Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Data Extraction
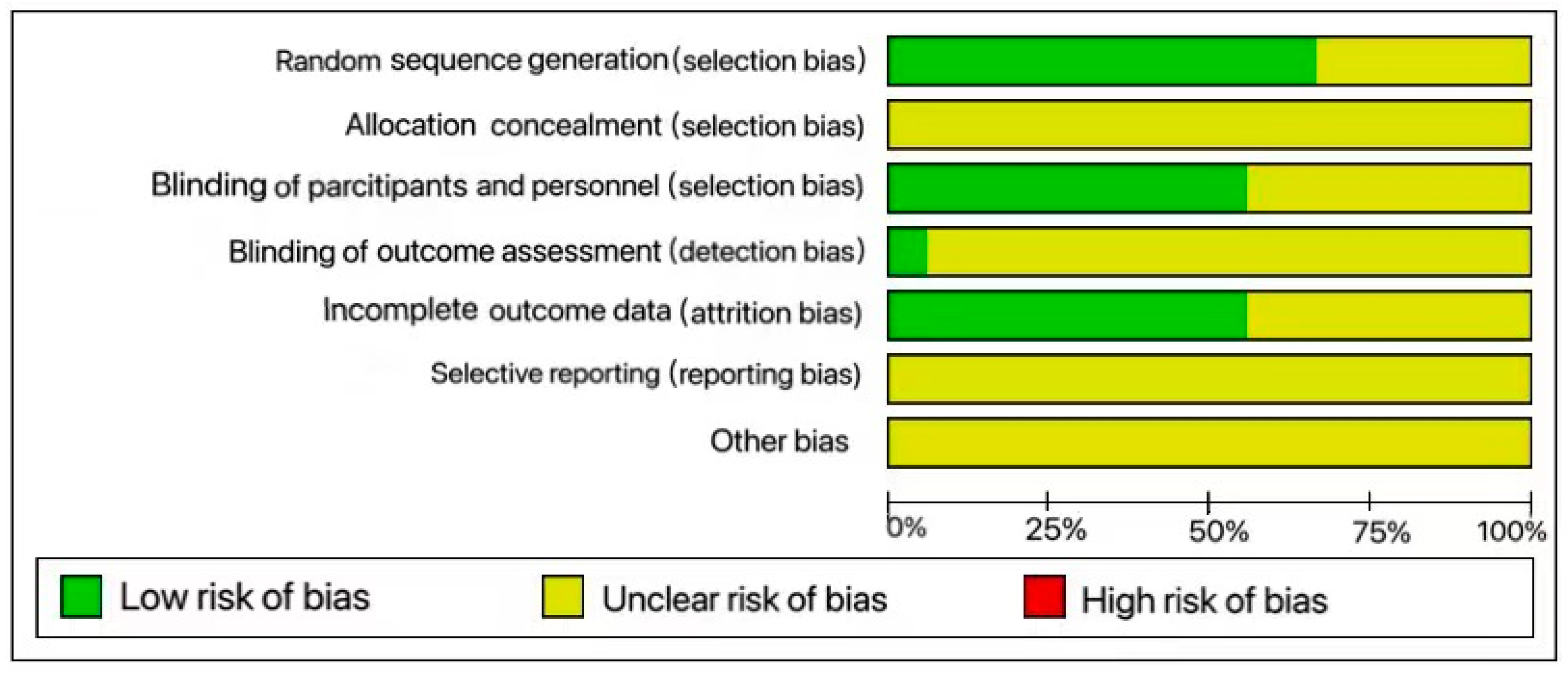
2.5. Risk of Bias Assessment
3. Results and Discussion
3.1. Search Results
3.2. Study Characteristics
3.3. Isolation and Characterization of MSCs
3.4. In Vitro Studies
3.5. In Vivo Studies
3.6. In Vitro and In Vivo Findings
3.6.1. Osteogenesis
3.6.2. Bone Regeneration
3.6.3. Angiogenesis
3.6.4. Vascularization
3.7. Therapeutic Effects of DMOG on Disease-Induced Bone Loss and Osteonecrosis
3.8. Role of DMOG on Chondrogenesis
3.9. Immunomodulatory Effects of DMOG
3.10. Controversies in the Promotion of DMOG on Bone Tissue Regeneration
3.11. Secondary Results
3.12. Prospect and Deficiency
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kushioka, J.; Chow, S.K.; Toya, M.; Tsubosaka, M.; Shen, H.; Gao, Q.; Li, X.; Zhang, N.; Goodman, S.B. Bone regeneration in inflammation with aging and cell-based immunomodulatory therapy. Inflamm. Regen. 2023, 43, 29. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Ding, Q.; Zhang, S.; Sun, S.; Liu, W.; Liu, J.; Han, X.; Ding, C. Flavonoid-Loaded Biomaterials in Bone Defect Repair. Molecules 2023, 28, 6888. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Tan, S.H.S.; Wong, J.R.Y.; Sim, S.J.Y.; Tjio, C.K.E.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater. Today Bio 2020, 7, 100067. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. Bone Healing Materials in the Treatment of Recalcitrant Nonunions and Bone Defects. Int. J. Mol. Sci. 2022, 23, 3352. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Rochlin, D.H.; Parsaei, Y.; Shetye, P.R.; Witek, L.; Leucht, P.; Rabbani, P.S.; Flores, R.L. Bone Tissue Engineering Strategies for Alveolar Cleft: Review of Preclinical Results and Guidelines for Future Studies. Cleft Palate Craniofac J. 2023, 60, 1450–1461. [Google Scholar] [CrossRef]
- Rodríguez-Merchán, E.C. A Review of Recent Developments in the Molecular Mechanisms of Bone Healing. Int. J. Mol. Sci. 2021, 22, 767. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Li, X.; Li, N.; Guo, T.; Cai, Y.; Yang, X.; Liang, J.; Sun, Y.; Fan, Y. A comparative study of autogenous, allograft and artificial bone substitutes on bone regeneration and immunotoxicity in rat femur defect model. Regen. Biomater. 2021, 8, rbaa040. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, V.S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B. An overview of translational research in bone graft biomaterials. J. Biomater. Sci. Polym. Ed. 2023, 34, 497–540. [Google Scholar] [CrossRef]
- Huber, J.; Griffin, M.F.; Longaker, M.T.; Quarto, N. Exosomes: A Tool for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 101–113. [Google Scholar] [CrossRef]
- Bouland, C.; Philippart, P.; Dequanter, D.; Corrillon, F.; Loeb, I.; Bron, D.; Lagneaux, L.; Meuleman, N. Cross-Talk Between Mesenchymal Stromal Cells (MSCs) and Endothelial Progenitor Cells (EPCs) in Bone Regeneration. Front. Cell Dev. Biol. 2021, 9, 674084. [Google Scholar] [CrossRef] [PubMed]
- Tatullo, M.; Codispoti, B.; Paduano, F.; Nuzzolese, M.; Makeeva, I. Strategic Tools in Regenerative and Translational Dentistry. Int. J. Mol. Sci. 2019, 20, 1879. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.S.; Marconi, G.D.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Shang, F.; Yu, Y.; Liu, S.; Ming, L.; Zhang, Y.; Zhou, Z.; Zhao, J.; Jin, Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact. Mater. 2021, 6, 666–683. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, H.R.; Sanchouli, M.; Mehrani, J.; Sabour, D. Potential of Bone-Marrow-Derived Mesenchymal Stem Cells for Maxillofacial and Periodontal Regeneration: A Narrative Review. Int. J. Dent. 2021, 2021, 4759492. [Google Scholar] [CrossRef] [PubMed]
- Adamička, M.; Adamičková, A.; Danišovič, L.; Gažová, A.; Kyselovič, J. Pharmacological Approaches and Regeneration of Bone Defects with Dental Pulp Stem Cells. Stem Cells Int. 2021, 2021, 4593322. [Google Scholar] [CrossRef]
- Halim, A.; Ariyanti, A.D.; Luo, Q.; Song, G. Recent Progress in Engineering Mesenchymal Stem Cell Differentiation. Stem Cell Rev. Rep. 2020, 16, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. S21), 92–102. [Google Scholar] [CrossRef]
- Bari, E.; Roato, I.; Perale, G.; Rossi, F.; Genova, T.; Mussano, F.; Ferracini, R.; Sorlini, M.; Torre, M.L.; Perteghella, S. Biohybrid Bovine Bone Matrix for Controlled Release of Mesenchymal Stem/Stromal Cell Lyosecretome: A Device for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 4064. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, T.; Ding, J.; Gu, H.; Wang, Q.; Wang, Y.; Zhang, D.; Gao, C. A reactive oxygen species-responsive hydrogel encapsulated with bone marrow derived stem cells promotes repair and regeneration of spinal cord injury. Bioact. Mater. 2023, 19, 550–568. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chen, H.; Wang, G.; Lyu, J.; Liu, Y.; Lin, S.; Zhou, M.; Jiang, X. CGRP-Loaded Porous Microspheres Protect BMSCs for Alveolar Bone Regeneration in the Periodontitis Microenvironment. Adv. Healthc. Mater. 2023, 12, e2301366. [Google Scholar] [CrossRef] [PubMed]
- Huan, Y.; Zhou, D.; Wu, X.; He, X.; Chen, H.; Li, S.; Jia, B.; Dou, Y.; Fei, X.; Wu, S.; et al. 3D bioprinted autologous bone particle scaffolds for cranioplasty promote bone regeneration with both implanted and native BMSCs. Biofabrication 2023, 15, 025016. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Madhoun, W.; Graham, E.M.; Hendrycks, R.; Renouard, M.; Hu, M.S. Stem Cells Regenerating the Craniofacial Skeleton: Current State-Of-The-Art and Future Directions. J. Clin. Med. 2020, 9, 3307. [Google Scholar] [CrossRef] [PubMed]
- Farmani, A.R.; Nekoofar, M.H.; Ebrahimi-Barough, S.; Azami, M.; Najafipour, S.; Moradpanah, S.; Ai, J. Preparation and In Vitro Osteogenic Evaluation of Biomimetic Hybrid Nanocomposite Scaffolds Based on Gelatin/Plasma Rich in Growth Factors (PRGF) and Lithium-Doped 45s5 Bioactive Glass Nanoparticles. J. Polym. Environ. 2023, 31, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.D.; Hu, C.H.; Liu, A.Q.; Zheng, C.X.; Xuan, K.; Jin, Y. Stem cell-based bone regeneration in diseased microenvironments: Challenges and solutions. Biomaterials 2019, 196, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Guo, Y.; Li, C.; Li, S.; Wan, X. Small Molecules that Promote Self-Renewal of Stem Cells and Somatic Cell Reprogramming. Stem Cell Rev. Rep. 2020, 16, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Farzin, A.; Hassan, S.; Ebrahimi-Barough, S.; Ai, A.; Hasanzadeh, E.; Goodarzi, A.; Ai, J. A facile two step heat treatment strategy for development of bioceramic scaffolds for hard tissue engineering applications. Mater. Sci. Eng. C 2019, 105, 110009. [Google Scholar] [CrossRef]
- Costa, M.H.G.; Serra, J.; McDevitt, T.C.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol. J. 2021, 16, e2000389. [Google Scholar] [CrossRef]
- Zhou, B.; Ge, T.; Zhou, L.; Jiang, L.; Zhu, L.; Yao, P.; Yu, Q. Dimethyloxalyl Glycine Regulates the HIF-1 Signaling Pathway in Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2020, 16, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ghadge, S.; Doppelhammer, M.; Van Pham, T.; Kühlenthal, S.; Franz, W.M.; Zaruba, M.M. Inhibition of Prolyl Hydroxylase as a Novel Therapeutic Target for Hif-Mediated Sdf-1 Activation and Stem Cell Homing in the Ischemic Heart. Circulation 2013, 128, A16652. [Google Scholar]
- Archacka, K.; Grabowska, I.; Mierzejewski, B.; Graffstein, J.; Górzyńska, A.; Krawczyk, M.; Różycka, A.M.; Kalaszczyńska, I.; Muras, G.; Stremińska, W.; et al. Hypoxia preconditioned bone marrow-derived mesenchymal stromal/stem cells enhance myoblast fusion and skeletal muscle regeneration. Stem Cell Res. Ther. 2021, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Zhang, Z.; Ye, D.; Tang, A.; Deng, L.; Han, W.; Zhao, J.; Wang, S.; Zhang, W.; Zhu, C.; et al. Repair of critical-sized rat calvarial defects using genetically engineered bone marrow-derived mesenchymal stem cells overexpressing hypoxia-inducible factor-1α. Stem Cells 2011, 29, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Liu, Y.; Yang, Z.; Aimaijiang, M.; Ma, R.; Yang, Y.; Zhang, Y.; Zhou, Y. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 11201. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Zhao, Y.; Chen, X.; Xiao, Y.; Bao, C. HIF signaling: A new propellant in bone regeneration. Biomater. Adv. 2022, 138, 212874. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.L. Role of prolyl hydroxylase/HIF-1 signaling in vascular calcification. Clin. Kidney J. 2023, 16, 205–209. [Google Scholar] [CrossRef]
- Korbecki, J.; Simińska, D.; Gąssowska-Dobrowolska, M.; Listos, J.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. Chronic and Cycling Hypoxia: Drivers of Cancer Chronic Inflammation through HIF-1 and NF-κB Activation: A Review of the Molecular Mechanisms. Int. J. Mol. Sci. 2021, 22, 10701. [Google Scholar] [CrossRef]
- Ding, H.; Gao, Y.S.; Hu, C.; Wang, Y.; Wang, C.G.; Yin, J.M.; Sun, Y.; Zhang, C.Q. HIF-1α transgenic bone marrow cells can promote tissue repair in cases of corticosteroid-induced osteonecrosis of the femoral head in rabbits. PLoS ONE 2013, 8, e63628. [Google Scholar] [CrossRef]
- Zou, D.; Zhang, Z.; He, J.; Zhu, S.; Wang, S.; Zhang, W.; Zhou, J.; Xu, Y.; Huang, Y.; Wang, Y.; et al. Repairing critical-sized calvarial defects with BMSCs modified by a constitutively active form of hypoxia-inducible factor-1α and a phosphate cement scaffold. Biomaterials 2011, 32, 9707–9718. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Gao, Y.S.; Wang, Y.; Hu, C.; Sun, Y.; Zhang, C. Dimethyloxaloylglycine increases the bone healing capacity of adipose-derived stem cells by promoting osteogenic differentiation and angiogenic potential. Stem Cells Dev. 2014, 23, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Z.; Wei, J.; Yu, Y.; Luo, J.; Zhou, J.; Li, Y.; Zheng, X.; Tang, W.; Liu, L.; et al. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-regulation of Hif-1α. Int. J. Biol. Sci. 2018, 14, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, S.; Song, W.Q.; Gao, Y.S.; Guan, J.J.; Wang, Y.; Sun, Y.; Zhang, C.Q. Dimethyloxaloylglycine improves angiogenic activity of bone marrow stromal cells in the tissue-engineered bone. Int. J. Biol. Sci. 2014, 10, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Qi, X.; Ding, H.; Yuan, H.; Xie, Z.; Chen, C.; Li, X.; Zhang, C.; Huang, Y. Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration. Int. J. Biol. Sci. 2016, 12, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Li, Q.; An, J.H.; Chae, H.K.; Yang, J.I.; Ryu, M.O.; Nam, A.; Song, W.J.; Youn, H.Y. Enhanced angiogenic activity of dimethyloxalylglycine-treated canine adipose tissue-derived mesenchymal stem cells. J. Vet. Med. Sci. 2019, 81, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Wang, J.A.; Ji, X.Y.; Yu, S.P.; Wei, L. Preconditioning of bone marrow mesenchymal stem cells by prolyl hydroxylase inhibition enhances cell survival and angiogenesis in vitro and after transplantation into the ischemic heart of rats. Stem Cell Res. Ther. 2014, 5, 111. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shahba, A.G.; Gebraad, A.; Kaur, S.; Paananen, R.O.; Peltoniemi, H.; Seppänen-Kaijansinkko, R.; Mannerström, B. Proangiogenic Hypoxia-Mimicking Agents Attenuate Osteogenic Potential of Adipose Stem/Stromal Cells. Tissue Eng. Regen. Med. 2020, 17, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Li, M.Y.; Lin, Y.W.; Yin, F.L.; Shan, H.J.; Wu, T.Y. Dimethyl Oxalylglycine Activates Tendon-Derived Stem Cells to Promote Regeneration of Achilles Tendon Rupture in Rats via HIF-1α. Adv. Ther. 2023, 6, 2200164. [Google Scholar] [CrossRef]
- Sinha, K.M.; Tseng, C.; Guo, P.; Lu, A.; Pan, H.; Gao, X.; Andrews, R.; Eltzschig, H.; Huard, J. Hypoxia-inducible factor 1α (HIF-1α) is a major determinant in the enhanced function of muscle-derived progenitors from MRL/MpJ mice. FASEB J. 2019, 33, 8321–8334. [Google Scholar] [CrossRef]
- Imran Khan, M. Exploration of metabolic responses towards hypoxia mimetic DMOG in cancer cells by using untargeted metabolomics. Saudi J. Biol. Sci. 2022, 29, 103426. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Lai, Z.G.; Fang, Z.L.; Xing, S.; Hui, K.; Hao, C.; Jin, Q.; Qi, Z.; Shen, W.J.; Dong, Q.N.; et al. Dimethyloxalylglycine prevents bone loss in ovariectomized C57BL/6J mice through enhanced angiogenesis and osteogenesis. PLoS ONE 2014, 9, e112744. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.H.; Song, W.Q.; Zhang, C.Q.; Yin, J.M. Dimethyloxaloylglycine increases bone repair capacity of adipose-derived stem cells in the treatment of osteonecrosis of the femoral head. Exp. Ther. Med. 2016, 12, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y. Expression of the HIF-1α/VEGF pathway is upregulated to protect alveolar bone density reduction in nasal-obstructed rats. Histol. Histopathol. 2024, 18701. [Google Scholar] [CrossRef]
- Taheem, D.K.; Foyt, D.A.; Loaiza, S.; Ferreira, S.A.; Ilic, D.; Auner, H.W.; Grigoriadis, A.E.; Jell, G.; Gentleman, E. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors. Stem Cells 2018, 36, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Wei, D.; Tang, K.; Wang, Q.; Estill, J.; Yao, L.; Wang, X.; Chen, Y.; Yang, K. The use of GRADE approach in systematic reviews of animal studies. J. Evid. Based Med. 2016, 9, 98–104. [Google Scholar] [CrossRef]
- Weng, T.; Zhou, L.; Yi, L.; Zhang, C.; He, Y.; Wang, T.; Ju, Y.; Xu, Y.; Li, L. Delivery of dimethyloxalylglycine in calcined bone calcium scaffold to improve osteogenic differentiation and bone repair. Biomed. Mater. 2021, 16, 035008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.Y.; Pei, X.; Li, Y.H.; Feng, H.; He, Z.H.; Xie, W.J.; Pei, X.B.; Zhu, Z.; Wan, Q.B.; et al. One-Pot Facile Encapsulation of Dimethyloxallyl Glycine by Nanoscale Zeolitic Imidazolate Frameworks-8 for Enhancing Vascularized Bone Regeneration. Adv. Healthc. Mater. 2023, 12, e2202317. [Google Scholar] [CrossRef]
- Shi, M.; Zhou, Y.; Shao, J.; Chen, Z.; Song, B.; Chang, J.; Wu, C.; Xiao, Y. Stimulation of osteogenesis and angiogenesis of hBMSCs by delivering Si ions and functional drug from mesoporous silica nanospheres. Acta Biomater. 2015, 21, 178–189. [Google Scholar] [CrossRef]
- Zhou, X.; Qian, Y.; Chen, L.; Li, T.; Sun, X.; Ma, X.; Wang, J.; He, C. Flowerbed-Inspired Biomimetic Scaffold with Rapid Internal Tissue Infiltration and Vascularization Capacity for Bone Repair. ACS Nano 2023, 17, 5140–5156. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.J.; Ma, X.J.; Li, S.K.; Li, T.; Li, Z.H.; Qian, Y.H.; Shafiq, M.; Wang, J.W.; Zhou, X.J.; He, C.L. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Sun, T.; Liu, K.; Chen, C.; Wen, W.; Ding, S.; Liu, M.; Zhou, C.; Luo, B. Highly Elastic and Anisotropic Wood-Derived Composite Scaffold with Antibacterial and Angiogenic Activities for Bone Repair. Adv. Healthc. Mater. 2023, 12, e2300122. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; He, X.; Zhu, M.; Zhu, Y.F. Design of bioglasses/PDLLA scaffolds with responsive drug delivery in ultrasonic-assisted bone repair. Mater. Lett. 2023, 342, 134295. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Chang, J.; Xiao, Y. Delivery of dimethyloxallyl glycine in mesoporous bioactive glass scaffolds to improve angiogenesis and osteogenesis of human bone marrow stromal cells. Acta Biomater. 2013, 9, 9159–9168. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, S.; Hosseini, S.; Mostafaei, F.; Sayahpour, F.A.; Baghaban Eslaminejad, M. 3D-porous β-tricalcium phosphate-alginate-gelatin scaffold with DMOG delivery promotes angiogenesis and bone formation in rat calvarial defects. J. Mater. Sci. Mater. Med. 2018, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Zippusch, S.; Besecke, K.F.W.; Helms, F.; Klingenberg, M.; Lyons, A.; Behrens, P.; Haverich, A.; Wilhelmi, M.; Ehlert, N.; Böer, U. Chemically induced hypoxia by dimethyloxalylglycine (DMOG)-loaded nanoporous silica nanoparticles supports endothelial tube formation by sustained VEGF release from adipose tissue-derived stem cells. Regen. Biomater. 2021, 8, rbab039. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Canciani, B.; Cirillo, F.; Anastasia, L.; Peretti, G.M.; Mangiavini, L. Effect of Chemically Induced Hypoxia on Osteogenic and Angiogenic Differentiation of Bone Marrow Mesenchymal Stem Cells and Human Umbilical Vein Endothelial Cells in Direct Coculture. Cells 2020, 9, 757. [Google Scholar] [CrossRef]
- Jin, X.Y.; Han, D.; Tao, J.; Huang, Y.J.; Zhou, Z.H.; Zhang, Z.; Qi, X.; Jia, W.T. Dimethyloxallyl Glycine-Incorporated Borosilicate Bioactive Glass Scaffolds for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Curr. Drug Deliv. 2019, 16, 565–576. [Google Scholar] [CrossRef]
- Qi, X.; Liu, Y.; Ding, Z.Y.; Cao, J.Q.; Huang, J.H.; Zhang, J.Y.; Jia, W.T.; Wang, J.; Liu, C.S.; Li, X.L. Synergistic effects of dimethyloxallyl glycine and recombinant human bone morphogenetic protein-2 on repair of critical-sized bone defects in rats. Sci. Rep. 2017, 7, 42820. [Google Scholar] [CrossRef]
- Zarkesh, I.; Halvaei, M.; Ghanian, M.H.; Bagheri, F.; Sayahpour, F.A.; Azami, M.; Mohammadi, J.; Baharvand, H.; Baghaban Eslaminejad, M. Scalable and cost-effective generation of osteogenic micro-tissues through the incorporation of inorganic microparticles within mesenchymal stem cell spheroids. Biofabrication 2019, 12, 015021. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.; Lawson, H.D.; Overholt, K.J.; Damodaran, K.; Gottardi, R.; Acharya, A.P.; Little, S.R. Synthesis and characterization of CaSr-Metal Organic Frameworks for biodegradable orthopedic applications. Sci. Rep. 2019, 9, 13024. [Google Scholar] [CrossRef] [PubMed]
- Sathy, B.N.; Daly, A.; Gonzalez-Fernandez, T.; Olvera, D.; Cunniffe, G.; McCarthy, H.O.; Dunne, N.; Jeon, O.; Alsberg, E.; Donahue, T.L.H.; et al. Hypoxia mimicking hydrogels to regulate the fate of transplanted stem cells. Acta Biomater. 2019, 88, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, X.; Chen, H.; Bao, D.; Su, X.; Wei, L.; Hu, N.; Huang, W.; Xiang, Z. Hypoxia-mimicking scaffolds with controlled release of DMOG and PTHrP to promote cartilage regeneration via the HIF-1α/YAP signaling pathway. Int. J. Biol. Macromol. 2023, 226, 716–729. [Google Scholar] [CrossRef]
- Ji, X.; Shao, H.; Li, X.; Ullah, M.W.; Luo, G.; Xu, Z.; Ma, L.; He, X.; Lei, Z.; Li, Q.; et al. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials 2022, 285, 121530. [Google Scholar] [CrossRef]
- Falcon, J.M.; Chirman, D.; Veneziale, A.; Morman, J.; Bolten, K.; Kandel, S.; Querido, W.; Freeman, T.; Pleshko, N. DMOG Negatively Impacts Tissue Engineered Cartilage Development. Cartilage 2021, 13 (Suppl. S2), 722s–733s. [Google Scholar] [CrossRef]
- Moore, K.A.; Lemischka, I.R. Stem cells and their niches. Science 2006, 311, 1880–1885. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef]
- Regan, J.N.; Lim, J.; Shi, Y.; Joeng, K.S.; Arbeit, J.M.; Shohet, R.V.; Long, F. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc. Natl. Acad. Sci. USA 2014, 111, 8673–8678. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Gomes, R.S.; Yeoh, K.K.; Perbellini, F.; Malandraki-Miller, S.; Ambrose, L.; Heather, L.C.; Faggian, G.; Schofield, C.J.; Davies, K.E.; et al. Preconditioning of Cardiosphere-Derived Cells With Hypoxia or Prolyl-4-Hydroxylase Inhibitors Increases Stemness and Decreases Reliance on Oxidative Metabolism. Cell Transplant. 2016, 25, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, L. Unique bone marrow blood vessels couple angiogenesis and osteogenesis in bone homeostasis and diseases. Ann. N. Y. Acad. Sci. 2020, 1474, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hendriks, M.; Chatzis, A.; Ramasamy, S.K.; Kusumbe, A.P. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J. Bone Miner. Res. 2020, 35, 2103–2120. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jain, M.; Alimperti, S. Bone Microvasculature: Stimulus for Tissue Function and Regeneration. Tissue Eng. Part B Rev. 2021, 27, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Y.; Wei, C. Nanoparticles based composite coatings with tunable vascular endothelial growth factor and bone morphogenetic protein-2 release for bone regeneration. J. Biomed. Mater. Res. A 2023, 111, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, H.; Fu, H.; Hu, Y.; Fang, W.; Liu, J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered 2022, 13, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, V.; Martín-Moldes, Z.; Deck, A.; Torres-Sanchez, R.; Valat, A.; Cairns, D.; Li, C.; Kaplan, D.L. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 2021, 276, 120995. [Google Scholar] [CrossRef]
- Woo, K.M.; Jung, H.M.; Oh, J.H.; Rahman, S.U.; Kim, S.M.; Baek, J.H.; Ryoo, H.M. Synergistic effects of dimethyloxalylglycine and butyrate incorporated into α-calcium sulfate on bone regeneration. Biomaterials 2015, 39, 1–14. [Google Scholar] [CrossRef]
- Janjić, K.; Lilaj, B.; Moritz, A.; Agis, H. Formation of spheroids by dental pulp cells in the presence of hypoxia and hypoxia mimetic agents. Int. Endod. J. 2018, 51 (Suppl. 2), e146–e156. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.D.; Cvikl, B.; Gruber, R.; Watzek, G.; Agis, H. Prolyl hydroxylase inhibitors increase the production of vascular endothelial growth factor in dental pulp-derived cells. J. Endod. 2012, 38, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Nadine, S.; Fernandes, I.J.; Correia, C.R.; Mano, J.F. Close-to-native bone repair via tissue-engineered endochondral ossification approaches. iScience 2022, 25, 105370. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.; Wei, T.; Sun, Q.; Midgley, A.C.; Huang, Z.; Wang, T.; Shafiq, M.; Zhi, D.; Si, J.; Yan, H.; et al. The effect of hypoxia-mimicking responses on improving the regeneration of artificial vascular grafts. Biomaterials 2021, 271, 120746. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Furusho, H.; Hirota, K.; Sasaki, H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int. J. Oral. Sci. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L.; et al. Stabilization of HIF-1alpha alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nan, H.; Chiou, Y.S.; Zhan, Z.; Lobie, P.E.; Hu, C. Selective Formation of Osteogenic and Vasculogenic Tissues for Cartilage Regeneration. Adv. Healthc. Mater. 2023, 12, e2202008. [Google Scholar] [CrossRef]
- Muresan, G.C.; Hedesiu, M.; Lucaciu, O.; Boca, S.; Petrescu, N. Effect of Vitamin D on Bone Regeneration: A Review. Medicina (Kaunas) 2022, 58, 1337. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.Y.; Chu, X.Y.; Zheng, C.Y.; Luan, Y.Y.; He, X.; Yang, K.; Zhang, D.L. VEGF-Loaded Heparinised Gelatine-Hydroxyapatite-Tricalcium Phosphate Scaffold Accelerates Bone Regeneration via Enhancing Osteogenesis-Angiogenesis Coupling. Front. Bioeng. Biotechnol. 2022, 10, 915181. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Whole Aspect of Runx2 Functions in Skeletal Development. Int. J. Mol. Sci. 2022, 23, 5776. [Google Scholar] [CrossRef]
- Xie, T.; Zhong, H.; Jin, Y.; Liu, X.; Chen, F.; Xiang, K.; Wu, S. Research on Runx2 gene induced differentiation of human amniotic mesenchymal stem cells into ligament fibroblasts in vitro and promotion of tendon-bone healing in rabbits. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2023, 37, 1523–1532. [Google Scholar]
- Bao, M.; Chen, Y.; Liu, J.T.; Bao, H.; Wang, W.B.; Qi, Y.X.; Lv, F. Extracellular matrix stiffness controls VEGF(165) secretion and neuroblastoma angiogenesis via the YAP/RUNX2/SRSF1 axis. Angiogenesis 2022, 25, 71–86. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, M.; Weng, H. Induction of the mitochondrial NDUFA4L2 protein by HIF-1a regulates heart regeneration by promoting the survival of cardiac stem cell. Biochem. Biophys. Res. Commun. 2018, 503, 2226–2233. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Q.; Zhang, Y.; Dai, M.; Jiang, Y.; Wang, H.; Yu, M.; Jing, W.; Tian, W. Metabolic reprogramming by HIF-1 activation enhances survivability of human adipose-derived stem cells in ischaemic microenvironments. Cell Prolif. 2017, 50, e12363. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, L.; Li, Q.; Lai, Y. HIF-1α protects osteoblasts from ROS-induced apoptosis. Free Radic. Res. 2022, 56, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Léniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef]
- Li, J.; Fan, L.; Yu, Z.; Dang, X.; Wang, K. The effect of deferoxamine on angiogenesis and bone repair in steroid-induced osteonecrosis of rabbit femoral heads. Exp. Biol. Med. (Maywood) 2015, 240, 273–280. [Google Scholar] [CrossRef]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Y.; Lan, Y.; Wu, Y.; Li, J.; Xu, X. CoCl(2)-Induced hypoxia promotes hPDLSCs osteogenic differentiation through AKT/mTOR/4EBP-1/HIF-1α signaling and facilitates the repair of alveolar bone defects. Cell Biol. Int. 2024. Online ahead of print. [Google Scholar]
- Yu, X.; Wan, Q.; Ye, X.; Cheng, Y.; Pathak, J.L.; Li, Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell Mol. Biol. Lett. 2019, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Molecular Basis beyond Interrelated Bone Resorption/Regeneration in Periodontal Diseases: A Concise Review. Int. J. Mol. Sci. 2023, 24, 4599. [Google Scholar] [CrossRef] [PubMed]
- Brylka, L.J.; Schinke, T. Chemokines in Physiological and Pathological Bone Remodeling. Front. Immunol. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Duda, G.N.; Geissler, S.; Checa, S.; Tsitsilonis, S.; Petersen, A.; Schmidt-Bleek, K. The decisive early phase of bone regeneration. Nat. Rev. Rheumatol. 2023, 19, 78–95. [Google Scholar] [CrossRef]
- Hao, S.; Wang, M.; Yin, Z.; Jing, Y.; Bai, L.; Su, J. Microenvironment-targeted strategy steers advanced bone regeneration. Mater. Today Bio 2023, 22, 100741. [Google Scholar] [CrossRef] [PubMed]
- Murugaiyan, K.; Amirthalingam, S.; Hwang, N.S.; Jayakumar, R. Role of FGF-18 in Bone Regeneration. J. Funct. Biomater. 2023, 14, 36. [Google Scholar] [CrossRef]
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An ossifying landscape: Materials and growth factor strategies for osteogenic signalling and bone regeneration. Curr. Opin. Biotechnol. 2022, 73, 355–363. [Google Scholar] [CrossRef]
- Mangione, F.; Salmon, B.; EzEldeen, M.; Jacobs, R.; Chaussain, C.; Vital, S. Characteristics of Large Animal Models for Current Cell-Based Oral Tissue Regeneration. Tissue Eng. Part B Rev. 2022, 28, 489–505. [Google Scholar] [CrossRef] [PubMed]

| MSCs Source/Origin | Isolation Method | Whether to Identify | Characterization Method | MSCs Makers | Three-Line Differentiation | Reference |
|---|---|---|---|---|---|---|
| Human BMSCs | - | YES | Flow cytometry | CD90, CD105, CD73 (+), CD34, CD45 (-) | - | [55] |
| Human BMSCs | - | NO | - | - | - | [60] |
| Human BMSCs | Density gradient centrifugation | NO | - | - | - | [65] |
| Human BMSCs | Enzyme digestion of tissue mass | YES | Flow cytometry | CD73, CD90, CD105 (+), CD80, HLA-DR, CD14, CD34, CD45 (-) | - | [31] |
| Human BMSCs | - | YES | Flow cytometry, identification of three-line differentiation | CD73, CD90, CD106 (+), CD34, CD45 (-) | Osteogenesis, Adipogenesis, Chondrogenesis | [70] |
| Human BMSCs | - | NO | - | - | - | [72] |
| Human BMSCs | - | NO | - | - | - | [73] |
| Human BMSCs | - | NO | - | - | - | [74] |
| Rat BMSCs | Enzyme digestion of tissue mass | NO | - | - | - | [43] |
| Rat BMSCs | - | NO | - | - | - | [58] |
| Rat BMSCs | Enzyme digestion of tissue mass | NO | - | - | - | [44] |
| Rat BMSCs | - | NO | - | - | - | [64] |
| Rat BMSCs | - | NO | - | - | - | [77] |
| Rat BMSCs | Enzyme digestion of tissue mass | YES | Flow cytometry | CD90 (+), CD34, CD45 (-) | - | [47] |
| Rat BMSCs | Lysate separation | NO | - | - | - | [32] |
| Mouse BMSCs | - | NO | - | - | - | [63] |
| Rabbit BMSCs | Density gradient centrifugation | NO | - | - | - | [76] |
| Pig BMSCs | Enzyme digestion of tissue mass | NO | - | - | - | [75] |
| Rat ADSCs | Enzyme digestion of tissue mass | YES | Flow cytometry, identification of three-line differentiation | CD29, CD44, CD90 (+), CD31, CD34, CD35 (-) | Osteogenesis Adipogenesis Chondrogenesis | [42] |
| Rat ADSCs | Enzyme digestion of tissue mass | YES | Identification of three-line differentiation | - | Osteogenesis Adipogenesis Chondrogenesis | [66] |
| Rat ADSCs | - | NO | - | - | - | [67] |
| Rabbit ADSCs | Enzyme digestion of tissue mass | NO | - | - | - | [53] |
| Human ADSCs | Enzymatic digestion and mechanical treatment | YES | Flow cytometry, identification of three-line differentiation | CD73, CD90, CD105 (+), CD14, CD19, CD45, HLA-DR (-) | Osteogenesis Adipogenesis Chondrogenesis | [48] |
| Human ADSCs | Enzyme digestion of tissue mass | NO | - | - | - | [69] |
| Dog ADSCs | Enzyme digestion of tissue mass | YES | Flow cytometry, identification of three-line differentiation | CD29, CD73 (+), CD34, CD45 (-) | Osteogenesis Adipogenesis Chondrogenesis | [46] |
| Human PDLSCs | Enzyme digestion of tissue mass | YES | Flow cytometry | CD29, CD44, CD90 (+), CD34, CD45 (-) | - | [68] |
| Human iPSCs | - | - | - | CD73, CD90, CD105 (+), CD34, CD45, HLA-DR (-) | Osteogenesis Adipogenesis Chondrogenesis | [45] |
| Stem Cells Conditioning Method | DMOG Concentration | Scaffold | Treatment/Groups | Analysis | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Rat ADSCs conditioned with DMOG in different concentrations under regular medium for 24 h and then using osteogenic medium for 1, 3, 7, 14, 21 days | 200, 500 and 1000 μM | - | 0 μM, 200 μM, 500 μM, and 1000 μM DMOG | WB, qRT-PCR, ALP, ARS | HIF-1α, VEGF protein secretion ↑, Runx-2, OCN, ALP, Collagen-I gene expression ↑, ALP activity ↑ by DMOG, 1000 μM max. | [42] |
| Rat ADSCs conditioned with DMOG under regular medium for 48 h and then using osteogenic medium for 7,21 days | 500 μM | - | Control group, DMOG group | ALP, ARS, WB, tube formation | HIF-1α, VEGF protein secretion ↑, ALP activity ↑, mineralized nodule ↑, increased tubule formation by DMOG. | [43] |
| Human ADSCs conditioned with DMOG using osteogenic medium for 7, 14 days | 100, 200, 500 μM | - | Control group, 500 μM DMOG group, 200 μM Baicalein group | WB, qRT-PCR, ALP, ARS | HIF-1α, VEGF protein secretion ↑, ALP activity , mineralized nodule , ALP, BMP-2, Runx-2, Collagen-I gene expression by DMOG. | [48] |
| Rat BMSCs was cultured with DMOG using osteogenic medium for 7, 21 days. | 100, 500, 1000, 2000 μM | - | 0 μM,500 μM DMOG | WB, qRT-PCR, ALP, ARS | HIF-1α, VEGF, Runx-2, OCN, ALP, Collagen-I protein secretion ↑, HIF-1α, VEGF, Runx-2, OCN, ALP, Collagen-I gene expression ↑, ALP activity ↑, mineralized nodule ↑ by DMOG. | [54] |
| Rat BMSCs was cultured on different scaffolds using osteogenic medium for 7 days | 4 M | CBC | CBC group, Collagen/CBC group and DMOG/Collagen/CBC group | qRT-PCR | ALP, Runx-2, OCN, VEGF gene expression ↑ by DMOG. | [58] |
| BMSCs was cultured on different scaffolds using osteogenic medium for 7, 21 days | - | ZIF8 | Control group ZIF8 group ZIF8/DMOG group | WB, ALP, ARS, tube formation | ALP, Collagen-I, P-ERK-1/2, HIF-1α, VEGF-a, eNOS protein secretion ↑, ALP activity ↑ mineralized nodule ↑, increased tubule formation by DMOG. | [59] |
| Human BMSCs was cultured on different scaffolds using osteogenic medium for 7, 14 days | 35 μg/mL | MSN | MSN group, DMOG/MSN group | WB, qRT-PCR, ALP | OCN, Runx-2, VEGF protein secretion ↑, Runx-2, OCN, OPN, VEGF gene expression ↑, ALP activity ↑ by DMOG. | [60] |
| BMSCs was cultured on different scaffolds using osteogenic medium for 7, 14, 21 days | - | MSN, PLGA, PCL | Control group, PLGA-PCL group, DMOG-MSN/PLGA-PCL group | WB, qRT-PCR, ALP, ARS, tube formation | Runx-2, Collagen-I, OPN protein secretion ↑, Runx-2, ALP, OPN, VEGF, bFGF gene expression ↑, ALP activity ↑, mineralized nodule ↑, increased tubule formation by DMOG. | [61] |
| BMSCs was cultured on different scaffolds using osteogenic medium for 7, 14, 21 days | - | GP, MSN | Control group, GP group, and DMOG-MSN/GP group | WB, qRT-PCR, ALP, ARS, tube formation | Runx-2, Collagen-I, OPN, OCN, HIF-1α, KDR, eNOS, VEGF gene expression ↑, ALP activity ↑, mineralized nodule, increased tubule formation by DMOG. | [62] |
| Mouse BMSCs was cultured on different scaffolds using osteogenic medium for 7 days | - | MBG | MBG group, DMOG-MBG group | WB, qRT-PCR | HIF-1α, VEGF protein secretion ↑, ALP, OPN, OCN gene expression ↑ by DMOG. | [65] |
| Rat ADSCs was cultured on different scaffolds using osteogenic medium for 7, 14 days | - | Sodium alginate-gelatin-β-tricalcium phosphate | Control group, Scaffolds group, Scaffolds-DMOG group | WB, qRT-PCR | VEGF protein secretion ↑, ALP, OCN, Runx2, CD31, KDR, CD133 gene expression ↑ by DMOG. | [66] |
| Rat ADSCs was cultured on different scaffolds using osteogenic medium for 7, 14, 21 days | 1 mM | carrageenan nanocomposite hydrogel | Control group, hydrogel group, DMOG-hydrogel group | ALP, ARS, IHC, tube formation | ALP, OPN, Collagen-I protein secretion ↑, ALP activity ↑, mineralized nodule ↑, increased tubule formation by DMOG. | [67] |
| Human PDLSCs was cultured on different scaffolds using osteogenic medium for 7, 14, 21 days | - | PLGA | PLGA, DMOG-PLGA | ALP, qRT-PCR, tube formation | Runx-2, BSP, OPN, OCN, VEGF, CD31, SCF, PLGF gene expression ↑, ALP activity ↑, increased tubule formation by DMOG. | [68] |
| Rat BMSCs conditioned with DMOG using regular medium for 1, 3, 7, 14, 21 days | 200, 500 and 1000 μM | - | 0 μM, 200 μM, 500 μM, and 1000 μM DMOG | WB, qRT-PCR, ELISA | HIF-1α, VEGF, SDF-1, PLGF, bFGF protein secretion ↑, VEGF, SDF-1, PLGF, bFGF gene expression ↑ by DMOG. | [44] |
| Human iPSCs conditioned with DMOG using regular medium for 3, 7 days | 1000 μM | - | 0 μM,1000 μM DMOG | WB, qRT-PCR, tube formation | HIF-1α,VEGF protein secretion↑,VEGF, SDF-1, PLGF, bFGF gene expression ↑, increased tubule formation by DMOG. | [45] |
| Dog ADSCs conditioned with DMOG using regular medium for 12, 24, 72 h | 0.1, 0.5 mM | - | 0 mM, 0.1 mM, 0.5 mM DMOG | WB, qRT-PCR, ELISA, tube formation | HIF-1α,VEGF protein secretion ↑,VEGF gene expression ↑ last for 72 h, bFGF gene expression ↑ at 48 h, but ↓ at 72 h, HGF gene expression ↑ at 6 h, but ↓ at 12 h, Ang-1 ↓ by DMOG, increased tubule formation by DMOG. | [46] |
| Human ADSCs conditioned with regular medium for 3, 6, 9 days. Human ADSCs conditioned with NPSNPs for 4, 7 days | 50, 100 and 500 μM | NPSNPs | 0 μM, 100 μM and 500 μM Control group, NPSNPs, 50 μM DMOG NPSNPs, and 100 μM DMOG NPSNPs | ELISA, tube formation | VEGF protein secretion ↑, increased tubule formation by DMOG. | [69] |
| The direct coculture method at ratio 1:1 was used to cultivate human BMSCs and HUVECs together with osteogenic medium for 2, 9 days | 0.5 mM | - | Control group, DMOG group | ELISA, qPCR | VEGF protein secretion ↑, VEGF gene expression ↑ by DMOG. | [70] |
| Human BMSCs conditioned with different scaffolds using osteogenic medium for 7 days | 1 mg/mL | PHMG | PHMG group, PHMG-DMOG group, PHMG-BMP2 group, PHMG-DMOG-BMP2 group | WB, qRT-PCR | HIF-1α, VEGF protein secretion ↑, HIF-1α, VEGF gene expression ↑, ALP, Collagen-I, Runx-2 gene expression no up-regulation, ALP, Collagen-I, Runx-2 gene expression ↑ by DMOG is used in conjunction with BMP-2. | [72] |
| Human BMSCs conditioned with different scaffolds for 1, 7 and 14 days | 5 mg/mL | CMPs | Mesosphere group, GMP-mesosphere group, CMPs-mesosphere group, DMOG-CMPs-mesosphere group | qRT-PCR, ALP, IHC, histology | Runx2, Sox9, OSX, ALP, OCN, OPN, VEGF, KDR gene expression ↑, IHC for Collagen-I, OCN, VEGF ↑, ALP activity ↑, in CMPs group and DMOG-CMPs group, there was no significant difference between the two groups. | [73] |
| Human BMSCs conditioned with different scaffolds for 7, 14 and 21 days | 0.001, 0.01, 0.1 mole | Ca-Sr-MOFs | Ca-Sr-MOFs group, DMOG-Ca-Sr-MOFs group, Osteogenic medium group | qRT-PCR | Runx-2, OCN, Collagen-I gene expression by DMOG. | [74] |
| Animal Model | Bone Defect Model | Scaffold/Vehicle | DMOG Concen-tration | Treatment Groups | Time Point | Analysis | Outcome | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Sprague Dawley (male, 21-month-old, 500–600 g) | One 5 mm diameter right mandible defects | GS | 0.5 mM | GS group, GS-BMSCs group, GS-DMOG-BMSCs (BMSCs were preconditioned with DMOG for 48 h) | 8 and 12 weeks | micro CT, histology, and IHC | BV and BV/TV ratio ↑, IHC for OCN, Runx-2, CD31 VEGF ↑, new bone formation by DMOG. | [43] | |
| Rat | Two 5 mm diameter calvarial defects | β-TCP scaffolds | 1000 μM | β-TCP group, β-TCP-BMSCs group, β-TCP-DMOG-BMSCs group (BMSCs were pretreated with DMOG for 72 h) | 8 weeks | micro CT, microfilm perfusion, histology and IHC | BV/TV ratio ↑, IHC for CD31 ↑, new bone and vessel formation by DMOG. | [44] | |
| New Zealand white rabbits (male, 6 months old, 2.5–3 kg) | One 5 mm diameter and 8 mm depth critical-sized condyle defect | Collagen-CBC | 4 M | Collagen-CBC group, DMOG-Collagen-CBC group | 12 weeks | micro CT, histology and IHC | BV/TV ratio ↑, Tb.Sp ↓, new bone formation by DMOG. There was no difference in expression of CD31 and Runx-2 between the with or without DMOG groups. | [58] | |
| Rat | A critical-sized cranial defect | ZIF-8, SA hydrogel | - | Control group, ZIF-SA group, ZIF-DMOG-SA group | 2 and 4 weeks | micro CT, histology and IF | BV/TV ratio ↑, Tb.n ↓, Tb.Th ↑, IF for BMP-2, OPN, OCN, CD31, HIF-1α, VEGF-a ↑, new bone formation by DMOG. | [59] | |
| Rat | Calvarial defects | MSN, PLGA, PCL | - | Control group, PLGA-PCL group, DMOG-MSN/PLGA-PCL group, SrHA/PLGA-PCL group, and DMOG-MSN/SrHA/PLGA-PCL group | 4, 8 and 12 weeks | micro CT, microfilm perfusion, histology and IF | BMD ↑, BV/TV ratio ↑, IF for CD31, HIF-1α and OCN ↑, new bone and vessel formation by DMOG and Sr ion. DMOG and Sr ion have synergistic effect. | [61] | |
| Rat | Calvarial defects | GP, MSN | - | Control group, GP group, DMOG-MSN/GP group, BFP1-MSN/GP group, DB-MSN/GP group | 4, 6 and 12 weeks | micro CT, microfilm perfusion, histology and IF | BV/TV ratio ↑, IF for CD31, HIF-1α, α-SMA and OCN ↑, new bone and vessel formation by DMOG and BFP-1. DMOG and BFP-1 have synergistic effect. | [62] | |
| Sprague Dawley rats (male, adult, 250–300 g) | Two 5 mm diameter calvarial defects | β-TCP scaffolds | 1000 μM | β-TCP group, β-TCP-hiPSC-MSCs group, and β-TCP with DMOG-hiPSC-MSCs group (hiPSCs were treated with DMOG for 72 h) | 2, 4, 6 and 8 weeks | micro CT, microfil perfusion, histology, IHC and sequential fluorescent labeling | BV/TV ratio ↑, IHC for CD31, HIF-1α and VEGF ↑, new bone and vessel formation by DMOG. | [45] | |
| Sprague Dawley rats (male, adult, 250–300 g) | Two 5 mm diameter calvarial defects | PHMG | 1 mg/mL | PHMG group, PHMG-DMOG group, PHMG-BMP2 group, and PHMG-DMOG-BMP2 group | 2, 4, 6 and 8 weeks | micro CT, microfilm perfusion, histology, IHC and sequential fluorescent labeling | BV/TV ratio ↑, BMD ↑, IHC for CD31, OCN ↑, new bone and vessel formation by DMOG-BMP2. | [72] | |
| Sprague Dawley rats (7–8 weeks, 250–300 g) | calvarial defects | CMP | 5 mg/mL | Control group, trapper without mesosphere group, trapper loaded with mesosphere group, and trapper loaded with DCMP mesosphere group | 8 weeks | micro CT, histology | new bone and vessel formation by DMOG. | [73] | |
| Animal Model | Bone-Related Disease | Scaffold/Vehicle | Concentration of DMOG | Treatment Groups | Time Point Analysis | Analysis | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Two-month-old female C57BL/6J mice | OVX | - | 5 mg/kg, 20 mg/kg | Sham group, OVX group, OVX + 5 mg/kg/day DMOG group, and OVX + 20 mg/kg/day DMOG group. | 4 weeks | micro CT, microfilm perfusion, mechanical testing, histology, TRAP, fluorochrome labeling, bone histomorphometry, and ELISA | BMD, BV/TV, Tb. Th, Tb. N, Tb.Sp , new bone and new blood vessels form by DMOG. There was no significant difference in serum levels of CTX and TRAP staining cells between DMOG-treated OVX mice and untreated mice. | [52] |
| New Zealand rabbits (weighing 2.5–3 kg and aged 2–3 months) | ONFH | Beaver Nano hydrogel | 1000 µM | Controls group, core decompression group, core decompression + ADSCs group and core decompression + DMOG-treated ADSCs group. | 4 weeks | micro CT, histology, and IHC | BMD, BV/TV , IHC for HIF-1α, CD31 new bone and new blood vessels form by DMOG. | [53] |
| Wistar rats (3 weeks old, male) | OSAHS nasal obstructed | - | 2 mg | Control group, PBS group, DMOG group | 2 weeks | micro CT, histology, IHC, WB, and qRT-PCR | BV/TV b,IHC, qRT-PCR, WB for HIF-1α, VEGF, ALP, Runx2, OCN, Collagen-I and new bone and new blood vessels form by DMOG. | [54] |
| Stem Cells Conditioning Method | Animal Model | Bone Defect Model | DMOG Concentration | Scaffold | Treatment/Groups | Analysis | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Human BMSCs conditioned with chondroblast medium for 5,7,14,21 days | - | - | 200 μM | - | Control group, 100 μM CoCl2 group, 50 μM DFX group and 200 μM DMOG | qRT-PCR, alcian blue staining, glycosaminoglycan quantification | VEGFA, PKG1, EGLN, Sox-9 gene expression ↑, MMP-13 gene expression ↓ by DMOG, DMOG promotes cartilage formation. | [55] |
| Pig BMSCs conditioned in alginate hydrogel with chondroblast medium for 7 days. Different alginate hydrogel implanted defect for 4 and 12 weeks | Balb/C nude mice | Two subcutaneous pockets (one in the shoulder level and one in the hip level) | 2.1, 4.2, 6.3 mg/mL | alginate hydroge-l | 0, 2.1, 4.2, 6.3 mg/mL alginate hydrogel group, alginate hydrogel-DMOG group | qRT-PCR, micro CT,histology, IHC, gl-ycosaminoglycan and collagen content | Collagen-II, aggrecan gene expression ↑, MMP-13 gene expression ↓, IHC for Collagen-II ↑ by DMOG, DMOG promotes cartilage formation. | [75] |
| Rabbit BMSCs conditioned in scaffold with chondroblast medium for 4 weeks. Different scaffold implanted defect for 4 and 8 weeks. | 7-week-old male athymic nude mice | Subcutaneous pocket on the back on each side of the incision | 100, 200, 500, and 1000 μM | PLGA PLLA | 0 μM, 100 μM, 200 μM, 500 μM, and 1000 μM DMOG PLLA group, PLLA/PLGA-DMOG group, PLLA/PLGA-PTHrP group, and PLLA/PLGA-DP group | qRT-PCR, WB, histology, toluidine blue, safranin O-fast green staining and IHC | Sox-9, aggrecan, Collagen-II gene expression ↑, MMP-13 gene expression ↓, Collagen-II, aggrecan protein secretion ↑, IHC for Collagen-II ↑ by DMOG, DMOG promotes cartilage formation. | [76] |
| Rat BMSCs conditioned in scaffold with chondroblast medium for 7 days. Different scaffold implanted defect for 6, 12 and 18 weeks. | Sprague Dawley rats (male, 300 g) | Femoral trochlear defect (2 mm diameter and 2 mm depth) | 25, 50 μg/mL DMOG | CS, HPCH | Control group, HPCH group, 25 μg/mL DMOG-HPCP group, 50 μg/mL DMOG-HPCP group Control group, CS-PMS group, HD/CS-PMS group, CSK-PMS group, and HD/ CSK-PMS group | qRT-PCR, WB,histology,toluidine blue, safranin O-fast green staining, IHC and micro CT | Collagen-II, Sox-9 protein secretion ↑, Sox-9, aggrecan, Collagen-II gene expression ↑, Collagen-X gene expression ↓ by DMOG, DMOG promotes cartilage formation. | [77] |
| Chondrocytes and SYN-MSCs were cultured in chondrogenic induction ratio of 1:4 on particulate-engineered scaffolds containing DMOG for 4 weeks and 6 weeks | - | - | 200 μM | Particulate-engineered scaffolds | Untreated group, treated DMOG group | histology (alcian blue and H&E staining), biochemistry, spectral imaging compositional analysis, attenuated total reflection spectroscopy, mechanical assessment | DMOG did not translate to overall increased extracellular matrix deposition, and negatively affected the mechanical competency of the engineered cartilage. | [78] |
| Stem Cells Type | Functions/Signaling Pathways | Ref. |
|---|---|---|
| Human iPSCs,BMSCs | PI3K/Akt | [45,61,62] |
| BMSCs | PI3K/Akt, MAPK | [59] |
| Mouse BMSCs | YAP/TAZ | [63,68] |
| Murine mesenchymal C3H10T1/2 clone 8 cells | Wnt/β-catenin | [52] |
| Pig BMSCs | S-mad | [75] |
| Rabbit BMSCs | HIF-1α/YAP | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Q.; Fei, X.; Zhang, H.; Zhu, X.; Ruan, J. Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 3879. https://doi.org/10.3390/ijms25073879
Dong Q, Fei X, Zhang H, Zhu X, Ruan J. Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review. International Journal of Molecular Sciences. 2024; 25(7):3879. https://doi.org/10.3390/ijms25073879
Chicago/Turabian StyleDong, Qiannan, Xiuzhi Fei, Hengwei Zhang, Ximei Zhu, and Jianping Ruan. 2024. "Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review" International Journal of Molecular Sciences 25, no. 7: 3879. https://doi.org/10.3390/ijms25073879
APA StyleDong, Q., Fei, X., Zhang, H., Zhu, X., & Ruan, J. (2024). Effect of Dimethyloxalylglycine on Stem Cells Osteogenic Differentiation and Bone Tissue Regeneration—A Systematic Review. International Journal of Molecular Sciences, 25(7), 3879. https://doi.org/10.3390/ijms25073879






