Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review
Abstract
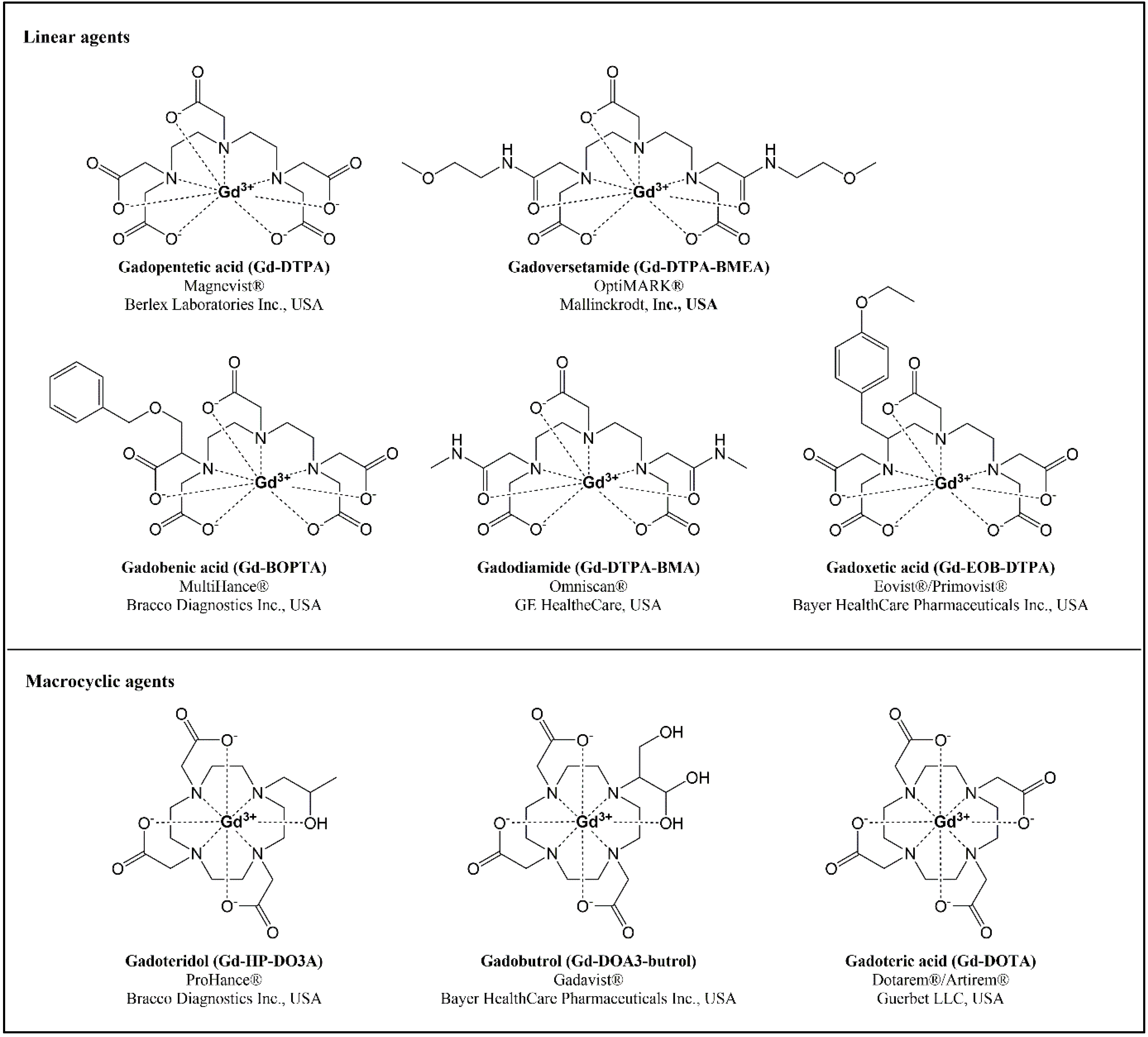
1. Introduction
2. Gd (III) Mechanisms of Toxicity
3. Concerns about the Use of GBCAs
4. Final Considerations
Funding
Acknowledgments
Conflicts of Interest
References
- de Haen, C. Conception of the first magnetic resonance imaging contrast agents: A brief history. Top. Magn. Reson. Imaging 2001, 12, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lohrke, J.; Frisk, A.L.; Frenzel, T.; Schockel, L.; Rosenbruch, M.; Jost, G.; Lenhard, D.C.; Sieber, M.A.; Nischwitz, V.; Kuppers, A.; et al. Histology and Gadolinium Distribution in the Rodent Brain After the Administration of Cumulative High Doses of Linear and Macrocyclic Gadolinium-Based Contrast Agents. Investig. Radiol. 2017, 52, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Robertson, A.G.; Hill, L.R.; Rendina, L.M. Synthesis and tumour cell uptake studies of gadolinium(III)-phosphonium complexes. Sci. Rep. 2021, 11, 598. [Google Scholar] [CrossRef]
- Mohammadi, E.; Amanlou, M.; Ebrahimi, S.; Hamedani, M.P.; Mahrooz, A.; Mehravi, B.; Emami, B.A.; Aghasadeghi, M.R.; Bitarafan-Rajabi, A.; Akbar, H.R.P.A.; et al. Cellular uptake, imaging and pathotoxicological studies of a novel Gd[iii]-DO3A-butrol nano-formulation. RSC Adv. 2014, 4, 45984–45994. [Google Scholar] [CrossRef]
- Mundim, J.S.; Lorena, S.d.C.; Abensur, H.; Elias, R.M.; Moysés, R.M.A.; Castro, M.C.M.; Romão, J.E., Jr. Fibrose sistêmica nefrogênica: Uma complicação grave do uso do gadolínio em pacientes com insuficiência renal. Rev. Assoc. Med. Bras. 2009, 55, 220–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Gregorio, E.; Gianolio, E.; Stefania, R.; Barutello, G.; Digilio, G.; Aime, S. On the fate of MRI Gd-based contrast agents in cells. Evidence for extensive degradation of linear complexes upon endosomal internalization. Anal. Chem. 2013, 85, 5627–5631. [Google Scholar] [CrossRef] [PubMed]
- Grobner, T. Gadolinium—A specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol. Dial. Transplant. 2006, 21, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, K.; Tamada, T.; Kanki, A.; Watanabe, S.; Nishimura, H.; Tanimoto, D.; Higashi, H.; Yamamoto, A. Tissue gadolinium deposition in renally impaired rats exposed to different gadolinium-based MRI contrast agents: Evaluation with inductively coupled plasma mass spectrometry (ICP-MS). Magn. Reson. Imaging 2013, 31, 1412–1417. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: Relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Mercantepe, T.; Tumkaya, L.; Celiker, F.B.; Topal Suzan, Z.; Cinar, S.; Akyildiz, K.; Mercantepe, F.; Yilmaz, A. Effects of gadolinium-based MRI contrast agents on liver tissue. J. Magn. Reson. Imaging 2018, 48, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Do, C.; Barnes, J.L.; Tan, C.; Wagner, B. Type of MRI contrast, tissue gadolinium, and fibrosis. Am. J. Physiol. Renal Physiol. 2014, 307, F844–F855. [Google Scholar] [CrossRef] [PubMed]
- Damme, N.M.; Fernandez, D.P.; Wang, L.M.; Wu, Q.; Kirk, R.A.; Towner, R.A.; McNally, J.S.; Hoffman, J.M.; Morton, K.A. Analysis of retention of gadolinium by brain, bone, and blood following linear gadolinium-based contrast agent administration in rats with experimental sepsis. Magn. Reson. Med. 2020, 83, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Moon, W.J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Kanal, E.; Tweedle, M.F. Residual or retained gadolinium: Practical implications for radiologists and our patients. Radiology 2015, 275, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Sherry, A.D.; Caravan, P.; Lenkinski, R.E. Primer on gadolinium chemistry. J. Magn. Reson. Imaging 2009, 30, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Bazari, H.; Avery, L.L.; Koreishi, A.F. Case records of the Massachusetts General Hospital. Case 6-2008. A 46-year-old woman with renal failure and stiffness of the joints and skin. N. Engl. J. Med. 2008, 358, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Parant, M.; Sohm, B.; Flayac, J.; Perrat, E.; Chuburu, F.; Cadiou, C.; Rosin, C.; Cossu-Leguille, C. Impact of gadolinium-based contrast agents on the growth of fish cells lines. Ecotoxicol. Environ. Saf. 2019, 182, 109385. [Google Scholar] [CrossRef] [PubMed]
- Broome, D.R. Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: A summary of the medical literature reporting. Eur. J. Radiol. 2008, 66, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Elmholdt, T.R.; Pedersen, M.; Jorgensen, B.; Sondergaard, K.; Jensen, J.D.; Ramsing, M.; Olesen, A.B. Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: A case-control study from Denmark. Br. J. Dermatol. 2011, 165, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Reilly, R.F. Risk for nephrogenic systemic fibrosis with gadoteridol (ProHance) in patients who are on long-term hemodialysis. Clin. J. Am. Soc. Nephrol. 2008, 3, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Elmholdt, T.R.; Jorgensen, B.; Ramsing, M.; Pedersen, M.; Olesen, A.B. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus 2010, 3, 285–287. [Google Scholar] [CrossRef]
- Wollanka, H.; Weidenmaier, W.; Giersig, C. NSF after Gadovist exposure: A case report and hypothesis of NSF development. Nephrol. Dial. Transplant. 2009, 24, 3882–3884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clases, D.; Fingerhut, S.; Jeibmann, A.; Sperling, M.; Doble, P.; Karst, U. LA-ICP-MS/MS improves limits of detection in elemental bioimaging of gadolinium deposition originating from MRI contrast agents in skin and brain tissues. J. Trace Elem. Med. Biol. 2019, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A. Immunotoxic potential of nanoparticles of cerium oxide and gadolinium oxide in human monocyte (THP-1) cells. J. King Saud Univ.–Sci. 2022, 34, 102291. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Tsushima, Y.; Koibuchi, N. Gadolinium-based contrast agent accelerates the migration of astrocyte via integrin alphavbeta3 signaling pathway. Sci. Rep. 2022, 12, 5850. [Google Scholar] [CrossRef] [PubMed]
- Chanana, P.; Uosef, A.; Vaughn, N.; Suarez-Villagran, M.; Ghobrial, R.M.; Kloc, M.; Wosik, J. The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages. Cells 2022, 11, 757. [Google Scholar] [CrossRef]
- Cobanoglu, H. Assessment of genetic damage induced by gadolinium-based radiocontrast agents. J. Trace Elem. Med. Biol. 2022, 70, 126914. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Takanezawa, Y.; Ohshiro, Y.; Uraguchi, S.; Kiyono, M. Effects of chemical forms of gadolinium on the spleen in mice after single intravenous administration. Biochem. Biophys. Rep. 2022, 29, 101217. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.F.; Yang, J.S.; Chiu, Y.J.; Tsai, C.W.; Bau, D.T.; Chang, W.S. Gadodiamide Induced Autophagy and Apoptosis in Human Keratinocytes. Vivo 2022, 36, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Uosef, A.; Subuddhi, A.; Lu, A.; Ubelaker, H.V.; Karmonik, C.; Wosik, J.; Ghobrial, R.M.; Kloc, M. 7T MRI and molecular studies of Dotarem (gadoterate meglumine) retention in macrophages. J. Magn. Reson. Open 2022, 12–13, 100085. [Google Scholar] [CrossRef]
- Algieri, C.; Trombetti, F.; Pagliarani, A.; Fabbri, M.; Nesci, S. The inhibition of gadolinium ion (Gd3+) on the mitochondrial F1FO-ATPase is linked to the modulation of the mitochondrial permeability transition pore. Int. J. Biol. Macromol. 2021, 184, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Baykara, M.; Ozcan, M.; Bilgen, M.; Kelestimur, H. Interference of gadolinium dechelated from MR contrast agents by calcium signaling in neuronal cells of GnRH. J. Cell. Physiol. 2021, 236, 2139–2143. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.A.; Apaydin, M.; Armagan, G.; Taskiran, D. Evaluation of toxicity of gadolinium-based contrast agents on neuronal cells. Acta Radiol. 2021, 62, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kartamihardja, A.A.P.; Amalia, S.N.; Sekiguchi, A.; Bhattarai, A.; Taketomi-Takahashi, A.; Motegi, S.I.; Koyama, H.; Tsushima, Y. Neutrophil elastase in the development of nephrogenic systemic fibrosis (NSF)-like skin lesion in renal failure mouse model. PLoS ONE 2021, 16, e0259211. [Google Scholar] [CrossRef]
- Kartamihardja, A.A.P.; Ariyani, W.; Hanaoka, H.; Taketomi-Takahashi, A.; Koibuchi, N.; Tsushima, Y. The Role of Ferrous Ion in the Effect of the Gadolinium-Based Contrast Agents (GBCA) on the Purkinje Cells Arborization: An In Vitro Study. Diagnostics 2021, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, S.; Wang, J.; Han, C.; Yu, N.; Liu, Q.; Wang, W.; Xu, K. Potential toxicity evaluation and comparison within multiple mice organs after repeat injections of linear versus macrocyclic gadolinium-based contrast agents: A comprehensive and time course study. Toxicol. Lett. 2021, 350, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Sousa, N.R.; Rocha, S.; Santos-Silva, A.; Coimbra, S.; Valente, M.J. Cellular and molecular pathways underlying the nephrotoxicity of gadolinium. Toxicol. Sci. 2022, 186, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, V.; Kose Ozlece, H.; Fatih Bozkurt, M.; Ozkul, B.; Erbas, O. Repeated gadoteric acid and gadobutrol exposure causes deterioration of behavior and memory functions in rats: MRI, histopathological and biochemical evidence. Brain Res. 2021, 1754, 147256. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.F.; Yang, J.S.; Tsai, F.J.; Lu, C.C.; Chiu, Y.J.; Tsai, S.C. In Vitro Toxicological Assessment of Gadodiamide in Normal Brain SVG P12 Cells. Vivo 2021, 35, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wen, T.; Yang, A.; Zhang, X.; Chen, B.; Meng, J.; Liu, J.; Gu, N.; Xu, H. A Contrast Examination of Proinflammatory Effects on Kidney Function for gamma-Fe2O3 NP and Gadolinium Dimeglumine. Int. J. Nanomed. 2021, 16, 2271–2282. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H. Gadolinium Oxide Nanoparticles Induce Toxicity in Human Endothelial HUVECs via Lipid Peroxidation, Mitochondrial Dysfunction and Autophagy Modulation. Nanomaterials 2020, 10, 1675. [Google Scholar] [CrossRef]
- Bloomer, S.A.; Moyer, E.D.; Brown, K.E.; Kregel, K.C. Aging results in accumulation of M1 and M2 hepatic macrophages and a differential response to gadolinium chloride. Histochem. Cell Biol. 2020, 153, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Nong, Q.; Chen, X.; Hu, L.; Huang, Y.; Luan, T.; Liu, H.; Chen, B. Identification and characterization of Gd-binding proteins in NIH-3T3 cells. Talanta 2020, 219, 121281. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.L.; Farris, A.F.; Rashid, N.; Chan, K.M.; Rajab, N.F. In vitro toxicological assessment of gadolinium (III) chloride in V79-4 fibroblasts. Genes. Environ. 2020, 42, 22. [Google Scholar] [CrossRef] [PubMed]
- Supawat, B.; Moungthong, P.; Chanloi, C.; Jindachai, N.; Tima, S.; Kothan, S.; Udomtanakunchai, C.; Tungjai, M. Effects of gadolinium-based magnetic resonance imaging contrast media on red blood cells and K562 cancer cells. J. Trace Elem. Med. Biol. 2020, 62, 126640. [Google Scholar] [CrossRef]
- Takanezawa, Y.; Nakamura, R.; Kusaka, T.; Ohshiro, Y.; Uraguchi, S.; Kiyono, M. Significant contribution of autophagy in mitigating cytotoxicity of gadolinium ions. Biochem. Biophys. Res. Commun. 2020, 526, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.; Alrokayan, S. Toxicity Mechanism of Gadolinium Oxide Nanoparticles and Gadolinium Ions in Human Breast Cancer Cells. Curr. Drug Metab. 2019, 20, 907–917. [Google Scholar] [CrossRef]
- Baykara, M.; Ozcan, M.; Bilgen, M.; Kelestimur, H. Effects of gadolinium and gadolinium chelates on intracellular calcium signaling in sensory neurons. Neurosci. Lett. 2019, 707, 134295. [Google Scholar] [CrossRef] [PubMed]
- Celiker, F.B.; Tumkaya, L.; Mercantepe, T.; Beyazal, M.; Turan, A.; Beyazal Polat, H.; Suzan, Z.T.; Inecikli, M.F.; Akyildiz, K.; Yilmaz, A. Effects of Gadodiamide and Gadoteric Acid on Rat Kidneys: A Comparative Study. J. Magn. Reson. Imaging 2019, 49, 382–389. [Google Scholar] [CrossRef]
- Bower, D.V.; Richter, J.K.; von Tengg-Kobligk, H.; Heverhagen, J.T.; Runge, V.M. Gadolinium-Based MRI Contrast Agents Induce Mitochondrial Toxicity and Cell Death in Human Neurons, and Toxicity Increases with Reduced Kinetic Stability of the Agent. Investig. Radiol. 2019, 54, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Do, C.; Ford, B.; Lee, D.Y.; Tan, C.; Escobar, P.; Wagner, B. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol. Appl. Pharmacol. 2019, 375, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Do, C.; Drel, V.; Tan, C.; Lee, D.; Wagner, B. Nephrogenic Systemic Fibrosis Is Mediated by Myeloid C-C Chemokine Receptor 2. J. Investig. Dermatol. 2019, 139, 2134–2143.e2. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, J.; He, X.; Deng, J.; Dong, F.; Wang, K.; Yu, S. Gadolinium chloride promotes proliferation of HEK293 human embryonic kidney cells by activating EGFR/PI3K/Akt and MAPK pathways. Biometals 2019, 32, 683–693. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Chen, Y.F.; Hsiao, C.Y.; Huang, C.W.; Lu, C.C.; Tsai, S.C.; Yang, J.S. Caspase-dependent apoptotic death by gadolinium chloride (GdCl3) via reactive oxygen species production and MAPK signaling in rat C6 glioma cells. Oncol. Rep. 2019, 41, 1324–1332. [Google Scholar] [CrossRef]
- Wang, S.; Hesse, B.; Roman, M.; Stier, D.; Castillo-Michel, H.; Cotte, M.; Suuronen, J.P.; Lagrange, A.; Radbruch, H.; Paul, F.; et al. Increased Retention of Gadolinium in the Inflamed Brain After Repeated Administration of Gadopentetate Dimeglumine: A Proof-of-Concept Study in Mice Combining ICP-MS and Micro- and Nano-SR-XRF. Investig. Radiol. 2019, 54, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Hu, X.; Zheng, J.; Xia, F.; Wang, N.; Liao, H.; Liu, Y.; Kim, D.; Liu, J.; Li, F.; et al. Toxicological Risk Assessments of Iron Oxide Nanocluster- and Gadolinium-Based T1MRI Contrast Agents in Renal Failure Rats. ACS Nano 2019, 13, 6801–6812. [Google Scholar] [CrossRef] [PubMed]
- Beyazal Celiker, F.; Tumkaya, L.; Mercantepe, T.; Turan, G.; Yilmaz, A.; Beyazal, M.; Turan, A.; Inecikli, M.F.; Kosem, M. The effect of gadolinium-based contrast agents on rat testis. Andrologia 2018, 50, e13031. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, A.R.; Mishriki, S.; Kammann, T.; Sahu, R.P.; Geng, F.; Puri, I.K. Gadopentatic acid affects in vitro proliferation and doxorubicin response in human breast adenocarcinoma cells. Biometals 2018, 31, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Friebe, B.; Godenschweger, F.; Fatahi, M.; Speck, O.; Roggenbuck, D.; Reinhold, D.; Reddig, A. The potential toxic impact of different gadolinium-based contrast agents combined with 7-T MRI on isolated human lymphocytes. Eur. Radiol. Exp. 2018, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.I.; Chen, H.J.; Lu, C.W.; Ho, Y.C.; Wu, J.L.; Liu, S.H.; Hsiao, J.K. Exposure of Macrophages to Low-Dose Gadolinium-Based Contrast Medium: Impact on Oxidative Stress and Cytokines Production. Contrast Media Mol. Imaging 2018, 2018, 3535769. [Google Scholar] [CrossRef] [PubMed]
- Alarifi, S.; Ali, H.; Alkahtani, S.; Alessia, M.S. Regulation of apoptosis through bcl-2/bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int. J. Nanomed. 2017, 12, 4541–4551. [Google Scholar] [CrossRef]
- Knoepp, F.; Bettmer, J.; Fronius, M. Gadolinium released by the linear gadolinium-based contrast-agent Gd-DTPA decreases the activity of human epithelial Na+ channels (ENaCs). Biochim. Biophys. Acta Biomembr. 2017, 1859, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Baksa, V.; Kiss, A.; Turani, M.; Banfalvi, G. Gadolinium induced effects on mammalian cell motility, adherence and chromatin structure. Apoptosis 2017, 22, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Hayashi, S.; Hamasaki, Y.; Hatamochi, A. Effects of Gadodiamide on cell proliferation and collagen production in cultured human dermal fibroblasts. Arch. Dermatol. Res. 2016, 308, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.F.; Huang, C.W.; Chiang, J.H.; Tsai, F.J.; Hsu, Y.M.; Lu, C.C.; Hsiao, C.Y.; Yang, J.S. Gadolinium chloride elicits apoptosis in human osteosarcoma U-2 OS cells through extrinsic signaling, intrinsic pathway and endoplasmic reticulum stress. Oncol. Rep. 2016, 36, 3421–3426. [Google Scholar] [CrossRef] [PubMed]
- Bose, C.; Megyesi, J.K.; Shah, S.V.; Hiatt, K.M.; Hall, K.A.; Karaduta, O.; Swaminathan, S. Evidence Suggesting a Role of Iron in a Mouse Model of Nephrogenic Systemic Fibrosis. PLoS ONE 2015, 10, e0136563. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 2015, 9, 12425–12435. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lauber, C.; Bossaller, L.; Abujudeh, H.H.; Vladimer, G.I.; Christ, A.; Fitzgerald, K.A.; Latz, E.; Gravallese, E.M.; Marshak-Rothstein, A.; Kay, J. Gadolinium-based compounds induce NLRP3-dependent IL-1beta production and peritoneal inflammation. Ann. Rheum. Dis. 2015, 74, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, Y.; Lee, S.; Choi, Y.J.; Chung, H.W. Enhanced cytotoxic and genotoxic effects of gadolinium following ELF-EMF irradiation in human lymphocytes. Drug Chem. Toxicol. 2014, 37, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, A.; Yao, P.; Sun, X.; Chen, C.; Mo, C.; Shi, L.; Chen, Y.; Liu, Q. Gadolinium promoted proliferation in mouse embryo fibroblast NIH3T3 cells through Rac and PI3K/Akt signaling pathways. Biometals 2014, 27, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Jimenez, S.A. Induction of a type I interferon signature in normal human monocytes by gadolinium-based contrast agents: Comparison of linear and macrocyclic agents. Clin. Exp. Immunol. 2014, 175, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Bose, C.; Shah, S.V.; Hall, K.A.; Hiatt, K.M. Gadolinium contrast agent-induced CD163+ ferroportin+ osteogenic cells in nephrogenic systemic fibrosis. Am. J. Pathol. 2013, 183, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Bleavins, K.; Perone, P.; Naik, M.; Rehman, M.; Aslam, M.N.; Dame, M.K.; Meshinchi, S.; Bhagavathula, N.; Varani, J. Stimulation of fibroblast proliferation by insoluble gadolinium salts. Biol. Trace Elem. Res. 2012, 145, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.V.; Shimizu, M.H.; Rodrigues, L.P.; Leite, C.C.; Andrade, L.; Seguro, A.C. N-acetylcysteine protects rats with chronic renal failure from gadolinium-chelate nephrotoxicity. PLoS ONE 2012, 7, e39528. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Tan, C.; Barnes, J.L.; Ahuja, S.; Davis, T.L.; Gorin, Y.; Jimenez, F. Nephrogenic systemic fibrosis: Evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am. J. Pathol. 2012, 181, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Jimenez, S.A. Gadolinium compounds signaling through TLR4 and TLR7 in normal human macrophages: Establishment of a proinflammatory phenotype and implications for the pathogenesis of nephrogenic systemic fibrosis. J. Immunol. 2012, 189, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Angeli, J.K.; Ramos, D.B.; Casali, E.A.; Souza, D.O.; Sarkis, J.J.; Stefanon, I.; Vassallo, D.V.; Furstenau, C.R. Gadolinium increases the vascular reactivity of rat aortic rings. Braz. J. Med. Biol. Res. 2011, 44, 445–452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, X.D.; Xia, Q.; Yuan, L.; Huang, H.F.; Yang, X.D.; Wang, K. Gadolinium triggers unfolded protein responses (UPRs) in primary cultured rat cortical astrocytes via promotion of an influx of extracellular Ca2+. Cell Biol. Toxicol. 2011, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Soukup, J.M.; Dailey, L.A.; Richards, J.; Deng, Z.; Abraham, J.L. Gadolinium exposure disrupts iron homeostasis in cultured cells. J. Biol. Inorg. Chem. 2011, 16, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Long, X.H.; Yang, P.Y.; Liu, Q.; Yao, J.; Wang, Y.; He, G.H.; Hong, G.Y.; Ni, J.Z. Metabolomic profiles delineate potential roles for gadolinium chloride in the proliferation or inhibition of Hela cells. Biometals 2011, 24, 663–677. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, S.; Bains, S.; Johnson, C.; Idee, J.M.; Factor, C.; Jestin, G.; Fretellier, N.; Morcos, S.K. Gadolinium contrast agent associated stimulation of human fibroblast collagen production. Investig. Radiol. 2011, 46, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Yamanaka, M.; Ishikawa, O. New insights into the mechanism of abnormal calcification in nephrogenic systemic fibrosis—Gadolinium promotes calcium deposition of mesenchymal stem cells and dermal fibroblasts. J. Dermatol. Sci. 2011, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zou, X.M.; Huang, J.; Zhang, T.L.; Wang, K. Gadolinium inhibits prostate cancer PC3 cell migration and suppresses osteoclast differentiation in vitro. Cell Biol. Int. 2011, 35, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, B.; Kehlbach, R.; Hemsen, J.; Bebin, J.; Bantleon, R.; Schwenzer, N.; Spira, D.; Claussen, C.D.; Wiskirchen, J. Effects of magnetic resonance imaging contrast agents on human umbilical vein endothelial cells and evaluation of magnetic resonance imaging contrast media-triggered transforming growth factor-beta induction in dermal fibroblasts (HSF) as a model for nephrogenic systemic fibrosis. Investig. Radiol. 2011, 46, 71–76. [Google Scholar] [CrossRef]
- Xia, Q.; Feng, X.; Huang, H.; Du, L.; Yang, X.; Wang, K. Gadolinium-induced oxidative stress triggers endoplasmic reticulum stress in rat cortical neurons. J. Neurochem. 2011, 117, 38–47. [Google Scholar] [CrossRef]
- Bhagavathula, N.; Dame, M.K.; DaSilva, M.; Jenkins, W.; Aslam, M.N.; Perone, P.; Varani, J. Fibroblast response to gadolinium: Role for platelet-derived growth factor receptor. Investig. Radiol. 2010, 45, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Del Galdo, F.; Wermuth, P.J.; Addya, S.; Fortina, P.; Jimenez, S.A. NFkappaB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: Possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann. Rheum. Dis. 2010, 69, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Gou, B.D.; Bian, S.; Zhang, T.L.; Wang, K. Gadolinium-promoted precipitation of calcium phosphate is associated with profibrotic activation of RAW 264.7 macrophages. Toxicol. Vitr. 2010, 24, 1743–1749. [Google Scholar] [CrossRef]
- Li, J.X.; Liu, J.C.; Wang, K.; Yang, X.G. Gadolinium-containing bioparticles as an active entity to promote cell cycle progression in mouse embryo fibroblast NIH3T3 cells. J. Biol. Inorg. Chem. 2010, 15, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xia, Q.; Yuan, L.; Yang, X.; Wang, K. Impaired mitochondrial function and oxidative stress in rat cortical neurons: Implications for gadolinium-induced neurotoxicity. Neurotoxicology 2010, 31, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Bhagavathula, N.; DaSilva, M.; Aslam, M.N.; Dame, M.K.; Warner, R.L.; Xu, Y.; Fisher, G.J.; Johnson, K.J.; Swartz, R.; Varani, J. Regulation of collagen turnover in human skin fibroblasts exposed to a gadolinium-based contrast agent. Investig. Radiol. 2009, 44, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.J.; Li, J.X.; Yang, X.G.; Wang, K. Gadolinium-promoted cell cycle progression with enhanced S-phase entry via activation of both ERK and PI3K signaling pathways in NIH 3T3 cells. J. Biol. Inorg. Chem. 2009, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Wei, L.; Wu, H.; Li, W.; Wu, Y.; Li, X.; Ni, J.; Pei, F. Biochemical effects of gadolinium chloride in rats liver and kidney studied by 1H NMR metabolomics. J. Rare Earths 2009, 27, 280–287. [Google Scholar] [CrossRef]
- Moriconi, F.; Ahmad, G.; Ramadori, P.; Malik, I.; Sheikh, N.; Merli, M.; Riggio, O.; Dudas, J.; Ramadori, G. Phagocytosis of gadolinium chloride or zymosan induces simultaneous upregulation of hepcidin- and downregulation of hemojuvelin- and Fpn-1-gene expression in murine liver. Lab. Investig. 2009, 89, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Steger-Hartmann, T.; Raschke, M.; Riefke, B.; Pietsch, H.; Sieber, M.A.; Walter, J. The involvement of pro-inflammatory cytokines in nephrogenic systemic fibrosis—A mechanistic hypothesis based on preclinical results from a rat model treated with gadodiamide. Exp. Toxicol. Pathol. 2009, 61, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; DaSilva, M.; Warner, R.L.; Deming, M.O.; Barron, A.G.; Johnson, K.J.; Swartz, R.D. Effects of gadolinium-based magnetic resonance imaging contrast agents on human skin in organ culture and human skin fibroblasts. Investig. Radiol. 2009, 44, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wermuth, P.J.; Del Galdo, F.; Jimenez, S.A. Induction of the expression of profibrotic cytokines and growth factors in normal human peripheral blood monocytes by gadolinium contrast agents. Arthritis Rheum. 2009, 60, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.C.; Kuhlmann, M.K.; Kohlbacher, S.; Scheer, M.; Grgic, A.; Heckmann, M.B.; Uder, M. Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: In vitro study. Radiology 2007, 242, 425–434. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Dergunova, M.A.; Alekseenko, T.V.; Zhanaeva, S.Y.; Filyushina, E.E.; Filatova, T.G. Intralysosomal accumulation of gadolinium and lysosomal damage during selective depression of liver macrophages in vivo. Bull. Exp. Biol. Med. 2006, 142, 391–394. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, L.; Yang, X.; Wang, K. La3+, Gd3+ and Yb3+ induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem. Biol. Interact. 2003, 146, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Greisberg, J.K.; Wolf, J.M.; Wyman, J.; Zou, L.; Terek, R.M. Gadolinium inhibits thymidine incorporation and induces apoptosis in chondrocytes. J. Orthop. Res. 2001, 19, 797–801. [Google Scholar] [CrossRef]
- Yongxing, W.; Xiaorong, W.; Zichun, H. Genotoxicity of lanthanum (III) and gadolinium (III) in human peripheral blood lymphocytes. Bull. Environ. Contam. Toxicol. 2000, 64, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Hancox, J.C. Gadolinium inhibits Na+-Ca2+ exchanger current in guinea-pig isolated ventricular myocytes. Br. J. Pharmacol. 2000, 130, 485–488. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bales, P.J.; Zerbes, M.; Powis, D.A.; Marley, P.D. Effect of Gd3+ on bradykinin-induced catecholamine secretion from bovine adrenal chromaffin cells. Br. J. Pharmacol. 1999, 128, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.M.; Feranchak, A.P.; Davison, A.K.; Schwiebert, E.M.; Fitz, J.G. Evidence for Gd3+ inhibition of membrane ATP permeability and purinergic signaling. Am. J. Physiol. 1999, 277, G1222–G1230. [Google Scholar] [CrossRef] [PubMed]
- Adding, L.C.; Bannenberg, G.L.; Gustafsson, L.E. Gadolinium chloride inhibition of pulmonary nitric oxide production and effects on pulmonary circulation in the rabbit. Pharmacol. Toxicol. 1998, 83, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Tapia, G.; Videla, L.A. Effects of the Kupffer cell inactivator gadolinium chloride on rat liver oxygen uptake and content of mitochondrial cytochromes. FEBS Lett. 1998, 426, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Badger, D.A.; Kuester, R.K.; Sauer, J.M.; Sipes, I.G. Gadolinium chloride reduces cytochrome P450: Relevance to chemical-induced hepatotoxicity. Toxicology 1997, 121, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; Wilson, S.A.; Batchelor, J.; Reid, A.; Rees, J.; Harpur, E. Gadolinium chloride toxicity in the rat. Toxicol. Pathol. 1997, 25, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.M.; Zhang, J.X.; Clemens, M.G.; Diehl, A.M. Gadolinium chloride alters the acinar distribution of phagocytosis and balance between pro- and anti-inflammatory cytokines. Shock 1996, 6, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ruttinger, D.; Vollmar, B.; Wanner, G.A.; Messmer, K. In vivo assessment of hepatic alterations following gadolinium chloride-induced Kupffer cell blockade. J. Hepatol. 1996, 25, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Mizgerd, J.P.; Molina, R.M.; Stearns, R.C.; Brain, J.D.; Warner, A.E. Gadolinium induces macrophage apoptosis. J. Leukoc. Biol. 1996, 59, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Laine, M.; Arjamaa, O.; Vuolteenaho, O.; Ruskoaho, H.; Weckstrom, M. Block of stretch-activated atrial natriuretic peptide secretion by gadolinium in isolated rat atrium. J. Physiol. 1994, 480 Pt 3, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, B.; Enyeart, J.J. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J. Physiol. 1993, 469, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.R.; Lindhorst, S.M.; Welsh, C.T.; Maravilla, K.R.; Herring, M.N.; Braun, K.A.; Thiers, B.H.; Davis, W.C. High Levels of Gadolinium Deposition in the Skin of a Patient with Normal Renal Function. Investig. Radiol. 2016, 51, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, I.A.; Roos, R.; van der Molen, A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2018, 28, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Bussi, S.; Coppo, A.; Celeste, R.; Fanizzi, A.; Fringuello Mingo, A.; Ferraris, A.; Botteron, C.; Kirchin, M.A.; Tedoldi, F.; Maisano, F. Macrocyclic MR contrast agents: Evaluation of multiple-organ gadolinium retention in healthy rats. Insights Imaging 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Gonzalez-Cuyar, L.F.; Murata, K.; Fligner, C.; Dills, R.; Hippe, D.; Maravilla, K.R. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients with Normal Renal Function. Investig. Radiol. 2016, 51, 447–453. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Dai, D.; Schroeder, D.; Jentoft, M.E.; Murray, D.L.; Kadirvel, R.; Eckel, L.J.; Kallmes, D.F. Comparison of Gadolinium Concentrations within Multiple Rat Organs after Intravenous Administration of Linear versus Macrocyclic Gadolinium Chelates. Radiology 2017, 285, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, D.A.; Aracki-Trenkic, A.; Vojinovic, S.; Benedeto-Stojanov, D.; Ljubisavljevic, S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: Correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur. Radiol. 2016, 26, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Rossi Espagnet, M.C.; Bernardi, B.; Pasquini, L.; Figa-Talamanca, L.; Toma, P.; Napolitano, A. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr. Radiol. 2017, 47, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Perri, M.; Marsecano, C.; Vellucci, V.; Michelini, G.; Barile, A.; Di Cesare, E. Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: A retrospective study in 158 multiple sclerosis (MS) patients. Radiol. Med. 2018, 123, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Bjornerud, A.; Vatnehol, S.A.S.; Larsson, C.; Due-Tonnessen, P.; Hol, P.K.; Groote, I.R. Signal Enhancement of the Dentate Nucleus at Unenhanced MR Imaging after Very High Cumulative Doses of the Macrocyclic Gadolinium-based Contrast Agent Gadobutrol: An Observational Study. Radiology 2017, 285, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Blaichman, J.I.; Costa, A.F.; Glikstein, R.; Hurrell, C.; James, M.; Jabehdar Maralani, P.; Shabana, W.; Tang, A.; Tsampalieros, A.; et al. Gadolinium-Based Contrast Agents in Kidney Disease: Comprehensive Review and Clinical Practice Guideline Issued by the Canadian Association of Radiologists. Can. Assoc. Radiol. J. 2018, 69, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Bussi, S.; Fouillet, X.; Morisetti, A. Toxicological assessment of gadolinium release from contrast media. Exp. Toxicol. Pathol. 2007, 58, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, M.; Caravan, P. The biological fate of gadolinium-based MRI contrast agents: A call to action for bioinorganic chemists. Metallomics 2019, 11, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, E. Revisiting the Pharmacokinetic Profiles of Gadolinium-Based Contrast Agents: Differences in Long-Term Biodistribution and Excretion. Investig. Radiol. 2016, 51, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Bussi, S.; Coppo, A.; Bonafe, R.; Rossi, S.; Colombo Serra, S.; Penard, L.; Kirchin, M.A.; Maisano, F.; Tedoldi, F. Gadolinium Clearance in the First 5 Weeks After Repeated Intravenous Administration of Gadoteridol, Gadoterate Meglumine, and Gadobutrol to rats. J. Magn. Reson. Imaging 2021, 54, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Bussi, S.; Coppo, A.; Botteron, C.; Fraimbault, V.; Fanizzi, A.; De Laurentiis, E.; Colombo Serra, S.; Kirchin, M.A.; Tedoldi, F.; Maisano, F. Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J. Magn. Reson. Imaging 2018, 47, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Rosa, E.; Diaferia, C.; Rossi, F.; Tesauro, D.; Accardo, A. Systematic overview of soft materials as a novel frontier for MRI contrast agents. RSC Adv. 2020, 10, 27064–27080. [Google Scholar] [CrossRef] [PubMed]
- Narmani, A.; Farhood, B.; Haghi-Aminjan, H.; Mortezazadeh, T.; Aliasgharzadeh, A.; Mohseni, M.; Najafi, M.; Abbasi, H. Gadolinium nanoparticles as diagnostic and therapeutic agents: Their delivery systems in magnetic resonance imaging and neutron capture therapy. J. Drug Deliv. Sci. Technol. 2018, 44, 457–466. [Google Scholar] [CrossRef]
- Mueller, R.; Moreau, M.; Yasmin-Karim, S.; Protti, A.; Tillement, O.; Berbeco, R.; Hesser, J.; Ngwa, W. Imaging and Characterization of Sustained Gadolinium Nanoparticle Release from Next Generation Radiotherapy Biomaterial. Nanomaterials 2020, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., 2nd; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Simeckova, P.; Hubatka, F.; Kotoucek, J.; Turanek Knotigova, P.; Masek, J.; Slavik, J.; Kovac, O.; Neca, J.; Kulich, P.; Hrebik, D.; et al. Gadolinium labelled nanoliposomes as the platform for MRI theranostics: In vitro safety study in liver cells and macrophages. Sci. Rep. 2020, 10, 4780. [Google Scholar] [CrossRef] [PubMed]
- Gibby, W.; Parish, W.; Merrill, R.M.; Fernandez, D.; Anderson, C.R.; Merchel, E.; Parr, R. The use of a binary chelate formulation: Could gadolinium based linear contrast agents be rescued by the addition of zinc selective chelates? Magn. Reson. Imaging 2019, 58, 76–81. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | Main Findings |
|---|---|---|
| Akhtar et al., 2022 [25] | Human monocytes (THP-1 cell line) exposed to nanoparticles (NPs) of CeO2 or Gd2O3 | Gd2O3 NPs showed increased cytotoxic, pro-inflammatory (↑IL-1β and TNFα), and oxidative (↑ROS and TBARS, ↓GSH) potential, compared to CeO2 NPs; cell death induced by Gd2O3 NPs appears as apoptosis-independent (no effect on Bax-Bcl2 or caspase 3 activity), contrarily to CeO2 NPs |
| Ariyani et al., 2022 [26] | Rat glioma cells (C6 cell line), human astrocytoma cells (U87MG cell line), and primary cultures of mouse cerebral cortex astrocytes, exposed to Omniscan™ (gadodiamide), Magnescope® (gadoteric acid), Magnevist® (gadopentetic acid), or Gadovist® (gadobutrol) | All GBCAs acted via integrin αvβ3, leading to increased astrocytes migration, focal adhesion, and F-actin rearrangement, through activation of FAK/ERK1/2/Akt and Rho family of GTPases signaling pathways |
| Chanana et al., 2022 [27] | Mouse peritoneal macrophages isolated from C57BL/6 (H-2b) mice and murine leukemia transformed mouse macrophages (RAW 264.7 cell line) exposed to Dotarem® (gadoteric acid) in the presence of a static magnetic field gradient | Gadoteric acid appeared to affect actin polymerization, leading to macrophage elongation and relocation of organelles; enhanced pro-inflammatory M1 phenotype (↑iNOS and CD80) and decreased anti-inflammatory M2 phenotype (↓FcεRI); the magnetic field gradient had an opposite effect |
| Cobanoglu 2022 [28] | Human peripheral blood lymphocytes exposed to Dotarem® (gadoteric acid) and OptiMARK® (gadoversetamide) | Gadoversetamide, but not gadoteric acid, showed genotoxic and cytotoxic potential (↑frequency of micronuclei, nucleoplasmic bridges and nuclear buds, ↓cytostasis) |
| Nakamura et al., 2022 [29] | BALB/c male mice treated with a single administration of Omniscan™ (gadodiamide), Gadovist® (gadobutrol), or Gd (III) in the form of Gd(NO3)3 or GdCl3 | Tissue deposition of gadolinium varied with the chemical forms tested—higher levels for Gd(NO3)3, spleen enlargement and iron deposition for Gd (III)-treated mice |
| Tsai et al., 2022 [30] | Human keratinocytes (HaCaT cell line) exposed to gadodiamide | Apoptotic cell death (↑caspase 3 activity, ↓Bcl-2, ↑Bax) and autophagic activation (↑autophagic vacuoles and acidic lysosomes); autophagy potentiated apoptotic cell death |
| Uosef et al., 2022 [31] | Mouse macrophages treated with Dotarem® (gadoteric acid) | Macrophages retained Gd (III) for at least 7 days after exposure; this retention downregulated the expression of RhoA, mTORC1, and mTORC2 proteins, and dysregulated the expression level of organelle markers |
| Algieri et al., 2021 [32] | Mitochondrial (MT) fractions from swine hearts (Susscrofa domesticus) exposed to GdCl3 | GdCl3 inhibited both MT Ca2+- and Mg2+-activated F1FO-ATPase and desensitized the permeability transition pore to Ca2+ by binding to F1 |
| Baykara et al., 2021 [33] | Mouse hypothalamic neurons (GT1-7 cell line) treated with Omniscan™ (gadodiamide) or Dotarem® (gadoteric acid) | The amount of gadolinium released from gadodiamide was higher (versus gadoteric acid), leading to a higher impact in Ca2+ signaling |
| Erdoğan et al., 2021 [34] | Human neuroblastoma cells (SH-SY5Y cell line) exposed to Dotarem® (gadoteric acid), Gadovist® (gadobutrol), Omniscan™ (gadodiamide), Primovist® (gadoxectic acid), Magnevist® (gadopentetic acid), or OptiMARK™ (gadoversetamide) | Both linear and macrocyclic GBCAs triggered neuronal cell death through activation of apoptosis (↑Bax/Bcl-2 ratio); neurotoxicity was more prominent in cells exposed to linear GBCAs |
| Kartamihardja et al., 2021 [35] | Renal failure mouse model (kidney electrocoagulation) exposed for three weeks to Omniscan™ (gadodiamide) and Magnevist® (gadopentetic acid), three times per week | Gadodiamide showed higher skin gadolinium retention than gadopentetic acid, and more prominent pro-fibrotic potential (↑Collagen 1α, CTGF, TGFβ, αSMA, and IL-6); both GBCAs, especially gadodiamide, increased skin infiltration of CD3+ T cells and CD68+ macrophages, and (skin) expression and (serum) activity of neutrophil elastase |
| Kartamihardja et al., 2021 [36] | Primary mouse pups’ cerebellar cultures exposed to Magnevist® (gadopentetic acid) or Gadovist® (gadobutrol), in the presence or absence of iron (II) | Both GBCAs augmented dendrite arborization; iron (II) potentiated this effect only with gadopentetic acid |
| Kong et al., 2021 [37] | ICR female mice treated with repeated administrations of Magnevist® (gadopentetic acid), Dotarem® (gadoteric acid), Omniscan™ (gadodiamide), or Gadavist® (gadobutrol) for 3–5 weeks, followed by a recovery period of 1–5 weeks | Gadodiamide caused vacuolar changes in renal tubular epithelium; linear GBCAs increased leukocyte count after 5 weeks of exposure and induced higher gadolinium tissue deposition (cerebellum, liver, kidney, femur, skin, and peripheral nerve) compared to macrocyclic GBCAs |
| Reis Sousa et al., 2021 [38] | Human proximal tubular cells (HK-2 cell line) exposed to GdCl3 | GdCl3 induced disruption of oxidative status (↓TAS and GSH, ↑GSSG and NRF2), MT dysfunction (↑Ca2+, ↓ΔΨm and ATP), cell death by apoptosis (↑caspase 3, ↓Bcl-2), switching to necrosis (↑LDH leakage) at higher levels, and autophagic activation (↑p62); disturbance of lipid metabolism (↑ACACA, CPT1A, and neutral red uptake) increased expression of modulators of inflammation, hypoxia, and fibrosis (↑NFκB, IL-6 and 1β, TGFβ, OPN, and HIF-1α) at low to subtoxic concentrations |
| Solmaz et al., 2021 [39] | Male Sprague Dawley rats treated repeatedly for 3 weeks with Gadovist® (gadobutrol), Clariscan® (gadoteric acid), and Dotarem® (gadoteric acid); evaluation after a recovery period of 1 week | Repeated exposure to GBCAs caused hippocampal gliosis and increased oxidative stress and inflammation in the brain (↑LPO and TNFα, ↓SOD activity); neurotoxicity of gadobutrol was relatively lower than that of gadoteric acid |
| Tsai et al., 2021 [40] | Human fetal normal glial cells (SVG P12 cell line) exposed to Omniscan™ (gadodiamide) | Apoptotic cell death (↓Bcl-2 and -XL, ↑Bax and BAD, ↑cytochrome c, Apaf-1, and cleaved-caspase 3 and 9) and autophagic activation (↑autophagic vacuoles and acid lysosomes, ↑LC3-I/II turnover, beclin-1, autophagy-related proteins -5, and -14); autophagy potentiated cell death |
| Xie et al., 2021 [41] | Healthy mice treated with repeated doses of γ-Fe2O3 NPs and gadopentetic acid (Gd-DTPA) | Proinflammatory responses elicited by Gd-DTPA were stronger than for γ-Fe2O3 NPs (↑IL-1β, -6, -18, TNFα, CRP, and ferritin) |
| Akhtar et al., 2020 [42] | Human umbilical vein endothelial cells (HUVEC cell line) exposed to Gd2O3 NPs | Gd2O3 NPs acted as inducer of oxidative stress (↑TBARS, ROS and LPO, ↓GSH), MT dysfunction (↑MT membrane potential), and autophagy (↑acidic lysosomes and autophagic vacuoles), and revealed apoptotic (↑caspase 3 and annexinV) and necrotic potentials |
| Bloomer et al., 2020 [43] | Hepatic macrophages of young (6 months) and aged (24 months) Fischer 344 rats evaluated 2 days after exposure to GdCl3 | In aged animals, GdCl3 shifted liver macrophage polarization towards the anti-inflammatory M2 phenotype (↓iNOS+ cells). |
| Nong et al., 2020 [44] | Mouse embryo fibroblasts (NIH-3T3 cell line) exposed to gadodiamide or GdCl3 | Inhibition of cell growth, more pronounced with GdCl3; tubulin filaments appeared as potential gadolinium-binding proteins, which might lead to impaired microtubule assembling |
| Siew et al., 2020 [45] | Chinese hamster lung fibroblasts (V79-4 cell line) exposed to GdCl3 | Cell death and no significant DNA damage, although showing clastogenic potential (↑micronuclei frequency) |
| Supawat et al., 2020 [46] | K562 cancer cells and red blood cells exposed to gadoteric acid, gadopentetic acid, or gadobenic acid | Gadoteric acid and gadobenic acid decreased cell viability in K562 cancer cells in a concentration-dependent manner |
| Takanezawa et al., 2020 [47] | Human embryonic kidney cells (HEK293 cell line), lung carcinoma epithelial cells (A549 cell line), neuroblastoma cells (SH-SY5Y cell line), and mouse embryonic fibroblasts (MEF cell line) exposed to Gd(NO3)3 or GdCl3 | Gd (III) reduced cell viability in all cell lines, triggered ER stress, and activated autophagy (↑LC3-II), which appears as cytoprotective against Gd (III) toxicity |
| Akhtar et al., 2019 [48] | Human breast cancer cells (MCF-7 cell line) exposed to Gd2O3 NPs or to GdCl3 | Gd2O3 NPs and GdCl3 induced cytotoxicity (↑LDH leakage), oxidative damage (↑TBARS, ROS, GSH), and autophagic activation (↑autophagic vacuoles and acidic lysosomes); cell death was apoptosis-dependent (↑Bax/Bcl2 ratio) for GdCl3 and apoptosis-independent for Gd2O3 NPs |
| Baykara et al., 2019 [49] | Primary cultures of dorsal root ganglion neuron exposed to gadolinium, Omniscan™ (gadodiamide), Dotarem® (gadoteric acid), Gadovist® (gadobutrol), or MultiHance® (gadobenic acid) | Ca2+ levels within neurons decreased, as ionic currents were blocked by Gd (III) released from the chelates, in accordance with their stability (gadobutrol < gadobenic acid ≈ gadodiamide; no effect from gadoteric acid) |
| Beyazal Celiker et al., 2019 [50] | Male Sprague Dawley rats treated with repeated administrations of Dotarem® (gadoteric acid) or Omniscan™ (gadodiamide) for 5 weeks; evaluation after a recovery period of 5 weeks | Gadodiamide promoted higher kidney interstitial fibrosis, amyloid deposits, and vasocongestion, while gadoteric acid led to greater renal leukocytic infiltration and tubules atrophy; both GBCAs increased caspase 3 expression |
| Bower et al., 2019 [51] | Differentiated human neuroblastoma cells (SH-SY5Y cell line) exposed to Omniscan™ (gadodiamide), Magnevist® (gadopentetic acid), Primovist® (gadoxetic acid), MultiHance® (gadobenic acid), Dotarem® (gadoteric acid), Gadovist® (gadobutrol), or ProHance® (gadoteridol) | GBCAs triggered cell death by apoptosis, with reduction of the ΔΨm and of the oxidative respiratory function; disturbances were dependent on the stability of the GBCA, being more pronounced for linear GBCAs |
| Do et al., 2019 [52] | Female C57 black mice exposed to repeated administrations of Omniscan™ (gadodiamide) for 4 weeks | Impaired renal function, associated with myeloid cell infiltration and renal fibrosis (↑fibronectin, CCR2, and αSMA); metabolic dysfunction was also induced, with particular impact on renal lipid metabolism; obesity appeared to amplify these effects |
| Do et al., 2019 [53] | Female C57 black mice exposed to repeated administrations of Omniscan™ (gadodiamide) for 8 weeks | Skin fibrosis mediated by CCR2 (↑fibronectin, collagen I, CCR2, CCL2) |
| Pan et al., 2019 [54] | Human embryonic kidney cells (HEK293 cell line) treated with GdCl3 | Proliferation of HEK293 cells (increased DNA synthesis and activation of EGFR/Akt/ERK signaling pathways; pro-fibrotic/pro-inflammatory changes (↑TGFβ and its receptor, TNFα, TIMP-1, and integrins αV and β1)) |
| Tsai et al., 2019 [55] | Rat glioma C6 cells treated with GdCl3 | Cell death by apoptosis (↑caspases 3, 8, and 9 activity, ROS and Ca2+, ↓ΔΨm); down-regulation of the mitogen-activated protein kinases pathway |
| Wang et al., 2019 [56] | SJL/J mice, healthy or with autoimmune encephalomyelitis, exposed to repeated administrations of gadopentetic acid for 4 days | Ongoing inflammation favored retention of Gd (III) in the brain tissue |
| Weng et al., 2019 [57] | Adenine-induced renal failure rat model treated with repeated administrations of gadodiamide for 5 days; human normal liver cells (L02 cell line), human embryonic kidney cells (HEK293 cell line), mouse fibroblasts (3T6 cell line), and mouse macrophages (RAW264.7 cell line), exposed to gadodiamide | Skin fibrosis, oxidative stress, and inflammation (↑αSMA and TGFβ1, heme oxygenase-1, NOX4, CCL2, IL-1β and TNFα) in renal failure rats; in vitro exposure of macrophages showed upregulation of markers of fibrosis and inflammation (↑αSMA and TGFβ1, IL-1β and TNFα), and of fibrosis (↑αSMA) in fibroblast exposed to the supernatant of exposed macrophages; at the highest concentrations, promoted cell death in normal liver and kidney cells and in macrophages |
| Beyazal Celiker et al., 2018 [58] | Male Sprague Dawley rats treated with repeated administrations of Dotarem® (gadoteric acid) or Omniscan™ (gadodiamide) for 5 weeks | Both showed toxic effects on testis tissue, inducing apoptosis (↑caspase 3 and Ca2+) and reducing testosterone levels |
| Fattah et al., 2018 [59] | Human breast cancer (MCF-7 cell line), mammary epithelial (Hs 578T cell line), and epithelial-like triple-negative breast cancer cells (MDA-MB-231 cell line) exposed to gadopentetic acid | Triggered cell proliferation of MCF-7 cells at low concentrations and cell death, as well as cell migration, at higher levels |
| Friebe et al., 2018 [60] | Lymphocytes from healthy donors incubated with Gadovist® (gadobutrol), Dotarem® (gadoteric acid), Omniscan™ (gadodiamide), Magnograf® (gadopentetic acid), or Primovist® (gadoxetic acid), either alone or combined with ultra-high-field 7-T magnetic resonance imaging exposure | Only linear GBCAs showed a dose-dependent increase in apoptosis (↑annexinV+ cells) and a decrease in DNA synthesis, independent of additional 7-T magnetic resonance imaging co-exposure |
| Mercantepe et al., 2018 [10] | Male Sprague Dawley rats exposed repeatedly to Omniscan™ (gadodiamide) or Dotarem® (gadoteric acid) for 20 days | Both triggered hepatocellular necrosis, portal inflammation, and apoptosis (↑caspase 3); no changes occurred in total antioxidant/oxidant capacity |
| Weng et al., 2018 [61] | Macrophages exposed to low levels of Omniscan® (gadodiamide), Primovist® (gadoxetic acid), Magnevist® (gadopentetic acid), Gadovist® (gadobutrol), or GdCl3 | GdCl3 and GBCAs had no effect on cell viability, but promoted MT dysfunction and oxidative stress (↓ΔΨm, and ↑ROS); GBCAs also triggered an inflammatory response (↑nitrate/nitrite, prostaglandin E2, IL-6, ↓IL-10) |
| Alarifi et al., 2017 [62] | Human neuroblastoma cells (SH-SY5Y cell line) exposed to Gd2O3 NPs | Cell death by apoptosis (↑caspase 3, ↓ΔΨm and Bcl2/Bax ratio), DNA damage, and oxidative stress (↑ROS, LPO, SOD and catalase, ↓GSH) |
| Knoepp et al., 2017 [63] | Xenopus laevis oocytes heterologously expressing human epithelial Na+-channels exposed to GdCl3, Magnevist® (gadopentetic acid), Dotarem® (gadoteric acid), or their chelates | GdCl3 triggered changes in epithelial Na+-channels-mediated currents and appeared to act on at least two binding sites; Gd (III) released from the linear GBCAs, but not from gadoteric acid, was sufficient to interfere with the channels’ activity |
| Nagy et al., 2017 [64] | Human skin keratinocytes (HaCaT cell line), human limbal stem cells (HuLi cell line), colorectal adenocarcinoma (CaCO2 cell line), murine squamous carcinoma (SCC cell line), and Indian muntjac cells (IM cell line) exposed to GdCl3 | Loss of cellular motility, premature chromatin condensation, and highly condensed chromatin, consistent with apoptotic cell death |
| Ozawa et al., 2016 [65] | Normal human dermis-derived fibroblasts incubated with Omniscan™ (gadodiamide) | Increased fibroblast growth, with increased DNA synthesis |
| Tsai et al., 2016 [66] | Human osteosarcoma cells (U-2 OS cell line) exposed to GdCl3 | Apoptotic cell death mediated by death receptors, mitochondria, and ER stress (↑caspases 3, 4, 8, and 9 activity, Fas and its ligand, cytochrome c, Apaf-1, GADD153, GRP78, Ca2+, ↓ΔΨm) |
| Bose et al., 2015 [67] | Male BALB/c mice with a two-step surgical 5/6 nephrectomy, exposed to repeated administrations of Omniscan™ (gadodiamide), with or without deferiprone, for 22 days; evaluations after 16 weeks; human peripheral blood mononuclear cells exposed to Omniscan™ (gadodiamide), with or without deferiprone | Renal failure mice exposed to gadodiamide developed nephrogenic systemic fibrosis; infiltration of ferroportin-expressing fibrocyte-like cells and iron accumulation in the skin; these effects were less pronounced in gadodiamide plus deferiprone-treated group; gadodiamide also prompted release of catalytic iron in vitro |
| Chen et al., 2015 [68] | BALB/c mice exposed to a single dose of gadopentetic acid for 24 h | Reduced circulating leukocytes and triggered an inflammatory response (↑IL-6 and TNFα); it also induced damage in the lungs, kidneys, and spleen |
| Schmidt-Lauber et al., 2015 [69] | Bone marrow derived macrophages from C57BL/6, Nlrp3−/−, and Asc−/− mice incubated with Omniscan™ (gadodiamide), gadopentetic acid, or GdCl3; male C57BL/6 and Nlrp3−/− mice intraperitoneally injected with a single dose of gadopentetic acid | Free Gd (III) and GBCAs induced the secretion of IL-1β in wild type mice-derived macrophages, through the activation of the inflammasome; Gd-containing compounds exhibited higher potential to activate anti-inflammatory M2 macrophages; the inflammatory response in vivo was also dependent on engagement of the inflammasome |
| Cho et al., 2014 [70] | Human lymphocytes exposed to GdCl3 | Genotoxicity (↑micronuclei frequency and DNA damage), apoptotic cell death, and oxidative stress (↑ROS); extremely low-frequency electromagnetic fields potentiated these effects |
| Do et al., 2014 [11] | Human foreskin fibroblasts incubated with Omniscan™ (gadodiamide) or ProHance® (gadoteridol); Female Fisher 344 rats with renal failure (5/6 nephrectomy) exposed to repeated doses of the GBCA for 4 weeks | In vitro, GBCAs triggered fibrosis (↑fibronectin, TGFβ, and αSMA); in vivo, gadodiamide led to greater skin fibrosis (↑fibronectin) and dermal cellularity than gadoteridol; gadoteridol induced higher expression of skin TGFβ and fibronectin accumulation in the liver; both agents led to proximal renal tubule vacuolization |
| Shen et al., 2014 [71] | Mouse embryo fibroblasts (NIH3T3 cell line) exposed to GdCl3 | Cell proliferation via Rac, PI3K/Akt, and integrin-mediated signaling pathways |
| Wermuth and Jimenez 2014 [72] | Human dermal fibroblasts incubated with supernatants of human peripheral blood mononuclear cells treated with gadopentetic acid, Omiscan™ (gadodiamide), Dotarem® (gadoteric acid), MultiHance® (gadobenic acid), ProHance® (gadoteridol), OptiMARK® (gadoversetamide), or non-chelated Gd (III) | GBCA exposure led to variable expressions of profibrotic and proinflammatory cytokines in monocytes, more pronounced for linear agents (↑IL-4, -6, -13, TGFβ, and VEGF); overall increase in gene expression of cytokines, chemokines, genes involved in the activation of NFκB and interferon-responsive genes was also observed in Gd-treated monocytes; fibroblast showed a profibrotic phenotype (↑types I and III collagen, fibronectin, and αSMA) |
| Swaminathan et al., 2013 [73] | Human peripheral blood mononuclear cells exposed to Omniscan™ (gadodiamide); skin biopsy specimens from NSF patients (for confirmatory purposes) | Differentiation of mononuclear cells into collagen-secreting cells, with increased expression of iron metabolism proteins, angiogenic and osteoblast-lineage markers; these types of cell were also present in skin biopsies of NSF patients |
| Bleavins et al., 2012 [74] | Human dermal fibroblasts and epidermal keratinocytes isolated from neonatal foreskin exposed to Gd (III) salts, Magnevist® (gadopentetic acid), MultiHance® (gadobenic acid), Omniscan™ (gadodiamide), or non-clinical gadodiamide | Gd (III) salts attached to fibroblasts surface; proliferation was stimulated at lower concentrations via MAPK and PI3K signaling pathways, while cytotoxicity was seen at higher levels; GBCAs, but not the salts, also showed proliferative potential in fibroblasts under low-Ca2+ conditions, more evident for gadodiamide; no effects were observed in keratinocytes |
| Pereira et al., 2012 [75] | Male Wistar rats without or with renal failure (5/6 nephrectomy), exposed to a single dose of Dotarem® (gadoteric acid) | Rats with renal failure showed a decreased renal function (↓GFR, ↑proteinuria, decrease in total iron binding capacity, increased serum ferritin, transferrin oversaturation, and increased plasmatic TBARS); treatment with the antioxidant N-acetylcysteine ameliorated these effects; rats with normal renal function showed no effects when treated with gadoteric acid compared to controls |
| Wagner et al., 2012 [76] | Female Fischer 344 rats with renal failure (5/6 nephrectomy) treated with repeated administrations of gadodiamide for 4 weeks | Skin presenting bone marrow-derived cells, with increased expression of αSMA, and with profibrotic (↑fibronectin, collagen IV, cathepsin L), and pro-oxidant phenotypes (↑superoxide, NOX4) |
| Wermuth & Jimenez 2012 [77] | Human embryonic kidney cells (HEK293 cell line) expressing one of different human TLRs or NLRs, and macrophages differentiated from human peripheral blood mononuclear cells exposed to Dotarem® (gadoteric acid), MultiHance® (gadobenic acid), ProHance® (gadoteridol), OptiMARK® (gadoversetamide), Omniscan™ (gadodiamide), non-clinical gadodiamide or gadopentetic acid, or non-chelated Gd (III) | Non-chelated Gd (III), gadoteric and gadobenic acid, as well as both gadodiamide formulations, induced NFκB activation via TLR4 and 7, more pronounced with the latter two; this stimulation of TLR resulted in a strong profibrotic/pro-inflammatory response in macrophages treated with Omniscan™ and gadodiamide (↑CXCL10, 11, and 12, CCL2 8 and 9, IL-4 and -6, TGFβ, and VEGF) |
| Angeli et al., 2011 [78] | Aortic rings of Wistar rats incubated with GdCl3 | Blockade of ADP and ATP hydrolysis through stimulation of angiotensin II receptor type 1 |
| Feng et al., 2011 [79] | Primary cultures of cortical astrocytes, isolated from neonatal Sprague Dawley rats, treated with GdCl3 | Ca2+ influx; no effects on cytotoxicity, potentially due to the activation of unfolded protein responses, as a consequence of triggered ER stress |
| Ghio et al., 2011 [80] | Human alveolar macrophages, human monocytes (THP-1 cell line), primary and immortalized (BEAS-2B cell line) human normal bronchial epithelial cells exposed to GdCl3 or Omniscan™ (gadodiamide) | A concentration-dependent uptake of Gd (III) was observed for all cell types, for both GdCl3 and gadodiamide; co-exposure of cells to GdCl3 and ferric ammonium citrate increased iron levels compared to incubation with each compound alone; in BEAS-2B cells, GdCl3 triggered increased production of IL-18, and co-exposure with ferric ammonium citrate led to increased ferritin levels |
| Long et al., 2011 [81] | Human adenocarcinoma cells (HeLa cell line) exposed to GdCl3 | Cell proliferation and increased lipid and amino acid metabolisms at low concentrations, while promoting cell death and disrupting the metabolism of lipids, amino acids, and carbohydrates at higher concentrations |
| MacNeil et al., 2011 [82] | Primary human keratinocytes and dermal fibroblasts exposed to Gd-EDTA, Omniscan™ (gadodiamide), or Dotarem® (gadoteric acid) | Gd-EDTA and gadodiamide stimulated both fibroblast and keratinocyte viability at lower concentrations and induced cell death at higher levels; they also stimulated collagen production in fibroblasts, but not in keratinocytes |
| Okada et al., 2011 [83] | Mouse pre-osteoblastic cells (MC3T3-E1 cell line), human adipose tissue-derived mesenchymal stem cells, human subcutaneous preadipocytes, and human dermal fibroblasts, exposed to GdCl3 | Cell differentiation in all cell types and Ca2+ deposition, leading to abnormal calcification; downregulation of type I collagen was also observed in fibroblasts |
| Wang et al., 2011 [84] | Prostate cancer cells (DU145 and PC3 cell lines) exposed to GdCl3 | Inhibition of PC3 cell viability via apoptosis (↑annexinV), as well as cell migration in both cell lines, which was mediated by the inactivation of both ERK1/2 and p38 MAPK pathways; increase in Ca2+ levels; all effects appear to be regulated upstream by the PTx-sensitive Gi protein signaling pathway; suppression of cell-induced osteoclast differentiation via the RANKL/RANK/OPG pathway |
| Wiesinger et al., 2011 [85] | Human umbilical vein endothelial cells (HUVECs) and human dermal fibroblasts (HSF 1 cells) were exposed to Gadovist® (gadobutrol), Magnevist® (gadopentetic acid), MultiHance® (gadobenic acid), or Omniscan™ (gadodiamide), as well as the manganese- and the iron-based contrast agents Teslascan® and Resovist® | Gadodiamide and Teslascan® showed antiproliferative effect in HUVECs, which was rapidly compensated; HSF 1 cells showed no effect on TGFβ levels after exposure to the GBCAs |
| Xia et al., 2011 [86] | Primary cultured rat cortical neurons exposed to GdCl3 | Cytotoxicity in neurons, with increased Ca2+ levels, through oxidative injury (↑ROS) and ER stress-related signal transduction |
| Bhagavathula et al., 2010 [87] | Human dermal fibroblasts and intact skin in organ culture exposed to GdCl3 | Increased cell proliferation in fibroblasts, possibly involving MAPK/PI3K signaling pathways; upregulation of MMP-1 and TIMP-1 in both cells and skin culture; increased type 1 collagen deposition in the skin |
| Del Galdo et al., 2010 [88] | Human monocyte-derived macrophages incubated with Omniscan™ (gadodiamide) | Stimulated macrophage activation, with NFκB-dependent expression, and increased chemokines production (↑CCL2 and 8, CXCL10 and 11) and iNOS |
| Gou et al., 2010 [89] | Mouse macrophages (RAW 264.7 cell line) treated with GdCl3 | No effect on macrophage viability; trigger of profibrotic/pro-inflammatory responses (↑TGFβ1 and IL-6) via the activation of protein kinase C and ERK1/2 signaling pathways |
| Li et al., 2010 [90] | Mouse embryo fibroblasts (NIH3T3 cell line) treated with Gd-containing particles | Promoted G1/S cell cycle progression through the activation of ERK and Akt signaling pathways; increased levels of serum in media led to the formation of smaller particles that exert a stronger effect on cell cycle |
| Feng et al., 2010 [91] | Primary cultures of embryonic cortical neurons exposed to GdCl3 | Cell death by apoptosis (↓MT activity, ΔΨm and ATP, ↑cytochrome c, and caspase 3), oxidative stress (↑ROS), and DNA fragmentation |
| Bhagavathula et al., 2009 [92] | Human dermal fibroblasts treated with Omniscan™ (gadodiamide) | Increased production of MMP-1 and TIMP-1 and increased type I collagen deposition, without affecting type I procollagen production |
| Fu et al., 2009 [93] | Mouse embryo fibroblasts (NIH-3T3 cell line) exposed to GdCl3 | Increased cell growth, promoting G1/S cell cycle progression (↑cyclin A, B, and D), which appears to be mediated by activation of both ERK and PI3K signaling pathways |
| Liao et al., 2009 [94] | Male Wistar rats treated with a single dose of GdCl3 | Liver damage with disrupted carbohydrate metabolism (↓glycogen, ↑succinate, lactate, alanine, and betaine); no histological evidence of kidney damage, but with changes in renal metabolic profile |
| Moriconi et al., 2009 [95] | Male Wistar rats and C3H/HeJ endotoxin-resistant mice injected intraperitoneally with a single dose of GdCl3 | Phagocytosis dysregulated the hepatic iron metabolism (↑hepcidin, ↓hemojuvelin, and ferroportin-1); these changes might be mediated by the locally produced acute-phase-cytokines (↑IL-1β and -6, TNFα) |
| Steger-Hartmann et al., 2009 [96] | Male Wistar rats treated either once, three, or eight times with a daily administration of Omniscan™ (gadodiamide) | A decrease in reticulocyte and an increase in monocyte counts; a decrease in albumin/globulin ratio; histological signs of renal damage and dermal fibrosis; Gd (III) was detectable in the skin, femur, and liver; trigger a pro-inflammatory response, which appears to increase vascular permeability (↑OPN, VEGF, CXCL2, CCL1 and 3, TNFα, and TIMP-1) |
| Varani et al., 2009 [97] | Human dermal fibroblasts and human skin in organ culture, isolated from adult volunteers, treated with Omniscan™ (gadodiamide), Magnevist® (gadopentetic acid), MultiHance® (gadobenic acid), or Prohance® (gadoteridol) | GBCA exposure increased fibroblast proliferation, accompanied by increased production of MMP-1 and TIMP-1, but not of type I procollagen; similar effects were observed with gadodiamide exposure in ex vivo skin |
| Wermuth et al., 2009 [98] | Human peripheral blood monocytes incubated with Omniscan™ (gadodiamide), Gd-DTPA, or GdCl3 | The three compounds stimulated a pro-inflammatory/profibrotic response (↑IL-4, 6, and 13, interferon γ, TGFβ, VEGF, αSMA, and type I collagen) |
| Heinrich et al., 2007 [99] | Pig kidney proximal tubular cells (LLC-PK1 cell line) incubated with Magnevist® (gadopentetic acid), MultiHance® (gadobenic acid), Dotarem® (gadoteric acid), or Omniscan™ (gadodiamide) | All GBCAs induced concentration-dependent cell death; induction of necrosis and apoptosis was more evident for gadopentetic and gadobenic acid |
| Korolenko et al., 2006 [100] | Male CBA mice administered with a single dose of GdCl3 | GdCl3 accumulated in liver macrophages lysosomes, leading to damage and a decrease in macrophage density |
| Liu et al., 2003 [101] | Mitochondria isolated from Laca mice liver and human normal liver cells (7701 cell line) exposed to lanthanides | Disruption of MT function (↑MT swelling and membrane fluidity, and ↓ΔΨm); induction of apoptosis (↑cytochrome c release) with potential involvement of oxidative stress (↑ROS) |
| Greisberg et al., 2001 [102] | Cultured bovine chondrocytes, isolated from articular cartilage, exposed to Omniscan™ (gadodiamide) | Adverse changes in chondrocyte metabolism (↑matrix production, ↓cellular proliferation, ↑apoptosis) |
| Yongxing et al., 2000 [103] | Human peripheral blood lymphocytes, from a healthy male adult, exposed to Gd(NO3)3 | DNA damage (↑micronuclei frequency, single stranded DNA breaks and unscheduled DNA synthesis) |
| Zhang et al., 2000 [104] | Single ventricular myocytes, isolated from hearts of male guineapigs, exposed to GdCl3 | Non-voltage dependent inhibitory effect on both inward and outward ionic current, which appears to reflect gradual Gd (III) accumulation at the binding site of the Na+-Ca2+ exchanger protein that carries the current |
| Bales et al., 1999 [105] | Bovine adrenal chromaffin cells treated with Gd (III) | Enhancement of the Ca2+ -mediated catecholamine secretion by inhibiting Ca2+ efflux |
| Roman et al., 1999 [106] | Primary cultured rat hepatocytes and rat hepatoma cells (HTC cell line) exposed to Gd (III) | High inhibition of ATP release in liver cells, suggesting that Gd (III) might be an effective inhibitor of ATP-permeable channels |
| Adding et al., 1998 [107] | Male New Zealand white rabbits infused with GdCl3 for 25 min | Decrease in pulmonary vascular resistance, which appears to be partly due to inhibition of NO formation |
| Ferreira et al., 1998 [108] | Liver mitochondria isolated from male Sprague Dawley rats treated with a single dose of GdCl3 | A reversible decrease in liver O2 consumption, accompanied by a decline in MT cytochromes c1 and c |
| Badger et al., 1997 [109] | Liver microsomes and hepatocytes isolated from control male and female Sprague Dawley rats and rats administered with a single dose of GdCl3 | GdCl3 treatment reduced the activity of total hepatic microsomal cytochrome P450 and aniline hydroxylase; it also reduced the susceptibility of hepatocytes to the cytotoxicity induced by CCl4, but not by CdCl2 |
| Spencer et al., 1997 [110] | Male and female Sprague Dawley rats treated with a single administration of GdCl3 | Deposition in capillary beds of the lung and kidney, and in the liver and spleen, with signs of necrosis in both organs; phagocytosis by the mononuclear phagocytic system was also observed |
| Rai et al., 1996 [111] | Rats treated with a single dose of GdCl3 | Distribution of Kupfer cells in the liver and changes in their phenotype towards a more pro-inflammatory one (↑TNFα, ↓IL-10) |
| Ruttinger et al., 1996 [112] | Male Sprague Dawley rats treated with a single dose of GdC13 | Lower phagocytic activity of Kupfer cells, which may be related to the increased inflammatory response (↑TNFα and IL-6) |
| Mizgerd et al., 1996 [113] | Rat alveolar macrophages exposed to GdCl3 | Cell death by apoptosis |
| Laine et al., 1994 [114] | Rat atrial preparations, from male Sprague Dawley rats, incubated with GdCl3 | Blocked voltage-gated calcium channels and inhibited stretch-activated atrial natriuretic peptide secretion |
| Mlinar and Enyeart 1993 [115] | Rat and human medullary thyroid carcinoma cells (6-23 (clone 6) and TT cell lines, respectively) exposed to trivalent metal cations | GdCl3 blocked the current through T-type voltage gated calcium channel by occlusion of the channel pore, and in a voltage-independent way |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, S.; Rocha, S.; Sousa, N.R.; Catarino, C.; Belo, L.; Bronze-da-Rocha, E.; Valente, M.J.; Santos-Silva, A. Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review. Int. J. Mol. Sci. 2024, 25, 4071. https://doi.org/10.3390/ijms25074071
Coimbra S, Rocha S, Sousa NR, Catarino C, Belo L, Bronze-da-Rocha E, Valente MJ, Santos-Silva A. Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review. International Journal of Molecular Sciences. 2024; 25(7):4071. https://doi.org/10.3390/ijms25074071
Chicago/Turabian StyleCoimbra, Susana, Susana Rocha, Nícia Reis Sousa, Cristina Catarino, Luís Belo, Elsa Bronze-da-Rocha, Maria João Valente, and Alice Santos-Silva. 2024. "Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review" International Journal of Molecular Sciences 25, no. 7: 4071. https://doi.org/10.3390/ijms25074071
APA StyleCoimbra, S., Rocha, S., Sousa, N. R., Catarino, C., Belo, L., Bronze-da-Rocha, E., Valente, M. J., & Santos-Silva, A. (2024). Toxicity Mechanisms of Gadolinium and Gadolinium-Based Contrast Agents—A Review. International Journal of Molecular Sciences, 25(7), 4071. https://doi.org/10.3390/ijms25074071









