Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet
Abstract
:1. Introduction
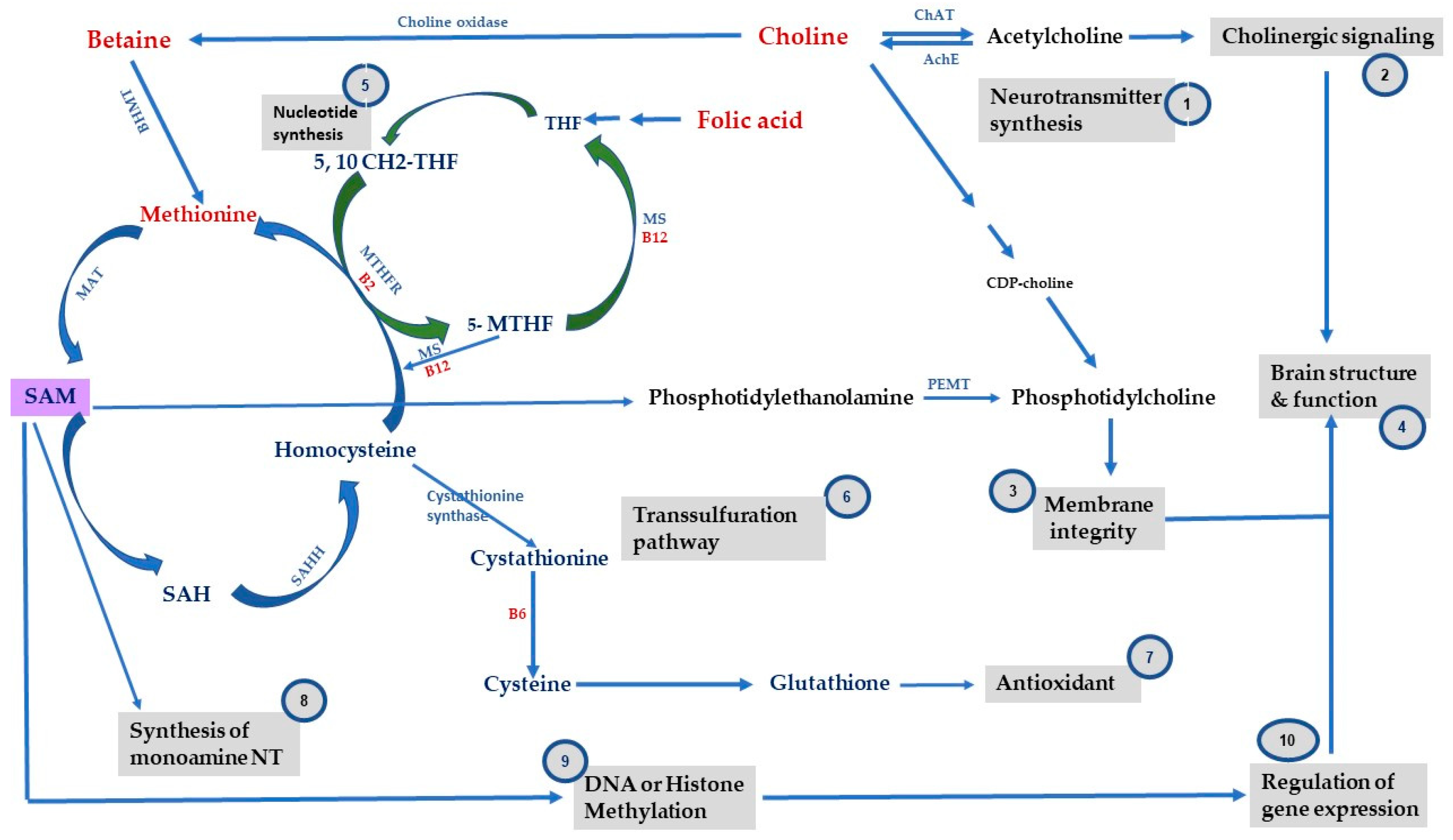
2. One-Carbon Metabolism, Methyl-Donor Micronutrients and Epigenetic Mechanisms
3. The Role of Key Methyl-Donor Micronutrients and Epigenetic Mechanisms in Metabolic and Mental Health Disorders
3.1. Folate
3.2. Choline
3.3. VitB
3.4. Methionine and SAM
4. Recommendation and Future Considerations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander Arguello, P.; Addington, A.; Borja, S.; Brady, L.; Dutka, T.; Gitik, M.; Koester, S.; Meinecke, D.; Merikangas, K.; McMahon, F.J.; et al. From Genetics to Biology: Advancing Mental Health Research in the Genomics ERA. Mol. Psychiatry 2019, 24, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.D. A Brief Tour of Epidemiologic Epigenetics and Mental Health. Curr. Opin. Psychol. 2019, 27, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Mental Disorders. Available online: https://www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed on 4 January 2024).
- Mental Illness—National Institute of Mental Health (NIMH). Available online: https://www.nimh.nih.gov/health/statistics/mental-illness (accessed on 4 January 2024).
- Overweight & Obesity Statistics—NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Available online: https://www.niddk.nih.gov/health-information/health-statistics/overweight-obesity (accessed on 28 February 2024).
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 February 2024).
- Dauncey, M.J. Recent Advances in Nutrition, Genes and Brain Health. Proc. Nutr. Soc. 2012, 71, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional Psychiatry: Towards Improving Mental Health by What You Eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the Brain: From Adaptation to Disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Gabbianelli, R.; Damiani, E. Epigenetics and Neurodegeneration: Role of Early-Life Nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic Signatures Underlying Inflammation: An Interplay of Nutrition, Physical Activity, Metabolic Diseases, and Environmental Factors for Personalized Nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Ptak, C.; Petronis, A. Epigenetic Approaches to Psychiatric Disorders. Dialogues Clin. Neurosci. 2010, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Peña, C.J.; Kundakovic, M.; Mitchell, A.; Akbarian, S. Epigenetic Basis of Mental Illness. Neuroscientist 2016, 22, 447–463. [Google Scholar] [CrossRef]
- Klengel, T.; Pape, J.; Binder, E.B.; Mehta, D. The Role of DNA Methylation in Stress-Related Psychiatric Disorders. Neuropharmacology 2014, 80, 115–132. [Google Scholar] [CrossRef]
- Holdgate, G.A.; Bardelle, C.; Lanne, A.; Read, J.; O’Donovan, D.H.; Smith, J.M.; Selmi, N.; Sheppard, R. Drug Discovery for Epigenetics Targets. Drug Discov. Today 2022, 27, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27259202/ (accessed on 6 March 2024).
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Q.; Chang, C. Epigenetics in Health and Disease. Adv. Exp. Med. Biol. 2020, 1253, 3–55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and Epigenetics: An Interplay of Dietary Methyl Donors, One-Carbon Metabolism and DNA Methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Friso, S.; Udali, S.; De Santis, D.; Choi, S.-W. One-Carbon Metabolism and Epigenetics. Mol. Asp. Med. 2017, 54, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mentch, S.J.; Locasale, J.W. One-Carbon Metabolism and Epigenetics: Understanding the Specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A. Methyl Donors, Epigenetic Alterations, and Brain Health: Understanding the Connection. Int. J. Mol. Sci. 2023, 24, 2346. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Jiang, X. One Carbon Metabolism and Early Development: A Diet-Dependent Destiny. Trends Endocrinol. Metab. 2021, 32, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Georgieff, M.K.; Committee on Nutrition. Advocacy for Improving Nutrition in the First 1000 Days to Support Childhood Development and Adult Health. Pediatrics 2018, 141, e20173716. [Google Scholar] [CrossRef]
- Chen, Y.; Baram, T.Z. Toward Understanding How Early-Life Stress Reprograms Cognitive and Emotional Brain Networks. Neuropsychopharmacology 2016, 41, 197–206. [Google Scholar] [CrossRef]
- Georgieff, M.K. Early Life Nutrition and Brain Development: Breakthroughs, Challenges and New Horizons. Proc. Nutr. Soc. 2023, 82, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Moody, L.; Chen, H.; Pan, Y.-X. Early-Life Nutritional Programming of Cognition-The Fundamental Role of Epigenetic Mechanisms in Mediating the Relation between Early-Life Environment and Learning and Memory Process. Adv. Nutr. 2017, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- A Functional Transsulfuration Pathway in the Brain Links to Glutathione Homeostasis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/17005561/ (accessed on 24 January 2024).
- Neural Tube Defects, Folic Acid and Methylation—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24048206/ (accessed on 24 January 2024).
- McGarel, C.; Pentieva, K.; Strain, J.J.; McNulty, H. Emerging Roles for Folate and Related B-Vitamins in Brain Health across the Lifecycle. Proc. Nutr. Soc. 2015, 74, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary Choline Deficiency Alters Global and Gene-Specific DNA Methylation in the Developing Hippocampus of Mouse Fetal Brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Kennedy, B.C.; Pisansky, M.T.; Won, K.-J.; Gewirtz, J.C.; Simmons, R.A.; Georgieff, M.K. Prenatal Choline Supplementation Diminishes Early-Life Iron Deficiency-Induced Reprogramming of Molecular Networks Associated with Behavioral Abnormalities in the Adult Rat Hippocampus. J. Nutr. 2016, 146, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, A.; Kerek, R.; Pourié, G.; Helle, D.; Guéant, J.-L.; Daval, J.-L.; Bossenmeyer-Pourié, C. Late Maternal Folate Supplementation Rescues from Methyl Donor Deficiency-Associated Brain Defects by Restoring Let-7 and miR-34 Pathways. Mol. Neurobiol. 2017, 54, 5017–5033. [Google Scholar] [CrossRef] [PubMed]
- Carlin, J.; George, R.; Reyes, T.M. Methyl Donor Supplementation Blocks the Adverse Effects of Maternal High Fat Diet on Offspring Physiology. PLoS ONE 2013, 8, e63549. [Google Scholar] [CrossRef] [PubMed]
- McCoy, C.R.; Jackson, N.L.; Day, J.; Clinton, S.M. Genetic Predisposition to High Anxiety- and Depression-like Behavior Coincides with Diminished DNA Methylation in the Adult Rat Amygdala. Behav. Brain Res. 2017, 320, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Pannia, E.; Huot, P.S.P.; Sánchez-Hernández, D.; Kubant, R.; Dodington, D.W.; Ward, W.E.; Bazinet, R.P.; Anderson, G.H. Methyl Vitamins Contribute to Obesogenic Effects of a High Multivitamin Gestational Diet and Epigenetic Alterations in Hypothalamic Feeding Pathways in Wistar Rat Offspring. Mol. Nutr. Food Res. 2015, 59, 476–489. [Google Scholar] [CrossRef]
- Choline and Folic Acid in Diets Consumed during Pregnancy Interact to Program Food Intake and Metabolic Regulation of Male Wistar Rat Offspring—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/33561219/ (accessed on 24 January 2024).
- Hammoud, R.; Pannia, E.; Kubant, R.; Metherel, A.; Simonian, R.; Pausova, Z.; Anderson, G.H. High Choline Intake during Pregnancy Reduces Characteristics of the Metabolic Syndrome in Male Wistar Rat Offspring Fed a High Fat But Not a Normal Fat Post-Weaning Diet. Nutrients 2021, 13, 1438. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Li, D.; Li, N.; Dindot, S.V.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Nutrition, Epigenetics, and Metabolic Syndrome. Antioxid. Redox Signal 2012, 17, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional Psychiatry: The Present State of the Evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal Folate and Choline Levels and Brain and Cognitive Development in Children: A Critical Narrative Review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef] [PubMed]
- Villamor, E.; Rifas-Shiman, S.L.; Gillman, M.W.; Oken, E. Maternal Intake of Methyl-Donor Nutrients and Child Cognition at 3 Years of Age. Paediatr. Perinat. Epidemiol. 2012, 26, 328–335. [Google Scholar] [CrossRef]
- Bekdash, R.A. Neuroprotective Effects of Choline and Other Methyl Donors. Nutrients 2019, 11, 2995. [Google Scholar] [CrossRef]
- Penner-Goeke, S.; Binder, E.B. Epigenetics and Depression. Dialogues Clin. Neurosci. 2019, 21, 397–405. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Kato, T. Epigenetics in Mood Disorders. Environ. Health Prev. Med. 2008, 13, 16–24. [Google Scholar] [CrossRef]
- Perez-Polo, J.R. Epigenetics: Stress and Disease. Int. J. Dev. Neurosci. 2017, 62, 54–55. [Google Scholar] [CrossRef]
- Lintas, C. Linking Genetics to Epigenetics: The Role of Folate and Folate-Related Pathways in Neurodevelopmental Disorders. Clin. Genet. 2019, 95, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.; Mariotti, V.; Iofrida, C.; Pellegrini, S. Genes and Aggressive Behavior: Epigenetic Mechanisms Underlying Individual Susceptibility to Aversive Environments. Front. Behav. Neurosci. 2018, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Bacon, E.R.; Brinton, R.D. Epigenetics of the Developing and Aging Brain: Mechanisms That Regulate Onset and Outcomes of Brain Reorganization. Neurosci. Biobehav. Rev. 2021, 125, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, C.N.; Brown, E.C.; Mar, M.-H.; Albright, C.D.; Nadeau, M.R.; Zeisel, S.H. Folic Acid Deficiency during Late Gestation Decreases Progenitor Cell Proliferation and Increases Apoptosis in Fetal Mouse Brain. J. Nutr. 2004, 134, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, C.N.; Johnson, A.R.; Zeisel, S.H. Dietary Choline Reverses Some, but Not All, Effects of Folate Deficiency on Neurogenesis and Apoptosis in Fetal Mouse Brain. J. Nutr. 2010, 140, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Saber Cherif, L.; Pourié, G.; Geoffroy, A.; Julien, A.; Helle, D.; Robert, A.; Umoret, R.; Guéant, J.-L.; Bossenmeyer-Pourié, C.; Daval, J.-L. Methyl Donor Deficiency during Gestation and Lactation in the Rat Affects the Expression of Neuropeptides and Related Receptors in the Hypothalamus. Int. J. Mol. Sci. 2019, 20, 5097. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Sánchez-Hernández, D.; Reza-López, S.A.; Huot, P.S.P.; Kim, Y.-I.; Anderson, G.H. High Folate Gestational and Post-Weaning Diets Alter Hypothalamic Feeding Pathways by DNA Methylation in Wistar Rat Offspring. Epigenetics 2013, 8, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.G.; Shehata, G.A.; Ali, R.M.; Makboul, R.; Abd Allah, E.S.H.; Abd El-Rady, N.M. Folic Acid Ameliorates Neonatal Isolation-Induced Autistic like Behaviors in Rats: Epigenetic Modifications of BDNF and GFAP Promotors. Appl. Physiol. Nutr. Metab. 2021, 46, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and Folate Concentrations during Pregnancy and Insulin Resistance in the Offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Karat, S.C.; Yajnik, C.S.; Fall, C.H.D. Association between Maternal Folate Concentrations during Pregnancy and Insulin Resistance in Indian Children. Diabetologia 2014, 57, 110–121. [Google Scholar] [CrossRef]
- Xie, R.-H.; Liu, Y.-J.; Retnakaran, R.; MacFarlane, A.J.; Hamilton, J.; Smith, G.; Walker, M.C.; Wen, S.W. Maternal Folate Status and Obesity/Insulin Resistance in the Offspring: A Systematic Review. Int. J. Obes. 2016, 40, 1–9. [Google Scholar] [CrossRef]
- Deshmukh, U.; Katre, P.; Yajnik, C.S. Influence of Maternal Vitamin B12 and Folate on Growth and Insulin Resistance in the Offspring. Nestle Nutr. Inst. Workshop Ser. 2013, 74, 145–154; discussion 154–156. [Google Scholar] [CrossRef]
- Kadam, I.; Dalloul, M.; Hausser, J.; Huntley, M.; Hoepner, L.; Fordjour, L.; Hittelman, J.; Saxena, A.; Liu, J.; Futterman, I.D.; et al. Associations between Nutrients in One-Carbon Metabolism and Fetal DNA Methylation in Pregnancies with or without Gestational Diabetes Mellitus. Clin. Epigenetics 2023, 15, 137. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Samblas, M.; Milagro, F.I.; Zulet, M.A.; Mansego, M.L.; Riezu-Boj, J.I.; Martinez, J.A. Association of Low Dietary Folate Intake with Lower CAMKK2 Gene Methylation, Adiposity, and Insulin Resistance in Obese Subjects. Nutr. Res. 2018, 50, 53–62. [Google Scholar] [CrossRef]
- Derbyshire, E.; Obeid, R. Choline, Neurological Development and Brain Function: A Systematic Review Focusing on the First 1000 Days. Nutrients 2020, 12, 1731. [Google Scholar] [CrossRef]
- Zeisel, S.H. Metabolic Crosstalk between Choline/1-Carbon Metabolism and Energy Homeostasis. Clin. Chem. Lab. Med. 2012, 51, 467–475. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Needed for Normal Development of Memory. J. Am. Coll. Nutr. 2000, 19 (Suppl. S5), 528S–531S. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Clinical Nutrigenetic/Nutrigenomic Approaches for Identification of Functions and Dietary Requirements. World Rev. Nutr. Diet. 2010, 101, 73–83. [Google Scholar] [CrossRef]
- Zeisel, S.H.; da Costa, K.-A. Choline: An Essential Nutrient for Public Health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Bekdash, R.A. Choline and the Brain: An Epigenetic Perspective. Adv. Neurobiol. 2016, 12, 381–399. [Google Scholar] [CrossRef]
- Hammoud, R.; Pannia, E.; Kubant, R.; Liao, C.-S.; Ho, M.; Yang, N.V.; Chatterjee, D.; Caudill, M.A.; Malysheva, O.V.; Pausova, Z.; et al. Maternal Choline Intake Programs Hypothalamic Energy Regulation and Later-Life Phenotype of Male Wistar Rat Offspring. Mol. Nutr. Food Res. 2020, 64, e1901178. [Google Scholar] [CrossRef]
- Zeisel, S.H. The Supply of Choline Is Important for Fetal Progenitor Cells. Semin. Cell Dev. Biol. 2011, 22, 624–628. [Google Scholar] [CrossRef]
- Freedman, R.; Ross, R.G. Prenatal Choline and the Development of Schizophrenia. Shanghai Arch. Psychiatry 2015, 27, 90–102. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Ju, G.; Zhang, X.; Xu, Q.; Liu, S.; Yu, Y.; Shi, J.; Boyle, S.; Wang, Z.; et al. A Study of the PEMT Gene in Schizophrenia. Neurosci. Lett. 2007, 424, 203–206. [Google Scholar] [CrossRef]
- Bjelland, I.; Tell, G.S.; Vollset, S.E.; Konstantinova, S.; Ueland, P.M. Choline in Anxiety and Depression: The Hordaland Health Study2. Am. J. Clin. Nutr. 2009, 90, 1056–1060. [Google Scholar] [CrossRef]
- Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; D’Alessandro, A.; Wyrwa, A.; Noonan, K.; Christians, U.; Nakimuli-Mpungu, E.; Zeisel, S.H.; Law, A.J.; et al. Black American Maternal Prenatal Choline, Offspring Gestational Age at Birth, and Developmental Predisposition to Mental Illness. Schizophr. Bull. 2020, 47, 896–905. [Google Scholar] [CrossRef]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA Signaling Molecules KCC2, NKCC1, and GAD1 in Cortical Development and Schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef]
- Chen, V.; Schwartz, J.L.; Cho, C.E. Folate and Choline: Does It Take Two to Tango in Early Programming of Disease? Lifestyle Genom. 2023, 16, 177–191. [Google Scholar] [CrossRef]
- Sahara, Y.; Matsuzawa, D.; Ishii, D.; Fuchida, T.; Goto, T.; Sutoh, C.; Shimizu, E. Paternal Methyl Donor Deficient Diets during Development Affect Male Offspring Behavior and Memory-Related Gene Expression in Mice. Dev. Psychobiol. 2019, 61, 17–28. [Google Scholar] [CrossRef]
- Saunderson, E.A.; Spiers, H.; Mifsud, K.R.; Gutierrez-Mecinas, M.; Trollope, A.F.; Shaikh, A.; Mill, J.; Reul, J.M.H.M. Stress-Induced Gene Expression and Behavior Are Controlled by DNA Methylation and Methyl Donor Availability in the Dentate Gyrus. Proc. Natl. Acad. Sci. USA 2016, 113, 4830–4835. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation-Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Cruz-Rodríguez, J.; Díaz-López, A.; Canals-Sans, J.; Arija, V. Maternal Vitamin B12 Status during Pregnancy and Early Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2023, 15, 1529. [Google Scholar] [CrossRef]
- van de Lagemaat, E.E.; de Groot, L.C.P.G.M.; van den Heuvel, E.G.H.M. Vitamin B12 in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef]
- Ata, F.; Bint I Bilal, A.; Javed, S.; Shabir Chaudhry, H.; Sharma, R.; Fatima Malik, R.; Choudry, H.; Bhaskaran Kartha, A. Optic Neuropathy as a Presenting Feature of Vitamin B-12 Deficiency: A Systematic Review of Literature and a Case Report. Ann. Med. Surg. 2020, 60, 316–322. [Google Scholar] [CrossRef]
- Sangle, P.; Sandhu, O.; Aftab, Z.; Anthony, A.T.; Khan, S. Vitamin B12 Supplementation: Preventing Onset and Improving Prognosis of Depression. Cureus 2020, 12, e11169. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef]
- Skarupski, K.A.; Tangney, C.; Li, H.; Ouyang, B.; Evans, D.A.; Morris, M.C. Longitudinal Association of Vitamin B-6, Folate, and Vitamin B-12 with Depressive Symptoms among Older Adults over Time123. Am. J. Clin. Nutr. 2010, 92, 330–335. [Google Scholar] [CrossRef]
- Levine, J.; Stahl, Z.; Sela, B.-A.; Ruderman, V.; Shumaico, O.; Babushkin, I.; Osher, Y.; Bersudsky, Y.; Belmaker, R.H. Homocysteine-Reducing Strategies Improve Symptoms in Chronic Schizophrenic Patients with Hyperhomocysteinemia. Biol. Psychiatry 2006, 60, 265–269. [Google Scholar] [CrossRef]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low Serum Vitamin B-12 and Folate Concentrations and Low Thiamin and Riboflavin Intakes Are Inversely Associated with Greater Adiposity in Mexican American Children. J. Nutr. 2014, 144, 2027–2033. [Google Scholar] [CrossRef]
- Baltaci, D.; Deler, M.H.; Turker, Y.; Ermis, F.; Iliev, D.; Velioglu, U. Evaluation of Serum Vitamin B12 Level and Related Nutritional Status among Apparently Healthy Obese Female Individuals. Niger. J. Clin. Pract. 2017, 20, 99–105. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Mora, F.; Rodríguez-Jiménez, R.; Fernandez-Rojo, S.; et al. Nutrition, Epigenetics, and Major Depressive Disorder: Understanding the Connection. Front. Nutr. 2022, 9, 867150. [Google Scholar] [CrossRef] [PubMed]
- Kaner, G.; Soylu, M.; Yüksel, N.; Inanç, N.; Ongan, D.; Başmısırlı, E. Evaluation of Nutritional Status of Patients with Depression. Biomed. Res. Int. 2015, 2015, 521481. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Smith, R.; Collins, C.E.; Mossman, D.; Wong-Brown, M.W.; Chan, E.-C.; Evans, T.-J.; Attia, J.R.; Smith, T.; Butler, T.; et al. Methyl-Donor and Cofactor Nutrient Intakes in the First 2–3 Years and Global DNA Methylation at Age 4: A Prospective Cohort Study. Nutrients 2018, 10, 273. [Google Scholar] [CrossRef]
- Gao, J.; Cahill, C.M.; Huang, X.; Roffman, J.L.; Lamon-Fava, S.; Fava, M.; Mischoulon, D.; Rogers, J.T. S-Adenosyl Methionine and Transmethylation Pathways in Neuropsychiatric Diseases Throughout Life. Neurotherapeutics 2018, 15, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Coppen, A.; Bolander-Gouaille, C. Treatment of Depression: Time to Consider Folic Acid and Vitamin B12. J. Psychopharmacol. 2005, 19, 59–65. [Google Scholar] [CrossRef]
- Otero-Losada, M.E.; Rubio, M.C. Acute Changes in 5-HT Metabolism after S-Adenosyl-L-Methionine Administration. Gen. Pharmacol. 1989, 20, 403–406. [Google Scholar] [CrossRef]
- Losada, M.E.; Rubio, M.C. Acute Effects of S-Adenosyl-L-Methionine on Catecholaminergic Central Function. Eur. J. Pharmacol. 1989, 163, 353–356. [Google Scholar] [CrossRef]
- Mischoulon, D.; Fava, M. Role of S-Adenosyl-L-Methionine in the Treatment of Depression: A Review of the Evidence. Am. J. Clin. Nutr. 2002, 76, 1158S–1161S. [Google Scholar] [CrossRef]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, Folate, Methylation, and Monoamine Metabolism in Depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef]
- Weaver, I.C.G.; Champagne, F.A.; Brown, S.E.; Dymov, S.; Sharma, S.; Meaney, M.J.; Szyf, M. Reversal of Maternal Programming of Stress Responses in Adult Offspring through Methyl Supplementation: Altering Epigenetic Marking Later in Life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef]
- De Berardis, D.; Marini, S.; Serroni, N.; Rapini, G.; Iasevoli, F.; Valchera, A.; Signorelli, M.; Aguglia, E.; Perna, G.; Salone, A.; et al. S-Adenosyl-L-Methionine Augmentation in Patients with Stage II Treatment-Resistant Major Depressive Disorder: An Open Label, Fixed Dose, Single-Blind Study. Sci. World J. 2013, 2013, 204649. [Google Scholar] [CrossRef] [PubMed]
- Kelsoe, J.R.; Tolbert, L.C.; Crews, E.L.; Smythies, J.R. Kinetic Evidence for Decreased Methionine Adenosyltransferase Activity in Erythrocytes from Schizophrenics. J. Neurosci. Res. 1982, 8, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Bottiglieri, T.; Schaefer, C.A.; Quesenberry, C.P.; Liu, L.; Bresnahan, M.; Susser, E.S. Elevated Prenatal Homocysteine Levels as a Risk Factor for Schizophrenia. Arch. Gen. Psychiatry 2007, 64, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, J.B.; Susser, E.; Kundakovic, M.; Kresovich, J.K.; Davey Smith, G.; Relton, C.L. Prenatal Nutrition, Epigenetics and Schizophrenia Risk: Can We Test Causal Effects? Epigenomics 2012, 4, 303–315. [Google Scholar] [CrossRef]
- Zhubi, A.; Veldic, M.; Puri, N.V.; Kadriu, B.; Caruncho, H.; Loza, I.; Sershen, H.; Lajtha, A.; Smith, R.C.; Guidotti, A.; et al. An Upregulation of DNA-Methyltransferase 1 and 3a Expressed in Telencephalic GABAergic Neurons of Schizophrenia Patients Is Also Detected in Peripheral Blood Lymphocytes. Schizophr. Res. 2009, 111, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Cheng, K.; Russo, A.; Smith, C.L.; Faraone, S.V.; Wilcox, M.; Shafa, R.; Glatt, S.J.; Nguyen, G.; Ponte, J.F.; et al. Hypermethylation of the Reelin (RELN) Promoter in the Brain of Schizophrenic Patients: A Preliminary Report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 134B, 60–66. [Google Scholar] [CrossRef]
- Naninck, E.F.G.; Oosterink, J.E.; Yam, K.-Y.; de Vries, L.P.; Schierbeek, H.; van Goudoever, J.B.; Verkaik-Schakel, R.-N.; Plantinga, J.A.; Plosch, T.; Lucassen, P.J.; et al. Early Micronutrient Supplementation Protects against Early Stress-Induced Cognitive Impairments. FASEB J. 2017, 31, 505–518. [Google Scholar] [CrossRef]
| Methyl-Donor Micronutrients | Condition | Effects | References |
|---|---|---|---|
| Folate | Maternal supplementation with folate from GD13-GD20 in rats | Normalized Let-7a and miR-34 levels at GD20 and normalized the expression of genes related to embryonic development, cell migration, axonal guidance, and vesicular trafficking | [33] |
| Deficiency in folate and VitB12 in rats | Altered expression of metabolic genes in the rat hypothalamus | [30] | |
| Folate deficiency in mice from GD11-GD17 | Decrease in NSC proliferation and differentiation and increase in neuronal apoptosis in fetal mouse brain | [52] | |
| Supplementation of high folate diet to Wistar rat offspring | Decrease in Pomc methylation and improved glucose response of offspring to glucose load | [55] | |
| Folate Supplementation in neonatal isolation rat model | Hypomethylation of BDNF and increase in its expression + mitigated autistic and anxiety-like behavior in offspring | [56] | |
| Supplementation with high methyl-vitamins gestational diet (B12, B6, folate) in Wistar rats | Hypermethylation of hypothalamic Pomc and 5-Htr2a + leptin and insulin resistance in offspring | [36] | |
| High maternal folate and low VitB12 in pregnant women | Insulin resistance and higher adiposity in children at age 6 | [57] | |
| High maternal folate but not VitB12 in pregnant women | Insulin resistance in children at age of 5, 9.5, and 13.5 | [58] | |
| High maternal folate and low VitB12 in pregnant women | Insulin resistance and higher adiposity in children at age 6 | [60] | |
| Low dietary intake of folate in obese females | Insulin resistance and decrease in methylation of metabolic-related gene Camkk2 in WBCs | [62] | |
| Choline | Prenatal choline supplementation from GD11-GD18 in iron-deficient rats | Mitigated the effects of iron deficiency on hippocampal genes associated with autism and schizophrenia | [32] |
| High maternal choline intake in Wistar rats | Increase in hypothalamic expression of Npy in pups at birth + higher food intake and body weight gain postweaning in offspring | [69] | |
| Low choline intake from GD12-GD17 in rodents | Disruption of fetal progenitor cell differentiation and changes in methylation | [70] | |
| Paternal supplementation of FMCD diet in F0 mice | Anxiety-like behavior and impairment in memory consolidation with modest PP1c methylation in male F1 offspring | [77] | |
| Maternal choline intake in women with gestational diabetes | Cord blood CRH methylation and decrease in plasma cortisol levels | [61] | |
| Low choline levels in plasma in the Hardland Health Study | Anxiety in adults | [73] | |
| Low plasma choline at 16-week gestation in women | Dysregulated cerebral P50 inhibition in infant Increased predisposition to mental illness | [74] | |
| VitB12 | Increase intake of VitB12 and B6 in humans | Reduced depressive-like symptoms in adults | [85] |
| Intake of folate and VitB12 in humans | Improved symptoms in schizophrenics | [86] | |
| Reduced folate and VitB12 levels in children | Associated with obesity in Mexican American children | [87] | |
| Low dietary intake of folate and B12 in humans | Associated with MDD individuals | [89] | |
| Methionine/SAM | SAM supplementation in rats prior to a forced swimming test | Increase in methylation of immediate early genes with elevated levels of Dnmt3a in DG granule neurons and reduced stress response | [78] |
| Maternal L-methionine supplementation in rats | Normalized methylation of NGFA binding site on GR promoter in offspring | [98] | |
| Supplementation of methionine and B vitamins from PD2-PD9 in mice subjected to early life stress (ES) | Prevented ES-induced rise in corticosterone and rescued ES-induced cognitive impairments in offspring | [105] | |
| Elevated plasma homocysteine levels and reduced levels of SAM, folate, and monoamine metabolites in CSF of patients | Observed in MDD patients | [97] | |
| SAM supplementation in MDD patients | Clinical improvements in MDD patients | [99] | |
| Elevated homocysteine levels during third trimester of pregnancy | Increased risk of schizophrenia in offspring | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekdash, R.A. Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. Int. J. Mol. Sci. 2024, 25, 4036. https://doi.org/10.3390/ijms25074036
Bekdash RA. Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. International Journal of Molecular Sciences. 2024; 25(7):4036. https://doi.org/10.3390/ijms25074036
Chicago/Turabian StyleBekdash, Rola A. 2024. "Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet" International Journal of Molecular Sciences 25, no. 7: 4036. https://doi.org/10.3390/ijms25074036
APA StyleBekdash, R. A. (2024). Epigenetics, Nutrition, and the Brain: Improving Mental Health through Diet. International Journal of Molecular Sciences, 25(7), 4036. https://doi.org/10.3390/ijms25074036





