Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. CDS Region Clone of Ovine JAK2 and Nucleotide Sequence Analysis
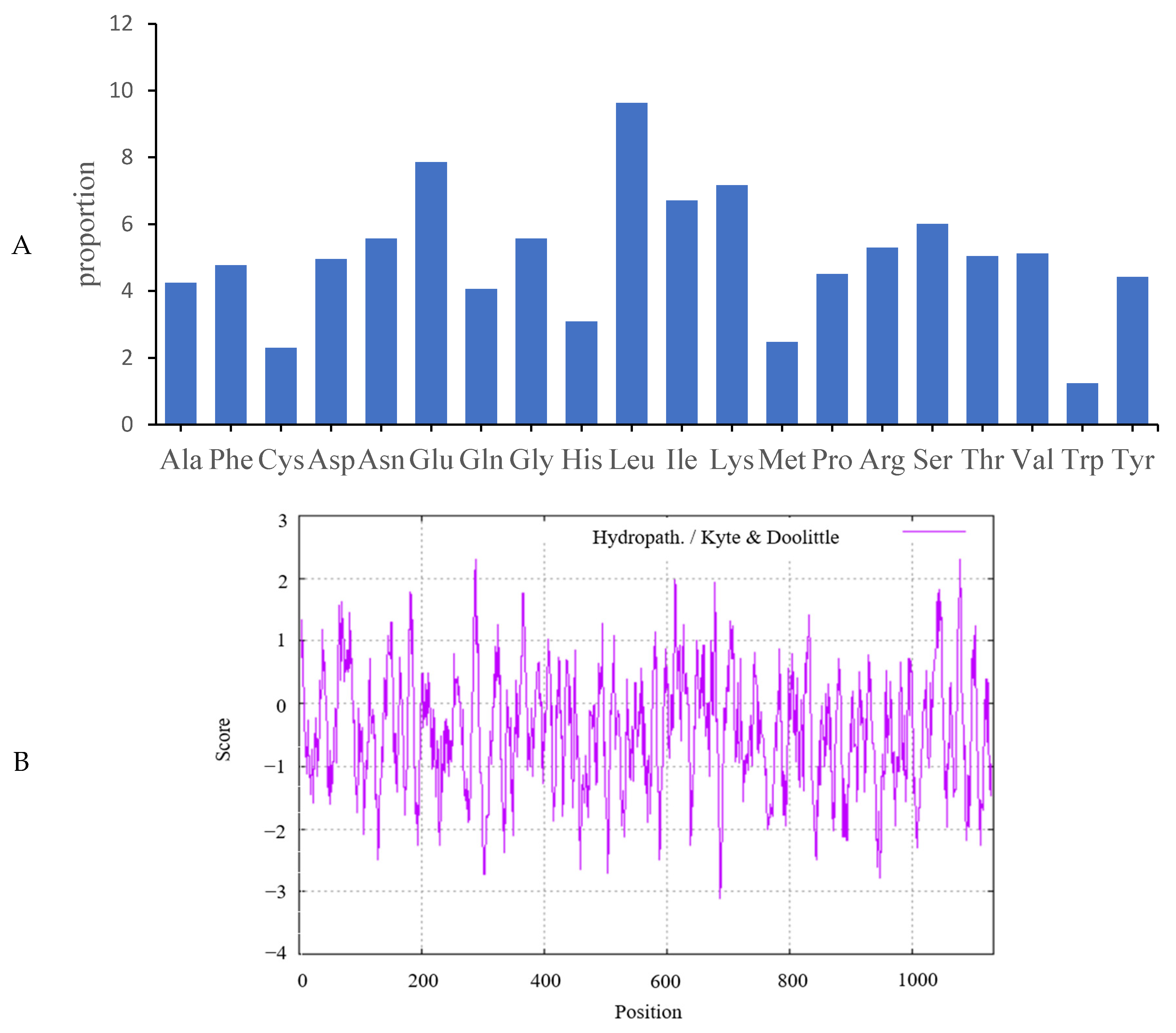
2.2. Analysis of Predicted Ovine JAK2 Protein Characteristics
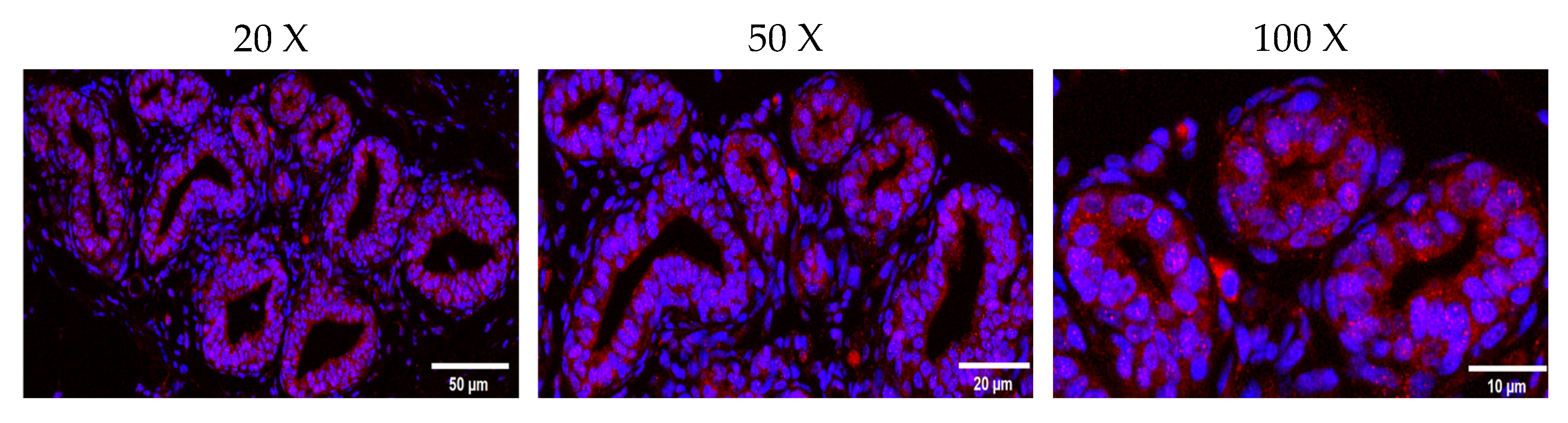
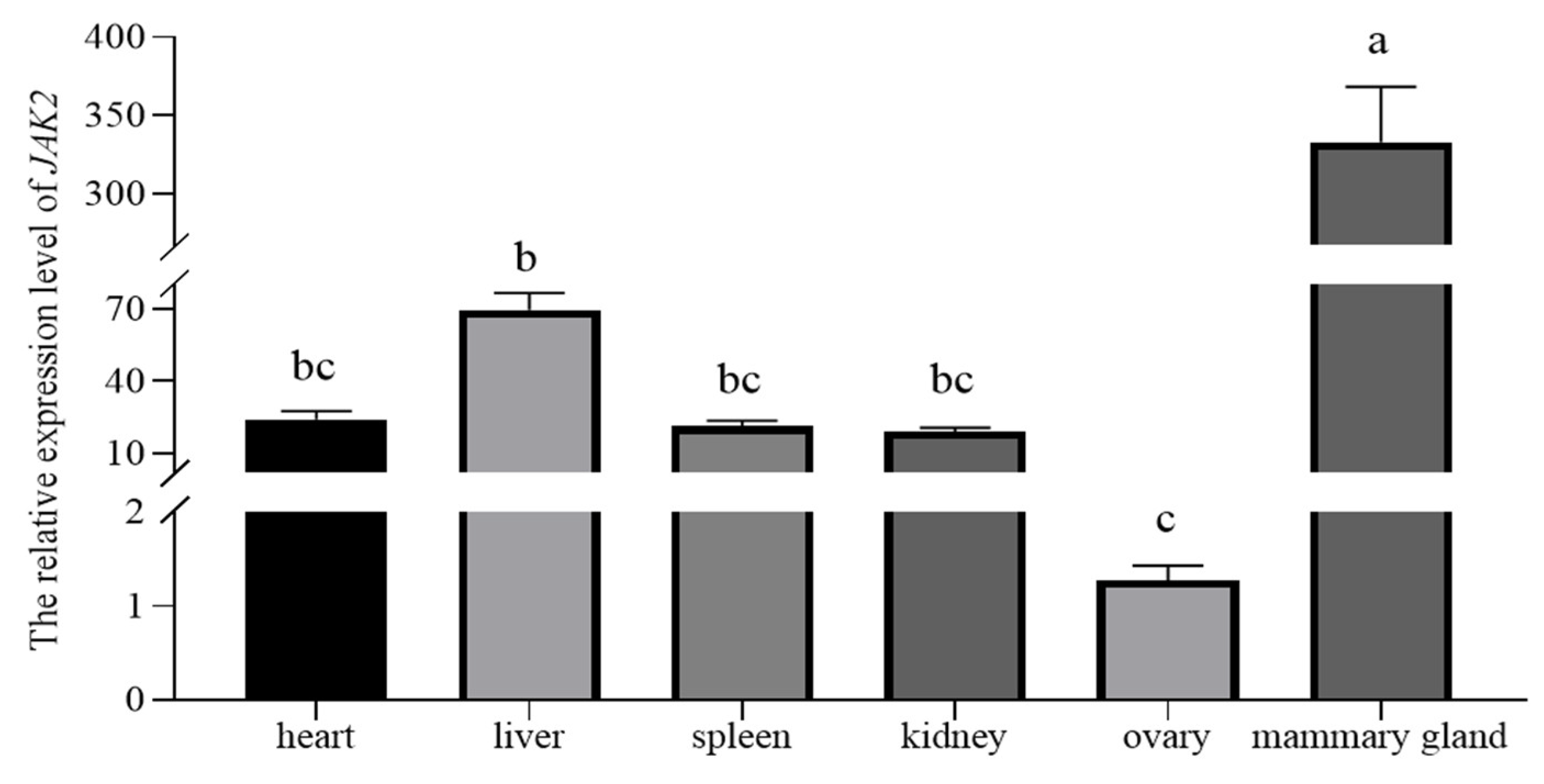
2.3. Localization of JAK2 in the Ovine Mammary Gland and Its Expression in Tissue
2.4. Culture and Identification of OMECs
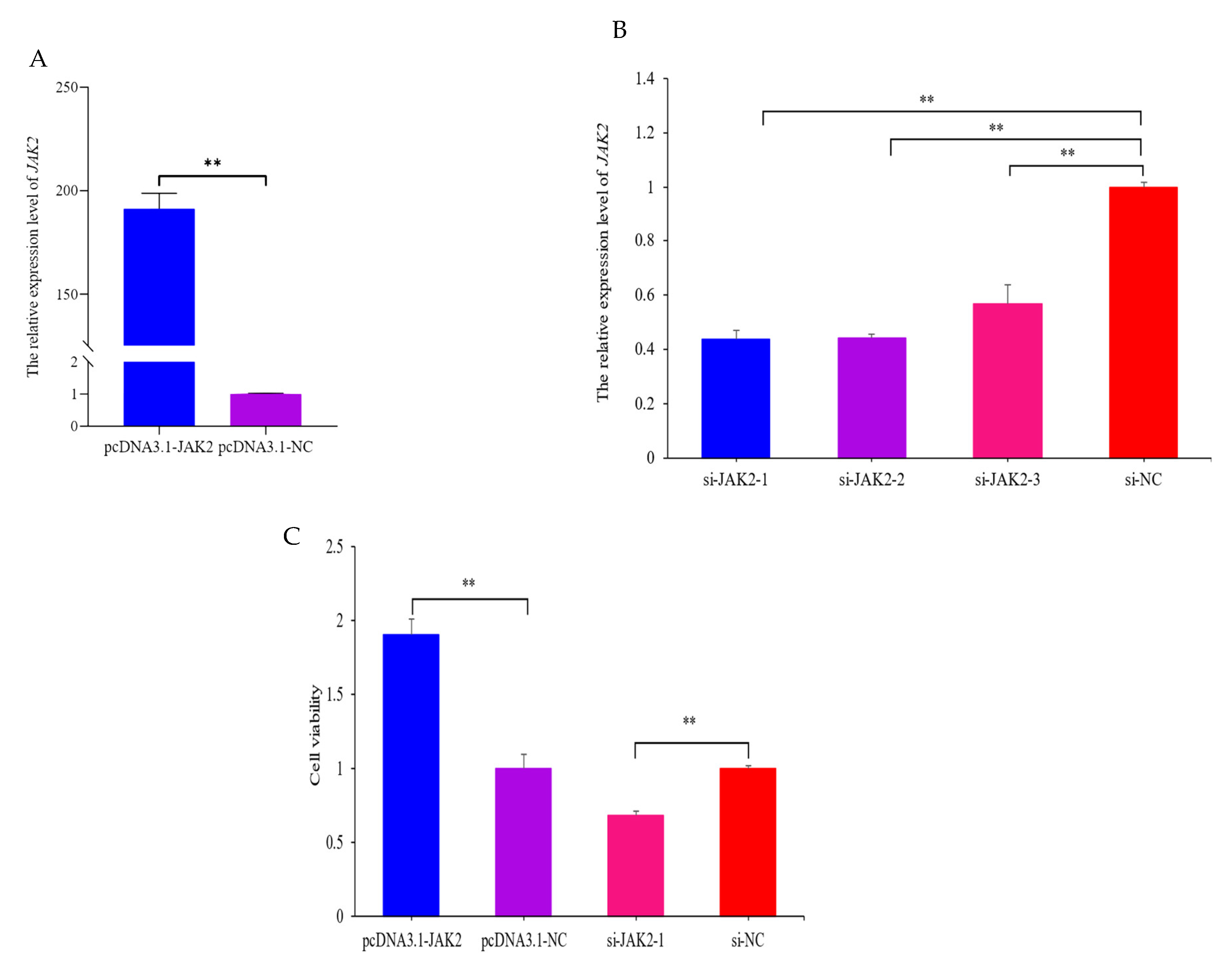
2.5. JAK2 Increases the Viability of OMECs
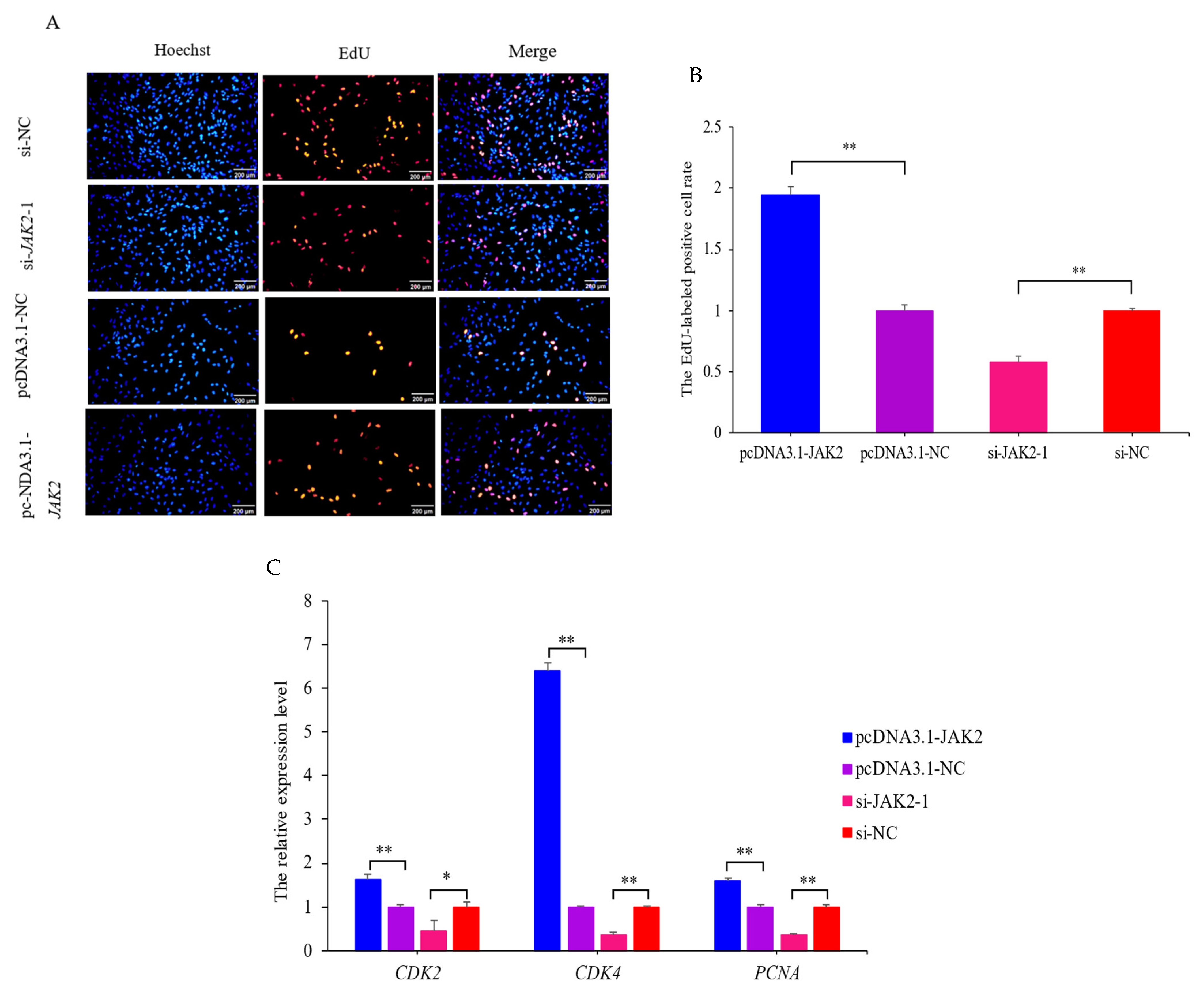
2.6. JAK2 Promotes the Proliferation of OMECs
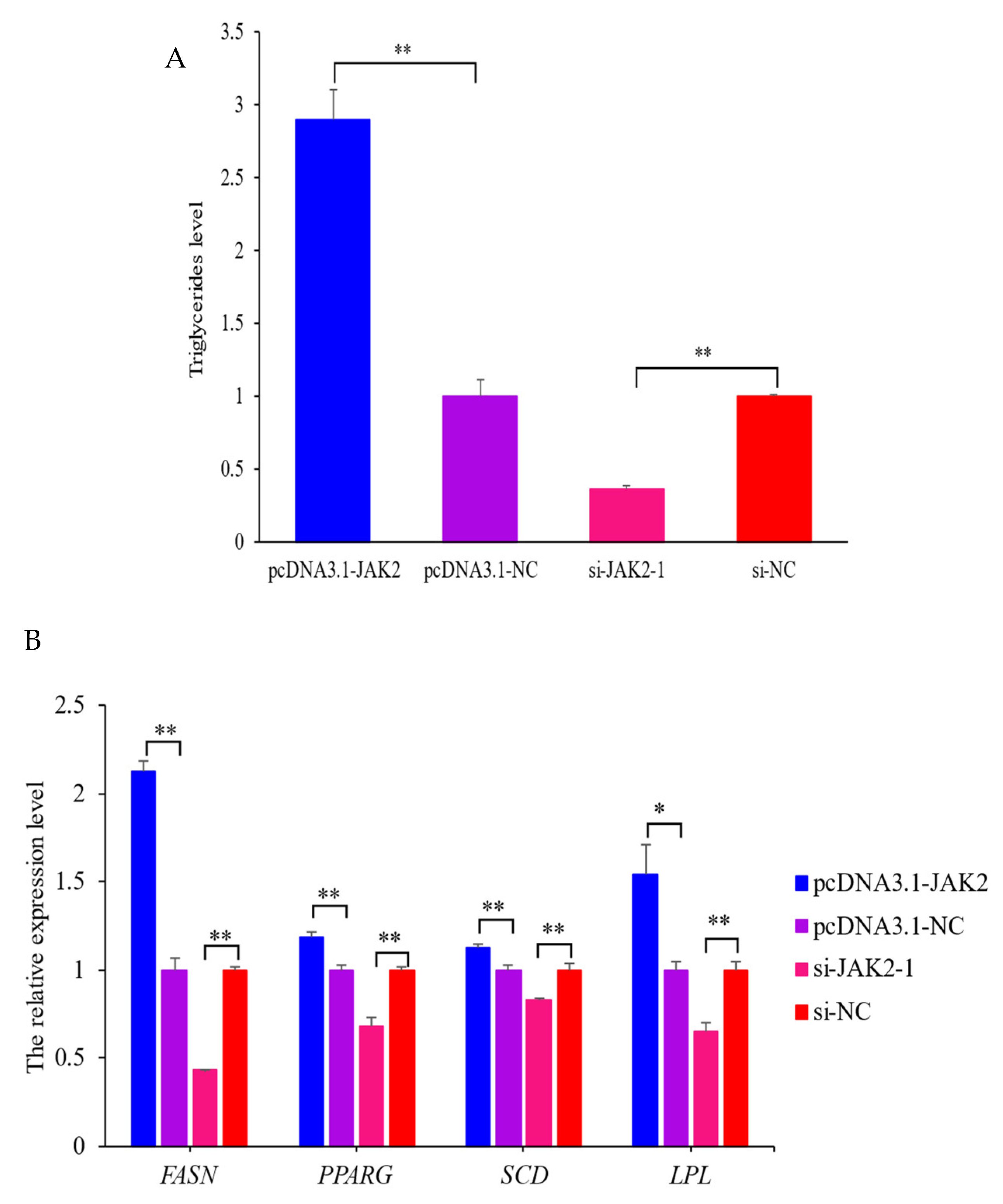
2.7. Effect of JAK2 on Milk Fat Synthesis
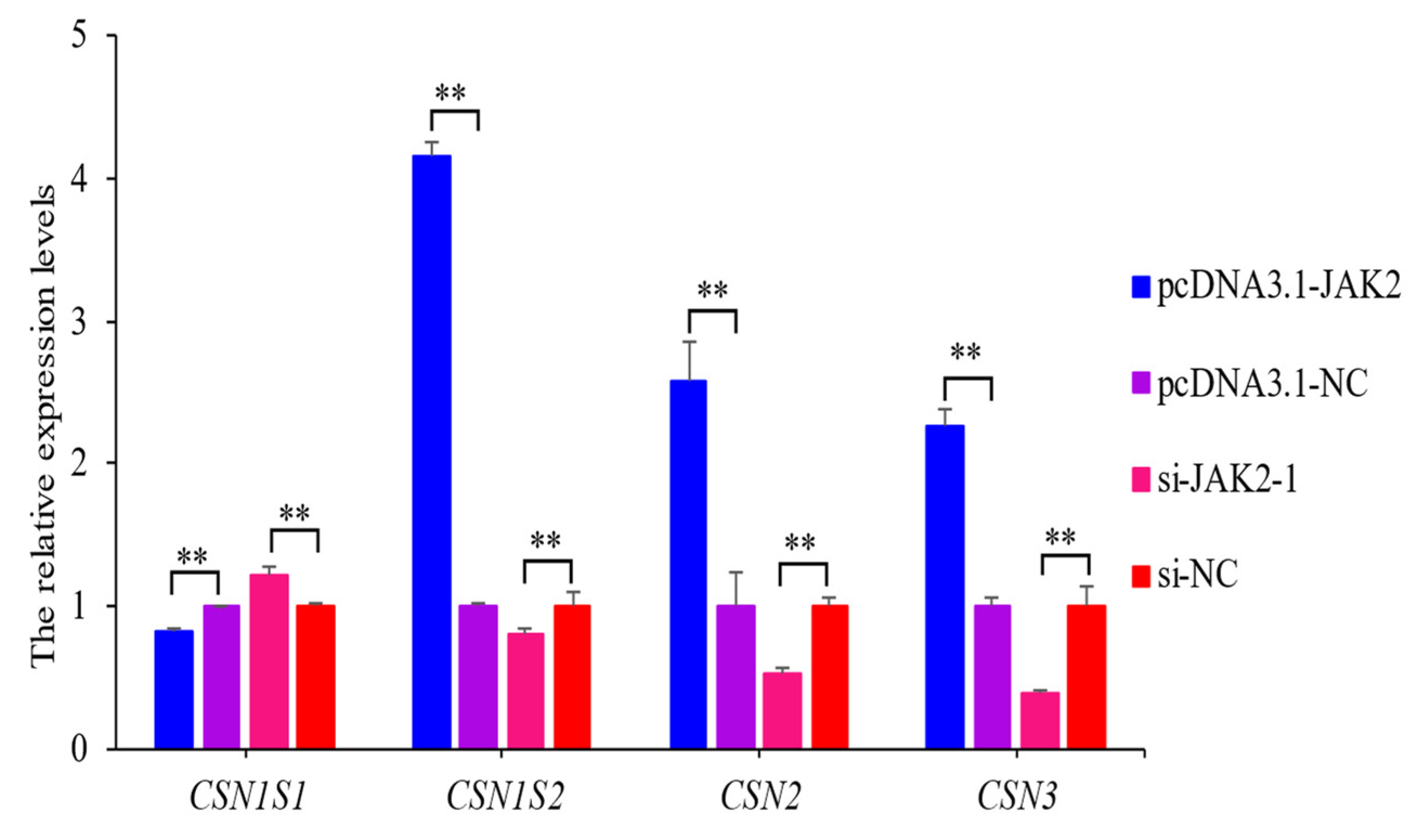
2.8. JAK2 Regulates the Expression of Casein Genes
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Molecular Clone and Characteristics Analysis of Ovine JAK2
4.3. RNA Extraction and Reverse Transcription–Quantitative PCR
4.4. Localization of JAK2 in Ovine Mammary Glands
4.5. Isolation, Culture, and Identification of OMECs
4.6. Construction of Over-Expressed and Small Interfered JAK2 Vectors
4.7. Cell Transfection, EdU, and CCK-8 Assays
4.8. Triglyceride Content Detection
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richert, M.M.; Schwertfeger, K.L.; Ryder, J.W.; Anderson, S.M. An atlas of mouse mammary gland development. J. Mammary Gland. Biol. Neoplasia 2000, 5, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Boutinaud, M.; Guinard-Flamenta, J.; Jammes, H. The number and activity of mammary epithelial cells, determining factors for milk production. Reprod. Nutr. Dev. 2004, 44, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Miyoshi, K.; Robinson, G.W.; Grimm, S.L.; Rosen, J.M.; Neubauer, H.; Pfeffer, K.; Hennighausen, L. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 2002, 16, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Li, M.; Li, Q.Z.; Lu, L.M.; Tong, H.L.; Gao, X.J. Stat5a increases lactation of dairy cow mammary gland epithelial cells cultured in vitro. In Vitro Cell Dev. Biol. Anim. 2012, 48, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Liu, X.; Li, H.; Lin, X.; Yan, Z.; Cao, Q.; Zhao, M.; Xu, Z.; Wang, Z. Menin Modulates Mammary Epithelial Cell Numbers in Bovine Mammary Glands Through Cyclin D1. J. Mammary Gland. Biol. Neoplasia 2017, 22, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Y.; Ma, L.; Wang, J.; Guo, W.; Cheng, J.; Hu, G.; Fu, S.; Liu, J. Niacin stimulates EPH4EV mammary epithelial cell proliferation and mammary gland development in pubertal mice through activation of AKT/mTOR and ERK1/2 signaling pathways. Cell Tissue Res. 2021, 384, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Khan, F.B.; Ali, S.A.; Abdullah, A.; Sharma, A.K.; Yadav, V.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Sahoo, A.K.; et al. Molecular complexity of mammary glands development: A review of lactogenic differentiation in epithelial cells. Artif. Cells Nanomed. Biotechnol. 2023, 51, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Khan, A.; Xiao, J.; Ma, Y.; Ma, J.; Gao, J.; Cao, Z. Role of the JAK-STAT Pathway in Bovine Mastitis and Milk Production. Animals 2020, 10, 2107. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Qi, Y.; Zhang, X.; Wu, Z.; Chen, J.; Chen, F.; Guan, W.; Zhang, S. Regulation of the JAK2-STAT5 Pathway by Signaling Molecules in the Mammary Gland. Front. Cell Dev. Biol. 2020, 8, 604896. [Google Scholar] [CrossRef]
- Paten, A.M.; Duncan, E.J.; Pain, S.J.; Peterson, S.W.; Kenyon, P.R.; Blair, H.T.; Dearden, P.K. Functional development of the adult ovine mammary gland—Insights from gene expression profiling. BMC Genom. 2015, 16, 748. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, W.; Zhang, G.; Xu, S.; Gao, Q.; Yang, Z. MicroRNA-204 targets JAK2 in breast cancer and induces cell apoptosis through the STAT3/BCl-2/survivin pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 5017–5025. [Google Scholar] [PubMed]
- Geng, Z.; Shan, X.; Lian, S.; Wang, J.; Wu, R. LPS-induced SOCS3 antagonizes the JAK2-STAT5 pathway and inhibits β-casein synthesis in bovine mammary epithelial cells. Life Sci. 2021, 278, 119547. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Fang, Z.; Guan, Y.; Chen, X.; Loor, J.J.; Jia, H.; Dong, J.; Wang, Y.; Zuo, R.; Liu, G.; et al. High levels of fatty acids inhibit β-casein synthesis through suppression of the JAK2/STAT5 and mTOR signaling pathways in mammary epithelial cells of cows with clinical ketosis. J. Dairy. Res. 2020, 87, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, M. Association of a genetic marker at the bovine Janus kinase 2 locus (JAK2/RsaI) with milk production traits of four cattle breeds. J. Dairy. Res. 2015, 82, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Wang, D.; Liu, L.; Usman, T.; Wen, H.; Zhang, R.; Liu, S.; Shi, L.; Mi, S.; Xiao, W.; et al. Significant genetic effects of JAK2 and DGAT1 mutations on milk fat content and mastitis resistance in Holsteins. J. Dairy. Res. 2019, 86, 388–393. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Garnsworthy, P.C.; Mann, G.E.; Sinclair, L.A. Reducing dietary protein in dairy cow diets: Implications for nitrogen utilization, milk production, welfare and fertility. Animal 2014, 8, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, S.; Zou, Y.; Zhao, F.Q.; Liu, J.; Liu, H. Lysine Stimulates Protein Synthesis by Promoting the Expression of ATB0,+ and Activating the mTOR Pathway in Bovine Mammary Epithelial Cells. J. Nutr. 2018, 148, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Qu, B.; Zhen, Z.; Yuan, X.; Zhang, L.; Zhang, M. Leucine Promotes Milk Synthesis in Bovine Mammary Epithelial Cells via the PI3K-DDX59 Signaling. J. Agric. Food Chem. 2019, 67, 8884–8895. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.V.; Wood, D.L.; Baldwin, R.; Mcleod, K.; Paape, M.J. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bST. J. Dairy. Sci. 2001, 84, 2177–2187. [Google Scholar] [CrossRef]
- Rawson, P.; Stockum, C.; Peng, L.; Manivannan, B.; Lehner, K.; Ward, H.E.; Berry, S.D.; Davis, S.R.; Snell, R.G.; McLauchlan, D.; et al. Metabolic proteomics of the liver and mammary gland during lactation. J. Proteom. 2012, 75, 4429–4435. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Capuco, A.V.; Ellis, S.E.; Hale, S.A.; Long, E.; Erdman, R.A.; Zhao, X.; Paape, M.J. Lactation persistency: Insights from mammary cell proliferation studies. J. Anim. Sci. 2003, 81 (Suppl. S3), 18–31. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, J.; Hu, J.; Liu, X.; Li, S.; Lu, Y.; Zhen, H.; Li, M.; Zhao, Z.; Luo, Y. MicroRNA-200b Regulates the Proliferation and Differentiation of Ovine Preadipocytes by Targeting p27 and KLF9. Animals 2021, 11, 2417. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhen, H.; Liu, Y.; Li, L.; Luo, Y.; Liu, X.; Li, S.; Hao, Z.; Li, M.; Hu, L.; et al. Tissue-Specific Expression of Circ_015343 and Its Inhibitory Effect on Mammary Epithelial Cells in Sheep. Front. Vet. Sci. 2022, 9, 919162. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lv, S.X. The effect of JAK2 knockout on inhibition of liver tumor growth by inducing apoptosis, autophagy and anti-proliferation via STATs and PI3K/AKT signaling pathways. Biomed. Pharmacother. 2016, 84, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Duan, L.; Wu, W.; Li, W.; Zhao, L.; Li, A.; Lu, X.; He, X.; Dong, Z.; Liu, K.; et al. Vortioxetine hydrobromide inhibits the growth of gastric cancer cells in vivo and in vitro by targeting JAK2 and SRC. Oncogenesis 2023, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Creamer, B.A.; Triplett, A.A.; Wagner, K.U. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol. Endocrinol. 2007, 8, 1877–1892. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Creamer, B.A.; Sakamoto, K.; Schmidt, J.W.; Triplett, A.A.; Moriggl, R.; Wagner, K.U. Stat5 promotes survival of mammary epithelial cells through transcriptional activation of a distinct promoter in Akt1. Mol. Cell Biol. 2010, 12, 2957–2970. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 5, 83–98. [Google Scholar] [CrossRef]
- Rädler, P.D.; Wehde, B.L.; Wagner, K.U. Crosstalk between STAT5 activation and PI3K/AKT functions in normal and transformed mammary epithelial cells. Mol. Cell Endocrinol. 2017, 451, 31–39. [Google Scholar] [CrossRef]
- Keller, A.; Wingelhofer, B.; Peter, B.; Bauer, K.; Berger, D.; Gamperl, S.; Reifinger, M.; Cerny-Reiterer, S.; Moriggl, R.; Willmann, M.; et al. The JAK2/STAT5 signaling pathway as a potential therapeutic target in canine mastocytoma. Vet. Comp. Oncol. 2018, 16, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Kollmann, S.; Pickem, J.; Hoelbl-Kovacic, A.; Sexl, V. STAT5A and STAT5B-Twins with Different Personalities in Hematopoiesis and Leukemia. Cancers 2019, 11, 1726. [Google Scholar] [CrossRef] [PubMed]
- Kung, M.H.; Lee, Y.J.; Hsu, J.T.; Huang, M.C.; Ju, Y.T. A functional study of proximal goat β-casein promoter and intron 1 in immortalized goat mammary epithelial cells. J. Dairy. Sci. 2015, 98, 3859–3875. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Chen, Y.; Luo, J.; Huang, L.; Tian, H.; Li, C.; Loor, J.J. Negative regulation of αS1-casein (CSN1S1) improves β-casein content and reduces allergy potential in goat milk. J. Dairy. Sci. 2020, 103, 9561–9572. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Qiu, L.; Zhu, W.; Huang, L.; Tu, X.; Miao, Y. CEBPA-Regulated Expression of SOCS1 Suppresses Milk Protein Synthesis through mTOR and JAK2-STAT5 Signaling Pathways in Buffalo Mammary Epithelial Cells. Foods 2023, 12, 708. [Google Scholar] [CrossRef]
- Huang, Y.L.; Zhao, F.; Luo, C.C.; Zhang, X.; Si, Y.; Sun, Z.; Zhang, L.; Li, Q.Z.; Gao, X.J. SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro. Molecules 2013, 18, 12987–13002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Luo, J.; Wang, W.; Yu, K.; Wang, H.B.; Shi, H.B.; Sun, Y.T.; Lin, X.Z.; Li, J. Inhibition of FASN reduces the synthesis of medium-chain fatty acids in goat mammary gland. Animal 2014, 8, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A.C.; Bovenhuis, H.; Visker, M.H.; van Arendonk, J.A. Genome-wide association of milk fatty acids in Dutch dairy cattle. BMC Genet. 2011, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chang, G.; Xu, T.; Zhao, H.; Zhang, K.; Shen, X. Feeding a High Concentrate Diet Down-Regulates Expression of ACACA, LPL and SCD and Modifies Milk Composition in Lactating Goats. PLoS ONE 2015, 10, e0130525. [Google Scholar] [CrossRef]
- Shi, H.B.; Luo, J.; Yao, D.W.; Zhu, J.J.; Xu, H.F.; Shi, H.P.; Loor, J.J. Peroxisome proliferator-activated receptor-γ stimulates the synthesis of monounsaturated fatty acids in dairy goat mammary epithelial cells via the control of stearoyl-coenzyme A desaturase. J. Dairy. Sci. 2013, 96, 7844–7853. [Google Scholar] [CrossRef]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Memisoglu, A.; Hu, F.B.; Hankinson, S.E.; Manson, J.E.; De Vivo, I.; Willett, W.C.; Hunter, D.J. Interaction between a peroxisome proliferator-activated receptor gamma gene polymorphism and dietary fat intake in relation to body mass. Hum. Mol. Genet. 2003, 12, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.M.; Flavelle, S.; Bureau, A.; Glass, H.C.; Fujiwara, T.M.; Wirrell, E.; Davey, K.; Chudley, A.E.; Scott, J.N.; McLeod, D.R.; et al. Homozygous deletion of the very low density lipoprotein receptor gene causes autosomal recessive cerebellar hypoplasia with cerebral gyral simplification. Am. J. Hum. Genet. 2005, 77, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, H.; Wang, X.; Yin, E.; Chen, N.; Kang, L.; Zhao, X.; Ma, Y. Cloning, Molecular Characterization and Expression Patterns of DMRTC2 Implicated in Germ Cell Development of Male Tibetan Sheep. Int. J. Mol. Sci. 2020, 2, 2448. [Google Scholar] [CrossRef]
- Hao, Z.; Luo, Y.; Wang, J.; Hickford, J.G.H.; Zhou, H.; Hu, J.; Liu, X.; Li, S.; Shen, J.; Ke, N.; et al. MicroRNA-432 inhibits milk fat synthesis by targeting SCD and LPL in ovine mammary epithelial cells. Food Funct. 2021, 12, 9432–9442. [Google Scholar] [CrossRef]


| Name | Forward (5′→3′) | Reverse (5′→3′) | Amplicon Size (bp) |
|---|---|---|---|
| JAK2 | CTTGGGAAAGCTGAGGAGGA | CGATAGGTTCTGTTGCTGCC | 260 |
| CDK2 | AGAAGTGGTGGCGCTTAAAA | TCTTGAGATCCTGGTGCAGA | 185 |
| CDK4 | GCTTTTGAGCATCCCAATGT | AGGTCTTGGTCCACATGCTC | 107 |
| PCNA | GAACCTCACCAGCATGTCCAA | TTCACCAGAAGGCATCTTTACT | 220 |
| FASN | GGGCTCCACCACCGTGTTCCA | GCTCTGCTGGGCCTGCAGCTG | 226 |
| SCD | CGACGTGGCTTTTTCTTCTC | GATGAAGCACAACAGCAGGA | 165 |
| PPARG | CCGCTGACCAAAGCAAAG | GGAGCGAAACTGACACCC | 189 |
| LPL | AGGACACTTGCCACCTCATTC | TTGGAGTCTGGTTCCCTCTTGTA | 169 |
| CSN3 | CAACAGAGACCAGTTGCACTAA | ACTTGGCAGGCACAGCATTT | 130 |
| CSN2 | TCCCACAAAACATCCTGCCT | GGGAAGGGCATTTCCTTGTG | 127 |
| CSN1S1 | AGCACCAAGGACTCTCTCCA | TGACGAACTGCTTCCAGCTT | 182 |
| CSN1S2 | AATCTGCTGAAGTTGCCCCA | TGGGCCTTGATACAGATACTGG | 131 |
| β-actin | AGCCTTCCTTCCTGGGCATGGA | GGACAGCACCGTGTTGGCGTAGA | 113 |
| Name | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| si-JAK2-1 | GCAAAUAGAUCCAGUCCUATT | UAGGACUGGAUCUAUUUGCTT |
| si-JAK2-2 | GCAAAUCAAGAAGGCGCAATT | UUGCGCCUUCUUGAUUUGCTT |
| si-JAK2-3 | CCAGCUUUCUCACAAACAUTT | AUGUUUGUGAGAAAGCUGGTT |
| si-NC | UUCUCCGAACGUGUCACGUdTdT | ACGUGACACGUUCGGAGAAdTdT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhen, H.; Wu, X.; Wang, J.; Luo, Y.; Hu, J.; Liu, X.; Li, S.; Li, M.; Shi, B.; et al. Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 4027. https://doi.org/10.3390/ijms25074027
Liu Y, Zhen H, Wu X, Wang J, Luo Y, Hu J, Liu X, Li S, Li M, Shi B, et al. Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells. International Journal of Molecular Sciences. 2024; 25(7):4027. https://doi.org/10.3390/ijms25074027
Chicago/Turabian StyleLiu, Yuan, Huimin Zhen, Xinmiao Wu, Jiqing Wang, Yuzhu Luo, Jiang Hu, Xiu Liu, Shaobin Li, Mingna Li, Bingang Shi, and et al. 2024. "Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells" International Journal of Molecular Sciences 25, no. 7: 4027. https://doi.org/10.3390/ijms25074027
APA StyleLiu, Y., Zhen, H., Wu, X., Wang, J., Luo, Y., Hu, J., Liu, X., Li, S., Li, M., Shi, B., Ren, C., Gu, Y., & Hao, Z. (2024). Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells. International Journal of Molecular Sciences, 25(7), 4027. https://doi.org/10.3390/ijms25074027






