Circadian Synchronization of Feeding Attenuates Rats’ Food Restriction-Induced Anxiety and Amygdalar Thyrotropin-Releasing Hormone Downregulation
Abstract
1. Introduction
2. Results
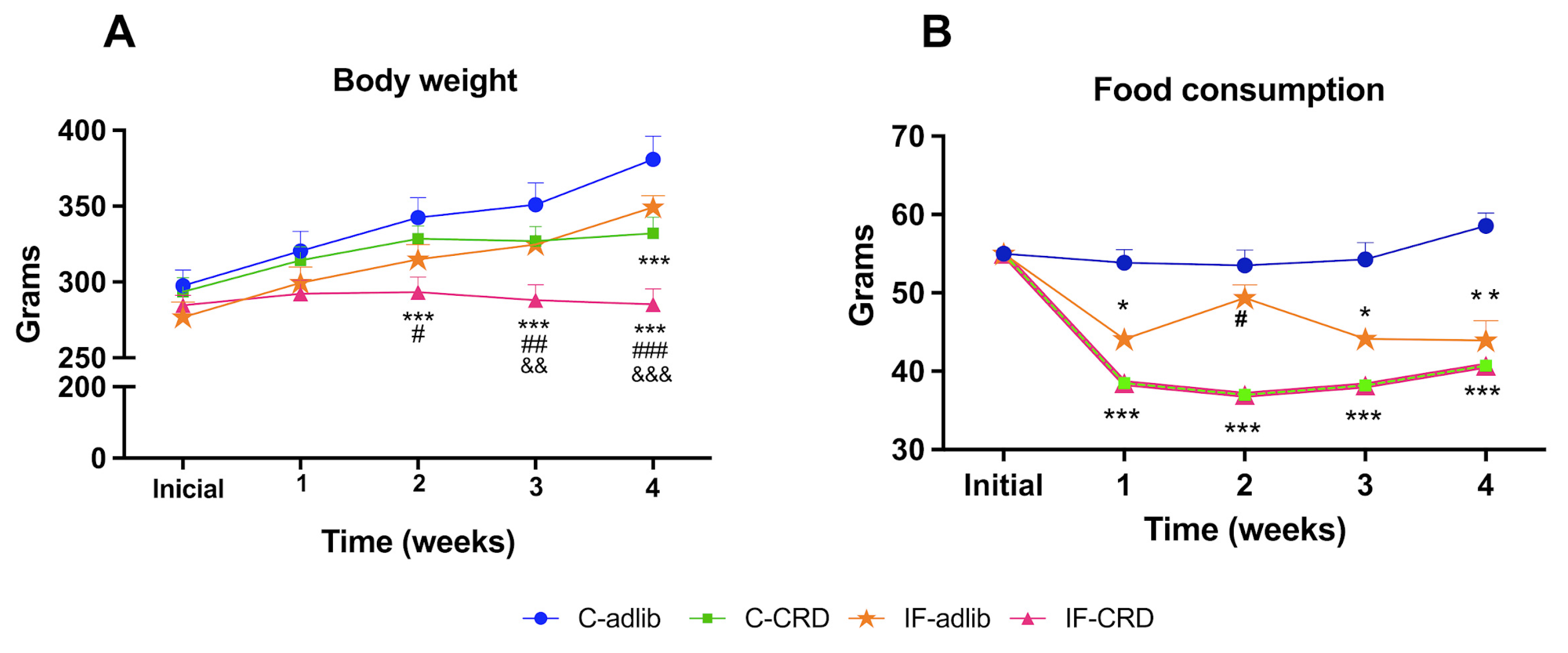
2.1. Body Weight
2.2. Weekly Food Intake
2.3. Corticosterone (CORT) Serum Levels
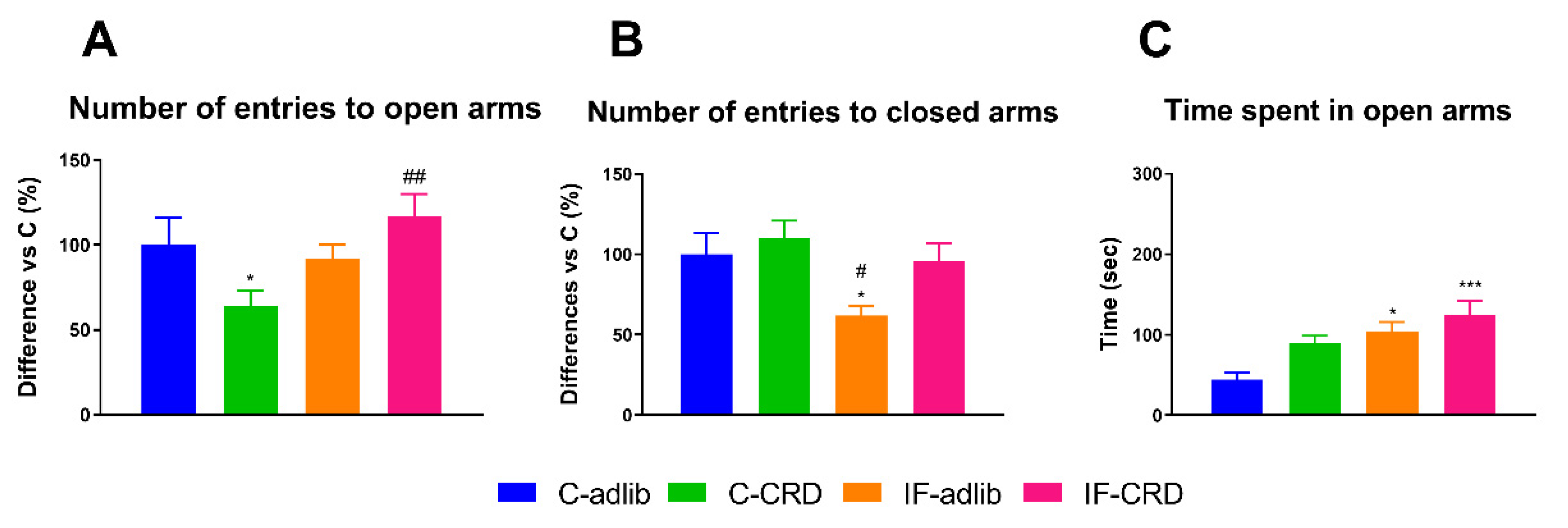
2.4. Elevated plus Maze Performance
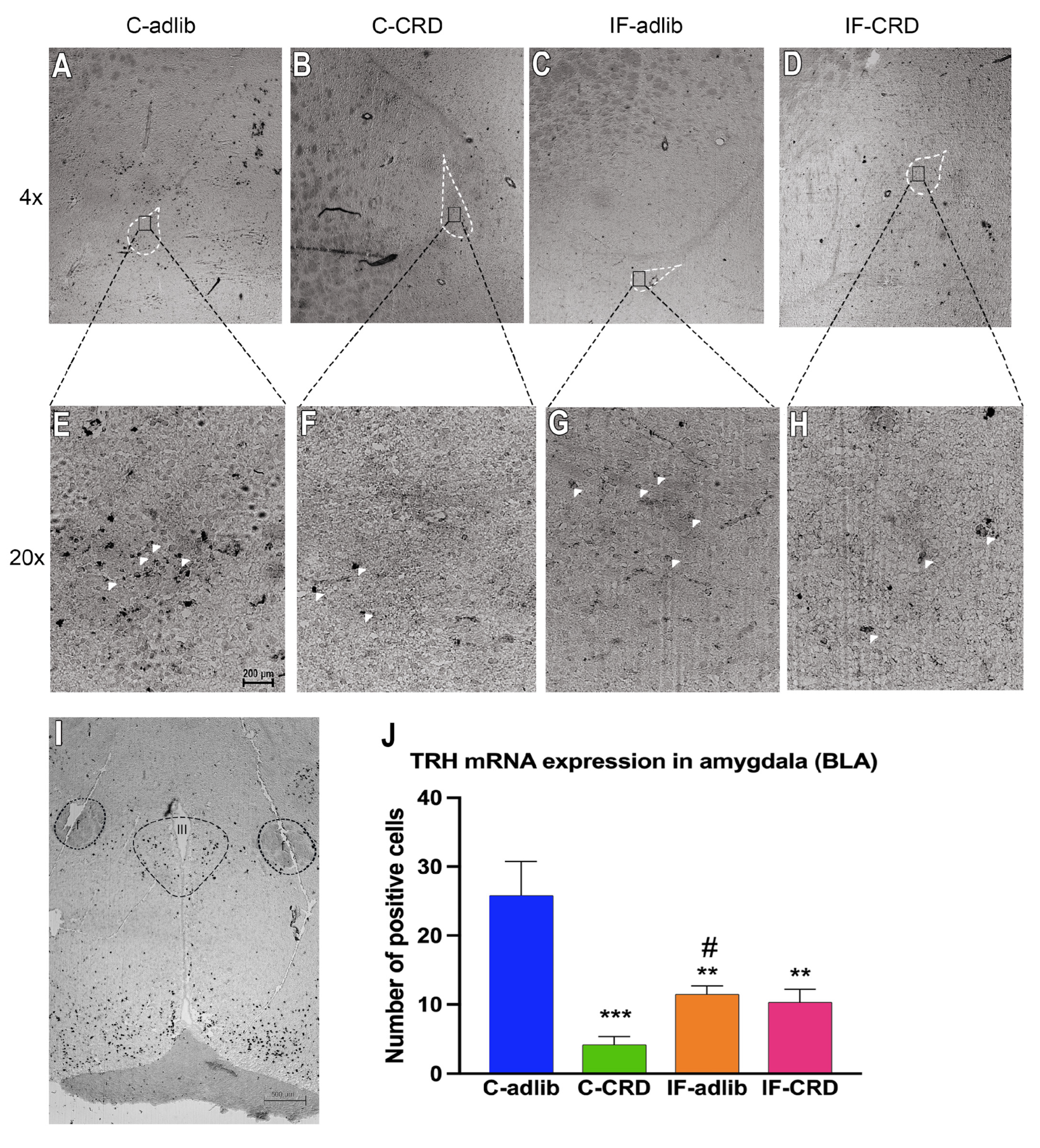
2.5. Pro-TRH mRNA Expression in the Amygdala
3. Discussion
4. Materials and Methods
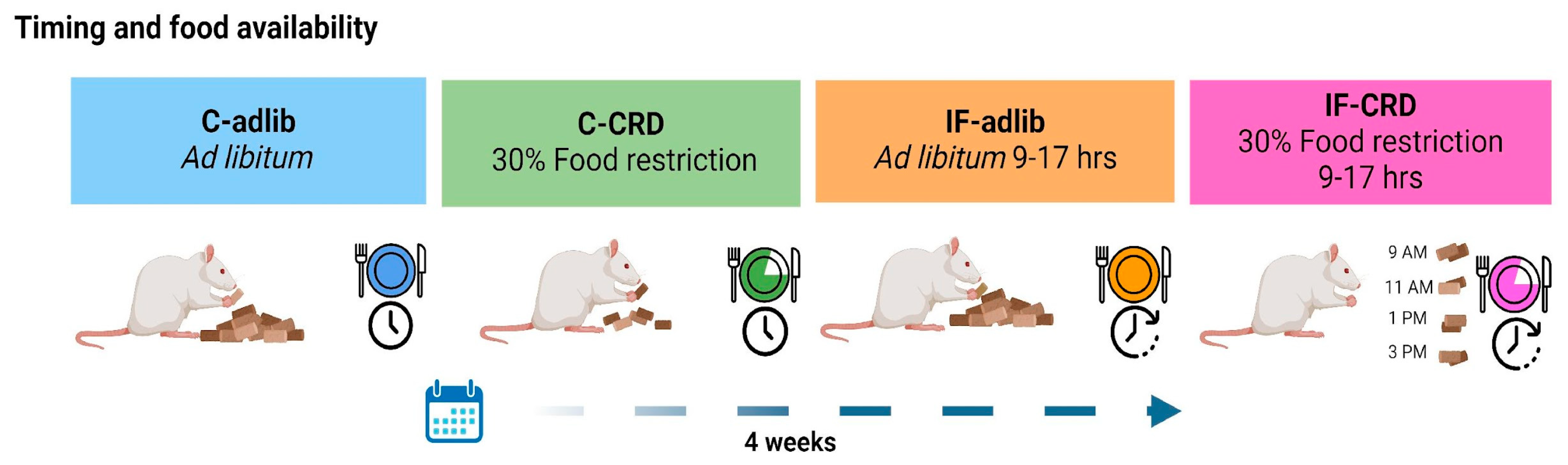
4.1. Body Weight and Food Consumption Registration
4.2. Anxiety-like Behavior Assessment
4.3. Corticosterone (CORT) Serum Levels
4.4. In Situ Hybridization Histochemistry Analysis
4.5. Image Analysis
4.6. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lykouras, L.; Michopoulos, J. Anxiety Disorders and Obesity. Psychiatrike 2011, 22, 307–313. [Google Scholar]
- Wang, X.L.; Wei, X.; Yuan, J.J.; Mao, Y.Y.; Wang, Z.Y.; Xing, N.; Gu, H.W.; Lin, C.H.; Wang, W.T.; Zhang, W.; et al. Downregulation of Fat Mass and Obesity-Related Protein in the Anterior Cingulate Cortex Participates in Anxiety- and Depression-Like Behaviors Induced by Neuropathic Pain. Front. Cell. Neurosci. 2022, 16, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P. Timing of Food Intake and Obesity: A Novel Association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Gómez-Abellán, P.; Garaulet, M. Timing of Breakfast, Lunch, and Dinner. Effects on Obesity and Metabolic Risk. Nutrients 2019, 11, 2624. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Aeschbach, D. Sleep and Anxiety: From Mechanisms to Interventions. Sleep Med. Rev. 2022, 61, 101583. [Google Scholar] [CrossRef]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P. The Role of Insufficient Sleep and Circadian Misalignment in Obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Zemdegs, J.; Quesseveur, G.; Jarriault, D.; Pénicaud, L.; Fioramonti, X.; Guiard, B. High Fat Diet-Induced Metabolic Disorders Impairs Serotonergic Function and Anxiety-like Behaviours in Mice. Br. J. Pharmacol. 2015, 173, 2095–2110. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhou, Y.; Du, H.; Zhang, C.; Zhao, Z.; Chen, Y.; Zhou, Z.; Mei, J.; Wu, W.; et al. High Fat Diet-Induced Obesity Leads to Depressive and Anxiety-like Behaviors in Mice via AMPK/MTOR-Mediated Autophagy. Exp. Neurol. 2022, 348, 113949. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, D.E.; Teegarden, S.L.; Hedin, A.D.; Jensen, C.L.; Bale, T.L. Caloric Restriction Experience Reprograms Stress and Orexigenic Pathways and Promotes Binge Eating. J. Neurosci. 2010, 30, 16399–16407. [Google Scholar] [CrossRef]
- Agnihothri, R.V.; Courville, A.B.; Linderman, J.D.; Smith, S.; Brychta, R.; Remaley, A.; Chen, K.Y.; Simchowitz, L.; Celi, F.S. Moderate Weight Loss Is Sufficient to Affect Thyroid Hormone Homeostasis and Inhibit Its Peripheral Conversion. Thyroid 2014, 24, 19–26. [Google Scholar] [CrossRef]
- Hartmann-Boyce, J.; Cobiac, L.J.; Theodoulou, A.; Oke, J.L.; Butler, A.R.; Scarborough, P.; Bastounis, A.; Dunnigan, A.; Byadya, R.; Hobbs, F.D.R.; et al. Weight Regain after Behavioural Weight Management Programmes and Its Impact on Quality of Life and Cost Effectiveness: Evidence Synthesis and Health Economic Analyses. Diabetes Obes. Metab. 2023, 25, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Van Haasteren, G.A.C.; Linkels, E.; Klootwijk, W.; Van Toor, H.; Rondeel, J.M.M.; Themmen, A.P.N.; De Jong, F.H.; Valentijn, K.; Vaudry, H.; Bauer, K.; et al. Starvation-Induced Changes in the Hypothalamic Content of Prothyrotrophin-Releasing Hormone (ProTRH) MRNA and the Hypothalamic Release of ProTRH-Derived Peptides: Role of the Adrenal Gland. J. Endocrinol. 1995, 145, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Salas, E.; González, A.; Amaya, M.I.; de Gortari, P. Accumbal TRH Is Downstream of the Effects of Isolation Stress on Hedonic Food Intake in Rats. Nutr. Neurosci. 2021, 24, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, A.; Zhang, L. Role of the Hypothalamus in the Neuroendocrine Regulation of Body Weight and Composition during Energy Deficit. Obes. Rev. 2012, 13, 234–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef]
- Chawla, S.; Beretoulis, S.; Deere, A.; Radenkovic, D. The Window Matters: A Systematic Review of Time Restricted Eating Strategies in Relation to Cortisol and Melatonin Secretion. Nutrients 2021, 13, 2525. [Google Scholar] [CrossRef]
- Gutiérrez-Mariscal, M.; de Gortari, P.; López-Rubalcava, C.; Martínez, A.; Joseph-Bravo, P. Analysis of the Anxiolytic-like Effect of TRH and the Response of Amygdalar TRHergic Neurons in Anxiety. Psychoneuroendocrinology 2008, 33, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Gnocchi, D.; Bruscalupi, G. Circadian Rhythms and Hormonal Homeostasis: Pathophysiological Implications. Biology 2017, 6, 10. [Google Scholar] [CrossRef]
- Starnes, A.N.; Jones, J.R. Inputs and Outputs of the Mammalian Circadian Clock. Biology 2023, 12, 508. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, L.; Mu, Z. Effects of Different Weight Loss Dietary Interventions on Body Mass Index and Glucose and Lipid Metabolism in Obese Patients. Medicine 2023, 102, e33254. [Google Scholar] [CrossRef] [PubMed]
- García-Luna, C.; Prieto, I.; Soberanes-Chávez, P.; Alvarez-Salas, E.; Torre-Villalvazo, I.; Matamoros-Trejo, G.; de Gortari, P. Effects of Intermittent Fasting on Hypothalamus–Pituitary–Thyroid Axis, Palatable Food Intake, and Body Weight in Stressed Rats. Nutrients 2023, 15, 1164. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.d.C.; Morais, G.P.; Ropelle, E.R.; de Moura, L.P.; Cintra, D.E.; Pauli, J.R.; de Freitas, E.C.; Rorato, R.; da Silva, A.S.R. Using Intermittent Fasting as a Non-Pharmacological Strategy to Alleviate Obesity-Induced Hypothalamic Molecular Pathway Disruption. Front. Nutr. 2022, 9, 858320. [Google Scholar] [PubMed]
- Shin, L.M.; Liberzon, I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Herhaus, B.; Ullmann, E.; Chrousos, G.; Petrowski, K. High/Low Cortisol Reactivity and Food Intake in People with Obesity and Healthy Weight. Transl. Psychiatry 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Bazhan, N.; Zelena, D. Food-Intake Regulation during Stress by the Hypothalamo-Pituitary-Adrenal Axis. Brain Res. Bull. 2013, 95, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Schwaber, J.S.; Kapp, B.S.; Higgins, G.A.; Rapp, P.R. Amygdaloid and Basal Forebrain Direct Connections with the Nucleus of the Solitary Tract and the Dorsal Motor Nucleus. J. Neurosci. 1982, 2, 1424–1438. [Google Scholar] [CrossRef] [PubMed]
- Babaev, O.; Piletti Chatain, C.; Krueger-Burg, D. Inhibition in the Amygdala Anxiety Circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Comeras, L.B.; Herzog, H.; Tasan, R.O. Neuropeptides at the Crossroad of Fear and Hunger: A Special Focus on Neuropeptide Y. In Annals of the New York Academy of Sciences; Wiley Periodicals, Inc.: Hoboken, NJ, USA, 2019; Volume 1455, pp. 59–80. [Google Scholar]
- Pekary, A.E.; Sattin, A. Increased TRH and TRH-like Peptide Release in Rat Brain and Peripheral Tissues during Proestrus/Estrus. Peptides 2014, 52, 1–10. [Google Scholar] [CrossRef]
- Pekary, A.E.; Sattin, A.; Lloyd, R.L. Ketamine Modulates TRH and TRH-like Peptide Turnover in Brain and Peripheral Tissues of Male Rats. Peptides 2015, 69, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Fossey, M.D.; Lydiard, R.B.; Ballenger, J.C.; Laraia, M.T.; Bissette, G.T.; Nemeroff, C.B. Cerebrospinal Fluid Thyrotropin-Releasing Hormone Concentrations in Patients with Anxiety Disorders. J. Neuropsychiatry Clin. Neurosci. 1993, 5, 335–337. [Google Scholar] [PubMed]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Himanshu; Dharmila; Sarkar, D.; Nutan. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-Anxiety Effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open: Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.M.; Basu, C. Optimization of a Digoxigenin-Based Immunoassay System for Gene Detection in Arabidopsis Thaliana. J. Biomol. Tech. 2009, 20, 96–100. [Google Scholar] [PubMed]
- Luehrsen, K.R.; Davidson, S.; Lee, Y.J.; Rouhani, R.; Soleimani, A.; Raich, T.; Cain, C.A.; Collarini, E.J.; Yamanishi, D.T.; Pearson, J.; et al. High-Density Hapten Labeling and HRP Conjugation of Oligonucleotides for Use as in Situ Hybridization Probes to Detect MRNA Targets in Cells and Tissues. J. Histochem. Cytochem. 2000, 48, 133–145. [Google Scholar] [CrossRef]
- Alvarez-Salas, E.; Mengod, G.; García-Luna, C.; Soberanes-Chávez, P.; Matamoros-Trejo, G.; de Gortari, P. Mct8 and Trh Co-Expression throughout the Hypothalamic Paraventricular Nucleus Is Modified by Dehydration-Induced Anorexia in Rats. Neuropeptides 2016, 56, 33–40. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soberanes-Chávez, P.; Trujillo-Barrera, J.; de Gortari, P. Circadian Synchronization of Feeding Attenuates Rats’ Food Restriction-Induced Anxiety and Amygdalar Thyrotropin-Releasing Hormone Downregulation. Int. J. Mol. Sci. 2024, 25, 5857. https://doi.org/10.3390/ijms25115857
Soberanes-Chávez P, Trujillo-Barrera J, de Gortari P. Circadian Synchronization of Feeding Attenuates Rats’ Food Restriction-Induced Anxiety and Amygdalar Thyrotropin-Releasing Hormone Downregulation. International Journal of Molecular Sciences. 2024; 25(11):5857. https://doi.org/10.3390/ijms25115857
Chicago/Turabian StyleSoberanes-Chávez, Paulina, Jariz Trujillo-Barrera, and Patricia de Gortari. 2024. "Circadian Synchronization of Feeding Attenuates Rats’ Food Restriction-Induced Anxiety and Amygdalar Thyrotropin-Releasing Hormone Downregulation" International Journal of Molecular Sciences 25, no. 11: 5857. https://doi.org/10.3390/ijms25115857
APA StyleSoberanes-Chávez, P., Trujillo-Barrera, J., & de Gortari, P. (2024). Circadian Synchronization of Feeding Attenuates Rats’ Food Restriction-Induced Anxiety and Amygdalar Thyrotropin-Releasing Hormone Downregulation. International Journal of Molecular Sciences, 25(11), 5857. https://doi.org/10.3390/ijms25115857





