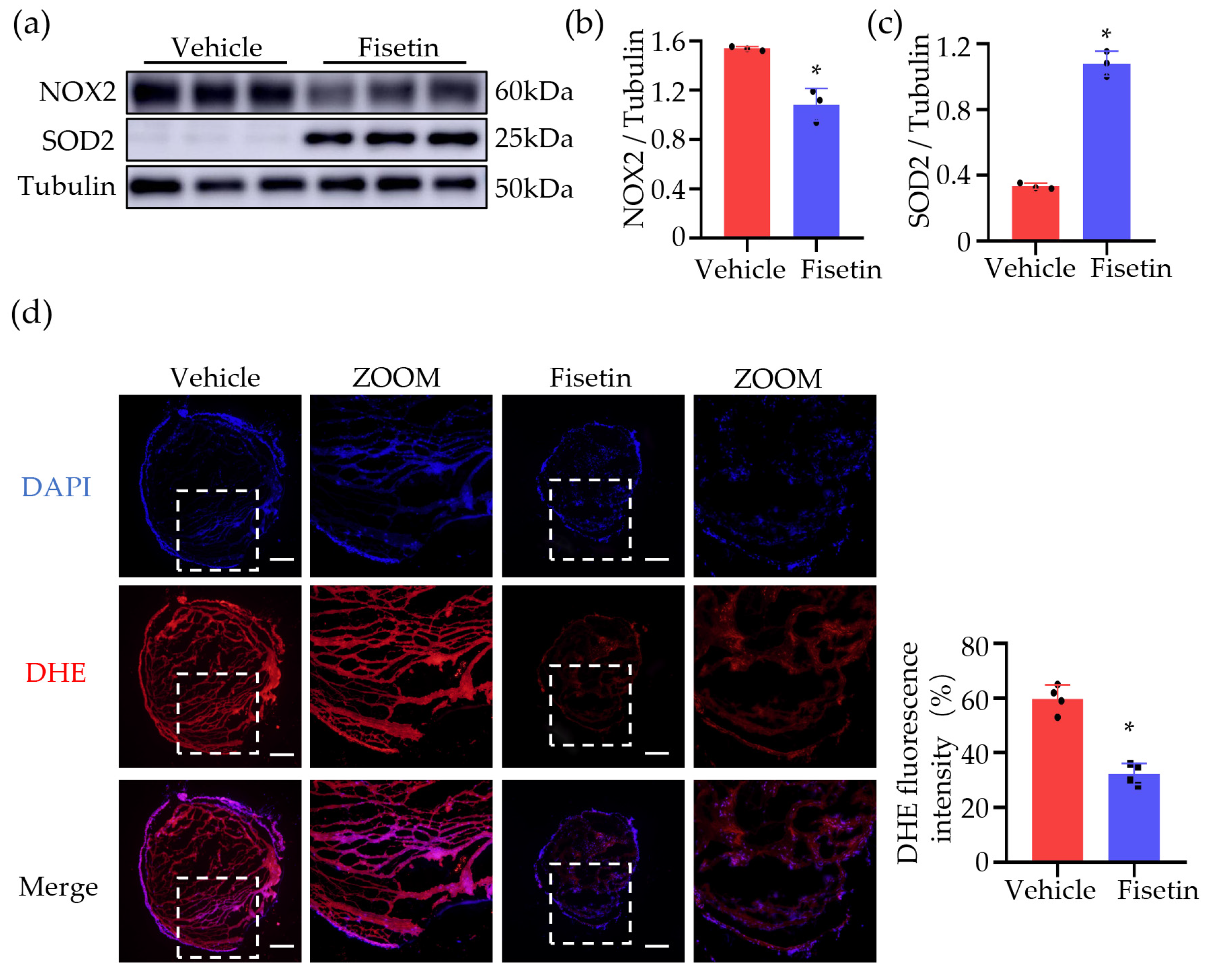
2.7. Fisetin Reduced ROS in DVT Mouse Model
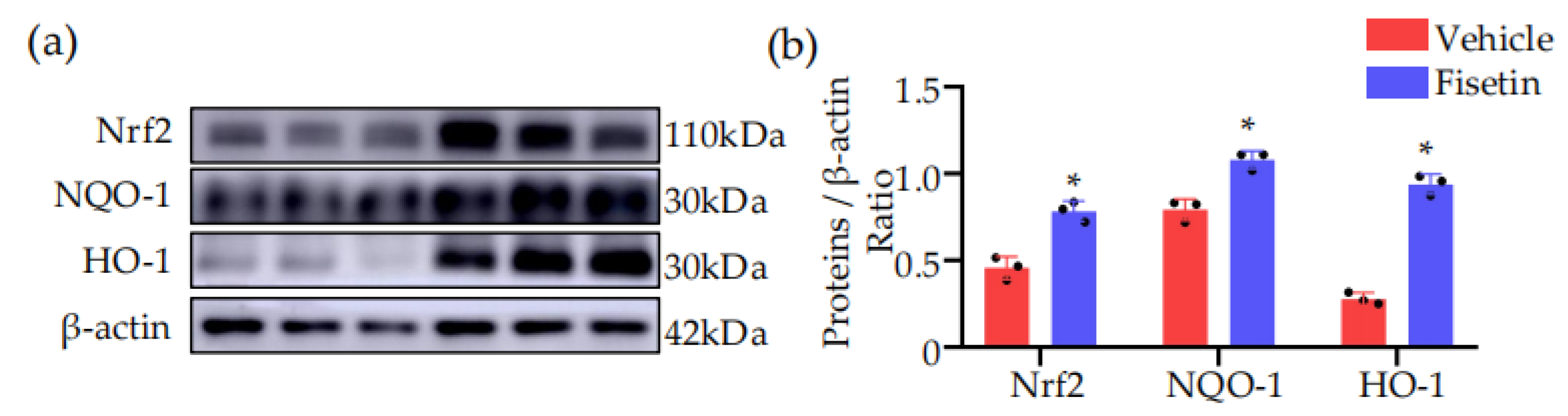
2.8. Fisetin Activated NRF2-Mediated Antioxidant Pathway in DVT Mouse Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice
4.3. Deep Vein Thrombosis (DVT) Surgery in Mice
4.4. Experimental Groups
4.5. Hematoxylin and Eosin (HE) and Masson Staining
4.6. Cell Culture
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Immunohistochemistry
4.9. Western Blot
4.10. THP-1 Monocytes Adhesion Assays
4.11. Transwell Migration Assays
4.12. Dihydroethidium Staining
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pai, R.Z.; Fang, Q.; Tian, G.; Zhu, B.; Ge, X. Expression and role of interleukin-1β and associated biomarkers in deep vein thrombosis. Exp. Ther. Med. 2021, 22, 1366. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.J.; Roy, T.L. Catheter-Directed Interventions for the Treatment of Lower Extremity Deep Vein Thrombosis. Life 2022, 12, 1984. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Deng, M.; Nie, W.; Zou, D.; Wu, H.; Xu, D. Naringenin Inhibits Platelet Activation and Arterial Thrombosis Through Inhibition of Phosphoinositide 3-Kinase and Cyclic Nucleotide Signaling. Front. Pharmacol. 2021, 12, 722257. [Google Scholar] [CrossRef] [PubMed]
- Brouns, S.L.N.; van Geffen, J.P.; Campello, E.; Swieringa, F.; Spiezia, L.; van Oerle, R.; Provenzale, I.; Verdoold, R.; Farndale, R.W.; Clemetson, K.J.; et al. Platelet-primed interactions of coagulation and anticoagulation pathways in flow-dependent thrombus formation. Sci. Rep. 2020, 10, 11910. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Lee, S.; Oh, J.-M.; Lee, M.-S.; Yoon, K.-H.; Park, B.H.; Kim, J.W.; Song, H.; Kim, S.-H. Anti-inflammatory activity of fisetin in human mast cells (HMC-1). Pharmacol. Res. 2007, 55, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, W.; Feng, X.; Yang, F.; Qin, H.; Wu, S.; Hou, D.-X.; Chen, J. Nrf2–ARE Signaling Acts as Master Pathway for the Cellular Antioxidant Activity of Fisetin. Molecules 2019, 24, 708. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Piani, F.; Crescimanno, C.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Modulation of NRF2/KEAP1 Signaling in Preeclampsia. Cells 2023, 12, 1545. [Google Scholar] [CrossRef]
- Xia, L.; Ma, W.; Afrashteh, A.; Sajadi, M.A.; Fakheri, H.; Valilo, M. The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer. Biochem. Med. 2023, 33, 030504. [Google Scholar] [CrossRef]
- Shi, W.; Shi, Y.; Peng, Y.; Gu, J. Circle or semi–circle hyper–intensity on T1 high–resolution isovolumetric examination (THRIVE) indicates the young age of experimentally induced caval thrombus. J. Thromb. Thrombolysis 2021, 52, 628–634. [Google Scholar] [CrossRef]
- Gutmann, C.; Siow, R.; Gwozdz, A.M.; Saha, P.; Smith, A. Reactive Oxygen Species in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 1918. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Li, Y.; Shan, J. Study on the correlation between high density lipoprotein and lower extremities deep venous thrombosis in patients undergoing hip arthroplasty. Phlebol. J. Venous Dis. 2022, 37, 516–521. [Google Scholar] [CrossRef]
- Yuriditsky, E.; Narula, N.; Jacobowitz, G.R.; Moreira, A.L.; Maldonado, T.S.; Horowitz, J.M.; Sadek, M.; Barfield, M.E.; Rockman, C.B.; Garg, K. Histologic assessment of lower extremity deep vein thrombus from patients undergoing percutaneous mechanical thrombectomy. J. Vasc. Surg.: Venous Lymphat. Disord. 2022, 10, 18–25. [Google Scholar] [CrossRef]
- Fuentes, E.; Gibbins, J.M.; Holbrook, L.M.; Palomo, I. NADPH oxidase 2 (NOX2): A key target of oxidative stress-mediated platelet activation and thrombosis. Trends Cardiovasc. Med. 2018, 28, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Pan, L.; Chen, L.; Wang, Y.; Liu, X.; You, L.; Jia, Y.; Hu, C. Protective effect of 7,3’,4’-flavon-3-ol (fisetin) on acetaminophen-induced hepatotoxicity in vitro and in vivo. Phytomedicine 2019, 58, 152865. [Google Scholar] [CrossRef]
- Ehren, J.L.; Maher, P. Concurrent regulation of the transcription factors NRF2 and ATF4 mediates the enhancement of glutathione levels by the flavonoid fisetin. Biochem. Pharmacol. 2013, 85, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Kumar, J.M.; Sistla, R. Fisetin, a dietary flavonoid, ameliorates experimental colitis in mice: Relevance of NF-κB signaling. J. Nutr. Biochem. 2016, 28, 171–182. [Google Scholar] [CrossRef]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic Downregulation of Nrf2 Activity via ERK Contributes to Oxidative Stress–Induced Insulin Resistance in Cardiac Cells In Vitro and In Vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Ko, W.C.; Shieh, J.M.; Wu, W.B. P38 MAPK and NRF2 Activation Mediated Naked Gold Nanoparticle Induced Heme Oxygenase-1 Expression in Rat Aortic Vascular Smooth Muscle Cells. Arch. Med. Res. 2020, 51, 388–396. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, F.; Zheng, H.; Whitman, S.A.; Lin, Y.; Zhang, Z.; Zhang, N.; Zhang, D.D. NRF2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-κB-mediated inflammatory response. Kidney Int. 2014, 85, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, S.K.; Farris, D.M.; Diaz, J.A.; Myers, D.D., Jr.; Wakefield, T.W. Mouse complete stasis model of inferior vena cava thrombosis. J. Vis. Exp. 2011, 15, 2783. [Google Scholar] [CrossRef]
- Ahmad, A.; Ali, T.; Rehman, S.U.; Kim, M.O. Phytomedicine-Based Potent Antioxidant, Fisetin Protects CNS-Insult LPS-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment. J. Clin. Med. 2019, 8, 850. [Google Scholar] [CrossRef] [PubMed]
- Yalniz, Y.; Yunusoğlu, O.; Berköz, M.; Demirel, M.E. Effects of fisetin on ethanol-induced rewarding properties in mice. Am. J. Drug Alcohol Abus. 2024, 50, 75–83. [Google Scholar] [CrossRef]
- Bertin, F.; Rys, R.N.; Mathieu, C.; Laurance, S.; Lemarié, C.A.; Blostein, M.D. Natural killer cells induce neutrophil extracellular trap formation in venous thrombosis. J. Thromb. Haemost. 2019, 17, 403–414. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| Gapdh-F | CTGAACGGGAAGCTCACTG |
| Gapdh-R | CATACTTGGCAGGTTTCTCCAG |
| β-actin-F | CGGTTCCGATGCCCTGAGGCTCTT |
| β-actin-R | CGTCACACTTCATGATGGAATTGA |
| Tnf-α-F | ATCGGTCCCCAAAGGGATGA |
| Tnf-α-R | GGTGGTTTGCTACGACGTG |
| Il-1β-F | GAAATGCCACCTTTTGACAGTG |
| Il-1β-R | TGGATGCTCTCATCAGGACAG |
| Il-6-F | TCTATACCACTTCACAAGTCGGA |
| Il-6-R | GAATTGCCATTGCACAACTCTTT |
| Ccl2-F | GCATCCACGTGTTGGCTC |
| Ccl2-R | CTCCAGCCTACTCATTGGGATCA |
| Psgl-1-F | AGGAGATAAGATGGCTGGTGC |
| Psgl-1-R | GCTTTCTCGGCTTCATCTGC |
| Lam-1-F | CCTCTGTTACACAGCTTCTTGC |
| Lam-1-R | GTACCCCACATCACAGTTGC |
| Lfa-1-F | TGTCTCGAACGTGTGACCAG |
| Lfa-1-R | CGTTGCCCTTGATACATTCCTG |
| Ccl3-F | TCCCAGCCAGGTGTCATTTTCC |
| Ccl3-R | TCAGGCATTCAGTTCCAGGTCA |
| Mmp-9-F | TCCTGGCTCTCCTGGCTTTC |
| Mmp-9-R | TAGCGGTACAAGTATGCCTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Lu, Q. Fisetin Alleviates Inflammation and Oxidative Stress in Deep Vein Thrombosis via MAPK and NRF2 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 3724. https://doi.org/10.3390/ijms25073724
Liu H, Lu Q. Fisetin Alleviates Inflammation and Oxidative Stress in Deep Vein Thrombosis via MAPK and NRF2 Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(7):3724. https://doi.org/10.3390/ijms25073724
Chicago/Turabian StyleLiu, Hao, and Qiulun Lu. 2024. "Fisetin Alleviates Inflammation and Oxidative Stress in Deep Vein Thrombosis via MAPK and NRF2 Signaling Pathway" International Journal of Molecular Sciences 25, no. 7: 3724. https://doi.org/10.3390/ijms25073724
APA StyleLiu, H., & Lu, Q. (2024). Fisetin Alleviates Inflammation and Oxidative Stress in Deep Vein Thrombosis via MAPK and NRF2 Signaling Pathway. International Journal of Molecular Sciences, 25(7), 3724. https://doi.org/10.3390/ijms25073724





