Complement MASP-1 Modifies Endothelial Wound Healing
Abstract
:1. Introduction
2. Results
2.1. rMASP-1 Alters the Expression of Wound-Healing-Related and Angiogenesis-Related Genes
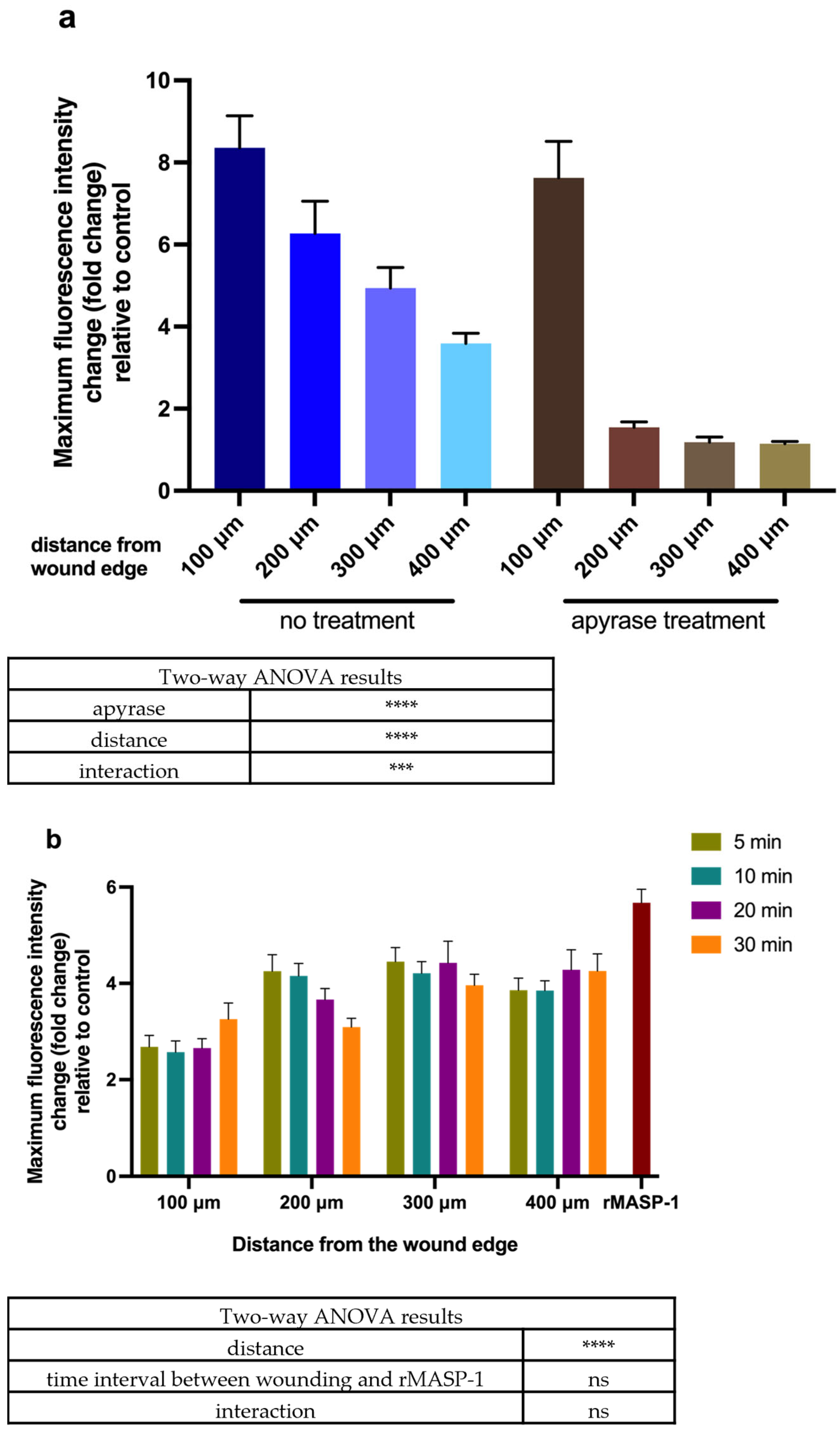
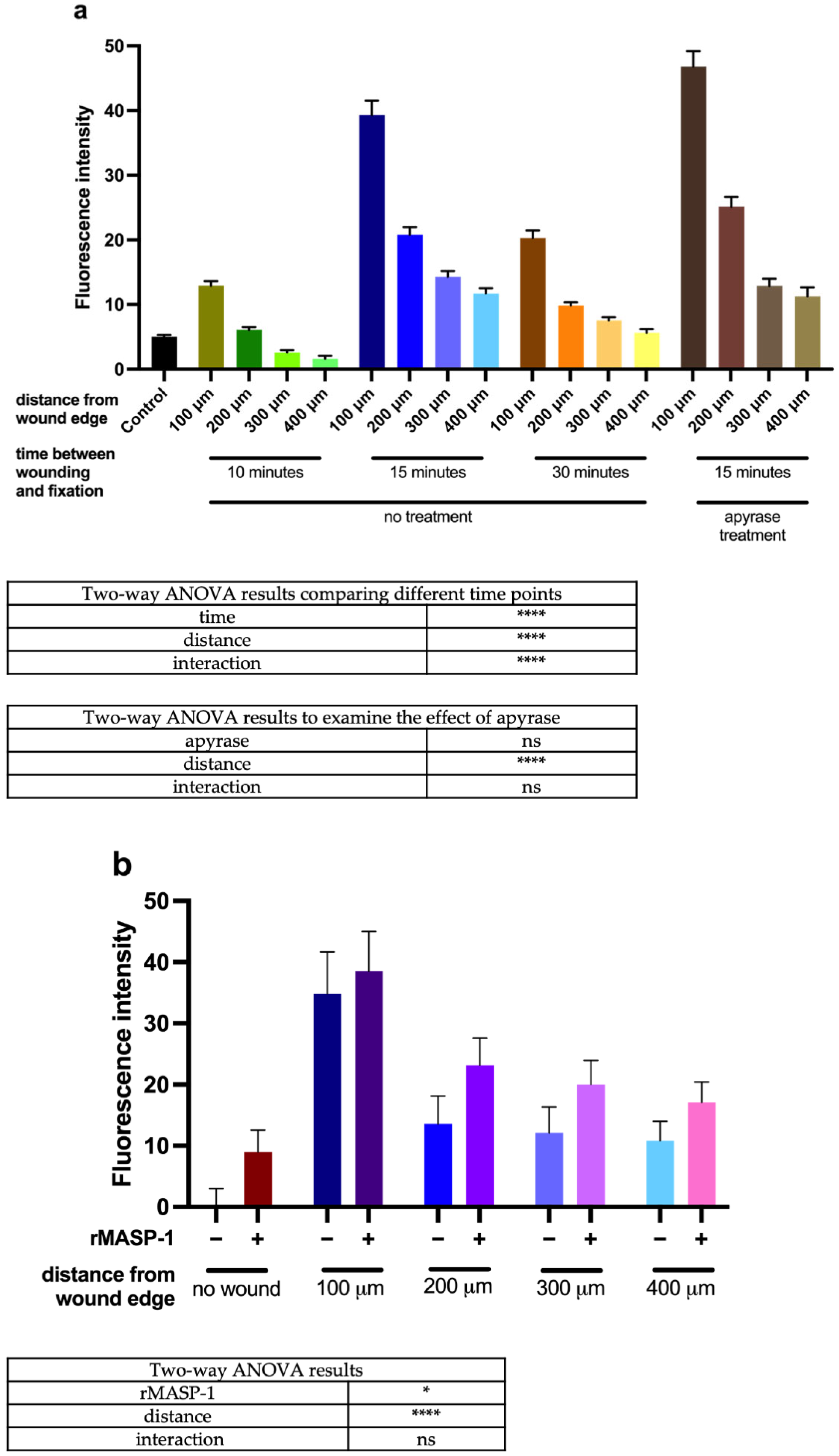
2.2. Calcium Wave Propagation
2.3. Changes in CREB Phosphorylation and NFκB Activation
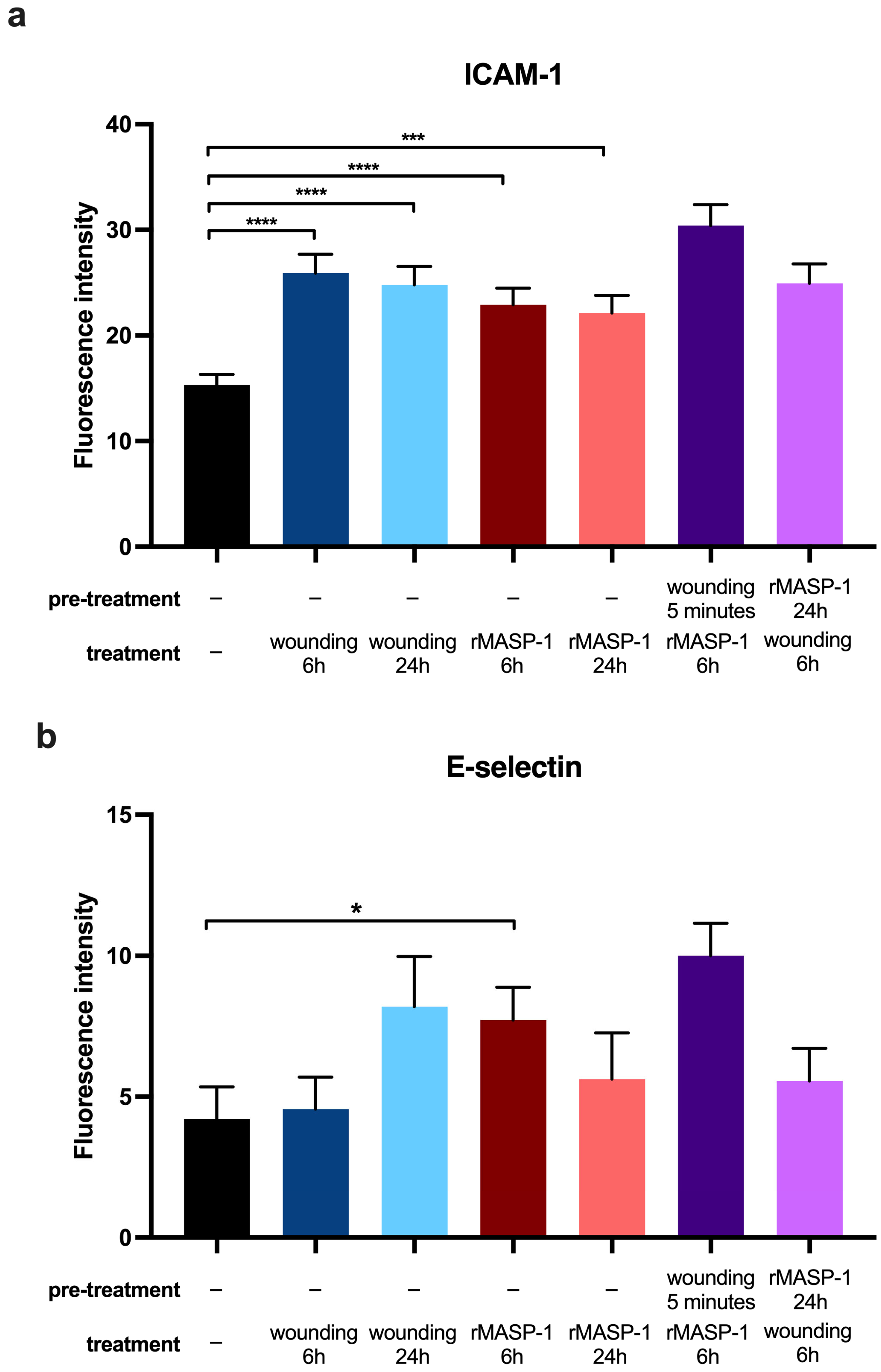
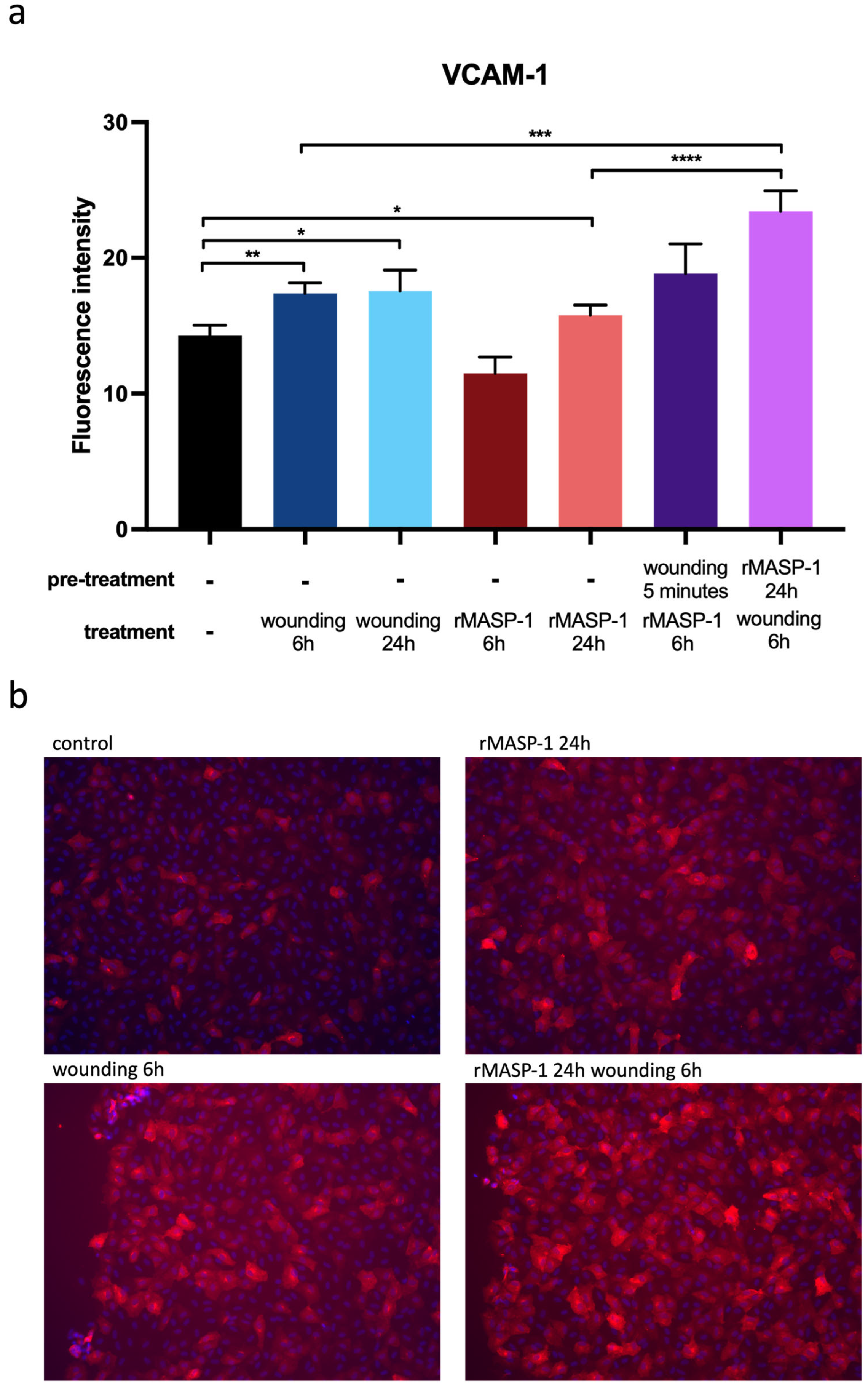
2.4. Expression of Adhesion Molecules
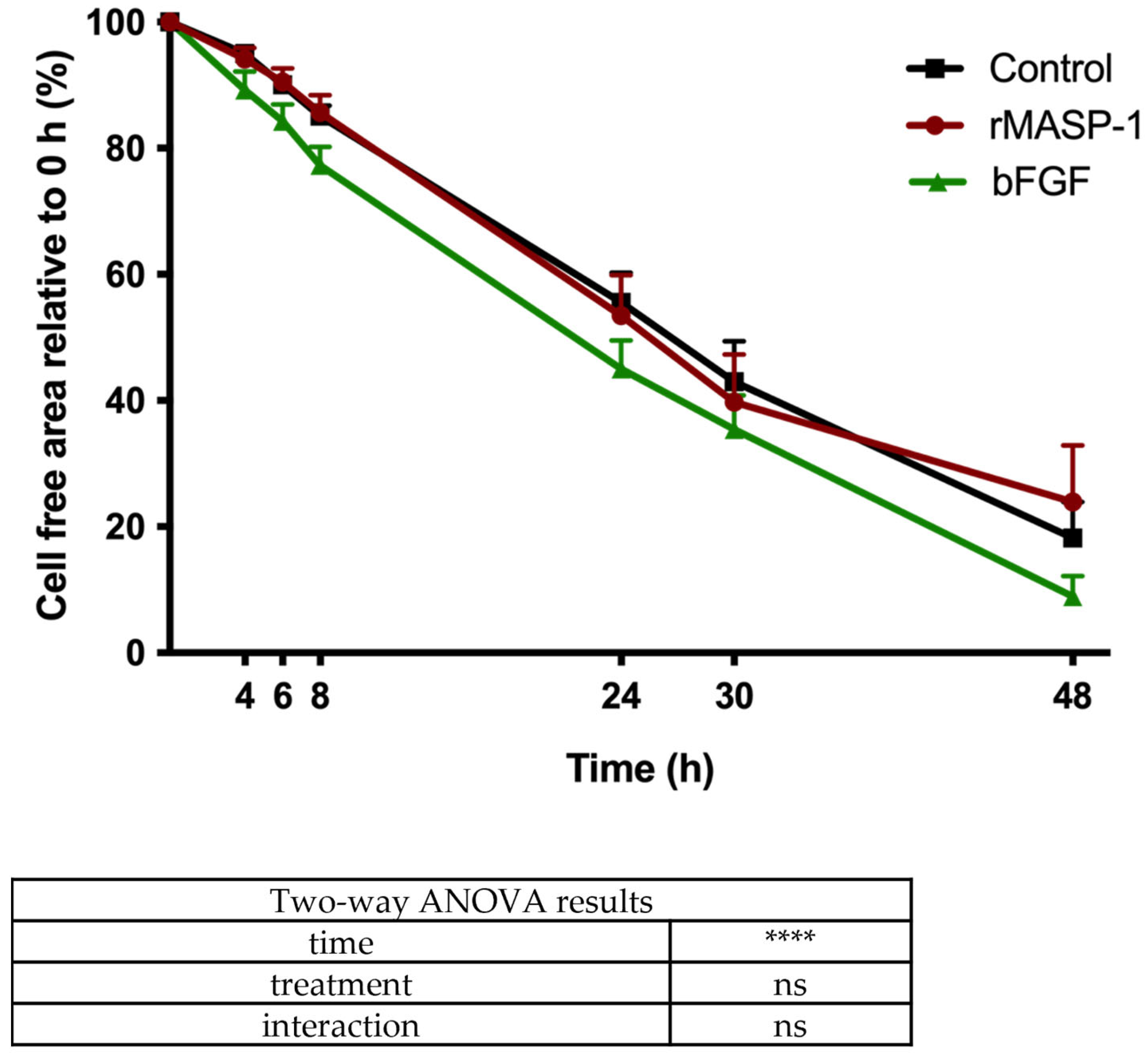
2.5. Wound-Healing Assay
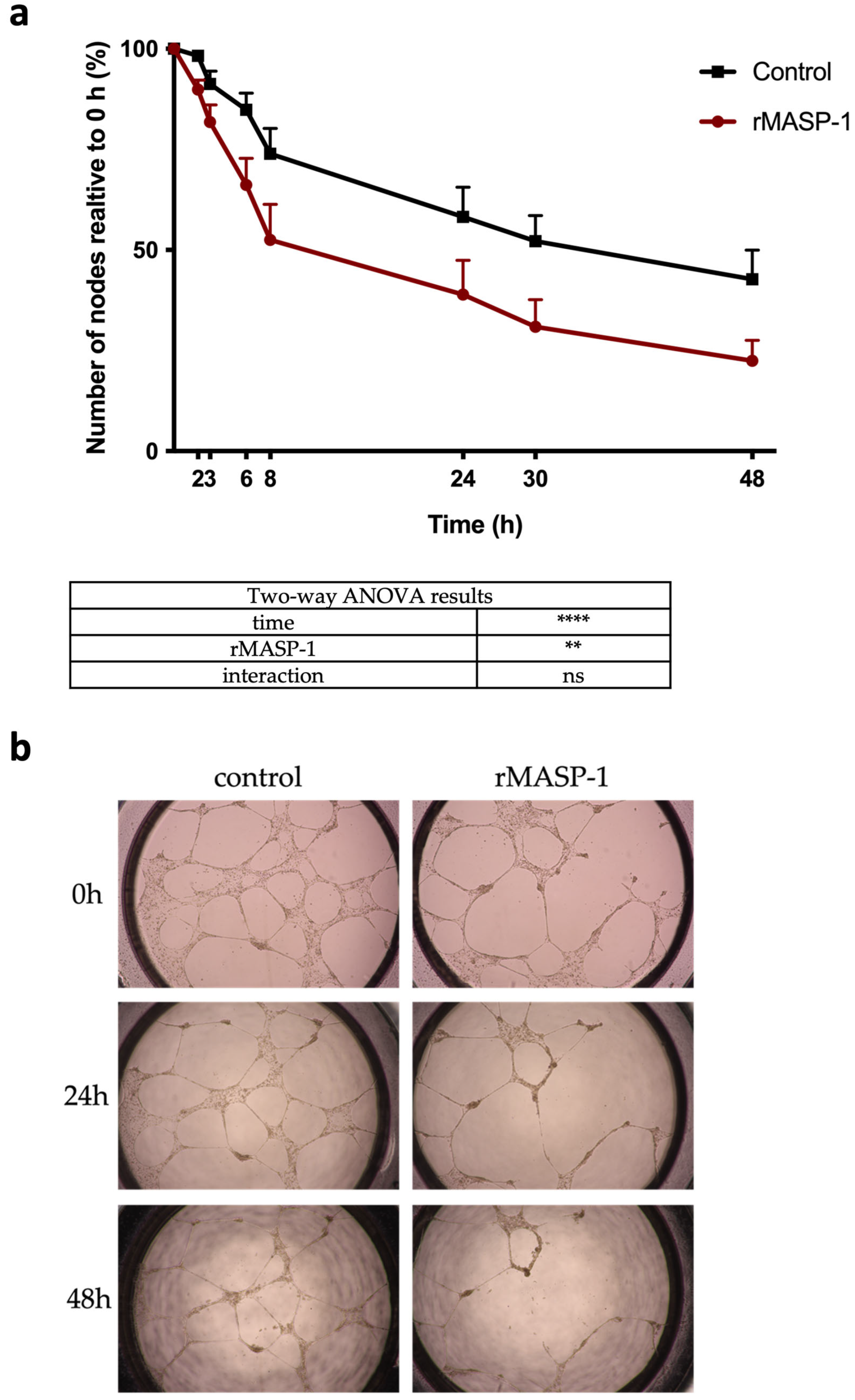
2.6. Capillary Networks on Matrigel®
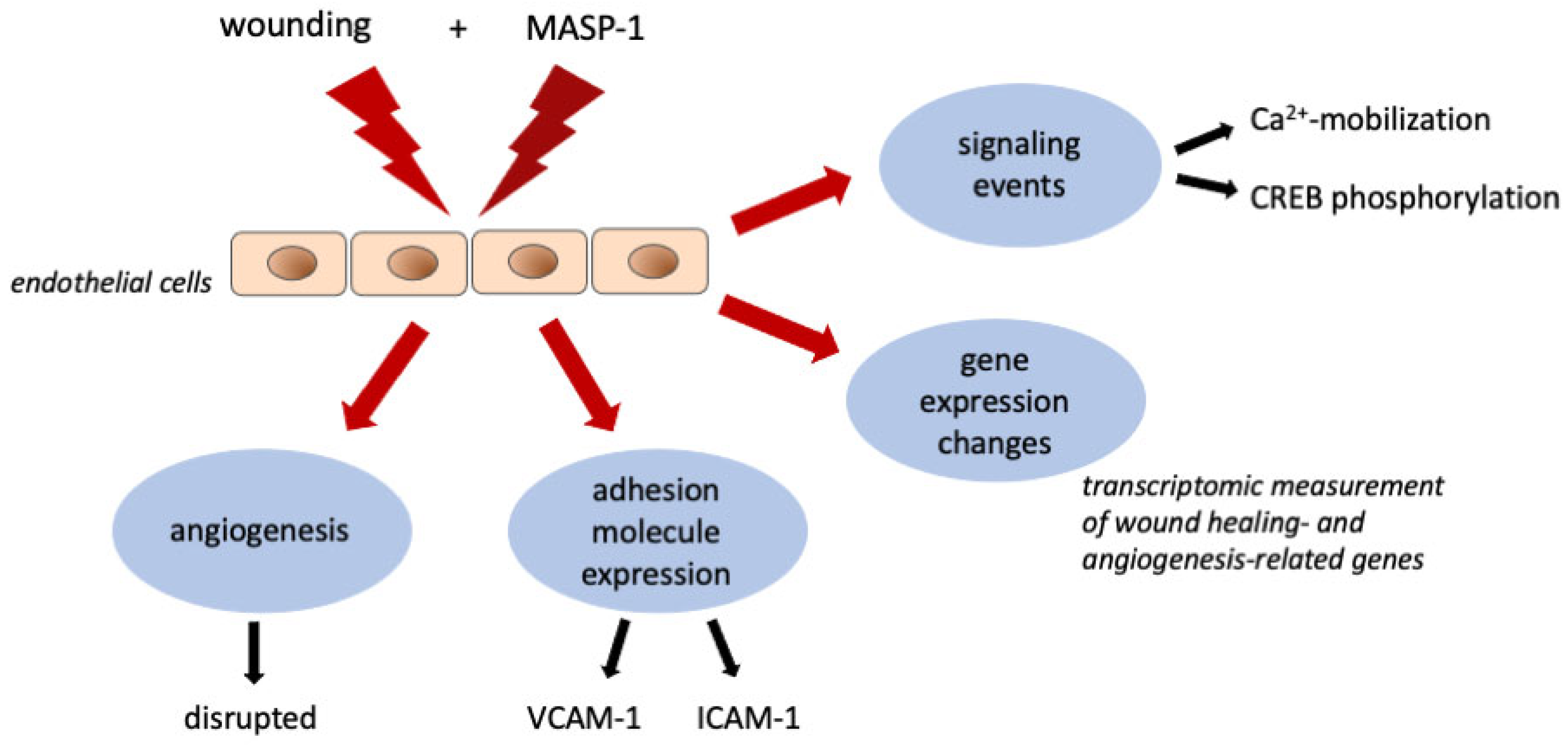
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation and Culturing of Human Umbilical Vein Endothelial Cells (HUVECs)
4.3. Gene Set Enrichment Analysis (GSEA)
4.4. Intracellular Ca2+ Mobilization Assay
4.5. Measurement of CREB Phosphorylation and NFκB Activation by Fluorescent Microscopy
4.6. Wound-Healing Assay
4.7. Capillary Network Integrity—Matrigel® Assay
4.8. Visualization of Adhesion Molecules by Fluorescent Microscopy
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henriksen, M.L.; Brandt, J.; Andrieu, J.-P.; Nielsen, C.; Jensen, P.H.; Holmskov, U.; Jorgensen, T.J.D.; Palarasah, Y.; Thielens, N.M.; Hansen, S. Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J. Immunol. 2013, 191, 6117–6127. [Google Scholar] [CrossRef] [PubMed]
- Garred, P.; Genster, N.; Pilely, K.; Bayarri-Olmos, R.B.; Rosbjerg, A.; Ma, Y.J.; Skjoedt, M.O. A journey through the lectin pathway of complement-MBL and beyond. Immunol. Rev. 2016, 274, 74–97. [Google Scholar] [CrossRef]
- Héja, D.; Harmat, V.; Fodor, K.; Wilmanns, M.; Dobó, J.; Kékesi, K.A.; Závodszky, P.; Gál, P.; Pál, G. Monospecific inhibitors show that both mannan-binding lectin-associated serine protease-1 (MASP-1) and -2 Are essential for lectin pathway activation and reveal structural plasticity of MASP-2. J. Biol. Chem. 2012, 287, 20290–20300. [Google Scholar] [CrossRef] [PubMed]
- Schwaner, E.; Németh, Z.; Jani, P.K.; Kajdácsi, E.; Debreczeni, M.L.; Doleschall, Z.; Dobó, J.; Gál, P.; Rigó, J.; András, K.; et al. Transcriptome analysis of inflammation-related gene expression in endothelial cells activated by complement MASP-1. Sci. Rep. 2017, 7, 10462. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, M.; Makó, V.; Beinrohr, L.; Doleschall, Z.; Prohászka, Z.; Cervenak, L.; Závodszky, P.; Gál, P. Complement protease MASP-1 activates human endothelial cells: PAR4 activation is a link between complement and endothelial function. J. Immunol. 2009, 183, 3409–3416. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.K.; Kajdácsi, E.; Megyeri, M.; Dobó, J.; Doleschall, Z.; Futosi, K.; Tímár, C.I.; Mócsai, A.; Makó, V.; Gál, P.; et al. MASP-1 induces a unique cytokine pattern in endothelial cells: A novel link between complement system and neutrophil granulocytes. PLoS ONE 2014, 9, e87104. [Google Scholar] [CrossRef] [PubMed]
- Jani, P.K.; Schwaner, E.; Kajdácsi, E.; Debreczeni, M.L.; Ungai-Salánki, R.; Dobó, J.; Doleschall, Z.; Rigó, J.; Geiszt, M.; Szabó, B.; et al. Complement MASP-1 enhances adhesion between endothelial cells and neutrophils by up-regulating E-selectin expression. Mol. Immunol. 2016, 75, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Debreczeni, M.L.; Németh, Z.; Kajdácsi, E.; Schwaner, E.; Makó, V.; Masszi, A.; Doleschall, Z.; Rigó, J.; Walter, F.R.; Deli, M.A.; et al. MASP-1 Increases Endothelial Permeability. Front. Immunol. 2019, 10, 991. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Debreczeni, M.L.; Kajdácsi, E.; Dobó, J.; Gál, P.; Cervenak, L. Cooperation of Complement MASP-1 with Other Proinflammatory Factors to Enhance the Activation of Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 9181. [Google Scholar] [CrossRef]
- Demeter, F.; Kajdacsi, Z.N.E.; Bihari, G.; Dobó, J.; Gál, P.; Cervenak, L. Detrimental synergistic effects of hypoxia and complement MASP-1 in endothelial cells as a model for atherosclerosis-related diseases. Manuscript under publication. 2024. [Google Scholar]
- Velnar, T.; Gradisnik, L. Tissue Augmentation in Wound Healing: The Role of Endothelial and Epithelial Cells. Med. Arch. 2018, 72, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Tsirogianni, A.K.; Moutsopoulos, N.M.; Moutsopoulos, H.M. Wound healing: Immunological aspects. Injury 2006, 37 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, S.; Perego, C.; Zangari, R.; De Blasio, D.; Oggioni, M.; De Nigris, F.; Snider, F.; Garred, P.; Ferrante, A.M.R.; De Simoni, M.-G. Lectin Pathway of Complement Activation Is Associated with Vulnerability of Atherosclerotic Plaques. Front. Immunol. 2017, 8, 288. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.E.; Montalto, M.C.; Stahl, G.L. Inhibition of mannose-binding lectin reduces postischemic myocardial reperfusion injury. Circulation 2001, 104, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Cazander, G.; Jukema, G.N.; Nibbering, P.H. Complement activation and inhibition in wound healing. Clin. Dev. Immunol. 2012, 2012, 534291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sarda, A.; Bhalla, S.; Goyal, A.; Kulshreshta, V. The effect of recent trauma on serum complement activation and serum C3 levels correlated with the injury severity score. Indian J. Med. Microbiol. 2004, 22, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.S.; Ignatius, A.; Köhl, J.; Mannes, M.; Braun, C.K. Complement in trauma-Traumatised complement? Br. J. Pharmacol. 2021, 178, 2863–2879. [Google Scholar] [CrossRef]
- Demer, L.L.; Wortham, C.M.; Dirksen, E.R.; Sanderson, M.J.; Melchior, B.; Frangos, J.A.; Moldobaeva, A.; Jenkins, J.; Wagner, E.; Platoshyn, O.; et al. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. Am. J. Physiol. 1993, 264 Pt 2, H2094-102. [Google Scholar] [CrossRef]
- D’Hondt, C.; Ponsaerts, R.; Srinivas, S.P.; Vereecke, J.; Himpens, B. Reduced intercellular communication and altered morphology of bovine corneal endothelial cells with prolonged time in cell culture. Curr. Eye Res. 2009, 34, 454–465. [Google Scholar] [CrossRef]
- Junkin, M.; Lu, Y.; Long, J.; Deymier, P.A.; Hoying, J.B.; Wong, P.K. Mechanically induced intercellular calcium communication in confined endothelial structures. Biomaterials 2013, 34, 2049–2056. [Google Scholar] [CrossRef]
- Atkinson, B.T.; Jasuja, R.; Chen, V.M.; Nandivada, P.; Furie, B.; Furie, B.C. Laser-induced endothelial cell activation supports fibrin formation. Blood 2010, 116, 4675–4683. [Google Scholar] [CrossRef] [PubMed]
- Kameritsch, P.; Pogoda, K.; Ritter, A.; Münzing, S.; Pohl, U. Gap junctional communication controls the overall endothelial calcium response to vasoactive agonists. Cardiovasc. Res. 2012, 93, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Togo, T. Cell membrane disruption stimulates NO/PKG signaling and potentiates cell membrane repair in neighboring cells. PLoS ONE 2012, 7, e42885. [Google Scholar] [CrossRef] [PubMed]
- Togo, T. Long-term potentiation of wound-induced exocytosis and plasma membrane repair is dependent on cAMP-response element-mediated transcription via a protein kinase C- and p38 MAPK-dependent pathway. J. Biol. Chem. 2004, 279, 44996–45003. [Google Scholar] [CrossRef] [PubMed]
- Lindner, V.; Collins, T. Expression of NF-kappa B and I kappa B-alpha by aortic endothelium in an arterial injury model. Am. J. Pathol. 1996, 148, 427–438. [Google Scholar] [PubMed]
- Chaudhuri, V.; Potts, B.R.; Karasek, M.A. Mechanisms of microvascular wound repair I. Role of mitosis, oxygen tension, and I-kappa B. Vitr. Cell. Dev. Biol. 2006, 42, 308–313. [Google Scholar]
- Nagaoka, T.; Kaburagi, Y.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Steeber, D.A.; Tedder, T.F.; Sato, S. Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am. J. Pathol. 2000, 157, 237–247. [Google Scholar] [CrossRef]
- Bachmann, L.; Kasper, M.; Hauck, J.G.; Dreßler, J.; Müller, E. Time dependence of the expression of ICAM-1 (CD 54) in human skin wounds. Int. J. Leg. Med. 1997, 110, 299–304. [Google Scholar]
- O’Brien, K.D.; Allen, M.D.; O McDonald, T.; Chait, A.; Harlan, J.M.; Fishbein, D.; McCarty, J.; Ferguson, M.; Hudkins, K.; Benjamin, C.D. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J. Clin. Investig. 1993, 92, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Dreßler, J.; Bachmann, L.; Koch, R.; Müller, E. Estimation of wound age and VCAM-1 in human skin. Int. J. Leg. Med. 1999, 112, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Kucharzewska, P.; Christianson, H.C.; Sköld, S.; Löfstedt, T.; Johansson, M.C.; Mörgelin, M.; Bengzon, J.; Ruf, W.; Belting, M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13147–13152. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.J.; Suh, D.Y.; Hunt, T.K.; Coughlin, S.R. Mice lacking the thrombin receptor, PAR1, have normal skin wound healing. Am. J. Pathol. 1997, 151, 1199–1204. [Google Scholar] [PubMed]
- Chan, B.; Merchan, J.R.; Kale, S.; Sukhatme, V.P. Antiangiogenic property of human thrombin. Microvasc. Res. 2003, 66, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, T.M.; Vara, D.S.; Wheeler-Jones, C.P.; Pula, G. Expression of protease-activated receptor 1 and 2 and anti-tubulogenic activity of protease-activated receptor 1 in human endothelial colony-forming cells. PLoS ONE 2014, 9, e109375. [Google Scholar] [CrossRef] [PubMed]
- Sinno, H.; Prakash, S. Complements and the wound healing cascade: An updated review. Plast. Surg. Int. 2013, 2013, 146764. [Google Scholar] [CrossRef] [PubMed]
- Bossi, F.; Tripodo, C.; Rizzi, L.; Bulla, R.; Agostinis, C.; Guarnotta, C.; Munaut, C.; Baldassarre, G.; Papa, G.; Zorzet, S.; et al. C1q as a unique player in angiogenesis with therapeutic implication in wound healing. Proc. Natl. Acad. Sci. USA 2014, 111, 4209–4214. [Google Scholar] [CrossRef] [PubMed]
- Balslev, E.; Thomsen, H.K.; Danielsen, L.; Sheller, J.; Garred, P. The terminal complement complex is generated in chronic leg ulcers in the absence of protectin (CD59). APMIS 1999, 107, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, H.I.; Krijnen, P.A.; Ulrich, M.M.; de Jong, E.; van Zuijlen, P.P.; Niessen, H.W. The role of complement in the acute phase response after burns. Burns 2017, 43, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, N.; de Leij, L.; Duis, H.J.T. Immunohistopathological appearance of three different types of injury in human skin. Inflamm. Res. 2001, 50, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ambrus, G.; Gál, P.; Kojima, M.; Szilágyi, K.; Balczer, J.; Antal, J.; Gráf, L.; Laich, A.; Moffatt, B.E.; Schwaeble, W.; et al. Natural substrates and inhibitors of mannan-binding lectin-associated serine protease-1 and -2: A study on recombinant catalytic fragments. J. Immunol. 2003, 170, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Dobó, J.; Harmat, V.; Beinrohr, L.; Sebestyén, E.; Závodszky, P.; Gál, P. MASP-1, a promiscuous complement protease: Structure of its catalytic region reveals the basis of its broad specificity. J. Immunol. 2009, 183, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Megyeri, M.; Harmat, V.; Major, B.; Végh, Á.; Balczer, J.; Héja, D.; Szilágyi, K.; Datz, D.; Pál, G.; Závodszky, P.; et al. Quantitative characterization of the activation steps of mannan-binding lectin (MBL)-associated serine proteases (MASPs) points to the central role of MASP-1 in the initiation of the complement lectin pathway. J. Biol. Chem. 2013, 288, 8922–8934. [Google Scholar] [CrossRef]
- Megyeri, M.; Jani, P.K.; Kajdácsi, E.; Dobó, J.; Schwaner, E.; Major, B.; Rigó, J.; Závodszky, P.; Thiel, S.; Cervenak, L.; et al. Serum MASP-1 in complex with MBL activates endothelial cells. Mol. Immunol. 2014, 59, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Producer | Catalog Number | Dilution |
|---|---|---|---|
| Mouse anti-human E-selectin | Invitrogen | BMS110 | 1:500 |
| Mouse anti-human ICAM-1 | Invitrogen | BMS108 | 1:500 |
| Mouse anti-human VCAM-1 | BD Pharmingen | 555,645 | 1:500 |
| Rabbit anti-human NFκB p65 | Santa-Cruz Biotechnology | sc-372 | 1:200 |
| Rabbit anti-human phosphor-CREB | Cell Signaling Technology Inc. | 9198 | 1:200 |
| Alexa568 conjugated goat anti-mouse IgG | Invitrogen | A-11004 | 1:500 |
| Alexa568 conjugated goat anti-rabbit mouse IgG | Invitrogen | A-11036 | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, Z.; Demeter, F.; Dobó, J.; Gál, P.; Cervenak, L. Complement MASP-1 Modifies Endothelial Wound Healing. Int. J. Mol. Sci. 2024, 25, 4048. https://doi.org/10.3390/ijms25074048
Németh Z, Demeter F, Dobó J, Gál P, Cervenak L. Complement MASP-1 Modifies Endothelial Wound Healing. International Journal of Molecular Sciences. 2024; 25(7):4048. https://doi.org/10.3390/ijms25074048
Chicago/Turabian StyleNémeth, Zsuzsanna, Flóra Demeter, József Dobó, Péter Gál, and László Cervenak. 2024. "Complement MASP-1 Modifies Endothelial Wound Healing" International Journal of Molecular Sciences 25, no. 7: 4048. https://doi.org/10.3390/ijms25074048
APA StyleNémeth, Z., Demeter, F., Dobó, J., Gál, P., & Cervenak, L. (2024). Complement MASP-1 Modifies Endothelial Wound Healing. International Journal of Molecular Sciences, 25(7), 4048. https://doi.org/10.3390/ijms25074048






