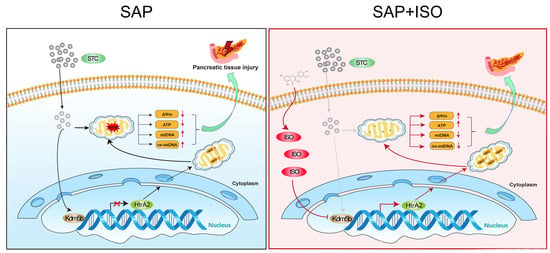
Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway
Abstract
:1. Introduction
2. Results
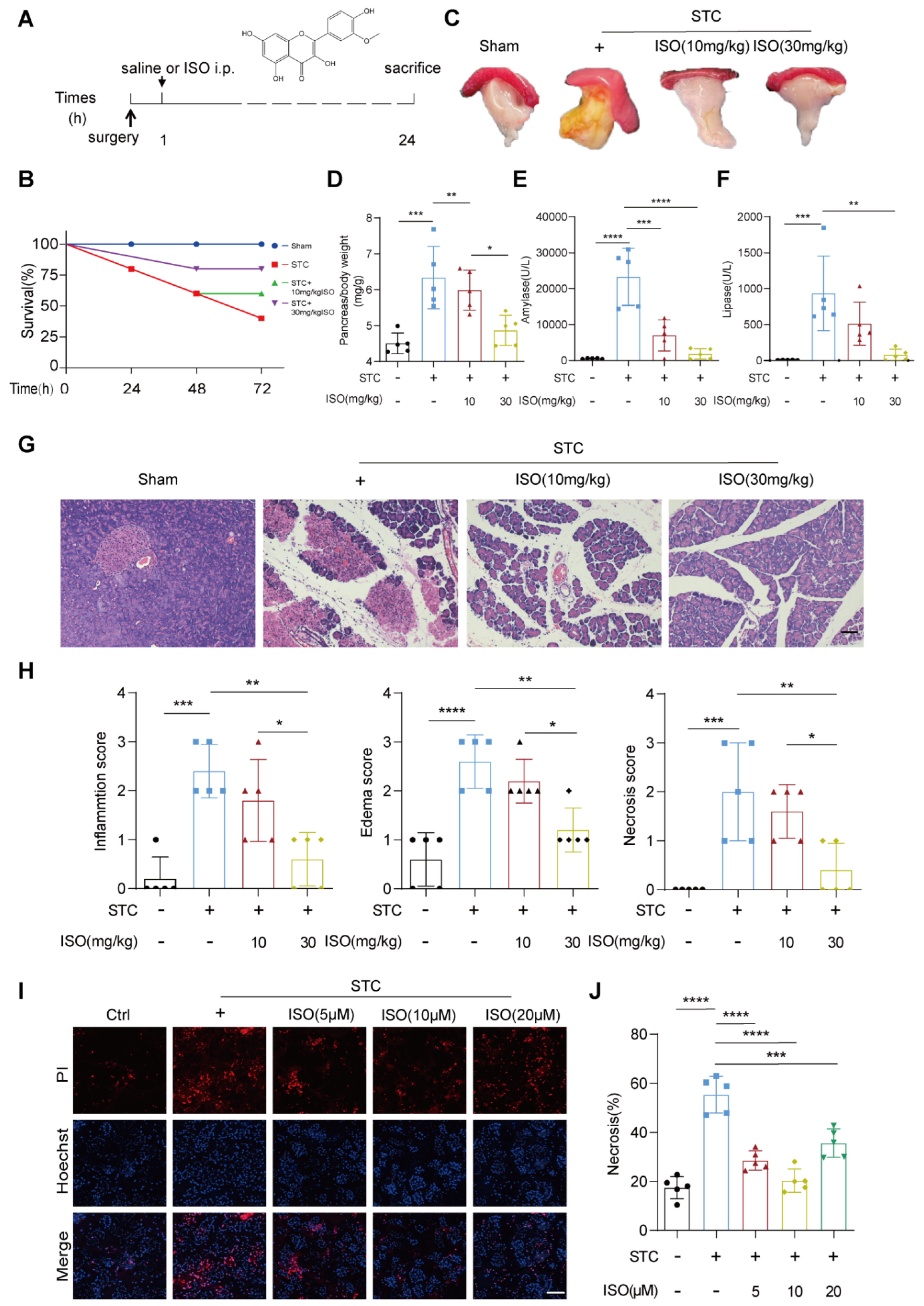
2.1. ISO Treatment Mitigates STC and L-Arginine-Induced Pancreatic Injury
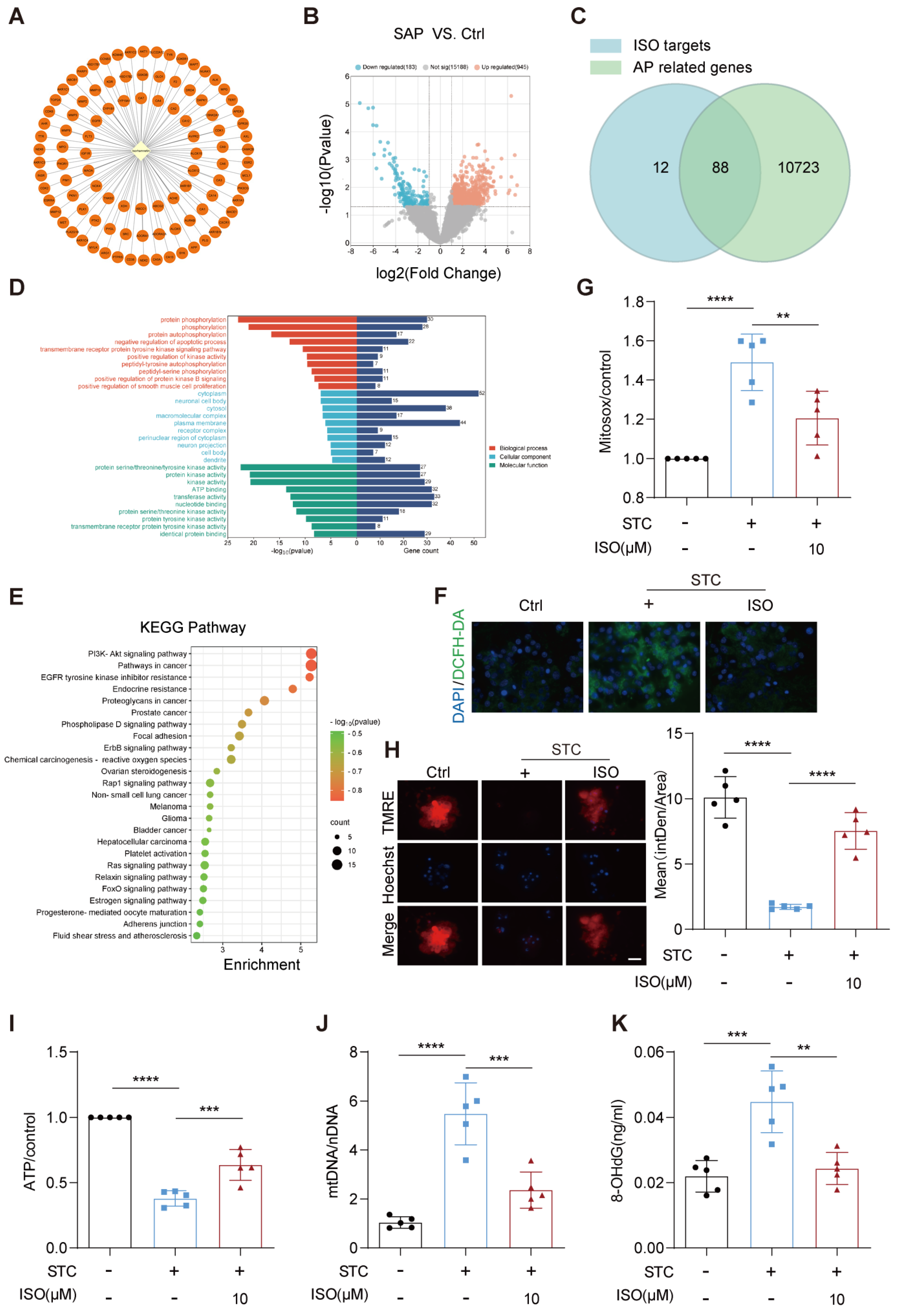
2.2. ISO Administration Improves ROS Generation and Mitochondrial Dysfunction in STC-Treated Primary Pancreatic Acinar Cells
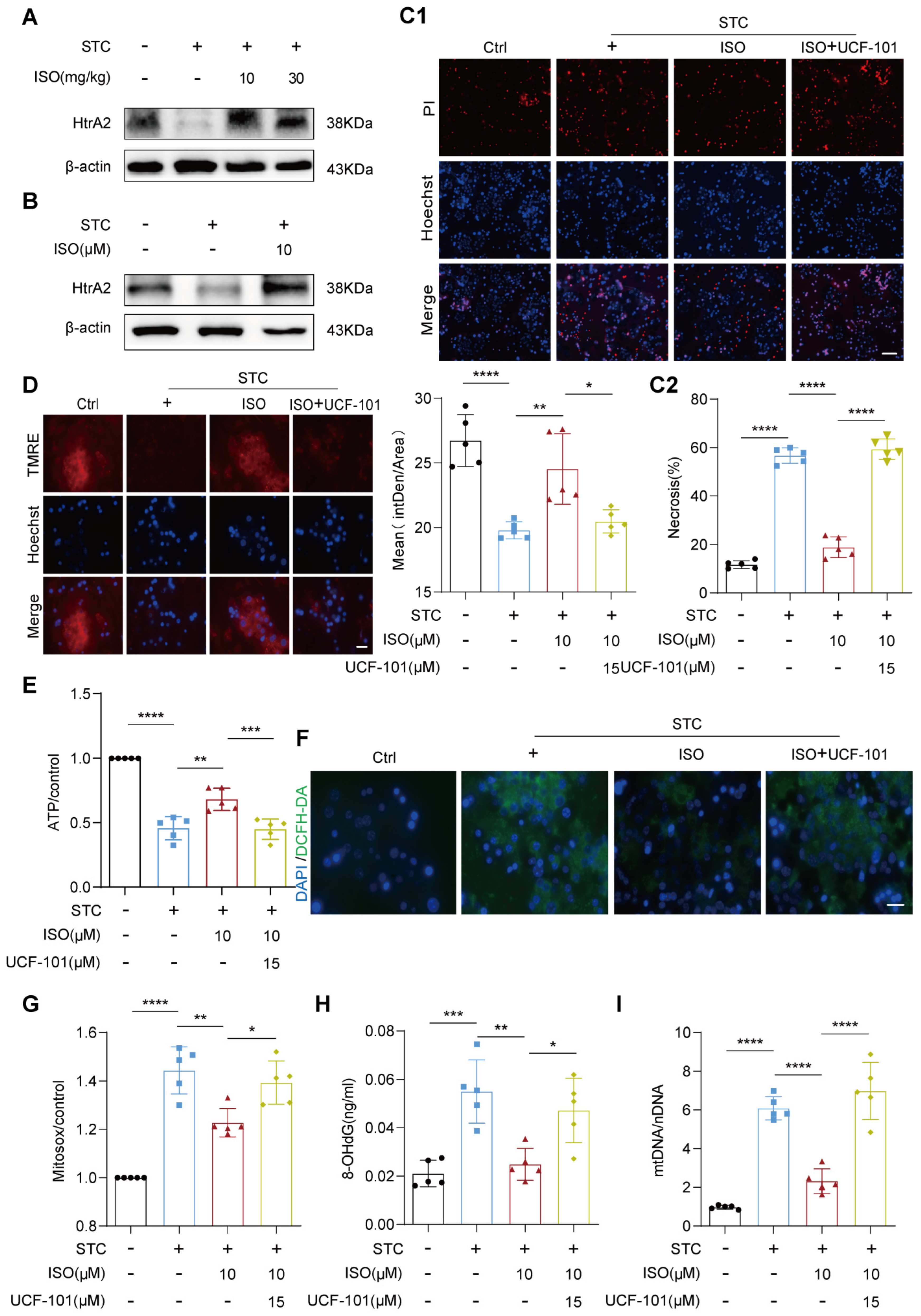
2.3. HtrA2 Is Essential for ISO’s Protective Effect on STC-Induced Mitochondrial Dysfunction
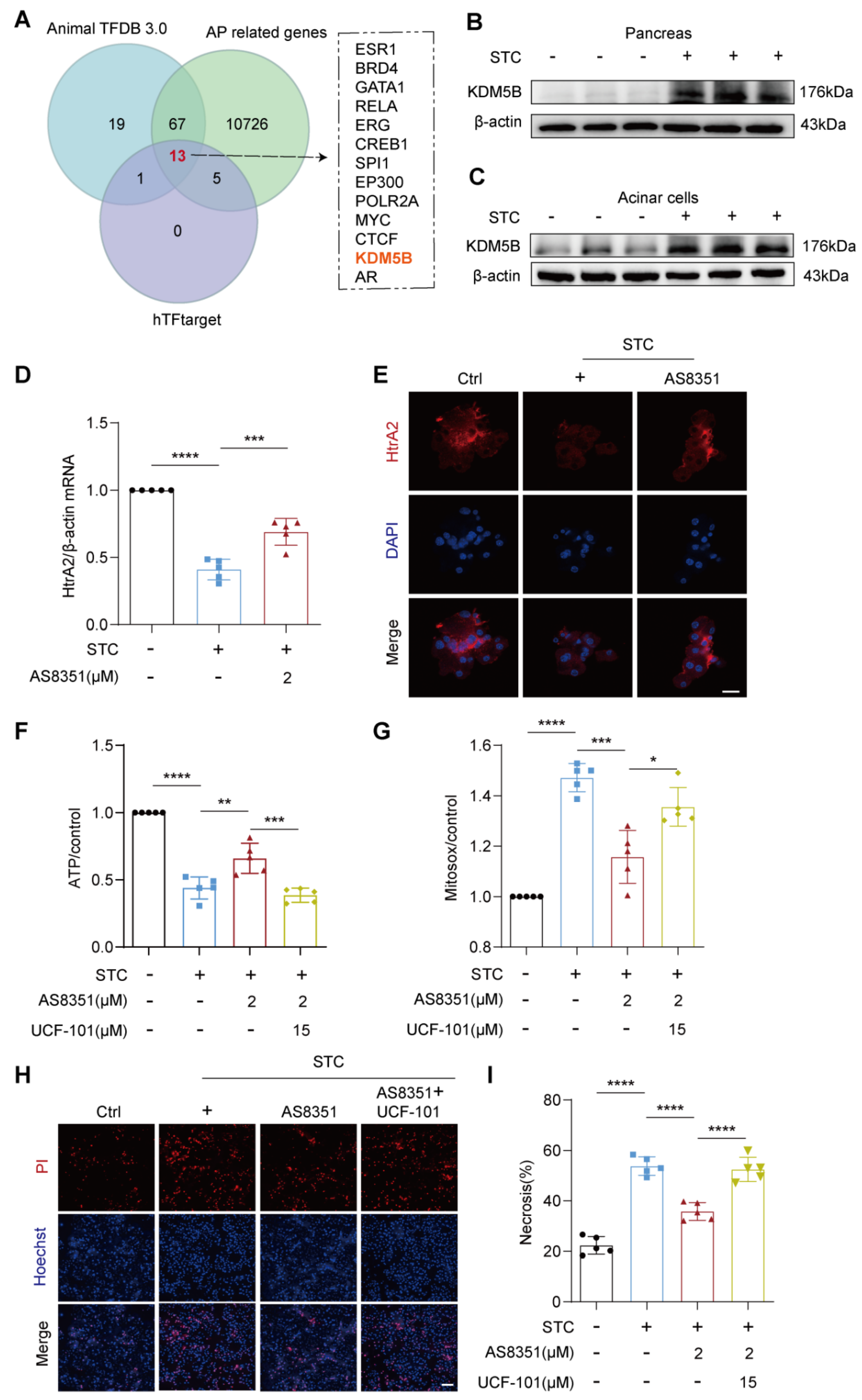
2.4. KDM5B Regulates HtrA2 Protein Expression in Pancreatic Acinar Cells during SAP
2.5. ISO Functions as a Potential Inhibitor for KDM5B
3. Discussion
4. Methods and Materials
4.1. Experimental Animals
4.2. SAP Model Establishment and Experimental Design
4.3. Primary Pancreatic Acinar Cells Isolation
4.4. Histological Examination
4.5. Serum Amylase and Lipase Measurement
4.6. Propidium Iodide (PI) Staining
4.7. Potential ISO Targets Prediction
4.8. Targeted Pathway Analysis
4.9. Intracellular ROS Examination
4.10. Mitochondrial Superoxide Assessment
4.11. Cellular Fractionation and Cytosolic mtDNA Measurement
4.12. RT-qPCR Analysis
4.13. 8-OHdG Production Measurement
4.14. Western Blotting Analysis
4.15. Immunofluorescence Staining
4.16. Molecular Docking
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iannuzzi, J.P.; King, J.A.; Leong, J.H.; Quan, J.; Windsor, J.W.; Tanyingoh, D.; Coward, S.; Forbes, N.; Heitman, S.J.; Shaheen, A.A.; et al. Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis. Gastroenterology 2022, 162, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Lazzarin, G.; Romano, L.; Coletti, G.; Di Sibio, A.; Vicentini, V.; Fatayer, M.W.A.; Schietroma, M.; Pessia, B.; Leone, M.; Carlei, F.; et al. Branch Duct—IPMN and PanIN, in IgG4-Autoimmune pancreatitis: A case report. Clin. Case Rep. 2020, 8, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Singh, V.P. Organ failure due to systemic injury in acute pancreatitis. Gastroenterology 2019, 156, 2008–2023. [Google Scholar] [CrossRef] [PubMed]
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Mederos, M.A.; Reber, H.A.; Girgis, M.D. Acute Pancreatitis: A Review. JAMA 2021, 325, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, O.V.; Gerasimenko, J.V. Mitochondrial function and malfunction in the pathophysiology of pancreatitis. Pflugers Arch. 2012, 464, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Maléth, J.; Hegyi, P. Ca2+ toxicity and mitochondrial damage in acute pancreatitis: Translational overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150425. [Google Scholar] [CrossRef] [PubMed]
- Pandol, S.J.; Gottlieb, R.A. Calcium, mitochondria and the initiation of acute pancreatitis. Pancreatology 2022, 22, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Gukovskaya, A.S.; Pandol, S.J. Acute pancreatitis: A multifaceted set of organelle and cellular interactions. Gastroenterology 2019, 156, 1941–1950. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Y.; Li, S.; Dong, Y.; Wan, C.; Yu, X.; Xin, G.; Wei, Z.; Li, F.; Wang, Y.; et al. Deoxyarbutin attenuates severe acute pancreatitis via the htra2/pgc-1α pathway. Free Radic. Res. 2022, 56, 651–665. [Google Scholar] [CrossRef]
- Piplani, H.; Marek-Iannucci, S.; Sin, J.; Hou, J.; Takahashi, T.; Sharma, A.; de Freitas Germano, J.; Waldron, R.T.; Saadaeijahromi, H.; Song, Y.; et al. Simvastatin induces autophagic flux to restore cerulein-impaired phagosome-lysosome fusion in acute pancreatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165530. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, K.M.; Morais, T.C.; de Melo, T.S.; de Castro Brito, G.A.; de Andrade, G.M.; Rao, V.S.; Santos, F.A. The natural flavonoid quercetin ameliorates cerulein-induced acute pancreatitis in mice. Biol. Pharm. Bull. 2010, 33, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Sheng, B.; Zhao, L.; Zang, X.; Zhen, J.; Liu, Y.; Bian, W.; Chen, W. Quercetin inhibits caerulein-induced acute pancreatitis through regulating mir-216b by targeting map2k6 and neat1. Inflammopharmacology 2021, 29, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, H.; Liu, Z.; Liu, D.; Yin, D.; He, M. Protective effects of isorhamnetin on cardiomyocytes against anoxia/reoxygenation-induced injury is mediated by sirt1. J. Cardiovasc. Pharmacol. 2016, 67, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, Y. Effects of isorhamnetin on adipocyte mitochondrial biogenesis and ampk activation. Molecules 2018, 23, 1853. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, P.; Madácsy, T.; Varga, Á.; Maléth, J. Intracellular Ca2+ signalling in the pathogenesis of acute pancreatitis: Recent Advances and Translational Perspectives. Int. J. Mol. Sci. 2020, 21, 4005. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, D.; Wu, Y.; Wei, X.; Wang, Z.; Wang, W.; Zhang, S.; Yang, H.; Yi, M.; Liu, H. P53 mediated transcription of omi/htra2 in aging myocardium. Biochem. Biophys. Res. Commun. 2019, 519, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, L.; Wu, Y.; Wei, X.; Wang, W.; Zhang, S.; Yi, M.; Li, J.; Liu, H.; Ma, X. Heat shock factor 1-mediated transcription activation of omi/htra2 induces myocardial mitochondrial apoptosis in the aging heart. Aging 2019, 11, 8982–8997. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Liang, Q.Q.; Zhang, H.R.; Chen, S.Y.; Xu, L.H.; Zeng, B.; Xu, R.; Shi, F.L.; Ouyang, D.Y.; Zha, Q.B.; et al. Baicalin inhibits necroptosis by decreasing oligomerization of phosphorylated mlkl and mitigates caerulein-induced acute pancreatitis in mice. Int. Immunopharmacol. 2022, 108, 108885. [Google Scholar] [CrossRef]
- Liang, X.; Hu, C.; Liu, C.; Yu, K.; Zhang, J.; Jia, Y. Dihydrokaempferol (dhk) ameliorates severe acute pancreatitis (sap) via keap1/nrf2 pathway. Life Sci. 2020, 261, 118340. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.D.; Liu, Y.Y.; Li, D.J.; Yang, S.J.; Wang, Q.F.; Liu, Y.N.; Li, M.K.; Mei, C.P.; Cui, H.N.; Chen, S.Y.; et al. Galangin ameliorates severe acute pancreatitis in mice by activating the nuclear factor e2-related factor 2/heme oxygenase 1 pathway. Biomed. Pharmacother. 2021, 144, 112293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, H.; Huang, C.; Fan, J.; Mei, Q.; Lu, Y.; Lou, L.; Wang, X.; Zeng, Y. Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through tlr4/myd88/p38 mapk and ers inhibition. Pancreatology 2018, 18, 742–752. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, J.; Chen, J.; Liu, J.; Lu, Y.; Huang, C.; Hu, G.; Wang, X.; Zeng, Y. Therapeutic effects of quercetin on early inflammation in hypertriglyceridemia-related acute pancreatitis and its mechanism. Pancreatology 2016, 16, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Okamoto, T. Safety of quercetin for clinical application (review). Int. J. Mol. Med. 2005, 16, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, K.; Nelson, A.L.; Nishimura, H.; Rutledge, J.C.; Ravuri, S.K.; Bahney, C.; Philippon, M.J.; Huard, J. Therapeutic potential of senolytic agent quercetin in osteoarthritis: A systematic review and meta-analysis of preclinical studies. Ageing Res. Rev. 2023, 90, 101989. [Google Scholar] [CrossRef] [PubMed]
- Vringer, E.; Tait, S.W.G. Mitochondria and cell death-associated inflammation. Cell Death Differ. 2023, 30, 304–312. [Google Scholar] [CrossRef]
- Odinokova, I.V.; Sung, K.F.; Mareninova, O.A.; Hermann, K.; Evtodienko, Y.; Andreyev, A.; Gukovsky, I.; Gukovskaya, A.S. Mechanisms regulating cytochrome c release in pancreatic mitochondria. Gut 2009, 58, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, M.; Wang, X.; Bu, Q.; Wang, Q.; Su, W.; Li, L.; Zhou, H.; Lu, L. Xbp1 deficiency promotes hepatocyte pyroptosis by impairing mitophagy to activate mtdna-cgas-sting signaling in macrophages during acute liver injury. Redox Biol. 2022, 52, 102305. [Google Scholar] [CrossRef]
- Bruce, J.I.E.; Sánchez-Alvarez, R.; Sans, M.D.; Sugden, S.A.; Qi, N.; James, A.D.; Williams, J.A. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat. Commun. 2021, 12, 4386. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Jin, C.Y.; Kim, C.H.; Yoo, Y.H.; Choi, S.H.; Kim, G.Y.; Yoon, H.M.; Park, H.T.; Choi, Y.H. Isorhamnetin alleviates lipopolysaccharide-induced inflammatory responses in bv2 microglia by inactivating nf-κb, blocking the tlr4 pathway and reducing ros generation. Int. J. Mol. Med. 2019, 43, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, J.V.; Gryshchenko, O.; Ferdek, P.E.; Stapleton, E.; Hébert, T.O.; Bychkova, S.; Peng, S.; Begg, M.; Gerasimenko, O.V.; Petersen, O.H. Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl. Acad. Sci. USA 2013, 110, 13186–13191. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xu, H.; Wang, X.; Zhu, H.; Ge, J.; Ren, J.; Zhang, Y. Fundc1 protects against doxorubicin-induced cardiomyocyte panoptosis through stabilizing mtdna via interaction with tufm. Cell Death Dis. 2022, 13, 1020. [Google Scholar] [CrossRef]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA fragments exit mitochondria via mptp- and vdac-dependent channels to activate nlrp3 inflammasome and interferon signaling. Immunity 2022, 55, 1370–1385.e1378. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, T.; Zhou, Q.; Li, F.; Liu, T.; Zhang, K.; Wen, A.; Feng, L.; Shu, X.; Tian, S.; et al. Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 3784. https://doi.org/10.3390/ijms25073784
Li X, Wang T, Zhou Q, Li F, Liu T, Zhang K, Wen A, Feng L, Shu X, Tian S, et al. Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway. International Journal of Molecular Sciences. 2024; 25(7):3784. https://doi.org/10.3390/ijms25073784
Chicago/Turabian StyleLi, Xiaojuan, Tao Wang, Qilong Zhou, Fan Li, Ting Liu, Kun Zhang, Ao Wen, Lijuan Feng, Xiaoling Shu, Simin Tian, and et al. 2024. "Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway" International Journal of Molecular Sciences 25, no. 7: 3784. https://doi.org/10.3390/ijms25073784