D-Allulose Reduces Hypertrophy and Endoplasmic Reticulum Stress Induced by Palmitic Acid in Murine 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Results
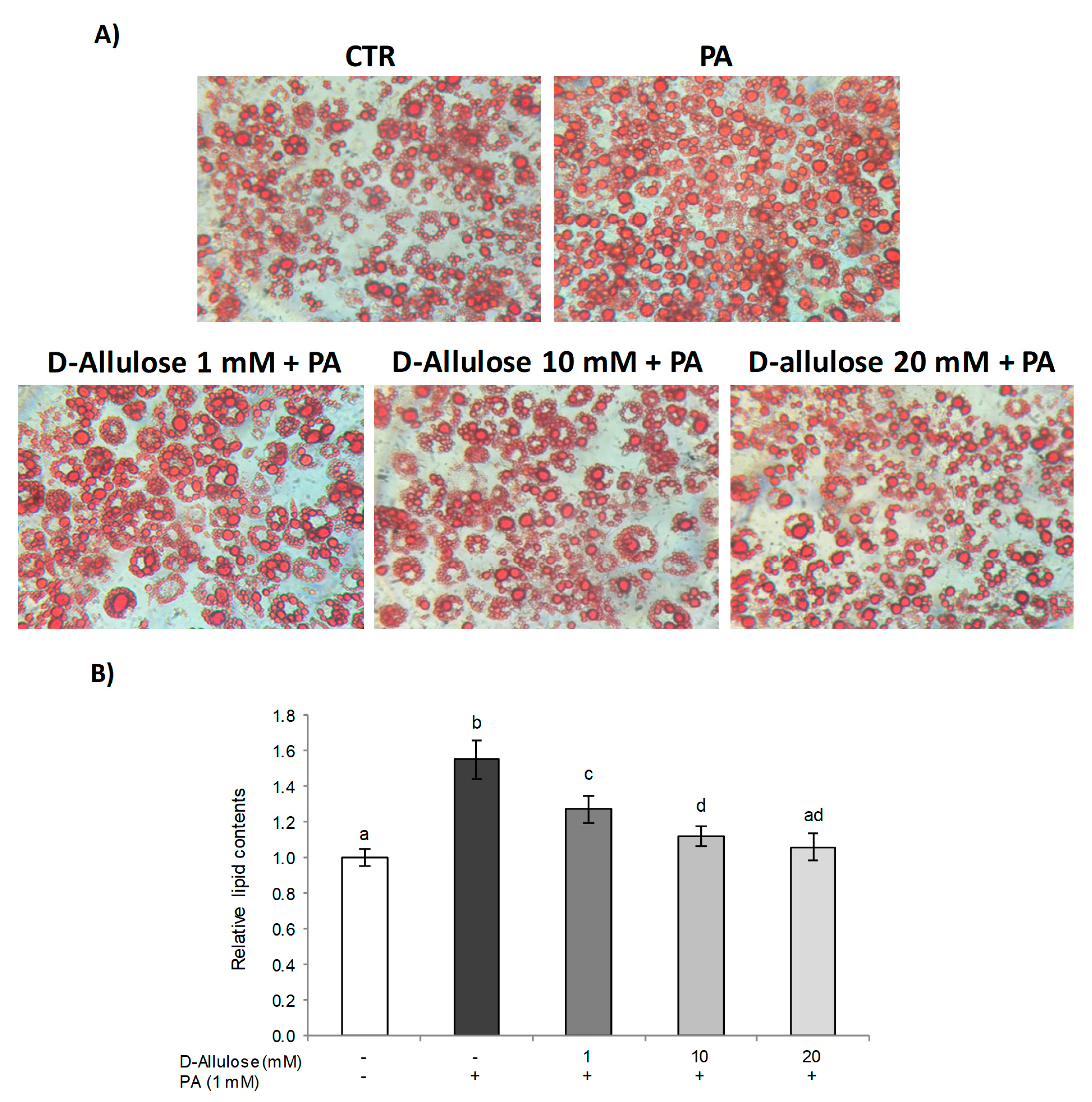
2.1. D-Allulose Protects against PA-Induced Hypertrophy
2.2. D-Allulose Prevents NF-kB Pathway Activation Induced by PA in Adipocytes
2.3. D-Allulose Protects against PA-Induced Oxidative Stress and Activates the Nrf2 Pathway in Hypertrophic Adipocytes
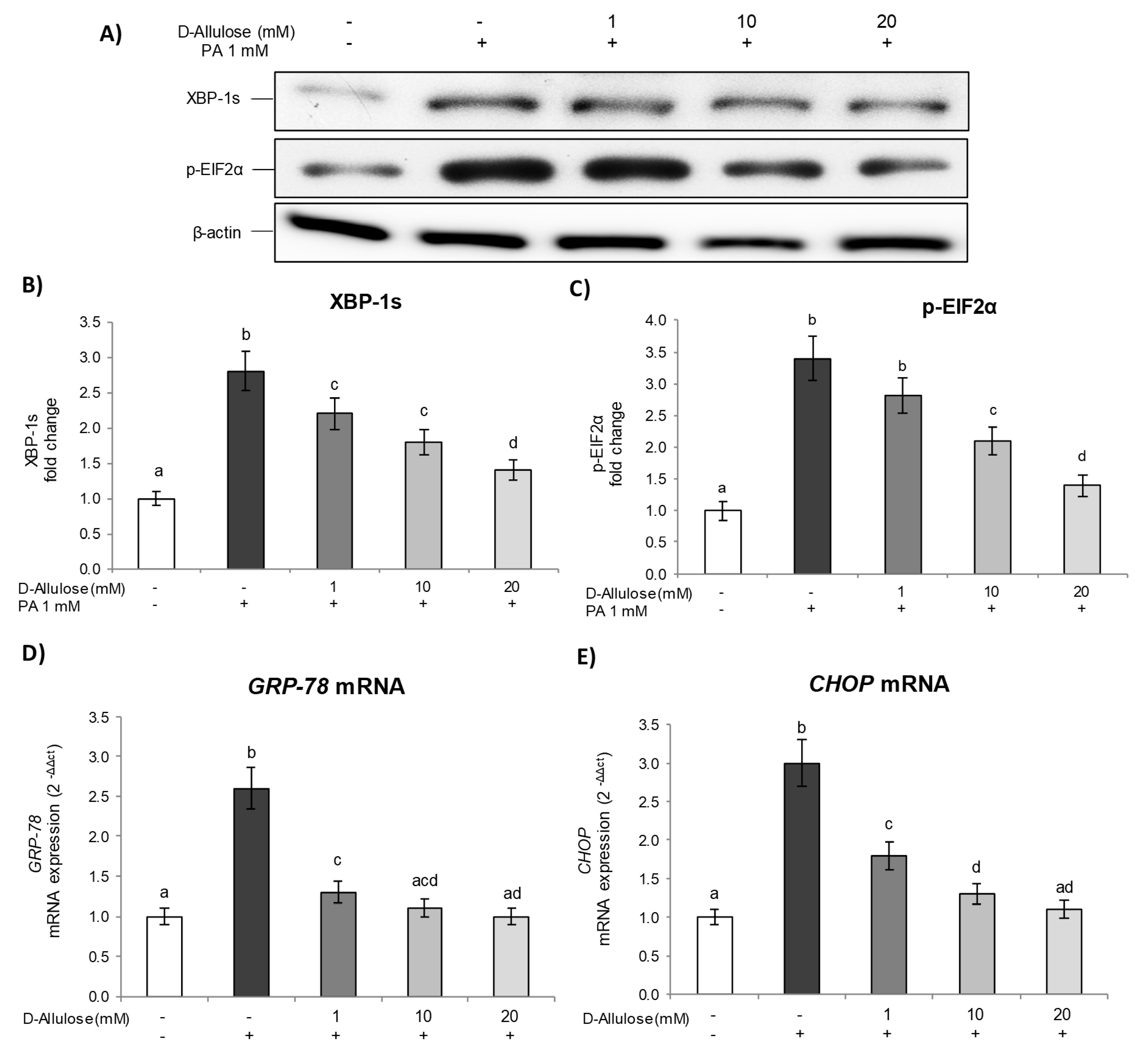
2.4. D-Allulose Prevents ER Stress in Hypertrophic Adipocytes
2.5. D-Allulose Ameliorates Adipocyte Dysfunction Induced by PA
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatments
4.3. Oil Red O Staining
4.4. Cell Lysate Preparation
4.5. Immunoblotting
4.6. Real-Time PCR
4.7. ROS Measurement via Dichlorodihydro-Fluorescein Diacetate Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, J.; Cascalho, A.; Goodchild, R.E. The ins and outs of endoplasmic reticulum-controlled lipid biosynthesis. EMBO Rep. 2017, 18, 1905–1921. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Smith, M.; Tokuda, M. The role of artificial and natural sweeteners in reducing the consumption of table sugar: A narrative review. Clin. Nutr. ESPEN 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Mortensen, A. Sweeteners permitted in the European Union: Safety aspects. Scand. J. Food Nutr. 2006, 50, 104–116. [Google Scholar] [CrossRef]
- Smith, A.; Avery, A.; Ford, R.; Yang, Q.; Goux, A.; Mukherjee, I.; Neville, D.C.A.; Jethwa, P. Rare sugars: Metabolic impacts and mechanisms of action: A scoping review. Br. J. Nutr. 2021, 128, 389–406. [Google Scholar] [CrossRef]
- Ahmed, A.; Khan, T.A.; Dan Ramdath, D.; Kendall, C.W.C.; Sievenpiper, J.L. Rare sugars and their health effects in humans: A systematic review and narrative synthesis of the evidence from human trials. Nutr. Rev. 2022, 80, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Whistler, R.L.; Singh, P.P.; Lake, W.C. D-Psicose metabolism in the rat. Carbohydr. Res. 1974, 34, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Hayashi, N.; Yamada, T.; Yoshikawa, Y.; Miyazato, S.; Kishimoto, Y.; Okuma, K.; Tokuda, M.; Izumori, K. Failure of d-psicose absorbed in the small intestine to metabolize into energy and its low large intestinal fermentability in humans. Metabolism 2010, 59, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Maeng, H.J.; Yoon, J.H.; Chun, K.H.; Kim, S.T.; Jang, D.J.; Park, J.E.; Kim, Y.H.; Kim, S.B.; Kim, Y.C. Metabolic Stability of D-Allulose in Biorelevant Media and Hepatocytes: Comparison with Fructose and Erythritol. Foods 2019, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Sendo, M.; Dezaki, K.; Hira, T.; Sato, T.; Nakata, M.; Goswami, C.; Aoki, R.; Arai, T.; Kumari, P.; et al. GLP-1 release and vagal afferent activation mediate the beneficial metabolic and chronotherapeutic effects of D-allulose. Nat. Commun. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Peng, C.; Lee, H.Y.; Park, S.A.; Hoang, T.H.; Kim, J.H.; Sa, S.; Kim, G.E.; Han, J.S.; Chae, H.J. D-allulose ameliorates adiposity through the AMPK-SIRT1-PGC-1α pathway in HFD-induced SD rats. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Moon, S.; Kim, Y.H.; Choi, K. Inhibition of 3T3-L1 Adipocyte Differentiation by D-allulose. Biotechnol. Bioprocess Eng. 2020, 25, 22–28. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Han, Y.; Choi, B.R.; Kim, S.Y.; Kim, S.B.; Kim, Y.H.; Kwon, E.Y.; Choi, M.S. Gastrointestinal Tolerance of D-Allulose in Healthy and Young Adults. A Non-Randomized Controlled Trial. Nutrients 2018, 10, 2010. [Google Scholar] [CrossRef]
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. 3), 23–33. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Sankhla, M.; Sharma, T.K.; Mathur, K.; Rathor, J.S.; Butolia, V.; Gadhok, A.K.; Vardey, S.K.; Sinha, M.; Kaushik, G.G. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin. Lab. 2012, 58, 385–392. [Google Scholar] [PubMed]
- Naomi, R.; Teoh, S.H.; Embong, H.; Balan, S.S.; Othman, F.; Bahari, H.; Yazid, M.D. The Role of Oxidative Stress and Inflammation in Obesity and Its Impact on Cognitive Impairments—A Narrative Review. Antioxidants 2023, 12, 1071. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Celik, C.; Lee, S.Y.T.; Yap, W.S.; Thibault, G. Endoplasmic reticulum stress and lipids in health and diseases. Prog. Lipid Res. 2023, 89, 101198. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. The bidirectional link between HDL and COVID-19 infections. J. Lipid Res. 2021, 62, 100067. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, X.D.; Li, Z. Recent Advances Regarding the Physiological Functions and Biosynthesis of D-Allulose. Front. Microbiol. 2022, 13, 881037. [Google Scholar] [CrossRef] [PubMed]
- Tak, J.; Bok, M.; Rho, H.; Park, J.H.; Lim, Y.; Chon, S.; Lim, H. Effect of diabetes-specific oral nutritional supplements with allulose on weight and glycemic profiles in overweight or obese type 2 diabetic patients. Nutr. Res. Pract. 2023, 17, 241–256. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Zhang, T.; Lu, M.; Jiang, B. Anti-obesity potential of rare sugar d-psicose by regulating lipid metabolism in rats. Food Funct. 2019, 10, 2417–2425. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Obese Adipocytes Have Altered Redox Homeostasis with Metabolic Consequences. Antioxidants 2023, 12, 1449. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Lebeaupin, C.; Wu, N.N.; Kaufman, R.J.; Ren, J. ER stress and inflammation crosstalk in obesity. Med. Res. Rev. 2023, 43, 5–30. [Google Scholar] [CrossRef]
- Gao, C.L.; Zhu, C.; Zhao, Y.P.; Chen, X.H.; Ji, C.B.; Zhang, C.M.; Zhu, J.G.; Xia, Z.K.; Tong, M.L.; Guo, X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010, 320, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Mooradian, A.D.; Haas, M.J.; Onstead-Haas, L.; Tani, Y.; Iida, T.; Tokuda, M. Naturally occurring rare sugars are free radical scavengers and can ameliorate endoplasmic reticulum stress. Int. J. Vitam. Nutr. Res. 2020, 90, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-E.; Kim, S.J.; Kim, H.-J.; Sung, M.-K. d-Psicose, a sugar substitute, suppresses body fat deposition by altering networks of inflammatory response and lipid metabolism in C57BL/6J-ob/ob mice. J. Funct. Foods 2017, 28, 265–274. [Google Scholar] [CrossRef]
- Daniel, H.; Hauner, H.; Hornef, M.; Clavel, T. Allulose in human diet: The knowns and the unknowns. Br. J. Nutr. 2022, 128, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Yoshimura, T.; Imachi, H.; Kobayashi, T.; Saheki, T.; Sato, S.; Saheki, N.; Jiang, W.; Murao, K. A Pilot Study on the Efficacy of a Diabetic Diet Containing the Rare Sugar D-Allulose in Patients with Type 2 Diabetes Mellitus: A Prospective, Randomized, Single-Blind, Crossover Study. Nutrients 2023, 15, 2802. [Google Scholar] [CrossRef]
- Yuma, T.; Tokuda, M.; Nishimoto, N.; Yokoi, H.; Izumori, K. Allulose for the attenuation of postprandial blood glucose levels in healthy humans: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0281150. [Google Scholar] [CrossRef]
- Franchi, F.; Yaranov, D.M.; Rollini, F.; Rivas, A.; Rivas Rios, J.; Been, L.; Tani, Y.; Tokuda, M.; Iida, T.; Hayashi, N.; et al. Effects of D-allulose on glucose tolerance and insulin response to a standard oral sucrose load: Results of a prospective, randomized, crossover study. BMJ Open Diabetes Res. Care 2021, 9, e001939. [Google Scholar] [CrossRef]
- Tanaka, M.; Kanasaki, A.; Hayashi, N.; Iida, T.; Murao, K. Safety and efficacy of a 48-week long-term ingestion of D-allulose in subjects with high LDL cholesterol levels. Fundam. Toxicol. Sci. 2020, 7, 15–31. [Google Scholar] [CrossRef]
- Molonia, M.S.; Occhiuto, C.; Muscarà, C.; Speciale, A.; Ruberto, G.; Siracusa, L.; Cristani, M.; Saija, A.; Cimino, F. Effects of a pinitol-rich Glycyrrhiza glabra L. leaf extract on insulin and inflammatory signaling pathways in palmitate-induced hypertrophic adipocytes. Nat. Prod. Res. 2022, 36, 4768–4775. [Google Scholar] [CrossRef]
- Molonia, M.S.; Speciale, A.; Muscarà, C.; Salamone, F.L.; Saija, A.; Cimino, F. Low concentrations of α-lipoic acid reduce palmitic acid-induced alterations in murine hypertrophic adipocytes. Nat. Prod. Res. 2024, 38, 916–925. [Google Scholar] [CrossRef]
- Molonia, M.S.; Muscarà, C.; Speciale, A.; Salamone, F.L.; Costa, G.; Vento, G.; Saija, A.; Cimino, F. Low concentrations of antimony impair adipogenesis and endoplasmic reticulum homeostasis during 3T3-L1 cells differentiation. Food Chem. Toxicol. 2023, 181, 114107. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Molonia, M.S.; Muscarà, C.; Speciale, A.; Salamone, F.L.; Toscano, G.; Saija, A.; Cimino, F. The p-Phthalates Terephthalic Acid and Dimethyl Terephthalate Used in the Manufacture of PET Induce In Vitro Adipocytes Dysfunction by Altering Adipogenesis and Thermogenesis Mechanisms. Molecules 2022, 27, 7645. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Li, G.; You, L.; Ma, Y.; Jin, L.; Ma, J.; Li, X.; Li, M.; Liu, H. MiR-185 inhibits 3T3-L1 cell differentiation by targeting SREBP-1. Biosci. Biotechnol. Biochem. 2017, 81, 1747–1754. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Gao, P.; Chen, J.; Yu, C.; Zong, C.; Lu, S.; Li, X.; Ma, X.; Liu, Y.; et al. The Effect of Growth Hormone on Lipid Accumulation or Maturation in Adipocytes. Cell. Physiol. Biochem. 2016, 39, 2135–2148. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Brand, M.; Zenke, Y.; Tashiro, S.; Groudine, M.; Igarashi, K. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network. Proc. Natl. Acad. Sci. USA 2004, 101, 1461–1466. [Google Scholar] [CrossRef]
- Kavalakatt, S.; Khadir, A.; Madhu, D.; Koistinen, H.A.; Al-Mulla, F.; Tuomilehto, J.; Abubaker, J.; Tiss, A. Urocortin 3 overexpression reduces ER stress and heat shock response in 3T3-L1 adipocytes. Sci. Rep. 2021, 11, 15666. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.Y.; Jeon, E.J.; Leem, J.; Park, J.H.; Cho, H. Regulation of Adipsin Expression by Endoplasmic Reticulum Stress in Adipocytes. Biomolecules 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Molonia, M.S.; Quesada-Lopez, T.; Speciale, A.; Muscarà, C.; Saija, A.; Villarroya, F.; Cimino, F. In Vitro Effects of Cyanidin-3-O-Glucoside on Inflammatory and Insulin-Sensitizing Genes in Human Adipocytes Exposed to Palmitic Acid. Chem. Biodivers. 2021, 18, e2100607. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bashllari, R.; Molonia, M.S.; Muscarà, C.; Speciale, A.; Wilde, P.J.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside protects intestinal epithelial cells from palmitate-induced lipotoxicity. Arch. Physiol. Biochem. 2023, 129, 379–386. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molonia, M.S.; Salamone, F.L.; Speciale, A.; Saija, A.; Cimino, F. D-Allulose Reduces Hypertrophy and Endoplasmic Reticulum Stress Induced by Palmitic Acid in Murine 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2024, 25, 4059. https://doi.org/10.3390/ijms25074059
Molonia MS, Salamone FL, Speciale A, Saija A, Cimino F. D-Allulose Reduces Hypertrophy and Endoplasmic Reticulum Stress Induced by Palmitic Acid in Murine 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2024; 25(7):4059. https://doi.org/10.3390/ijms25074059
Chicago/Turabian StyleMolonia, Maria Sofia, Federica Lina Salamone, Antonio Speciale, Antonella Saija, and Francesco Cimino. 2024. "D-Allulose Reduces Hypertrophy and Endoplasmic Reticulum Stress Induced by Palmitic Acid in Murine 3T3-L1 Adipocytes" International Journal of Molecular Sciences 25, no. 7: 4059. https://doi.org/10.3390/ijms25074059
APA StyleMolonia, M. S., Salamone, F. L., Speciale, A., Saija, A., & Cimino, F. (2024). D-Allulose Reduces Hypertrophy and Endoplasmic Reticulum Stress Induced by Palmitic Acid in Murine 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 25(7), 4059. https://doi.org/10.3390/ijms25074059








