High-Resolution Frequency-Domain Spectroscopic and Modeling Studies of Photosystem I (PSI), PSI Mutants and PSI Supercomplexes
Abstract
1. Introduction
2. High-Resolution Site-Selective (Low-Temperature) Frequency-Domain Spectroscopies
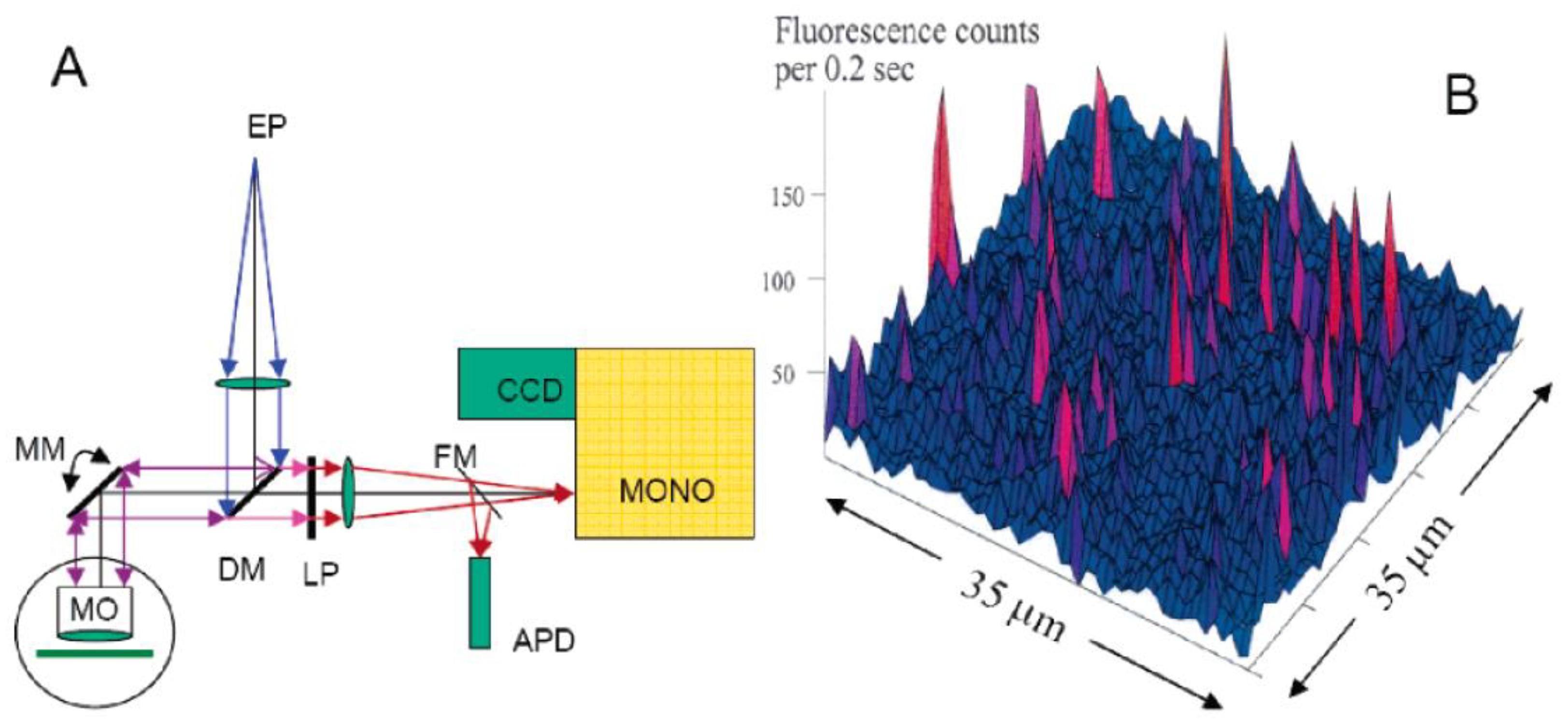
2.1. Single Photosynthetic Complex Spectroscopy (SPCS)
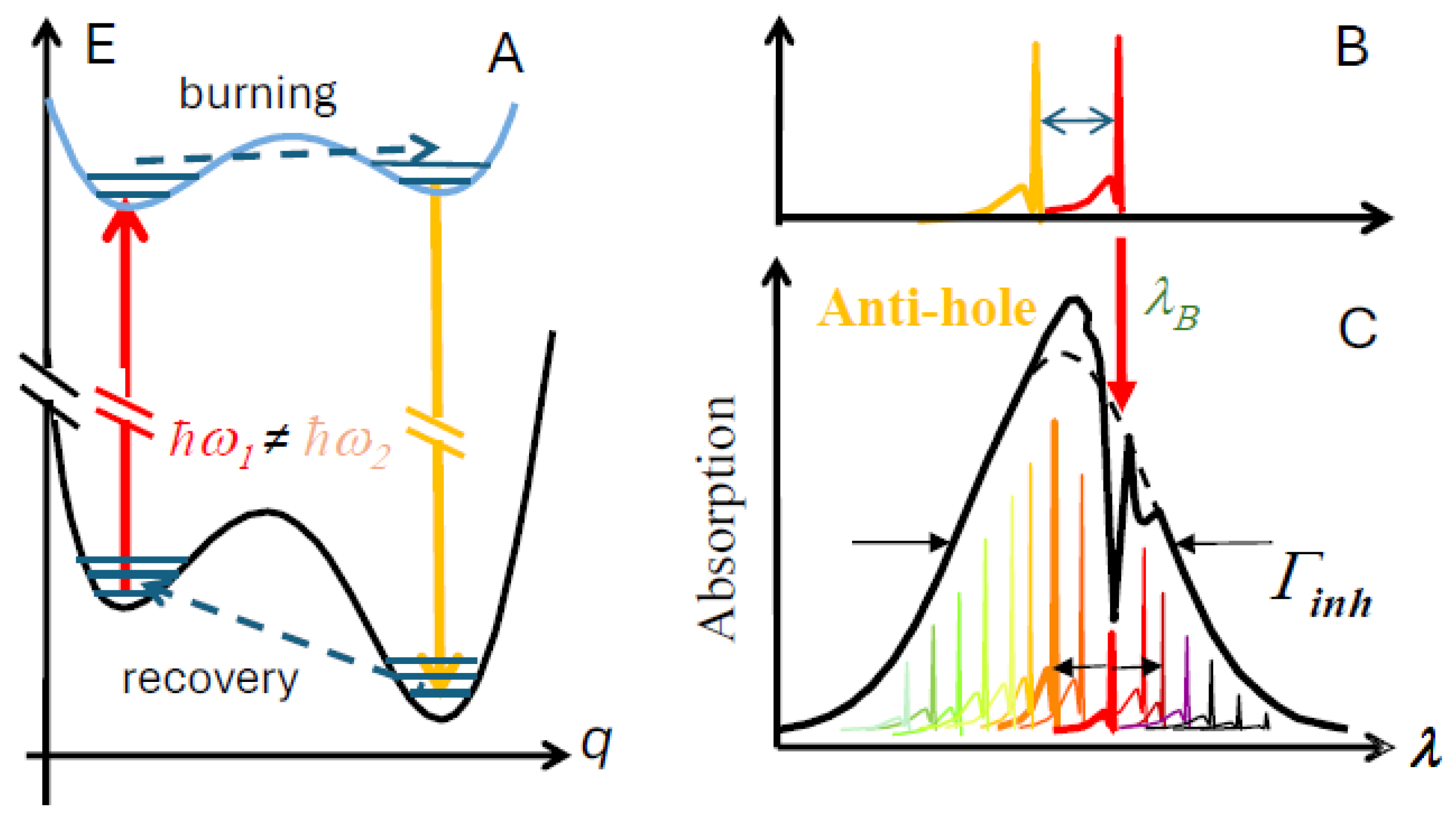
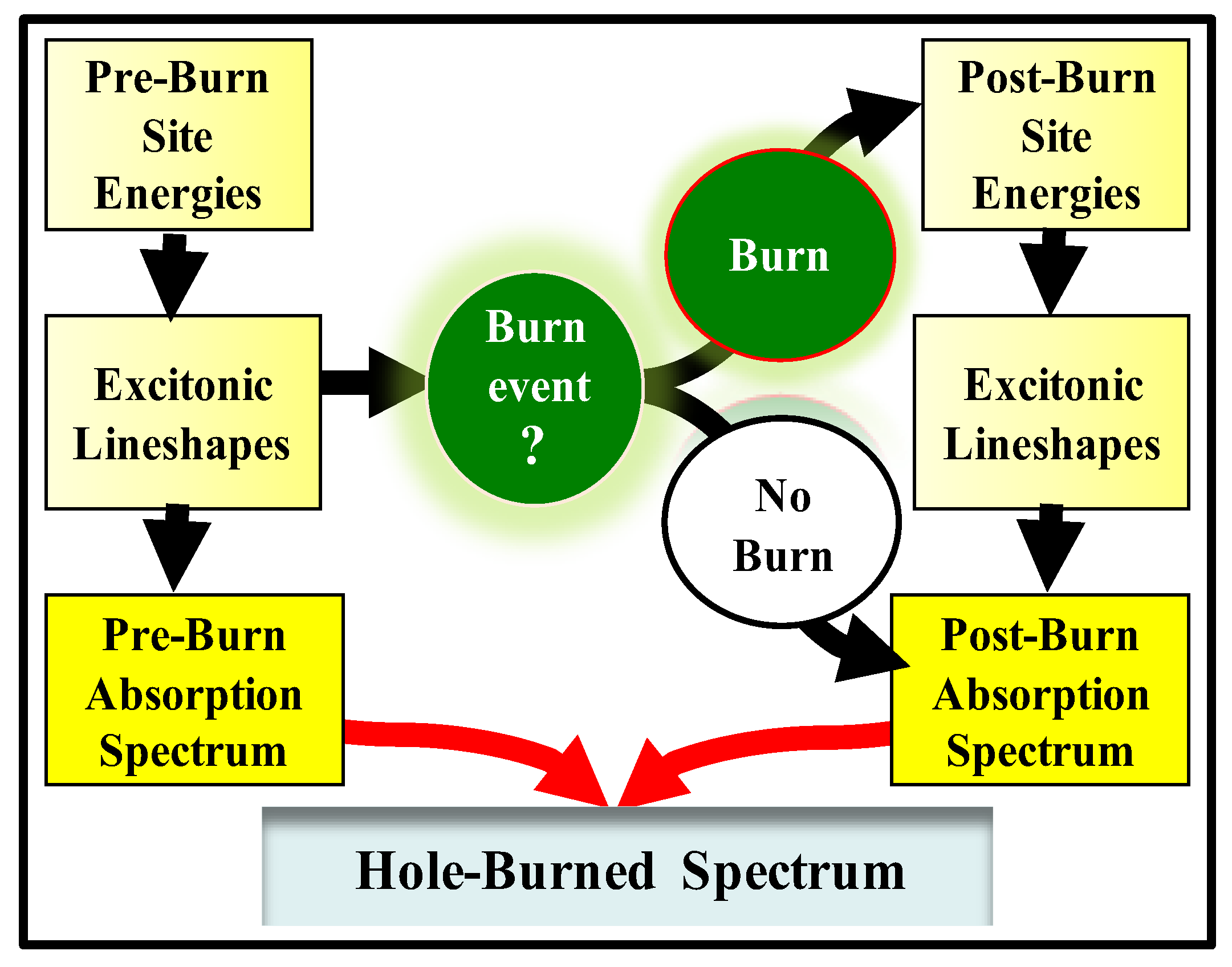
2.2. Hole-Burning (HB) Spectroscopies
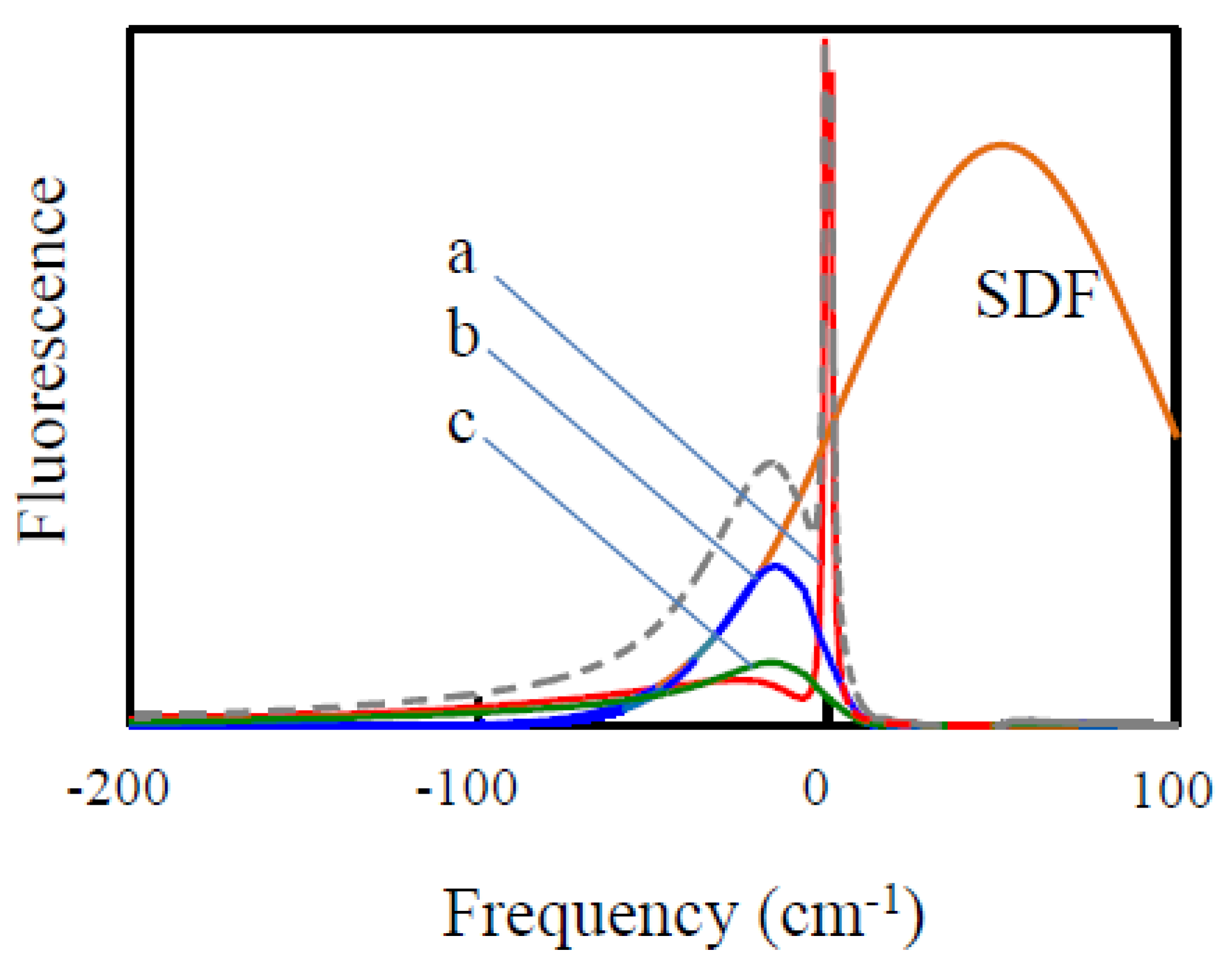
2.3. Fluorescence Line Narrowing (FLN) Spectroscopy and Delta FLN (∆FLN)
3. Structure and Function of PSI: Plants vs. Cyanobacteria, Synechocystis PCC 6803 vs. Thermosynechococcus elongatus; Supercomplexes in Iron-Deficient Environments
4. Applications of Frequency Domain Methodologies to PSI and Its Mutants
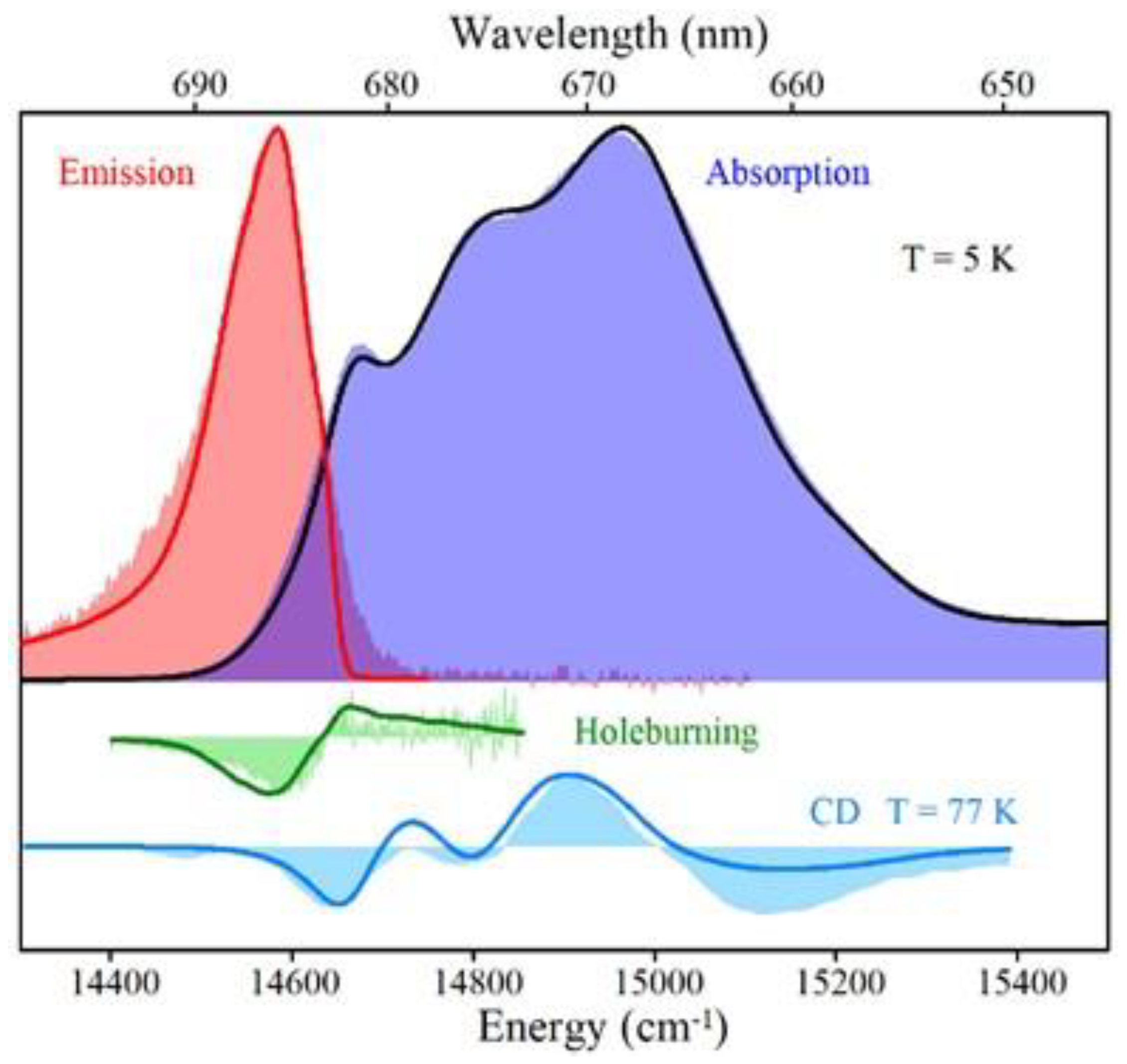
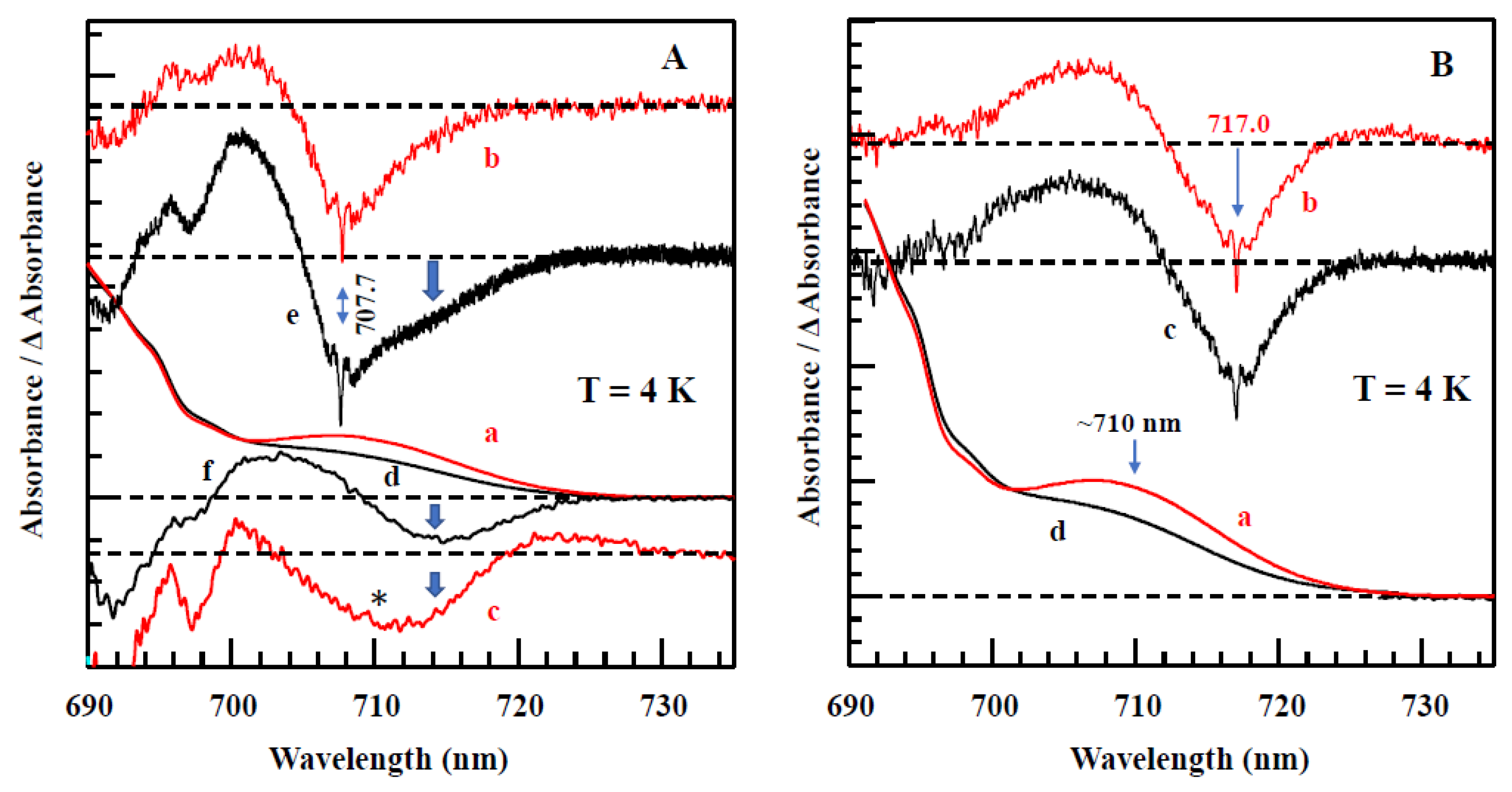
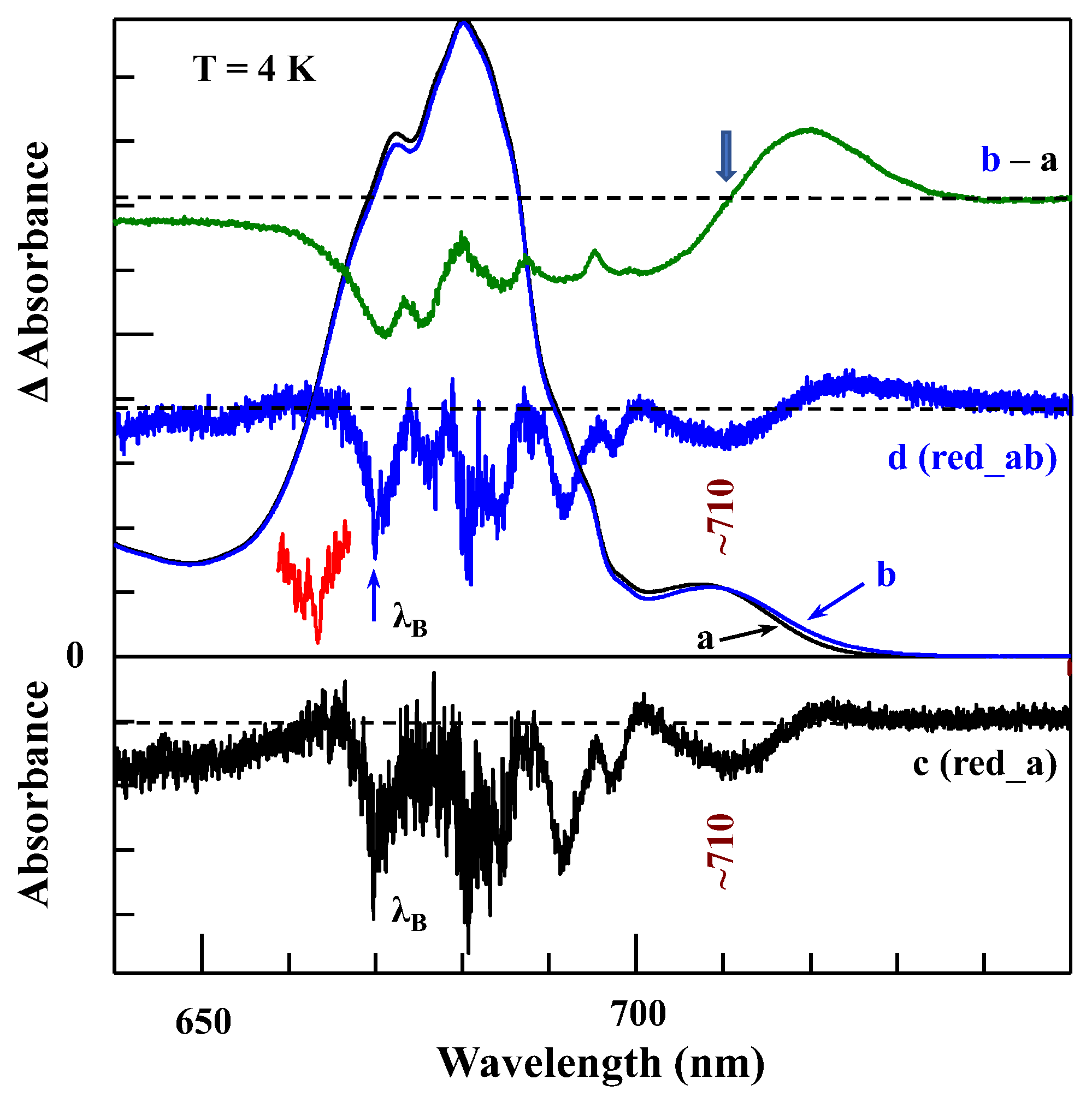
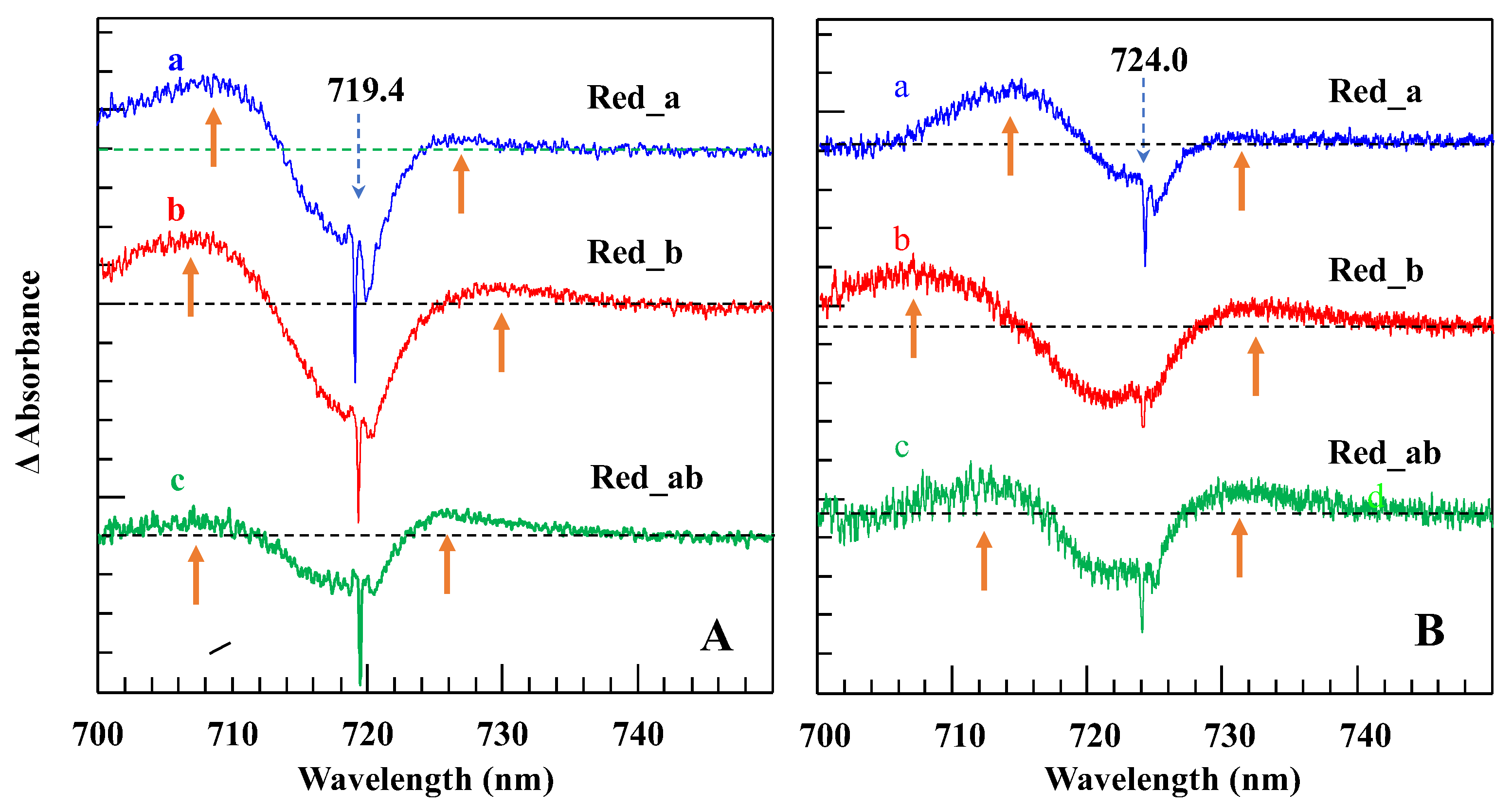
4.1. Low-Temperature Absorption, Emission, and HB Spectra
| WT Synechocystis | Red_a Mutant Synechocystis | Red_b Mutant Synechocystis | Red_ab Mutant Synechocystis | WT T. elongatus (from Refs [63,67]) |
|---|---|---|---|---|
| C706 a (B31-B32) | C710 b (B31-B32-B33) | C706 a (B31-B32) | C710 b (B31-B32-B33) | C710 b (B31-B32-B33) |
| C714 c (B37-B38) | C714 c (B37-B38) | C714 c (B37-B38) | C714 c (B37-B38) | C715 d (B37-B38) |
| C707 a (His95-B7-A31-A32) | C707 a (His95-B7-A31-A32) | C716 e (Gln 95-B7–A31–A32) | C716 f (Gln 95-B7–A31–A32) | C719 g (Gln94-B7–A31–A32) |
| WT PSI and Mutant Synechocystis | Low-Energy Emitting States/Stokes Shift (cm−1) | Assignment of Fluorescence Bands |
|---|---|---|
| WT | C706/119 C707 C714/150 | F712 (minor) b F722 (major) |
| Red_b mutant | C706/119 C714/144 C716 a | F712 (minor) F722 (major) F735 (<1%) |
| Red_a mutant | C714/150 C710 c/292 | F722 (~53%) F725 (~47%) |
| Red_ab mutant | C710/292 C714/150 C716 a | F725 (~80%) F722 (~12%) F735 (~8%) |
4.2. Possible Energy Transfer Pathways in PSI Complex, PSI Mutants, and Resulting Fluorescence Maxima
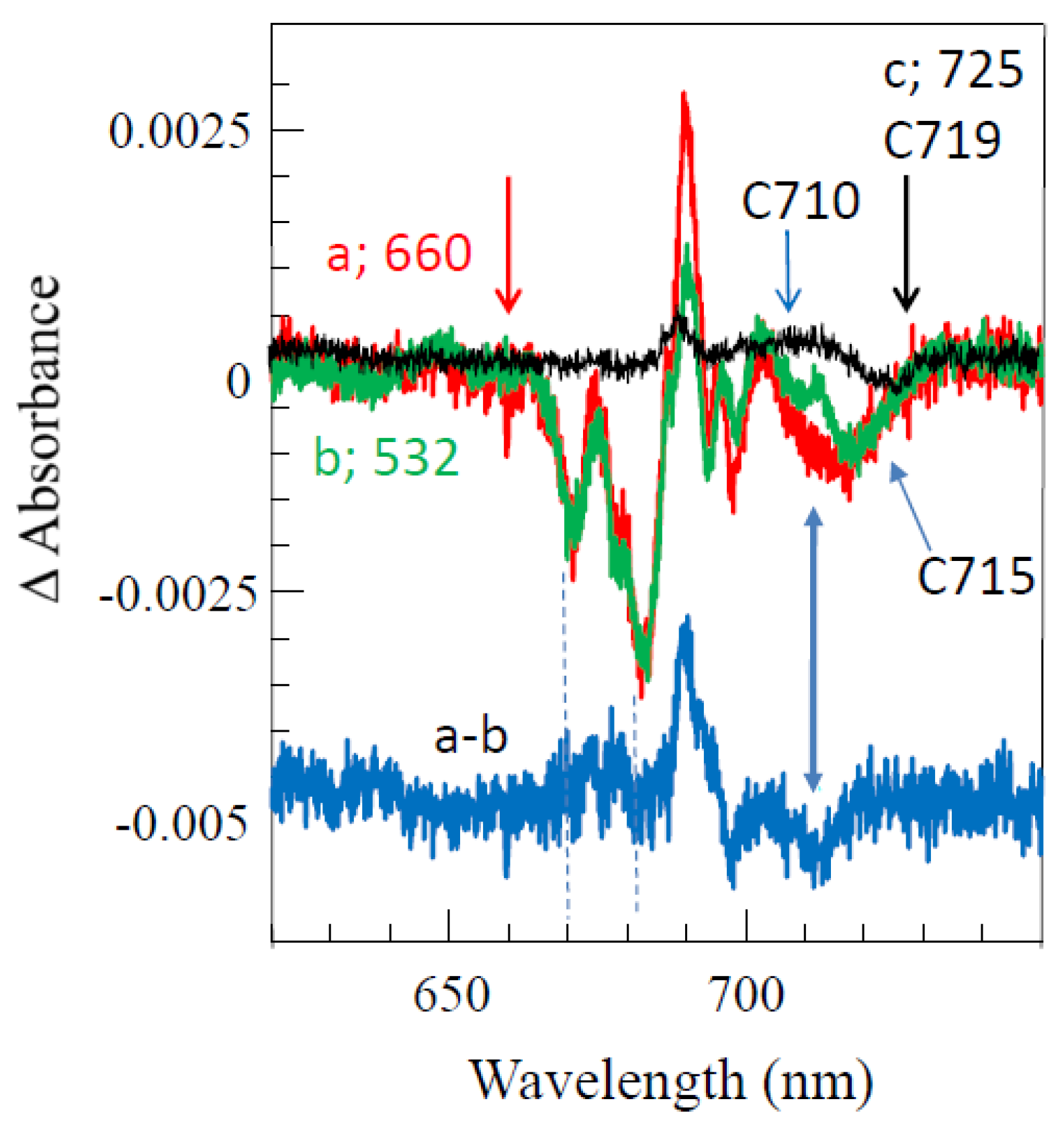
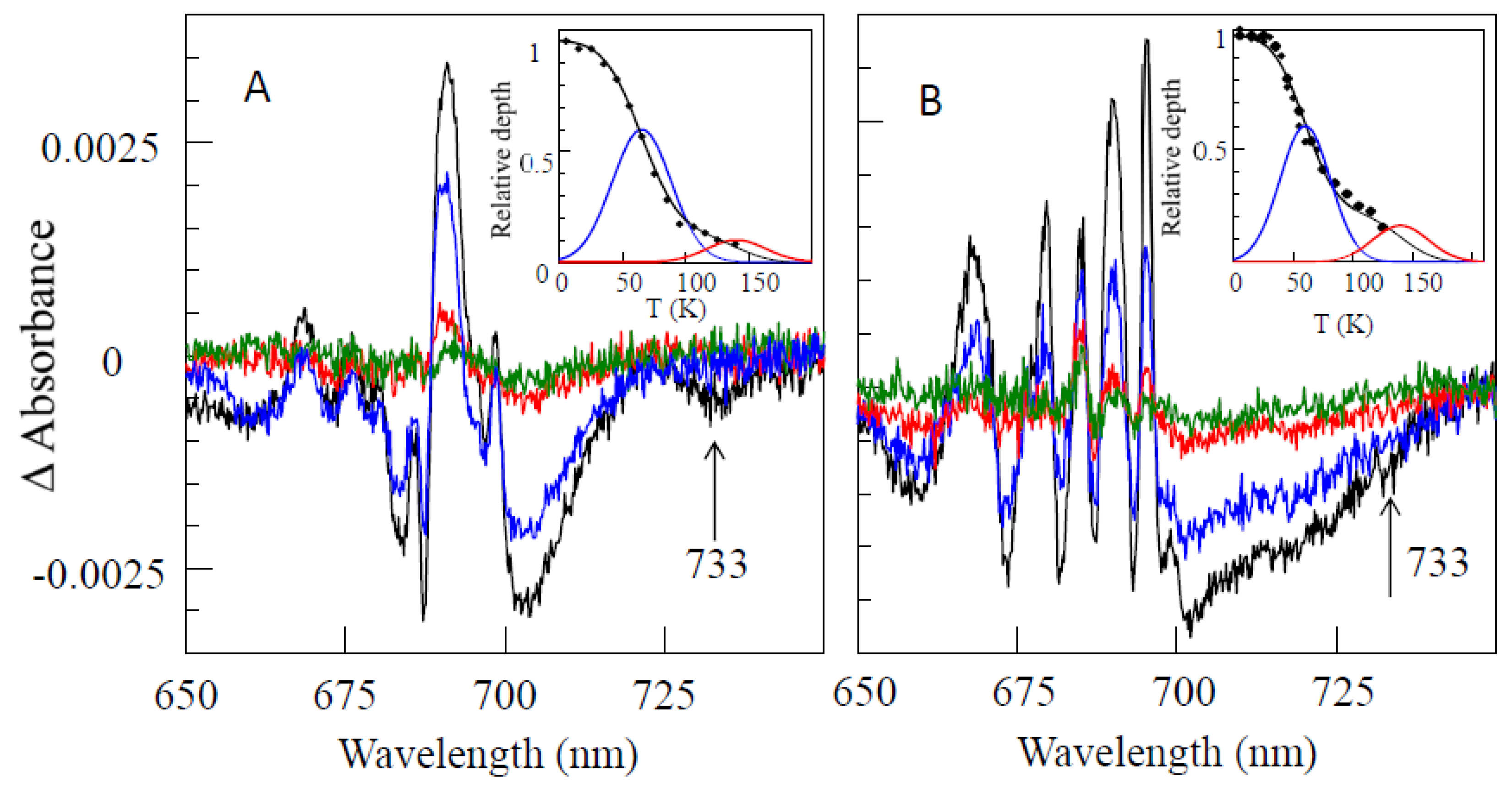
4.3. Persistent Holes Associated with Primary Charge Separation in the PSI3 RC with Low-Energy Excitation
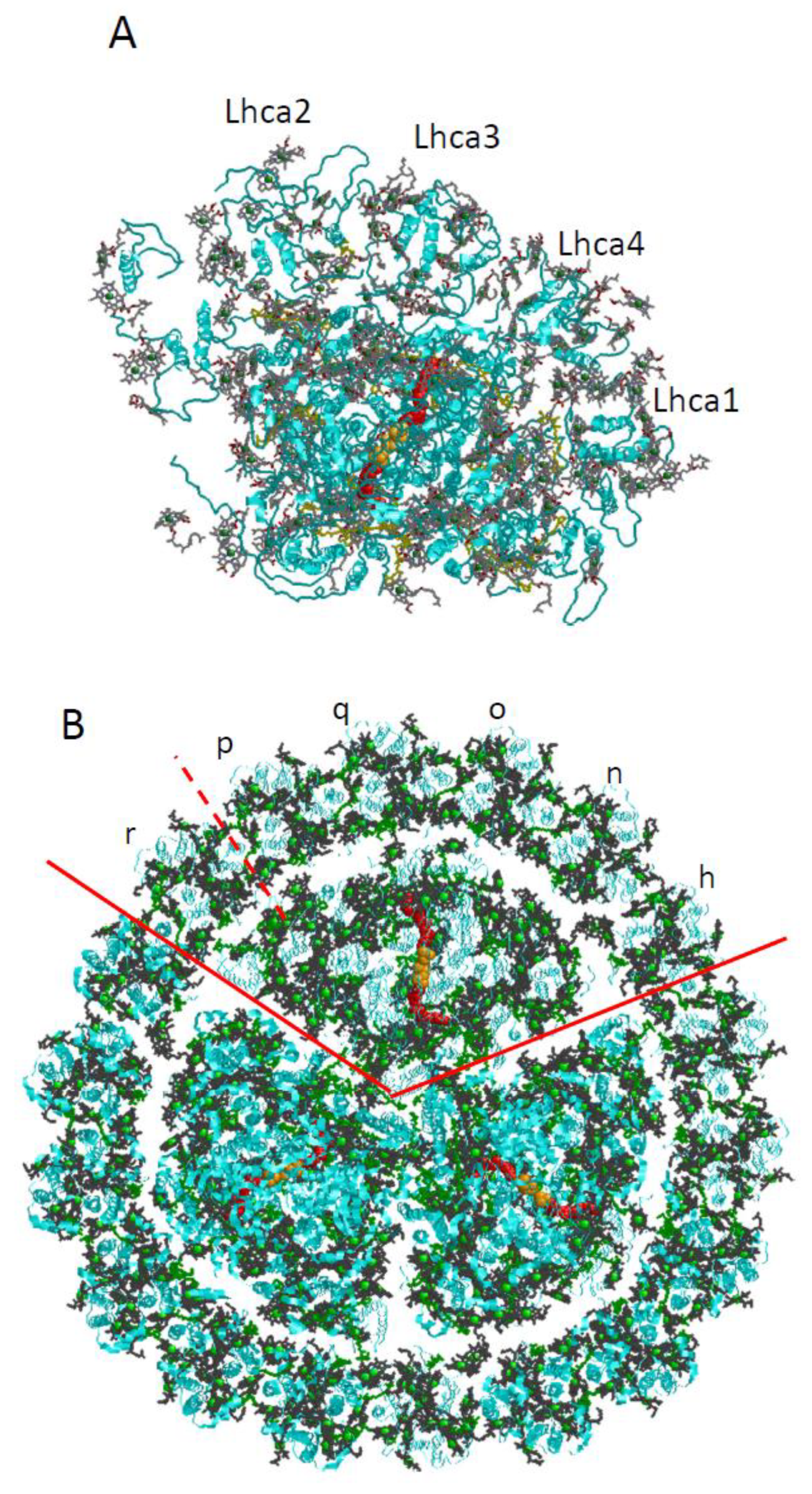
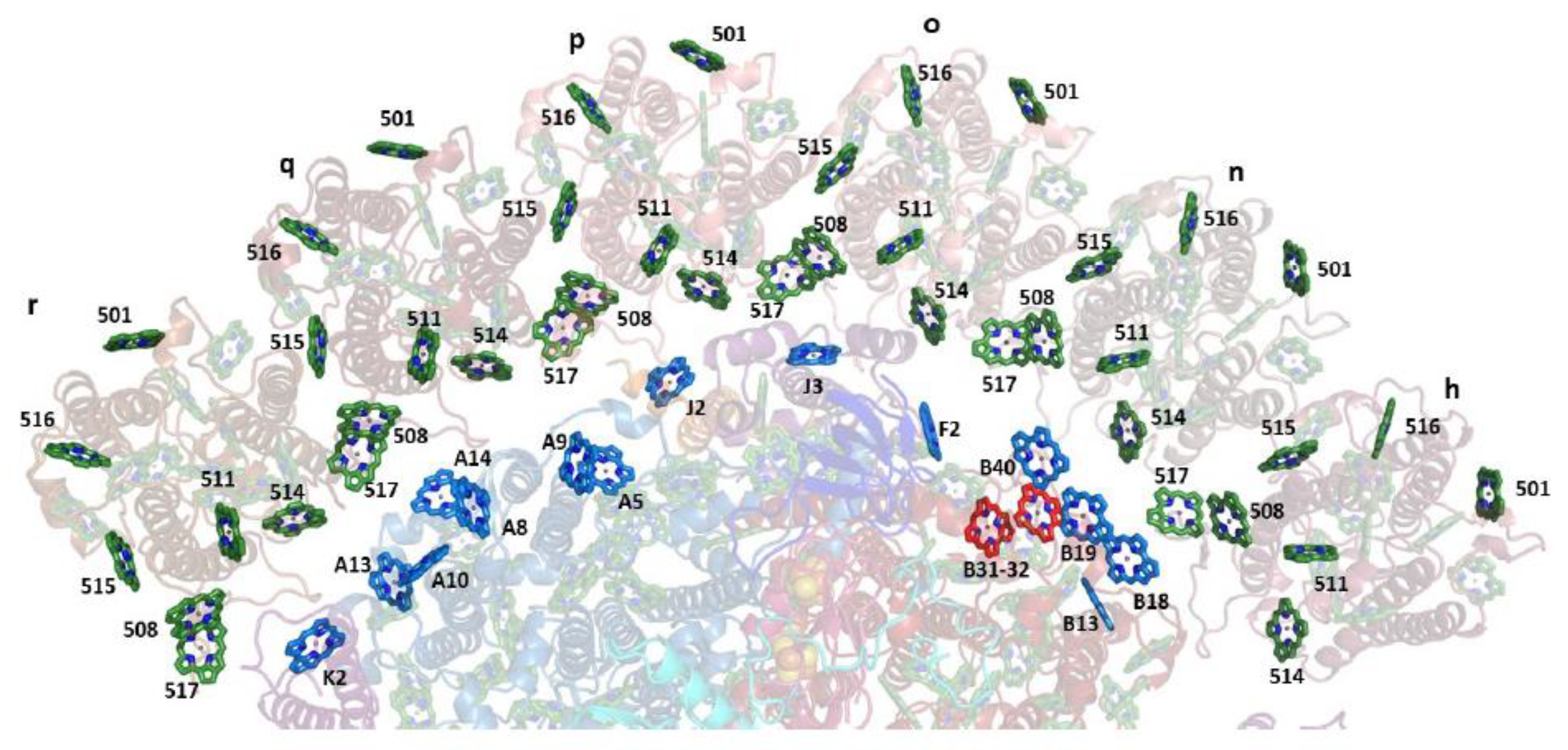
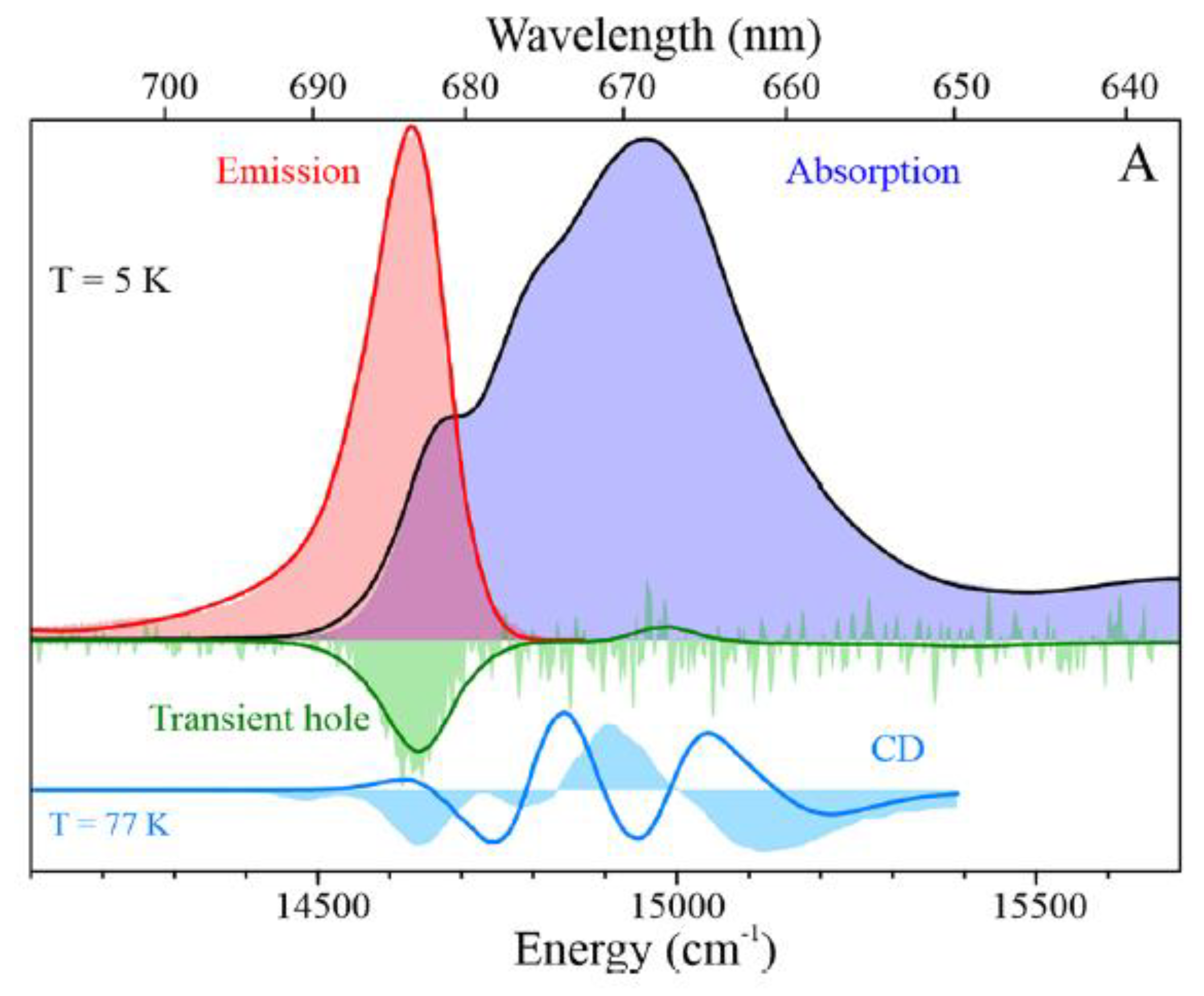
5. Applications of Frequency Domain Methodologies to PSI3–IsiA18 Supercomplexes and IsiA Monomers
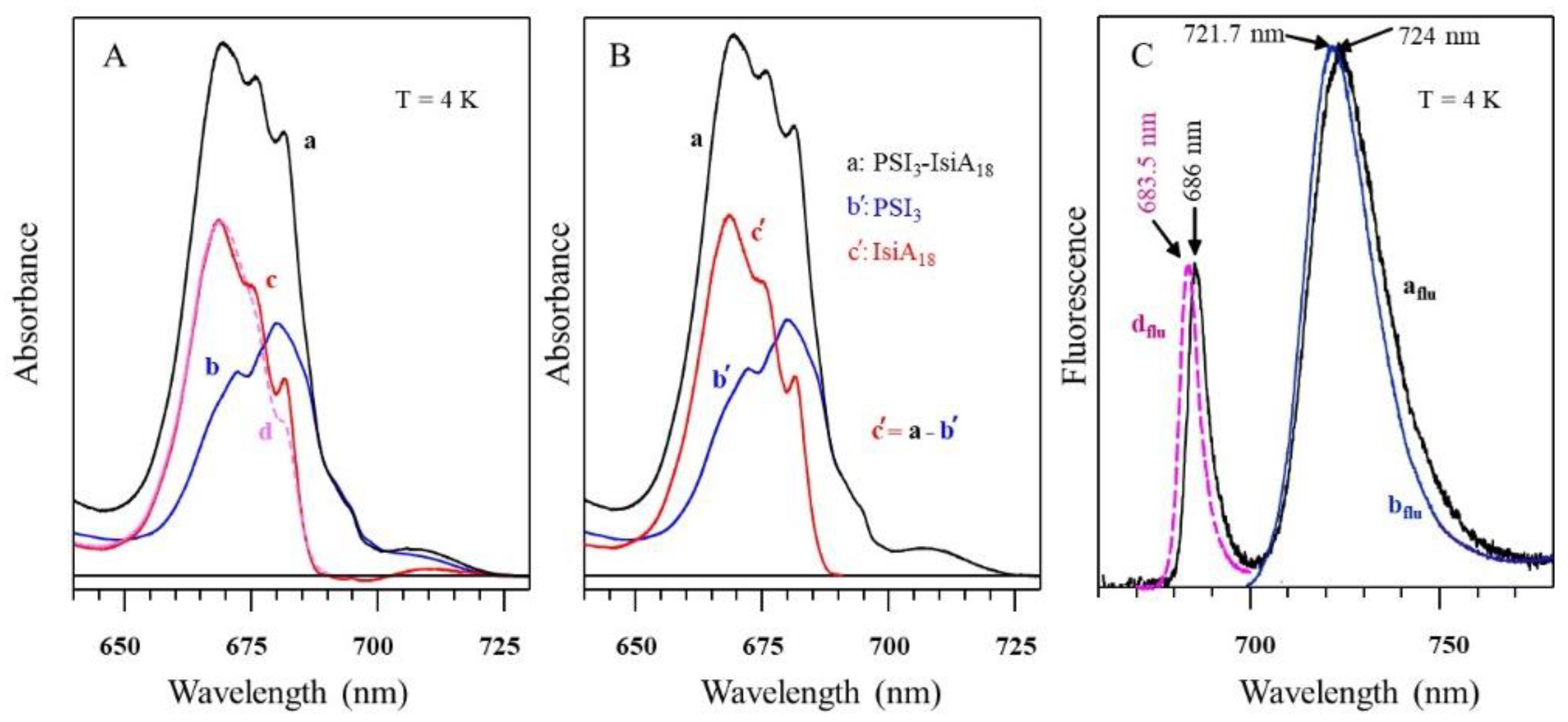
5.1. Comparison of Low-Temperature Emission and Absorption Spectra for Isolated IsiA Monomers, PSI3 Trimer, and PSI3–IsiA18 Supercomplex
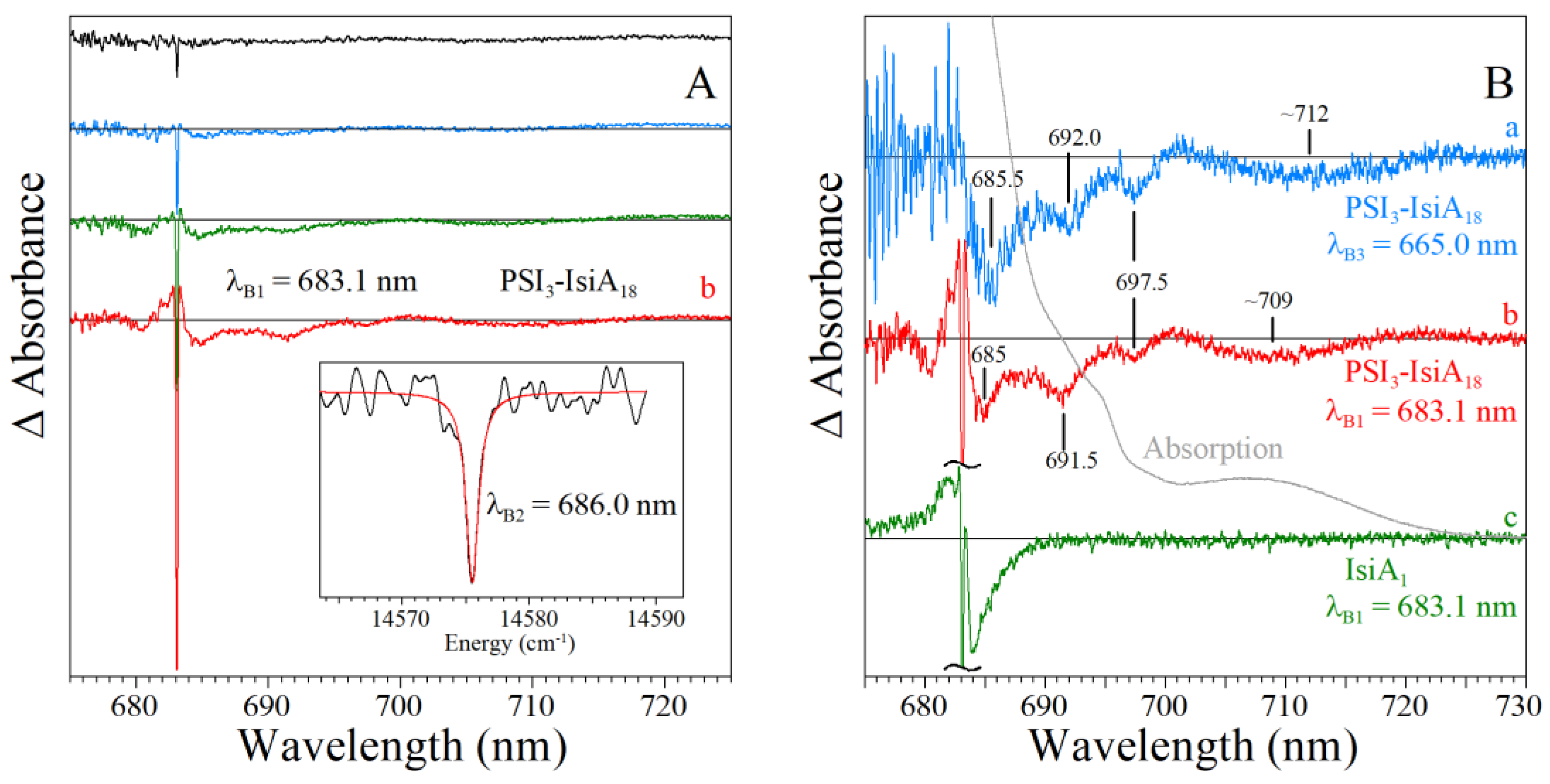
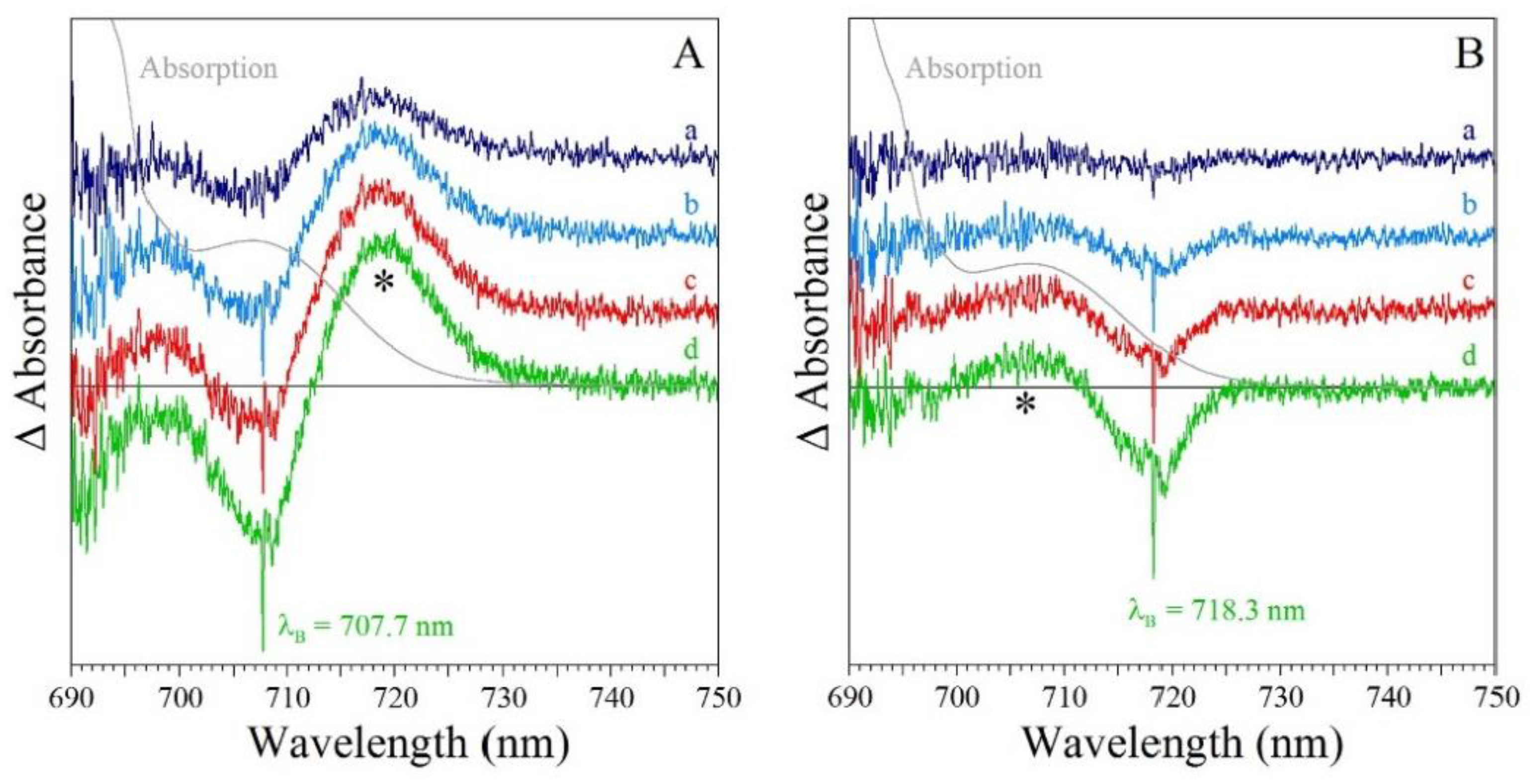
5.2. Hole-Burned Spectra
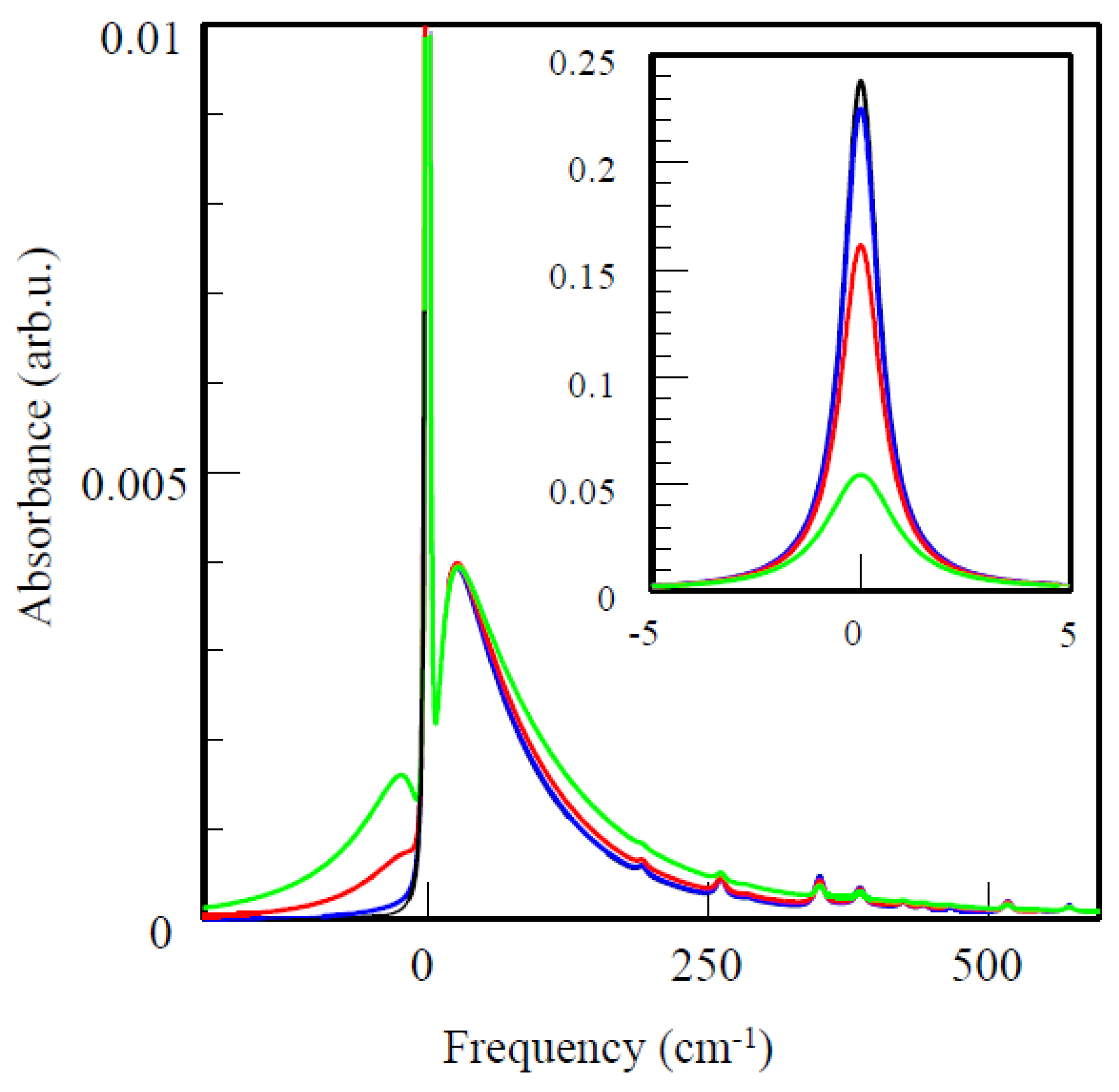
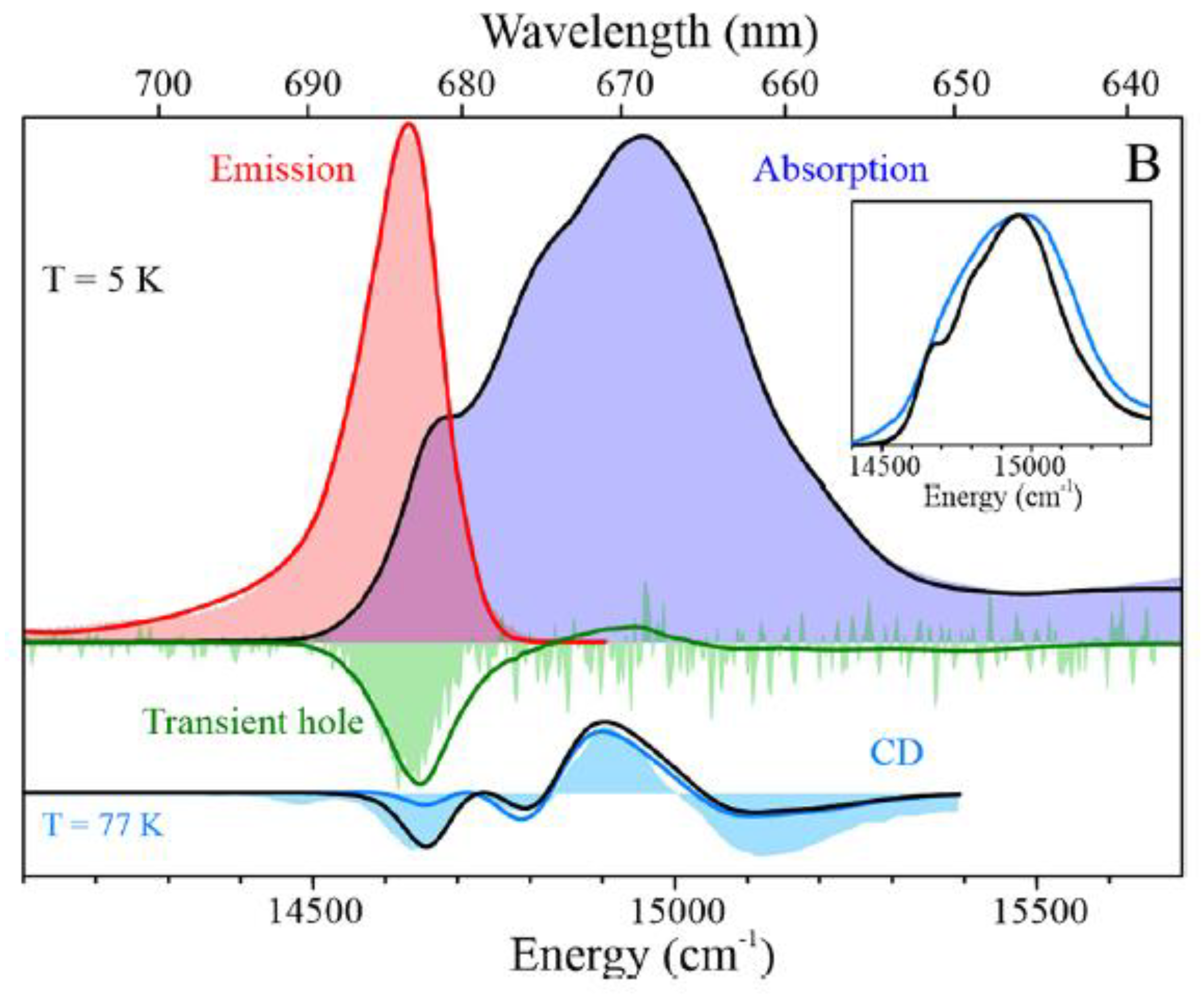
5.3. Modeling of Absorption, Emission, Transient Holes, and CD Spectra of IsiA Monomer
5.4. Modeling of Absorption, Emission, Transient Holes, and CD Spectra of the IsiA18 Ring
5.5. Most Likely Scenario of EET from the IsiA18 Ring to the PSI3 Core: Major Core Entry Points
6. Concluding Remarks
7. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DES | two-dimensional electronic spectroscopy |
| B(Chl) | Bacterio(chlorophyll) |
| Car | carotenoid |
| CD | circular dichroism |
| Chl(s) | chlorophyll(s) |
| Cryo-EM | cryogenic electron microscopy |
| CW | continuous wave |
| DFT | density functional theory |
| ∆FLN | difference fluorescence line-narrowing (spectroscopy) |
| EET | excitation energy transfer |
| el-ph | electron–phonon (coupling) |
| ET | electron transfer |
| FT | Fourier transform |
| FLN | fluorescence line-narrowing |
| Γhom | homogeneous width |
| Γinh | inhomogeneous width; width of the SDF |
| Gln | glutamine |
| HB | (spectral) hole-burning |
| His | histidine |
| Jph(ω) | phonon spectral density |
| Jvib(ω) | vibrational spectral density |
| LH2 | light-harvesting complex 2 (of purple bacteria) |
| LHCII | light-harvesting complex II of green plants |
| ML | machine learning |
| NPHB | non-photochemical (spectral) hole burning |
| P700 | primary electron donor of Photosystem I |
| PC | photosynthetic complex, pigment–protein complex |
| PSI | Photosystem I |
| PSI3 | trimeric cyanobacterial Photosystem I core |
| PSI3–IsiA18 | PSI–IsiA supercomplex |
| PSII | Photosystem II |
| PSB | phonon sideband |
| RC | reaction center |
| Red_a mutation | insertion of four amino acids coordinating the additional Chl B33 |
| Red_b mutation | His95 was mutated to Gln95 of Synechocystis |
| Red_ab mutation | both of the above |
| S | el-ph coupling strength (Huang–Rhys factor) |
| SDF | site distribution function |
| SPCS | single pigment–protein complex spectroscopy |
| Synechocystis | Cyanobacterium Synechocystis (PCC 6803 unless specified otherwise) |
| T | temperature |
| T. elongatus | Cyanobacterium Thermosynechococcus elongatus |
| TLS | two-level system (double-well potential) |
| WT | wild-type |
| ZPH | zero-phonon hole |
| ZPL | zero-phonon line |
References
- Saer, R.G.; Blankenship, R.E. Light harvesting in phototrophic bacteria: Structure and function. Biochem. J. 2017, 474, 2107–2131. [Google Scholar] [CrossRef]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis, 3rd ed.; Wiley: Chichester, UK, 2021. [Google Scholar]
- Reimers, J.R.; Biczysko, M.; Bruce, D.; Coker, D.F.; Frankcombe, T.J.; Hashimoto, H.; Hauer, J.; Jankowiak, R.; Kramer, T.; Linnanto, J.; et al. Challenges facing an understanding of the nature of low-energy excited states in photosynthesis. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1627–1640. [Google Scholar] [CrossRef]
- Savikhin, S. Ultrafast Optical Spectroscopy of Photosystem I. In Photosystem I: The Light-Driven Plastocyanin: Ferredoxin Oxidoreductase; Golbeck, J., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 155–175. [Google Scholar]
- Lee, Y.; Gorka, M.; Golbeck, J.H.; Anna, J.M. Ultrafast energy transfer involving the red chlorophylls of cyanobacterial Photosystem I probed through two-dimensional electronic spectroscopy. J. Am. Chem. Soc. 2018, 140, 1631–11638. [Google Scholar] [CrossRef]
- Savikhin, S.; Jankowiak, R. Mechanism of primary charge separation in photosynthetic reaction centers. In Biophysics of Photosynthesis; Golbeck, J., van der Est, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 193–240. [Google Scholar]
- Melkozernov, A.N.; Bibby, T.S.; Lin, S.; Barber, J.; Blankenship, R.E. Time-resolved absorption and emission show that the CP43′ antenna ring of iron-stressed Synechocystis sp. PCC6803 is efficiently coupled to the Photosystem I reaction center core. Biochemistry 2003, 42, 3893–3903. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Orf, G.S.; Blankenship, R.E.; Savikhin, S. Near shot-noise limited time-resolved circular dichroism pump-probe spectrometer. Rev. Sci. Instrum. 2018, 89, 033104. [Google Scholar] [CrossRef]
- Mascoli, V.; Novoderezhkin, V.; Liguori, N.; Xu, P.; Croce, R. Design principles of solar light harvesting in plants: Functional architecture of the monomeric antenna CP29. Biochim. Bipophys. Acta-Bioenerg. 2020, 1861, 148156. [Google Scholar] [CrossRef]
- Molotokaite, E.; Remelli, W.; Casazza, A.P.; Zucchelli, G.; Polli, D.; Cerullo, G.; Santabarbara, S. Trapping dynamics in Photosystem I—Light harvesting complex I of higher plants is governed by the competition between excited state diffusion from low energy states and photochemical charge separation. J. Phys. Chem. B 2017, 121, 9816–9830. [Google Scholar] [CrossRef]
- Akhtar, P.; Caspy, I.; Nowakowski, P.J.; Malavath, T.; Nelson, N.; Tan, H.-S.; Lambrev, P.H. Two-dimensional electronic spectroscopy of a minimal Photosystem I complex reveals the rate of primary charge separation. J. Am. Chem. Soc. 2021, 143, 14601–14612. [Google Scholar] [CrossRef]
- Jankowiak, R.; Rancova, O.; Chen, J.; Kell, A.; Saer, R.G.; Beatty, J.T.; Abramavičius, D. Mutation-induced changes in the protein environment and site energies in the (M) L214G mutant of the Rhodobacter sphaeroides bacterial reaction center. J. Phys. Chem. B 2016, 120, 7859–7871. [Google Scholar] [CrossRef]
- Fuller, F.D.; Ogilvie, J.P. Experimental implementations of two-dimensional Fourier transform electronic spectroscopy. Ann. Rev. Phys. Chem. 2015, 66, 667–690. [Google Scholar] [CrossRef]
- Zakutauskaitė, K.; Mačernis, M.; Nguyen, H.H.; Ogilvie, J.P.; Abramavičius, D. Extracting the excitonic Hamiltonian of a chlorophyll dimer from broadband two-dimensional electronic spectroscopy. J. Chem. Phys. 2023, 158, 015103. [Google Scholar] [CrossRef]
- Song, Y.; Sechrist, R.; Nguyen, H.H.; Johnson, W.; Abramavičius, D.; Redding, K.; Ogilvie, J.P. Excitonic structure and charge separation in the heliobacterial reaction center probed by multispectral multidimensional spectroscopy. Nat. Commun. 2021, 12, 2801. [Google Scholar] [CrossRef]
- Sardjan, A.S.; Westerman, F.P.; Ogilvie, J.P.; Jansen, T.L.C. Observation of ultrafast coherence transfer and degenerate states with polarization-controlled two-dimensional electronic spectroscopy. J. Phys. Chem. B 2020, 124, 9420–9427. [Google Scholar] [CrossRef]
- Niedringhaus, A.; Policht, V.R.; Sechrist, R.; Konar, A.; Laible, P.D.; Bocian, D.F.; Holten, D.; Kirmaier, C.; Ogilvie, J.P. Primary processes in the bacterial reaction center probed by two-dimensional electronic spectroscopy. Proc. Natl. Acad. Sci. USA 2018, 115, 3563–3568. [Google Scholar] [CrossRef]
- Myers, J.A.; Lewis, K.L.M.; Fuller, F.D.; Tekavec, P.F.; Yocum, C.F.; Ogilvie, J.P. Two-dimensional electronic spectroscopy of the D1-D2-cyt b559 Photosystem II reaction center complex. Phys. Chem. Lett. 2010, 1, 2774–2780. [Google Scholar] [CrossRef]
- Jonas, D.M. Two-dimensional femtosecond spectroscopy. Annu. Rev. Phys. Chem. 2003, 54, 425–464. [Google Scholar] [CrossRef]
- Cho, M.H. Coherent two-dimensional optical spectroscopy. Chem Rev. 2008, 108, 1331–1418. [Google Scholar] [CrossRef]
- Gelzinis, A.; Valkunas, L.; Fuller, F.D.; Ogilvie, J.P.; Mukamel, S.; Abramavičius, D. Tight-binding model of the Photosystem II reaction center: Application to two-dimensional electronic spectroscopy. New J. Phys. 2013, 15, 075013. [Google Scholar] [CrossRef]
- Yang, M.; Fleming, G.R. Two-color three-pulse photon echoes as a probe of electronic coupling in molecular complexes. J. Chem. Phys. 1999, 110, 2983–2990. [Google Scholar] [CrossRef]
- Read, E.L.; Lee, H.; Fleming, G.R. Photon echo studies of photosynthetic light harvesting. Photosynth. Res. 2009, 101, 233–243. [Google Scholar] [CrossRef][Green Version]
- Agarwal, R.; Prall, B.S.; Rizvi, A.H.; Yang, M.; Fleming, G.R. Two-color three pulse photon echo peak shift spectroscopy. J. Chem. Phys. 2002, 116, 6243–6252. [Google Scholar] [CrossRef]
- Agarwal, R.; Krueger, B.P.; Scholes, G.D.; Yang, M.; Yom, J.; Metsand, L.; Fleming, G.R. Ultrafast energy transfer in LHC-II revealed by three-pulse photon echo peak shift measurements. J. Phys. Chem. B 2000, 104, 2908–2918. [Google Scholar] [CrossRef]
- Mančal, T.; Fleming, G.R. Probing electronic coupling in excitonically coupled heterodimer complexes by two-color three-pulse photon echoes. J. Chem. Phys. 2004, 121, 10556–10565. [Google Scholar] [CrossRef]
- Zhang, W.M.; Meier, T.; Chernyak, V.; Mukamel, S. Exciton-migration and three-pulse femtosecond optical spectroscopy of photosynthetic antenna complexes. J. Chem. Phys. 1998, 108, 7763–7774. [Google Scholar] [CrossRef]
- Duan, H.G.; Prokhorenko, V.I.; Cogdell, R.J.; Ashraf, K.; Stevens, A.L.; Thorwart, M.; Miller, R.J.D. Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer. Proc. Natl. Acad. Sci. USA 2017, 114, 8493–8498. [Google Scholar] [CrossRef]
- Ishizaki, A.; Fleming, G.R. On the adequacy of the Redfield equation and related approaches to the study of quantum dynamics in electronic energy transfer. J. Chem. Phys. 2009, 130, 234110. [Google Scholar] [CrossRef]
- Maiuri, M.; Garavelli, M.; Cerullo, G. Ultrafast spectroscopy: State of the art and open challenges. J. Am. Chem. Soc. 2020, 142, 3–15. [Google Scholar] [CrossRef]
- Oliver, T.A.A. Recent advances in multidimensional ultrafast spectroscopy. R. Soc. Open Sci. 2018, 5, 171425. [Google Scholar] [CrossRef]
- van Stokkum, I.H.M.; Larsen, D.S.; van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 2004, 1657, 82–104. [Google Scholar] [CrossRef]
- Jankowiak, R. Fundamental aspects of fluorescence line-narrowing spectroscopy. In Shpol’skii Spectroscopy and Other Site Selective Methods: Applications in Environmental Analysis, Bioanalytical Chemistry and Chemical Physics; Gooijer, C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 235–272. [Google Scholar]
- Jankowiak, R.; Reppert, M.; Zazubovich, V.; Pieper, J.; Reinot, T. Site selective and single complex laser-based spectroscopies: A window on excited state electronic structure, excitation energy transfer, and electron-phonon coupling of selected photosynthetic complexes. Chem. Rev. 2011, 111, 4546–4598. [Google Scholar] [CrossRef]
- Purchase, R.; Völker, S. Spectral hole burning: Examples from photosynthesis. Photosynth. Res. 2009, 101, 245–266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jankowiak, R.; Hayes, J.M.; Small, G.J. Spectral hole-burning spectroscopy in amorphous molecular solids and proteins. Chem. Rev. 1993, 93, 1471–1502. [Google Scholar] [CrossRef]
- Jankowiak, R. Invited Perspective, Probing electron transfer times in photosynthetic reaction centers by hole-burning spectroscopy. J. Phys. Chem. Lett. 2012, 3, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Völker, S. Spectral Hole-Burning in Crystalline and Amorphous Organic Solids. Optical Relaxation Processes at Low Temperature. In Relaxation Processes in Molecular Excited States; Fünfschilling, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 113–242. [Google Scholar]
- Moerner, W.E. (Ed.) Topics in Current Physics, Persistent Spectral Hole Burning: Science and Applications; Springer: New York, NY, USA, 1987; Volume 44. [Google Scholar]
- Zazubovich, V.; Jankowiak, R. Biophotonics of photosynthesis. In Photonics Series: Biological and Medical Photonics, Spectroscopy and Microscopy; Biophotonics, Andrews, D., Eds.; Wiley: Hoboken, NJ, USA, 2015; Volume 4, pp. 129–165. [Google Scholar]
- Berlin, Y.; Burin, A.; Friedrich, J.; Köhler, J. Spectroscopy of proteins at low temperature. Part I: Experiments with molecular ensembles. Phys. Life Rev. 2006, 3, 262–292. [Google Scholar] [CrossRef]
- Zazubovich, V. Fluorescence line narrowing and Δ-FLN spectra in the presence of excitation energy transfer between weakly coupled chromophores. J. Phys. Chem. B 2014, 118, 13535–13543. [Google Scholar] [CrossRef] [PubMed]
- Pieper, J.; Artene, P.; Rätsep, M.; Pajusalu, M.; Freiberg, A. Evaluation of electron-phonon coupling and spectral densities of pigment-protein complexes by line-narrowed optical spectroscopy. J. Phys. Chem. B 2018, 122, 9289–9301. [Google Scholar] [CrossRef]
- Kell, A.; Feng, X.; Reppert, M.; Jankowiak, R. On the shape of the phonon spectral density in photosynthetic complexes. J. Phys. Chem. B 2013, 117, 7317–7323. [Google Scholar] [CrossRef]
- Renger, T.; Marcus, R.A. On the relation of protein dynamics and exciton relaxation in pigment–protein complexes: An estimation of the spectral density and a theory for the calculation of optical spectra. J. Chem. Phys. 2002, 116, 9997–10019. [Google Scholar] [CrossRef]
- Berlin, Y.; Burin, A.; Friedrich, J.; Köhler, J. Low temperature spectroscopy of proteins. Part II: Experiments with single protein complexes. Phys. Life Rev. 2007, 4, 64–89. [Google Scholar] [CrossRef]
- Kondo, T.; Shibata, Y. Recent advances in single-molecule spectroscopy studies on light-harvesting processes in oxygenic photosynthesis. Biophys. Physicobiol. 2022, 19, e190013. [Google Scholar] [CrossRef]
- Kondo, T.; Chen, W.J.; Schlau-Cohen, G.S. Single-molecule fluorescence spectroscopy of photosynthetic systems. Chem. Rev. 2017, 117, 860–898. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, J.A.; Rätsep, M.; Jensen, P.E.; Scheller, H.V.; Croce, R.; Bassi, R.; Korppi-Tommola, J.E.I.; Freiberg, A. Red spectral forms of chlorophylls in green plant PSI—A site-selective and high-pressure spectroscopy study. J. Phys. Chem. B 2003, 107, 9086–9093. [Google Scholar] [CrossRef]
- Kim, Y.; Morozov, D.; Stadnytskyi, V.; Savikhin, S.; Slipchenko, L.V. Predictive first-principles modeling of a photosynthetic antenna protein: The Fenna–Matthews–Olson complex. J. Phys. Chem. Lett. 2020, 11, 1636–1643. [Google Scholar] [CrossRef]
- Adolphs, J.; Muh, F.; Madjet, M.; Schmidt am Busch, M.; Renger, T. Structure-based calculations of optical spectra of Photosystem I suggest an asymmetric light-harvesting process. J. Am. Chem. Soc. 2010, 132, 3331–3343. [Google Scholar] [CrossRef]
- Madjet, M.E.; Abdurahman, A.; Renger, T. Intermolecular coulomb couplings from ab initio electrostatic potentials: application to optical transitions of strongly coupled pigments in photosynthetic antennae and reaction centers. J. Phys. Chem. B 2006, 110, 17268–17281. [Google Scholar] [CrossRef] [PubMed]
- Renger, T.; Müh, F. Theory of excitonic couplings in dielectric media: Foundation of Poisson-TrEsp method and application to Photosystem I trimers. Photosynth. Res. 2012, 111, 47–52. [Google Scholar] [CrossRef]
- Dang, N.C.; Zazubovich, V.; Reppert, M.; Neupane, B.; Picorel, R.; Seibert, M.; Jankowiak, R. The CP43 proximal antenna complex of higher plant Photosystem II revisited: Modeling and hole burning study. J. Phys. Chem. B 2008, 112, 9921–9933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reppert, M.; Acharya, K.; Neupane, B.; Jankowiak, R. Lowest electronic states of the CP47 antenna protein complex of Photosystem II: Simulation of optical spectra and revised structural assignments. J. Phys. Chem. B 2010, 114, 11884–11898. [Google Scholar] [CrossRef]
- Khmelnitskiy, A.; Reinot, T.; Jankowiak, R. Impact of single point mutations on the excitonic structure and dynamics in Fenna–Matthews–Olson complex. J. Phys. Chem. Lett. 2018, 9, 3378–3386. [Google Scholar] [CrossRef]
- Reinot, T.; Khmelnitskiy, A.; Kell, A.; Jassas, M.; Jankowiak, R. Exciton lifetime distributions and population dynamics of the FMO protein complex from Prosthecochloris aestuarii. ACS Omega 2021, 6, 5990–6008. [Google Scholar] [CrossRef]
- Madjet, E.-A.A.; Müh, F.; Renger, T. Deciphering the influence of short-range electronic couplings on optical properties of molecular dimers: Application to “special pairs” in photosynthesis. J. Phys. Chem. B 2009, 113, 12603–12614. [Google Scholar] [CrossRef]
- Shrestha, K.; Virgil, K.A.; Jakubikova, E. Electronic absorption spectra of tetrapyrrole-based pigments via TD-DFT: A reduced orbital space study. J. Phys. Chem. A 2016, 120, 5816–5825. [Google Scholar] [CrossRef]
- Renger, T.; Trostmann, I.; Theiss, C.; Madjet, M.E.; Richter, M.; Paulsen, H.; Eichler, H.J.; Knorr, A.; Renger, G. Refinement of a structural model of a pigment-protein complex by accurate optical line shape theory and experiments. J. Phys. Chem. B 2007, 111, 10487–10501. [Google Scholar] [CrossRef]
- Adolphs, J.; Renger, T. ; How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys. J. 2006, 91, 2778–2797. [Google Scholar] [CrossRef]
- Riley, K.J.; Zazubovich, V.; Jankowiak, R. Frequency-domain spectroscopic study of the PSI-CP43′ supercomplex from the cyanobacterium Synechocystis PCC 6803 grown under iron stress condition. J. Phys. Chem. B 2006, 110, 22436–22446. [Google Scholar] [CrossRef]
- Khmelnitskiy, A.; Toporik, H.; Mazor, Y.; Jankowiak, R. On the red antenna states of Photosystem I mutants from cyanobacteria Synechocystis PCC 6803. J. Phys. Chem. B 2020, 124, 8504–8515. [Google Scholar] [CrossRef]
- Reinot, T.; Khmelnitskiy, A.; Zazubovich, V.; Toporik, H.; Mazor, Y.; Jankowiak, R. Frequency-domain spectroscopic study of the Photosystem I supercomplexes, isolated IsiA monomers, and the intact IsiA ring. J. Phys. Chem. B 2022, 126, 6891–6910. [Google Scholar] [CrossRef]
- Ratsep, M.; Johnson, T.W.; Chitnis, P.R.; Small, G.J. The red-absorbing chlorophyll a antenna states of Photosystem I: A hole-burning study of Synechocystis sp. PCC 6803 and its mutants. J. Phys. Chem. B 2000, 104, 836–847. [Google Scholar]
- Riley, K.J.; Reinot, T.; Jankowiak, R.; Fromme, P.; Zazubovich, V. Red antenna states of Photosystem I from cyanobacteria Synechocystis PCC 6803 and Thermosynechococcus elongatus: Single-complex spectroscopy and spectral hole-burning study. J. Phys. Chem. B 2007, 111, 286–292. [Google Scholar] [CrossRef]
- Herascu, N.; Hunter, M.S.; Shafiei, G.; Najafi, M.; Johnson, T.W.; Fromme, P.; Zazubovich, V. Spectral hole burning in cyanobacterial Photosystem I with P700 in oxidized and neutral states. J. Phys. Chem. B 2016, 120, 10483–10495. [Google Scholar]
- Feng, X.; Neupane, B.; Acharya, K.; Zazubovich, V.; Picorel, R.; Seibert, M.; Jankowiak, R. Spectroscopic study of the CP43′ complex and the PSI–CP43′ supercomplex of the cyanobacterium Synechocystis PCC 6803. J. Phys. Chem. B 2011, 115, 13339–13349. [Google Scholar] [CrossRef]
- Hsin, T.-M.; Zazubovich, V.; Hayes, J.M.; Small, G.J. Red antenna states of PS I of cyanobacteria: Stark effect and interstate energy transfer. J. Phys. Chem. B 2004, 108, 10515–10521. [Google Scholar] [CrossRef]
- Hayes, J.M.; Matsuzaki, S.; Rätsep, M.; Small, G.J. Red chlorophyll a antenna states of Photosystem I of the cyanobacterium Synechocystis sp. PCC 6803. J. Phys. Chem. B 2000, 104, 5625–5633. [Google Scholar] [CrossRef]
- Zazubovich, V.; Matsuzaki, S.; Johnson, T.W.; Hayes, J.M.; Chitnis, P.R.; Small, G.J. Red antenna states of Photosystem I from cyanobacterium Synechococcus elongatus: A spectral hole burning study. Chem. Phys. 2002, 275, 47–59. [Google Scholar] [CrossRef]
- Adolphs, J.; Berrer, M.; Renger, T. Hole-burning spectroscopy on excitonically coupled pigments in proteins: Theory meets experiment. J. Am. Chem. Soc. 2016, 138, 2993–3001. [Google Scholar] [CrossRef]
- Rebane, K.K. Impurity Spectra of Solids; Plenum: New York, NY, USA, 1970. [Google Scholar]
- Jang, S.; Silbey, R.J.; Kunz, R.; Hofmann, C.; Köhler, J. Is there elliptic distortion in the light harvesting complex 2 of purple bacteria? J. Phys. Chem. B 2011, 115, 12947–12953. [Google Scholar] [CrossRef]
- Hofmann, C.; Aartsma, T.J.; Michel, H.; Köhler, J. Direct observation of tiers in the energy landscape of a chromoprotein: A single-molecule study. Proc. Natl. Acad. Sci. USA 2003, 100, 15534–15538. [Google Scholar] [CrossRef]
- Hofmann, C.; Ketelaars, M.; Matsushita, M.; Michel, H.; Aartsma, T.J.; Köhler, J. Single-molecule study of the electronic couplings in a circular array of molecules: Light-harvesting-2 complex from Rhodospirillum molischianum. Phys. Rev. Lett. 2003, 90, 013004. [Google Scholar] [CrossRef]
- Novoderezhkin, V.I.; Rutkauskas, D.; van Grondelle, R. Dynamics of the emission spectrum of a single LH2 complex: Interplay of slow and fast nuclear motions. Biophys. J. 2006, 90, 2890–2902. [Google Scholar] [CrossRef]
- Janusonis, J.; Valkunas, L.; Rutkauskas, D.; van Grondelle, R. Spectral dynamics of individual bacterial light-harvesting complexes: Alternative disorder model. Biophys. J. 2008, 94, 1348–1358. [Google Scholar] [CrossRef][Green Version]
- Rutkauskas, D.; Novoderezhkin, V.; Cogdell, R.J.; van Grondelle, R. Fluorescence spectroscopy of conformational changes of single LH2 complexes. Biophys. J. 2005, 88, 422–435. [Google Scholar] [CrossRef]
- Krüger, T.P.J.; Novoderezhkin, V.I.; Ilioaia, C.; van Grondelle, R. Fluorescence spectral dynamics of single LHCII trimers. Biophys. J. 2010, 98, 3093–3101. [Google Scholar] [CrossRef]
- Krüger, T.P.J.; Wientjes, E.; Croce, R.; van Grondelle, R. Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc. Natl. Acad. Sci. USA 2011, 108, 13516–13521. [Google Scholar] [CrossRef]
- Krüger, T.P.J.; Ilioaia, C.; Valkunas, L.; van Grondelle, R. Fluorescence intermittency from the main plant light-harvesting complex: Sensitivity to the local environment. J. Phys. Chem. B 2011, 115, 5083–5095. [Google Scholar] [CrossRef]
- Schlau-Cohen, G.S.; Yang, H.-Y.; Krüger, T.P.J.; Xu, P.; Gwizdala, M.; van Grondelle, R.; Croce, R.; Moerner, W.E. Single-molecule identification of quenched and unquenched states of LHCII. J. Phys. Chem. Lett. 2015, 6, 860–867. [Google Scholar] [CrossRef]
- Brecht, M.; Skandary, S.; Hellmich, J.; Glöckner, C.; Konrad, A.; Hussels, M.; Meixner, A.J.; Zouni, A.; Schlodder, E. ; Spectroscopic properties of Photosystem II core complexes from Thermosynechococcus elongatus revealed by single-molecule experiments. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 773–781. [Google Scholar] [CrossRef]
- Elli, A.F.; Jelezko, F.; Tietz, C.; Studier, H.; Brecht, M.; Bittl, R.; Wrachtrup, J. Red pool chlorophylls of Photosystem I of the cyanobacterium Thermosynechococcus elongatus: A single-molecule study. Biochemistry 2006, 45, 1454–1458. [Google Scholar] [CrossRef]
- Brecht, M.; Studier, H.; Elli, A.F.; Jelezko, F.; Bittl, R. Assignment of red antenna states in Photosystem I from Thermosynechoccocus elongatus by single-molecule spectroscopy. Biochemistry 2007, 46, 799–806. [Google Scholar] [CrossRef]
- Brecht, M.; Radics, V.; Nieder, J.B.; Studier, H.; Bittl, R. Red antenna states of Photosystem I from Synechocystis sp. PCC 6803. Biochemistry 2008, 47, 5536–5543. [Google Scholar] [CrossRef]
- Brecht, M.; Radics, V.; Nieder, J.B.; Bittl, R. Protein dynamics-induced variation of excitation energy transfer pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 11857–11861. [Google Scholar] [CrossRef]
- Brecht, M.; Studier, H.; Radics, V.; Nieder, J.B.; Bittl, R. Spectral diffusion induced by proton dynamics in pigment-protein complexes. J. Am. Chem. Soc. 2008, 130, 17487–17493. [Google Scholar] [CrossRef]
- Jelezko, F.; Tietz, C.; Gerken, U.; Wrachtrup, J.; Bittl, R. Single-molecule spectroscopy on Photosystem I pigment-protein complexes. J. Phys. Chem. B 2000, 104, 8093–8096. [Google Scholar] [CrossRef]
- Skandary, S.; Konrad, A.; Hussels, M.; Meixner, A.J.; Brecht, M. Orientations between red antenna states of Photosystem I monomers from Thermosynechococcus elongatus revealed by single-molecule spectroscopy. J. Phys. Chem. B 2015, 119, 13888–13896. [Google Scholar] [CrossRef]
- Jana, S.; Du, T.; Nagao, R.; Noguchi, T.; Shibata, Y. Redox-state dependent blinking of single Photosystem I trimers at around liquid-nitrogen temperature. Biochim. Biophys. Acta Bioenerg. 2018, 1860, 30–40. [Google Scholar] [CrossRef]
- Harris, D.; Toporik, H.; Schlau-Cohen, G.S.; Mazor, Y. Energetic robustness to large scale structural fluctuations in a photosynthetic supercomplex. Nat. Comm. 2023, 14, 4650. [Google Scholar] [CrossRef]
- Grozdanov, D.; Herascu, N.; Reinot, T.; Jankowiak, R.; Zazubovich, V. Low-temperature protein dynamics of the B800 molecules in the LH2 light-harvesting complex: Spectral hole burning study and comparison with single photosynthetic complex spectroscopy. J. Phys. Chem. B 2010, 114, 3426–3438. [Google Scholar] [CrossRef]
- Anderson, P.W.; Halperin, B.I.; Varma, C.M. Anomalous low temperature thermal properties of glasses and spin glasses. Phil. Mag. 1972, 25, 1–9. [Google Scholar] [CrossRef]
- Phillips, W.A. Tunneling states in amorphous solids. J. Low Temp. Phys. 1972, 7, 351–360. [Google Scholar] [CrossRef]
- Najafi, M.; Herascu, N.; Seibert, M.; Picorel, R.; Jankowiak, R.; Zazubovich, V. Spectral hole burning, recovery, and thermocycling in chlorophyll–protein complexes: Distributions of barriers on the protein energy landscape. J. Phys. Chem. B 2012, 116, 11780–11790. [Google Scholar] [CrossRef]
- Damjanovic, A.; Vaswani, H.M.; Fromme, P.; Fleming, G.R. Chlorophyll excitations in Photosystem I of Synechococcus elongatus. J. Phys. Chem. B 2002, 106, 10251–10262. [Google Scholar] [CrossRef]
- Reppert, M. Modeling of resonant hole-burning spectra in excitonically coupled systems: The effects of energy-transfer broadening. J. Phys. Chem. Lett. 2011, 2, 2716–2721. [Google Scholar] [CrossRef]
- Toutounji, M. Homogeneous dephasing in photosynthetic bacterial reaction centers: Time correlation function approach. ChemPhysChem 2024, 25, e202300335. [Google Scholar] [CrossRef]
- Reinot, T.; Small, G.J. ; Modeling of dispersive nonphotochemical hole growth kinetics data: Al-phthalocyanine tetrasulphonate in hyperquenched glassy water. J. Chem. Phys. 2000, 113, 10207–10214. [Google Scholar] [CrossRef]
- Reinot, T.; Dang, N.C.; Small, G.J. A model for persistent hole burned spectra and hole growth kinetics that includes photoproduct absorption: Application to free base phthalocyanine in hyperquenched glassy ortho-dichlorobenzene at 5 K. J. Chem. Phys. 2003, 119, 10404–10414. [Google Scholar] [CrossRef]
- Hughes, J.L.; Picorel, R.; Seibert, M.; Krausz, E. Photophysical behavior and assignment of the low-energy chlorophyll states in the CP43 proximal antenna protein of higher plant Photosystem II. Biochemistry 2006, 45, 12345–12357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shafiei, G.; Levenberg, A.; Lujan, M.A.; Picorel, R.; Zazubovich, V. Simultaneous spectral hole burning involving two tiers of the protein energy landscape in cytochrome b6f. J. Phys. Chem. B 2019, 123, 10930–10938. [Google Scholar] [CrossRef]
- Zazubovich, V.; Jankowiak, R. How well does the hole-burning action spectrum represent the site-distribution function of the lowest-energy state in photosynthetic pigment–protein complexes. J. Phys. Chem. B 2019, 123, 6007–6013. [Google Scholar] [CrossRef]
- Trempe, A.; Levenberg, A.; Gonzalez Ortega, A.D.; Lujan, M.A.; Picorel, R.; Zazubovich, V. Effects of chlorophyll triplet states on the kinetics of spectral hole growth. J. Phys. Chem. B 2021, 125, 3278–3285. [Google Scholar] [CrossRef] [PubMed]
- Hartzler, D.A.; Niedzwiedzki, D.M.; Bryant, D.A.; Blankenship, R.E.; Pushkar, Y.; Savikhin, S. Triplet excited state energies and phosphorescence spectra of (bacterio) chlorophylls. J. Phys. Chem. B 2014, 118, 7221–7232. [Google Scholar] [CrossRef]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauβ, N. Three-dimensional Structure of Cyanobacterial Photosystem I at 2.5 Å Resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Fromme, P.; Jordan, P.; Krauß, N. Structure of photosystem I. Biochim. Biophys. Acta 2001, 1507, 5–31. [Google Scholar] [CrossRef]
- Grotjohann, I.; Fromme, P. Structure of cyanobacterial Photosystem I. Photosynth. Res. 2005, 85, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shem, A.; Frolow, F.; Nelson, N. Crystal structure of plant Photosystem I. Nature 2003, 426, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Amunts, A.; Drory, O.; Nelson, N. The structure of a plant Photosystem I supercomplex at 3.4 Å Resolution. Nature 2007, 447, 58–63. [Google Scholar] [CrossRef]
- Amunts, A.; Toporik, H.; Borovikova, A.; Nelson, N. Structure determination and improved model of plant Photosystem I. J. Biol. Chem. 2010, 285, 3478–3486. [Google Scholar] [CrossRef]
- Jolley, C.; Ben-Shem, A.; Nelson, N.; Fromme, P. Structure of plant Photosystem I revealed by theoretical modeling. J. Biol. Chem. 2005, 280, 33627–33636. [Google Scholar] [CrossRef]
- Mazor, Y.; Borovikova, A.; Nelson, N. The structure of plant Photosystem I supercomplex at 2.8 Å resolution. eLife 2015, 4, e07433. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.K.; Jolley, C.; Ben-Shem, A.; Fromme, P.; Nelson, N.; Croce, R.; Schulten, K. Comparison of the light-harvesting networks of plant and cyanobacterial Photosystem I. Biophys. J. 2005, 89, 1630–1642. [Google Scholar] [CrossRef]
- Saenger, W.; Jordan, P.; Krauß, N. The assembly of protein subunits and cofactors in Photosystem I. Curr. Opin. Struct. Biol. 2002, 12, 244–254. [Google Scholar] [CrossRef]
- Mazor, Y.; Nataf, D.; Toporik, H.; Nelson, N. Crystal structures of virus-like Photosystem I complexes from the mesophilic cyanobacterium Synechocystis PCC 6803. eLife 2014, 3, e01496. [Google Scholar] [CrossRef]
- Malavath, T.; Caspy, I.; Netzer-El, S.Y.; Klaiman, D.; Nelson, N. Structure and function of wild-type and subunit-depleted Photosystem I in Synechocystis. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Boekema, E.J.; Hifney, A.; Yakushevska, A.E.; Piotrowski, M.; Keegstra, W.; Berry, S.; Michel, K.-P.; Pistorius, E.K.; Kruip, J. A giant chlorophyll–protein complex induced by iron deficiency in cyanobacteria. Nature 2001, 412, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Bibby, T.S.; Nield, J.; Barber, J. Three-dimensional model and characterization of the iron stress-induced CP43′-Photosystem I supercomplex isolated from the cyanobacterium Synechocystis PCC 6803. J. Biol. Chem. 2001, 276, 43246–43252. [Google Scholar] [CrossRef] [PubMed]
- Akita, F.; Nagao, R.; Kato, K.; Nakajima, Y.; Yokono, M.; Ueno, Y.; Suzuki, T.; Dohmae, N.; Shen, J.-R.; Akimoto, S.; et al. Structure of a cyanobacterial Photosystem I surrounded by octadecameric IsiA antenna proteins. Comm. Biol. 2020, 3, 232. [Google Scholar] [CrossRef] [PubMed]
- Nagao, R.; Kato, K.; Hamaguchi, T.; Ueno, Y.; Tsuboshita, N.; Shimizu, S.; Furutani, M.; Ehira, S.; Nakajima, Y.; Kawakami, K.; et al. Structure of a monomeric photosystem I core associated with iron-stress-induced-A proteins from Anabaena sp. PCC 7120. Nat. Commun. 2023, 14, 920. [Google Scholar] [CrossRef] [PubMed]
- Toporik, H.; Li, J.; Williams, D.; Chiu, P.L.; Mazor, Y. The structure of the stress-induced photosystem I–IsiA antenna supercomplex. Nat. Struct. Mol. Biol. 2019, 26, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, P.; Balog-Vig, F.; Kuntam, S.; Tóth, S.A.; Lambrev, P.H. Function of iron-stress-induced protein A in cyanobacterial cells with monomeric and trimeric Photosystem I. Plant Phys. 2024, 194, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Melkozernov, A.N.; Barber, J.; Blankenship, R.E. Light harvesting in Photosystem I supercomplexes. Biochemistry 2006, 45, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Semchonok, D.A.; Webber-Birungi, M.T.; Ehira, S.; Kondo, K.; Narikawa, R.; Ohmori, M.; Boekema, E.J.; Ikeuchi, M. Attachment of phycobilisomes in an antenna–photosystem I supercomplex of cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2512–2517. [Google Scholar] [CrossRef]
- Chitnis, V.P.; Chitnis, P.R. PsaL subunit is required for the formation of Photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1993, 336, 330–334. [Google Scholar] [CrossRef]
- Müller, M.G.; Slavov, C.; Luthra, R.; Redding, K.E.; Holzwarth, A.R. Independent initiation of primary electron transfer in the two branches of the photosystem I reaction center. Proc. Natl. Acad. Sci. USA 2010, 107, 4123–4128. [Google Scholar] [CrossRef] [PubMed]
- Brettel, K.; Leibl, W. Electron transfer in photosystem I. Biochim. Biophys. Acta. 2001, 1507, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Hastings, G.; Kleinherenbrink, F.A.M.; Lin, S.; McHugh, T.; Blankenship, R.E. Observation of the reduction and reoxidation of the primary electron acceptor in photosystem I. Biochemistry 1994, 33, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Savikhin, S.; Xu, W.; Chitnis, P.R.; Struve, W.S. Ultrafast primary processes in PS I from Synechocystis sp. PCC 6803: Roles of P700 and A0. Biophys. J. 2000, 79, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- White, N.T.H.; Beddard, G.S.; Thorne, J.R.G.; Feehan, T.M.; Keyes, T.E.; Heathcote, P. Primary charge separation and energy transfer in the photosystem I reaction center of higher plants. J. Phys. Chem. 1996, 100, 12086–12099. [Google Scholar] [CrossRef]
- Schlodder, E.; Shubin, V.V.; El-Mohsnawy, E.; Rögner, M.; Karapetyan, N.V. Steady-state and transient polarized absorption spectroscopy of Photosystem I complexes from the cyanobacteria Arthrospira platensis and Thermosynechococcus elongatus. Biochim. Biophys. Acta 2007, 1767, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Witt, H.; Bordignon, E.; Carbonera, D.; Dekker, J.P.; Karapetyan, N.V.; Teutloff, C.; Webber, A.; Lubitz, W.; Schlodder, E. Species-specific differences of the spectroscopic properties of P700. J. Biol. Chem. 2003, 278, 46760–46771. [Google Scholar] [CrossRef] [PubMed]
- Dashdorj, N.; Xu, W.; Martinsson, P.; Chitnis, P.R.; Savikhin, S. Electrochromic shift of chlorophyll absorption in Photosystem I from Synechocystis sp. PCC 6803: A probe of optical and dielectric properties around the secondary electron acceptor. Biophys. J. 2004, 86, 3121–3130. [Google Scholar] [CrossRef]
- Pålsson, L.-O.; Flemming, C.; Gobets, B.; van Grondelle, R.; Dekker, J.P.; Schlodder, E. Energy transfer and charge separation in Photosystem I: P700 oxidation upon selective excitation of the long-wavelength antenna chlorophylls of Synechococcus elongatus. Biophys. J. 1998, 74, 2611–2622. [Google Scholar] [CrossRef]
- Morosinotto, T.; Breton, J.; Bassi, R.; Croce, R. The nature of a chlorophyll ligand in Lhca proteins determines the far-red fluorescence emission typical of Photosystem I. J. Biol. Chem. 2003, 278, 49223–49229. [Google Scholar] [CrossRef]
- Wientjes, E.; Croce, R. The light-harvesting complexes of higher-plant Photosystem I: Lhca1/4 and Lhca2/3 form two red-emitting heterodimers. Biochem. J. 2011, 433, 477–485. [Google Scholar] [CrossRef]
- Croce, R.; van Amerongen, H. Light-harvesting in Photosystem I. Photosynth. Res. 2013, 116, 153–166. [Google Scholar] [CrossRef]
- Elias, E.; Brache, K.; Schäfers, J.; Croce, R. Coloring outside the lines: Exploiting pigment–protein synergy for far-red absorption in plant light-harvesting complexes. J. Am. Chem. Soc. 2024, 146, 3508–3520. [Google Scholar] [CrossRef]
- Schlodder, E.; Hussels, M.; Çetin, M.; Karapetyan, N.V.; Brecht, M. Fluorescence of the various red antenna states in Photosystem I complexes from cyanobacteria is affected differently by the redox state of P700. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Palsson, L.O.; Dekker, J.P.; Schlodder, E.; Monshouwer, R.; van Grondelle, R. Polarized site-selective fluorescence spectroscopy of the long-wavelength emitting chlorophylls in isolated Photosystem I particles of Synechococcus Elongatus. Photosynth. Res. 1996, 48, 239–246. [Google Scholar] [CrossRef]
- Gobets, B.; van Stokkum, I.H.M.; van Mourik, F.; Dekker, J.P.; van Grondelle, R. Excitation wavelength dependence of the fluorescence finetics in Photosystem I particles from Synechocystis PCC 6803 and Synechococcus Elongatus. Biophys. J. 2003, 85, 3883–3898. [Google Scholar] [CrossRef]
- Brüggemann, B.; Sznee, K.; Novoderezhkin, V.; van Grondelle, R.; May, V. From structure to dynamics: Modeling exciton dynamics in the photosynthetic antenna PS1. J. Phys. Chem. B 2004, 108, 13536–13546. [Google Scholar] [CrossRef]
- Byrdin, M.; Jordan, P.; Krauβ, N.; Fromme, P.; Stehlik, D.; Schlodder, E. Light harvesting in Photosystem I: Modeling based on the 2.5-A structure of Photosystem I from Synechococcus elongatus. Biophys. J. 2002, 83, 433–457. [Google Scholar] [CrossRef]
- Yin, S.; Dahlbom, M.G.; Canfield, P.J.; Hush, N.S.; Kobayashi, R.; Reimers, J.R. Assignment of the Qy absorption spectrum of Photosystem-I from Thermosynechococcus elongatus based on CAMB3LYP calculations at the PW91-optimized protein structure. J. Phys. Chem. B 2007, 111, 9923–9930. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.K.; Lu, D.; Ritz, T.; Park, S.; Fromme, P.; Schulten, K. Robustness and optimality of light harvesting in cyanobacterial Photosystem I. J. Phys. Chem. B 2002, 106, 7948–7960. [Google Scholar] [CrossRef]
- Shibata, Y.; Yamagishi, A.; Kawamoto, S.; Noji, T.; Itoh, S. Kinetically distinct three red chlorophylls in Photosystem I of Thermosynechococcus elongatus revealed by femtosecond time-resolved fluorescence spectroscopy at 15 K. J. Phys. Chem. B 2010, 114, 2954–2963. [Google Scholar] [CrossRef]
- Vaitekontis, S.; Trinkunas, G.; Valkunas, L. Red chlorophylls in the exciton model of Photosystem I. Photosynth. Res. 2005, 86, 185–201. [Google Scholar] [CrossRef]
- Balaban, T.S.; Fromme, P.; Holzwarth, A.R.; Krauβ, N.; Prokhorenko, V.I. Relevance of the diastereotopic ligation of magnesium atoms of chlorophylls in Photosystem I. Biochim. Biophys. Acta 2002, 1556, 197–207. [Google Scholar] [CrossRef]
- Kato, K.; Hamaguchi, T.; Nagao, R.; Kawakami, K.; Ueno, Y.; Suzuki, T.; Uchida, H.; Murakami, A.; Nakajima, Y.; Yokono, M.; et al. Structural basis for the absence of low-energy chlorophylls in a photosystem I trimer from Gloeobacter violaceus. eLife 2022, 11, e73990. [Google Scholar] [CrossRef]
- Canfield, P.; Dahlbom, M.G.; Reimers, J.R.; Hush, N.S. Density-functional geometry optimization of the 150 000-atom Photosystem-I trimer. J. Chem. Phys. 2006, 124, 024301. [Google Scholar] [CrossRef]
- Young, A.J.; Frank, H.A. Energy transfer reactions involving carotenoids: Quenching of chlorophyll fluorescence. J. Photochem. Photobiol. B Biol. 1996, 36, 3–15. [Google Scholar] [CrossRef]
- Guikema, J.A.; Sherman, L.A. Chlorophyll-protein organization of membranes from the Cyanobacterium Anacystis Nidulans. Arch. Biochem. Biophys. 1983, 220, 155–166. [Google Scholar] [CrossRef]
- Burnap, R.L.; Troyan, T.; Sherman, L.A. The highly abundant chlorophyll-protein complex of iron-deficient Synechococcus Sp. PCC7942 (CP43′) is encoded by the IsiA gene. Plant Physiol. 1993, 103, 893–902. [Google Scholar] [CrossRef]
- Laudenbach, D.E.; Straus, N.A. Characterization of a cyanobacterial iron stress-induced gene similar to PsbC. J. Bacteriol. 1988, 170, 5018–5026. [Google Scholar] [CrossRef]
- Andrizhiyevskaya, E.G.; Schwabe, T.M.E.; Germano, M.; D’Haene, S.; Kruip, J.; van Grondelle, R.; Dekker, J.P. Spectroscopic properties of PSI–IsiA supercomplexes from the cyanobacterium Synechococcus PCC 7942. Biochim. Biophys. Acta Bioenerg. 2002, 1556, 265–272. [Google Scholar] [CrossRef]
- Cao, P.; Cao, D.; Si, L.; Su, X.; Tian, L.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structural basis for energy and electron transfer of the Photosystem I–IsiA–flavodoxin supercomplex. Nat. Plants 2020, 6, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Guedeney, G.; Hagemann, M.; Yeremenko, N.; Matthijs, H.C.P.; Jeanjean, R. The Chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett. 2005, 579, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Sandström, S.; Park, Y.-I.; Öquist, G.; Gustafsson, P. CP43′, the IsiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus Sp. PCC 7942. Photochem. Photobiol. 2007, 74, 431–437. [Google Scholar] [CrossRef]
- Ihalainen, J.A.; D’Haene, S.; Yeremenko, N.; van Roon, H.; Arteni, A.A.; Boekema, E.J.; van Grondelle, R.; Matthijs, H.C.P.; Dekker, J.P. Aggregates of the chlorophyll-binding protein IsiA (CP43′) dissipate energy in cyanobacteria. Biochemistry 2005, 44, 10846–10853. [Google Scholar] [CrossRef]
- Umena, Y.; Kawakami, K.; Shen, J.-R.; Kamiya, N. Crystal structure of oxygen-evolving Photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Gobets, B.; van Stokkum, I.H.M.; Rogner, M.; Kruip, J.; Schlodder, E.; Karapetyan, N.V.; Dekker, J.P.; van Grondelle, R. Time-resolved fluorescence emission measurements of Photosystem I particles of various cyanobacteria: A unified compartmental model. Biophys. J. 2001, 81, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Toporik, H.; Khmelnitskiy, A.; Dobson, D.; Riddle, R.; Williams, D.; Lin, S.; Jankowiak, R.; Mazor, Y. The structure of a red-shifted Photosystem I reveals a red site in the core antenna. Nat. Commun. 2020, 11, 5279. [Google Scholar] [CrossRef] [PubMed]
- Bixon, M.; Jortner, J. Electronic relaxation in large molecules. J. Chem. Phys. 1969, 50, 4061–4070. [Google Scholar] [CrossRef]
- Cupellini, L.; Caprasecca, S.; Guido, C.A.; Müh, F.; Renger, T.; Mennucci, B. Coupling to charge transfer states is the key to modulate the optical bands for efficient light harvesting in purple bacteria. J. Phys. Chem. Lett. 2018, 9, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Miloslavina, Y.; Wehner, A.; Lambrev, P.H.; Wientjes, E.; Reus, M.; Garab, G.; Croce, R.; Holzwarth, A.R. Far-red fluorescence: A direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett. 2008, 582, 3625–3631. [Google Scholar] [CrossRef]
- Kell, A.; Feng, X.; Lin, C.; Yang, Y.; Li, J.; Reus, M.; Holzwarth, A.R.; Jankowiak, R. Charge-transfer character of the low-energy Chl a Qy absorption band in aggregated light harvesting complexes II. J. Phys. Chem. B 2014, 118, 6086–6091. [Google Scholar] [CrossRef]
- Gobets, B.; van Grondelle, R. Energy transfer and trapping in Photosystem I. Biochim. Biophys. Acta Bioenerg. 2001, 1507, 80–99. [Google Scholar] [CrossRef]
- Schlodder, E.; Lendzian, F.; Meyer, J.; Çetin, M.; Brecht, M.; Renger, T.; Karapetyan, N.V. Long-wavelength limit of photochemical energy conversion in Photosystem I. J. Am. Chem. Soc. 2014, 136, 3904–3918. [Google Scholar] [CrossRef] [PubMed]
- Moqvist, F.; Mamedov, F.; Styring, S. Defining the far-red limit of Photosystem I. The primary charge separation is functional to 840 nm. J. Biol. Chem. 2014, 289, 24630–24639. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A Simplex method for function minimization. Comp. J. 1965, 7, 308–313. [Google Scholar]
- Gradinaru, C.C.; Özdemir, S.; Gülen, D.; van Stokkum, I.H.M.; van Grondelle, R.; van Amerongen, H. The flow of excitation energy in LHCII monomers: Implications for the structural model of the major plant antenna. Biophys. J. 1998, 75, 3064–3077. [Google Scholar] [CrossRef]
- Rivadossi, A.; Zucchelli, G.; Garlaschi, F.; Jennings, R.C. The importance of PS I chlorophyll red forms in light-harvesting by leaves. Photosynth. Res. 1999, 60, 209–215. [Google Scholar] [CrossRef]
- Croce, R.; Zucchelli, G.; Garlaschi, F.M.; Bassi, R.; Jennings, R.C. Excited state equilibration in the Photosystem I—Light-harvesting I complex: P700 is almost isoenergetic with its antenna. Biochemistry 1996, 35, 8572–8579. [Google Scholar] [CrossRef]
- Koehne, B.; Elli, G.; Jennings, R.C.; Wilhelm, C.; Trissl, H.W. Spectroscopic and molecular characterization of a long wavelength absorbing antenna of Ostreobium sp. Biochim. Biophys. Acta Bioenerg. 1999, 1412, 94–107. [Google Scholar] [CrossRef]
- Trissl, H.W. Long-wavelength absorbing antenna pigments and heterogeneous absorption bands concentrate excitons and increase absorption cross section. Photosynth. Res. 1993, 35, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Khruschev, S.S.; Plyusnina, T.Y.; Antal, T.K.; Pogosyan, S.I.; Riznichenko, G.Y.; Rubin, A.B. ; Machine learning methods for assessing photosynthetic activity: Environmental monitoring applications. Biophys. Rev. 2022, 14, 821–842. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.; Kumar Cherukuri, A.; Doddrell, N.H.; Priya Doss, C.G.; Simkin, A.J.; Ramamoorthy, S. Machine learning in photosynthesis: Prospects on sustainable crop development. Plant Sci. 2023, 335, 111795. [Google Scholar] [CrossRef] [PubMed]
- Häse, F.; Valleau, S.; Pyzer-Knapp, E.; Aspuru-Guzik, A. Machine learning exciton dynamics. Chem. Sci. 2016, 7, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Häse, F.; Kreisbeck, C.; Aspuru-Guzik, A. Machine learning for quantum dynamics: Deep learning of excitation energy transfer properties. Chem. Sci. 2017, 8, 8419–8426. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazubovich, V.; Jankowiak, R. High-Resolution Frequency-Domain Spectroscopic and Modeling Studies of Photosystem I (PSI), PSI Mutants and PSI Supercomplexes. Int. J. Mol. Sci. 2024, 25, 3850. https://doi.org/10.3390/ijms25073850
Zazubovich V, Jankowiak R. High-Resolution Frequency-Domain Spectroscopic and Modeling Studies of Photosystem I (PSI), PSI Mutants and PSI Supercomplexes. International Journal of Molecular Sciences. 2024; 25(7):3850. https://doi.org/10.3390/ijms25073850
Chicago/Turabian StyleZazubovich, Valter, and Ryszard Jankowiak. 2024. "High-Resolution Frequency-Domain Spectroscopic and Modeling Studies of Photosystem I (PSI), PSI Mutants and PSI Supercomplexes" International Journal of Molecular Sciences 25, no. 7: 3850. https://doi.org/10.3390/ijms25073850
APA StyleZazubovich, V., & Jankowiak, R. (2024). High-Resolution Frequency-Domain Spectroscopic and Modeling Studies of Photosystem I (PSI), PSI Mutants and PSI Supercomplexes. International Journal of Molecular Sciences, 25(7), 3850. https://doi.org/10.3390/ijms25073850






