Comparative Genomic Analysis of PEBP Genes in Cucurbits Explores the Interactors of Cucumber CsPEBPs Related to Flowering Time
Abstract
1. Introduction
2. Results
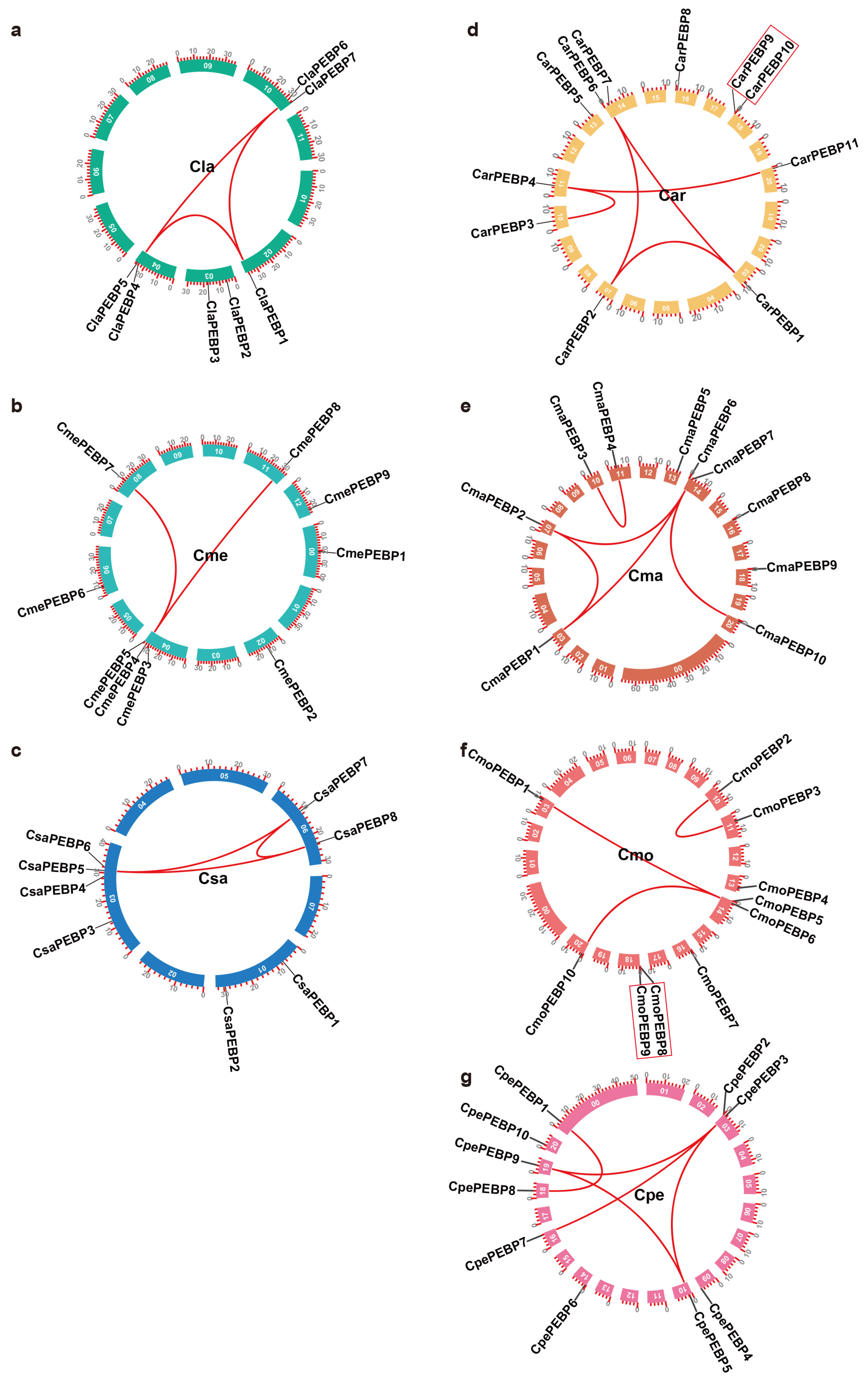
2.1. Identification of PEBP Family Genes in Seven Cucurbit Crops
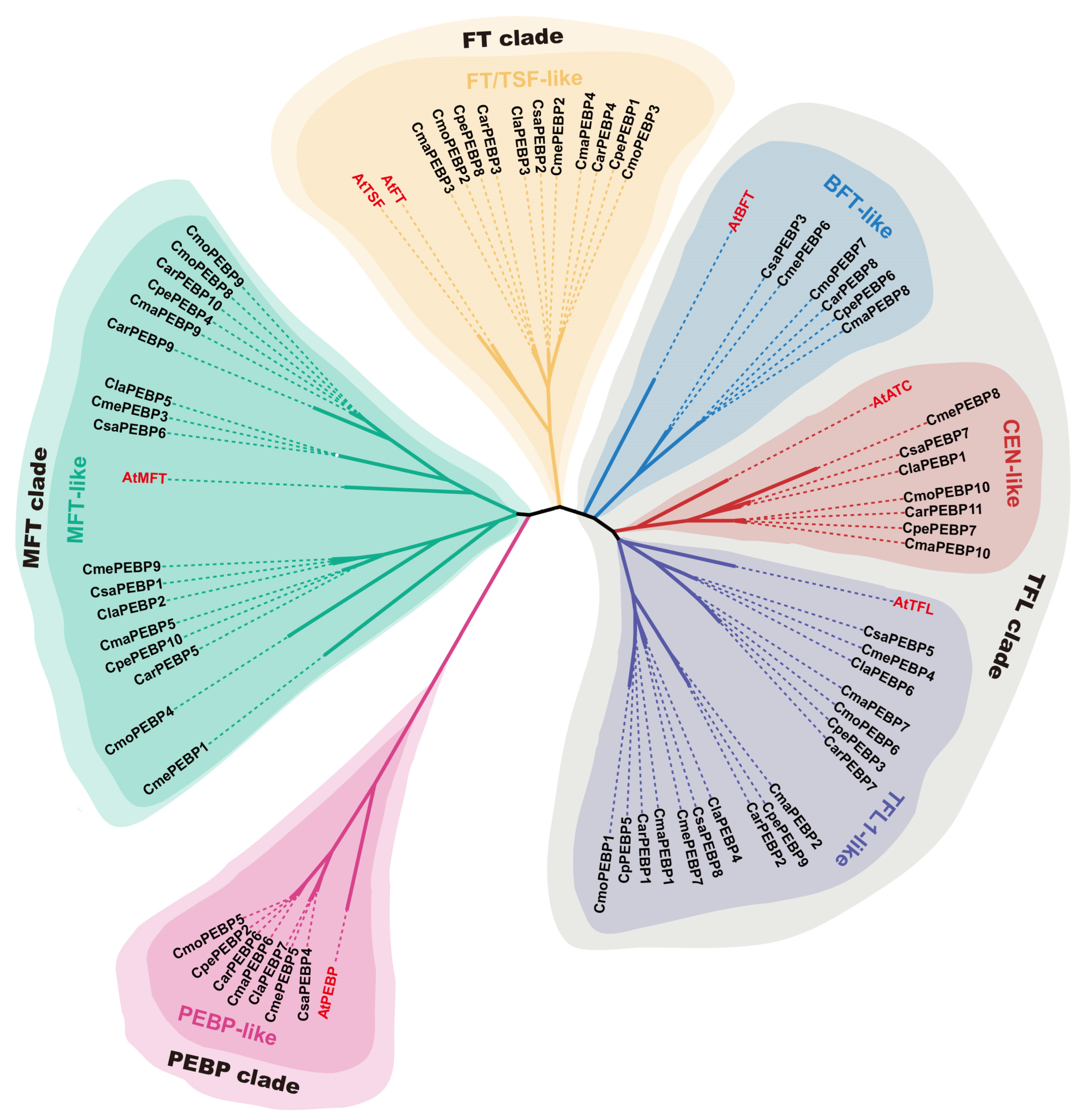
2.2. Evolution of PEBP Genes in Arabidopsis and Seven Cucurbit Crops
2.3. Comparative Analysis of the PEBP Family Gene Structure
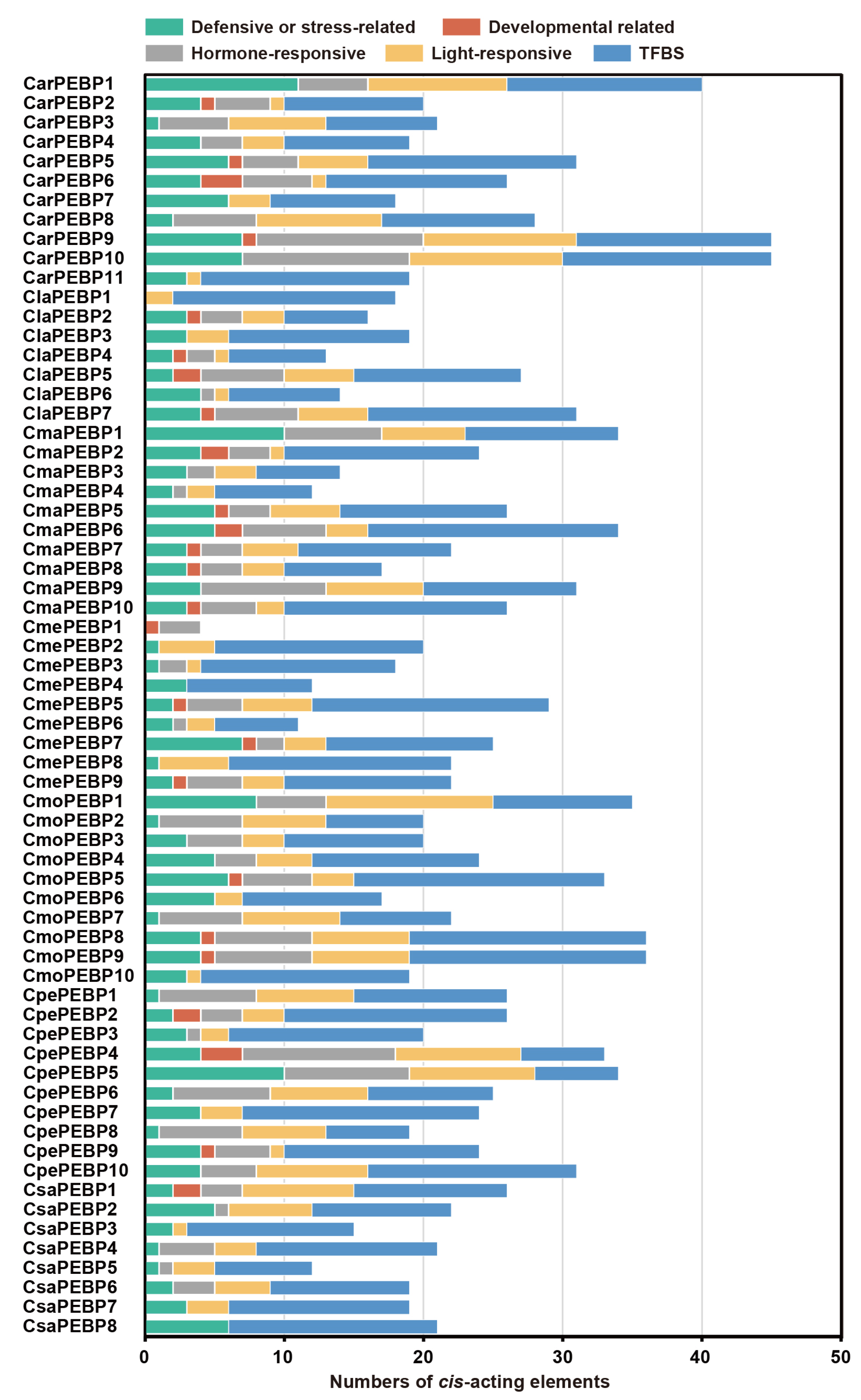
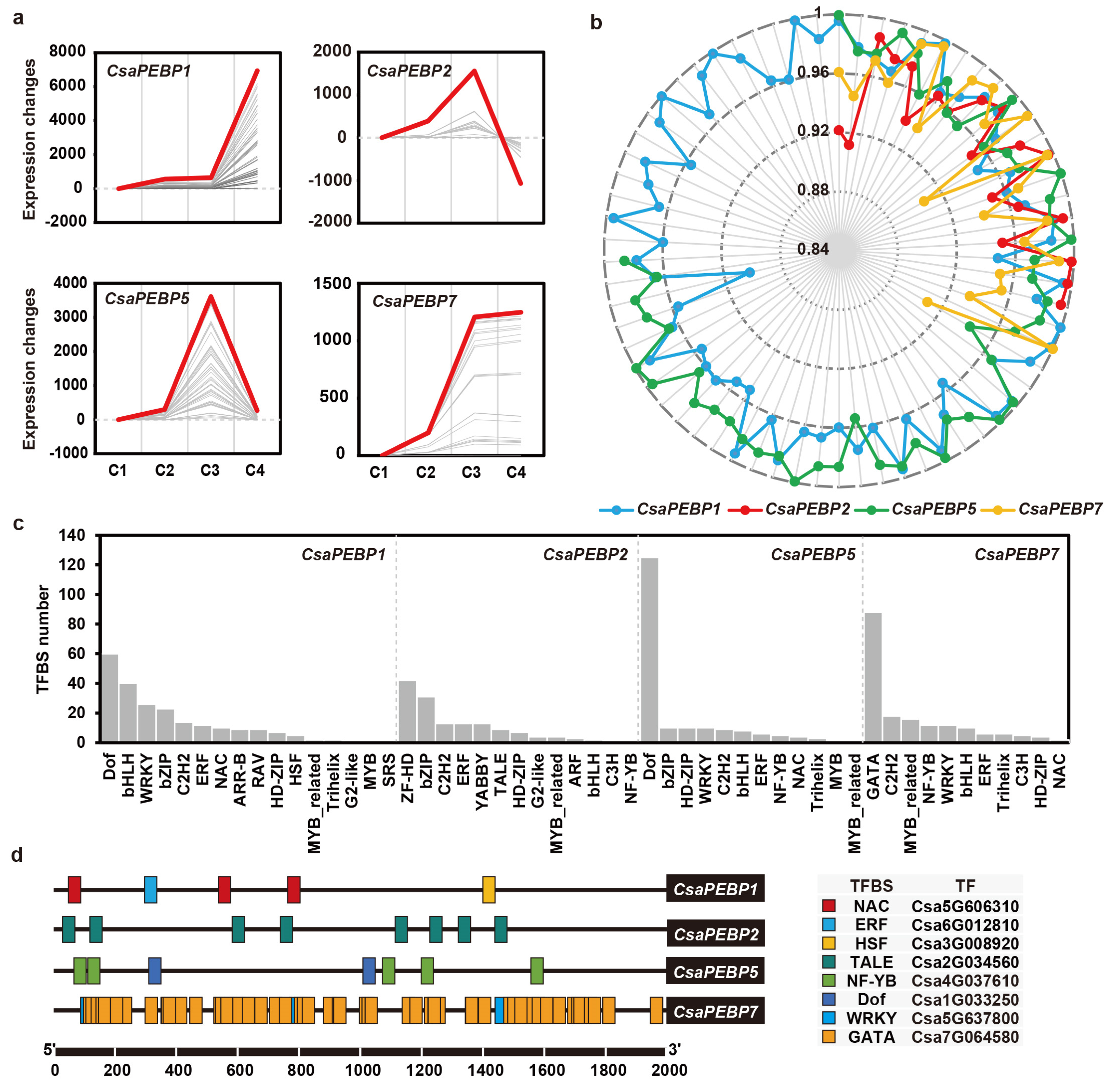
2.4. Cis-Acting Elements in the PEBP Promoter Regions
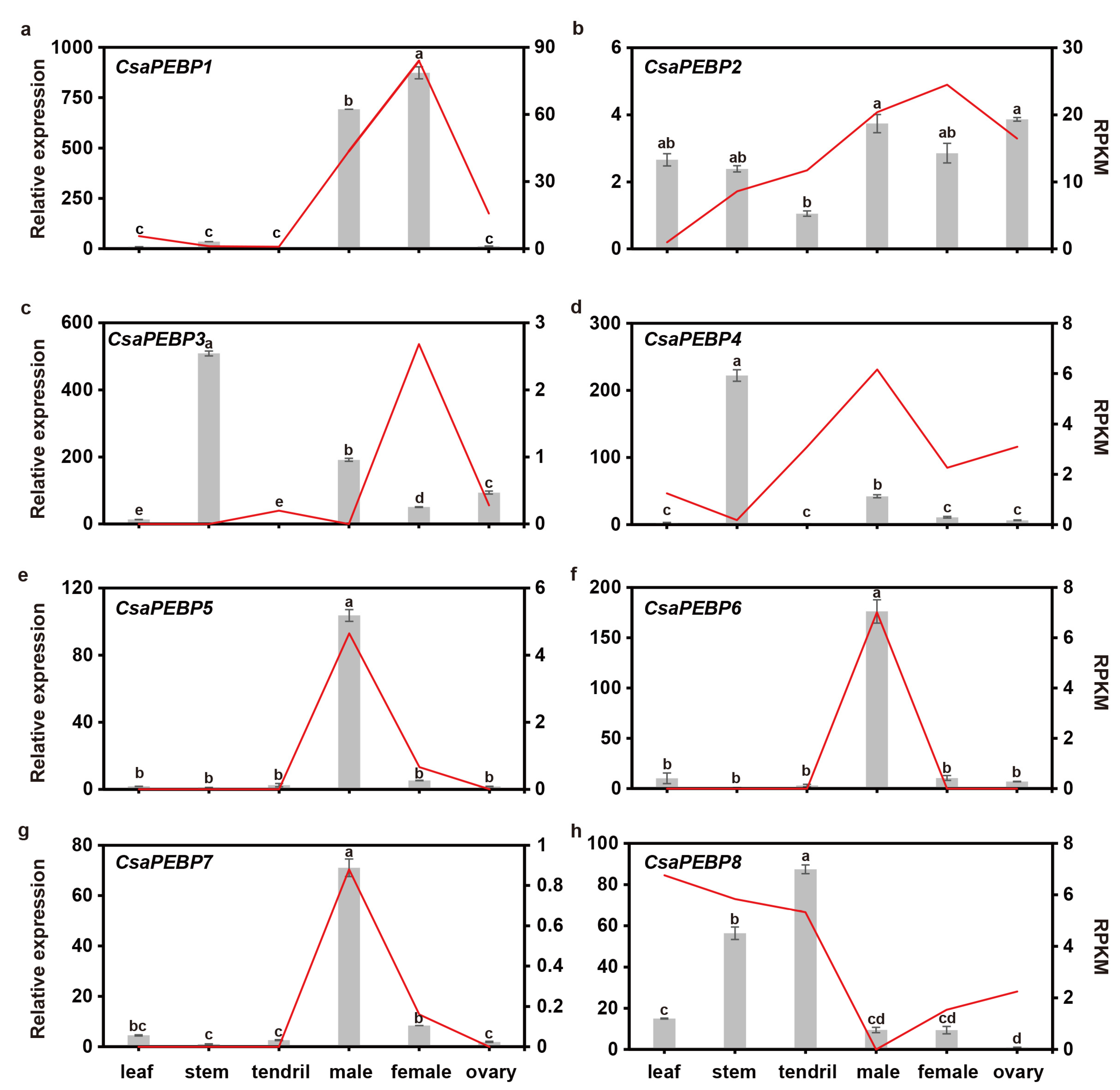
2.5. Spatio-Temporal Expression Patterns of CsaPEBP Genes
2.6. Screening for TFs That Interact with CsaPEBPs
3. Discussion
3.1. Conservation of the PEBP Family in Seven Cucurbits
3.2. Functional Diversification of Cucumber PEBP Genes
3.3. Novel TFs That Potentially Interact with CsaPEBPs
4. Materials and Methods
4.1. Search for PEBP Family Members in Cucurbits
4.2. Multiple Alignments, Phylogenetic and Synteny Relationships Analysis
4.3. Gene Localization and Structure Analysis
4.4. Investigation of Cis-Elements in the Promoter Region
4.5. Plant Sampling, RNA Preparation and RT-qPCR Analysis
4.6. Clustering of Time-Series and Correlation Analysis Based on RNA-Seq Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karlgren, A.; Gyllenstrand, N.; Källman, T.; Sundström, J.F.; Moore, D.; Lascoux, M.; Lagercrantz, U. Evolution of the PEBP gene family in plants: Functional diversification in seed plant evolution. Plant Physiol. 2011, 156, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shi, J.; Wu, J.; Zhang, J.; Chen, H.; Li, Y.; Liu, S.; Wu, Y.; Tian, Z.; Cao, X.; et al. A modified HLA-A*0201-restricted CTL epitope from human oncoprotein (hPEBP4) induces more efficient antitumor responses. Cell. Mol. Immunol. 2018, 15, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Kobayashi, Y.; Goto, K.; Abe, M.; Araki, T. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol. 2005, 46, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Chung, K.S.; Jung, S.H.; Yoo, S.Y.; Lee, J.S.; Ahn, J.H. BROTHER OF FT AND TFL1 (BFT) has TFL1-like activity and functions redundantly with TFL1 in inflorescence meristem development in Arabidopsis. Plant J. 2010, 63, 241–253. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Kardailsky, I.; Lee, J.S.; Weigel, D.; Ahn, J.H. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1). Mol. Cells 2004, 17, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Liu, C.; Hou, X.; Yu, H. MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 2010, 22, 1733–1748. [Google Scholar] [CrossRef]
- Banfield, M.J.; Brady, R.L. The structure of Antirrhinum centroradialis protein (CEN) suggests a role as a kinase regulator. J. Mol. Biol. 2000, 297, 1159–1170. [Google Scholar] [CrossRef]
- Ahn, J.H.; Miller, D.; Winter, V.J.; Banfield, M.J.; Lee, J.H.; Yoo, S.Y.; Henz, S.R.; Brady, R.L.; Weigel, D. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J. 2006, 25, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Shen, Y.; Chang, H.C.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C.; et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef]
- Cao, S.; Kumimoto, R.W.; Gnesutta, N.; Calogero, A.M.; Mantovani, R.; Holt, B.F. A distal CCAAT/NUCLEAR FACTOR Y complex promotes chromatin looping at the FLOWERING LOCUS T promoter and regulates the timing of flowering in Arabidopsis. Plant Cell 2014, 26, 1009–1017. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.; Hu, Y.; Huang, J.; Zhao, Y.; Zhang, L.; Huang, C.H.; Ma, H. Phylotranscriptomics in Cucurbitaceae reveal multiple whole-genome duplications and key morphological and molecular innovations. Mol. Plant 2020, 13, 1117–1133. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Zheng, Y.; Guo, J.; Yuan, S.; Fu, A.; Bai, C.; Zhao, X.; Zheng, S.; Wen, C.; et al. Cucurbitaceae genome evolution, gene function and molecular breeding. Hortic. Res. 2022, 9, uhab057. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.K.; Belanger, H.; Lee, Y.J.; Varkonyi-Gasic, E.; Taoka, K.; Miura, E.; Xoconostle-Cázares, B.; Gendler, K.; Jorgensen, R.A.; Phinney, B.; et al. FLOWERING LOCUS T protein may act as the long-distance florigenic signal in the cucurbits. Plant Cell 2007, 19, 1488–1506. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lin, T.; Klein, J.; Wang, S.; Qi, J.; Zhou, Q.; Sun, J.; Zhang, Z.; Weng, Y.; Huang, S. QTL-seq identifies an early flowering QTL located near Flowering Locus T in cucumber. Theor. Appl. Genet. 2014, 127, 1491–1499. [Google Scholar] [CrossRef]
- Wen, C.; Zhao, W.; Liu, W.; Yang, L.; Wang, Y.; Liu, X.; Xu, Y.; Ren, H.; Guo, Y.; Li, C.; et al. CsTFL1 inhibits determinate growth and terminal flower formation through interaction with CsNOT2a in cucumber. Development 2019, 146, dev180166. [Google Scholar] [CrossRef] [PubMed]
- Gimode, W.; Clevenger, J.; Mcgregor, C. Fine-mapping of a major quantitative trait locus Qdff3-1 controlling flowering time in watermelon. Mol. Breed. 2020, 40, 3. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Yan, P.; Huang, S.; Fei, Z.; Lin, K. RNA-Seq improves annotation of protein-coding genes in the cucumber genome. BMC Genom. 2011, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Qiao, L.; Chen, J.; Rong, Y.; Zhao, Y.; Cui, X.; Xu, J.; Hou, X.; Dong, C.H. Arabidopsis REM16 acts as a B3 domain transcription factor to promote flowering time via directly binding to the promoters of SOC1 and FT. Plant J. 2020, 103, 1386–1398. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Y.; Huang, X.; Gong, Y.; Wang, S.; Dai, Z. Genome-wide identification of flowering time genes in cucurbit plants and revealed a gene ClGA2/KS associate with adaption and flowering of watermelon. Mol. Biol. Rep. 2020, 47, 1057–1065. [Google Scholar] [CrossRef]
- Chardon, F.; Damerval, C. Phylogenomic analysis of the PEBP gene family in cereals. J. Mol. Evol. 2005, 61, 579–590. [Google Scholar] [CrossRef]
- Danilevskaya, O.N.; Meng, X.; Hou, Z.; Ananiev, E.V.; Simmons, C.R. A genomic and expression compendium of the expanded PEBP gene family from maize. Plant Physiol. 2008, 146, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, L.; Kohnen, M.V.; Xiong, B.; Zhen, X.; Liao, J.; Oka, Y.; Zhu, Q.; Gu, L.; Lin, C.; et al. Identification and characterization of the PEBP family genes in moso bamboo (Phyllostachys heterocycla). Sci. Rep. 2019, 9, 14998. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Li, Y.; Li, Z.; Qi, J.; Lin, T.; Yang, X.; Zhang, Z.; Huang, S. FLOWERING LOCUS T improves cucumber adaptation to higher latitudes. Plant Physiol. 2020, 182, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jia, X.; Yang, Z.; Fu, Q.; Yang, H.; Xu, X. Genome-wide identification of PEBP gene family in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 9185. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, Y.; Chen, F.; Jiang, J. Functional diversification and molecular mechanisms of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in horticultural plants. Mol. Hortic. 2022, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, P.; Yan, X.; Wang, J.; Cheng, T.; Zhang, Q. Genome-wide characterization of PEBP family genes in nine Rosaceae tree species and their expression analysis in P. mume. BMC Ecol. Evol. 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xuan, L.; Jiang, Y.; Yu, H. Regulation by FLOWERING LOCUS T and TERMINAL FLOWER 1 in flowering time and plant architecture. Small Struct. 2021, 2, 2000125. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Q.; Lin, R. The B3-domain transcription factor VAL1 regulates the floral transition by repressing FLOWERING LOCUS T. Plant Physiol. 2019, 181, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Sun, X.; Yu, L.; Wang, E.; Cheng, Z.; Liu, H.; Jiang, P.; Qin, J.; Begum, S.; Song, B. Transcription factor StABI5-like 1 binding to the FLOWERING LOCUS T homologs promotes early maturity in potato. Plant Physiol. 2022, 189, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Zhang, H.; Chen, L.; Yu, D. ERF1 delays flowering through direct inhibition of FLOWERING LOCUS T expression in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 1712–1723. [Google Scholar] [CrossRef]
- Paysan-Lafosse, T.; Blum, M.; Chuguransky, S.; Grego, T.; Pinto, B.L.; Salazar, G.A.; Bileschi, M.L.; Bork, P.; Bridge, A.; Colwell, L.; et al. InterPro in 2022. Nucleic Acids Res. 2023, 51, D418–D427. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Ge, J.; Wang, Q.; Chen, G.; Kassegne, K.; Zhang, H.; Yu, J.; Tang, J.; Wang, B.; Lu, F.; Cao, J.; et al. Immunogenicity and antigenicity of a conserved fragment of the rhoptry-associated membrane antigen of Plasmodium vivax. Parasites Vectors 2022, 15, 428. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Chow, C.N.; Yang, C.W.; Wu, N.Y.; Wang, H.T.; Tseng, K.C.; Chiu, Y.H.; Lee, T.Y.; Chang, W.C. PlantPAN 4.0: Updated database for identifying conserved non-coding sequences and exploring dynamic transcriptional regulation in plant promoters. Nucleic Acids Res. 2024, 52, D1569–D1578. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Luo, X.; Li, H.; Gao, Q.; Zhang, L.; Teng, Y.; Zhao, Q.; Zuo, Z.; Ren, J. IBS 2.0: An upgraded illustrator for the visualization of biological sequences. Nucleic Acids Res. 2022, 50, W420–W426. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Zhao, W.; Song, X.; Lu, M.; Li, X.; Li, X.; Liu, R.; Yan, L.; Zhang, X. Integration of hormonal and nutritional cues orchestrates progressive corolla opening. Plant Physiol. 2016, 171, 1209–1229. [Google Scholar] [CrossRef]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinform. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Zhu, Z.; Lin, X.; Shen, X.; Yang, T.; Wang, H.; Zhou, X. Comparative Genomic Analysis of PEBP Genes in Cucurbits Explores the Interactors of Cucumber CsPEBPs Related to Flowering Time. Int. J. Mol. Sci. 2024, 25, 3815. https://doi.org/10.3390/ijms25073815
Fan L, Zhu Z, Lin X, Shen X, Yang T, Wang H, Zhou X. Comparative Genomic Analysis of PEBP Genes in Cucurbits Explores the Interactors of Cucumber CsPEBPs Related to Flowering Time. International Journal of Molecular Sciences. 2024; 25(7):3815. https://doi.org/10.3390/ijms25073815
Chicago/Turabian StyleFan, Lianxue, Ziyi Zhu, Xiaoru Lin, Xia Shen, Tianjiao Yang, Haixin Wang, and Xiuyan Zhou. 2024. "Comparative Genomic Analysis of PEBP Genes in Cucurbits Explores the Interactors of Cucumber CsPEBPs Related to Flowering Time" International Journal of Molecular Sciences 25, no. 7: 3815. https://doi.org/10.3390/ijms25073815
APA StyleFan, L., Zhu, Z., Lin, X., Shen, X., Yang, T., Wang, H., & Zhou, X. (2024). Comparative Genomic Analysis of PEBP Genes in Cucurbits Explores the Interactors of Cucumber CsPEBPs Related to Flowering Time. International Journal of Molecular Sciences, 25(7), 3815. https://doi.org/10.3390/ijms25073815




