Cancer Stem Cells from Definition to Detection and Targeted Drugs
Abstract
:1. Introduction
2. General Concept of Cancer Stem Cells
2.1. Cancer Stem Cells: Origin and Detailed Characteristics
| Features | Normal Stem Cells | Cancer/Leukaemia Stem Cells |
|---|---|---|
| Localisation | In almost all physiological tissues | Periphery of the tumour |
| Composition | Hierarchical structure | Hierarchical structure |
| Characteristics | Primitive or undifferentiated precursors | Initiate and reconstitute tumour lesions |
| Function | To maintain tissue homeostasis | To maintain the unlimited growth of tumours and their morphological diversity |
| Self-renewal | Potent | Potent (tumour re-creation by metastasis) |
| Differentiation pattern | Pluripotent (differentiate into different kinds of normal cells) | Pluripotent (differentiate into different kinds of cancer cells) |
| Cell differentiation | Balanced | Dysregulated |
| Cell division | Mostly asymmetric | Subpopulations of CSCs: * Early-stage CSCs—mostly asymmetric * Late-stage CSCs—mostly symmetric |
| Cell cycle phase | G0/G1 phase | Ability to switch into any phase (mostly slow-cycling behaviour) |
| Proliferation index | Low, unlimited and well-controlled | Varied and uncontrolled |
| Morphology characteristics | High nuclear-to-cytoplasmic ratios | High nuclear-to-cytoplasmic ratios |
| Migration ability | High | High |
| Cell phenotypic potential (cell plasticity) | Stable | Heterogeneous |
| Partner of sld five 1 detection | Negative | Positive |
| Pro-angiogenic property | Limited | Unlimited |
| Drug sensitivity | Moderate | Strong resistance |
| Selected surface markers | CD24+, CD34+, CD44+, CD90+, CD133+ | CD24−/low, CD34+, CD44+, CD90+, CD133+, ALDH1high, ESA, EpCAM, side population cells |
| Immunosuppressive effect | Negative | Positive (via paracrine manner) |
| Survival rate | Prolong | Enhances their survival in an autocrine manner |
| Apoptosis | Antiapoptotic phenotype | Antiapoptotic phenotype (mediated by IL-4) |
| Chromosomal abnormality | Normal karyotype | Subpopulations of CSCs: * Early-stage CSCs—normal karyotype * Late-stage CSCs—an abnormal chromosome number |
| Telomerase activity | Potent | Potent |
| Histone H3 demethylation | Positive | Positive |
| Expression of Oct4, Notch, Sox1 genes | Positive | Positive |
| DNA repair ability | Potent | Potent |
| Genetic stability | Normal | Lost |
| Presence in peripheral blood | Trace amounts | Trace amounts |
| % of cells in specific tissue | 0.01 | 0.02–25 |
2.2. The Importance of the Tissue-Specific Microenvironment for the Maintenance of CSCs/LSCs
2.3. Immunophenotypic Fingerprints of CSCs/LSCs
2.4. Detection of CSCs/LSCs

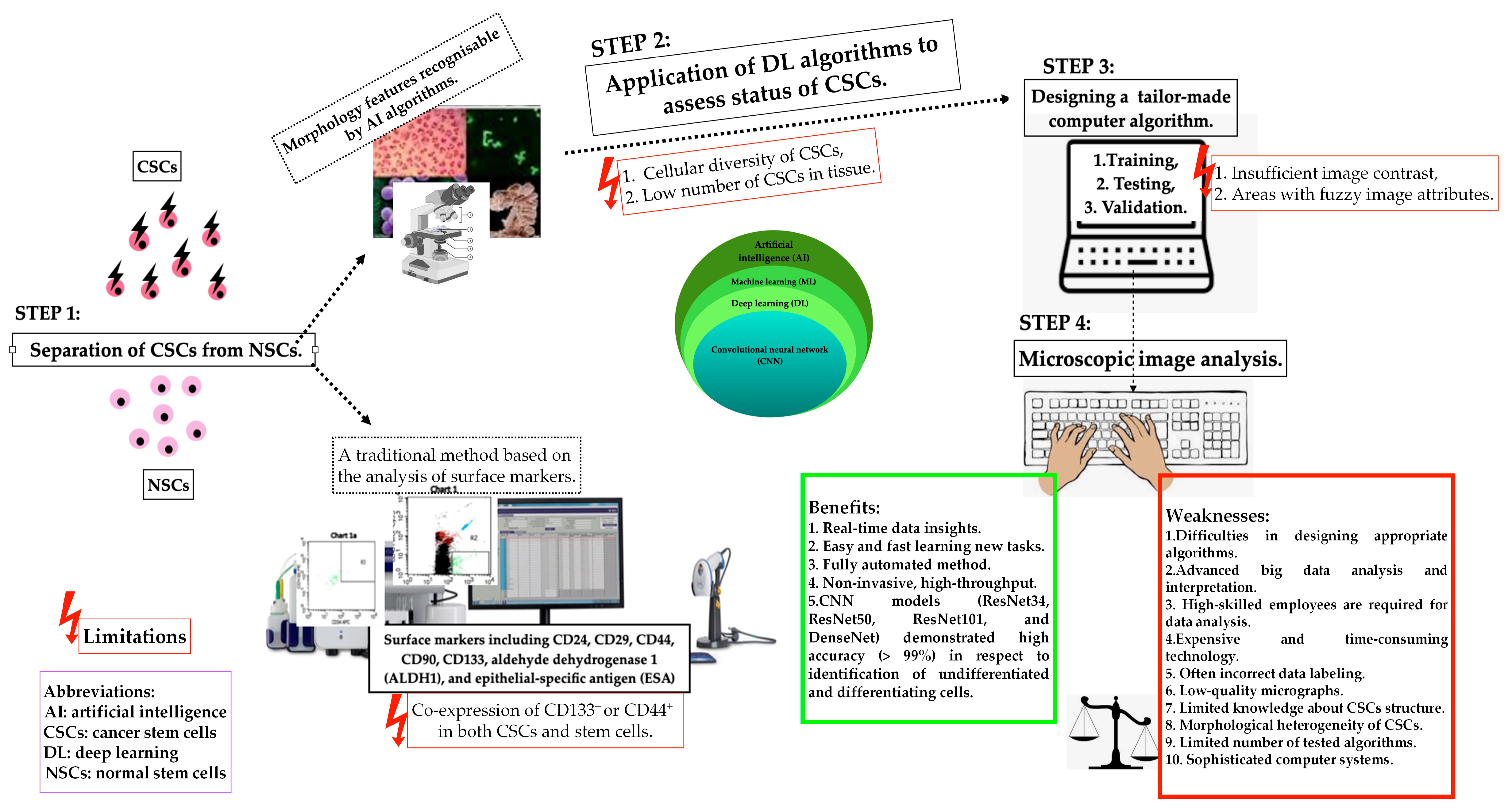
2.5. The Space for Artificial Intelligence in Cancer Stem Cell Detection
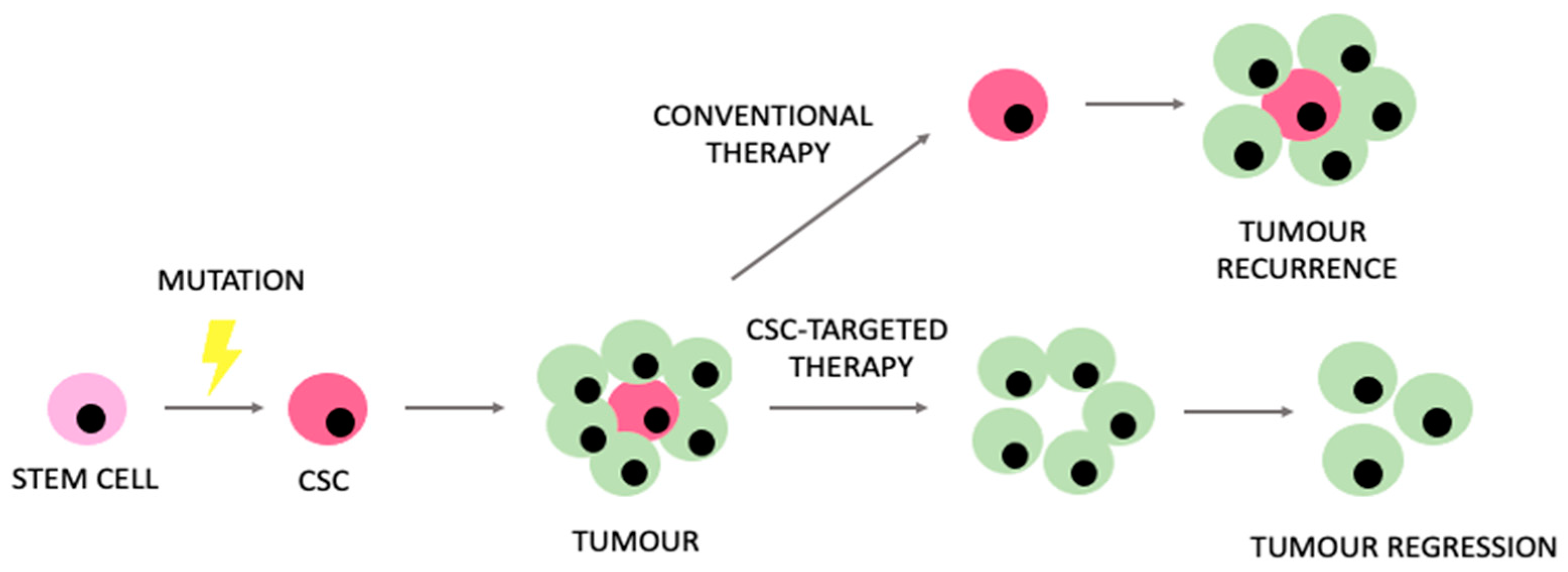
3. Cancer and Leukaemia Stem Cells in Disease Recurrence
4. Perspectives and Modern Therapeutic Strategies Targeting CSCs in Solid Tumours
5. Agents That Target Leukaemia Stem Cells in Haematological Malignancies
5.1. Agents Targeting Wnt and Hedgehog Pathways
5.2. Agents Targeting NF-κB and Notch Pathways
5.3. Polyphenols
5.4. Other Natural Compounds and Derivatives
6. Top 10 Reasons Why This Manuscript Is Important in the Oncology Field
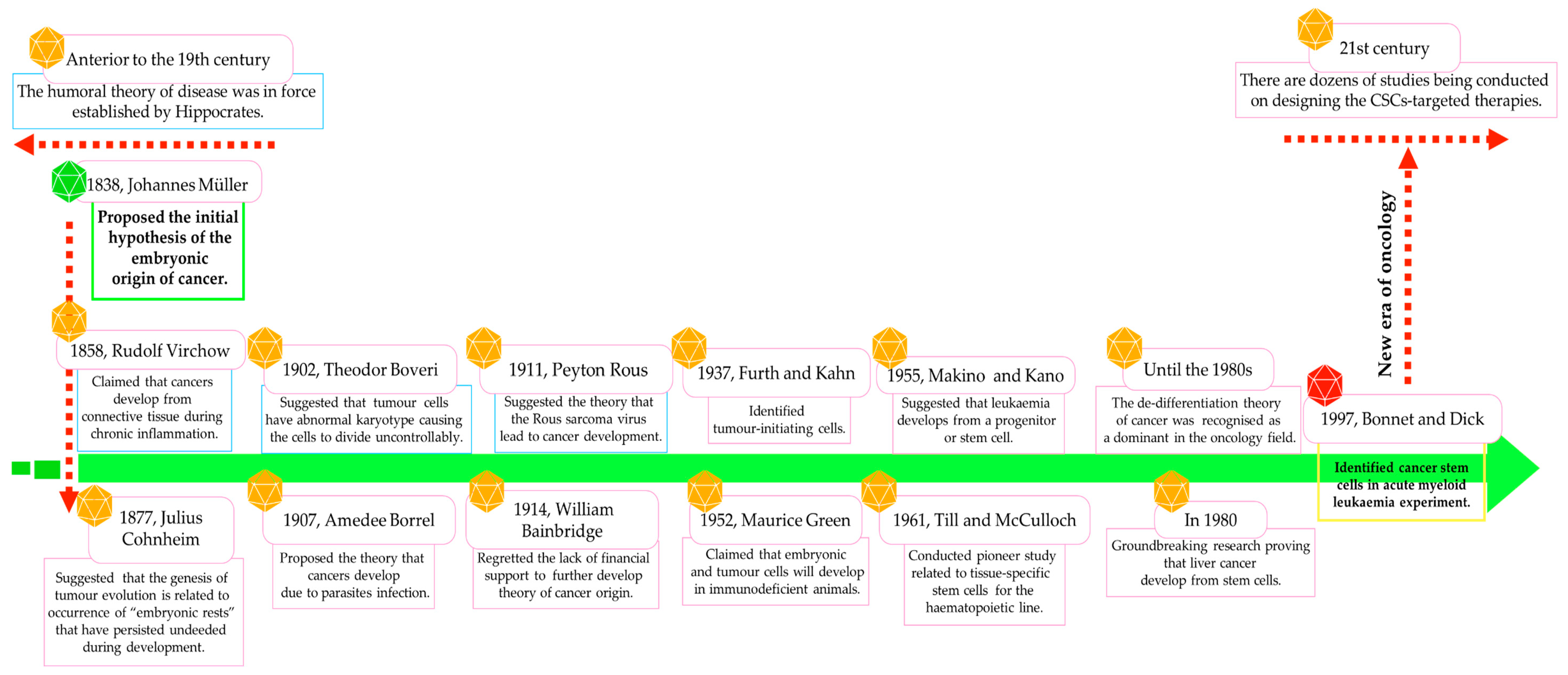
- Provides a historical graphical overview of research on the nature of CSCs/LSCs.
- Provides a clear, extensive, tabular presentation of the differences between normal stem cells and CSCs.
- Underlines the role of the tumour microenvironment in maintaining the pro-tumorigenic ability of CSCs/LSCs.
- Provides an organised summary of the knowledge regarding the functional methods of CSC/LSC detection including the application, benefits, and weaknesses of selected methods.
- Specifies the usefulness of the new technologies, including artificial intelligence and deep learning, in CSC examination.
- Provides an overview of the immunophenotypes of CSCs and LSCs in solid tumours and haematological malignancies.
- Discusses key characteristics of early-stage (pre-neoplastic) and late-stage (pro-metastatic) cancer and leukaemia stem cells.
- Indicates the importance of CSCs and LSCs in the recurrence of selected solid and non-solid cancers.
- Provides a concise analysis of the perspectives and modern therapeutic strategies targeting CSCs in solid tumours.
- Provides a broad analysis of candidate drugs for regulating LSCs in haematological malignancies, taking into account particular Wnt, Hedgehog, NF-κB, and Notch signalling pathways.
7. Methodology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC) Website. Available online: https://gco.iarc.fr/ (accessed on 22 January 2024).
- Afify, S.M.; Seno, M. Conversion of Stem Cells to Cancer Stem Cells: Undercurrent of Cancer Initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Trosko, J.E. On the Potential Origin and Characteristics of Cancer Stem Cells. Carcinogenesis 2021, 42, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Fontana, F.; Raimondi, M.; Limonta, P. Cancer Stem Cells-Key Players in Tumor Relapse. Cancers 2021, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Capp, J.P. Cancer Stem Cells: From Historical Roots to a New Perspective. J. Oncol. 2019, 2019, 5189232. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Guzmán, M.E.; Bigoni-Ordóñez, G.D.; Ibáñez Hernández, M.; Ortiz-Sánchez, E. Cancer stem cell impact on clinical oncology. World J. Stem Cells 2018, 10, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.; Afify, S.M.; Nair, N.; Kumon, K.; Osman, A.; Du, J.; Mansour, H.; Abu Quora, H.A.; Nawara, H.M.; Satoh, A.; et al. Hematopoietic Cells Derived from Cancer Stem Cells Generated from Mouse Induced Pluripotent Stem Cells. Cancers 2019, 12, 82. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Chen, Y.S.; Chen, F.R.; Xi, S.Y.; Chen, Z.P. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro Oncol. 2017, 19, 1109–1118. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Li, X.; Park, S. Deep learning models for cancer stem cell detection: A brief review. Front. Immunol. 2023, 14, 1214425. [Google Scholar] [CrossRef]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Masciale, V.; Grisendi, G.; Banchelli, F.; D’Amico, R.; Maiorana, A.; Sighinolfi, P.; Stefani, A.; Morandi, U.; Dominici, M.; Aramini, B. Isolation and Identification of Cancer Stem-Like Cells in Adenocarcinoma and Squamous Cell Carcinoma of the Lung: A Pilot Study. Front. Oncol. 2019, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.A.; Hughes, B.G.M. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl. Lung Cancer Res. 2015, 4, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Masciale, V.; Banchelli, F.; Grisendi, G.; D’Amico, R.; Maiorana, A.; Stefani, A.; Morandi, U.; Stella, F.; Dominici, M.; Aramini, B. The Influence of Cancer Stem Cells on the Risk of Relapse in Adenocarcinoma and Squamous Cell Carcinoma of the Lung: A Prospective Cohort Study. Stem Cells Transl. Med. 2022, 11, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Riedl, J.M.; Moik, F.; Esterl, T.; Kostmann, S.M.; Gerger, A.; Jost, P.J. Molecular diagnostics tailoring personalized cancer therapy-an oncologist’s view. Virchows Arch. 2023, 484, 169–179. [Google Scholar] [CrossRef]
- Sell, S. History of cancer stem cells. In Regulatory Networks in Stem Cells; Rajasekhar, V.K., Vemuri, M.C., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 495–503. [Google Scholar]
- Bonnet, D.; Dick, J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Toh, T.B.; Lim, J.J.; Chow, E.K. Epigenetics in cancer stem cells. Mol. Cancer 2017, 16, 29. [Google Scholar] [CrossRef]
- Osman, A.; Afify, S.M.; Hassan, G.; Fu, X.; Seno, A.; Seno, M. Revisiting Cancer Stem Cells as the Origin of Cancer-Associated Cells in the Tumor Microenvironment: A Hypothetical View from the Potential of iPSCs. Cancers 2020, 12, 879. [Google Scholar] [CrossRef]
- Lapidot, T.; Sirard, C.; Vormoor, J.; Murdoch, B.; Hoang, T.; Caceres-Cortes, J.; Minden, M.; Paterson, B.; Caligiuri, M.A.; Dick, J.E. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994, 367, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Gadre, P.; Nitsure, N.; Mazumdar, D.; Gupta, S.; Ray, K. The rates of stem cell division determine the cell cycle lengths of its lineage. iScience 2021, 24, 103232. [Google Scholar] [CrossRef] [PubMed]
- Moore, N.; Lyle, S. Quiescent, slow-cycling stem cell populations in cancer: A review of the evidence and discussion of significance. J. Oncol. 2011, 2011, 396076. [Google Scholar] [CrossRef] [PubMed]
- Aida, S.; Okugawa, J.; Fujisaka, S.; Kasai, T.; Kameda, H.; Sugiyama, T. Deep Learning of Cancer Stem Cell Morphology Using Conditional Generative Adversarial Networks. Biomolecules 2020, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.N.; Al-Karim, S.; Bora, R.S.; Chaudhary, A.G.; Saini, K.S. Cancer stem cells: A challenging paradigm for designing targeted drug therapies. Drug Discov. Today 2015, 20, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Barreto, I.V.; Pessoa, F.M.C.P.; Machado, C.B.; Pantoja, L.D.C.; Ribeiro, R.M.; Lopes, G.S.; Amaral de Moraes, M.E.; de Moraes Filho, M.O.; de Souza, L.E.B.; Burbano, R.M.R.; et al. Leukemic Stem Cell: A Mini-Review on Clinical Perspectives. Front. Oncol. 2022, 12, 931050. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Nigam, M.; Kunjwal, S.S.; Sergey, P.; Mishra, A.P.; Sharifi-Rad, J. Cancer Stem Cells: From an Insight into the Basics to Recent Advances and Therapeutic Targeting. Stem Cells Int. 2022, 2022, 9653244. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Cannot Target What Cannot Be Seen: Molecular Imaging of Cancer Stem Cells. Int. J. Mol. Sci. 2023, 24, 1524. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Fang, Y.; Lyu, Z.; Zhu, Y.; Yang, L. Exploring the dynamic interplay between cancer stem cells and the tumor microenvironment: Implications for novel therapeutic strategies. J. Transl. Med. 2023, 21, 686. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Marchand, T.; Pinho, S. Leukemic Stem Cells: From Leukemic Niche Biology to Treatment Opportunities. Front. Immunol. 2021, 12, 775128. [Google Scholar] [CrossRef]
- Schepers, K.; Campbell, T.B.; Passegue, E. Normal and leukemic stem cell niches: Insights and therapeutic opportunities. Cell Stem Cell 2015, 16, 254–267. [Google Scholar] [CrossRef]
- Nair, N.; Anna Sanchez Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef]
- Beier, D.; Hau, P.; Proescholdt, M.; Lohmeier, A.; Wischhusen, J.; Oefner, P.J.; Aigner, L.; Brawanski, A.; Bogdahn, U.; Beier, C.P. CD133+ and CD133− glioblastomaderived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007, 67, 4010–4015. [Google Scholar] [CrossRef]
- Matsuda, S.; Yan, T.; Mizutani, A.; Sota, T.; Hiramoto, Y.; Prieto-Vila, M.; Chen, L.; Satoh, A.; Kudoh, T.; Kasai, T.; et al. Cancer stem cells maintain a hierarchy of differentiation by creating their niche. Int. J. Cancer 2014, 135, 27–36. [Google Scholar] [CrossRef]
- Zarychta, E.; Ruszkowska-Ciastek, B. Cooperation between Angiogenesis, Vasculogenesis, Chemotaxis, and Coagulation in Breast CancerMetastases Development: Pathophysiological Point of View. Biomedicines 2022, 10, 300. [Google Scholar] [CrossRef]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef]
- Bai, J.; Chen, W.B.; Zhang, X.Y.; Kang, X.N.; Jin, L.J.; Zhang, H.; Wang, Z.Y. HIF-2α regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J. Stem Cells 2020, 12, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Won, C.; Kim, B.H.; Yi, E.H.; Choi, K.J.; Kim, E.K.; Jeong, J.M.; Lee, J.H.; Jang, J.J.; Yoon, J.H.; Jeong, W.I.; et al. Signal transducer and activator of transcription 3-mediated CD133 up-regulation contributes to promotion of hepatocellular carcinoma. Hepatology 2015, 62, 1160–1173. [Google Scholar] [CrossRef]
- Colmone, A.; Amorim, M.; Pontier, A.L.; Wang, S.; Jablonski, E.; Sipkins, D.A. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008, 322, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Chen, J.L. Understanding of leukemic stem cells and their clinical implications. Mol. Cancer. 2017, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Khaldoyanidi, S.K.; Hindoyan, A.; Stein, A.; Subklewe, M. Leukemic stem cells as a target for eliminating acute myeloid leukemia: Gaps in translational research. Crit. Rev. Oncol. Hematol. 2022, 175, 103710. [Google Scholar] [CrossRef]
- Konopleva, M.Y.; Jordan, C.T. Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J. Clin. Oncol. 2011, 29, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, A.; Blatt, K.; Cerny-Reiterer, S.; Sadovnik, I.; Herrmann, H.; Marian, B.; Grunt, T.W.; Zielinski, C.C.; Valent, P. Cancer stem cells in basic science and in translational oncology: Can we translate into clinical application? J. Hematol. Oncol. 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Powell, K.; Li, L. Breast Cancer Stem Cells: Biomarkers, Identification and Isolation Methods, Regulating Mechanisms, Cellular Origin, and Beyond. Cancers 2020, 12, 3765. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Ansary, J.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M.; et al. The Molecular Basis of Different Approaches for the Study of Cancer Stem Cells and the Advantages and Disadvantages of a Three-Dimensional Culture. Molecules 2021, 26, 2615. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Enciso-Benavides, J.; Alfaro, L.; Castañeda-Altamirano, C.; Rojas, N.; González-Cabeza, J.; Enciso, N.; Riesco, F.; Castillo, M.; Enciso, J. Biological characteristics of a sub-population of cancer stem cells from two triple-negative breast tumour cell lines. Heliyon 2021, 7, e07273. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.-J.; Lee, J.W.; Son, B.H.; Kim, S.-B.; Ahn, J.H.; Noh, W.C.; Gong, G. Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast 2011, 20, 78–85. [Google Scholar] [CrossRef]
- Marcato, P.; Dean, C.A.; Pan, D.; Araslanova, R.; Gillis, M.; Joshi, M.; Helyer, L.; Pan, L.; Leidal, A.; Gujar, S.; et al. Aldehyde dehydrogenase activity of breast cancer stem cells is primarily due to isoform ALDH1A3 and its expression is predictive of metastasis. Stem Cells 2011, 29, 32–45. [Google Scholar] [CrossRef]
- Li, X.; Strietz, J.; Bleilevens, A.; Stickeler, E.; Maurer, J. Chemotherapeutic Stress Influences Epithelial–Mesenchymal Transition and Stemness in Cancer Stem Cells of Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 404. [Google Scholar] [CrossRef]
- Xu, N.; Li, X.; Watanabe, M.; Ueki, H.; Hu, H.; Li, N.; Araki, M.; Wada, K.; Xu, A.; Liu, C.; et al. Induction of cells with prostate cancer stem-like properties from mouse induced pluripotent stem cells via conditioned medium. Am. J. Cancer Res. 2018, 8, 1624–1632. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, Y.; Jin, Z.; Li, X.; Li, B.; Xu, P.; Huang, P.; Liu, C. A Convenient and Effective Strategy for the Enrichment of Tumor-Initiating Cell Properties in Prostate Cancer Cells. Tumour Biol. 2016, 37, 11973–11981. [Google Scholar] [CrossRef] [PubMed]
- Hurt, E.M.; Kawasaki, B.T.; Klarmann, G.J.; Thomas, S.B.; Farrar, W.L. CD44+ CD24− Prostate Cells Are Early Cancer Progenitor/Stem Cells That Provide a Model for Patients with Poor Prognosis. Br. J. Cancer 2008, 98, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Su, Y.; Mei, Y.; Leng, Q.; Leng, B.; Liu, Z.; Stass, S.A.; Jiang, F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab. Investig. 2010, 90, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, E.; Soner, B.C.; Ozdil, B.; Guven, M. CD133+/CD44+ prostate cancer stem cells exhibit embryo-like behavior patterns. Acta Histochem. 2021, 123, 151743. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Zheng, P.S. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget 2013, 4, 2462–2475. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sánchez, E.; Santiago-López, L.; Cruz-Domínguez, V.B.; Toledo-Guzmán, M.E.; Hernández-Cueto, D.; Muñiz-Hernández, S.; Garrido, E.; Cantú De León, D.; García-Carrancá, A. Characterization of cervical cancer stem cell-like cells: Phenotyping, stemness, and human papilloma virus co-receptor expression. Oncotarget 2016, 7, 31943–31954. [Google Scholar] [CrossRef]
- Zamulaeva, I.A.; Selivanova, E.I.; Matchuk, O.N.; Krikunova, L.I.; Mkrtchyan, L.S.; Kulieva, G.Z.; Kaprin, A.D. Quantitative Changes in the Population of Cancer Stem Cells after Radiation Exposure in a Dose of 10 Gy as a Prognostic Marker of Immediate Results of the Treatment of Squamous Cell Cervical Cancer. Bull. Exp. Biol. Med. 2019, 168, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Vishnoi, K.; Mahata, S.; Verma, G.; Srivastava, Y.; Masaldan, S.; Roy, B.G.; Bharti, A.C.; Das, B.C. Cervical Cancer Stem Cells Selectively Overexpress HPV Oncoprotein E6 that Controls Stemness and Self-Renewal through Upregulation of HES1. Clin. Cancer Res. 2016, 22, 4170–4184. [Google Scholar] [CrossRef]
- Li, X.; Ding, J.; Li, N.; Liu, W.; Ding, F.; Zheng, H.; Ning, Y.; Wang, H.; Liu, R.; Ren, S. Synthesis and biological evaluation of celastrol derivatives as anti-ovarian cancer stem cell agents. Eur. J. Med. Chem. 2019, 179, 667–679. [Google Scholar] [CrossRef]
- Curley, M.D.; Therrien, V.A.; Cummings, C.L.; Sergent, P.A.; Koulouris, C.R.; Friel, A.M.; Roberts, D.J.; Seiden, M.V.; Scadden, D.T.; Rueda, B.R.; et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells 2009, 27, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Long, B.; Sullivan, P.; McClellan, S.; Finan, M.A.; Reed, E.; Shevde, L.; Rocconi, R.P. CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin. Exp. Metastasis 2012, 29, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Liu, S.; Roh, M.; Vatan, L.; Szeliga, W.; Wei, S.; Banerjee, M.; Mao, Y.; Kotarski, J.; Wicha, M.S.; et al. Expression of aldehyde dehydrogenase and CD133 defines ovarian cancer stem cells. Int. J. Cancer 2012, 130, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Balch, C.; Chan, M.W.; Lai, H.C.; Matei, D.; Schilder, J.M.; Yan, P.S.; Huang, T.H.; Nephew, K.P. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008, 68, 4311–4320. [Google Scholar] [CrossRef] [PubMed]
- Folkins, C.; Shaked, Y.; Man, S.; Tang, T.; Lee, C.R.; Zhu, Z.; Hoffman, R.M.; Kerbel, R.S. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009, 69, 7243–7251. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Galdieri, L.; Jash, A.; Malkova, O.; Mao, D.D.; DeSouza, P.; Chu, Y.E.; Salter, A.; Campian, J.L.; Naegle, K.M.; Brennan, C.W.; et al. Defining phenotypic and functional heterogeneity of glioblastoma stem cells by mass cytometry. JCI Insight 2021, 6, e128456. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, E.; Tatar, C.; Oktem, G. Triptolide inhibits CD133+/CD44+ colon cancer stem cell growth and migration through triggering apoptosis and represses epithelial-mesenchymal transition via downregulating expressions of snail, slug, and twist. J. Cell Biochem. 2020, 121, 3313–3324. [Google Scholar] [CrossRef]
- Yang, M.H.; Imrali, A.; Heeschen, C. Circulating cancer stem cells: The importance to select. Chin. J. Cancer Res. 2015, 27, 437–449. [Google Scholar] [CrossRef]
- Jing, F.; Kim, H.J.; Kim, C.H.; Kim, Y.J.; Lee, J.H.; Kim, H.R. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int. J. Oncol. 2015, 46, 1582–1588. [Google Scholar] [CrossRef]
- Huang, G.C.; Su, C.Y.; Chang, Y.C.; Chen, Y.J.; Fang, H.W. Establishment of surface marker expression profiles for colorectal cancer stem cells under different conditions. Transl. Cancer Res. 2020, 9, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Tsunekuni, K.; Konno, M.; Haraguchi, N.; Koseki, J.; Asai, A.; Matsuoka, K.; Kobunai, T.; Takechi, T.; Doki, Y.; Mori, M.; et al. CD44/CD133-Positive Colorectal Cancer Stem Cells are Sensitive to Trifluridine Exposure. Sci. Rep. 2019, 9, 14861. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Zeng, Z.; Bai, B.; Zhu, J.; Song, Z. The prognostic value of CSCs biomarker CD133 in NSCLC: A meta-analysis. Oncotarget 2016, 7, 56526–56539. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.P.; Spinola, M.; Dodge, M.; Raso, M.G.; Behrens, C.; Gao, B.; Schuster, K.; Shao, C.; Larsen, J.E.; Sullivan, L.A.; et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010, 70, 9937–9948. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.-C.; Chao, Y.-J.; Hsieh, M.-H.; Tung, H.-L.; Wang, H.-C.; Shan, Y.-S. Low CD8+ T Cell Infiltration and High PD-L1 Expression Are Associated with Level of CD44+/CD133+ Cancer Stem Cells and Predict an Unfavorable Prognosis in Pancreatic Cancer. Cancers 2019, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Yasuchika, K.; Ishii, T.; Katayama, H.; Yoshitoshi, E.Y.; Ogiso, S.; Kita, S.; Yasuda, K.; Fukumitsu, K.; Mizumoto, M.; et al. Keratin 19, a Cancer Stem Cell Marker in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2015, 21, 3081–3091. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Han, T.; Yang, P.; Wang, R.; Li, H.; Zhang, J.; Zhou, X. MicroRNA-28-5p Regulates Liver Cancer Stem Cell Expansion via IGF-1 Pathway. Stem Cells Int. 2019, 2019, 8734362. [Google Scholar] [CrossRef]
- Kahraman, D.C.; Kahraman, T.; Cetin-Atalay, R. Targeting PI3K/Akt/mTOR Pathway Identifies Differential Expression and Functional Role of IL8 in Liver Cancer Stem Cell Enrichment. Mol. Cancer Ther. 2019, 18, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Škerlová, J.; Král, V.; Kachala, M.; Fábry, M.; Bumba, L.; Svergun, D.I.; Tošner, Z.; Veverka, V.; Řezáčová, P. Molecular mechanism for the action of the anti-CD44 monoclonal antibody MEM-85. J. Struct. Biol. 2015, 191, 214–223. [Google Scholar] [CrossRef]
- Chinn, S.B.; Darr, O.A.; Owen, J.H.; Bellile, E.; McHugh, J.B.; Spector, M.E.; Papagerakis, S.M.; Chepeha, D.B.; Bradford, C.R.; Carey, T.E.; et al. Cancer stem cells: Mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck 2015, 37, 317–326. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, C.; Liu, X.; Fang, F.; Liu, S.; Liao, X.; Tao, S.; Mai, H. Characterisation of a subpopulation of CD133+ cancer stem cells from Chinese patients with oral squamous cell carcinoma. Sci. Rep. 2020, 10, 8875. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Gao, W.; Li, F.; Bo, Y.; Zhu, M.; Fu, R.; Liu, Q.; Wen, S.; Wang, B. Identification and characterization of CD133+ CD44+ cancer stem cells from human laryngeal squamous cell carcinoma cell lines. J. Cancer 2017, 8, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Terwijn, M.; Zeijlemaker, W.; Kelder, A.; Rutten, A.P.; Snel, A.N.; Scholten, W.J.; Pabst, T.; Verhoef, G.; Löwenberg, B.; Zweegman, S.; et al. Leukemic stem cell frequency: A strong biomarker for clinical outcome in acute myeloid leukemia. PLoS ONE 2014, 9, e107587. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Liedtke, M.; Gentles, A.J.; Cleary, M.L. CD93 Marks a Non-Quiescent Human Leukemia Stem Cell Population and Is Required for Development of MLL-Rearranged Acute Myeloid Leukemia. Cell Stem Cell 2015, 17, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.L.; Lu, J.; Hollands, C.G.; Alsostovar, L.; Murali, S.; Reid, J.C.; Ye, W.; Vandersluis, S.; Johnson, P.; ElRafie, A.; et al. Leukemic progenitor compartment serves as a prognostic measure of cancer stemness in patients with acute myeloid leukemia. Cell Rep. Med. 2023, 4, 101108. [Google Scholar] [CrossRef] [PubMed]
- Zeijlemaker, W.; Kelder, A.; Wouters, R.; Valk, P.J.M.; Witte, B.I.; Cloos, J.; Ossenkoppele, G.J.; Schuurhuis, G.J. Absence of Leukaemic CD34+ Cells in Acute Myeloid Leukaemia is of High Prognostic Value: A Longstanding Controversy Deciphered. Br. J. Haematol. 2015, 171, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Vergez, F.; Green, A.S.; Tamburini, J.; Sarry, J.E.; Gaillard, B.; Cornillet-Lefebvre, P.; Pannetier, M.; Neyret, A.; Chapuis, N.; Ifrah, N.; et al. High Levels of CD34+CD38low/−CD123+ Blasts are Predictive of an Adverse Outcome in Acute Myeloid Leukemia: A Groupe Ouest-Est Des Leucemies Aigues Et Maladies Du Sang (GOELAMS) Study. Haematologica 2011, 96, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.K.; Noh, E.K.; Ju, L.J.; Sung, J.Y.; Jeong, Y.K.; Cheon, J.; Koh, S.J.; Min, Y.J.; Choi, Y.; Jo, J.C. CD45dimCD34+CD38−CD133+ cells have the potential as leukemic stem cells in acute myeloid leukemia. BMC Cancer 2020, 20, 285. [Google Scholar] [CrossRef]
- Ågerstam, H.; Hansen, N.; von Palffy, S.; Sanden, C.; Reckzeh, K.; Karlsson, C.; Lilljebjörn, H.; Landberg, N.; Askmyr, M.; Högberg, C.; et al. IL1RAP Antibodies Block IL-1-Induced Expansion of Candidate CML Stem Cells and Mediate Cell Killing in Xenograft Models. Blood 2016, 128, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Warfvinge, R.; Geironson, L.; Sommarin, M.N.E.; Lang, S.; Karlsson, C.; Roschupkina, T.; Stenke, L.; Stentoft, J.; Olsson-Strömberg, U.; Hjorth-Hansen, H.; et al. Single-cell molecular analysis defines therapy response and immunophenotype of stem cell subpopulations in CML. Blood 2017, 129, 2384–2394. [Google Scholar] [CrossRef]
- Kinstrie, R.; Horne, G.A.; Morrison, H.; Irvine, D.; Munje, C.; Castañeda, E.G.; Moka, H.A.; Dunn, K.; Cassels, J.E.; Parry, N.; et al. CD93 is expressed on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Leukemia 2020, 34, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Houshmand, M.; Simonetti, G.; Circosta, P.; Gaidano, V.; Cignetti, A.; Martinelli, G.; Saglio, G.; Gale, R.P. Chronic myeloid leukemia stem cells. Leukemia 2019, 33, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.; Sadovnik, I.; Cerny-Reiterer, S.; Rülicke, T.; Stefanzl, G.; Willmann, M.; Hoermann, G.; Bilban, M.; Blatt, K.; Herndlhofer, S.; et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 2014, 123, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.V.; Diamanti, P.; Evely, R.S.; Kearns, P.R.; Blair, A. Expression of CD133 on leukemia-initiating cells in childhood ALL. Blood 2009, 113, 3287–3296. [Google Scholar] [CrossRef]
- Ji, H.; Chen, L.; Dai, Y.; Sun, X.; Li, X.; Wang, Q.; Ma, D.; Du, D.; Zhao, P.; Wang, Y. Aberrant expression of CD133 and CD82 in patients with pediatric acute lymphoblastic leukemia and the clinical significance. Oncol. Lett. 2017, 14, 5811–5818. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Yoshida, S.; Saito, Y.; Doi, T.; Nagatoshi, Y.; Fukata, M.; Saito, N.; Yang, S.M.; Iwamoto, C.; Okamura, J.; et al. CD34+CD38+CD19+ as well as CD34+CD38−CD19+ cells are leukemia-initiating cells with self-renewal capacity in human B-precursor ALL. Leukemia 2008, 22, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Watanabe, T.; Saito, Y.; Kuroki, Y.; Hijikata, A.; Takagi, M.; Tomizawa, D.; Eguchi, M.; Eguchi-Ishimae, M.; Kaneko, A.; et al. Identification of CD34+ and CD34− leukemia-initiating cells in MLL-rearranged human acute lymphoblastic leukemia. Blood 2015, 125, 967–980. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Tao, J.L.; Fu, R.; Wang, H.Q.; Jiang, H.J.; Yue, L.Z.; Zhang, W.; Liu, H.; Shao, Z.H. Increased CD34+CD38−CD123+ cells in myelodysplastic syndrome displaying malignant features similar to those in AML. Int. J. Hematol. 2014, 100, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Tehranchi, R.; Woll, P.S.; Anderson, K.; Buza-Vidas, N.; Mizukami, T.; Mead, A.J.; Astrand-Grundström, I.; Strömbeck, B.; Horvat, A.; Ferry, H.; et al. Persistent malignant stem cells in del(5q) myelodysplasia in remission. N. Engl. J. Med. 2010, 363, 1025–1037. [Google Scholar] [CrossRef]
- Will, B.; Zhou, L.; Vogler, T.O.; Ben-Neriah, S.; Schinke, C.; Tamari, R.; Yu, Y.; Bhagat, T.D.; Bhattacharyya, S.; Barreyro, L.; et al. Stem and progenitor cells in myelodysplastic syndromes show aberrant stage-specific expansion and harbor genetic and epigenetic alterations. Blood 2012, 120, 2076–2086. [Google Scholar] [CrossRef]
- Gao, M.; Bai, H.; Jethava, Y.; Wu, Y.; Zhu, Y.; Yang, Y.; Xia, J.; Cao, H.; Franqui-Machin, R.; Nadiminti, K.; et al. Identification and characterization of tumor-initiating cells in multiple myeloma. J. Natl. Cancer Inst. 2020, 112, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, H.; Chen, P.; Shen, X.; Zhang, B.; Liu, J.; Peng, H.; Xiao, X. Identification and Characterization of Multiple Myeloma Stem Cell-Like Cells. Cancers 2021, 13, 3523. [Google Scholar] [CrossRef] [PubMed]
- Matsui, W.; Wang, Q.; Barber, J.P.; Brennan, S.; Smith, B.D.; Borrello, I.; Mcniece, I.; Lin, L.; Ambinder, R.F.; Peacock, C.; et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008, 68, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Samart, P.; Rojanasakul, Y.; Issaragrisil, S.; Luanpitpong, S. A novel E-cadherin/SOX9 axis regulates cancer stem cells in multiple myeloma by activating Akt and MAPK pathways. Exp. Hematol. Oncol. 2022, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- McCarron, M.J.; Park, P.W.; Fooksman, D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood 2017, 129, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Reghunathan, R.; Bi, C.; Liu, S.C.; Loong, K.T.; Chung, T.H.; Huang, G.; Chng, W.J. Clonogenic multiple myeloma cells have shared stemness signature associated with patient survival. Oncotarget 2013, 4, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Greve, B.; Kelsch, R.; Spaniol, K.; Eich, H.T.; Götte, M. Flow cytometry in cancer stem cell analysis and separation. Cytom. Part A 2012, 81, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Borlak, J. Advances in Liver Cancer Stem Cell Isolation and their Characterization. Stem Cell Rev. Rep. 2021, 17, 1215–1238. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Czarnecka, A.M.; Helbrecht, I.; Bartnik, E.; Lian, F.; Szczylik, C. Current approaches in identification and isolation of human renal cell carcinoma cancer stem cells. Stem Cell Res. Ther. 2015, 6, 178. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Cheaito, K.; Chalhoub, R.M.; Hadadeh, O.; Monzer, A.; Ballout, F.; El-Hajj, A.; Mukherji, D.; Liu, Y.N.; Daoud, G.; et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Front. Oncol. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Chambost, A.J.; Berabez, N.; Cochet-Escartin, O.; Ducray, F.; Gabut, M.; Isaac, C.; Martel, S.; Idbaih, A.; Rousseau, D.; Meyronet, D.; et al. Machine learning-based detection of label-free cancer stem-like cell fate. Sci. Rep. 2022, 12, 19066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Wang, X.; Tang, Z.; Li, S.; Hu, Y.; Zong, X.; Wu, X.; Bu, Z.; Wu, A.; et al. The extent of inflammatory infiltration in primary cancer tissues is associated with lymphomagenesis in immunodeficient mice. Sci. Rep. 2015, 5, 9447. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.A.; Alaa Edeen, M.; Shedid, E.M.; Kamal, A.S.S.; Warda, M.M.; Mamdouh, F.; Khedr, S.A.; Soltan, M.A.; Jeon, H.W.; Zaki, M.S.A.; et al. Targeting Cancer Stem Cells as the Key Driver of Carcinogenesis and Therapeutic Resistance. Int. J. Mol. Sci. 2023, 24, 1786. [Google Scholar] [CrossRef] [PubMed]
- Dobbin, Z.C.; Landen, C.N. Isolation and characterization of potential cancer stem cells from solid human tumors--potential applications. Curr. Protoc. Pharmacol. 2013, 63, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; La Greca, A.; Möbbs, A.M.; Scarafía, M.A.; Santín Velazque, N.L.; Neiman, G.; Moro, L.N.; Luzzani, C.; Sevlever, G.E.; Guberman, A.S.; et al. Deep Learning Neural Networks Highly Predict Very Early Onset of Pluripotent Stem Cell Differentiation. Stem Cell Rep. 2019, 12, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Dillekås, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, Y.; Biekemitoufu, H.; Wang, H.; Li, C.; Zhang, W.; Ma, Y. Expression of Twist, Slug and Snail in esophageal squamous cell carcinoma and their prognostic significance. Oncol. Lett. 2021, 21, 184. [Google Scholar] [CrossRef]
- Khales, S.A.; Mozaffari-Jovin, S.; Geerts, D.; Abbaszadegan, M.R. TWIST1 activates cancer stem cell marker genes to promote epithelial-mesenchymal transition and tumorigenesis in esophageal squamous cell carcinoma. BMC Cancer 2022, 22, 1272. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Putz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al. ABCB5 maintains melanoma-initiating cells through a proinflammatory cytokine signaling circuit. Cancer Res. 2014, 74, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Láinez-González, D.; Serrano-López, J.; Alonso-Dominguez, J.M. Understanding the Notch Signaling Pathway in Acute Myeloid Leukemia Stem Cells: From Hematopoiesis to Neoplasia. Cancers 2022, 14, 1459. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.T.; Zhuan-Sun, Y.X.; Zhuang, Y.Y.; Wei, S.L.; Tang, J.; Chen, W.B.; Zhang, S.N. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int. J. Oncol. 2012, 41, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, P.; Mennillo, F.; De Rosa, A.; Giordano, C.; Rossi, M.; Benedetti, G.; Magrini, R.; Pericot Mohr, G.L.; Miragliotta, V.; Magnoni, L.; et al. In vitro and in vivo characterization of a novel Hedgehog signaling antagonist in human glioblastoma cell lines. Int. J. Cancer. 2012, 131, E33–E44. [Google Scholar] [CrossRef] [PubMed]
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic stem cells: Sources, niches, and vital pathways. Cell Stem Cell. 2014, 14, 306–321. [Google Scholar] [CrossRef]
- Ng, S.W.K.; Mitchell, A.; Kennedy, J.A.; Chen, W.C.; McLeod, J.; Ibrahimova, N.; Arruda, A.; Popescu, A.; Gupta, V.; Schimmer, A.D.; et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 2016, 540, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Jeremias, I. A rare subgroup of leukemia stem cells harbors relapse-inducing potential in acute lymphoblastic leukemia. Exp. Hematol. 2019, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Cruz, Y.; Celis, A.; Ceballos-Cancino, G.; Vazquez-Santillan, K.; Espinosa, M.; Zampedri, C.; Bahena, I.; Ruiz, V.; Maldonado, V.; Melendez-Zajgla, J. Basal-Type Breast Cancer Stem Cells Over-Express Chromosomal Passenger Complex Proteins. Cells 2020, 9, 709. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Y.; Hao, J.; Guenter, R.; Lathia, J.; Beck, A.W.; Hattaway, R.; Hurst, D.; Wang, Q.J.; Liu, Y.; et al. Development of an arteriolar niche and self-renewal of breast cancer stem cells by lysophosphatidic acid/protein kinase D signaling. Commun. Biol. 2021, 4, 780. [Google Scholar] [CrossRef]
- Tran, T.T.; Lee, K. JAG1 Intracellular Domain Enhances AR Expression and Signaling and Promotes Stem-like Properties in Prostate Cancer Cells. Cancers 2022, 14, 5714. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-C.; Chuang, I.-C.; Yang, Y.-C.; Chuang, P.-C.; Lin, H.; Ou, Y.-C.; Chang Chien, C.-C.; Huang, H.-S.; Kang, H.-Y. Low P16INK4A Expression Associated with High Expression of Cancer Stem Cell Markers Predicts Poor Prognosis in Cervical Cancer after Radiotherapy. Int. J. Mol. Sci. 2018, 19, 2541. [Google Scholar] [CrossRef] [PubMed]
- Iżycka, N.; Zaborowski, M.P.; Ciecierski, Ł.; Jaz, K.; Szubert, S.; Miedziarek, C.; Rezler, M.; Piątek-Bajan, K.; Synakiewicz, A.; Jankowska, A.; et al. Cancer Stem Cell Markers—Clinical Relevance and Prognostic Value in High-Grade Serous Ovarian Cancer (HGSOC) Based on The Cancer Genome Atlas Analysis. Int. J. Mol. Sci. 2023, 24, 12746. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoia, A.; Jemma, A.; Redaelli, S.; Silva, M.; Bentivegna, A.; Lavitrano, M.; Conconi, D. AhRR and PPP1R3C: Potential Prognostic Biomarkers for Serous Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 11455. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.M.; Zgheib, N.B.; Bush, S., 2nd; de Eulis, T.; Cortese, A.; Mollo, A.; Lirette, S.T.; Denning, K.; Valluri, J.; Claudio, P.P. Clinical relevance of cancer stem cell chemotherapeutic assay for recurrent ovarian cancer. Transl. Oncol. 2020, 13, 100860. [Google Scholar] [CrossRef] [PubMed]
- Vora, P.; Venugopal, C.; Salim, S.K.; Tatari, N.; Bakhshinyan, D.; Singh, M.; Seyfrid, M.; Upreti, D.; Rentas, S.; Wong, N.; et al. The Rational Development of CD133-Targeting Immunotherapies for Glioblastoma. Cell Stem Cell 2020, 26, 832–844.e6. [Google Scholar] [CrossRef] [PubMed]
- Hattermann, K.; Flüh, C.; Engel, D.; Mehdorn, H.M.; Synowitz, M.; Mentlein, R.; Held-Feindt, J. Stem cell markers in glioma progression and recurrence. Int. J. Once. 2016, 49, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.J.; McCoy, M.J.; Lee-Pullen, T.F.; Anyaegbu, C.C.; Hemmings, C.; Bulsara, M.K.; Platell, C.F. The Prognostic and Predictive Value of SOX2+ Cell Densities in Patients Treated for Colorectal Cancer. Cancers 2020, 12, 1110. [Google Scholar] [CrossRef]
- Obermayr, E.; Koppensteiner, N.; Heinzl, N.; Schuster, E.; Holzer, B.; Fabikan, H.; Weinlinger, C.; Illini, O.; Hochmair, M.; Zeillinger, R. Cancer Stem Cell-Like Circulating Tumor Cells Are Prognostic in Non-Small Cell Lung Cancer. J. Pers. Med. 2021, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Kou, H.-W.; Hsu, C.-P.; Lo, C.-H.; Hwang, T.-L. Identification and Clinical Significance of Pancreatic Cancer Stem Cells and Their Chemotherapeutic Drug Resistance. Int. J. Mol. Sci. 2023, 24, 7331. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Ghoshal, K.; Fernandez, S.; Li, L. Identification of a Subtype of Hepatocellular Carcinoma with Poor Prognosis Based on Expression of Genes within the Glucose Metabolic Pathway. Cancers 2019, 11, 2023. [Google Scholar] [CrossRef]
- Motegi, A.; Fujii, S.; Zenda, S.; Arahira, S.; Tahara, M.; Hayashi, R.; Akimoto, T. Impact of Expression of CD44, a Cancer Stem Cell Marker, on the Treatment Outcomes of Intensity Modulated Radiation Therapy in Patients With Oropharyngeal Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Sharaf, K.; Schirmer, M.; Leu, M.; Küffer, S.; Bertlich, M.; Ihler, F.; Haubner, F.; Canis, M.; Kitz, J. Role of cancer stem cell markers ALDH1, BCL11B, BMI-1, and CD44 in the prognosis of advanced HNSCC. Strahlenther. Onkol. 2021, 197, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Raju, K.L.; Augustine, D.; Patil, S. Prognostic Significance of ALDH1, Bmi1, and OCT4 Expression in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Cancer Control. 2020, 27, 1073274820904959. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; LaMere, M.; Stevens, B.M.; Ashton, J.M.; Myers, J.R.; O’Dwyer, K.M.; Liesveld, J.L.; Mendler, J.H.; Guzman, M.; Morrissette, J.D.; et al. Evolution of acute myelogenous leukemia stem cell properties after treatment and progression. Blood 2016, 128, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; McDonald, T.; Lin, A.; Chakraborty, S.; Huang, Q.; Snyder, D.S.; Bhatia, R. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood 2011, 118, 5565–5572. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, S.; Özdemir, E.Z.; Ziegenhain, C.; Tiedt, S.; Castro Alves, C.; Grunert, M.; Dworzak, M.; Lutz, C.; Turati, V.A.; Enver, T.; et al. Characterization of Rare, Dormant, and Therapy-Resistant Cells in Acute Lymphoblastic Leukemia. Cancer Cell 2016, 30, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Veerabathiran, R.; Pandian, A.; Sivamani, G. Targeting liver cancer stem cell through EpCAM therapy targeted with chemotherapy endorse enhanced progression in hepatocellular carcinoma. Egypt. Liver J. 2023, 13, 29. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, P.; Huang, J.; Qi, X.; Liang, Y.; Zhao, W.; Wang, H.; Lyu, J.; Zhu, H. Metabolomics, Transcriptome and Single-Cell RNA Sequencing Analysis of the Metabolic Heterogeneity between Oral Cancer Stem Cells and Differentiated Cancer Cells. Cancers 2024, 16, 237. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Chiu, L.-Y.; Tseng, J.-S.; Hsu, K.-H.; Chen, C.-H.; Sheu, G.-T.; Yang, T.-Y. Attenuation of PI3K-Akt-mTOR Pathway to Reduce Cancer Stemness on Chemoresistant Lung Cancer Cells by Shikonin and Synergy with BEZ235 Inhibitor. Int. J. Mol. Sci. 2024, 25, 616. [Google Scholar] [CrossRef]
- Santos, L.S.; Silva, V.R.; de Castro, M.V.L.; Dias, R.B.; Valverde, L.F.; Rocha, C.A.G.; Soares, M.B.P.; Quadros, C.A.; Dos Santos, E.R.; Oliveira, R.M.M.; et al. New ruthenium-xanthoxylin complex eliminates colorectal cancer stem cells by targeting the heat shock protein 90 chaperone. Cell Death Dis. 2023, 14, 832. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Santos, L.S.; de Castro, M.V.L.; Dias, R.B.; Valverde, L.F.; Rocha, C.A.G.; Soares, M.B.P.; Quadros, C.A.; Correa, R.S.; Batista, A.A.; et al. A novel ruthenium complex with 5-fluorouracil suppresses colorectal cancer stem cells by inhibiting Akt/mTOR signaling. Cell Death Discov. 2023, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Yang, C.; Chen, F.; Xiang, R.S.; Zhang, H.; Dai, S.Y.; Liu, J.; Lv, X.X.; Zhang, C.; Liu, X.T.; et al. ID1 expressing macrophages support cancer cell stemness and limit CD8+ T cell infiltration in colorectal cancer. Nat. Commun. 2023, 14, 7661. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, V.A.; Gharibi, S.; Hosseini Rizi, M.; Nekookar, A.; Mirhendi, H.; Rahimmalek, M.; Szumny, A. Satureja bachtiarica Induces Cancer Cell Death in Breast and Glioblastoma Cancer in 2D/3D Models and Suppresses Breast Cancer Stem Cells. Cells 2023, 12, 2713. [Google Scholar] [CrossRef] [PubMed]
- Gil-Gas, C.; Sánchez-Díez, M.; Honrubia-Gómez, P.; Sánchez-Sánchez, J.L.; Alvarez-Simón, C.B.; Sabater, S.; Sánchez-Sánchez, F.; Ramírez-Castillejo, C. Self-Renewal Inhibition in Breast Cancer Stem Cells: Moonlight Role of PEDF in Breast Cancer. Cancers 2023, 15, 5422. [Google Scholar] [CrossRef] [PubMed]
- Mouti, M.A.; Deng, S.; Pook, M.; Malzahn, J.; Rendek, A.; Militi, S.; Nibhani, R.; Soonawalla, Z.; Oppermann, U.; Hwang, C.I.; et al. KMT2A associates with PHF5A-PHF14-HMG20A-RAI1 subcomplex in pancreatic cancer stem cells and epigenetically regulates their characteristics. Nat. Commun. 2023, 14, 5685. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, J.; Wang, N.; Ghozlan, M.; Lebrun, J.-J. Transforming Growth Factor-β/Smad Signaling Inhibits Melanoma Cancer Stem Cell Self-Renewal, Tumor Formation and Metastasis. Cancers 2024, 16, 224. [Google Scholar] [CrossRef]
- Zeng, Z.; Fu, M.; Hu, Y.; Wei, Y.; Wei, X.; Luo, M. Regulation and signaling pathways in cancer stem cells: Implications for targeted therapy for cancer. Mol. Cancer. 2023, 22, 172. [Google Scholar] [CrossRef] [PubMed]
- Walcher, L.; Kistenmacher, A.K.; Suo, H.; Kitte, R.; Dluczek, S.; Strauß, A.; Blaudszun, A.R.; Yevsa, T.; Fricke, S.; Kossatz-Boehlert, U. Cancer Stem Cells-Origins and Biomarkers: Perspectives for Targeted Personalized Therapies. Front. Immunol. 2020, 11, 1280. [Google Scholar] [CrossRef]
- Pal, D.; Raj, K.; Nandi, S.S.; Sinha, S.; Mishra, A.; Mondal, A.; Lagoa, R.; Burcher, J.T.; Bishayee, A. Potential of Synthetic and Natural Compounds as Novel Histone Deacetylase Inhibitors for the Treatment of Hematological Malignancies. Cancers 2023, 15, 2808. [Google Scholar] [CrossRef] [PubMed]
- XPO1 Inhibitor Approved for Multiple Myeloma. Cancer Discov. 2019, 9, 1150–1151. [CrossRef] [PubMed]
- Chu, M.; Zheng, C.; Chen, C.; Song, G.; Hu, X.; Wang, Z.W. Targeting cancer stem cells by nutraceuticals for cancer therapy. Semin. Cancer Biol. 2022, 85, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Shafiee, S.; Burcher, J.T.; Lagoa, R.; Farzaei, M.H.; Bishayee, A. Anticancer Potential of Apigenin and Isovitexin with Focus on Oncogenic Metabolism in Cancer Stem Cells. Metabolites 2023, 13, 404. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Akhtar Siddiqui, J.; Anthony Sinha, R.; Raza, S. Targeting autophagy and lipid metabolism in cancer stem cells. Biochem. Pharmacol. 2023, 212, 115550. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Mohd Yusoff, N.; Zakaria, Z.; Widera, D.; Yahaya, B.H. Inhibition of NF-κB Signaling Reduces the Stemness Characteristics of Lung Cancer Stem Cells. Front. Oncol. 2018, 8, 166. [Google Scholar] [CrossRef]
- Stuart, S.A.; Minami, Y.; Wang, J.Y. The CML stem cell: Evolution of the progenitor. Cell Cycle 2009, 8, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Grainger, S.; Traver, D.; Willert, K. Wnt Signaling in Hematological Malignancies. Prog. Mol. Biol. Transl. Sci. 2018, 153, 321–341. [Google Scholar] [CrossRef]
- Zhou, H.; Mak, P.Y.; Mu, H.; Mak, D.H.; Zeng, Z.; Cortes, J.; Liu, Q.; Andreeff, M.; Carter, B.Z. Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo. Leukemia 2017, 31, 2065–2074. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, K.; Wu, Y.; Melendez, E.; Smbatyan, G.; Massiello, D.; Kahn, M. Characterization of Imatinib Resistant CML Leukemic Stem/Initiating Cells and Their Sensitivity to CBP/Catenin Antagonists. Curr. Mol. Pharmacol. 2018, 11, 113–121. [Google Scholar] [CrossRef]
- Zeng, X.; Zhao, H.; Li, Y.; Fan, J.; Sun, Y.; Wang, S.; Wang, Z.; Song, P.; Ju, D. Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy 2015, 11, 355–372. [Google Scholar] [CrossRef]
- Sadarangani, A.; Pineda, G.; Lennon, K.M.; Chun, H.J.; Shih, A.; Schairer, A.E.; Court, A.C.; Goff, D.J.; Prashad, S.L.; Geron, I.; et al. GLI2 inhibition abrogates human leukemia stem cell dormancy. J. Transl. Med. 2015, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-κB Pathway and Cancer Stem Cells. Cells 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Marques-da-Silva, D.; Diniz, M.; Daglia, M.; Bishayee, A. Molecular mechanisms linking environmental toxicants to cancer development: Significance for protective interventions with polyphenols. Semin. Cancer Biol. 2022, 80, 118–144. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.M.; Fan, M.; Yang, C.H.; Du, Z.; Sims, M.; Davidoff, A.M.; Pfeffer, L.M. Constitutive activation of signal transducer and activator of transcription 3 (STAT3) and nuclear factor κB signaling in glioblastoma cancer stem cells regulates the Notch pathway. J. Biol. Chem. 2013, 288, 26167–26176. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Khan, H.Y.; Muqbil, I.; Aboukameel, A.; Neggers, J.E.; Daelemans, D.; Mahipal, A.; Dyson, G.; Kamgar, M.; Al-Hallak, M.N.; et al. Preclinical Assessment with Clinical Validation of Selinexor with Gemcitabine and Nab-Paclitaxel for the Treatment of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Wanga, H.; Yanga, J.; Wanga, L. The potential of XPO1 inhibitors as a game changer in relapsed/refractory hematologic malignancies. Aging Pathobiol. Ther. 2020, 2, 109–113. [Google Scholar] [CrossRef]
- Hossain, F.; Sorrentino, C.; Ucar, D.A.; Peng, Y.; Matossian, M.; Wyczechowska, D.; Crabtree, J.; Zabaleta, J.; Morello, S.; Del Valle, L.; et al. Notch Signaling Regulates Mitochondrial Metabolism and NF-κB Activity in Triple-Negative Breast Cancer Cells via IKKα-Dependent Non-canonical Pathways. Front. Oncol. 2018, 8, 575. [Google Scholar] [CrossRef]
- Hsu, E.C.; Kulp, S.K.; Huang, H.L.; Tu, H.J.; Salunke, S.B.; Sullivan, N.J.; Sun, D.; Wicha, M.S.; Shapiro, C.L.; Chen, C.S. Function of Integrin-Linked Kinase in Modulating the Stemness of IL-6-Abundant Breast Cancer Cells by Regulating γ-Secretase-Mediated Notch1 Activation in Caveolae. Neoplasia 2015, 17, 497–508. [Google Scholar] [CrossRef]
- Schott, A.F.; Landis, M.D.; Dontu, G.; Griffith, K.A.; Layman, R.M.; Krop, I.; Paskett, L.A.; Wong, H.; Dobrolecki, L.E.; Lewis, M.T.; et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 2013, 19, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shimizu, F.; Hovinga, K.; Beal, K.; Karimi, S.; Droms, L.; Peck, K.K.; Gutin, P.; Iorgulescu, J.B.; Kaley, T.; et al. Molecular and Clinical Effects of Notch Inhibition in Glioma Patients: A Phase 0/I Trial. Clin. Cancer Res. 2016, 22, 4786–4796. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.; Marques-Da-Silva, D.; Lagoa, R. Towards the Development of Delivery Systems of Bioactive Compounds with Eyes Set on Pharmacokinetics. In Modeling and Control of Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–144. [Google Scholar]
- Lyu, C.; Stadlbauer, B.; Wang, L.; Buchner, A.; Pohla, H. Identification of a novel combination treatment strategy in clear cell renal cell carcinoma stem cells with shikonin and ipilimumab. Front. Immunol. 2023, 14, 1186388. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.; Sahoo, S.K.; Patra, P.; Mallik, S.; Zhao, Z. In silico ranking of phenolics for therapeutic effectiveness on cancer stem cells. BMC Bioinform. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wen, X.; Li, M.; Li, S.; Zhao, H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. Biofactors 2018, 44, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Ghaffari, S.H.; Shaiegan, M.; Nikogoftar Zarif, M.; Nikbakht, M.; Alimoghaddam, K.; Ghavamzadeh, A. Curcumin Veto the Effects of Osteopontin (OPN) Specific Inhibitor on Leukemic Stem Cell Colony Forming Potential via Promotion of OPN Overexpression. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 120–129. [Google Scholar] [PubMed]
- Li, Y.; Domina, A.; Lim, G.; Chang, T.; Zhang, T. Evaluation of curcumin, a natural product in turmeric, on Burkitt lymphoma and acute myeloid leukemia cancer stem cell markers. Future Oncol. 2018, 14, 2353–2360. [Google Scholar] [CrossRef]
- Panyajai, P.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S. Dietary Turmeric Bisdemethoxycurcumin Suppresses Wilms’ Tumor 1 and CD34 Protein Expressions in KG-1a Leukemic Stem Cells. Nutr. Cancer. 2019, 71, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Nirachonkul, W.; Ogonoki, S.; Thumvijit, T.; Chiampanichayakul, S.; Panyajai, P.; Anuchapreeda, S.; Tima, S.; Chiampanichayakul, S. CD123-Targeted Nano-Curcumin Molecule Enhances Cytotoxic Efficacy in Leukemic Stem Cells. Nanomaterials 2021, 11, 2974. [Google Scholar] [CrossRef]
- Kim, D.H.; Khan, H.; Ullah, H.; Hassan, S.T.S.; Šmejkal, K.; Efferth, T.; Mahomoodally, M.F.; Xu, S.; Habtemariam, S.; Filosa, R.; et al. MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol. Res. 2019, 147, 104346. [Google Scholar] [CrossRef]
- Biechonski, S.; Gourevich, D.; Rall, M.; Aqaqe, N.; Yassin, M.; Zipin-Roitman, A.; Trakhtenbrot, L.; Olender, L.; Raz, Y.; Jaffa, A.J.; et al. Quercetin alters the DNA damage response in human hematopoietic stem and progenitor cells via TopoII- and PI3K-dependent mechanisms synergizing in leukemogenic rearrangements. Int. J. Cancer 2017, 140, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocathechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.R.; Silva, E.; Calloway, E.; Kucuk, O.; Rossi, M.; McLemore, M.L. Genistein protects hematopoietic stem cells against G-CSF-induced DNA damage. Cancer Prev. Res. 2014, 7, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.W.; Min, Y.H.; Eom, J.I.; Kim, S.J.; Jeung, H.K.; Kim, J.S. Inhibition of CK2α and PI3K/Akt synergistically induces apoptosis of CD34+CD38− leukaemia cells while sparing haematopoietic stem cells. Anticancer Res. 2010, 30, 4625–4634. [Google Scholar] [PubMed]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin Inhibits Cancer Stem Cell-Like Phenotypes in Human Glioblastoma Cells via Suppression of c-Met Signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, X.; Cao, X.; Liu, L.; Qiu, Y.; Li, X.; Zhou, L.; Ning, Y.; Ren, K.; Cao, J. Isovitexin Inhibits Stemness and Induces Apoptosis in Hepatocellular Carcinoma SK-Hep-1 Spheroids by Upregulating miR-34a Expression. Anticancer. Agents Med. Chem. 2020, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.E.; Cuendet, M. Withaferin A induces cell death and differentiation in multiple myeloma cancer stem cells. Medchemcomm. 2016, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Sung, J.H.; Chung, N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol. Cell Biochem. 2014, 394, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, G.; Cai, Y.; Liu, R.; Xiong, X.; Gu, B.; He, W.; Liu, B.; Ren, Q.; Wu, J.; et al. A novel Apigenin derivative suppresses renal cell carcinoma via directly inhibiting wild-type and mutant MET. Biochem. Pharmacol. 2021, 190, 114620. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.; Clark, R.F.; Rupasinghe, H.P.V.; Hoskin, D.W.; Coombs, M.R.P. Phloridzin Docosahexaenoate Inhibits Spheroid Formation by Breast Cancer Stem Cells and Exhibits Cytotoxic Effects against Paclitaxel-Resistant Triple Negative Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 14577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Y.; Chen, K.; Shi, P.; Li, Y.; Deng, M.; Jiang, Z.; Wang, X.; Li, P.; Xu, B. The pan-Bcl2 Inhibitor AT101 Activates the Intrinsic Apoptotic Pathway and Causes DNA Damage in Acute Myeloid Leukemia Stem-Like Cells. Target. Oncol. 2017, 12, 677–687. [Google Scholar] [CrossRef] [PubMed]
- James, M.I.; Iwuji, C.; Irving, G.; Karmokar, A.; Higgins, J.A.; Griffin-Teal, N.; Thomas, A.; Greaves, P.; Cai, H.; Patel, S.R.; et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015, 364, 135–141. [Google Scholar] [CrossRef]
- Marques-Da-Silva, D.; Rodrigues, J.R.; Lagoa, R. Anthocyanins, Effects in Mitochondria and Metabolism. In Mitochondrial Physiology and Vegetal Molecules Therapeutic Potential of Natural Compounds on Mitochondrial Health; de Oliveira, M.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 267–300. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
| Cancer | Surface Marker and Cancer-Related Action |
|---|---|
| Breast cancer (BC) | |
| Implantation of only 100 cells with CD44+, CD24−/low, and lineage immunophenotypes led to breast cancer development [47]. | |
| The CD44+ and CD24−/low immunophenotypes were attributed to breast cancer stem cells [48]. | |
| Aggressive triple-negative breast cancer harbours CSCs with the phenotypes CD44+, CD24−/low, and ALDH1high [49]. | |
| Longer survival rate and lack of lymph node involvement were linked to the phenotype of CSCs CD44+ and CD24− [50]. | |
| Detection of ALDH1A3 was related to a pro-metastatic potential [51]. | |
| EpCAM+ and CD49f+ cell phenotypes in breast CSCs (classified as triple-negative) showed a high tumorigenic ability [52]. | |
| Prostate cancer (PC) | |
| Overexpression of CD44+ marker was associated with uncontrolled proliferation and self-renewal properties [53]. | |
| CD133+ and CD44+ cells were considered a subfraction of prostate CSCs [54]. | |
| CD44+ and CD24− cells demonstrated stem-like properties, including high tumorigenic ability [55]. | |
| ALDH1A1high cells showed high clonogenic and tumorigenic properties; this may serve as a prostate CSC-associated indicator [56]. | |
| CD133+ and CD44+ cells were able to form spheroids and showed embryo-like attributes [57]. | |
| Cervical cancer (CC) | |
| CD133+, CD44+, and ALDHhigh cells showed high cell division rate and self-renewal properties [58]. | |
| CD49+, AII+, p63+, CK-17+, and ALDHbright cells were considered a subfraction of putative cervical CSCs [59]. | |
| CD44+ and CD24− cells were considered a subfraction of cervical CSCs [60]. | |
| CD49f+, CD71−, and CD133+ cells were considered a subfraction of cervical CSCs [61]. | |
| Ovarian cancer (OC) | |
| CD133+, CD44+, and ALDHhigh cells showed high cell division rate and self-renewal properties [62]. | |
| CD133+ cells demonstrated a high tumorigenic property [63]. | |
| CD44+ and CD24− cells were considered a subfraction of ovarian CSCs [64]. | |
| CD133+ and ALDHhigh cells were considered a subfraction of ovarian CSCs [65]. | |
| CD44+ and cKIT+ cells were attributed a subfraction of ovarian CSCs [66]. | |
| Brain cancer (BnC) | |
| CD133+ cells demonstrated high cell division rate, self-renewal, and pro-angiogenic properties [67]. | |
| Only a few CD133+ cells were enough to generate a cancer [68]. | |
| A consensus on the CD133 marker has not been fully established, since tumours can also develop from CD133− cells in gliomas [34]. | |
| CD15+, CD44+, CD133+, and α6integrinhigh subpopulations demonstrated the highest ability for clonogenic self-renewal in vitro and increased in vivo tumorigenic capacity [69]. | |
| Colorectal cancer (CRC) | |
| CD133+ and CD44+ cells were able to form spheroids, migrate, and showed the EMT phenomenon [70]. | |
| The co-expression of CD26+, CD44+, CD133+ was associated with the development of new metastatic tumours [71]. | |
| The co-expression of CD44+ and CD133+ was associated with synchronous hepatic metastasis [72]. | |
| Colorectal CSCs were confirmed by single or co-expression of CD44+ and CD133+ surface markers [73]. | |
| CD133+ and CD44+ cells were able to form spheroids and were resistante to anticancer agents [74]. | |
| Lung cancer (LC) | |
| Higher CD133 expression was linked to undifferentiated tumours, lymph node involvement, and drug resistance [75]. | |
| ALDHhigh cells were associated with a higher risk of relapse in locally advanced NSCLC [13]. | |
| ALDHhigh cells were detected in NSCLC patients and cell lines [76]. | |
| Pancreatic cancer (PcC) | |
| The phenotypes CD24a+, EpCAM+, and CD133+ of CSCs were associated with a self-supporting model for integrity and maintenance, which promote malignancy [2]. | |
| Higher expression of CD44+ and CD133+ was associated with a higher risk of relapse and pro-metastatic potential [77]. | |
| Liver cancer (LrC) | |
| Overexpression of glypican-3, alpha fetoprotein, cytokeratin 19, CD44+, CD133+, and CD24+ were established as liver cancer markers [2,78]. | |
| The phenotypes CD24− and EpCAM+ were detected in primary HCC cells as well as primary HCC spheres [79]. | |
| CD133+ and EpCAM+ cells were able to create viable and dense spheres in comparison to their negative counterparts [80]. | |
| Head and neck squamous cell carcinoma (HNSCC) | |
| The CD44+ surface marker was confirmed in head and neck cancer [81]. | |
| The phenotypes CD44+and ALDHhigh of CSCs were linked to pro-metastatic potential. Additionally, size and advanced stage of primary tumours were associated with a higher number of CSCs [82]. | |
| Higher CD133+ expression was linked to higher growth rate, self-renewal ability, and drug resistance [83]. | |
| CD133+ and CD44+ cells showed high motility, colony formation ability, and potent resistance to anticancer treatment [84]. | |
| Acute myeloid leukaemia (AML) | |
| CD34+ and CD38− cells were able to initiate AML in a mouse model [16]. | |
| The phenotypes of CD34+, CD38− cells were considered as leukaemia stem-like cells [85]. | |
| The CD93+ marker was indicated on LSCs and is essential for development of MLL-rearranged (current name of the gene KMT2A) AML [86]. | |
| CD34+ cell population was considered as functional LSCs [87]. | |
| A higher incidence of recurrence was related to detection of CD34+ blasts [88]. | |
| The CD34+, CD38−/low, and CD123+ phenotypes of blasts were associated with worse overall survival [89]. | |
| Cells with the phenotypes CD45dim, CD34+, CD38−, and CD133+ were considered LSCs [90]. | |
| Chronic myeloid leukaemia (CML) | |
| CD25+ and IL-1RAP surface markers are specific for LSCs. Both antigens were associated with the activation of NF-kB and AKT signalling pathways, which enhanced proliferation of CML LSCs [91]. | |
| Cells with phenotypes CD45dim, CD34+, CD38−/low, and CD133+ were established as leukaemia-initiating cells [90]. | |
| Lin−, CD34+, CD38−/low, CD45RA−, cKIT−, and CD26+ cells were considered a subfraction of putative CML LSCs [92]. | |
| Lin−, CD34+, CD38−/low, CD90+, and CD93+ cells were considered a subfraction of chronic-phase CML LSCs [93]. | |
| CD25+ was identified as a CML indicator of LSCs and a suppressor of growth [94]. | |
| CD34+, CD38−/low, and CD26+ cells were considered a subfraction of CML LSCs [95]. | |
| Acute lymphoblastic leukaemia (ALL) | |
| Cells with the phenotypes CD133+, CD19−, and CD38−/low were considered LSCs [96]. | |
| The percentages of CD34+, CD133+ or CD34+, and CD82+ cells in ALL patients were higher than those in healthy volunteers [97]. | |
| Cells with phenotypes CD34+, CD38+, and CD19+, as well as CD34+, CD38−/low, and CD19+ cells, were considered LSCs with self-renewal ability [98]. | |
| In MLL(KMT2A)-AF4 patients, CD34+, CD38+, and CD19+ phenotypes and CD34− and CD19+ cells were able to trigger leukaemia, but in MLL(KMT2A)-AF9 patients, CD34− and CD19+ cells were considered LSCs [99]. | |
| Myelodysplastic syndromes (MDS) | |
| The phenotypes CD34+, CD38−/low, and CD123+ confirmed malignant clonal cells with abnormal differentiation, uncontrolled proliferation, and limited apoptosis [100]. | |
| CD34+, CD38−/low, and CD90+ cells demonstrated 5q deletion upon diagnosis and were selectively resistant to treatment [101]. | |
| Higher expressions of Lin−, CD34+, CD38−/low, CD90+, and CD45R− cells were shown in cases with the monosomy of chromosome 7 (−7) and deletion of the long arm of chromosome 20 (20q−) [102]. | |
| Multiple myeloma (MM) | |
| Positive expression of CD24 was considered a dominant marker of MM stem cells, and CD24+ cells showed self-renewal and drug resistance properties [103]. | |
| ALDHhigh cells had upregulated chromosomal instability genes associated with low drug sensitivity and high tumorigenic rate [104]. | |
| Clonotypic CD138−/low cells exhibited robust stemness characteristics, drug resistance, and anti-apoptotic potential and a higher ability to sustain in G0 and G1 cell cycle phases [105,106,107]. | |
| Cells with the phenotypes ALDHhigh and CD138−/low presented high potential to generate tumours [105,108]. |
| Features | Early-Stage (Pre-Tumorigenic) | Late-Stage (Pro-Metastatic) |
|---|---|---|
| Cell cycle regulation | Quiescent | Active |
| Cell division | Mostly asymmetric | Mostly symmetric |
| Self-renewal capacity | Potent | Potent |
| Mutation/chromosomal status | Normal | Abnormal (genetic instability) |
| Tumorigenic ability | Low | Potent |
| Clonogenic ability | Low | Potent |
| Migration ability | Low | Potent |
| Proangiogenic potential | Low | High |
| Resistance to anticancer treatment | Intrinsic | Both intrinsic and acquired |
| Name of Cancer | Sample | Key Findings |
|---|---|---|
| Breast cancer (BC) | ||
| MDA-MB-468 basal breast cancer cells. | CSCs showed notable changes, such as enrichment in transduction cascades linked to apoptosis, cellular growth, proliferation, and stemness. AURKB, INCENP, and BIRC5, among other coregulated chromosomal passenger proteins, were overexpressed in CSCs. Overexpression of BIRC5 boosted the population of CSCs in vitro and in vivo. This coregulated module was shown to be overexpressed in basal breast tumours and was also linked to relapse-free and overall survival in patients, according to analysis of previously reported cohorts [131]. | |
| Tumour samples from patients with ER+ breast cancer. | Breast CSCs are enriched in the arterial niche for human oestrogen receptor and interact with arterial endothelial cells; this interaction is driven by the lysophosphatidic acid/protein kinase D signalling pathway. This pathway promotes both EC arterial differentiation and self-renewal. Targeting the LPA/PKD-1-CD36 signalling pathway may inhibit tumour progression by disrupting the arterial niche and eradicate CSCs effectively [132]. | |
| Prostate cancer (PC) | ||
| LNCaP (CRL-1740), HEK 293T (CRL-11268), PC-3 (CRL-1435), and DU145 (HTB-81) cells. | Intracellular domain of JAG1 (JICD) enhances the androgen independence of androgen receptor signalling in prostate cancer cells and, by promoting PC stem-like cell characteristics, migration, and invasion of PC cells, also promotes carcinogenesis. JICD plays a role in the development of PC cells into advanced metastatic castration-resistant prostate cancers [133]. | |
| Cervical cancer (CC) | ||
| Cohort of 332 patients. | The five-year overall survival (OS) and disease-free survival (DFS) rates were longer in the P16INK4Ahigh expression group compared to the P16INK4Alow expression group. Five-year OS and DFS rates were shorter in the P16INK4Alow, SOX2high and P16INK4Alow, and ALDH1A1high groups, respectively, than in the P16INK4Ahigh, SOX2low and P16INK4Ahigh, and ALDH1A1low groups. A promising target for patients with cervical cancer is lower P16INK4A expression, which is linked to greater CSC markers and indicates worse future outcomes [134]. | |
| Ovarian cancer (OC) | ||
| Database with 558 ovarian cancer tumour samples. Data retrieval, clinical and pathological features, data pre-processing. | Higher platinum sensitivity was revealed by the mRNA expressions of ALDH1A1 and LGR5. POU5F1 mRNA expression identified tumours resistant to platinum. Longer OS was correlated with the expression of CD44 and EPCAM mRNA, while reduced OS was linked to the levels of THY1 mRNA and protein. The three factors EPCAM, LGR5, and CD44 have a beneficial impact on DFS. The median overall survival in the high-risk group was 9.1 months longer than in the low-risk group in a multivariate model based on CSC marker expression. The expression of ALDH1A1, CD44, EpCAM, LGR5, POU5F1, and THY1 in OC was proposed to predict treatment response and serve as prognostic markers for future outcomes [135]. | |
| Ovarian cancer cell lines Caov3, Ovcar5, and Ovcar8. | The expression of AhRR and PPP1R3C negatively correlates with the OS of patients with OC and progression-free survival. Increased expression of AhRR and PPP1R3C was maintained in some CSC subpopulations, strengthening their potential role in OC [136]. | |
| Cohort of 45 patients affected by third–fifth relapsed ovarian cancer. | Patients with recurrent OC treated with high cell-killing chemotherapy experienced improvements in median progression-free survival (PFS) corresponding to 5.4 months (third recurrence), 3.6 months (fourth recurrence), and 3.9 months (fifth recurrence). Additionally, they showed that patients who did not respond to treatment (CSC drug response test) had a 30 times greater risk of death compared to treatment responders [137]. | |
| Brain cancer (BnC) | ||
| Human glioblastoma (GBM) samples. | Immunoglobulin G (RW03-IgG), dual antigen T cell engager (DATE), and chimeric CD133-specific antigen receptor T cell (CART133) showed activity against patient-derived CD133+ GBM cells. CART133 cells demonstrated superior efficacy in patient-derived GBM xenograft models without causing adverse effects on normal CD133+ haematopoietic stem cells in humanised CD34+ mice [138]. | |
| Human astrocytomas of WHO grade I–IV. | Among astrocytomas, OCT4, MYC, and KLF4 mRNA expression increased with tumour malignancy, while in recurrent gliomas, MYC expression slightly decreased. Moreover, there was a positive correlation between different stem cell markers. Embryonic markers were detected at similar levels in glioma cell lines (long- and short-term cultures). Increased expression of KLF4 (and lower Nanog and OCT4) was observed after exposure to temozolomide [139]. | |
| Colorectal cancer (CRC) | Cohort of 797 patients with stage II and III colorectal cancer. | High SOX2+ cell density was not associated with poor overall survival. Furthermore, a significant improvement in survival was observed in all patients after treatment with 5-fluorouracil (FU) (regardless of SOX2+ cell density). SOX2 can predict response to oxaliplatin but not 5-FU treatment [140]. |
| Lung cancer (LC) | ||
| Cohort of 118 patients with non-small cell lung cancer. | In 53.7% of samples positive at the time of primary diagnosis, and 25.6% in the case of recurrence, the most prevalent transcript was EpCAM. EpCAM and CK19, NANOG, PROM1, TERT, CDH5, FAM83A, and PTHLH were associated with worse OS. Only CSC-specific NANOG and PROM1 were associated with outcomes at initial diagnosis and disease progression [141]. | |
| Cohort of 35 patients with non-small cell lung cancer. | CSC rate had no impact on the likelihood of a recurrence. In a secondary study, patients with locally advanced cancer and a greater prevalence of CSCs had a higher chance of disease recurrence; patients with early-stage disease did not show this association [13]. | |
| Pancreatic cancer (PnC) | ||
| Human pancreatic cancer cell line Capan-1, MIA PaCa-2, PANC-1, and BxPC-3 cells. | No significant differences were found in the effect of different concentrations of gemcitabine on CD44+ or EpCAM+ CSCs of different pancreatic ductal adenocarcinoma (PDAC) cell line cultures (BxPC-3, Capan-1, and PANC-1), nor between CSCs and non-CSCs. The expression of the ABCG2 transport protein was significantly higher in CD44+ and EpCAM+ CSCs of PDAC cell lines. Additionally, CSCs showed low anticancer drug sensitivity. Gemcitabine-resistant PnC cells were associated with epithelial–mesenchymal transition (EMT), a more aggressive and invasive phenotype of many solid tumours. Increased c-Met phosphorylation may also be associated with chemotherapy and EMT resistance and could be a chemotherapeutic target in PnC [142]. | |
| Liver cancer (LrC) | ||
| TCGA (The Cancer Genome Atlas) liver cancer RNA-seq (LIHC) data. | The expression of approximately 30% of genes involved in the glucose metabolism pathway was found dysregulated, with downregulation in hepatocellular carcinoma. Differentially expressed genes are associated with advanced clinical stage and poor prognosis. Furthermore, clustering analysis of differentially expressed genes revealed a subset of patients with a worse prognosis, including reduced OS, disease-specific survival, and recurrence-free survival. This aggressive subtype significantly increased expression of stemness-related genes and downregulated metabolic genes, also increasing immune infiltration, which contribute to poor prognosis [143]. | |
| Head and neck squamous cell carcinoma (HNSCC) | ||
| Cohort of 58 patients. | Progression-free survival was shorter for patients with CD44 positive expression of CSCs [144]. | |
| Cohort of 85 patients with advanced stage HNSCC. | Patients with high CD44 expression showed worse future outcomes, regardless of the survival model application [145]. | |
| Cohort of 40 patients. | High expression of ALDH1 was associated with lymph node involvement and shorter survival rate. This observation confirms the existence of an elevated number of stem-like cells with invasion ability, which are able to promote lymph node metastasis [146]. | |
| Acute myeloid leukaemia (AML) | ||
| Cohort of 121 patients. | Overall survival was shorter for patients with higher enumeration of leukaemia progenitor population [87]. | |
| Cohort of 250 patients. | In CD34+ AML subjects, the percentage of the CD34+ and CD38−/low cells at diagnosis was associated with shorter patient survival [85]. | |
| Bone marrow aspirates were analysed from 87 patients and 27 healthy donors. | In AML patients, a higher percentage of CD45dim, CD34+, CD38−/low, and CD133+ cells (≥40%) was considered an independent prognostic factor for overall survival. Additionally, the immunophenotypes of CD45dim, CD34+, CD38−/low, and CD133+ cells allowed for discrimination between LSCs and normal haematopoietic stem cells, as well as emerging as a promising therapeutic approach in AML [90]. | |
| Bone marrow samples were analysed from 111 AML de novo diagnosed patients. | A high percentage (>1%) of CD34+, CD38−/low, and CD123+ cells was associated with poor disease-free survival, overall survival, and treatment failure, regardless of the patient’s cytogenetic profile [25]. | |
| Bone marrow or peripheral blood samples were analysed from 25 AML patients. | After disease recurrence, 9 to 90 times higher LSC activity was observed, independently of the surface markers applied to specify the LSCs. Recurrence after standard chemotherapy was associated with accumulation of more phenotypically composite LSCs. This observation may explain drug resistance and shorter survival rate in patients who relapse after initial treatment [147]. | |
| Chronic myeloid leukaemia (CML) | ||
| Bone marrow aspirates were analysed from 20 CML patients. | CD34+ and CD38−/low stem cells constitute a dominant reservoir of residual BCR-ABL+ cells in patients in remission on imatinib mesylate therapy. The probability of disease relapse is associated with the number of LSCs among the residual BCR-ABL+ cells, re-initiating the leukaemia ability of residual BCR-ABL+ cells, and diversity of bone marrow niches that control leukaemia cell growth [148]. | |
| Acute lymphoblastic leukaemia (ALL) | ||
| In vivo model (mouse bone marrow) | Established an infrequent, long-term quiescent subfraction called label-retaining cells (LRCs) manifesting the unfavourable phenotype of dormancy, in vivo drug insensitivity, and re-initiating leukaemia ability. LRCs are useful as a substitute for recurrence-promoting cells in cases for developing treatment to limit relapse [149]. | |
| Bone marrow aspirates were analysed from 59 ALL patients. | CD82 and CD133 expression at the time of ALL diagnosis was higher in respect to the controls. The hyperdiploid karyotype was associated with upregulation of CD133 mRNA. CD82 and CD133 overexpression was linked to the development of ALL progression. Also, CD133 and CD82 were suggested a therapeutic strategy in paediatric ALL [97]. | |
| Myelodysplastic syndrome (MDS) | ||
| Eight patients with MDS. | 5q deletions of CD34+, CD38−/low, and CD90+ cells demonstrated at diagnosis were selectively resistant to treatment at the time of complete clinical and cytogenetic remission and based on follow-up, all patients had recurrence during continued lenalidomide treatment with confirmed clinical and cytogenetic progression [101]. | |
| Multiple myeloma (MM) | ||
| Bone marrow aspirates were analysed from 137 MM patients. | Patients with a high preliminary percentage of CD24+ MM cells had more bone lytic lesions and worse progression-free survival and overall survival. Tumorigenic ability of CD24+ cells was confirmed in vivo after injection of only 10 cells from MM cell lines. Furthermore, CD24+ MM cells exhibited higher expression of iPS/ES genes, including NANOG, OCT4, and SOX2 [103]. | |
| E-cadherin-depleted cells in human MM-derived cell lines RPMI 8226 and NCI-H929. | In MM CSCs, loss of E-cadherin led to either G0/G1 or G2/M blockade, depending on the cellular milieu, by regulating its crucial cell cycle mediators in each phase, and also limited the side population phenotype. A new regulatory system of MM CSCs through the E-cadherin/SOX9 axis could contribute to the long-term cell survival and outgrowth associated with recurrent/refractory MM [106]. | |
| Blood and bone marrow were analysed from 16 MM patients. | CD138−/low cells demonstrated insensitivity to four drugs, including the corticosteroid dexamethasone and the thalidomide analogue lenalidomide. CD138−/low cells presented greater drug efflux ability and vital intracellular drug detoxification efficacy [105]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszkowska-Ciastek, B.; Kwiatkowska, K.; Marques-da-Silva, D.; Lagoa, R. Cancer Stem Cells from Definition to Detection and Targeted Drugs. Int. J. Mol. Sci. 2024, 25, 3903. https://doi.org/10.3390/ijms25073903
Ruszkowska-Ciastek B, Kwiatkowska K, Marques-da-Silva D, Lagoa R. Cancer Stem Cells from Definition to Detection and Targeted Drugs. International Journal of Molecular Sciences. 2024; 25(7):3903. https://doi.org/10.3390/ijms25073903
Chicago/Turabian StyleRuszkowska-Ciastek, Barbara, Katarzyna Kwiatkowska, Dorinda Marques-da-Silva, and Ricardo Lagoa. 2024. "Cancer Stem Cells from Definition to Detection and Targeted Drugs" International Journal of Molecular Sciences 25, no. 7: 3903. https://doi.org/10.3390/ijms25073903
APA StyleRuszkowska-Ciastek, B., Kwiatkowska, K., Marques-da-Silva, D., & Lagoa, R. (2024). Cancer Stem Cells from Definition to Detection and Targeted Drugs. International Journal of Molecular Sciences, 25(7), 3903. https://doi.org/10.3390/ijms25073903










