The Formation and Function of the VTA Dopamine System
Abstract
:1. Development of the Dopamine System
1.1. Ontogeny of the Dopamine System
1.2. The Dopamine System in Invertebrates
2. Features of the Ventral Tegmental Area
2.1. Cell Types
2.2. Ion Channels and Receptors
2.2.1. Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels
2.2.2. Voltage-Gated Potassium Channel Subfamily Q (KCNQ)
2.2.3. Dopamine Receptor and Transporter
2.2.4. Serotonin Receptor and Transporter
2.2.5. Adrenergic Receptor
2.3. Neuropeptides and Receptors
2.3.1. Corticotropin-Releasing Factor
2.3.2. Neurotensin
2.3.3. Orexin and Dynorphin
2.3.4. Oxytocin
2.3.5. Cholecystokinin
3. Connectivity of the Ventral Tegmental Area
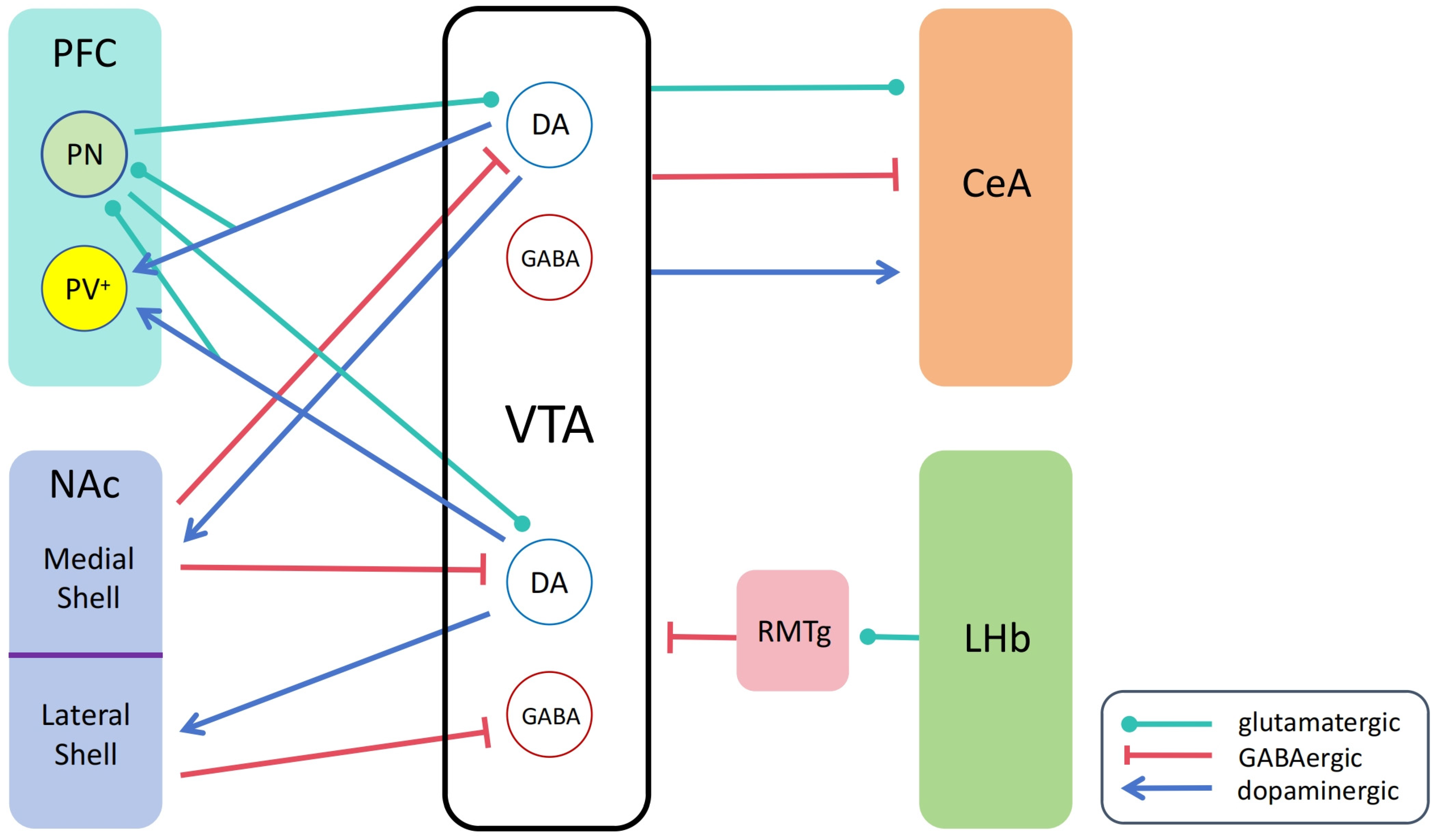
3.1. VTA and NAc
3.2. VTA and PFC
3.3. VTA and CeA
3.4. VTA and Lateral Habenula
4. Multiple Neuropsychiatric Disorders
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prasad, A.A.; Pasterkamp, R.J. Axon guidance in the dopamine system. Adv. Exp. Med. Biol. 2009, 651, 91–100. [Google Scholar] [CrossRef]
- Jin, X.; Costa, R.M. Start/stop signals emerge in nigrostriatal circuits during sequence learning. Nature 2010, 466, 457–462. [Google Scholar] [CrossRef]
- Bissonette, G.B.; Roesch, M.R. Development and function of the midbrain dopamine system: What we know and what we need to. Genes Brain Behav. 2016, 15, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Garritsen, O.; van Battum, E.Y.; Grossouw, L.M.; Pasterkamp, R.J. Development, wiring and function of dopamine neuron subtypes. Nat. Rev. Neurosci. 2023, 24, 134–152. [Google Scholar] [CrossRef]
- Ono, Y.; Nakatani, T.; Sakamoto, Y.; Mizuhara, E.; Minaki, Y.; Kumai, M.; Hamaguchi, A.; Nishimura, M.; Inoue, Y.; Hayashi, H.; et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: Midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 2007, 134, 3213–3225. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.; Pollock, J.D. Making connections: The development of mesencephalic dopaminergic neurons. Brain Res. Dev. Brain Res. 2003, 147, 3–21. [Google Scholar] [CrossRef]
- Adams, K.A.; Maida, J.M.; Golden, J.A.; Riddle, R.D. The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development 2000, 127, 1857–1867. [Google Scholar] [CrossRef]
- Brodski, C.; Weisenhorn, D.M.; Signore, M.; Sillaber, I.; Oesterheld, M.; Broccoli, V.; Acampora, D.; Simeone, A.; Wurst, W. Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J. Neurosci. 2003, 23, 4199–4207. [Google Scholar] [CrossRef]
- Howe, M.W.; Dombeck, D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Pereira Luppi, M.; Azcorra, M.; Caronia-Brown, G.; Poulin, J.F.; Gaertner, Z.; Gatica, S.; Moreno-Ramos, O.A.; Nouri, N.; Dubois, M.; Ma, Y.C.; et al. Sox6 expression distinguishes dorsally and ventrally biased dopamine neurons in the substantia nigra with distinctive properties and embryonic origins. Cell Rep. 2021, 37, 109975. [Google Scholar] [CrossRef]
- Poulin, J.F.; Caronia, G.; Hofer, C.; Cui, Q.; Helm, B.; Ramakrishnan, C.; Chan, C.S.; Dombeck, D.A.; Deisseroth, K.; Awatramani, R. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat. Neurosci. 2018, 21, 1260–1271. [Google Scholar] [CrossRef]
- Ikemoto, S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007, 56, 27–78. [Google Scholar] [CrossRef]
- Ioanas, H.I.; Saab, B.J.; Rudin, M. Whole-brain opto-fMRI map of mouse VTA dopaminergic activation reflects structural projections with small but significant deviations. Transl. Psychiatry 2022, 12, 60. [Google Scholar] [CrossRef]
- Brischoux, F.; Chakraborty, S.; Brierley, D.I.; Ungless, M.A. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 4894–4899. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Saunders, B.T.; Richard, J.M.; Margolis, E.B.; Janak, P.H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 2018, 21, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. I. Nigrosomes and the nigral matrix, a compartmental organization based on calbindin D(28K) immunohistochemistry. Brain 1999, 122 Pt 8, 1421–1436. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122 Pt 8, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Monzon-Sandoval, J.; Poggiolini, I.; Ilmer, T.; Wade-Martins, R.; Webber, C.; Parkkinen, L. Human-Specific Transcriptome of Ventral and Dorsal Midbrain Dopamine Neurons. Ann. Neurol. 2020, 87, 853–868. [Google Scholar] [CrossRef] [PubMed]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.W.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580.e19. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, E. Dopamine miracle: From brain homogenate to dopamine replacement. Movement Disord. 2002, 17, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Marsden, C.A. Dopamine: The rewarding years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S136–S144. [Google Scholar] [CrossRef] [PubMed]
- Callier, S.; Snapyan, M.; Le Crom, S.; Prou, D.; Vincent, J.D.; Vernier, P. Evolution and cell biology of dopamine receptors in vertebrates. Biol. Cell 2003, 95, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Beggs, K.T.; Mercer, A.R. Molecular biology of the invertebrate dopamine receptors. Arch. Insect Biochem. Physiol. 2005, 59, 103–117. [Google Scholar] [CrossRef]
- Aimon, S.; Katsuki, T.; Jia, T.Q.; Grosenick, L.; Broxton, M.; Deisseroth, K.; Sejnowski, T.J.; Greenspan, R.J. Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol. 2019, 17, e2006732. [Google Scholar] [CrossRef]
- Marquis, M.; Wilson, R.I. Locomotor and olfactory responses in dopamine neurons of the superior-lateral brain. Curr. Biol. 2022, 32, 5406–5414. [Google Scholar] [CrossRef]
- Kume, K.; Kume, S.; Park, S.K.; Hirsh, J.; Jackson, F.R. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 2005, 25, 7377–7384. [Google Scholar] [CrossRef]
- Ueno, T.; Tomita, J.; Tanimoto, H.; Endo, K.; Ito, K.; Kume, S.; Kume, K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 2012, 15, 1516–1523. [Google Scholar] [CrossRef]
- Waddell, S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010, 33, 457–464. [Google Scholar] [CrossRef]
- Yamamoto, S.; Seto, E.S. Dopamine Dynamics and Signaling in Drosophila: An Overview of Genes, Drugs and Behavioral Paradigms. Exp. Anim. Tokyo 2014, 63, 107–119. [Google Scholar] [CrossRef]
- Aso, Y.; Siwanowicz, I.; Bräcker, L.; Ito, K.; Kitamoto, T.; Tanimoto, H. Specific Dopaminergic Neurons for the Formation of Labile Aversive Memory. Curr. Biol. 2010, 20, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Joiner, W.J.; Crocker, A.; White, B.H.; Sehgal, A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 2006, 441, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Plaçais, P.Y.; Yamagata, N.; Pfeiffer, B.D.; Aso, Y.; Friedrich, A.B.; Siwanowicz, I.; Rubin, G.M.; Preat, T.; Tanimoto, H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 2012, 488, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Pitman, J.L.; McGill, J.J.; Keegan, K.P.; Allada, R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 2006, 441, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Yu, F.; Guo, A.K. Sleep Deprivation Specifically Impairs Short-term Olfactory Memory in Drosophila. Sleep 2009, 32, 1417–1424. [Google Scholar] [CrossRef]
- Ma, S.; Zhong, H.; Liu, X.; Wang, L. Spatial Distribution of Neurons Expressing Single, Double, and Triple Molecular Characteristics of Glutamatergic, Dopaminergic, or GABAergic Neurons in the Mouse Ventral Tegmental Area. J. Mol. Neurosci. 2023, 73, 345–362. [Google Scholar] [CrossRef]
- Bariselli, S.; Glangetas, C.; Tzanoulinou, S.; Bellone, C. Ventral tegmental area subcircuits process rewarding and aversive experiences. J. Neurochem. 2016, 139, 1071–1080. [Google Scholar] [CrossRef]
- Morales, M.; Root, D.H. Glutamate neurons within the midbrain dopamine regions. Neuroscience 2014, 282, 60–68. [Google Scholar] [CrossRef]
- Olson, V.G.; Nestler, E.J. Topographical organization of GABAergic neurons within the ventral tegmental area of the rat. Synapse 2007, 61, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A.; Onn, S.P. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci. 1989, 9, 3463–3481. [Google Scholar] [CrossRef]
- Margolis, E.B.; Toy, B.; Himmels, P.; Morales, M.; Fields, H.L. Identification of rat ventral tegmental area GABAergic neurons. PLoS ONE 2012, 7, e42365. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, N.B.; Bonci, A.; Calabresi, P.; Stefani, A.; Bernardi, G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur. J. Neurosci. 1995, 7, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ungless, M.A.; Grace, A.A. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012, 35, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Lim, B.K.; Malenka, R.C. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 2014, 76 Pt B, 351–359. [Google Scholar] [CrossRef]
- Cohen, J.Y.; Haesler, S.; Vong, L.; Lowell, B.B.; Uchida, N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 2012, 482, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Ungless, M.A.; Magill, P.J.; Bolam, J.P. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 2004, 303, 2040–2042. [Google Scholar] [CrossRef]
- Hnasko, T.S.; Hjelmstad, G.O.; Fields, H.L.; Edwards, R.H. Ventral tegmental area glutamate neurons: Electrophysiological properties and projections. J. Neurosci. 2012, 32, 15076–15085. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef]
- Faget, L.; Osakada, F.; Duan, J.; Ressler, R.; Johnson, A.B.; Proudfoot, J.A.; Yoo, J.H.; Callaway, E.M.; Hnasko, T.S. Afferent Inputs to Neurotransmitter-Defined Cell Types in the Ventral Tegmental Area. Cell Rep. 2016, 15, 2796–2808. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Eisch, A.J.; Tang, M.D.; Kaczmarek, L.K.; Nestler, E.J. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res. Mol. Brain Res. 2000, 81, 129–139. [Google Scholar] [CrossRef]
- Notomi, T.; Shigemoto, R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 2004, 471, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Franz, O.; Liss, B.; Neu, A.; Roeper, J. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons. Eur. J. Neurosci. 2000, 12, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
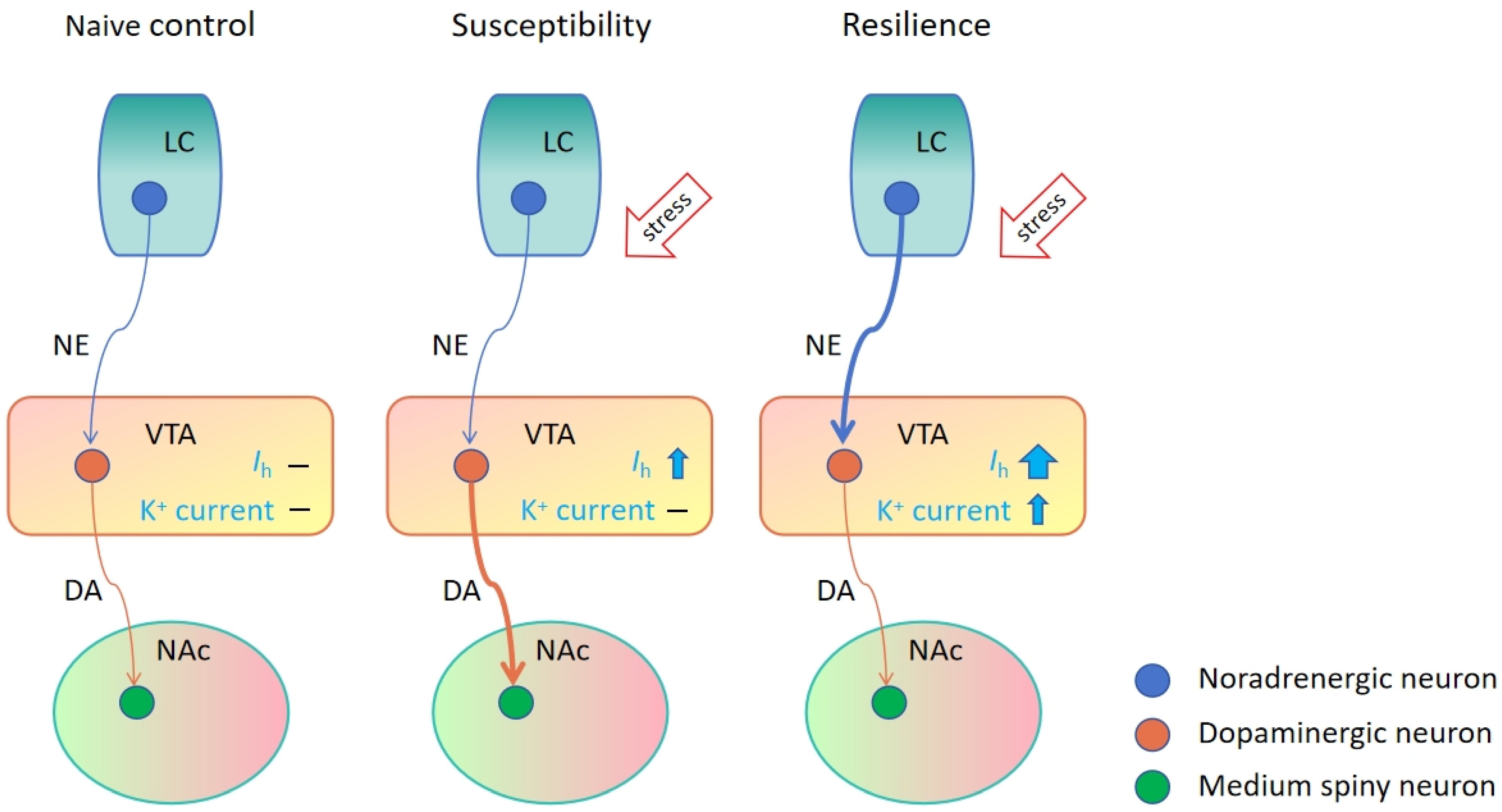
- Friedman, A.K.; Walsh, J.J.; Juarez, B.; Ku, S.M.; Chaudhury, D.; Wang, J.; Li, X.; Dietz, D.M.; Pan, N.; Vialou, V.F.; et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science 2014, 344, 313–319. [Google Scholar] [CrossRef]
- Juarez, B.; Han, M.H. Diversity of Dopaminergic Neural Circuits in Response to Drug Exposure. Neuropsychopharmacology 2016, 41, 2424–2446. [Google Scholar] [CrossRef]
- Lammel, S.; Hetzel, A.; Hackel, O.; Jones, I.; Liss, B.; Roeper, J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 2008, 57, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, H.; Neu, A.; Liss, B.; Roeper, J. Ih channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J. Neurosci. 2002, 22, 1290–1302. [Google Scholar] [CrossRef]
- Cao, J.L.; Covington, H.E., 3rd; Friedman, A.K.; Wilkinson, M.B.; Walsh, J.J.; Cooper, D.C.; Nestler, E.J.; Han, M.H. Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J. Neurosci. 2010, 30, 16453–16458. [Google Scholar] [CrossRef]
- Cai, M.; Zhu, Y.; Shanley, M.R.; Morel, C.; Ku, S.M.; Zhang, H.; Shen, Y.; Friedman, A.K.; Han, M.H. HCN channel inhibitor induces ketamine-like rapid and sustained antidepressant effects in chronic social defeat stress model. Neurobiol. Stress 2023, 26, 100565. [Google Scholar] [CrossRef]
- Ku, S.M.; Han, M.H. HCN Channel Targets for Novel Antidepressant Treatment. Neurotherapeutics 2017, 14, 698–715. [Google Scholar] [CrossRef]
- Lerche, C.; Scherer, C.R.; Seebohm, G.; Derst, C.; Wei, A.D.; Busch, A.E.; Steinmeyer, K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. J. Biol. Chem. 2000, 275, 22395–22400. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; Laplant, Q.; Graham, A.; Lutter, M.; Lagace, D.C.; et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- Li, L.; Sun, H.; Ding, J.; Niu, C.; Su, M.; Zhang, L.; Li, Y.; Wang, C.; Gamper, N.; Du, X.; et al. Selective targeting of M-type potassium Kv7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour. Br. J. Pharmacol. 2017, 174, 4277–4294. [Google Scholar] [CrossRef]
- Wang, H.R.; Hu, S.W.; Zhang, S.; Song, Y.; Wang, X.Y.; Wang, L.; Li, Y.Y.; Yu, Y.M.; Liu, H.; Liu, D.; et al. KCNQ Channels in the Mesolimbic Reward Circuit Regulate Nociception in Chronic Pain in Mice. Neurosci. Bull. 2021, 37, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Passmore, G.M. Neural KCNQ (Kv7) channels. Br. J. Pharmacol. 2009, 156, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M.; O’Malley, M.; Datta, S.; Ciraulo, D.A. The Kv7 potassium channel activator retigabine decreases alcohol consumption in rats. Am. J. Drug Alcohol Abuse 2014, 40, 244–250. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Grippon, S.; Kirkpatrick, P. Ezogabine (retigabine). Nat. Rev. Drug Discov. 2011, 10, 729–730. [Google Scholar] [CrossRef]
- Friedman, A.K.; Juarez, B.; Ku, S.M.; Zhang, H.; Calizo, R.C.; Walsh, J.J.; Chaudhury, D.; Zhang, S.; Hawkins, A.; Dietz, D.M.; et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat. Commun. 2016, 7, 11671. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Costi, S.; Morris, L.S.; Van Dam, N.T.; Kautz, M.; Whitton, A.E.; Friedman, A.K.; Collins, K.A.; Ahle, G.; Chadha, N.; et al. Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol. Psychiatry 2020, 25, 1323–1333. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Sig. Transd. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Palermo, G.; Giannoni, S.; Bellini, G.; Siciliano, G.; Ceravolo, R. Dopamine Transporter Imaging, Current Status of a Potential Biomarker: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11234. [Google Scholar] [CrossRef]
- McHugh, P.C.; Buckley, D.A. The Structure and Function of the Dopamine Transporter and its Role in CNS Diseases. Vitam. Horm. 2015, 98, 339–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Yang, Y.K.; Howes, O.; Lee, I.H.; Landau, S.; Yeh, T.L.; Chiu, N.T.; Chen, P.S.; Lu, R.B.; David, A.S.; et al. Striatal Dopamine Transporter Availability in Drug-Naive Patients With Schizophrenia: A Case-Control SPECT Study with [99mTc]-TRODAT-1 and a Meta-Analysis. Schizophr. Bull. 2013, 39, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Meyer-Lindenberg, A. Striatal Presynaptic Dopamine in Schizophrenia, Part I: Meta-Analysis of Dopamine Active Transporter (DAT) Density. Schizophr. Bull. 2013, 39, 22–32. [Google Scholar] [CrossRef]
- Gellynck, E.; Heyninck, K.; Andressen, K.W.; Haegeman, G.; Levy, F.O.; Vanhoenacker, P.; Van Craenenbroeck, K. The serotonin 5-HT7 receptors: Two decades of research. Exp. Brain Res. 2013, 230, 555–568. [Google Scholar] [CrossRef]
- Zhou, F.M.; Hablitz, J.J. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J. Neurophysiol. 1999, 82, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef]
- Sharp, T.; Barnes, N.M. Central 5-HT receptors and their function; present and future. Neuropharmacology 2020, 177, 108155. [Google Scholar] [CrossRef]
- Di Giovanni, G.; Di Matteo, V.; Pierucci, M.; Esposito, E. Serotonin-dopamine interaction: Electrophysiological evidence. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2008; Volume 172, pp. 45–71. [Google Scholar] [CrossRef]
- Vanbockstaele, E.J.; Cestari, D.M.; Pickel, V.M. Synaptic Structure and Connectivity of Serotonin Terminals in the Ventral Tegmental Area—Potential Sites for Modulation of Mesolimbic Dopamine Neurons. Brain Res. 1994, 647, 307–322. [Google Scholar] [CrossRef]
- Nagayasu, K. Serotonin transporter: Recent progress of in silico ligand prediction methods and structural biology towards structure-guided in silico design of therapeutic agents. J. Pharmacol. Sci. 2022, 148, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Yang, D.X.; Zhao, Z.Y.; Wen, P.C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.S.; Andersen, J.; Jorgensen, T.N.; Sorensen, L.; Eriksen, J.; Loland, C.J.; Stromgaard, K.; Gether, U. SLC6 Neurotransmitter Transporters: Structure, Function, and Regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef]
- Nalepa, I.; Kreiner, G.; Bielawski, A.; Rafa-Zablocka, K.; Roman, A. alpha1-Adrenergic receptor subtypes in the central nervous system: Insights from genetically engineered mouse models. Pharmacol. Rep. 2013, 65, 1489–1497. [Google Scholar] [CrossRef]
- Papay, R.; Gaivin, R.; Jha, A.; McCune, D.F.; McGrath, J.C.; Rodrigo, M.C.; Simpson, P.C.; Doze, V.A.; Perez, D.M. Localization of the mouse alpha1A-adrenergic receptor (AR) in the brain: α1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J. Comp. Neurol. 2006, 497, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Solecki, W.B.; Szklarczyk, K.; Pradel, K.; Kwiatkowska, K.; Dobrzanski, G.; Przewlocki, R. Noradrenergic signaling in the VTA modulates cocaine craving. Addict. Biol. 2018, 23, 596–609. [Google Scholar] [CrossRef]
- Tovar-Diaz, J.; Pomrenze, M.B.; Kan, R.; Pahlavan, B.; Morikawa, H. Cooperative CRF and alpha1 Adrenergic Signaling in the VTA Promotes NMDA Plasticity and Drives Social Stress Enhancement of Cocaine Conditioning. Cell Rep. 2018, 22, 2756–2766. [Google Scholar] [CrossRef]
- Velasquez-Martinez, M.C.; Santos-Vera, B.; Velez-Hernandez, M.E.; Vazquez-Torres, R.; Jimenez-Rivera, C.A. Alpha-1 Adrenergic Receptors Modulate Glutamate and GABA Neurotransmission onto Ventral Tegmental Dopamine Neurons during Cocaine Sensitization. Int. J. Mol. Sci. 2020, 21, 790. [Google Scholar] [CrossRef]
- El Mansari, M.; Guiard, B.P.; Chernoloz, O.; Ghanbari, R.; Katz, N.; Blier, P. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci. Ther. 2010, 16, e1–e17. [Google Scholar] [CrossRef]
- Guiard, B.P.; El Mansari, M.; Blier, P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol. Pharmacol. 2008, 74, 1463–1475. [Google Scholar] [CrossRef]
- Mejias-Aponte, C.A. Specificity and impact of adrenergic projections to the midbrain dopamine system. Brain Res. 2016, 1641, 258–273. [Google Scholar] [CrossRef]
- Park, J.W.; Bhimani, R.V.; Park, J. Noradrenergic Modulation of Dopamine Transmission Evoked by Electrical Stimulation of the Locus Coeruleus in the Rat Brain. ACS Chem. Neurosci. 2017, 8, 1913–1924. [Google Scholar] [CrossRef]
- Goertz, R.B.; Wanat, M.J.; Gomez, J.A.; Brown, Z.J.; Phillips, P.E.; Paladini, C.A. Cocaine increases dopaminergic neuron and motor activity via midbrain alpha1 adrenergic signaling. Neuropsychopharmacology 2015, 40, 1151–1162. [Google Scholar] [CrossRef]
- Isingrini, E.; Perret, L.; Rainer, Q.; Amilhon, B.; Guma, E.; Tanti, A.; Martin, G.; Robinson, J.; Moquin, L.; Marti, F.; et al. Resilience to chronic stress is mediated by noradrenergic regulation of dopamine neurons. Nat. Neurosci. 2016, 19, 560–563. [Google Scholar] [CrossRef]
- Ekstrand, M.I.; Nectow, A.R.; Knight, Z.A.; Latcha, K.N.; Pomeranz, L.E.; Friedman, J.M. Molecular profiling of neurons based on connectivity. Cell 2014, 157, 1230–1242. [Google Scholar] [CrossRef]
- Zhang, H.; Chaudhury, D.; Nectow, A.R.; Friedman, A.K.; Zhang, S.; Juarez, B.; Liu, H.; Pfau, M.L.; Aleyasin, H.; Jiang, C.; et al. α1- and β3-Adrenergic Receptor-Mediated Mesolimbic Homeostatic Plasticity Confers Resilience to Social Stress in Susceptible Mice. Biol. Psychiatry 2019, 85, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Douma, E.H.; de Kloet, E.R. Stress-induced plasticity and functioning of ventral tegmental dopamine neurons. Neurosci. Biobehav. Rev. 2020, 108, 48–77. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.A.; Mazei-Robison, M.S. Opioid-Induced Molecular and Cellular Plasticity of Ventral Tegmental Area Dopamine Neurons. Cold Spring Harb. Perspect. Med. 2021, 11, a039362. [Google Scholar] [CrossRef] [PubMed]
- Polter, A.M.; Kauer, J.A. Stress and VTA synapses: Implications for addiction and depression. Eur. J. Neurosci. 2014, 39, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Mazei-Robison, M.S.; Koo, J.W.; Friedman, A.K.; Lansink, C.S.; Robison, A.J.; Vinish, M.; Krishnan, V.; Kim, S.; Siuta, M.A.; Galli, A.; et al. Role for mTOR Signaling and Neuronal Activity in Morphine-Induced Adaptations in Ventral Tegmental Area Dopamine Neurons. Neuron 2011, 72, 977–990. [Google Scholar] [CrossRef]
- Georges, F.; Le Moine, C.; Aston-Jones, G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J. Neurosci. 2006, 26, 5720–5726. [Google Scholar] [CrossRef]
- Wanat, M.J.; Hopf, F.W.; Stuber, G.D.; Phillips, P.E.; Bonci, A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J. Physiol. 2008, 586, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shaham, Y.; Zitzman, D.; Azari, S.; Wise, R.A.; You, Z.B. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: A role in stress-induced relapse to drug seeking. J. Neurosci. 2005, 25, 5389–5396. [Google Scholar] [CrossRef] [PubMed]
- Rodaros, D.; Caruana, D.A.; Amir, S.; Stewart, J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 2007, 150, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Hauger, R.L.; Risbrough, V.; Brauns, O.; Dautzenberg, F.M. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol. Disord. Drug Targets 2006, 5, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Ungless, M.A.; Singh, V.; Crowder, T.L.; Yaka, R.; Ron, D.; Bonci, A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 2003, 39, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferro, P.; Morales, M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J. Comp. Neurol. 2008, 506, 616–626. [Google Scholar] [CrossRef]
- Holly, E.N.; DeBold, J.F.; Miczek, K.A. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: Modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology 2015, 232, 4469–4479. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.J.; Friedman, A.K.; Sun, H.; Heller, E.A.; Ku, S.M.; Juarez, B.; Burnham, V.L.; Mazei-Robison, M.S.; Ferguson, D.; Golden, S.A.; et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat. Neurosci. 2014, 17, 27–29. [Google Scholar] [CrossRef]
- Woodworth, H.L.; Brown, J.A.; Batchelor, H.M.; Bugescu, R.; Leinninger, G.M. Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides 2018, 68, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Perez-Bonilla, P.; Santiago-Colon, K.; Matasovsky, J.; Ramirez-Virella, J.; Khan, R.; Garver, H.; Fink, G.; Dorrance, A.M.; Leinninger, G.M. Activation of ventral tegmental area neurotensin Receptor-1 neurons promotes weight loss. Neuropharmacology 2021, 195, 108639. [Google Scholar] [CrossRef] [PubMed]
- Soden, M.E.; Yee, J.X.; Zweifel, L.S. Circuit coordination of opposing neuropeptide and neurotransmitter signals. Nature 2023, 619, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; van den Pol, A.N. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J. Neurosci. 2006, 26, 13037–13047. [Google Scholar] [CrossRef] [PubMed]
- Muschamp, J.W.; Hollander, J.A.; Thompson, J.L.; Voren, G.; Hassinger, L.C.; Onvani, S.; Kamenecka, T.M.; Borgland, S.L.; Kenny, P.J.; Carlezon, W.A., Jr. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. USA 2014, 111, E1648–E1655. [Google Scholar] [CrossRef]
- Baimel, C.; Lau, B.K.; Qiao, M.; Borgland, S.L. Projection-Target-Defined Effects of Orexin and Dynorphin on VTA Dopamine Neurons. Cell Rep. 2017, 18, 1346–1355. [Google Scholar] [CrossRef]
- Kallo, I.; Omrani, A.; Meye, F.J.; de Jong, H.; Liposits, Z.; Adan, R.A.H. Characterization of orexin input to dopamine neurons of the ventral tegmental area projecting to the medial prefrontal cortex and shell of nucleus accumbens. Brain Struct. Funct. 2022, 227, 1083–1098. [Google Scholar] [CrossRef]
- Thomas, C.S.; Mohammadkhani, A.; Rana, M.; Qiao, M.; Baimel, C.; Borgland, S.L. Optogenetic stimulation of lateral hypothalamic orexin/dynorphin inputs in the ventral tegmental area potentiates mesolimbic dopamine neurotransmission and promotes reward-seeking behaviours. Neuropsychopharmacology 2022, 47, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Priest, M.F.; Nasenbeny, J.; Lu, T.; Kozorovitskiy, Y. Biased Oxytocinergic Modulation of Midbrain Dopamine Systems. Neuron 2017, 95, 368–384.e5. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Stoop, R. Neuromodulation by oxytocin and vasopressin. Neuron 2012, 76, 142–159. [Google Scholar] [CrossRef]
- Tribollet, E.; Barberis, C.; Jard, S.; Dubois-Dauphin, M.; Dreifuss, J.J. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988, 442, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Mullis, K.; Kay, K.; Williams, D.L. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013, 1513, 85–91. [Google Scholar] [CrossRef]
- Angioni, L.; Cocco, C.; Ferri, G.L.; Argiolas, A.; Melis, M.R.; Sanna, F. Involvement of nigral oxytocin in locomotor activity: A behavioral, immunohistochemical and lesion study in male rats. Horm. Behav. 2016, 83, 23–38. [Google Scholar] [CrossRef]
- Song, Z.; Borland, J.M.; Larkin, T.E.; O’Malley, M.; Albers, H.E. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology 2016, 74, 164–172. [Google Scholar] [CrossRef]
- Musardo, S.; Contestabile, A.; Knoop, M.; Baud, O.; Bellone, C. Oxytocin neurons mediate the effect of social isolation via the VTA circuits. eLife 2022, 11, e73421. [Google Scholar] [CrossRef] [PubMed]
- Tanganelli, S.; Fuxe, K.; Antonelli, T.; O’Connor, W.T.; Ferraro, L. Cholecystokinin/dopamine/GABA interactions in the nucleus accumbens: Biochemical and functional correlates. Peptides 2001, 22, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Martinez Damonte, V.; Pomrenze, M.B.; Manning, C.E.; Casper, C.; Wolfden, A.L.; Malenka, R.C.; Kauer, J.A. Somatodendritic Release of Cholecystokinin Potentiates GABAergic Synapses Onto Ventral Tegmental Area Dopamine Cells. Biol. Psychiatry 2023, 93, 197–208. [Google Scholar] [CrossRef]
- Beier, K.T.; Gao, X.J.; Xie, S.; DeLoach, K.E.; Malenka, R.C.; Luo, L. Topological Organization of Ventral Tegmental Area Connectivity Revealed by Viral-Genetic Dissection of Input-Output Relations. Cell Rep. 2019, 26, 159–167.e6. [Google Scholar] [CrossRef]
- Yang, H.; de Jong, J.W.; Tak, Y.; Peck, J.; Bateup, H.S.; Lammel, S. Nucleus Accumbens Subnuclei Regulate Motivated Behavior via Direct Inhibition and Disinhibition of VTA Dopamine Subpopulations. Neuron 2018, 97, 434–449.e4. [Google Scholar] [CrossRef]
- Cui, X.; Tong, Q.; Xu, H.; Xie, C.; Xiao, L. A putative loop connection between VTA dopamine neurons and nucleus accumbens encodes positive valence to compensate for hunger. Prog. Neurobiol. 2023, 229, 102503. [Google Scholar] [CrossRef]
- Zhong, P.; Qin, L.; Yan, Z. Dopamine Differentially Regulates Response Dynamics of Prefrontal Cortical Principal Neurons and Interneurons to Optogenetic Stimulation of Inputs from Ventral Tegmental Area. Cereb. Cortex 2020, 30, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.B.; Sesack, S.R. Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J. Neurosci. 2000, 20, 3864–3873. [Google Scholar] [CrossRef] [PubMed]
- Sesack, S.R.; Carr, D.B.; Omelchenko, N.; Pinto, A. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann. N. Y. Acad. Sci. 2003, 1003, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Avegno, E.M.; Kasten, C.R.; Snyder, W.B., 3rd; Kelley, L.K.; Lobell, T.D.; Templeton, T.J.; Constans, M.; Wills, T.A.; Middleton, J.W.; Gilpin, N.W. Alcohol dependence activates ventral tegmental area projections to central amygdala in male mice and rats. Addict. Biol. 2021, 26, e12990. [Google Scholar] [CrossRef]
- Gatto, G.J.; McBride, W.J.; Murphy, J.M.; Lumeng, L.; Li, T.K. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol 1994, 11, 557–564. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Chen, S.; Zhang, Z.; Liu, Y.; Montardy, Q.; Tang, Y.; Wei, P.; Liu, N.; Li, L.; et al. A VTA GABAergic Neural Circuit Mediates Visually Evoked Innate Defensive Responses. Neuron 2019, 103, 473–488.e6. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yao, J.; Hu, Y.D.; Chen, H.Y.; Liu, P.C.; Wang, W.F.; Zeng, Y.H.; Zhuang, C.W.; Zeng, S.X.; Li, Y.P.; et al. Control of Behavioral Arousal and Defense by a Glutamatergic Midbrain-Amygdala Pathway in Mice. Front. Neurosci. 2022, 16, 850193. [Google Scholar] [CrossRef] [PubMed]
- Herkenham, M.; Nauta, W.J.H. Efferent Connections of the Habenular Nuclei in the Rat. J. Comp. Neurol. 1979, 187, 19–47. [Google Scholar] [CrossRef]
- Hong, S.; Jhou, T.C.; Smith, M.; Saleem, K.S.; Hikosaka, O. Negative Reward Signals from the Lateral Habenula to Dopamine Neurons Are Mediated by Rostromedial Tegmental Nucleus in Primates. J. Neurosci. 2011, 31, 11457–11471. [Google Scholar] [CrossRef]
- Bocklisch, C.; Pascoli, V.; Wong, J.C.; House, D.R.; Yvon, C.; de Roo, M.; Tan, K.R.; Luscher, C. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 2013, 341, 1521–1525. [Google Scholar] [CrossRef]
- Edwards, N.J.; Tejeda, H.A.; Pignatelli, M.; Zhang, S.; McDevitt, R.A.; Wu, J.; Bass, C.E.; Bettler, B.; Morales, M.; Bonci, A. Circuit specificity in the inhibitory architecture of the VTA regulates cocaine-induced behavior. Nat. Neurosci. 2017, 20, 438–448. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Surmeier, D.J. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011, 34, 441–466. [Google Scholar] [CrossRef] [PubMed]
- Kupchik, Y.M.; Brown, R.M.; Heinsbroek, J.A.; Lobo, M.K.; Schwartz, D.J.; Kalivas, P.W. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci. 2015, 18, 1230–1232. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.J.; Sun, S.B.; Hu, Y.; Zhang, H.F.; Sun, X.R. Neuropeptides Modulate Feeding via the Dopamine Reward Pathway. Neurochem. Res. 2023, 48, 2622–2643. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Schott, M.; la Fleur, S.E.; Barrot, M. Role of the striatal dopamine, GABA and opioid systems in mediating feeding and fat intake. Neurosci. Biobehav. Rev. 2022, 139, 104726. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, C.L.; Yang, S.; Jin, G.Z.; Bunney, B.S.; Shi, W.X. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J. Neurosci. 2007, 27, 5414–5421. [Google Scholar] [CrossRef] [PubMed]
- Risco, S.; Mediavilla, C. Orexin A in the ventral tegmental area enhances saccharin-induced conditioned flavor preference: The role of D1 receptors in central nucleus of amygdala. Behav. Brain Res. 2018, 348, 192–200. [Google Scholar] [CrossRef]
- Huang, M.; Wang, G.; Lin, Y.; Guo, Y.; Ren, X.; Shao, J.; Cao, J.; Zang, W.; Li, Z. Dopamine receptor D2, but not D1, mediates the reward circuit from the ventral tegmental area to the central amygdala, which is involved in pain relief. Mol. Pain 2022, 18, 17448069221145096. [Google Scholar] [CrossRef]
- Bressel, P.J.R.D.; McNally, G.P. The Role of the Lateral Habenula in Punishment. PLoS ONE 2014, 9, e111699. [Google Scholar] [CrossRef]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Yang, E.; Lee, J.; Kim, J.Y.; Yoo, H.; Park, H.S.; Jung, J.T.; Lee, D.; Chun, S.; Jo, Y.S.; et al. Neural mechanism of acute stress regulation by trace aminergic signalling in the lateral habenula in male mice. Nat. Commun. 2023, 14, 2435. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Lim, B.K.; Ran, C.; Huang, K.W.; Betley, M.J.; Tye, K.M.; Deisseroth, K.; Malenka, R.C. Input-specific control of reward and aversion in the ventral tegmental area. Nature 2012, 491, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Fan, R.F.; Liu, X.F.; Shen, X.F.; Liu, X.; Zhao, H. The convergence of aversion and reward signals in individual neurons of the mice lateral habenula. Exp. Neurol. 2021, 339, 113637. [Google Scholar] [CrossRef] [PubMed]
- Wilczkowski, M.; Karwowska, K.; Kielbinski, M.; Zajda, K.; Pradel, K.; Drwiega, G.; Rajfur, Z.; Blasiak, T.; Przewlocki, R.; Solecki, W.B. Recruitment of inhibitory neuronal pathways regulating dopaminergic activity for the control of cocaine seeking. Eur. J. Neurosci. 2022, 58, 4487–4501. [Google Scholar] [CrossRef]
- Pierucci, M.; Delicata, F.; Colangeli, R.; Gammazza, A.M.; Pitruzzella, A.; Casarrubea, M.; De Deurwaerdère, P.; Di Giovanni, G. Nicotine modulation of the lateral habenula/ventral tegmental area circuit dynamics: An electrophysiological study in rats. Neuropharmacology 2022, 202, 108859. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Ma, L.Y.; Zhang, X.Y.; Yue, L.P.; Wang, J.X.; Zheng, J.; Cui, S.; Liu, F.Y.; Wang, Z.Y.; Wan, Y.; et al. Deep brain stimulation in the lateral habenula reverses local neuronal hyperactivity and ameliorates depression-like behaviors in rats. Neurobiol. Dis. 2023, 180, 106069. [Google Scholar] [CrossRef]
- Brichta, L.; Greengard, P. Molecular determinants of selective dopaminergic vulnerability in Parkinson’s disease: An update. Front. Neuroanat. 2014, 8, 152. [Google Scholar] [CrossRef]
- Jiang, A.D.; Handley, R.R.; Lehnert, K.; Snell, R.G. From Pathogenesis to Therapeutics: A Review of 150 Years of Huntington’s Disease Research. Int. J. Mol. Sci. 2023, 24, 13021. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.S.; Parent, M.; Levine, M.S. The role of dopamine in huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef]
- Nakamura, S. Integrated pathophysiology of schizophrenia, major depression, and bipolar disorder as monoamine axon disorder. Front. Biosci. 2022, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychoph. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Grace, A.A.; Uliana, D.L. Insights into the Mechanism of Action of Antipsychotic Drugs Derived from Animal Models: Standard of Care versus Novel Targets. Int. J. Mol. Sci. 2023, 24, 12374. [Google Scholar] [CrossRef] [PubMed]
- Coddington, L.T.; Lindo, S.E.; Dudman, J.T. Mesolimbic dopamine adapts the rate of learning from action. Nature 2023, 614, 294–302. [Google Scholar] [CrossRef]
- Kutlu, M.G.; Zachry, J.E.; Melugin, P.R.; Cajigas, S.A.; Chevee, M.F.; Kelly, S.J.; Kutlu, B.; Tian, L.; Siciliano, C.A.; Calipari, E.S. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr. Biol. 2021, 31, 4748–4761.e8. [Google Scholar] [CrossRef]
- Syed, E.C.; Grima, L.L.; Magill, P.J.; Bogacz, R.; Brown, P.; Walton, M.E. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat. Neurosci. 2016, 19, 34–36. [Google Scholar] [CrossRef]
- Hong, S. Dopamine system: Manager of neural pathways. Front. Hum. Neurosci. 2013, 7, 854. [Google Scholar] [CrossRef]
| Neuropeptides | Functions | Animals | References |
|---|---|---|---|
| Corticotropin-releasing factor (CRF) | Stress-induced behaviors | Mouse, rat | Tovar-Diaz et al., 2018 [88]; Rodaros et al., 2007 [106]; Walsh et al., 2014 [111]; |
| Neurotensin (Nts) | Feeding inhibition | Mouse | Perez-Bonilla et al., 2021 [113]; Soden et al., 2023 [114]; |
| Orexin | Reward-seeking behaviors | Mouse | Muschamp et al., 2014 [116]; Thomas et al., 2022 [119]; |
| Oxytocin | Social behaviors | Mouse | Musardo et al., 2022 [127]; |
| Cholecystokinin (CCK) | Feeding and locomotion | Mouse | Martinez Damonte et al., 2023 [129]; |
| Connections |
Neurotransmitters
and Receptors | References |
|---|---|---|
|
NAcLat–VTA GABA neurons NAcMed–VTA NAcMed-projecting dopamine neurons NAcMed–VTA NAcLat-projecting dopamine neurons | GABA, GABA receptor GABA, GABAA receptor GABA, GABAB receptor | Yang et al., 2018 [131]; |
| VTA–NAc | Dopamine, dopamine receptor | Cui et al., 2023 [132]; |
| VTA–PFC | Dopamine, dopamine receptor Glutamate, glutamate receptor | Zhong et al., 2020 [133]; |
| PFC–VTA | Glutamate, glutamate receptor | Carr and Sesack, 2000 [134]; Sesack et al., 2003 [135]; |
| VTA–CeA | Dopamine, dopamine receptor GABA, GABA receptor Glutamate, glutamate receptor | Avegno et al., 2021 [136]; Gatto et al., 1994 [137]; Zhou et al., 2019 [138]; Chen et al., 2022 [139]; |
| LHb–RMTg–VTA | Glutamate, glutamate receptor GABA, GABA receptor | Herkenham and Nauta, 1979 [140]; Hong et al., 2011 [141]; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, G.; Hao, M.; Duan, J.; Han, M.-H. The Formation and Function of the VTA Dopamine System. Int. J. Mol. Sci. 2024, 25, 3875. https://doi.org/10.3390/ijms25073875
Hou G, Hao M, Duan J, Han M-H. The Formation and Function of the VTA Dopamine System. International Journal of Molecular Sciences. 2024; 25(7):3875. https://doi.org/10.3390/ijms25073875
Chicago/Turabian StyleHou, Guoqiang, Mei Hao, Jiawen Duan, and Ming-Hu Han. 2024. "The Formation and Function of the VTA Dopamine System" International Journal of Molecular Sciences 25, no. 7: 3875. https://doi.org/10.3390/ijms25073875
APA StyleHou, G., Hao, M., Duan, J., & Han, M.-H. (2024). The Formation and Function of the VTA Dopamine System. International Journal of Molecular Sciences, 25(7), 3875. https://doi.org/10.3390/ijms25073875







