A Conserved Intramolecular Ion-Pair Plays a Critical but Divergent Role in Regulation of Dimerization and Transport Function among the Monoamine Transporters
Abstract
1. Introduction
2. Results
2.1. Dimer Models of Monoamine Transporters
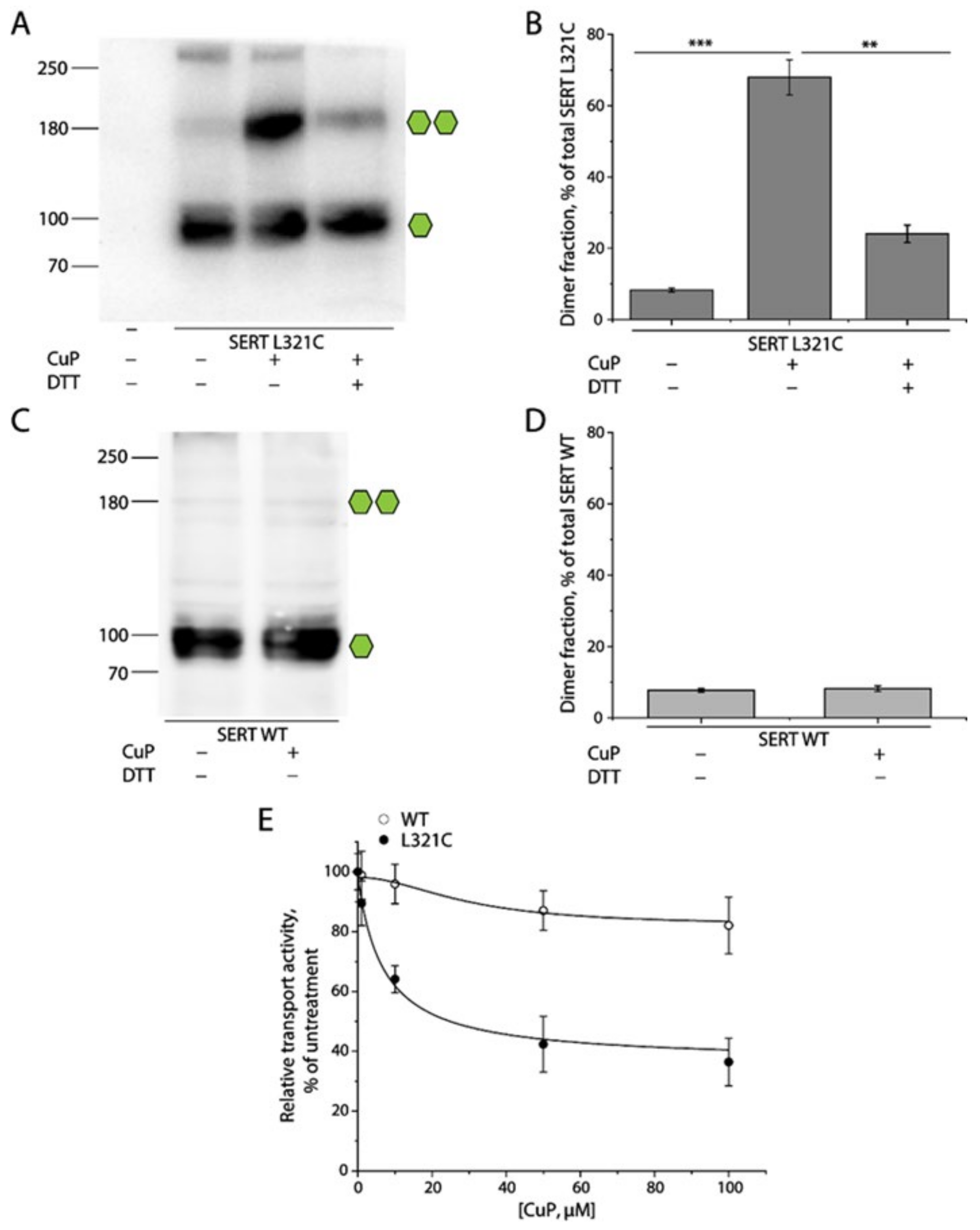
2.2. Oxidative Cross-Linking of SERT L321C and Its Functional Consequences
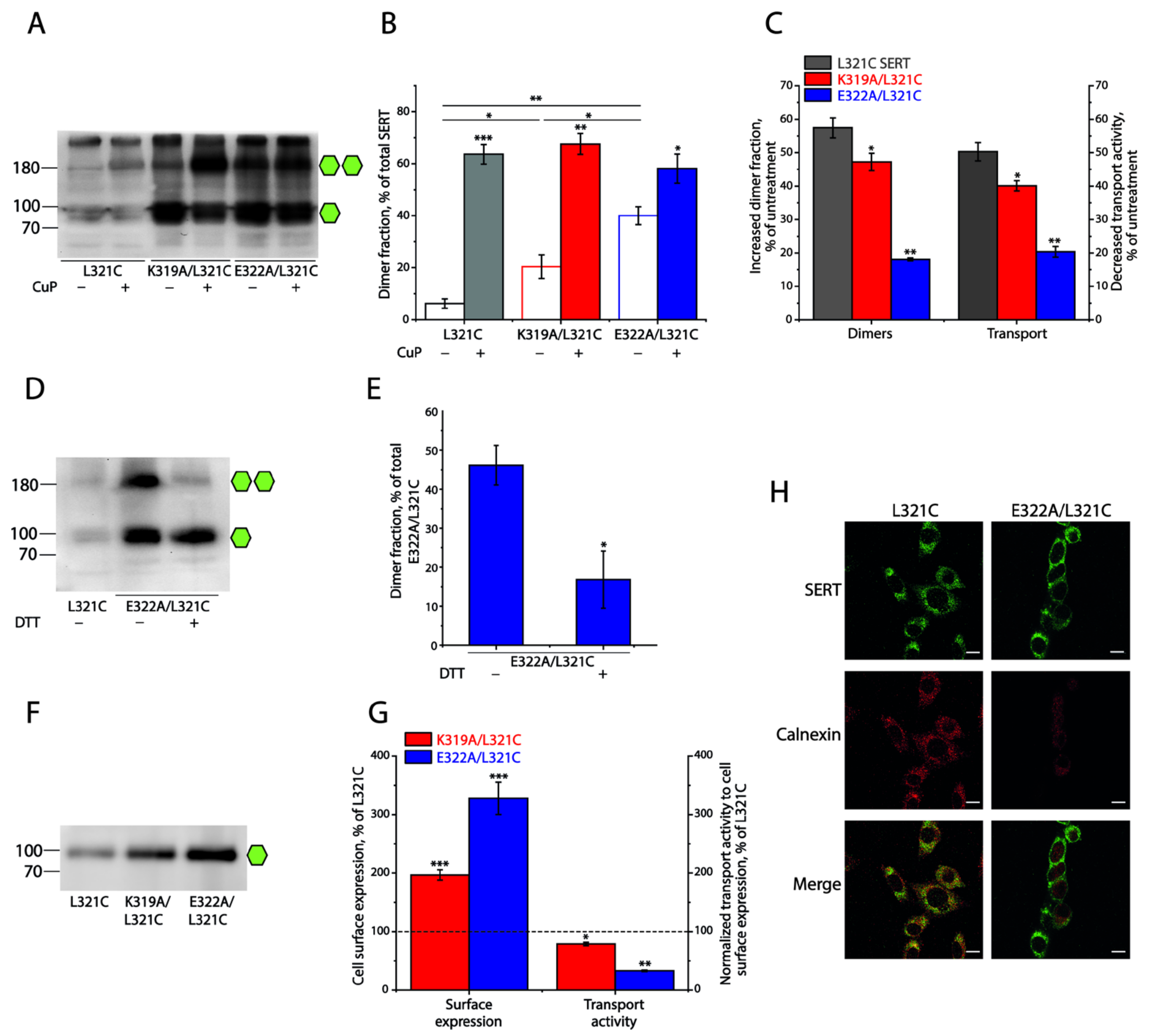
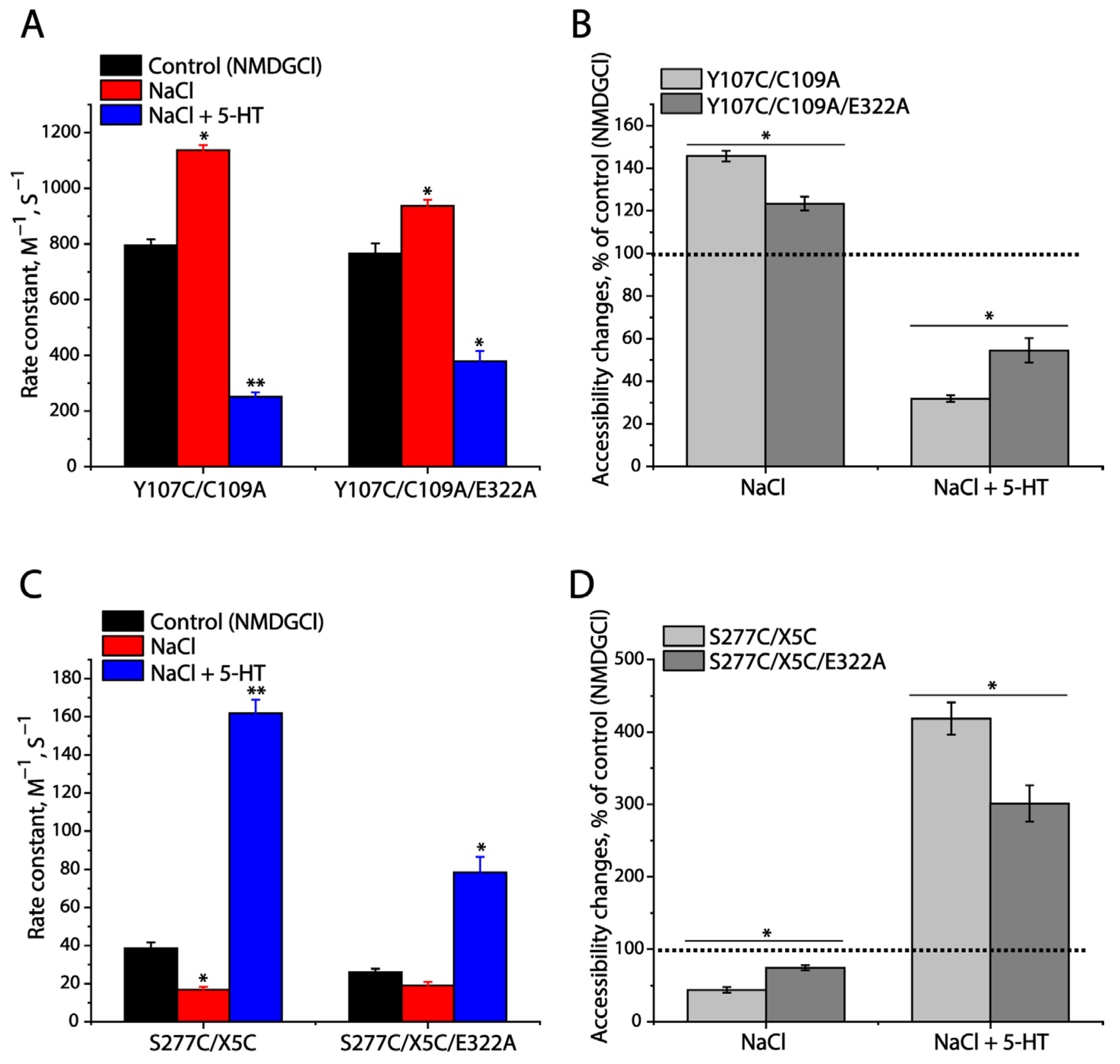
2.3. Influence of the Intramolecular K319-E322 Ion-Pair on CuP Cross-Linking of SERT L321C
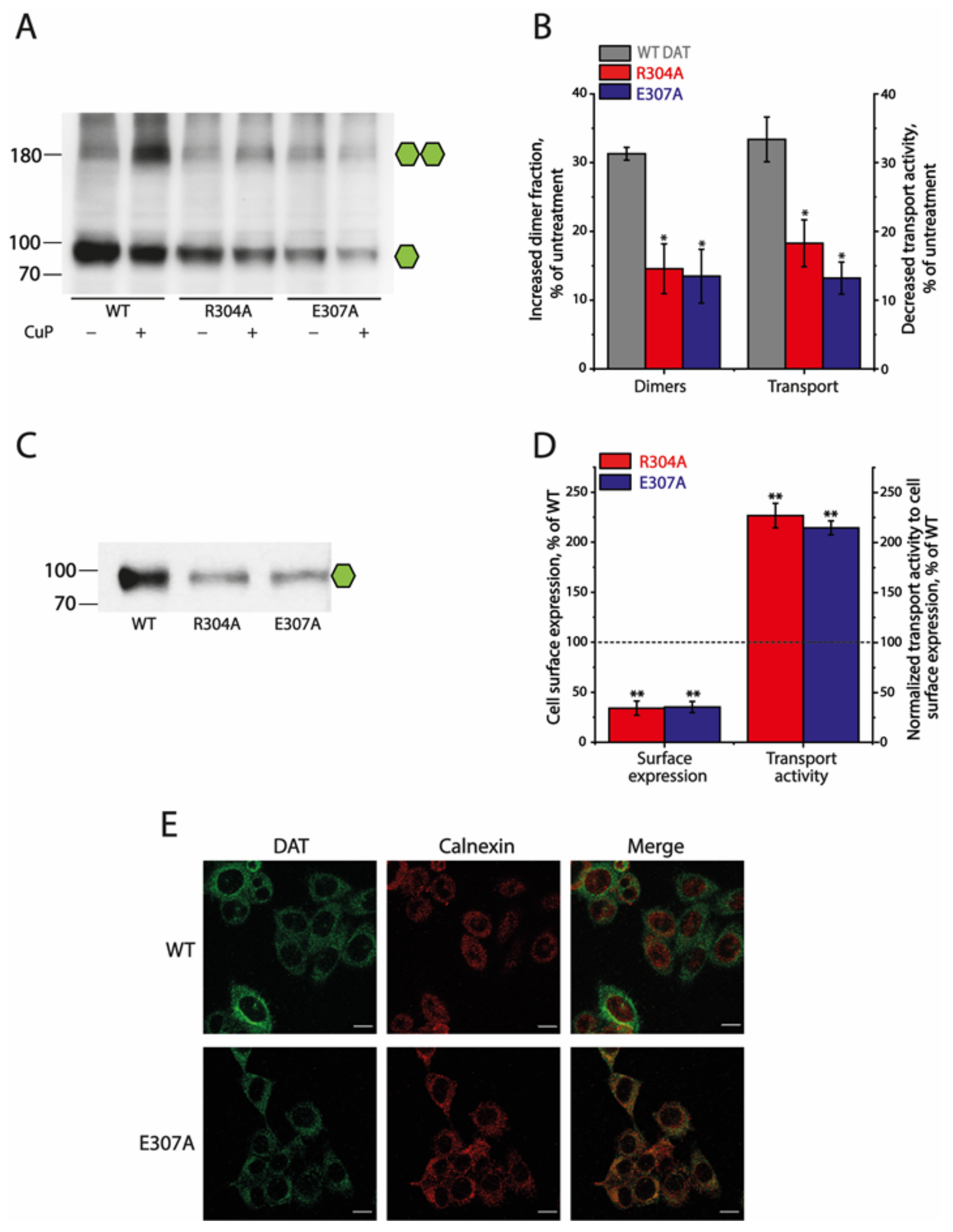
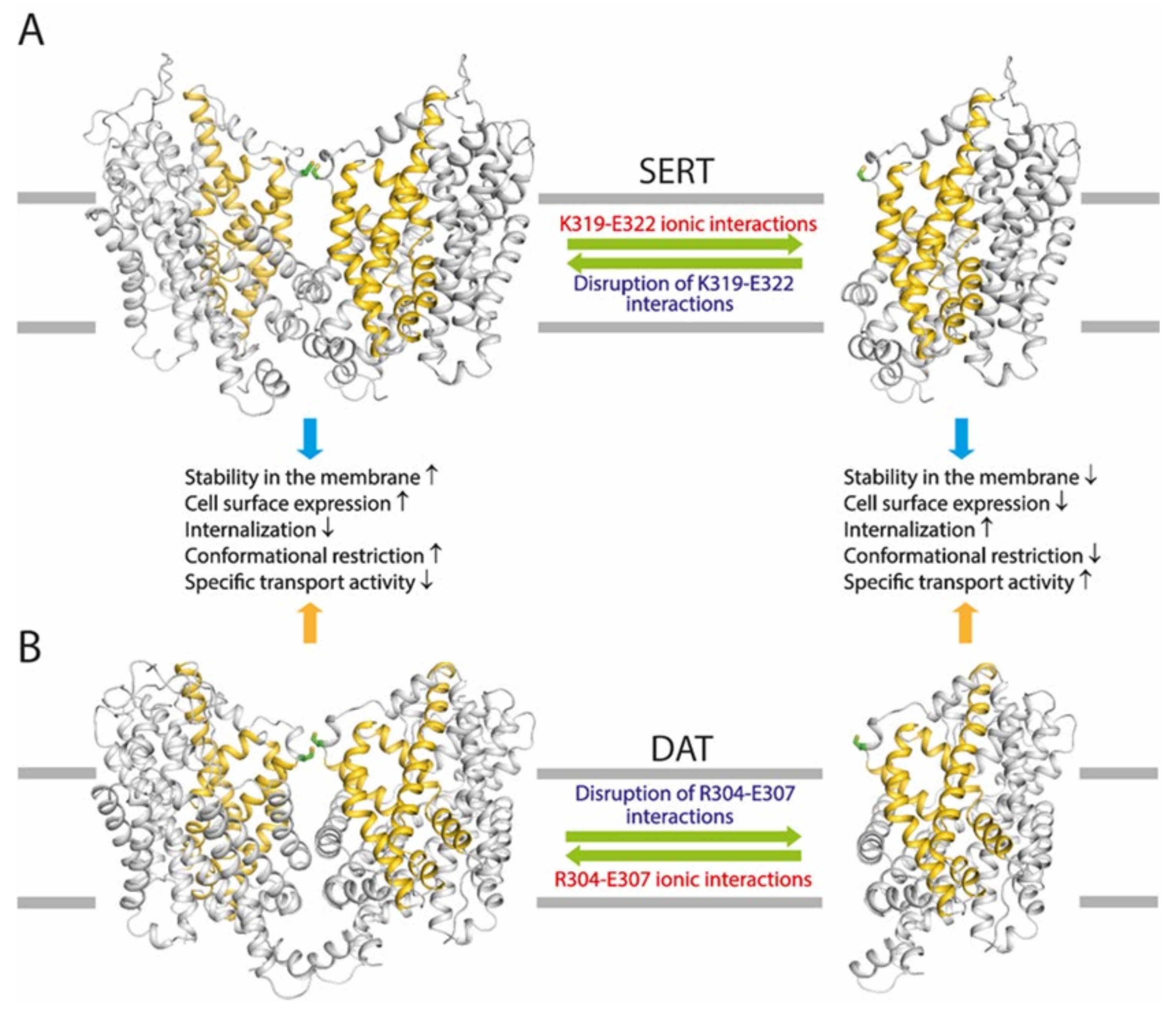
2.4. Effects of the Ion-Pair Interactions on DAT
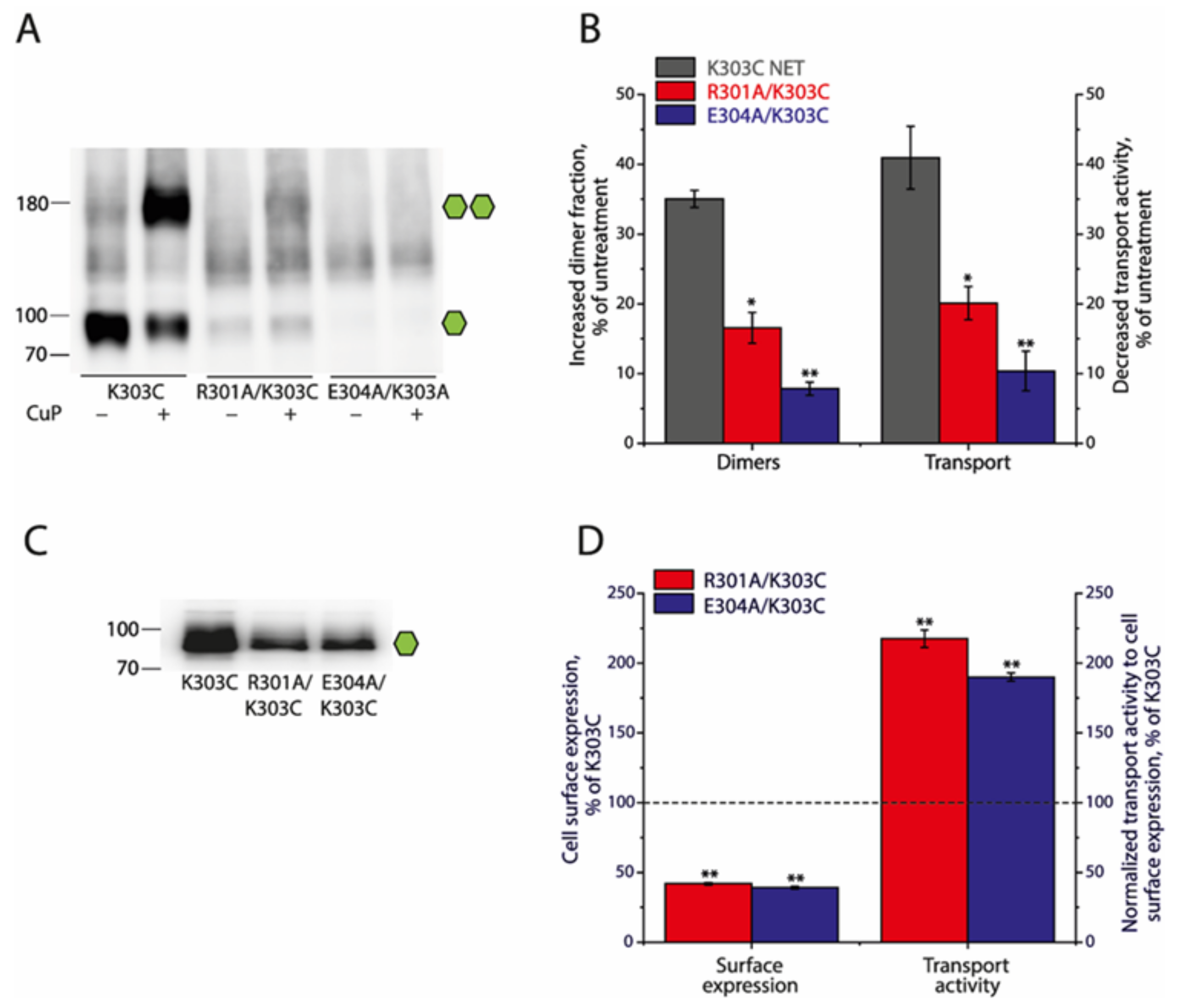
2.5. Effects of the Ion-Pair Interactions on NET
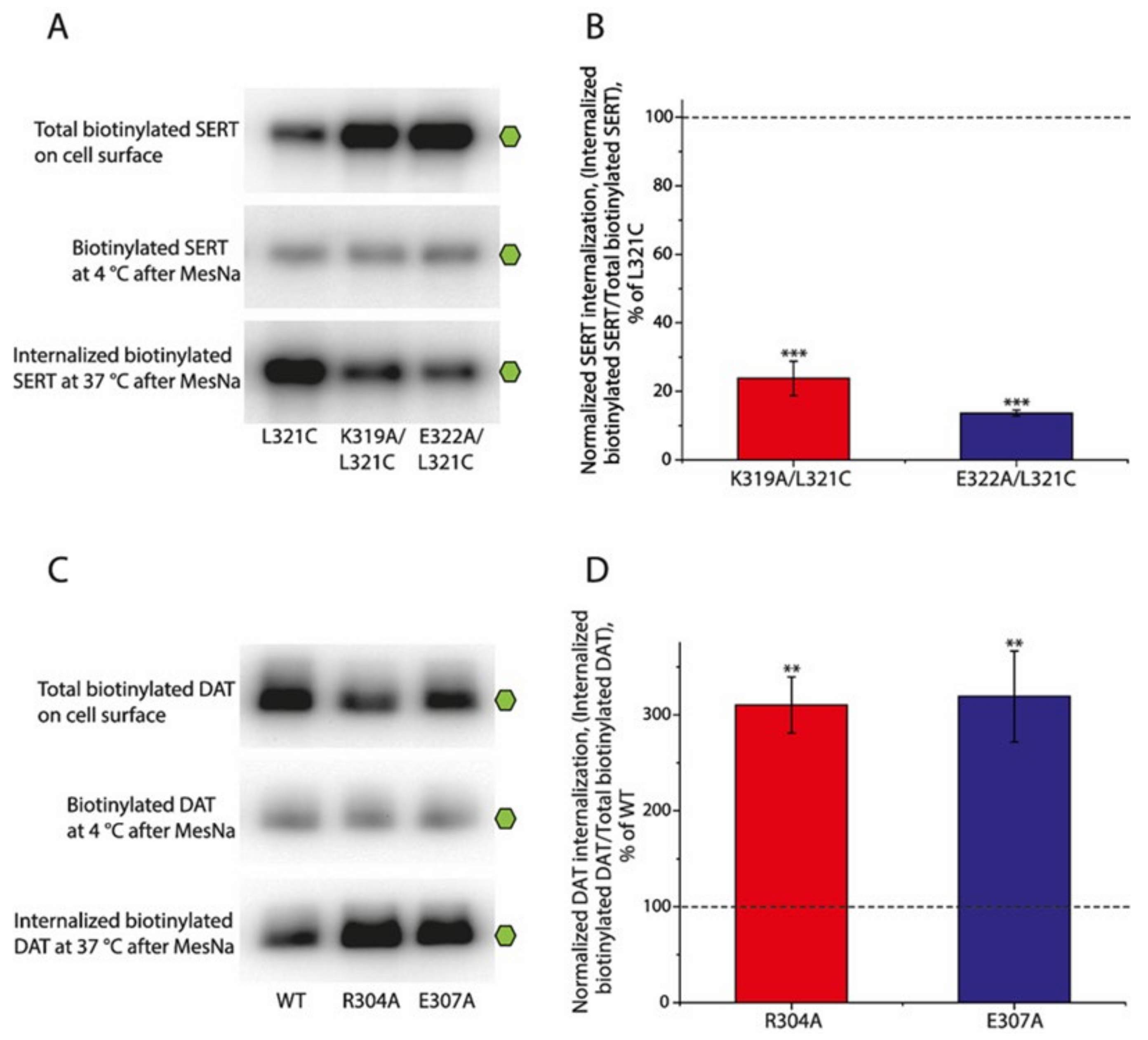
2.6. Effects of the Ion-Pair Interactions on Internalization (Endocytosis) of SERT and DAT
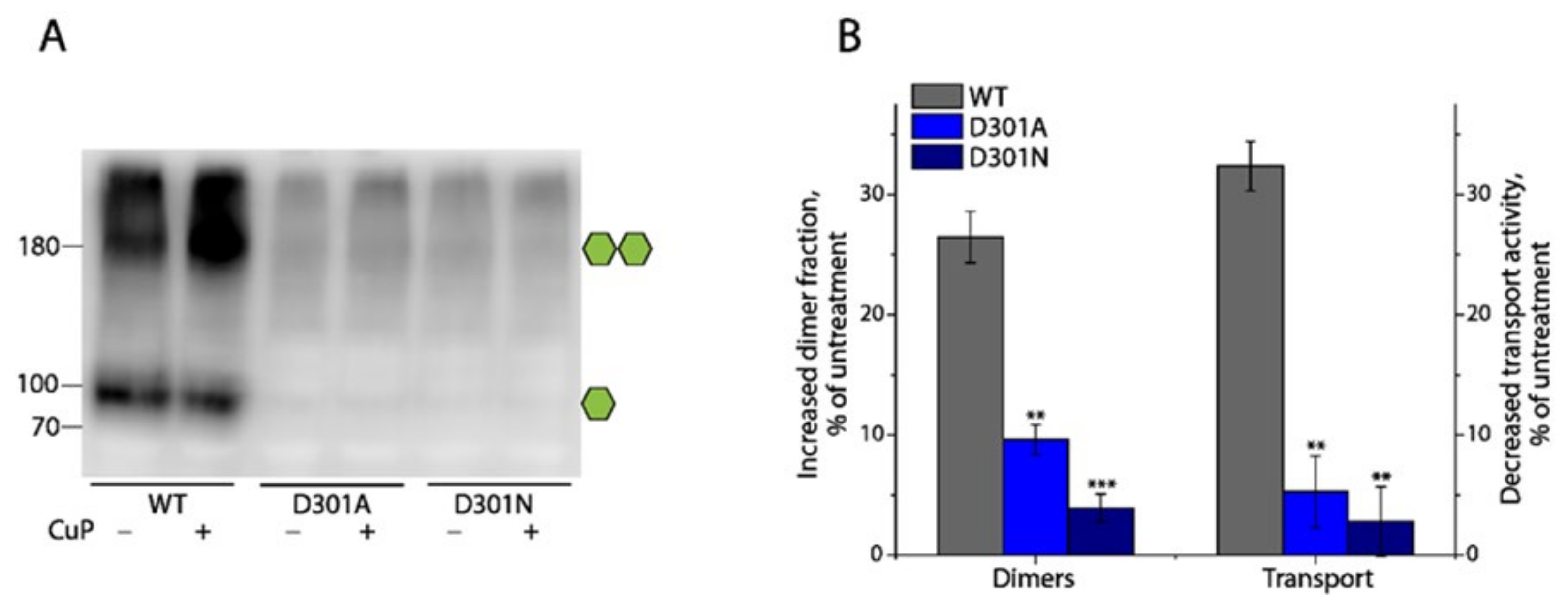
2.7. Effects of Substitutions of Asp301 on DAT
2.8. Effects of E322A Mutation on SERT Conformation
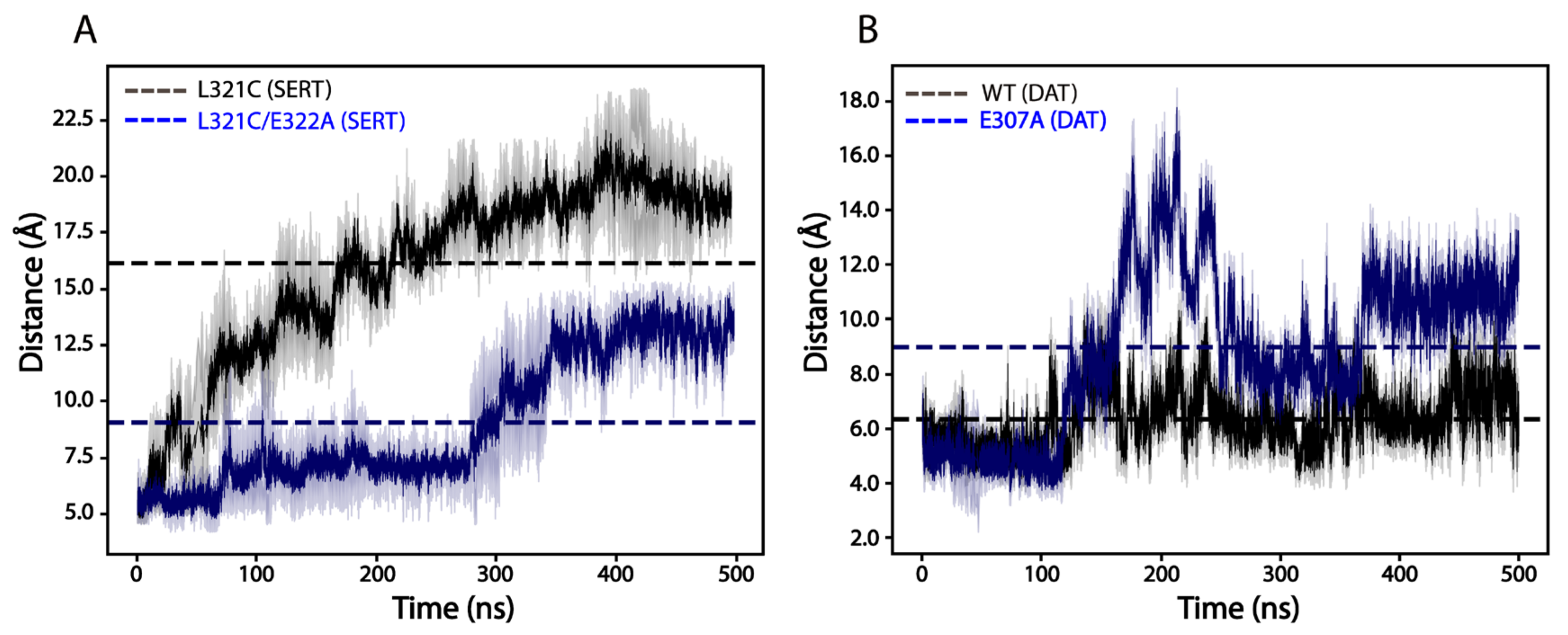
2.9. Effects of the Ion-Pair Mutation on the Dimeric Interface Interactions of SERTs or DATs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mutagenesis and Stable Cell Line Preparation
4.3. Homology Modeling and Dimer Docking of SERT, DAT, and NET
4.4. Oxidative Cross-Linking
4.5. Transport Assay
4.6. Immunoblot Analysis
4.7. Biotinylation
4.8. Reversible Biotinylation
4.9. Immunofluorescence Staining
4.10. Cell-Based SERT Cysteine Accessibility Measurements
4.11. Molecular Dynamics Simulations
4.12. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kristensen, A.S.; Andersen, J.; Jørgensen, T.N.; Sørensen, L.; Eriksen, J.; Loland, C.J.; Strømgaard, K.; Gether, U. SLC6 neurotransmitter transporters: Structure, function, and regulation. Pharmacol. Rev. 2011, 63, 585–640. [Google Scholar] [CrossRef]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S., Jr.; Clayton, P.T. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, G.; Krämer, R.; Blakely, R.D.; Murphy, D.L.; Verrey, F. The SLC6 transporters: Perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014, 466, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.H.; Reith, M.E.; Quick, M.W. Synaptic uptake and beyond: The sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004, 447, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Gether, U.; Andersen, P.H.; Larsson, O.M.; Schousboe, A. Neurotransmitter transporters: Molecular function of important drug targets. Trends Pharmacol. Sci. 2006, 27, 375–383. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mortensen, O.V. Overview of Monoamine Transporters. Curr. Protoc. Pharmacol. 2017, 79, 12.16.1–12.16.17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Bahar, I. Monoamine transporters: Structure, intrinsic dynamics and allosteric regulation. Nat. Struct. Mol. Biol. 2019, 26, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Navratna, V.; Gouaux, E. Insights into the mechanism and pharmacology of neurotransmitter sodium symporters. Curr. Opin. Struct. Biol. 2019, 54, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef]
- Coleman, J.A.; Yang, D.; Zhao, Z.; Wen, P.C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145. [Google Scholar] [CrossRef]
- Yang, D.; Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 2021, 7, eabl3857. [Google Scholar] [CrossRef] [PubMed]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Sitte, H.H.; Freissmuth, M. Oligomer formation by Na+-Cl−-coupled neurotransmitter transporters. Eur. J. Pharmacol. 2003, 479, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Das, A.K.; Luethi, D.; Szöllősi, D.; Schütz, G.J.; Reith, M.E.A.; Sitte, H.H.; Stockner, T. SLC6 transporter oligomerization. J. Neurochem. 2021, 157, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Jess, U.; Betz, H.; Schloss, P. The membrane-bound rat serotonin transporter, SERT1, is an oligomeric protein. FEBS Lett. 1996, 394, 44–46. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.E.; Carneiro, A.; Seamans, K.; Fiorentini, C.; Sweeney, A.; Yao, W.D.; Caron, M.G. Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J. Biol. Chem. 2003, 278, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, A.M.; Rudnick, G.; Kilic, F. Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters. J. Neurochem. 2003, 85, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar] [CrossRef]
- Hastrup, H.; Karlin, A.; Javitch, J.A. Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc. Natl. Acad. Sci. USA 2001, 98, 10055–10060. [Google Scholar] [CrossRef]
- Zhen, J.; Reith, M.E.A. Functional properties of dopamine transporter oligomers after copper linking. J. Neurochem. 2018, 144, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Ponzoni, L.; Sorkina, T.; Lee, J.Y.; Zhang, S.; Sorkin, A.; Bahar, I. Trimerization of dopamine transporter triggered by AIM-100 binding: Molecular mechanism and effect of mutations. Neuropharmacology 2019, 161, 107676. [Google Scholar] [CrossRef]
- Cheng, M.H.; Garcia-Olivares, J.; Wasserman, S.; DiPietro, J.; Bahar, I. Allosteric modulation of human dopamine transporter activity under conditions promoting its dimerization. J. Biol. Chem. 2017, 292, 12471–12482. [Google Scholar] [CrossRef] [PubMed]
- Sorkina, T.; Cheng, M.H.; Bagalkot, T.R.; Wallace, C.; Watkins, S.C.; Bahar, I.; Sorkin, A. Direct coupling of oligomerization and oligomerization-driven endocytosis of the dopamine transporter to its conformational mechanics and activity. J. Biol. Chem. 2021, 296, 100430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Uchendu, S.; Leone, V.; Bradshaw, R.T.; Sangwa, N.; Forrest, L.R.; Rudnick, G. Chloride-dependent conformational changes in the GlyT1 glycine transporter. Proc. Natl. Acad. Sci. USA 2021, 118, e2017431118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H.; Zhang, Y.W. Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1. Int. J. Mol. Sci. 2022, 23, 16157. [Google Scholar] [CrossRef]
- Forrest, L.R.; Zhang, Y.-W.; Jacobs, M.T.; Gesmonde, J.; Xie, L.; Honig, B.H.; Rudnick, G. Mechanism for alternating access in neurotransmitter transporters. Proc. Natl. Acad. Sci. USA 2008, 105, 10338–10343. [Google Scholar] [CrossRef] [PubMed]
- Kilic, F.; Rudnick, G. Oligomerization of serotonin transporter and its functional consequences. Proc. Natl. Acad. Sci. USA 2000, 97, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Sorkina, T.; Doolen, S.; Galperin, E.; Zahniser, N.R.; Sorkin, A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J. Biol. Chem. 2003, 278, 28274–28283. [Google Scholar] [CrossRef]
- Buchmayer, F.; Schicker, K.; Steinkellner, T.; Geier, P.; Stübiger, G.; Hamilton, P.J.; Jurik, A. Amphetamine actions at the serotonin transporter rely on the availability of phosphatidylinositol-4,5-bisphosphate. Proc. Natl. Acad. Sci. USA 2013, 110, 11642–11647. [Google Scholar] [CrossRef]
- Anderluh, A.; Hofmaier, T.; Klotzsch, E.; Kudlacek, O.; Stockner, T.; Sitte, H.H.; Schütz, G.J. Direct PIP(2) binding mediates stable oligomer formation of the serotonin transporter. Nat. Commun. 2017, 8, 14089. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.J.; Belovich, A.N.; Khelashvili, G.; Saunders, C.; Erreger, K.; Javitch, J.A.; Sitte, H.H.; Weinstein, H.; Matthies, H.J.G.; Galli, A. PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nat. Chem. Biol. 2014, 10, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Belovich, A.N.; Aguilar, J.I.; Mabry, S.J.; Cheng, M.H.; Zanella, D.; Hamilton, P.J.; Stanislowski, D.J.; Shekar, A.; Foster, J.D.; Bahar, I.; et al. A network of phosphatidylinositol (4,5)-bisphosphate (PIP2) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila melanogaster. Mol. Psychiatry 2021, 26, 4417–4430. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Morley, A.N.; Szöllősi, D.; Wassenaar, T.A.; Sitte, H.H.; Stockner, T. Dopamine transporter oligomerization involves the scaffold domain, but spares the bundle domain. PLoS Comput. Biol. 2018, 14, e1006229. [Google Scholar] [CrossRef] [PubMed]
- Elegheert, J.; Behiels, E.; Bishop, B.; Scott, S.; Woolley, R.E.; Griffiths, S.C.; Byrne, E.F.X.; Chang, V.T.; Stuart, D.I.; Jones, E.Y.; et al. Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins. Nat. Protoc. 2018, 13, 2991–3017. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Y.; Li, C.; Tang, Q.; Zhang, Y.W. Molecular docking and biochemical validation of (-)-syringaresinol-4-O-β-D-apiofuranosyl-(1→2)-β-D-glucopyranoside binding to an allosteric site in monoamine transporters. Front. Pharmacol. 2022, 13, 1018473. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Kozakov, D. New additions to the ClusPro server motivated by CAPRI. Proteins 2017, 85, 435–444. [Google Scholar] [CrossRef]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Proteins 2013, 81, 2159–2166. [Google Scholar] [CrossRef]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, L.B.; Chen, N.; Ramamoorthy, S.; Chi, L.; Cui, X.N.; Wang, L.C.; Reith, M.E. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 2004, 279, 21012–21020. [Google Scholar] [CrossRef]
- Chen, N.; Reith, M.E. Substrates dissociate dopamine transporter oligomers. J. Neurochem. 2008, 105, 910–920. [Google Scholar] [CrossRef]
- Li, M.; Chen, Q.; Zhang, Y.W. Determining Ligand and Ion-Induced Conformational Changes in Serotonin Transporter with Its Fluorescent Substrates. Int. J. Mol. Sci. 2022, 23, 10919. [Google Scholar] [CrossRef]
- Sato, Y.; Zhang, Y.W.; Androutsellis-Theotokis, A.; Rudnick, G. Analysis of transmembrane domain 2 of rat serotonin transporter by cysteine scanning mutagenesis. J. Biol. Chem. 2004, 279, 22926–22933. [Google Scholar] [CrossRef]
- Jayanthi, L.D.; Samuvel, D.J.; Blakely, R.D.; Ramamoorthy, S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol. Pharmacol. 2005, 67, 2077–2087. [Google Scholar] [CrossRef]
- Jayanthi, L.D.; Samuvel, D.J.; Ramamoorthy, S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J. Biol. Chem. 2004, 279, 19315–19326. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.T.; Zhang, Y.-W.; Campbell, S.D.; Rudnick, G. Ibogaine, a noncompetitive inhibitor of serotonin transport, acts by stabilizing the cytoplasm-facing state of the transporter. J. Biol. Chem. 2007, 282, 29441–29447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Rudnick, G. The cytoplasmic substrate permeation pathway of serotonin transporter. J. Biol. Chem. 2006, 281, 36213–36220. [Google Scholar] [CrossRef]
- Tavoulari, S.; Forrest, L.R.; Rudnick, G. Fluoxetine (Prozac) binding to serotonin transporter is modulated by chloride and conformational changes. J. Neurosci. 2009, 29, 9635–9643. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.; Belfon, K.; Ben-Shalom, I.; Berryman, J.; Brozell, S.; Cerutti, D.; Cheatham, T., III; Cisneros, G.; Cruzeiro, V.; et al. AMBER 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- David, A.C.; Walker, R.C.; Cheatham, T., III; Simmerling, C.; Roitberg, A.; Merz, K.M.; Luo, R.; Li, P.F.; Darden, T.; Celeste, S.; et al. AMBER 22 Reference Manual; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef] [PubMed]
- William, L.J.; Jayaraman, C.; Jeffry, D.M.; Roger, W.I.; Michael, L.K. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Cerutti, D.S.; Cisneros, G.A.; Cruzeiro, V.W.D.; Forouzesh, N. AmberTools. J. Chem. Inf. Model. 2023, 63, 6183–6191. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Huang, X.; Zhang, X.; Li, C.; Zhang, Y.-W. A Conserved Intramolecular Ion-Pair Plays a Critical but Divergent Role in Regulation of Dimerization and Transport Function among the Monoamine Transporters. Int. J. Mol. Sci. 2024, 25, 4032. https://doi.org/10.3390/ijms25074032
Chen S, Huang X, Zhang X, Li C, Zhang Y-W. A Conserved Intramolecular Ion-Pair Plays a Critical but Divergent Role in Regulation of Dimerization and Transport Function among the Monoamine Transporters. International Journal of Molecular Sciences. 2024; 25(7):4032. https://doi.org/10.3390/ijms25074032
Chicago/Turabian StyleChen, Sixiang, Xingyu Huang, Xintong Zhang, Chan Li, and Yuan-Wei Zhang. 2024. "A Conserved Intramolecular Ion-Pair Plays a Critical but Divergent Role in Regulation of Dimerization and Transport Function among the Monoamine Transporters" International Journal of Molecular Sciences 25, no. 7: 4032. https://doi.org/10.3390/ijms25074032
APA StyleChen, S., Huang, X., Zhang, X., Li, C., & Zhang, Y.-W. (2024). A Conserved Intramolecular Ion-Pair Plays a Critical but Divergent Role in Regulation of Dimerization and Transport Function among the Monoamine Transporters. International Journal of Molecular Sciences, 25(7), 4032. https://doi.org/10.3390/ijms25074032





