Amyloid-Forming Corpora Amylacea and Spheroid-Type Amyloid Deposition: Comprehensive Analysis Using Immunohistochemistry, Proteomics, and a Literature Review
Abstract
1. Introduction
2. Results
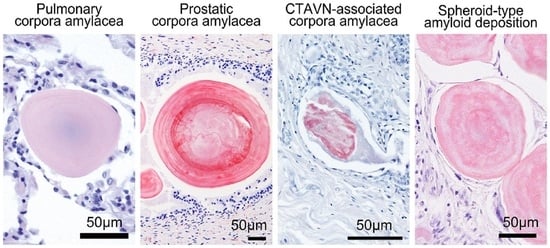
2.1. General Appearance of Pulmonary, Prostatic, and CTAVN-Associated CAs
2.2. General Appearance of Pulmonary, Prostatic, and CTAVN-Associated CAs
2.3. Proteomic and Immunohistochemical Features of Pulmonary CAs
2.4. Proteomic and Immunohistochemical Features of Prostatic CAs
2.5. Comparison of the Amino Acid Sequences of Proteins Commonly Identified in Pulmonary and Prostatic CAs
2.6. Proteomic and Immunohistochemical Features of CTAVN-Associated CAs
2.7. Proteomic and Immunohistochemical Features of STAD Associated with Systemic Amyloid Immunoglobulin Light Chain (AL) Amyloidosis
2.8. Literature Review of Previous Reports on STAD
3. Discussion
4. Materials and Methods
4.1. Tissue Samples
4.2. Histopathological Evaluation
4.3. Proteomics Analysis Using Mass Spectrometry
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Riba, M.; Del Valle, J.; Auge, E.; Vilaplana, J.; Pelegri, C. From corpora amylacea to wasteosomes: History and perspectives. Ageing Res. Rev. 2021, 72, 101484. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Gordon, R.E.; McBride, R.B.; Harpaz, N. Corpora amylacea in gastrointestinal leiomyomas: A clinical, light microscopic, ultrastructural and immunohistochemical study with comparison to hyaline globules. J. Clin. Pathol. 2013, 66, 951–955. [Google Scholar] [CrossRef]
- Hechtman, J.F.; Gordon, R.E.; Harpaz, N. Intramuscular corpora amylacea adjacent to ileal low-grade neuroendocrine tumours (typical carcinoids): A light microscopic, immunohistochemical and ultrastructural study. J. Clin. Pathol. 2013, 66, 566–572. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Yajima, N.; Katayama, Y.; Nomoto, K.; Nishida, N. Sex-dependent expression of prostatic markers and hormone receptors in cystic tumor of the atrioventricular node: A histopathological study of three cases. Pathol. Int. 2021, 71, 141–146. [Google Scholar] [CrossRef]
- DuPre, N.C.; Flavin, R.; Sfanos, K.S.; Unger, R.H.; To, S.; Gazeeva, E.; Fiorentino, M.; De Marzo, A.M.; Rider, J.R.; Mucci, L.A.; et al. Corpora amylacea in prostatectomy tissue and associations with molecular, histological, and lifestyle factors. Prostate 2018, 78, 1172–1180. [Google Scholar] [CrossRef]
- Dobashi, M.; Yuda, F.; Narabayashi, M.; Imai, Y.; Isoda, N.; Obata, K.; Umetsu, A.; Ohgushi, M. Histopathological study of corpora amylacea pulmonum. Histol. Histopathol. 1989, 4, 153–165. [Google Scholar]
- Sfanos, K.S.; Wilson, B.A.; De Marzo, A.M.; Isaacs, W.B. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 3443–3448. [Google Scholar] [CrossRef]
- Kanenawa, K.; Ueda, M.; Isoguchi, A.; Nomura, T.; Tsuda, Y.; Masuda, T.; Misumi, Y.; Yamashita, T.; Ando, Y. Histopathological and biochemical analyses of prostate corpora amylacea. Amyloid 2019, 26, 160–161. [Google Scholar] [CrossRef]
- David, R.; Hiss, Y. Corpora amylacea in mesothelioma of the atrioventricular node. J. Pathol. 1978, 124, 111–116. [Google Scholar] [CrossRef]
- Ichimata, S.; Hata, Y.; Abe, R.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Sekijima, Y.; Ehara, T.; Nishida, N. An autopsy case of amyloid tubulopathy exhibiting characteristic spheroid-type deposition. Virchows Arch. 2020, 477, 157–163. [Google Scholar] [CrossRef]
- Ichimata, S.; Aoyagi, D.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Uehara, T.; Shiozawa, S. A case of spheroid-type localized lactoferrin amyloidosis in the bronchus. Pathol. Int. 2019, 69, 235–240. [Google Scholar] [CrossRef]
- Kim, M.J.; McCroskey, Z.; Piao, Y.; Belcheva, A.; Truong, L.; Kurtin, P.J.; Ro, J.Y. Spheroid-type of AL amyloid deposition associated with colonic adenocarcinoma: A case report with literature review. Pathol. Int. 2018, 68, 123–127. [Google Scholar] [CrossRef]
- Buxbaum, J.N.; Dispenzieri, A.; Eisenberg, D.S.; Fandrich, M.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Westermark, P. Amyloid nomenclature 2022: Update, novel proteins, and recommendations by the International Society of Amyloidosis (ISA) Nomenclature Committee. Amyloid 2022, 29, 213–219. [Google Scholar] [CrossRef]
- Demirhan, B.; Bilezikci, B.; Kiyici, H.; Boyacioglu, S. Globular amyloid deposits in the wall of the gastrointestinal tract: Report of six cases. Amyloid 2002, 9, 42–46. [Google Scholar] [CrossRef]
- Hemmer, P.R.; Topazian, M.D.; Gertz, M.A.; Abraham, S.C. Globular amyloid deposits isolated to the small bowel: A rare association with AL amyloidosis. Am. J. Surg. Pathol. 2007, 31, 141–145. [Google Scholar] [CrossRef]
- Acebo, E.; Mayorga, M.; Fernando Val-Bernal, J. Primary amyloid tumor (amyloidoma) of the jejunum with spheroid type of amyloid. Pathology 1999, 31, 8–11. [Google Scholar] [CrossRef]
- Malhotra, A.; Venugopal, S.; Ravindra, S. Unique spheroid deposits of amyloid in an ampullary neuroendocrine tumour. Indian. J. Pathol. Microbiol. 2022, 65, 226–228. [Google Scholar]
- Diaz Del Arco, C.; Fernandez Acenero, M.J. Globular amyloidosis of the colon. Arab. J. Gastroenterol. 2018, 19, 96–99. [Google Scholar] [CrossRef]
- Martín-Arranz, E.; Pascual-Turrión, J.M.; Martín-Arranz, M.D.; Burgos, E.; Froilán-Torres, C.; Adán-Merino, L.; Lorenzo, A.; Segura-Cabral, J.M. Focal globular amyloidosis of the colon. An exceptional diagnosis. Rev. Esp. Enferm. Dig. 2010, 102, 555–556. [Google Scholar] [CrossRef][Green Version]
- Harris, J.C.; Zhang, Q.; Tondon, R.; Alipour, Z.; Stashek, K. Characterization of amyloidosis in the gastrointestinal tract with an emphasis on histologically distinct interstitial patterns of deposition and misinterpretations. Am. J. Surg. Pathol. 2024, 48, 302–308. [Google Scholar] [CrossRef]
- Makhlouf, H.R.; Goodman, Z.D. Globular hepatic amyloid: An early stage in the pathway of amyloid formation: A study of 20 new cases. Am. J. Surg. Pathol. 2007, 31, 1615–1621. [Google Scholar] [CrossRef]
- Agaram, N.; Shia, J.; Klimstra, D.S.; Lau, N.; Lin, O.; Erlandson, R.A.; Filippa, D.A.; Godwin, T.A. Globular hepatic amyloid: A diagnostic peculiarity that bears clinical significance. Hum. Pathol. 2005, 36, 845–849. [Google Scholar] [CrossRef]
- Pilgaard, J.; Fenger, C.; Schaffalitzky de Muckadell, O.B. Globular amyloid deposits in the liver. Histopathology 1993, 23, 479–480. [Google Scholar] [CrossRef]
- Osick, L.A.; Lee, T.P.; Pedemonte, M.B.; Jacob, L.; Chauhan, P.; Navarro, C.; Comer, G.M. Hepatic amyloidosis in intravenous drug abusers and AIDS patients. J. Hepatol. 1993, 19, 79–84. [Google Scholar] [CrossRef]
- French, S.W.; Schloss, G.T.; Stillman, A.E. Unusual amyloid bodies in human liver. Am. J. Clin. Pathol. 1981, 75, 400–402. [Google Scholar] [CrossRef]
- Chandan, V.S.; Shah, S.S.; Lam-Himlin, D.M.; Petris, G.D.; Mereuta, O.M.; Dogan, A.; Torbenson, M.S.; Wu, T.T. Globular hepatic amyloid is highly sensitive and specific for LECT2 amyloidosis. Am. J. Surg. Pathol. 2015, 39, 558–564. [Google Scholar] [CrossRef]
- Kumar, B.; Pant, B.; Kumar, V.; Negi, M. Sinonasal globular amyloidosis simulating malignancy: A rare presentation. Head. Neck Pathol. 2016, 10, 379–383. [Google Scholar] [CrossRef]
- Drut, R.; Giménez, P.O. Acinic cell carcinoma of salivary gland with massive deposits of globular amyloid. Int. J. Surg. Pathol. 2008, 16, 202–207. [Google Scholar] [CrossRef]
- Michaels, L.; Hyams, V.J. Amyloid in localised deposits and plasmacytomas of the respiratory tract. J. Pathol. 1979, 128, 29–38. [Google Scholar] [CrossRef]
- Pambuccian, S.E.; Horyd, I.D.; Cawte, T.; Huvos, A.G. Amyloidoma of bone, a plasma cell/plasmacytoid neoplasm. Report of three cases and review of the literature. Am. J. Surg. Pathol. 1997, 21, 179–186. [Google Scholar] [CrossRef]
- Bauer, W.H.; Kuzma, J.F. Solitary tumors of atypical amyloid (paramyloid). Am. J. Clin. Pathol. 1949, 19, 1097–1112. [Google Scholar] [CrossRef] [PubMed]
- Unal, F.; Hepgül, K.; Bayindir, C.; Bilge, T.; Imer, M.; Turantan, I. Skull base amyloidoma: Case report. J. Neurosurg. 1992, 76, 303–306. [Google Scholar] [CrossRef]
- Bommannan, B.K.K.; Sonai, M.; Sachdeva, M.U. Bone marrow amyloid spherulites in a case of AL amyloidosis. Blood Cells Mol. Dis. 2016, 58, 19–20. [Google Scholar] [CrossRef]
- Mantoo, S.; Hwang, J.S.; Chiang, G.S.; Tan, P.H. A rare case of localised AA-type amyloidosis of the ureter with spheroids of amyloid. Singapore Med. J. 2012, 53, e77–e79. [Google Scholar]
- Gibbons, D.; Lindberg, G.M.; Ashfaq, R.; Saboorian, M.H. Localized amyloidosis of the uterine cervix. Int. J. Gynecol. Pathol. 1998, 17, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Gondo, T.; Ishihara, T.; Kawano, H.; Uchino, F.; Takahashi, M.; Iwata, T.; Matsumoto, N.; Yokota, T. Localized amyloidosis in squamous cell carcinoma of uterine cervix: Electron microscopic features of nodular and star–like amyloid deposits. Virchows Arch. A Pathol. Anat. Histopathol. 1993, 422, 225–231. [Google Scholar] [CrossRef]
- Aho, H.J.; Talve, L.; Mäenpää, J. Acantholytic squamous cell carcinoma of the uterine cervix with amyloid deposition. Int. J. Gynecol. Pathol. 1992, 11, 150–155. [Google Scholar] [CrossRef]
- Kitamura, K.; Nakayama, T.; Ohata, K.; Wakasa, K.; Miki, Y. Computed tomography and magnetic resonance imaging appearance of prolactinoma with spheroid-type amyloid deposition. J. Comput. Assist. Tomogr. 2011, 35, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Bahadir, B.; Dusak, A.; Kalayci, M.; Edebali, N.; Acikgoz, B. Spherical amyloid deposition in a prolactin-producing pituitary adenoma. Neuropathology 2009, 29, 81–84. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Lee, S.K.; Kim, T.S. Squash smear findings of spherical amyloid in pituitary prolactinoma. A case report. Acta Cytol. 2004, 48, 447–450. [Google Scholar] [CrossRef]
- Hassan, T.; Ikeda, H.; Yoshimoto, T. Salmon roe-like amyloid deposition in a prolactinoma: A case report. Brain Tumor Pathol. 2003, 20, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Wiesli, P.; Brändle, M.; Brandner, S.; Kollias, S.S.; Bernays, R.L. Extensive spherical amyloid deposition presenting as a pituitary tumor. J. Endocrinol. Investig. 2003, 26, 552–555. [Google Scholar] [CrossRef]
- Hinton, D.R.; Polk, R.K.; Linse, K.D.; Weiss, M.H.; Kovacs, K.; Garner, J.A. Characterization of spherical amyloid protein from a prolactin-producing pituitary adenoma. Acta Neuropathol. 1997, 93, 43–49. [Google Scholar] [CrossRef]
- Kuratsu, J.; Matsukado, Y.; Miura, M. Prolactinoma of pituitary with associated amyloid-like substances. Case report. J. Neurosurg. 1983, 59, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, H.; Kitamura, A.; Kagawa, Y.; Hirayama, K.; Yoshino, T.; Kamiya, S. Localized amyloidosis in canine mammary tumors. Vet. Pathol. 2000, 37, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Alexeyev, O.; Zamotin, V.; Srivastava, V.; Shchukarev, A.; Brorsson, A.C.; Tartaglia, G.G.; Vogl, T.; Kayed, R.; Wingsle, G.; et al. Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLoS ONE 2009, 4, e5562. [Google Scholar] [CrossRef]
- Tekin, B.; Dasari, S.; Theis, J.D.; Vrana, J.A.; Murray, D.L.; Oglesbee, D.; Thompson, R.H.; Leibovich, B.C.; Boorjian, S.A.; Whaley, R.D.; et al. Mass spectrometry-based assessment of prostate cancer-associated crystalloids reveals enrichment for growth and differentiation factor 15. Hum. Pathol. 2023, 135, 35–44. [Google Scholar] [CrossRef]
- Cross, P.A.; Bartley, C.J.; McClure, J. Amyloid in prostatic corpora amylacea. J. Clin. Pathol. 1992, 45, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Palangmonthip, W.; Wu, R.; Tarima, S.; Bobholz, S.A.; LaViolette, P.S.; Gallan, A.J.; Iczkowski, K.A. Corpora amylacea in benign prostatic acini are associated with concurrent, predominantly low-grade cancer. Prostate 2020, 80, 687–697. [Google Scholar] [CrossRef]
- Riba, M.; Campo-Sabariz, J.; Tena, I.; Molina-Porcel, L.; Ximelis, T.; Calvo, M.; Ferrer, R.; Martin-Venegas, R.; Del Valle, J.; Vilaplana, J.; et al. Wasteosomes (corpora amylacea) of human brain can be phagocytosed and digested by macrophages. Cell Biosci. 2022, 12, 177. [Google Scholar] [CrossRef]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.; Simon, R.; Schubert, D.; et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef]
- Pioselli, B.; Salomone, F.; Mazzola, G.; Amidani, D.; Sgarbi, E.; Amadei, F.; Murgia, X.; Catinella, S.; Villetti, G.; De Luca, D.; et al. Pulmonary surfactant: A unique biomaterial with life-saving therapeutic applications. Curr. Med. Chem. 2022, 29, 526–590. [Google Scholar] [CrossRef]
- Gustafsson, M.; Thyberg, J.; Naslund, J.; Eliasson, E.; Johansson, J. Amyloid fibril formation by pulmonary surfactant protein C. FEBS Lett. 1999, 464, 138–142. [Google Scholar] [CrossRef]
- Yamanouchi, H.; Yoshinouchi, T.; Watanabe, R.; Fujita, J.; Takahara, J.; Ohtsuki, Y. Immunohistochemical study of a patient with diffuse pulmonary corpora amylacea detected by open lung biopsy. Intern. Med. 1999, 38, 900–903. [Google Scholar] [CrossRef][Green Version]
- Otzen, D.; Riek, R. Functional amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef]
- Ishii, W.; Matsuda, M.; Nakamura, N.; Katsumata, S.; Toriumi, H.; Suzuki, A.; Ikeda, S. Phenol Congo red staining enhances the diagnostic value of abdominal fat aspiration biopsy in reactive AA amyloidosis secondary to rheumatoid arthritis. Intern. Med. 2003, 42, 400–405. [Google Scholar] [CrossRef]
- Kametani, F.; Haga, S. Accumulation of carboxy-terminal fragments of APP increases phosphodiesterase 8B. Neurobiol. Aging 2015, 36, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Abe, R.; Katoh, N.; Takahashi, Y.; Takasone, K.; Yoshinaga, T.; Yazaki, M.; Kametani, F.; Sekijima, Y. Distribution of amyloidosis subtypes based on tissue biopsy site—Consecutive analysis of 729 patients at a single amyloidosis center in Japan. Pathol. Int. 2021, 71, 70–79. [Google Scholar] [CrossRef] [PubMed]






| Pulmonary CA Cases | Prostatic CA Cases | CTAVN Cases | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case # | Case 1 * | Case 2 | Case 3 | Case 1 * | Case 2 | Case 3 | Case 1 | Case 2 | Case 3 |
| Age | 75 | 90 | 85 | 75 | 88 | 45 | 36 | 45 | 76 |
| Sex | M | F | F | M | M | M | F | M | M |
| Cause of death | HyT | MN | Drowning | HyT | ACD | KA | SCD | SCD | SCD |
| Dialysis | None | None | None | None | None | None | None | None | None |
| Location | S/L | Concomitant Findings | Immunohistochemical Analysis | Proteomic Analysis |
|---|---|---|---|---|
| Stomach and small intestine [14] | S | Bronchiectasis, FMF, and renal failure | Pos: AA; Neg: β2MG, TTR, Igκ, Igλ, CD68 | NE |
| Small intestine [15,16] | L | Polypoid lesions | Pos: Igλ; Neg: AA, β2MG, TTR, Igκ [15] Pos: AP, AA, Igκ, Igλ (uneven) [16] | NE |
| Vater ampulla [17] | L | NET | NE | NE |
| Colon, TI [12] | L | Adenocarcinoma | NE | ALλ |
| Colon [18,19,20] | L | Ulcerative lesions [18] and rounded lesions [19] | Pos: Igλ [18] | NE |
| Liver [21,22,23,24,25,26] | S/L | Various diseases (See [21,22]) | Pos: AP, AA; Neg: Igκ, Igλ [21] Pos: none; Neg: AP, AA, UB, TTR, Igκ, Ig [23] Pos: AA; Neg: β2MG, TTR, Igκ, Igλ [24] Pos: LECT2 or Ig; Neg: AA, β2MG, TTR [26] | ALECT2 or AL [26] |
| Sino-nasal tract [27] | L | Nasal mass | Pos: Igκ and Igλ (κ > λ) | NE |
| Parotid gland [28] | L | Acinic cell carcinoma | NE | NE |
| URT [29] | S/L | Plasmacytoma | NE | NE |
| Bronchus [11] | L | Erythematous mass | Pos: Lac; Neg: AA, β2MG, TTR, Igκ, Igλ | ALac |
| Bone [30,31,32] | L | Myeloma, malignant lymphoma (see [30]) | Pos: Igκ or Igλ; Neg: AA [30] | NE |
| Bone marrow [33] | S | PCP | Pos: Igλ | NE |
| Ureter [34] | L | Hydronephrosis | NE (likely AA) | NE |
| Kidney [10] | S | PCP | Pos: Igλ; Neg: AA, β2MG, TTR, Igκ | ALλ |
| Uterine cervix [35,36,37] | L | Smooth mass [36], SCC [37,38] | Pos: AA; Neg: CK, Igκ, Igλ [35] Pos: CK; Neg: AA, TTR, Igκ, Igλ [36,37] | NE |
| Pituitary gland [38,39,40,41,42,43,44] | L | Prolactinoma | Pos: PRL; Neg: CK, vimentin, GFAP, GH, FSH, LH, TSH, ACTH, β-A4 [39,41,42] | NE |
| Breast [45] * | L | Mammary tumor | Pos: α-casein, Lac; Neg: AA, TTR, CK, Igκ, Igλ | NE |
| Prostatic-CA | Pulmonary-CA | CTAVN-CA | STAD | |
|---|---|---|---|---|
| Congophilia | Strong | Moderate–strong | Weak–moderate | Strong |
| Strength of the AGBR | Strong | Strong | Weak–moderate | Strong |
| Macrophages | Positive | Positive | Negative–positive | Positive |
| Presence of CPs | Positive | Positive | Negative | Negative |
| Presence of AAPs | Positive | Positive | Negative | Positive |
| p62-IR | Positive (focal) | Positive (focal) | Negative–positive (focal) | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichimata, S.; Hata, Y.; Yoshinaga, T.; Katoh, N.; Kametani, F.; Yazaki, M.; Sekijima, Y.; Nishida, N. Amyloid-Forming Corpora Amylacea and Spheroid-Type Amyloid Deposition: Comprehensive Analysis Using Immunohistochemistry, Proteomics, and a Literature Review. Int. J. Mol. Sci. 2024, 25, 4040. https://doi.org/10.3390/ijms25074040
Ichimata S, Hata Y, Yoshinaga T, Katoh N, Kametani F, Yazaki M, Sekijima Y, Nishida N. Amyloid-Forming Corpora Amylacea and Spheroid-Type Amyloid Deposition: Comprehensive Analysis Using Immunohistochemistry, Proteomics, and a Literature Review. International Journal of Molecular Sciences. 2024; 25(7):4040. https://doi.org/10.3390/ijms25074040
Chicago/Turabian StyleIchimata, Shojiro, Yukiko Hata, Tsuneaki Yoshinaga, Nagaaki Katoh, Fuyuki Kametani, Masahide Yazaki, Yoshiki Sekijima, and Naoki Nishida. 2024. "Amyloid-Forming Corpora Amylacea and Spheroid-Type Amyloid Deposition: Comprehensive Analysis Using Immunohistochemistry, Proteomics, and a Literature Review" International Journal of Molecular Sciences 25, no. 7: 4040. https://doi.org/10.3390/ijms25074040
APA StyleIchimata, S., Hata, Y., Yoshinaga, T., Katoh, N., Kametani, F., Yazaki, M., Sekijima, Y., & Nishida, N. (2024). Amyloid-Forming Corpora Amylacea and Spheroid-Type Amyloid Deposition: Comprehensive Analysis Using Immunohistochemistry, Proteomics, and a Literature Review. International Journal of Molecular Sciences, 25(7), 4040. https://doi.org/10.3390/ijms25074040