Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process
Abstract
1. Introduction
2. Skin Structure
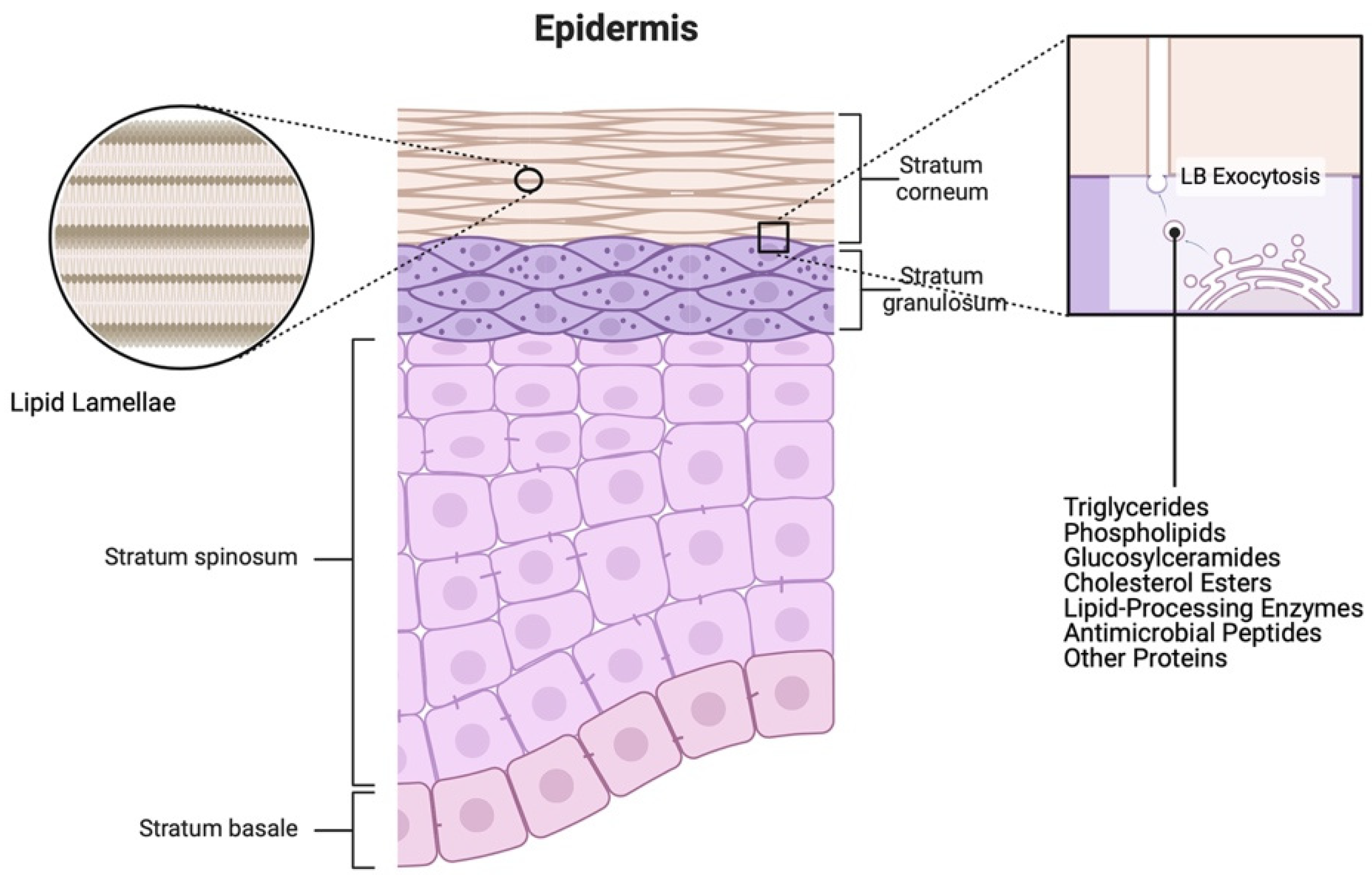
2.1. Epidermis
2.2. Dermis
2.3. Subcutaneous Tissue
3. Skin Function
3.1. Skin as a Protective Barrier
3.2. Skin as a Thermoregulator
3.3. Skin as a Sensory Organ
3.4. Skin as the Frontline of Defense
3.5. Skin as a Mediator of the Synthesis of Vitamin D
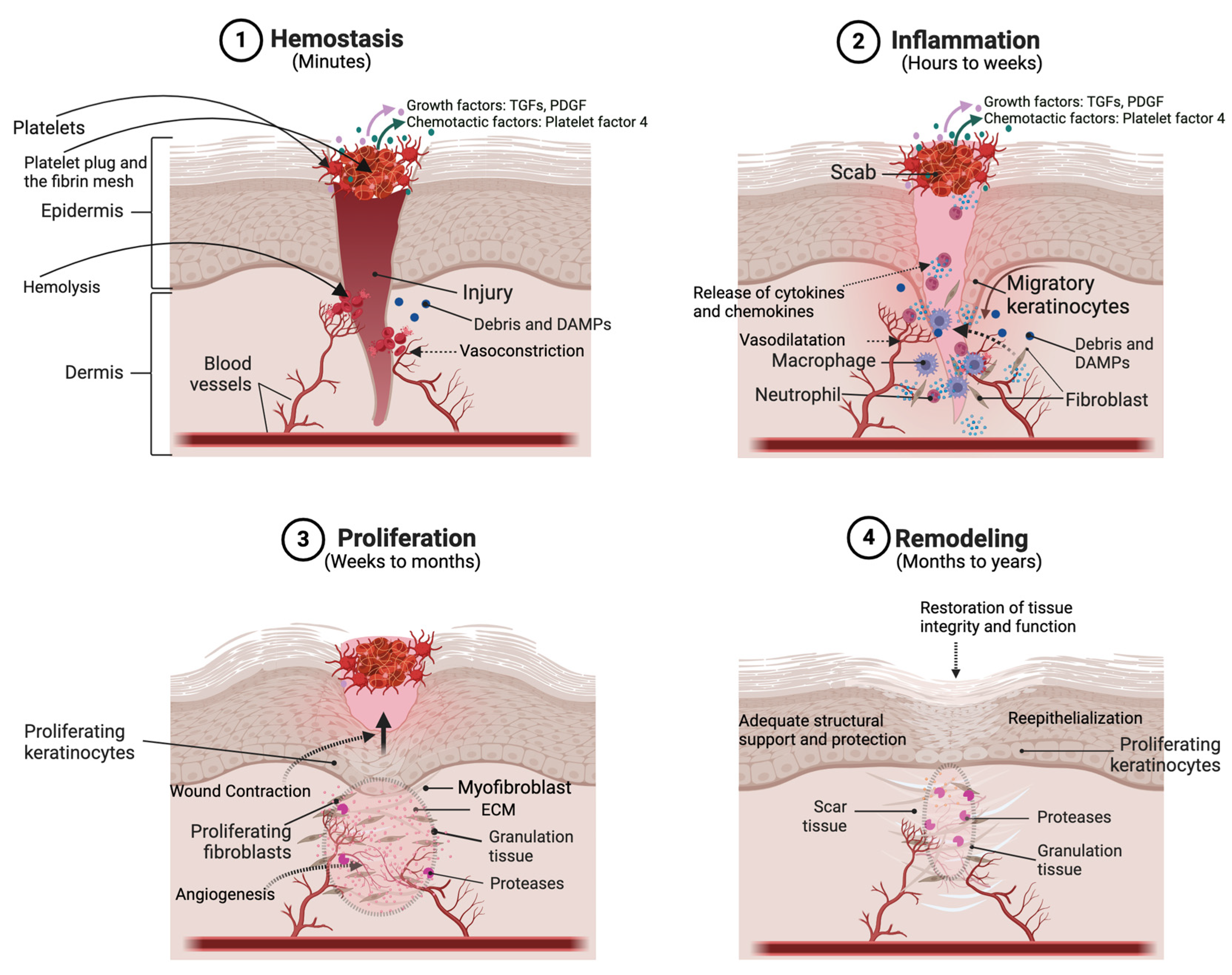
4. The Process of Cutaneous Wound Healing
4.1. Hemostasis
4.2. Inflammation
4.3. Proliferation
4.4. Remodeling
5. In Vitro Models of Skin Wound Healing
5.1. Single-Cell Models
5.2. Co-Culture System
5.3. Three-Dimensional In Vitro Models
5.4. Three-Dimensional Skin Equivalents
5.5. Three-Dimensional Bioprinting
5.6. Microfluidic Platforms
5.7. Advantages and Limitations of In Vitro Models
6. Ex Vivo Models or Skin Explant Culture Models
7. In Vivo Models of Skin Wound Healing
7.1. Rodent Models
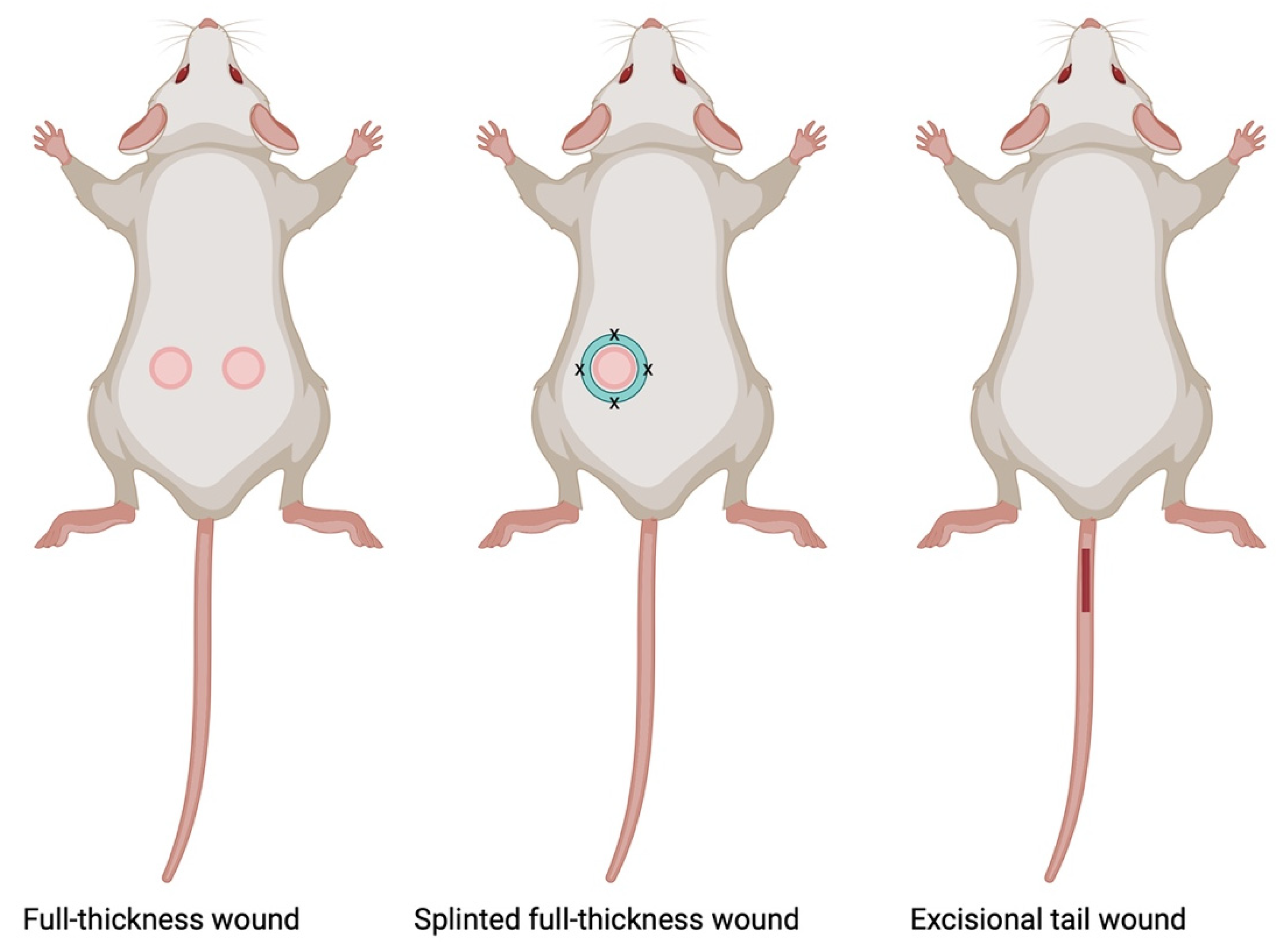
7.1.1. Full-Thickness Wound Model
7.1.2. Splinted Full-Thickness Wound Model
7.1.3. Tail Excisional Wound Model
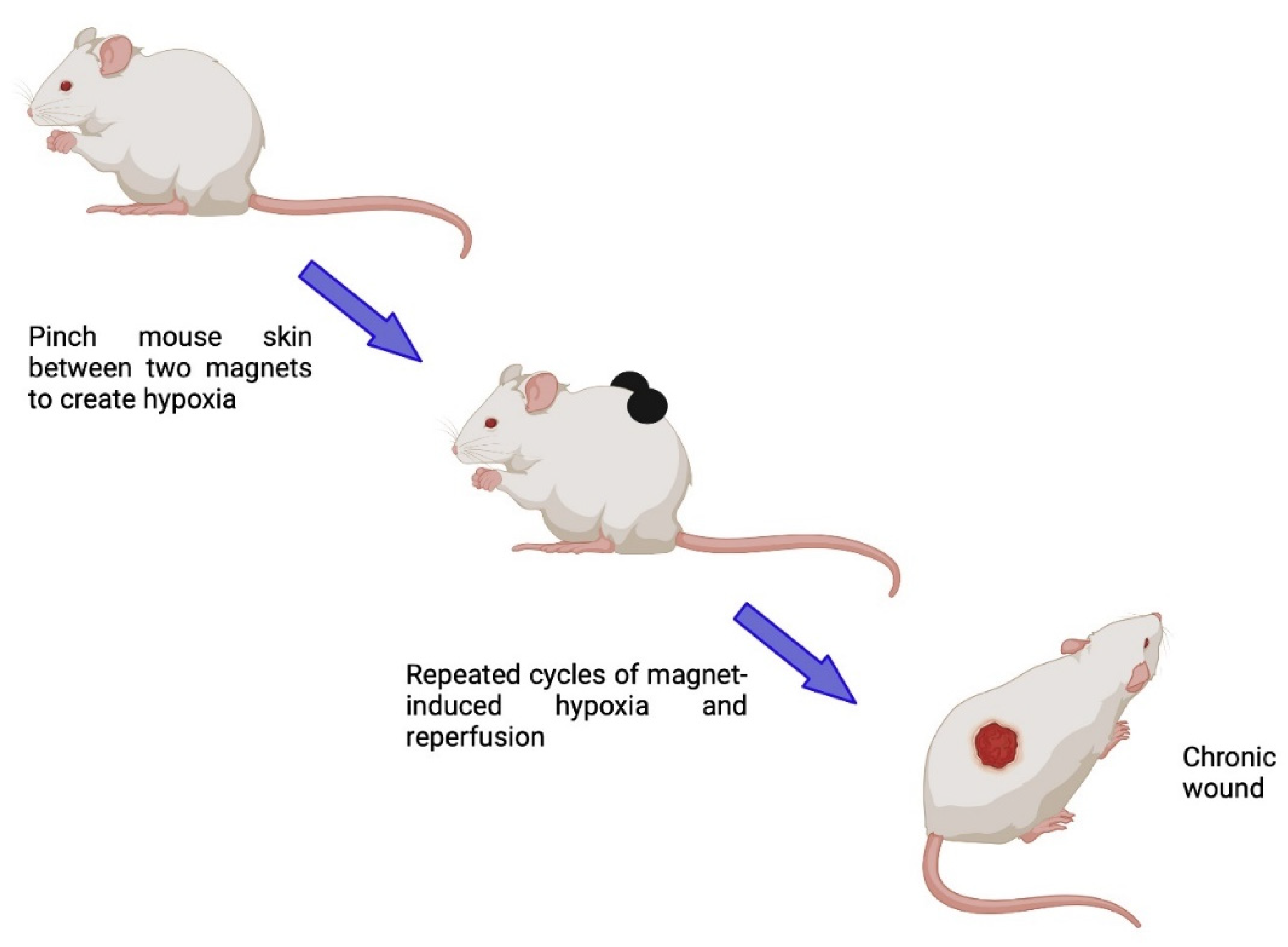
7.1.4. Chronic Wound Models
7.1.5. Special Considerations for Use in Conjunction with Different Wound Models
Genetically Modified (Transgenic) Models
Immunocompromised Model
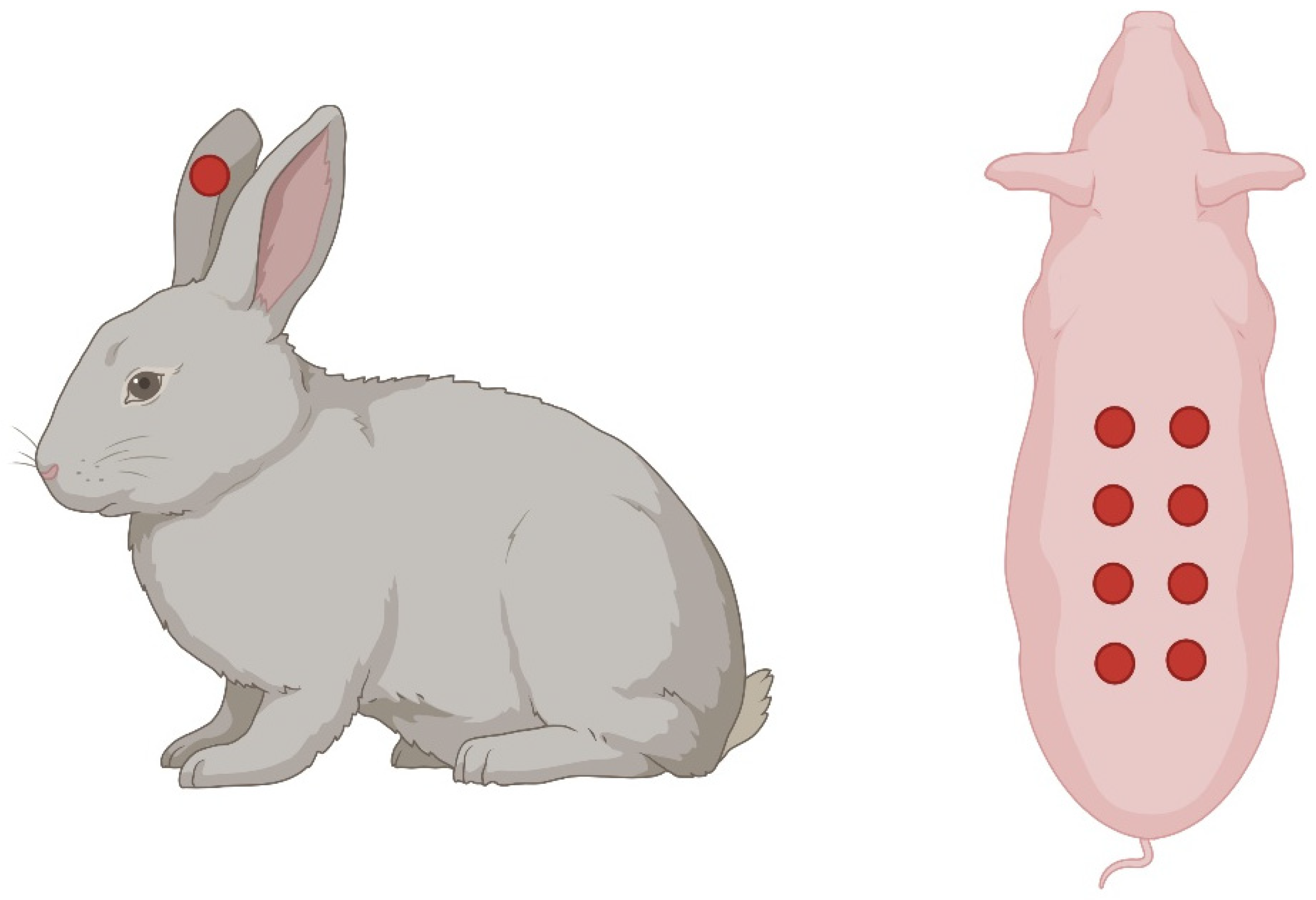
7.2. Rabbit Ear Model
7.3. Pig Skin Model
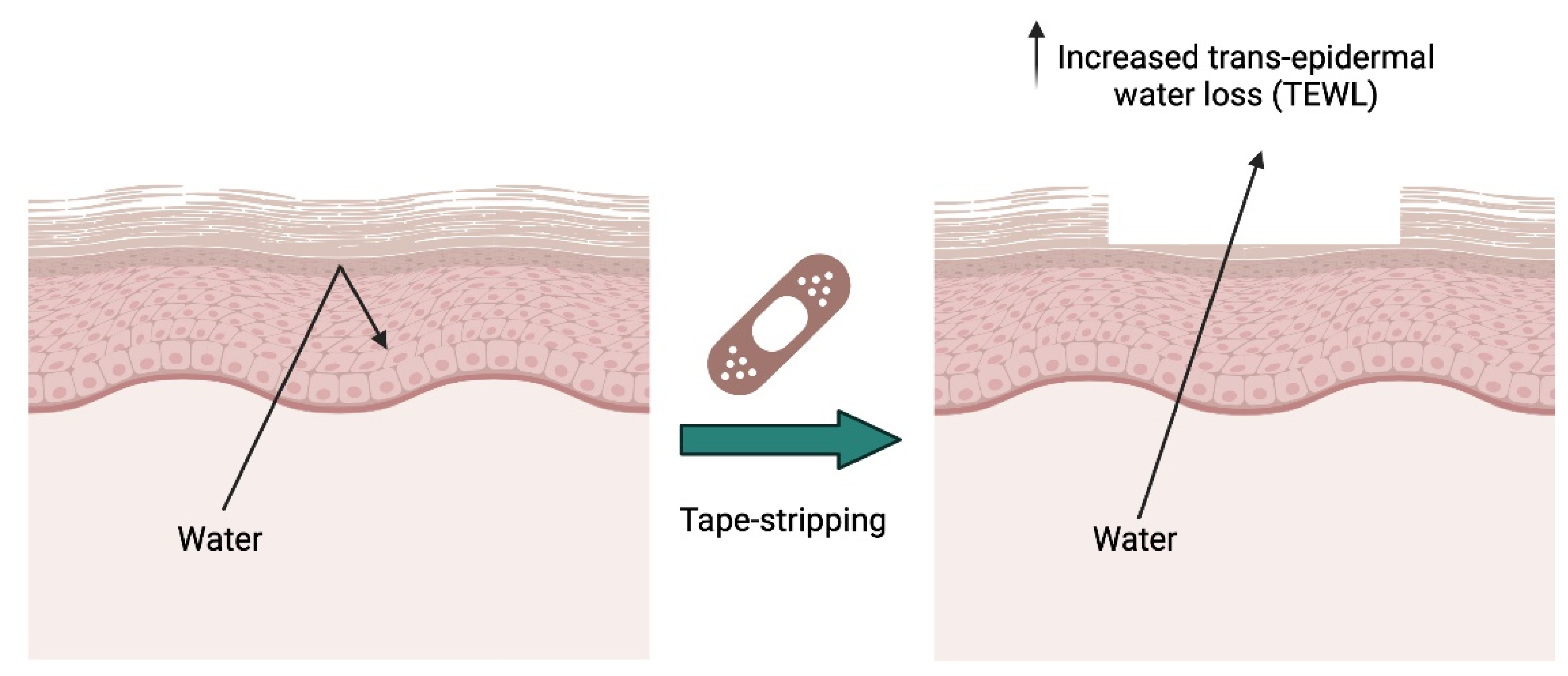
7.4. Superficial or Tape Stripping Model to Investigate Permeability Repair
8. Lipid Signals in Wound Healing
8.1. Lipids in Barrier Restoration and Skin Hydration
8.2. Lipids as Mediators of Inflammation
8.3. Lipid Mediators in the Resolution of the Immune Response (Inflammation)
8.4. Lipid Signaling in Angiogenesis
8.5. Lipids in Keratinocyte and Fibroblast Migration and Proliferation
8.6. Lipid Signals in the Remodeling Phase of Wound Healing
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The applied anatomy of human skin: A model for regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Wu, X. Molecular and cytoskeletal regulations in epidermal development. Semin. Cell Dev. Biol. 2017, 69, 18–25. [Google Scholar] [CrossRef]
- Elias, P.M. Epidermal lipids, barrier function, and desquamation. J. Investig. Dermatol. 1983, 80, 44s–49s. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, S.K.; Ahn, S.K. An update of the defensive barrier function of skin. Yonsei Med. J. 2006, 47, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Laly, A.C.; Sliogeryte, K.; Pundel, O.J.; Ross, R.; Keeling, M.C.; Avisetti, D.; Waseem, A.; Gavara, N.; Connelly, J.T. The keratin network of intermediate filaments regulates keratinocyte rigidity sensing and nuclear mechanotransduction. Sci. Adv. 2021, 7, eabd6187. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, L.I.; Choudhary, V.; Bollag, W.B. Updated Perspectives on Keratinocytes and Psoriasis: Keratinocytes are More Than Innocent Bystanders. Psoriasis 2022, 12, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Perone, P.; Deming, M.O.; Warner, R.L.; Aslam, M.N.; Bhagavathula, N.; Dame, M.K.; Voorhees, J.J. Impaired keratinocyte function on matrix metalloproteinase-1 (MMP-1) damaged collagen. Arch. Dermatol. Res. 2009, 301, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wasko, R.; Bridges, K.; Pannone, R.; Sidhu, I.; Xing, Y.; Naik, S.; Miller-Jensen, K.; Horsley, V. Langerhans cells are essential components of the angiogenic niche during murine skin repair. Dev. Cell 2022, 57, 2699–2713.e5. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Priya, A.; Chowdhary, M.; Batra, V.V.; Jyotsna; Nagarajan, P.; Gokhale, R.S.; Singh, A. Pigmented skin exhibits accelerated wound healing compared to the nonpigmented skin in Guinea pig model. iScience 2023, 26, 108159. [Google Scholar] [CrossRef] [PubMed]
- de Souza, K.S.; Cantaruti, T.A.; Azevedo, G.M., Jr.; Galdino, D.A.; Rodrigues, C.M.; Costa, R.A.; Vaz, N.M.; Carvalho, C.R. Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Exp. Dermatol. 2015, 24, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Song, B.; Chen, H.D.; Gao, X.H. Melanocytes and Skin Immunity. J. Investig. Dermatol. Symp. Proc. 2015, 17, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; Belpaire, A.; Speeckaert, M.; van Geel, N. The delicate relation between melanocytes and skin immunity: A game of hide and seek. Pigment Cell Melanoma Res. 2022, 35, 392–407. [Google Scholar] [CrossRef]
- Gao, F.L.; Jin, R.; Zhang, L.; Zhang, Y.G. The contribution of melanocytes to pathological scar formation during wound healing. Int. J. Clin. Exp. Med. 2013, 6, 609–613. [Google Scholar] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Ishizeki, K. Merkel cell development in the wound healing in the labial mucosa of adult rabbits. Arch. Histol. Jpn. 1981, 44, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Wulff, B.C. The Importance of Mast Cells in Dermal Scarring. Adv. Wound Care 2014, 3, 356–365. [Google Scholar] [CrossRef]
- Aitcheson, S.M.; Frentiu, F.D.; Hurn, S.E.; Edwards, K.; Murray, R.Z. Skin Wound Healing: Normal Macrophage Function and Macrophage Dysfunction in Diabetic Wounds. Molecules 2021, 26, 4917. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and Wound Repair: Positive Actions and Negative Reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.J.; Choudhary, V.; Merai, P.; Bollag, W.B. Preparation of primary cultures of mouse epidermal keratinocytes and the measurement of phospholipase D activity. Methods Mol. Biol. 2014, 1195, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Hattori, M.; Chamulitrat, W.; Ohno, Y.; Kihara, A. Skin permeability barrier formation by the ichthyosis-causative gene FATP4 through formation of the barrier lipid omega-O-acylceramide. Proc. Natl. Acad. Sci. USA 2020, 117, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans Cells-Programmed by the Epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.; Genehr, T.; Knuschke, P.; Pietzsch, J.; Meurer, M. UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J. Investig. Dermatol. 2001, 117, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Ducote, J.; Harmon, C.S. Biphasic effect of 1,25-dihydroxyvitamin D3 on primary mouse epidermal keratinocyte proliferation. J. Cell Physiol. 1995, 163, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, L.; Zhang, Y. Vitamin D and wound healing: Assessing skin barrier function and implications for chloasma treatment. Int. Wound J. 2024, 21, e14541. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z.; Lu, J.; Watsky, M. Effects of Topical 1,25 and 24,25 Vitamin D on Diabetic, Vitamin D Deficient and Vitamin D Receptor Knockout Mouse Corneal Wound Healing. Biomolecules 2023, 13, 1065. [Google Scholar] [CrossRef]
- Bikle, D.D. Role of vitamin D and calcium signaling in epidermal wound healing. J. Endocrinol. Investig. 2023, 46, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Boniakowski, A.E.; Kimball, A.S.; Jacobs, B.N.; Kunkel, S.L.; Gallagher, K.A. Macrophage-Mediated Inflammation in Normal and Diabetic Wound Healing. J. Immunol. 2017, 199, 17–24. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mayet, N.; Choonara, Y.E.; Kumar, P.; Tomar, L.K.; Tyagi, C.; Du Toit, L.C.; Pillay, V. A comprehensive review of advanced biopolymeric wound healing systems. J. Pharm. Sci. 2014, 103, 2211–2230. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Leibovich, S.J. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol. 2005, 15, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.A.; Saunus, J.M.; Ivanovski, S.; Walsh, L.J.; Farah, C.S. Accelerated wound healing phenotype in Interleukin 12/23 deficient mice. J. Inflamm. 2011, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Gill, S.E.; Parks, W.C. Metalloproteinases and their inhibitors: Regulators of wound healing. Int. J. Biochem. Cell Biol. 2008, 40, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol. Biol. 2020, 2109, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Magesh, K.T.; Sathyakumar, M.; Sivachandran, A.; Purushothaman, D.; Aravindhan, R. Evaluation of Wound Healing Property of the Ethanolic Extract of Glycyrrhiza Glabra on Vero Cell Lines Using In Vitro Scratch Assay Test. J. Pharm. Bioallied Sci. 2023, 15, S630–S635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Farhangfar, F.; Zimmer, M.; Zhang, Y. Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PLoS ONE 2012, 7, e40951. [Google Scholar] [CrossRef] [PubMed]
- Alencar-Silva, T.; Diaz-Martin, R.D.; Zonari, A.; Foyt, D.; Guiang, M.; Pogue, R.; Saldanha-Araujo, F.; Dias, S.C.; Franco, O.L.; Carvalho, J.L. The Combination of Synoeca-MP Antimicrobial Peptide with IDR-1018 Stimulates Proliferation, Migration, and the Expression of Pro-Regenerative Genes in Both Human Skin Cell Cultures and 3D Skin Equivalents. Biomolecules 2023, 13, 804. [Google Scholar] [CrossRef] [PubMed]
- Shamis, Y.; Hewitt, K.J.; Carlson, M.W.; Margvelashvilli, M.; Dong, S.; Kuo, C.K.; Daheron, L.; Egles, C.; Garlick, J.A. Fibroblasts derived from human embryonic stem cells direct development and repair of 3D human skin equivalents. Stem Cell Res. Ther. 2011, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Antezana, P.E.; Municoy, S.; Alvarez-Echazu, M.I.; Santo-Orihuela, P.L.; Catalano, P.N.; Al-Tel, T.H.; Kadumudi, F.B.; Dolatshahi-Pirouz, A.; Orive, G.; Desimone, M.F. The 3D Bioprinted Scaffolds for Wound Healing. Pharmaceutics 2022, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, C.; Li, Z.; Fu, X.; Huang, S. Advances in 3D skin bioprinting for wound healing and disease modeling. Regen. Biomater. 2023, 10, rbac105. [Google Scholar] [CrossRef] [PubMed]
- Ponmozhi, J.; Dhinakaran, S.; Varga-Medveczky, Z.; Fonagy, K.; Bors, L.A.; Ivan, K.; Erdo, F. Development of Skin-on-a-Chip Platforms for Different Utilizations: Factors to Be Considered. Micromachines 2021, 12, 294. [Google Scholar] [CrossRef]
- Shabestani Monfared, G.; Ertl, P.; Rothbauer, M. Microfluidic and Lab-on-a-Chip Systems for Cutaneous Wound Healing Studies. Pharmaceutics 2021, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Moles, C.M.; Bhattacharya, S.S.; LeSaint, M.; Dhamija, Y.; Le, L.D.; King, A.; Kidd, M.; Bouso, M.F.; Shaaban, A.; et al. Comparison of interleukin 10 homologs on dermal wound healing using a novel human skin ex vivo organ culture model. J. Surg. Res. 2014, 190, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Garcia, J.; Sebastian, A.; Alonso-Rasgado, T.; Bayat, A. Optimization of an ex vivo wound healing model in the adult human skin: Functional evaluation using photodynamic therapy. Wound Repair. Regen. 2015, 23, 685–702. [Google Scholar] [CrossRef]
- Cretu, B.E.; Dodi, G.; Gardikiotis, I.; Balan, V.; Nacu, I.; Stoica, I.; Stoleru, E.; Rusu, A.G.; Ghilan, A.; Nita, L.E.; et al. Bioactive Composite Cryogels Based on Poly (Vinyl Alcohol) and a Polymacrolactone as Tissue Engineering Scaffolds: In Vitro and In Vivo Studies. Pharmaceutics 2023, 15, 2730. [Google Scholar] [CrossRef]
- Jung, J.M.; Yoon, H.K.; Jung, C.J.; Jo, S.Y.; Hwang, S.G.; Lee, H.J.; Lee, W.J.; Chang, S.E.; Won, C.H. Cold Plasma Treatment Promotes Full-thickness Healing of Skin Wounds in Murine Models. Int. J. Low. Extrem. Wounds 2023, 22, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, N.; Indra, A.K.; Ganguli-Indra, G. Healing of Full-Thickness Murine Skin Wounds Containing Nanofibers Using Splints for Efficient Reepithelialization and to Avoid Contracture. Methods Mol. Biol. 2020, 2155, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kos, S.; Jesenko, T.; Blagus, T. In Vivo Wound Healing Model for Characterization of Gene Electrotransfer Effects in Mouse Skin. Methods Mol. Biol. 2024, 2773, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.J.; Henry, W.L., Jr.; Tran, H.L.; Albina, J.E.; Jamieson, A.M. Assessment of Acute Wound Healing using the Dorsal Subcutaneous Polyvinyl Alcohol Sponge Implantation and Excisional Tail Skin Wound Models. J. Vis. Exp. 2020, 157, e60653. [Google Scholar] [CrossRef]
- Falanga, V.; Schrayer, D.; Cha, J.; Butmarc, J.; Carson, P.; Roberts, A.B.; Kim, S.J. Full-thickness wounding of the mouse tail as a model for delayed wound healing: Accelerated wound closure in Smad3 knock-out mice. Wound Repair. Regen. 2004, 12, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, W.; Zhou, S.; Zhang, G.; He, J.; Li, Q. A Novel Model for Cutaneous Wound Healing and Scarring in the Rat. Plast. Reconstr. Surg. 2019, 143, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair. Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Kakanj, P.; Leptin, M.; Eming, S.A. Regulation of the Wound Healing Response during Aging. J. Investig. Dermatol. 2021, 141, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Martins-Green, M. Protocol to Create Chronic Wounds in Diabetic Mice. J. Vis. Exp. 2019, 151, e57656. [Google Scholar] [CrossRef]
- Stadler, I.; Zhang, R.Y.; Oskoui, P.; Whittaker, M.S.; Lanzafame, R.J. Development of a simple, noninvasive, clinically relevant model of pressure ulcers in the mouse. J. Investig. Surg. 2004, 17, 221–227. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr266. [Google Scholar] [CrossRef] [PubMed]
- Wilgus, T.A. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol. Res. 2008, 58, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Q.; Yang, W.; Wang, L.; Wang, J.; You, R.; Luo, Z.; Zhang, Q.; Yan, S. Development of a bioactive silk fibroin bilayer scaffold for wound healing and scar inhibition. Int. J. Biol. Macromol. 2024, 255, 128350. [Google Scholar] [CrossRef]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The pig as a model for human wound healing. Wound Repair Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Jarrett, P.; Nater, U.M.; Skoluda, N.; Broadbent, E. The effects of environmental enrichment on skin barrier recovery in humans: A randomised trial. Sci. Rep. 2020, 10, 9829. [Google Scholar] [CrossRef] [PubMed]
- Lovaszi, M.; Szegedi, A.; Zouboulis, C.C.; Torocsik, D. Sebaceous-immunobiology is orchestrated by sebum lipids. Dermatoendocrinology 2017, 9, e1375636. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Andrew, P.V.; Kay, L.J.; Pinnock, A.; Chittock, J.; Brown, K.; Williams, S.F.; Cork, M.J. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone skin. Br. J. Dermatol. 2022, 186, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Marchetti-Deschmann, M.; Kremslehner, C.; Schosserer, M. The Skin Epilipidome in Stress, Aging, and Inflammation. Front. Endocrinol. 2021, 11, 607076. [Google Scholar] [CrossRef] [PubMed]
- Ouweneel, A.B.; Thomas, M.J.; Sorci-Thomas, M.G. The ins and outs of lipid rafts: Functions in intracellular cholesterol homeostasis, microparticles, and cell membranes: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020, 61, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Vietri Rudan, M.; Watt, F.M. Mammalian Epidermis: A Compendium of Lipid Functionality. Front. Physiol. 2022, 12, 804824. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Role of lipids in the formation and maintenance of the cutaneous permeability barrier. Biochim. Biophys. Acta 2014, 1841, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Esser-von Bieren, J. Eicosanoids in tissue repair. Immunol. Cell Biol. 2019, 97, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.W.; Mannon, R.B.; Mannon, P.J.; Latour, A.; Oliver, J.A.; Hoffman, M.; Smithies, O.; Koller, B.H.; Coffman, T.M. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J. Clin. Investig. 1998, 102, 1994–2001. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Khan, F.; McLaren, M.; Bancroft, A.; Walker, M.; Belch, J.J. The effects of thromboxane receptor blockade on platelet aggregation and digital skin blood flow in patients with secondary Raynaud’s syndrome. Rheumatol. Int. 1991, 11, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Pierre, S.; Linke, B.; Suo, J.; Tarighi, N.; Del Turco, D.; Thomas, D.; Ferreiros, N.; Stegner, D.; Frolich, S.; Sisignano, M.; et al. GPVI and Thromboxane Receptor on Platelets Promote Proinflammatory Macrophage Phenotypes during Cutaneous Inflammation. J. Investig. Dermatol. 2017, 137, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.; Yoon, Y.S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Brandt, S.L.; Wang, S.; Dejani, N.N.; Klopfenstein, N.; Winfree, S.; Filgueiras, L.; McCarthy, B.P.; Territo, P.R.; Serezani, C.H. Excessive localized leukotriene B4 levels dictate poor skin host defense in diabetic mice. JCI Insight 2018, 3, e120220. [Google Scholar] [CrossRef] [PubMed]
- Brogliato, A.R.; Moor, A.N.; Kesl, S.L.; Guilherme, R.F.; Georgii, J.L.; Peters-Golden, M.; Canetti, C.; Gould, L.J.; Benjamim, C.F. Critical role of 5-lipoxygenase and heme oxygenase-1 in wound healing. J. Investig. Dermatol. 2014, 134, 1436–1445. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, F.R.; Sales-Campos, H.; Nardini, V.; da Costa, T.A.; Fonseca, M.T.C.; Junior, V.R.; Sorgi, C.A.; da Silva, J.S.; Chica, J.E.L.; Faccioli, L.H.; et al. The inhibition of 5-Lipoxygenase (5-LO) products leukotriene B4 (LTB(4)) and cysteinyl leukotrienes (cysLTs) modulates the inflammatory response and improves cutaneous wound healing. Clin. Immunol. 2018, 190, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 2011, 11, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Gangoiti, P.; Ouro, A.; Trueba, M.; Gomez-Munoz, A. Ceramide and ceramide 1-phosphate in health and disease. Lipids Health Dis. 2010, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.C.; Belury, M.; Ahijevych, K.; Blakely, W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008, 16, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Albina, J.E.; Gladden, P.; Walsh, W.R. Detrimental effects of an omega-3 fatty acid-enriched diet on wound healing. JPEN J. Parenter. Enter. Nutr. 1993, 17, 519–521. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Arantes, E.L.; Dragano, N.; Ramalho, A.; Vitorino, D.; de-Souza, G.F.; Lima, M.H.; Velloso, L.A.; Araujo, E.P. Topical Docosahexaenoic Acid (DHA) Accelerates Skin Wound Healing in Rats and Activates GPR120. Biol. Res. Nurs. 2016, 18, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Babaei, S.; Nakhaei, M.; Ansarihadipour, H.; Sakhaei, M.; Hosseini, S.; Nikgoftar, A. Omegaven Improves Skin Morphometric Indices in Diabetic Rat Model Wound Healing. J. Am. Coll. Clin. Wound Spec. 2017, 9, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Arun, S.N.; Xie, D.; Howard, A.C.; Zhong, Q.; Zhong, X.; McNeil, P.L.; Bollag, W.B. Cell wounding activates phospholipase D in primary mouse keratinocytes. J. Lipid Res. 2013, 54, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol Inhibits Toll-Like Receptor-Mediated Inflammation by Danger-Associated Molecular Patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Olala, L.O.; Xie, D.; Lu, X.; Qin, H.; Choudhary, V.; Patel, R.; Bogorad, D.; Estes, A.; Watsky, M. Dioleoylphosphatidylglycerol Accelerates Corneal Epithelial Wound Healing. Invest. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The Role of Platelet-Activating Factor in Chronic Inflammation, Immune Activation, and Comorbidities Associated with HIV Infection. AIDS Rev. 2015, 17, 191–201. [Google Scholar] [PubMed]
- Eming, S.A.; Brachvogel, B.; Odorisio, T.; Koch, M. Regulation of angiogenesis: Wound healing as a model. Prog. Histochem. Cytochem. 2007, 42, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Lange-Asschenfeldt, B.; Velasco, P.; Streit, M.; Hawighorst, T.; Pike, S.E.; Tosato, G.; Detmar, M. The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J. Investig. Dermatol. 2001, 117, 1036–1041. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ferreira, A.M.; Oberyszyn, T.M.; Bergdall, V.K.; Dipietro, L.A. Regulation of scar formation by vascular endothelial growth factor. Lab. Investig. 2008, 88, 579–590. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Barakat, M.; DiPietro, L.A. Angiogenesis in Wound Repair: Too Much of a Good Thing? Cold Spring Harb. Perspect. Biol. 2022, 14, a041225. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Aoki, H.; Mukhopadhyay, P.; Tsuge, T.; Yamamoto, H.; Matsumoto, N.M.; Toyohara, E.; Okubo, Y.; Ogawa, R.; Takabe, K. Sphingosine-1-Phosphate Facilitates Skin Wound Healing by Increasing Angiogenesis and Inflammatory Cell Recruitment with Less Scar Formation. Int. J. Mol. Sci. 2019, 20, 3381. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Estrada-Hernandez, T.; Paik, J.H.; Wu, M.T.; Venkataraman, K.; Brinkmann, V.; Claffey, K.; Hla, T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 2003, 278, 47281–47290. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Marsolais, D.; Rosen, H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat. Rev. Drug Discov. 2009, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, Y.; Du, W.; Qi, X.; Okamoto, Y.; Takuwa, N.; Yoshioka, K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J. Biol. Chem. 2010, 1, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.; Skoura, A.; Wu, M.T.; Casserly, B.; Harrington, E.O.; Hla, T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1312–1318. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B. Protein kinase Calpha puts the hand cuffs on epidermal keratinocyte proliferation. J. Investig. Dermatol. 2009, 129, 2330–2332. [Google Scholar] [CrossRef]
- Bollinger Bollag, W.; Bollag, R.J. 1,25-Dihydroxyvitamin D(3), phospholipase D and protein kinase C in keratinocyte differentiation. Mol. Cell Endocrinol. 2001, 177, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B.; Dodd, M.E.; Shapiro, B.A. Protein kinase D and keratinocyte proliferation. Drug News Perspect. 2004, 17, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Olala, L.O.; Kaddour-Djebbar, I.; Helwa, I.; Bollag, W.B. Protein kinase D1 deficiency promotes differentiation in epidermal keratinocytes. J. Dermatol. Sci. 2014, 76, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ernest Dodd, M.; Ristich, V.L.; Ray, S.; Lober, R.M.; Bollag, W.B. Regulation of protein kinase D during differentiation and proliferation of primary mouse keratinocytes. J. Investig. Dermatol. 2005, 125, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Bollag, W.B. Lipid signaling in keratinocytes: Lipin-1 plays a PArt. J. Lipid Res. 2016, 57, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Jung, J.Y.; Bae, I.H.; Kim, H.J.; Lee, T.R.; Shin, D.W. Lipin-1 expression is critical for keratinocyte differentiation. J. Lipid Res. 2016, 57, 563–573. [Google Scholar] [CrossRef]
- Kleuser, B.; Baumer, W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1456. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.O.; Choe, S.J.; Uchida, Y.; Kim, I.; Jeong, Y.; Park, K. Ginsenoside Rb1 Enhances Keratinocyte Migration by a Sphingosine-1-Phosphate-Dependent Mechanism. J. Med. Food 2018, 21, 1129–1136. [Google Scholar] [CrossRef]
- Eichholtz, T.; Jalink, K.; Fahrenfort, I.; Moolenaar, W.H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem. J. 1993, 291 Pt 3, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Monti, M.; Sesana, S.; Caputo, R.; Carelli, S.; Ghidoni, R. Ceramide composition of the psoriatic scale. Biochim. Biophys. Acta 1993, 1182, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Maus, K.D.; Stephenson, D.J.; Ali, A.N.; MacKnight, H.P.; Huang, H.J.; Serrats, J.; Kim, M.; Diegelmann, R.F.; Chalfant, C.E. Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1. J. Lipid Res. 2022, 63, 100187. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Bollag, W.B. De(C1P)hering the role of ceramide-1-phosphate levels in skin wound healing. J. Lipid Res. 2022, 63, 100231. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Seremwe, M.; Edwards, J.G.; Podolsky, R.; Bollag, W.B. Distinct effects of different phosphatidylglycerol species on mouse keratinocyte proliferation. PLoS ONE 2014, 9, e107119. [Google Scholar] [CrossRef]
- Konger, R.L.; Malaviya, R.; Pentland, A.P. Growth regulation of primary human keratinocytes by prostaglandin E receptor EP2 and EP3 subtypes. Biochim. Biophys. Acta 1998, 1401, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, T.; Liu, X.D.; Wen, F.Q.; Kim, H.J.; Takizawa, H.; Rennard, S.I. Prostaglandin D2 inhibits fibroblast migration. Eur. Respir. J. 2002, 19, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, D.S.; Brentnall, M.; Mietla, J.A.; Hoeferlin, L.A.; Diegelmann, R.F.; Boise, L.H.; Chalfant, C.E. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J. Lipid Res. 2014, 55, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Maibach, H.I. Role of ceramides in barrier function of healthy and diseased skin. Am. J. Clin. Dermatol. 2005, 6, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef]
- Herrera, B.S.; Kantarci, A.; Zarrough, A.; Hasturk, H.; Leung, K.P.; Van Dyke, T.E. LXA4 actions direct fibroblast function and wound closure. Biochem. Biophys. Res. Commun. 2015, 464, 1072–1077. [Google Scholar] [CrossRef]
- Liang, M.; Lv, J.; Jiang, Z.; He, H.; Chen, C.; Xiong, Y.; Zhu, X.; Xue, Y.; Yu, Y.; Yang, S.; et al. Promotion of Myofibroblast Differentiation and Tissue Fibrosis by the Leukotriene B(4) -Leukotriene B(4) Receptor Axis in Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1013–1025. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; He, R.; Kanaoka, Y.; ElKhal, A.; Kawamoto, S.; Lewis, C.N.; Austen, K.F.; Geha, R.S. Eosinophil-derived leukotriene C4 signals via type 2 cysteinyl leukotriene receptor to promote skin fibrosis in a mouse model of atopic dermatitis. Proc. Natl. Acad. Sci. USA 2012, 109, 4992–4997. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Griffith, S.; Chen, X.; Bollag, W.B. Pathogen-Associated Molecular Pattern-Induced TLR2 and TLR4 Activation Increases Keratinocyte Production of Inflammatory Mediators and is Inhibited by Phosphatidylglycerol. Mol. Pharmacol. 2020, 97, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shu, B.; Chen, L.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Prostaglandin E2 inhibits collagen synthesis in dermal fibroblasts and prevents hypertrophic scar formation in vivo. Exp. Dermatol. 2016, 25, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Warsinske, H.C.; Ashley, S.L.; Linderman, J.J.; Moore, B.B.; Kirschner, D.E. Identifying Mechanisms of Homeostatic Signaling in Fibroblast Differentiation. Bull. Math. Biol. 2015, 77, 1556–1582. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Pils, V.; Terlecki-Zaniewicz, L.; Schosserer, M.; Grillari, J.; Lammermann, I. The role of lipid-based signalling in wound healing and senescence. Mech. Ageing Dev. 2021, 198, 111527. [Google Scholar] [CrossRef] [PubMed]
| In Vitro Model Type | Description | Advantages | Disadvantages |
|---|---|---|---|
| Single-cell-type 2D Models | Single-cell types cultured to investigate basic cell signaling responses to injury and stress, typically created by “scratch wounding” techniques. |
|
|
| Co-culture Systems | Different cell types cultured together to investigate interactions and responses to injury; may be facilitated by Transwell systems for analyzing paracrine factors and/or chemo-tactic responses. |
|
|
| 3D In Vitro Models | Tissue architecture designed to replicate the physiological complexity of skin tissue, allowing assessment of wound contraction, migration, and matrix compaction in a 3D environment. |
|
|
| 3D Skin Equivalents | Advanced models incorporat- ing multiple cell types and lay-ers to mimic native skin architecture, providing insights into tissue regeneration and re-epithelialization. |
|
|
| 3D Bioprinting | Constructed patient-specific skin grafts with biomimetic structures. |
|
|
| Microfluidic Plat-forms | Microchannel designs to create cell-free wound areas for studying molecular processes in wound healing, including cell migration and interactions. |
|
|
| Ex Vivo Models | Living tissue samples harvested from organisms and cultured to study wound repair mech-anisms. |
|
|
| Model Type | Description | Advantages | Disadvantages |
|---|---|---|---|
| Rodent Models | |||
| a. Full-thickness wound model | Wounds created that pene-trate through the entire thickness of the skin in rodents. |
|
|
| b. Splinted full-thickness wound model | Similar to the full-thickness wound model, but involves the use of a splint to prevent wound contraction, allowing for granulation tissue formation and healing by re-epithelialization. |
|
|
| c. Tail excisional wound model | Similar to the full-thickness wound model, but wounds are created on the tail of rodents. |
|
|
| d. Chronic wound models | Models created produce chronic wounds, such as diabetic ulcers or pressure ulcers. |
|
|
| e. Genetically modified (transgenic) models | Models created in rodents with specific genetic alterations to study the role of particular genes in wound healing. |
|
|
| f. Immuno-compromised model | Models created in rodents with compromised immune systems to study wound healing in the absence of immune responses. |
|
|
| Other Models | |||
| Rabbit Ear Model | Wounds created in the thin skin of rabbit ears. |
|
|
| Pig Skin Model | Wounds created in pig skin for wound healing studies. |
|
|
| Superficial or tape stripping model | Superficial layers of the skin removed using tape strip-ping. |
|
|
| Lipid | Role and/or Skin Wound Healing Phase | Effect/Mechanism of Action of Wound Healing |
|---|---|---|
| Ceramide-1-phosphate (C1P) | Skin barrier maintenance | Maintains acidic pH to inhibit pathogenic microorganism growth [79]. Promotes β-defensin 2 expression, a vital antimicrobial peptide in human skin [80]. |
| Promotion of inflammation | Mediates inflammation through stimulation of cytosolic phospholipase A2 and the subsequent release of arachidonic acid and prostaglandin formation [96]. Serves as a checkpoint for phase transition from the inflammation to the proliferation phase [134,135]. | |
| Proliferation | Inhibits keratinocyte proliferation [134]. Plays a role in orderly migration of fibroblasts in the wound site [140]. | |
| Cholesterol | Skin barrier maintenance | Maintains skin barrier and integrity [141]. |
| Promotion of inflammation | Contributes to lipid raft synthesis, facilitating assembly of inflammation pathway signaling molecules [77,78]. | |
| Resolution of inflammation | Modulates inflammation by synthesizing steroid hormones like glucocorticoids, which regulate immune responses and control the intensity and duration of inflammation at the wound site [77]. | |
| Diacylglycerol | Proliferation phase | Activates protein kinases C and D, regulating keratinocyte proliferation and differentiation [122,123,124]. |
| Omega-3 polyunsaturated fatty acids (PUFAs) | Promotion of inflammation | Increase proinflammatory cytokine IL-1β levels and prolong wound closure time in healthy human skin (marine-derived omega-3 fatty acids) [97]. |
| Resolution of inflammation | Topical DHA treatment accelerates rat skin wound healing by activating GPR120, which reduces IL-1β expression and increases IL-6 levels [101]. Intraperitoneal administration of a fish-oil emulsion rich in EPA and DHA improved excisional skin wound healing in diabetic male rats [102]. | |
| Remodeling | Reduced wound strength due to changes in the fibroblastic or remodeling phases of wound healing [98]. | |
| Sphingosine-1-phosphate (S1P) | Promotion of inflammation | Promotes inflammation and regulates immune cell trafficking, vascular permeability, and pro-inflammatory cytokine production [95,129]. Increases the recruitment of T cells and macrophages [113]. |
| Resolution of inflammation | Modulates immune cell trafficking in inflamed tissues, promoting T cell retention (through S1PR1 and S1PR5) [142]. Promotes angiogenesis by enhancing the migration and sprouting of endothelial cells [113]. | |
| Proliferation | Promotes keratinocyte and fibroblast proliferation and migration [131]. | |
| Specialized Pro-resolving Mediators (SPMs) such as Resolvin D2 (RvD2) and Lipoxin A4 | Resolution of inflammation | Dampen inflammation and promote wound healing by reducing the production of pro-inflammatory cytokines [87,99]. Inhibit neutrophil infiltration, enhancing macrophage phagocytosis of apoptotic cells [99]. |
| Proliferation | Inhibit fibroblast proliferation and migration [143]. | |
| Remodeling | Modulate fibrosis during tissue remodeling [143]. | |
| Leukotrienes | Promotion of inflammation | Serve as chemoattractants, aiding in wound healing by recruiting white blood cells [90,91]. |
| Proliferation | LTB4 and LTC4 cause aberrant activation of fibroblasts, collagen synthesis, and fibrosis [144,145]. | |
| Phosphatidylglycerol (PG or DOPG) | Inflammation | Facilitates skin and corneal wound healing [104,105]. Dampens inflammation by inhibiting TLR2/4 pathway activation [146]. |
| Proliferation | Enhances keratinocyte proliferation [136]. | |
| Platelet-Activating Factor (PAF) | Promotion of inflammation | Promotes immune cell recruitment, enhancing leukocyte adhesion and extravasation, release of proinflammatory mediators, and vascular permeability [106]. |
| Resolution of inflammation | Promotes apoptosis of neutrophils and clearance of inflammatory cells and debris and regulates pro-inflammatory mediators [106]. | |
| Prostaglandin E2 (PGE2) | Promotion of inflammation | Contributes to vasodilation and increased vascular permeability and facilitates immune cell recruitment [87,138]. |
| Resolution of inflammation | Stimulates angiogenesis and inhibits production of pro-inflammatory cytokines and leukotrienes [138]. | |
| Proliferation | Promotes fibroblast and keratinocyte proliferation and migration [137]. | |
| Remodeling | Inhibits TGFβ1-induced collagen synthesis and reduces hypertrophic scar formation [147]. | |
| Prostaglandin D2 (PGD2) | Proliferation | Regulates/inhibits fibroblast migration [139]. |
| Remodeling | Regulates tissue regeneration preventing fibrosis or keloids [139]. | |
| Thromboxane A2 (TXA2) | Promotion of hemostasis | Amplifies platelet activation and irreversible aggregation for hemostasis [82]. |
| Promotion of inflammation | Induces the synthesis of pro-inflammatory cytokine intereukin-6 and PGE2 [85]. Inhibits the expression of the anti-inflammatory macrophage marker CD206 [85]. | |
| Lysophosphatidic Acid (LPA) | Proliferation | Promotes fibroblast migration and proliferation [129,130,131]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. https://doi.org/10.3390/ijms25073790
Choudhary V, Choudhary M, Bollag WB. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. International Journal of Molecular Sciences. 2024; 25(7):3790. https://doi.org/10.3390/ijms25073790
Chicago/Turabian StyleChoudhary, Vivek, Mrunal Choudhary, and Wendy B. Bollag. 2024. "Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process" International Journal of Molecular Sciences 25, no. 7: 3790. https://doi.org/10.3390/ijms25073790
APA StyleChoudhary, V., Choudhary, M., & Bollag, W. B. (2024). Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. International Journal of Molecular Sciences, 25(7), 3790. https://doi.org/10.3390/ijms25073790








