CdgB Regulates Morphological Differentiation and Toyocamycin Production in Streptomyces diastatochromogenes 1628
Abstract
1. Introduction
2. Results
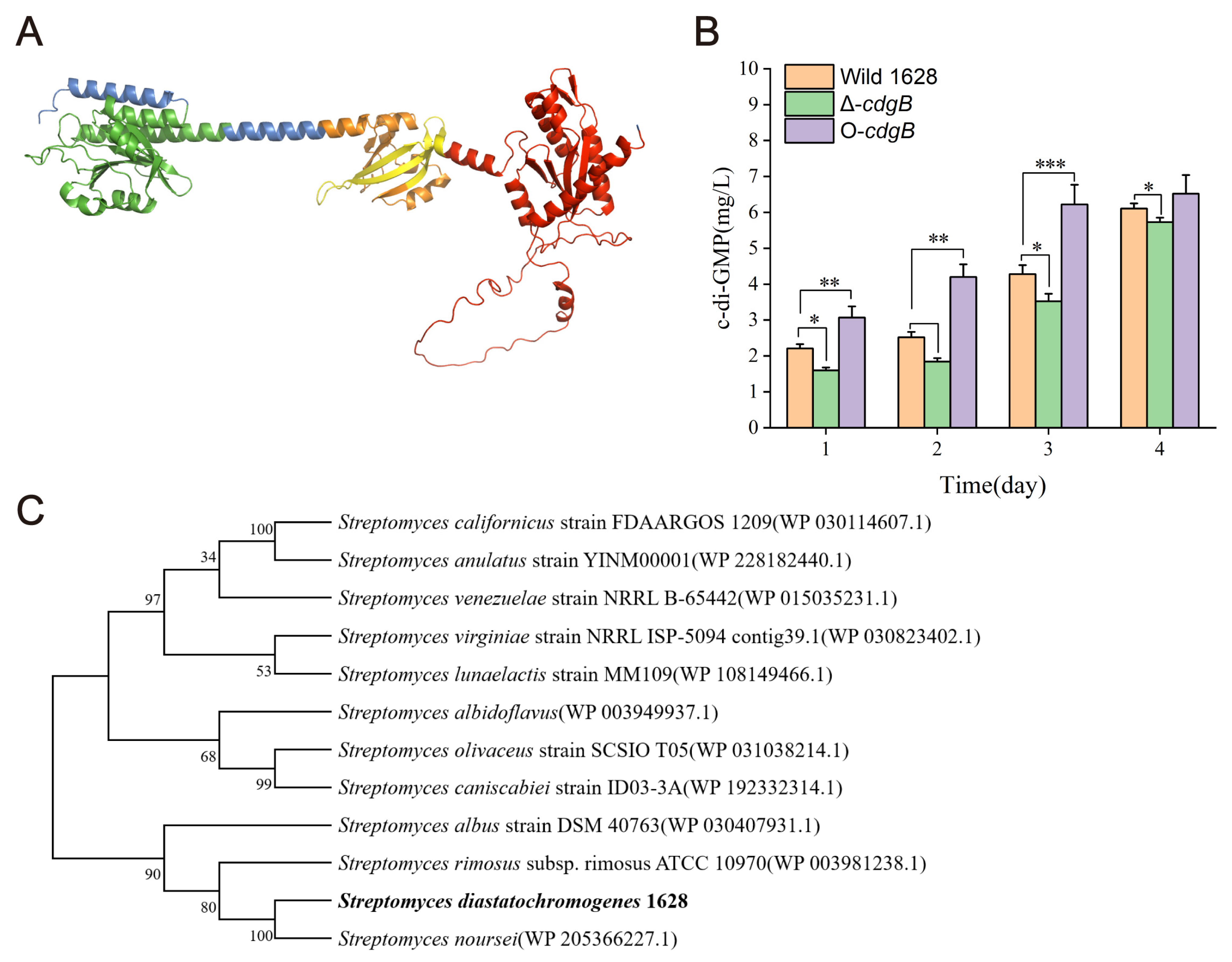
2.1. The Analysis of Phylogenetic Trees for CdgB and CdgB’s Function in c-di-GMP Synthesis in S. diastatochromogenes 1628
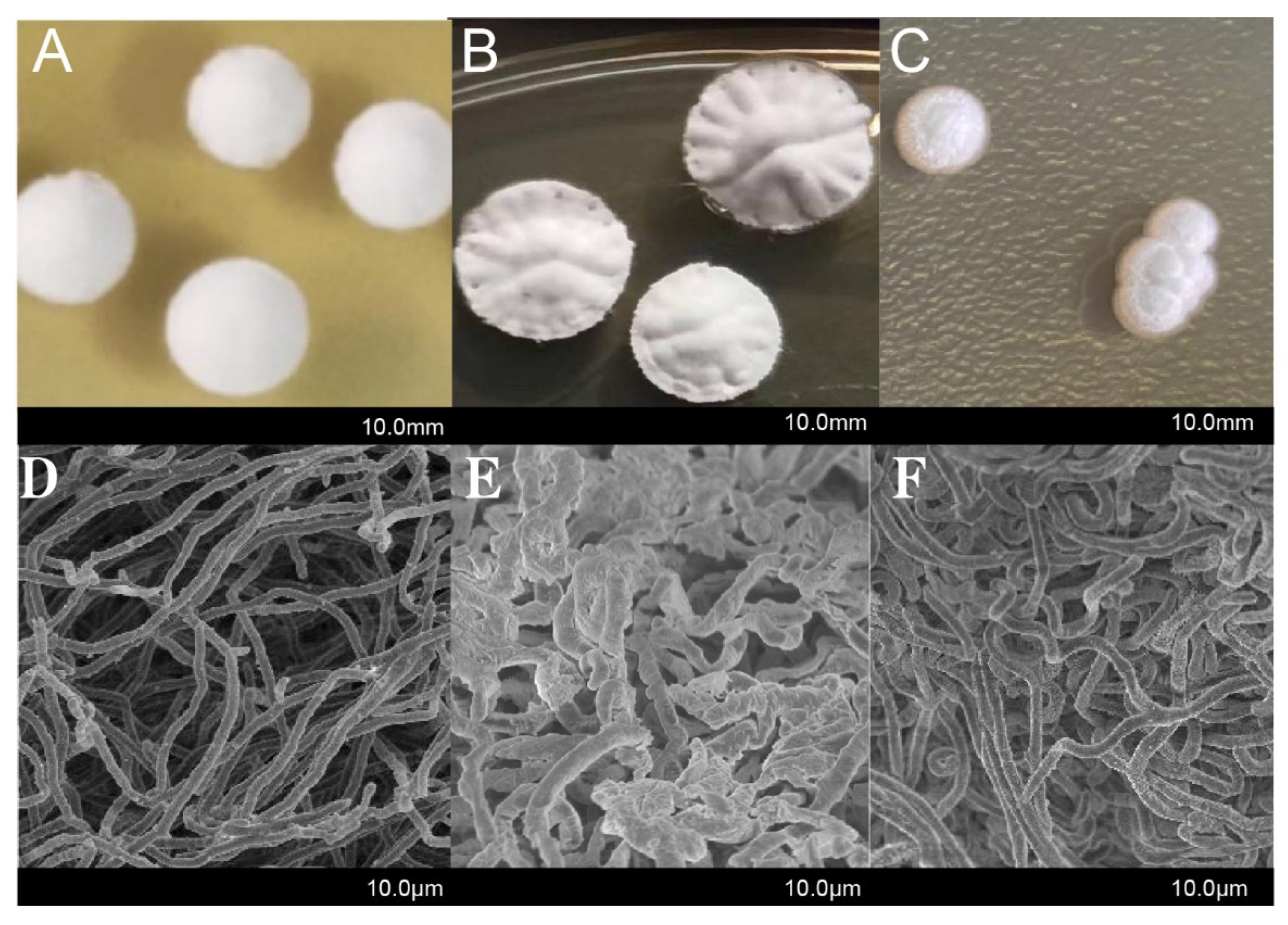
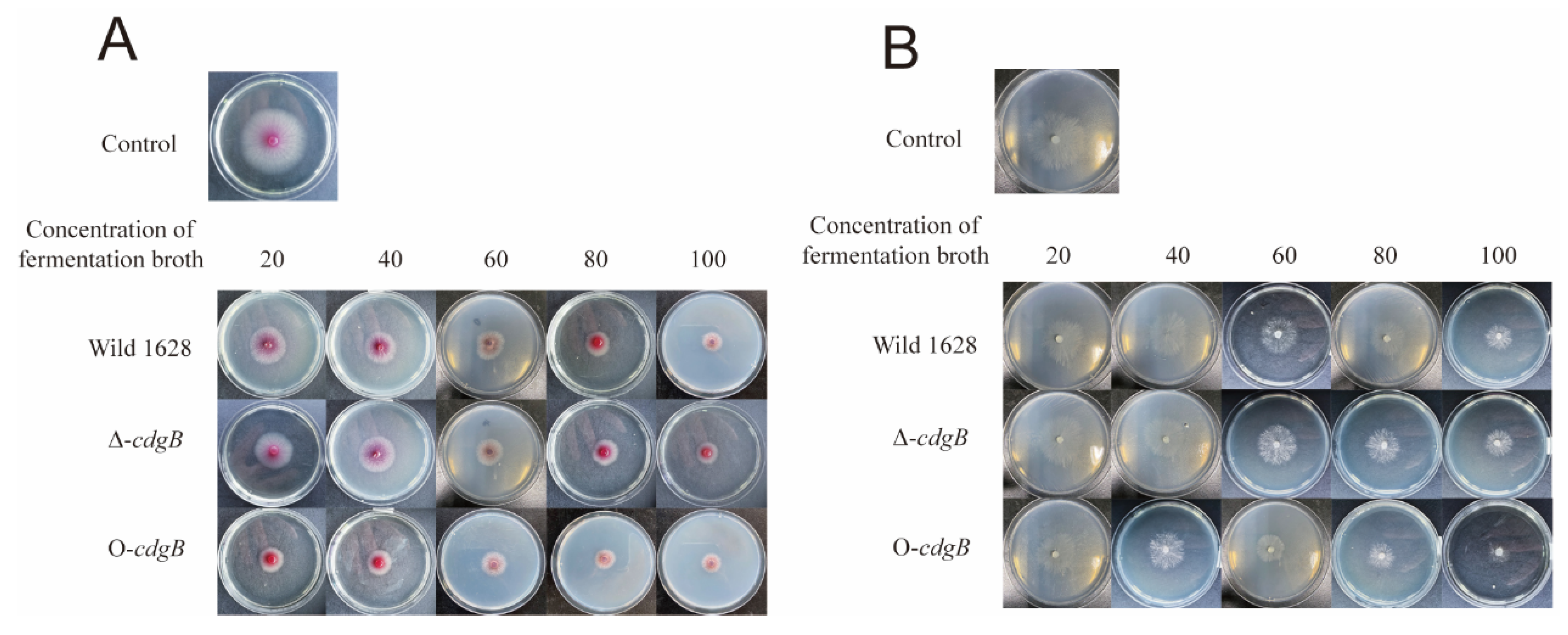
2.2. Effect of CdgB on the Morphological Characteristic of S. diastatochromogenes 1628
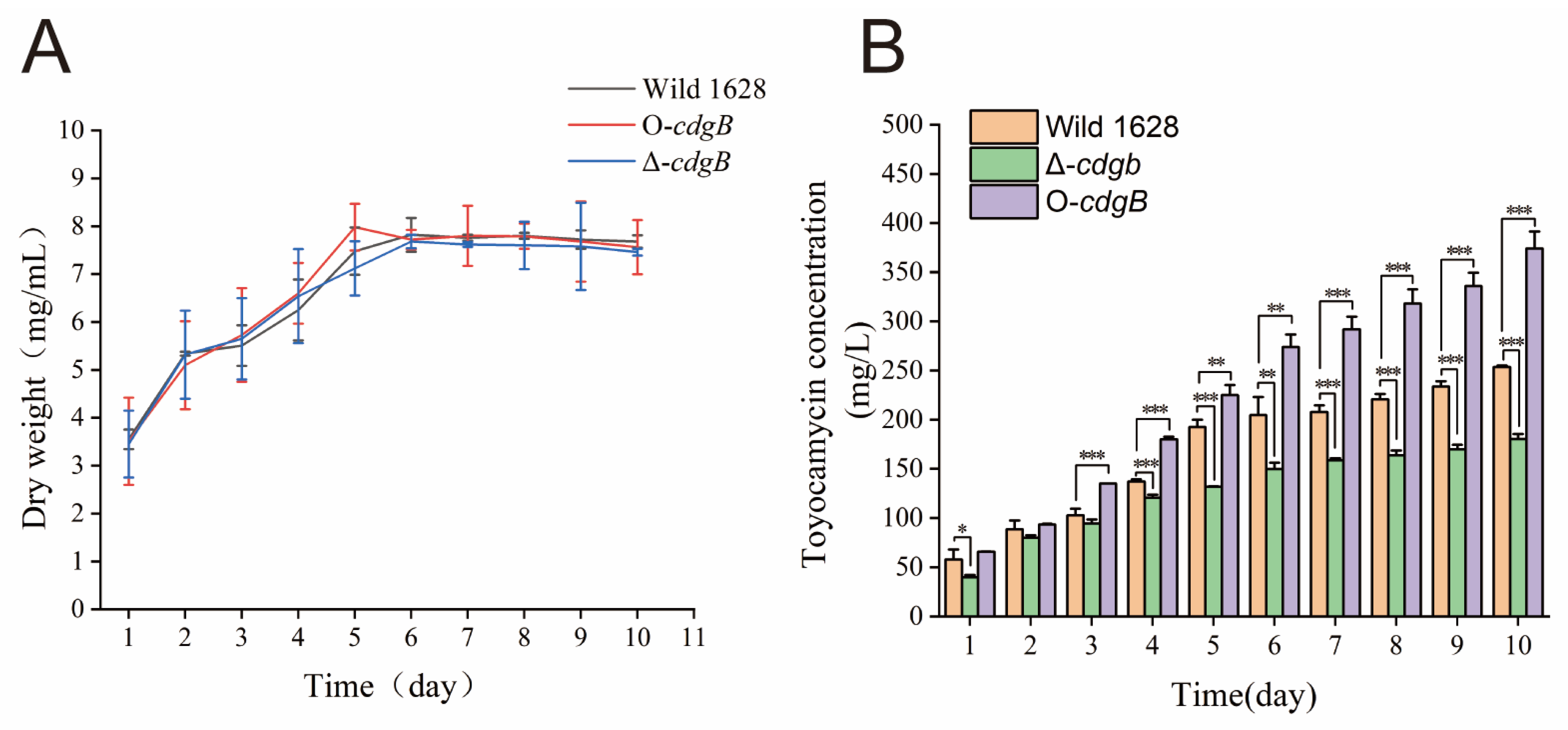
2.3. Effect of CdgB on Toyocamycin Production
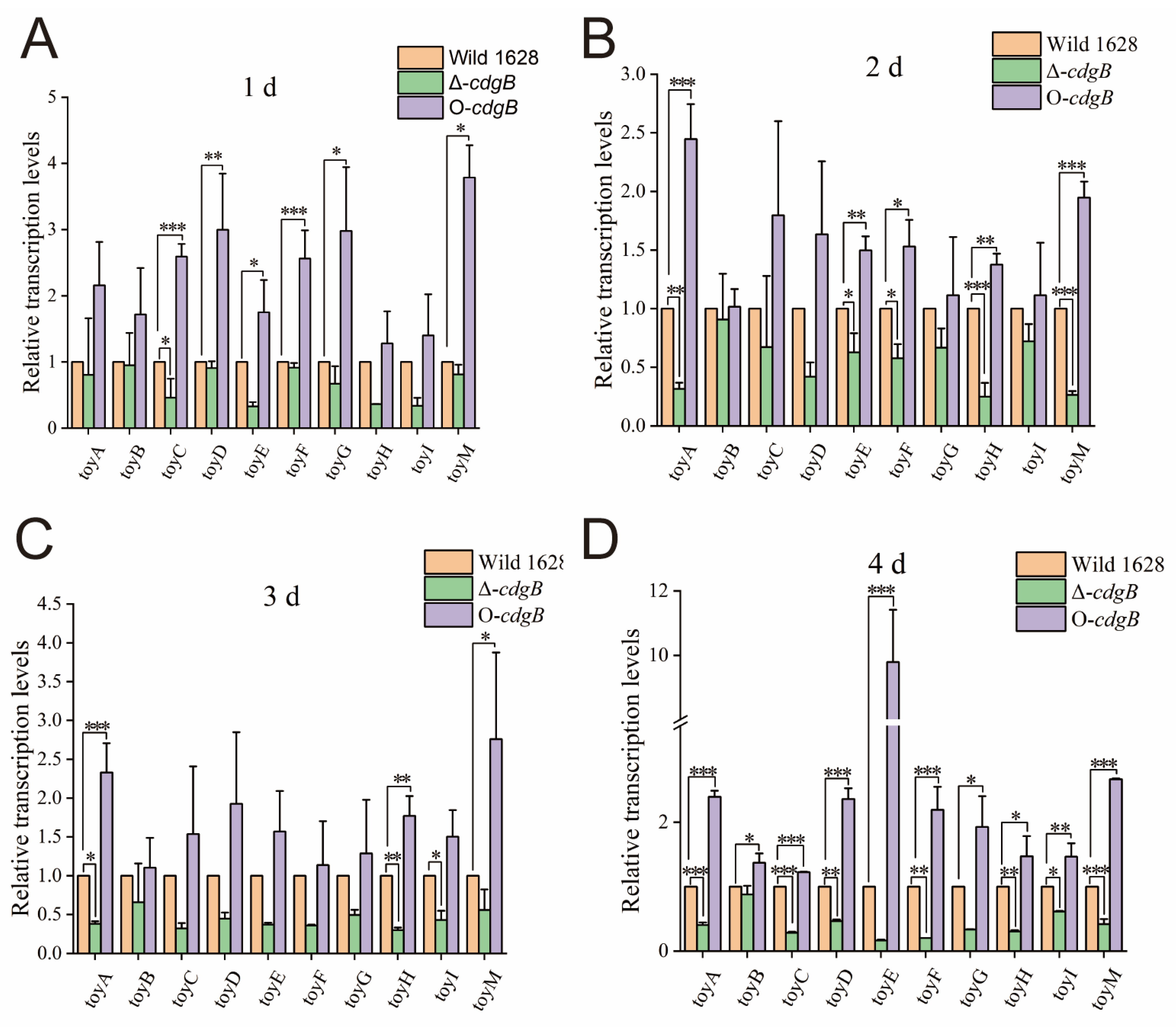
2.4. The Effect of CdgB on Toy Cluster Expression Level
2.5. Effect of CdgB on the Inhibitory Activity of S. diastatochromogenes 1628
3. Discussion
4. Materials and Methods
4.1. Strains, Plasmids, Primers, and Culture Conditions
4.2. The Phylogenetic Analysis of CdgB
4.3. Construction of cdgB Disruption and Overexpression Mutants
4.4. Morphological Assessment
4.5. The Effect of cdgB on Cell Growth and Toyocamycin Production
4.6. Intracellular c-di-GMP Extraction and Detection
4.7. The Transcriptional Level of Toy Clusters
4.8. The Antimicrobial Activity against Filamentous Fungi
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, J.X.; Song, Y.; Tang, G.; Ochi, K.; Shentu, X.P.; Yu, X.P. Substantial improvement of tetraene macrolide production in Streptomyces diastatochromogenes by cumulative drug resistance mutations. PLoS ONE 2020, 15, e0232927. [Google Scholar] [CrossRef]
- Pandey, S.; Djibo, R.; Darracq, A.; Calendo, G.; Zhang, H.; Henry, R.A.; Andrews, A.J.; Baylin, S.B.; Madzo, J.; Najmanovich, R.; et al. Selective CDK9 Inhibition by Natural Compound Toyocamycin in Cancer Cells. Cancers. 2022, 14, 3340. [Google Scholar] [CrossRef]
- He, Y.; Ding, Y.; Wu, Q.; Chen, M.; Zhao, S.; Zhang, J.; Wei, X.; Zhang, Y.; Bai, J.; Mo, S. Identification of the Potential Biological Preservative Tetramycin A-Producing Strain and Enhancing Its Production. Front. Microbiol. 2019, 10, 2925. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.C.; Rinehart, K.L., Jr. Polyene antibiotics. VII. Carbon-13 nuclear magnetic resonance evidence for cyclic hemiketals in the polyene antibiotics amphotericin B, nystatin A1, tetrin A, tetrin B, lucensomycin, and pimaricin1,2. J. Antibiot. 1976, 29, 1035–1042. [Google Scholar] [CrossRef]
- Liu, B.; Wei, Q.; Yang, M.; Shi, L.; Zhang, K.; Ge, B. Effect of toyF on wuyiencin and toyocamycin production by Streptomyces albulus CK-15. World J. Microbiol. 2022, 38, 65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, Z.; Xu, X.; Ma, Z.; Bechthold, A.; Yu, X. ToyA, a positive pathway-specific regulator for toyocamycin biosynthesis in Streptomyces diastatochromogenes 1628. Appl. Microbiol. Biotechnol. 2019, 103, 7071–7084. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Luo, S.; Ma, Z.; Bechthold, A.; Yu, X. AdpAsd, a Positive regulator for morphological development and toyocamycin biosynthesis in Streptomyces diastatochromogenes 1628. Curr. Microbiol. 2018, 75, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Hoskisson, P.A.; van Wezel, G.P. Streptomyces coelicolor . Trends Microbiol. 2019, 27, 468–469. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, L.; Tang, W.; Guo, L.; Gao, C.G.; Chen, X.; Liu, J.; Hu, G.; Liu, L. Metabolic engineering of Streptomyces to enhance the synthesis of valuable natural products. Eng. Microbiol. 2022, 2, 100022. [Google Scholar] [CrossRef]
- Tschowri, N.; Schumacher, M.A.; Schlimpert, S.; Chinnam, N.B.; Findlay, K.C.; Brennan, R.G.; Buttner, M.J. Tetrameric c-di-GMP Mediates Effective Transcription Factor Dimerization to Control Streptomyces Development. Cell 2014, 158, 1136–1147. [Google Scholar] [CrossRef]
- den Hengst, C.D.; Tran, N.T.; Bibb, M.J.; Chandra, G.; Leskiw, B.K.; Buttner, M.J. Genes essential for morphological development and antibiotic production in Streptomyces coelicolor are targets of BldD during vegetative growth. Mol. Microbiol. 2010, 78, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, K.A.; Schumacher, M.A.; Bush, M.J.; Bibb, M.J.; Chandra, G.; Holmes, N.A.; Zeng, W.; Henderson, M.; Zhang, H.; Findlay, K.C.; et al. c-di-GMP arms an anti-σ to control progression of multicellular differentiation in Streptomyces. Mol. Cell. 2020, 77, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, E.; Fortier, L.-C.; Malouin, F.; Burrus, V. C-di-GMP turn-over in Clostridium difficile is controlled by a plethora of diguanylate cyclases and phosphodiesterases. PLoS Genet. 2011, 7, e1002039. [Google Scholar] [CrossRef] [PubMed]
- Stelitano, V.; Giardina, G.; Paiardini, A.; Castiglione, N.; Cutruzzola, F.; Rinaldo, S. C-di-GMP hydrolysis by Pseudomonas aeruginosa HD-GYP phosphodiesterases: Analysis of the reaction mechanism and novel roles for pGpG. PLoS ONE 2013, 8, e74920. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Petersen, E.; Kulasekara, B.R.; Miller, S.I. A direct screen for c-di-GMP modulators reveals a Salmonella Typhimurium periplasmic L-arginine-sensing pathway. Sci. Signal. 2015, 8, ra57. [Google Scholar] [CrossRef] [PubMed]
- Makitrynskyy, R.; Tsypik, O.; Nuzzo, D.; Paululat, T.; Zechel, D.L.; Bechthold, A. Secondary nucleotide messenger c-di-GMP exerts a global control on natural product biosynthesis in Streptomycetes. Nucleic Acids Res. 2020, 48, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Fouhy, Y.; Lucey, J.F.; Dow, J.M. Cyclic di-GMP signaling in bacteria: Recent advances and new puzzles. J. Bacteriol. 2006, 188, 8327–8334. [Google Scholar] [CrossRef]
- Qi, Y.; Rao, F.; Luo, Z.; Liang, Z.-X. A flavin cofactor-binding PAS domain regulates c-di-GMP synthesis in AxDGC2 from Acetobacter xylinum. Biochemistry 2009, 48, 10275–10285. [Google Scholar] [CrossRef]
- Tran, N.T.; Den Hengst, C.D.; Gomez-Escribano, J.P.; Buttner, M.J. Identification and Characterization of CdgB, a diguanylate cyclase involved in developmental processes in Streptomyces coelicolor. J. Bacteriol. 2011, 193, 3100–3108. [Google Scholar] [CrossRef]
- Haist, J.; Neumann, S.A.; Al-Bassam, M.M.; Lindenberg, S.; Elliot, M.A.; Tschowri, N. Specialized and shared functions of diguanylate cyclases and phosphodiesterases in Streptomyces development. Mol. Microbiol. 2020, 114, 808–822. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, G.; Wang, G.; Jiang, W.; Li, L.; Lu, Y. Overexpression of the diguanylate cyclase CdgD blocks developmental transitions and antibiotic biosynthesis in Streptomyces coelicolor. Sci. China Life Sci. 2019, 62, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Al-Bassam, M.M.; Haist, J.; Neumann, S.A.; Lindenberg, S.; Tschowri, N. Expression patterns, genomic conservation and input into developmental regulation of the GGDEF/EAL/HD-GYP domain proteins in Streptomyces. Front. Microbiol. 2018, 9, 3100–3108. [Google Scholar] [CrossRef]
- Khan, F.; Jeong, G.-J.; Tabassum, N.; Kim, Y.-M. Functional diversity of c-di-GMP receptors in prokaryotic and eukaryotic systems. Cell Commun. Signal. 2023, 21, 2524. [Google Scholar] [CrossRef]
- Hull, T.D.; Ryu, M.-H.; Sullivan, M.J.; Johnson, R.C.; Klena, N.T.; Geiger, R.M.; Gomelsky, M.; Bennett, J.A. Cyclic di-GMP phosphodiesterases RmdA and RmdB are involved in regulating colony morphology and development in Streptomyces coelicolor. J. Bacteriol. 2012, 194, 4642–4651. [Google Scholar] [CrossRef] [PubMed]
- Rabyk, M.; Yushchuk, O.; Rokytskyy, I.; Anisimova, M.; Ostash, B. Genomic insights into evolution of AdpA family master regulators of morphological differentiation and secondary metabolism in Streptomyces. J. Mol. Evol. 2018, 86, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Hara, H.; Horinouchi, S.; Ohnishi, Y. Genome-wide distribution of AdpA, a global regulator for secondary metabolism and morphological differentiation in Streptomyces, revealed the extent and complexity of the AdpA regulatory network. DNA Res. 2012, 19, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, X.; Zhang, Z.; Shentu, X.; Yu, X. Physiology and transcriptional analysis of ppGpp-related regulatory effects in Streptomyces diastatochromogenes 1628. Microbiol. Spectr. 2022, 11, e01200-22. [Google Scholar] [CrossRef] [PubMed]
- López-García, M.T.; Yagüe, P.; González-Quiñónez, N.; Rioseras, B.; Manteca, A. The SCO4117 ECF Sigma Factor Pleiotropically Controls Secondary Metabolism and Morphogenesis in Streptomyces coelicolor. Front. Microbiol. 2018, 9, 312. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Ji, Y.; Niu, J.; Li, M. Expression of CYP107Z13 in Streptomyces lividans TK54 catalyzes the oxidation of avermectin to 4″-oxo-avermectin. Appl. Microbiol. Biotechnol. 2012, 93, 1957–1963. [Google Scholar] [CrossRef]
- Xu, Y.; Tan, G.; Ke, M.; Li, J.; Tang, Y.; Meng, S.; Niu, J.; Wang, Y.; Liu, R.; Wu, H.; et al. Enhanced lincomycin production by co-overexpression of metK1 and metK2 in Streptomyces lincolnensis. J. Ind. Microbiol. 2018, 45, 345–355. [Google Scholar]
- Ma, Z.; Tao, L.; Bechthold, A.; Shentu, X.; Bian, Y.; Yu, X. Overexpression of ribosome recycling factor is responsible for improvement of nucleotide antibiotic-toyocamycin in Streptomyces diastatochromogenes 1628. Appl. Microbiol. Biotechnol. 2014, 98, 5051–5058. [Google Scholar] [CrossRef] [PubMed]

| Strain or Plasmid | Description | Source or Reference |
|---|---|---|
| S. diastatochromogenes 1628 | Parental strain; toyocamycin producer | Our lab |
| Δ-cdgB | cdgB deletion mutant | This study |
| O-cdgB | cdgB overexpression mutant | This study |
| Fusarium oxysporum f. sp. cucumerinum | Plant pathogen | CICC 2532 |
| Rhizoctonia solani | Plant pathogen | CICC 40529 |
| E. coli JM109 | Host strain for cloning | Our lab |
| E. coli ET12567(PUZ8002) | Donor strain for conjugation | Our lab |
| pIB139 | Derivative of integrative plasmid pSET152, harboring a PermE * promoter | Our lab |
| pKC1139 | E. coli–Streptomyces shuttle vector | |
| pKC1139-cdgB | cdgB deletion vector based on pKC1139 | This study |
| pIB139-cdgB | cdgB gene under the control of promoter PermE* in pIB139 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, Z.; Yu, X.; Song, Y.; Shentu, X. CdgB Regulates Morphological Differentiation and Toyocamycin Production in Streptomyces diastatochromogenes 1628. Int. J. Mol. Sci. 2024, 25, 3878. https://doi.org/10.3390/ijms25073878
Wang R, Zhang Z, Yu X, Song Y, Shentu X. CdgB Regulates Morphological Differentiation and Toyocamycin Production in Streptomyces diastatochromogenes 1628. International Journal of Molecular Sciences. 2024; 25(7):3878. https://doi.org/10.3390/ijms25073878
Chicago/Turabian StyleWang, Rui, Zixuan Zhang, Xiaoping Yu, Yang Song, and Xuping Shentu. 2024. "CdgB Regulates Morphological Differentiation and Toyocamycin Production in Streptomyces diastatochromogenes 1628" International Journal of Molecular Sciences 25, no. 7: 3878. https://doi.org/10.3390/ijms25073878
APA StyleWang, R., Zhang, Z., Yu, X., Song, Y., & Shentu, X. (2024). CdgB Regulates Morphological Differentiation and Toyocamycin Production in Streptomyces diastatochromogenes 1628. International Journal of Molecular Sciences, 25(7), 3878. https://doi.org/10.3390/ijms25073878






