Abstract
Obesity and overweight are common and complex conditions influenced by multiple genetic and environmental factors. Several genetic variants located in the genes involved in clock systems and fat taste perception can affect metabolic health. In particular, the polymorphisms in CLOCK and BMAL1 genes were reported to be significantly related to cardiovascular disease, metabolic syndrome, sleep reduction, and evening preference. Moreover, genetic variants in the CD36 gene have been shown to be involved in lipid metabolism, regulation of fat intake, and body weight regulation. The aim of this study is to evaluate, for the first time, the association between variants in some candidate genes (namely, BMAL1 rs7950226 (G>A), CLOCK rs1801260 (A>G), CLOCK rs4864548 (G>A), CLOCK rs3736544 (G>A), CD36 rs1984112 (A>G), CD36 rs1761667 (G>A)) and overweight/obesity (OB) in pregnant women. A total of 163 normal-weight (NW) and 128 OB participants were included. A significant correlation was observed between A-allele in CLOCK rs4864548 and an increased risk of obesity (OR: 1.97; 95% CI 1.22–3.10, p = 0.005). In addition, we found that subjects carrying the haplotype of rs1801260-A, rs4864548-A, and rs3736544-G are likely to be overweight or obese (OR 1.47, 95% CI 1.03–2.09, p = 0.030), compared with those with other haplotypes. Moreover, a significant relation was observed between third-trimester lipid parameters and genetic variants—namely, CD36 rs1984112, CD36 rs1761667, BMAL1 rs7950226, and CLOCK rs1801260. A multivariate logistic regression model revealed that CLOCK rs4864548 A-allele carriage was a strong risk factor for obesity (OR 2.05, 95% CI 1.07–3.93, p = 0.029); on the other hand, greater adherence to Mediterranean diet (OR 0.80, 95% CI 0.65–0.98, p = 0.038) and higher HDL levels (OR 0.96, 95% CI 0.94–0.99, p = 0.021) were related to a reduced risk of obesity. Interestingly, an association between maternal CLOCK rs4864548 and neonatal birthweight was detected (p = 0.025). These data suggest a potential role of the polymorphisms in clock systems and in fat taste perception in both susceptibility to overweight/obesity and influencing the related metabolic traits in pregnant women.
1. Introduction
Overweight and obesity represent a significant public health issue related to an increased risk of chronic noncommunicable diseases (such as cardiovascular disease and diabetes mellitus). Both overweight and obesity are also the most common clinical conditions in women of reproductive age, representing relevant clinical entities that complicate pregnancies [1], and are known to be caused by gene–environment interactions. These conditions are linked with short- and long-term consequences for the mother [including increasing risk of gestational diabetes mellitus (GDM), hypertension, and preeclampsia] and their offspring [miscarriage, stillbirth, congenital anomalies, shoulder dystocia, macrosomia, and neonatal death] [2,3]. Metabolically, women with obesity present increased insulin resistance from the beginning of pregnancy, which may manifest during pregnancy as glucose intolerance and possible fetal overgrowth [1]. Synchronized circadian rhythms, which are closely related to health [4], consist of approximately 24 h intrinsic oscillations and are affected by the central/primary clock (located in the suprachiasmatic nucleus of the hypothalamus, SCN) and the periphery clock (located in the peripheral tissues) [5,6]. Circadian clocks are molecular machines that organize daily timing, orchestrating a wide range of molecular, physiological, and behavioral processes [7,8]. The molecular machinery of the circadian rhythm is made up of four main proteins, each of which regulates the expression of other downstream genes in a negative feedback loop. In detail, the core of this machinery is BMAL1 (Brain and Muscle ARNT-Like 1)–CLOCK (circadian locomotor output cycles kaput) heterodimer that induces the expression of PER and CRY. These transcription factors, in turn, inhibit the expression of BMAL1 and CLOCK. Thus, the lack of BMAL1 and CLOCK inhibits the expression of CRY and PER so that the cycle can begin again [9]. Pathological changes associated with the circadian gene have been linked to various phenotypes originating from disrupted physiological processes, including cancer and different types of tumors [10,11,12], metabolic diseases [13,14], cardiovascular diseases [15], sleep disorders [16], hypercholesterolemia, and hyperglycemia [17]. Moreover, several studies showed that dysfunction of the circadian clock may be a critical parameter contributing to the development of obesity [18]. Circadian gene variants are reported to be associated with BMI, increasing the risk of overweight and obesity by up to 1.5-fold [19,20,21,22,23]. Nevertheless, few studies have evaluated the relationship between circadian rhythms and reproduction, and circadian rhythm disruption has received less attention in pregnant women. Interestingly, there is growing interest in the role of circadian rhythms in metabolic regulation, as well as the lack of any studies on the role of clock genes and circadian rhythms in pregnant women. Thus, the investigated SNPs in the genes involved in clock systems (CLOCK and BMAL1) and taste genes (CD36) were selected in this study based on their genomic position and their function, according to several genome-wide association studies (GWAS) and the NCBI dbSNP database. In particular, rs7950226 (G>A) in the BMAL1 gene and rs1801260 (A>G), rs4864548 (G>A), and rs3736544 (G>A) in the CLOCK gene were reported to be significantly correlated with cardiovascular disease, obesity, metabolic syndrome, sleep reduction, and evening preference [22,23,24,25]. Moreover, rs1984112 (A>G) and rs1761667 (G>A) in the CD36 gene have been identified to be involved in lipid metabolism, regulation of fat intake, and body weight regulation [26,27,28,29].
Therefore, the aim of the present study is to investigate the association between variants in candidate genes—namely, BMAL1 rs7950226 (G>A), CLOCK rs1801260 (A>G), CLOCK rs4864548 (G>A), and CLOCK rs3736544 (G>A)—and overweight/obesity in 291 pregnant women. The genetic variants of the CD36 gene, reducing CD36 transcript, could explain the differences in fat perception and fat preferences [26,30]; on the other hand, the relationship between overweight/obesity and SNPs in CD36 among pregnant women has not been fully clarified. Therefore, two selected variants in CD36 (rs1984112 A>G and rs1761667 G>A) were also included in this study.
2. Results
2.1. Clinical and Anthropometric Data
The demographic, anthropometric, and clinical parameters of the two groups are presented in Table 1. A total of 291 pregnant women were included in the study: specifically, 163 NW (pre-pregnancy BMI = 21.4 ± 2.0) and 128 OB participants (pre-pregnancy BMI = 32.3 ± 6.4). The mean age of the women was 33.9 ± 5.1 years. There were statistical differences between the two groups regarding age and school education. In particular, the OB women showed lower mean age (33.2 ± 5.3 vs. 34.4 ± 5.0, p = 0.05) and lower educational status (p < 0.001) compared with controls. In addition, the OB group showed a higher BMI at the end of pregnancy (35.2 ± 6.6 vs. 25.9 ± 2.6, p < 0.0001) and a lower increase in average weight gain at delivery vs. the weight recorded before pregnancy than the women of the control group (p < 0.001). They also showed higher systolic (120.3 ± 12.8 vs. 113.5 ± 12.5, p < 0.001) and diastolic blood pressure (73.4 ± 9.5 vs. 69.2 ± 9.1, p = 0.001) when compared with controls. The cases group showed lower serum concentrations of HDL-c (62.0 ± 14.2 vs. 67.2 ± 15.2, p = 0.008) during the third trimester than the control group. A significant difference was detected between the two groups regarding the delivery—specifically, the cesarean sections in OB women were higher than in controls (p = 0.019). All participants underwent OGTT, and mean glucose values are presented in Table 1.

Table 1.
Demographic, anthropometric, and clinical data of NW and OB pregnant women expressed as mean and standard deviation (SD), absolute frequency (n), and column percentage (%).
The percentage of women in the two groups who had a diagnosis of GDM is summarized in Table 1. The OB group showed a higher occurrence of GDM than controls (OR:2.60; 95% CI 1.57–4.27, p < 0.001). All GDM women were informed of their hyperglycemic status, and they received the same nutritional advice in accordance with current recommendations. The NW women with GDM required a significantly lower insulin requirement than cases (p < 0.001).
2.2. Lifestyle Habits
Table 2 shows circadian attitude, Mediterranean diet adherence, smoking habits, and PA in the third trimester of pregnancy. The scores on the rMEQ showed that the sample comprised 33.8% morning type, 3.4% evening type, and 62.7% intermediate type. The distribution of the MEQ results demonstrated no significant differences between the two groups (Table 2). The median PREDIMED score was 9.1 and 8.4 for NW and OB women, respectively (p = 0.007). No differences in the PA between the groups were found. Smoking differed significantly between the cases and the control group (p = 0.004). In fact, 44.8% and 52.6% of former smokers and non-smokers were found in the cases group, respectively. Other comparisons between groups were not significant.

Table 2.
Lifestyle habits for cases and controls.
2.3. Newborn’s Data
The main neonatal anthropometric characteristics are shown in Table 3. Mean gestational age at delivery was statistically significantly lower in the OB group (p < 0.001) (Table 3). No other differences were found between the two groups.

Table 3.
Neonatal outcomes relative to NW and OB women.
2.4. Genotype Analysis
The genotype distributions of the polymorphisms tested based on additive, dominant, and recessive inheritance genetic models are shown in Table 4. A significant difference was detected in terms of genotype distribution of rs4864548 (G>A) in CLOCK between the OB group and controls (Table 4). In fact, the AG genotype was significantly more frequent among OB women compared with controls (53.9% vs. 36.2%, p = 0.009). Furthermore, using a dominant inheritance model ((AA+AG) vs. (GG)), the A-allele in CLOCK rs4864548 was found to be significantly overrepresented in OB cases compared with the controls (OR: 1.97; 95% CI 1.22–3.10, p = 0.005). For the other SNPs, in additive, dominant, and recessive inheritance genetic models, no significant differences in the genotype frequency were detected between the two groups. Furthermore, no significant association was found between the six genetic variants studied and GDM status.

Table 4.
Genotype distributions of the polymorphisms for cases and controls.
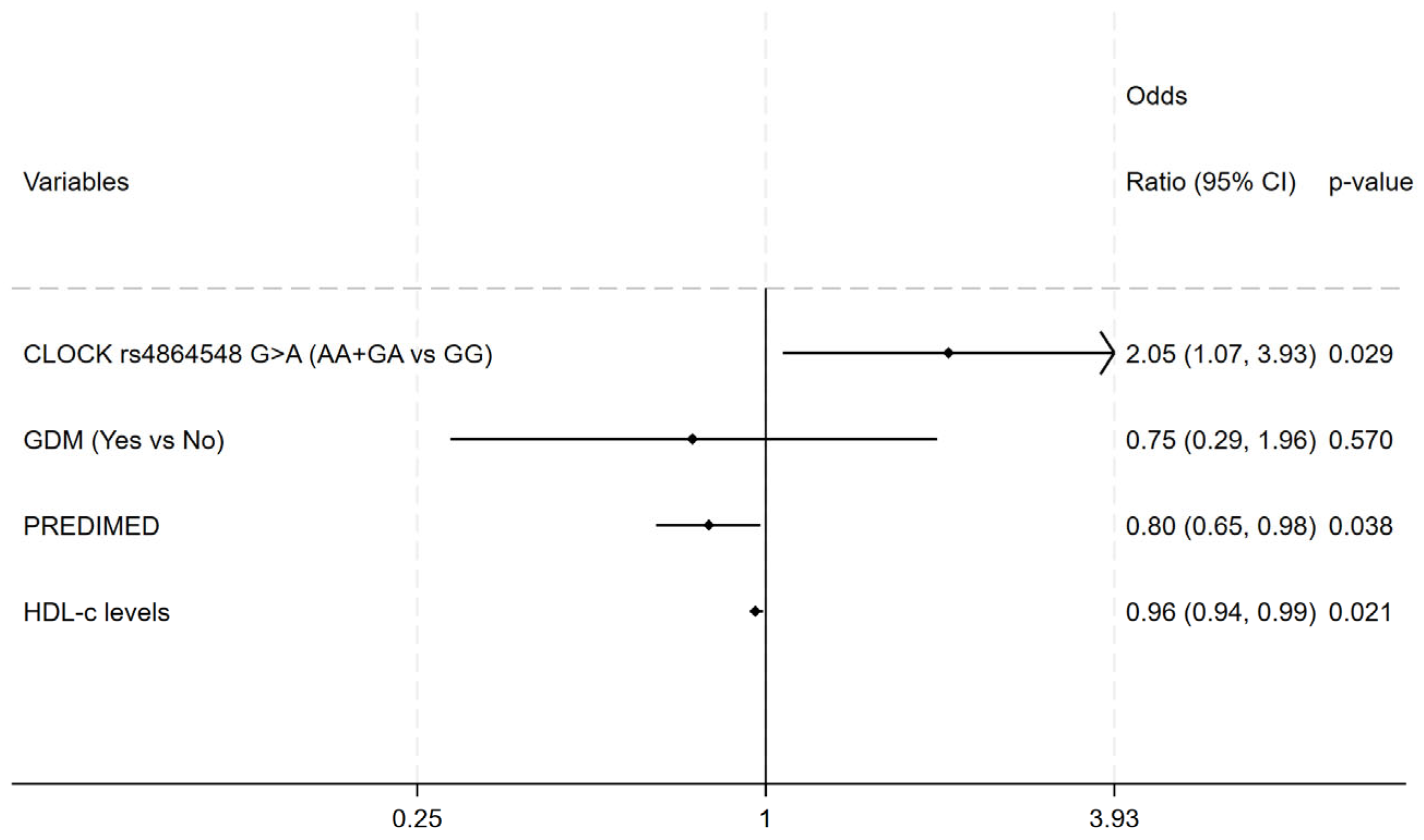
A significant relation between CD36 rs1984112 (A>G) with the third-trimester LDL-C as well as third-trimester TC in OB women was detected. In fact, G-allele carriers showed lower serum level of TC and LDL-C compared with A-allele carriers in both additive (for TC: AA 257.67 ± 52.03; AG 252.89 ± 50.48; GG 218.56 ± 54.75; p = 0.036; for LDL-c: AA 149.82 ± 43.23; AG 147.63 ± 40.08; GG 108.52 ± 46.65; p = 0.003) and recessive (for TC: GG 218.56 ± 54.76; AA+AG 254.59 ± 50.51; p = 0.011; for LDL-c: GG 108.52 ± 46.65; AA+AG 148.44 ± 41.03; p < 0.0001) genetic models. A significant relation was observed between CD36 rs1761667 (G>A) and third-trimester LDL-c as well as TC levels in both OB cases (for TC: GG 231.74 ± 49.93; AA+GA 256.43 ± 52.27, p = 0.028; for LDL-c: GG 126.45 ± 43.47; AA+GA 149.06 ± 43.07; p = 0.017) and controls (for TC: GG 229.00 ± 44.44; AA+GA 254.15 ± 50.11; p = 0.023; for LDL-c: GG 125.13 ± 43.42; AA+GA 146.18 ± 41.60; p = 0.032; for HDL-c: GG 61.08 ± 13.38; AA+GA 68.67 ± 15.26; p = 0.024). Then, an association was observed between BMAL1 rs7950226 (G>A) with third-trimester HDL-c levels of NW women in both additive (AA 72.77 ± 15.61; AG 64.31 ± 15.23; GG 66.90 ± 13.92; p = 0.047) and recessive (AA 72.76 ± 15.61; AA+GA 65.41 ± 14.67; p = 0.019) genetic models. Finally, a significant relation between CLOCK rs1801260 (A>G) and third-trimester TG levels (AA 195.31 ± 65.73, GG+AG 235.84 ± 101.22; p = 0.010) was found in the controls. In fact, the AA genotype showed lower TG levels compared with G-allele carriers in the dominant model. All the investigated genotype frequencies were within the Hardy–Weinberg equilibrium in both the cases and controls. In addition, we found that subjects carrying the haplotype of rs1801260-A, rs4864548-A, and rs3736544-G were likely to be OB compared with those with other haplotypes (36.3% vs. 27.9% p = 0.030; OR 1.47, 95% CI 1.03–2.09) Multivariate logistic regression analysis revealed that CLOCK rs4864548 A-allele carriage was a strong risk factor for obesity (OR 2.05, 95% CI 1.07–3.93, p = 0.029); on the other hand, greater adherence to the Mediterranean diet (OR 0.80, 95% CI 0.65–0.98, p = 0.038) and higher HDL levels (OR 0.96, 95% CI 0.94–0.99, p = 0.021) were related to a reduced risk of obesity (Figure 1). The Hosmer–Lemeshow test indicated a good fit of the model in describing the data (chi-square = 91.55, p = 0.439)

Figure 1.
Multivariate logistic regression model for obesity. Independent variables: CLOCK rs4864548, GDM, PREDIMED and HDL-C levels; Adjusted odds ratio (95% confidence interval) and p-value.
Finally, regarding neonatal outcomes, an association between maternal CLOCK rs4864548 and neonatal birthweight was detected in dominant genetic models. In detail, A- allele carriers showed lower neonatal birthweight compared with GG genotype carriers in the cases group (AA+AG: 3171.97 ± 447.44; GG: 3386.88 ± 422.64; p = 0.025).
3. Discussion
Obesity is a complex and multifactorial condition to which both lifestyle habits and genetic susceptibility contribute. The increased prevalence of women with overweight and obesity leads to an increase in pregnancies with obesity and, consequently, a global health burden that potentially has several adverse outcomes. CLOCK is a key component of the mammalian molecular clock within the pacemaker neurons of the SCN and plays an important role in fat and glucose metabolism in peripheral organs (including adipose tissue, muscle, and liver) [31,32]. Since circadian disruptions may contribute to different metabolic-related traits, several studies have evaluated the role of common SNPs located in genes involved in clock systems [18]. Previously, Deng et al. [33] identified, through a whole-genome scan and by comparison with earlier studies, the region 4q12, the chromosome location of the CLOCK gene, potentially important for obesity phenotypes. In addition, cross-sectional studies have shown associations between this locus and obesity, plasma glucose, hypertension, and T2D, also suggesting its role in cardiovascular risk. Given the emerging evidence showing that alteration in circadian rhythmicity can induce pathophysiological changes, in the present work, we evaluated for the first time the association between overweight/obesity in pregnant women and genetic variants involved with circadian regulation and fat preferences. In this study, we have shown that the A-allele in CLOCK rs4864548 is found to be significantly more frequent among OB women compared with the controls. In addition, subjects carrying the rs1801260-A/rs4864548-A/rs3736544-G haplotype are likely to be obese compared with those with other haplotypes. Moreover, our results confirm that SNPs in genes involved in the circadian clock and fat taste perception have been linked to third-trimester lipid profiles. As extensively reported in the literature, lipid absorption and metabolism are regulated by some of the core circadian rhythm genes [34,35,36,37,38]. Moreover, the possible interaction between the CD36 polymorphisms and lipid metabolism has been demonstrated [39]. CD36 receptor is attended in many biological processes, such as oral fat perception, inflammation, angiogenesis, atherosclerosis, immune regulation, and insulin resistance [40,41,42]. Genome-wide linkage scans have identified nearby regions of chromosome 7, where the CD36 gene is located, associated with TG, HDL-C, and TG/HDL ratio [43]. Therefore, genetic variants in fat taste preference are associated with cardiovascular risk factors, modulating lipid metabolism. In addition, CD36 contributes preferentially to the intake of some nutrients and adversely affects the consumption of others, suggesting interindividual variability in body weight regulation [26]. Future investigations must be conducted to shed light on the functional role of CD36, BMAL1, and CLOCK on lipid metabolism in pregnancy. Obesity prevention, particularly in pregnancy, which is a crucial stage in a woman’s life, has generally focused on modifiable risk factors. The literature supports the importance of lifestyle and dietary habits during pregnancy; in particular, the Mediterranean diet is positively related to maternal and neonatal outcomes [44,45,46,47,48]. In fact, it has been demonstrated that Mediterranean diet adherence is crucial for a correct gestational period with a protective effect on preterm delivery and glucose regulation [49]. In this view, our multivariate logistic regression model showed that CLOCK rs4864548 A-allele carriage was a strong risk factor for obesity. On the other hand, in agreement with the literature, the obesity risk decreased in pregnant women with higher HDL levels and higher adherence scores to the Mediterranean diet. These data strengthen the important role of modifiable risk factors, specifically Mediterranean dietary patterns, and suggest that targeted health and lifestyle interventions could attenuate genetic susceptibility. Therefore, lifestyle modifications, such as a higher Mediterranean diet adherence or smoking cessation, as well as physical activity, represent a promising strategy for reducing the risk of other complications in pregnancy and further in time. Pregnancy could be affected by epigenetic and environmental factors contributing to the complexity of the metabolic status of both mothers and their offspring [50]. An association between maternal CLOCK rs4864548 and neonatal birthweight was detected in this study. It is possible to assume that some effects of the regulatory genetic variants on body weight may be due to their effects on their expression level. Therefore, an interaction between CLOCK gene variants, which are located in regulatory sites, and lifestyle habits could also be possible; whereby, DNA methylation of this gene could induce the silencing of their expression and, eventually, consequences for the child and adult health. In conclusion, our study, for the first time, shows an association between CLOCK genetic variants and obesity in pregnant women, also strengthening the link between the rs1801260A/rs4864548-A/rs3736544-G haplotype of the core clock gene and the susceptibility to obesity. The present study has some limitations. Epigenetic alterations should be explored as potential molecular mechanisms of the effects occurring during pregnancy, and additional research identifying other plausible pathways is needed. Given the potential role of the genotype of CLOCK on gene expression, it is necessary to analyze the effect of genotypes on epigenetic mechanisms, particularly on DNA methylation levels. Future studies will be needed to understand the gene–diet interactions and how circadian disturbances during pregnancy may influence the development of chronic diseases, including diabetes, cardiovascular disease, and cancer, in both mothers and offspring.
4. Materials and Methods
4.1. Study Design and Participants
A total of 291 Caucasian pregnant women were recruited at the Diabetes, Nutrition, and Metabolism Unit and/or the Obstetrics and Gynaecology Clinic at the Hospital of University “Gabriele d’Annunzio” in Chieti-Pescara. This cross-sectional study received approval from the Ethics Committee of the Province di Chieti and Pescara (protocol code: richxrked; date of approval: 12 December 2019) in accordance with the Helsinki Declaration. All participants signed and gave back written informed consent prior to their participation in the study. The inclusion criteria consisted of pregnant women 18 years of age or older. The exclusion criteria: women suffering from pre-pregnancy diabetes, overt diabetes, or monogenic diabetes, and women with other chronic diseases. Overweight and obese (OB) pregnant women (cases) had pre-pregnancy body mass index (BMI) ≥ 25 and ≥30 kg/m2, respectively. Normal weight (NW) pregnant women (controls) were included if they reported pre-pregnancy body mass index (BMI) ≥ 18.5 and <25 kg/m2.
4.2. Anthropometric and Clinical Measurements
At the first medical examination, sociodemographic characteristics as well as clinical and anthropometric parameters were collected, including pre-pregnancy BMI and blood pressure.
In the third trimester of pregnancy, lipid profile and fasting plasma glucose were collected. At the end of pregnancy, BMI at the end of the pregnancy and gestational weight gain were recorded. Women’s body weight and height were measured using mechanical scales with a stadiometer while participants wore light clothing and no shoes. BMI was calculated by applying the following formula: weight in kilograms/(height in meters × height in meters). Gestational diabetes mellitus (GDM) diagnosis was proved both at the 16–18th and/or at the 24–28th weeks of gestation according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria [51]. In accordance with current guidelines, a blood glucose test was performed by the glucose reductase method, and HbA1c was measured according to the IFCC (International Federation of Clinical Chemistry and Laboratory Medicine) system [52].
In detail, the diagnosis of GDM was made by 75 g OGTT when any of the following plasma glucose levels were met or exceeded: fasting of 5.1 mmol/L (92 mg/dL), 10.0 mmol/L (180 mg/dL) at 1 h, and 8.5 mmol/L (153 mg/dL) at 2 h [51]. Furthermore, the rate of women needing insulin therapy was registered.
4.3. Lifestyle Questionnaires
Circadian typology (chronotype), Mediterranean diet adherence, smoking habits, and physical activity of the women were assessed in the third trimester of pregnancy. The interindividual circadian attitude known as chronotype was evaluated using a 5-item version of the Morningness–Eveningness Questionnaire (MEQr). The scale includes 5 questions taken from the original 19-item version of the MEQ, (the most widely used morningness measure) [53]. According to Italian cutoff criteria, MEQr distinguishes three different chronotypes defined as follows: (i) “morning” (19–25 score), (ii) “intermediate” (11–18 score), and (iii) “evening” (4–10 score) types [54]. In addition, the International Physical Activity Questionnaire (IPAQ-short version) was used to assess physical activity (PA) level [55] generating three different intensity levels (low, moderate, and high PA). The 14-point Mediterranean Diet Adherence Screener (MEDAS), previously validated in the PREDIMED study [56], was used to assess adherence to the Mediterranean diet (Med-Diet). The questionnaire consists of 14 items related to food commonly consumed in the Mediterranean area, recording the frequency of consumption of the food items in servings per day or week. Each question was scored 0 or 1, and the final PREDIMED score ranged from 0 to 14 [57]. Therefore, a score of less than or equal to 5 points indicates no adherence, 6 to 9 indicates medium adherence, and greater than or equal to 10 points indicates a maximum adherence to the Med-Diet.
4.4. Newborn’s Measurements
Clinical information relating to newborns was collected at birth from a review of clinical records. Data included mode of delivery, gestational age, sex, and anthropometric measurements such as weight, height, and head circumference. Apgar scores were recorded.
4.5. Genetic Analysis
The genetic analysis was conducted at the Laboratory of Molecular Genetics, School of Medicine and Health Sciences, “G. d’Annunzio” University of Chieti-Pescara. Venous blood from each woman was collected in an EDTA tube and stored in controlled conditions at +4 °C until DNA extraction. Genomic DNA was automatically extracted and purified from peripheral blood lymphocytes, using MagPurix Blood DNA Extraction Kit 200 and MagPurix 12 EVO instrument, according to the manufacturer’s instructions (Zinexts Life Science Corp. (New Taipei City, Taiwan)). Nucleic acids were quantified by measuring UV absorption using the NanoPhotometer NP80 instrument (Implen, Inc., Westlake Village, CA, USA). SNPs genotyping was conducted with real-time PCR and TaqMan chemistry using the QuantStudio 5 real-time instrument, according to the manufacturer’s instructions (Thermo Fisher, Waltham, MA, USA). Briefly, the TaqMan method can exploit multiple channels thanks to the use of fluorescent probes with different labels. The most used fluorochromes are FAM (6-carboxy-fluorescein) and VIC (4′,7-dichloro-5′,7′-dimethoxyl-fluorescein). FAM-VIC combined in a single reaction tube allows for multiple analyses simultaneously, detecting both the wild-type allele and mutant allele. The reaction MIX was prepared by the addition of 12.5 μL TaqPathTM ProAmpTM Master Mix, 1.25 μL TaqMan® SNP Genotyping Assay (20×) (Table 5), genomic DNA up to 11.25 μL, and nuclease-free water to a total volume of 25 μL. The reaction was optimized with 20 ng of starting DNA. PCR conditions were 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. After the amplification, the raw data were analyzed with Design & Analysis 2 (DA2) software.

Table 5.
The genetic variants selected in this study and the TaqMan SNP genotyping assay.
4.6. Statistical Analysis
Descriptive analysis was carried out using mean and standard deviation (SD) or median and interquartile range (IQR) for the quantitative variables and percentage values for the qualitative ones. Normality distribution for quantitative variables was assessed by the Shapiro–Wilk test. The association between categorical data was investigated by Pearson χ2 or Fisher’s exact test and the Student’s t-test for independent data or analysis of variance (ANOVA) for more than two groups of continuous data. In addition, the Bonferroni test was used for multiple comparisons. Crude odds ratio (ORs) and corresponding 95% confidence interval (CI) were calculated in order to quantify the risk associated with obesity. A multivariable logistic regression model was performed to identify the mutually adjusted effect among obesity and the independent variables chosen on the basis of (1) the statistical significance (univariate analysis, p ≤ 0.05); (2) the clinical judgment and their contribution to the model fit (likelihood-ratio test). The goodness of fit of the multivariable logistic regression model was assessed by the Hosmer–Lemeshow test. For each investigated locus, Hardy–Weinberg equilibrium was calculated (χ2 p > 0.05). A statistical significance was set at the level of ≤0.05 unless adjustment for multiple comparisons was needed. All analyses were performed using Stata software v18.0 MP (StataCorp, College Station, TX, USA) and haplotype frequencies were estimated by the Arlequin software (version 3.5) [58]. Haplotypes were composed of CLOCK variants (rs1801260, rs4864548, and rs3736544).
Author Contributions
M.F.: conceptualization; methodology; formal analysis; investigation; data curation; writing—original draft; review and editing. P.B.: methodology; software; formal analysis; data curation. P.C.: formal analysis; data curation; L.D.T.: data curation; D.G.: clinical evaluation; data curation. M.D.N.: methodology; software; formal analysis; validation; editing. L.S.: review and editing; supervision. E.V.: conceptualization; methodology; recruitment of patients and clinical evaluation and treatment; data curation; validation; review and editing; supervision; project administration; funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Italian Ministry of Health (Ministero della Salute, PNRR-Vitality, ECS00000041).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Province di Chieti and Pescara (date of approval: 12 December 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
MF was supported by the Fondazione Umberto Veronesi.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Giouleka, S.; Tsakiridis, I.; Koutsouki, G.; Kostakis, N.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Obesity in Pregnancy: A Comprehensive Review of Influential Guidelines. Obstet. Gynecol. Surv. 2023, 78, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Dinsmoor, M.J.; Ugwu, L.G.; Bailit, J.L.; Reddy, U.M.; Wapner, R.J.; Varner, M.W.; Thorp, J.M., Jr.; Caritis, S.N.; Prasad, M.; Tita, A.T.N.; et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Short-term neonatal outcomes of pregnancies complicated by maternal obesity. Am. J. Obstet. Gynecol. 2023, 5, 100874. [Google Scholar] [CrossRef]
- Loudon, A.S. Circadian biology: A 2.5 billion year old clock. Curr. Biol. 2012, 22, R570–R571. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M.; Foster, R.; Davis, F.; Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef]
- King, D.P.; Takahashi, J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000, 23, 713–742. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Gršković, P.; Korać, P. Circadian Gene Variants in Diseases. Genes 2023, 14, 1703. [Google Scholar] [CrossRef]
- Li, H.X. The role of circadian clock genes in tumors. OncoTargets Ther. 2019, 12, 3645–3660. [Google Scholar] [CrossRef]
- Kettner, N.M.; Katchy, C.A.; Fu, L. Circadian gene variants in cancer. Ann. Med. 2014, 46, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Morales-Santana, S.; Morell, S.; Leon, J.; Carazo-Gallego, A.; Jimenez-Lopez, J.C.; Morell, M. An Overview of the Polymorphisms of Circadian Genes Associated with Endocrine Cancer. Front. Endocrinol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Young, M.E. The Cardiomyocyte Circadian Clock: Emerging Roles in Health and Disease. Circ. Res. 2010, 106, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jain, M.K. Circadian regulation of cardiac metabolism. J. Clin. Investig. 2021, 131, e148276. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tang, X.; Gong, Z.; Zeng, W.; Hou, Q.; Lu, R. Circadian Rhythm Sleep Disorders: Genetics, Mechanisms, and Adverse Effects on Health. Front. Genet. 2022, 13, 875342. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Alessandrelli, E.; Notarangelo, S.; Stuppia, L.; Vitacolonna, E. Chrono-Nutrition: Circadian Rhythm and Personalized Nutrition. Int. J. Mol. Sci. 2023, 24, 2571. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. Genetic variants in human CLOCK associate with total energy intake and cytokine sleep factors in overweight subjects (GOLDN population). Eur. J. Hum. Genet. 2010, 18, 364–369. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.-C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.-Q.; Ordovas, J.M. CLOCK genetic variation and metabolic syndrome risk: Modulation by monounsaturated fatty acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef]
- Loria-Kohen, V.; Espinosa-Salinas, I.; Marcos-Pasero, H.; Lourenço-Nogueira, T.; Herranz, J.; Molina, S.; Reglero, G.; de Molina, A.R. Polymorphism in the CLOCK gene may influence the effect of fat intake reduction on weight loss. Nutrition 2016, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Circadian rhythms, food timing and obesity. Proc. Nutr. Soc. 2016, 75, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Rahati, S.; Qorbani, M.; Naghavi, A.; Nia, M.H.; Pishva, H. Association between CLOCK 3111 T/C polymorphism with ghrelin, GLP-1, food timing, sleep and chronotype in overweight and obese Iranian adults. BMC Endocr. Disord. 2022, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Borrelli, P.; Di Nicola, M.; Stuppia, L.; Vitacolonna, E. Genetic Variants in CD36 Involved in Fat Taste Perception: Association with Anthropometric and Clinical Parameters in Overweight and Obese Subjects Affected by Type 2 Diabetes or Dysglycemia-A Pilot Study. Nutrients 2023, 15, 4656. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Agrawal, C.G.; Banerjee, M. CD36 gene variants in early prediction of type 2 diabetes mellitus. Genet. Test. Mol. Biomark. 2015, 19, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.; Šerý, O.; Plesnik, J.; Daoudi, H.; Rouabah, A.; Rouabah, L.; Khan, N.A. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. 2015, 39, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, N.M.; El-Shabrawi, M.M.; Hassan, H.H. Association of cluster of differentiation 36 gene variant rs1761667 (G > A) with metabolic syndrome in Egyptian adults. Saudi Med. J. 2012, 33, 489–494. [Google Scholar]
- Keller, K.L.; Liang, L.C.; Sakimura, J.; May, D.; Van Belle, C.; Breen, C.; Driggin, E.; Tepper, B.J.; Lanzano, P.C.; Deng, L.; et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity 2012, 20, 1066–1073. [Google Scholar] [CrossRef]
- Williams, D.L.; Schwartz, M.W. Out of synch: Clock mutation causes obesity in mice. Cell Metab. 2005, 1, 355–356. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.W.; Deng, H.; Liu, Y.J.; Liu, Y.Z.; Xu, F.H.; Shen, H.; Conway, T.; Li, J.L.; Huang, Q.Y.; Davies, K.M.; et al. A genomewide linkage scan for quantitative-trait loci for obesity phenotypes. Am. J. Hum. Genet. 2002, 70, 1138–1151. [Google Scholar] [CrossRef]
- Pan, X.; Mota, S.; Zhang, B. Circadian Clock Regulation on Lipid Metabolism and Metabolic Diseases. Adv. Exp. Med. Biol. 2020, 1276, 53–66. [Google Scholar] [CrossRef]
- Gooley, J.J. Circadian regulation of lipid metabolism. Proc. Nutr. Soc. 2016, 75, 440–450. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Škrlec, I.; Milic, J.; Heffer, M.; Peterlin, B.; Wagner, J. Genetic variations in circadian rhythm genes and susceptibility for myocardial infarction. Genet. Mol. Biol. 2018, 41, 403–409. [Google Scholar] [CrossRef]
- Staels, B. When the clock stops ticking, metabolic syndrome explodes. Nat. Med. 2006, 12, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Arellano, L.E.; Salgado-Bernabé, A.B.; Guzmán-Guzmán, I.P.; Salgado-Goytia, L.; Muñoz-Valle, J.F.; Parra-Rojas, I. CD36 haplotypes are associated with lipid profile in normal-weight subjects. Lipids Health Dis. 2013, 12, 167. [Google Scholar] [CrossRef]
- Love-Gregory, L.; Sherva, R.; Schappe, T.; Qi, J.S.; McCrea, J.; Klein, S.; Connelly, M.A.; Abumrad, N.A. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 2011, 20, 193–201. [Google Scholar] [CrossRef]
- Love-Gregory, L.; Sherva, R.; Sun, L.; Wasson, J.; Schappe, T.; Doria, A.; Rao, D.C.; Hunt, S.C.; Klein, S.; Neuman, R.J.; et al. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum. Mol. Genet. 2008, 17, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef]
- Pravenec, M.; Kurtz, T.W. Molecular genetics of experimental hypertension and the metabolic syndrome: From gene pathways to new therapies. Hypertension 2007, 49, 941–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Renzo, L.; Marchetti, M.; Rizzo, G.; Gualtieri, P.; Monsignore, D.; Dominici, F.; Mappa, I.; Cavicchioni, O.; Aguzzoli, L.; De Lorenzo, A.; et al. Adherence to Mediterranean Diet and Its Association with Maternal and Newborn Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 8497. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Cinelli, G.; Dri, M.; Gualtieri, P.; Attinà, A.; Leggeri, C.; Cenname, G.; Esposito, E.; Pujia, A.; Chiricolo, G.; et al. Mediterranean Personalized Diet Combined with Physical Activity Therapy for the Prevention of Cardiovascular Diseases in Italian Women. Nutrients 2020, 12, 3456. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.M.; Wall, C.; Becroft, D.M.; Robinson, E.; Wild, C.J.; Mitchell, E.A. Maternal dietary patterns in pregnancy and the association with small-for-gestational-age infants. Br. J. Nutr. 2010, 103, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Barres, S.; Vrijheid, M.; Manzano-Salgado, C.B.; Valvi, D.; Martinez, D.; Iniguez, C.; Jimenez-Zabala, A.; Riano-Galan, I.; Navarrete Munoz, E.M.; Santa-Marina, L.; et al. The association of Mediterranean diet during pregnancy with longitudinal body mass index trajectories and cardiometabolic risk in early childhood. J. Pediatr. 2019, 206, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Starling, A.P.; Sauder, K.A.; Kaar, J.L.; Shapiro, A.L.; Siega-Riz, A.M.; Dabelea, D. Maternal dietary patterns during pregnancy are associated with newborn body composition. J. Nutr. 2017, 147, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Di Nunzio, M.; Bordoni, A.; Gori, D.; Lanari, M. Effect of Adherence to Mediterranean Diet during Pregnancy on Children’s Health: A Systematic Review. Nutrients 2019, 11, 997. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel; Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; de Leiva, A.; Hod, M.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hoelzel, W.; Weykamp, C.; Jeppsson, J.O.; Miedema, K.; Barr, J.R.; Goodall, I.; Hoshino, T.; John, W.G.; Kobold, U.; Little, R.; et al. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: A method-comparison study. Clin. Chem. 2004, 50, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian systems. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Natale, V.; Esposito, M.J.; Martoni, M.; Fabbri, M. Validity of the reduced version of the Morningness-Eveningness Questionnaire. Sleep Biol. Rhythms 2006, 4, 72–74. [Google Scholar] [CrossRef]
- Mannocci, A.; Di Thiene, D.; Del Cimmuto, A.; Masala, D.; Boccia, A.; De Vito, E.; La Torre, G. International physical activity questionnaire: Validation and assessment in an Italian sample. Ital. J. Public Health 2010, 7, 369–376. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2007, 1, 47–50. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).