Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines
Abstract
1. Introduction
2. Results
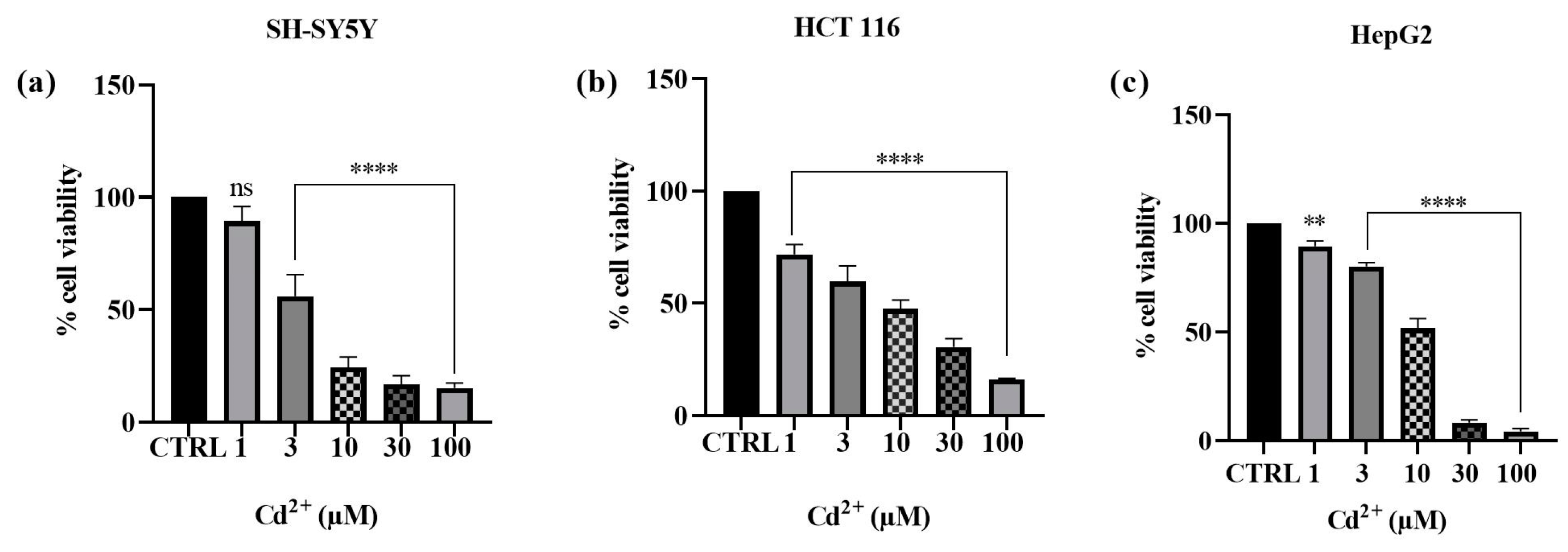
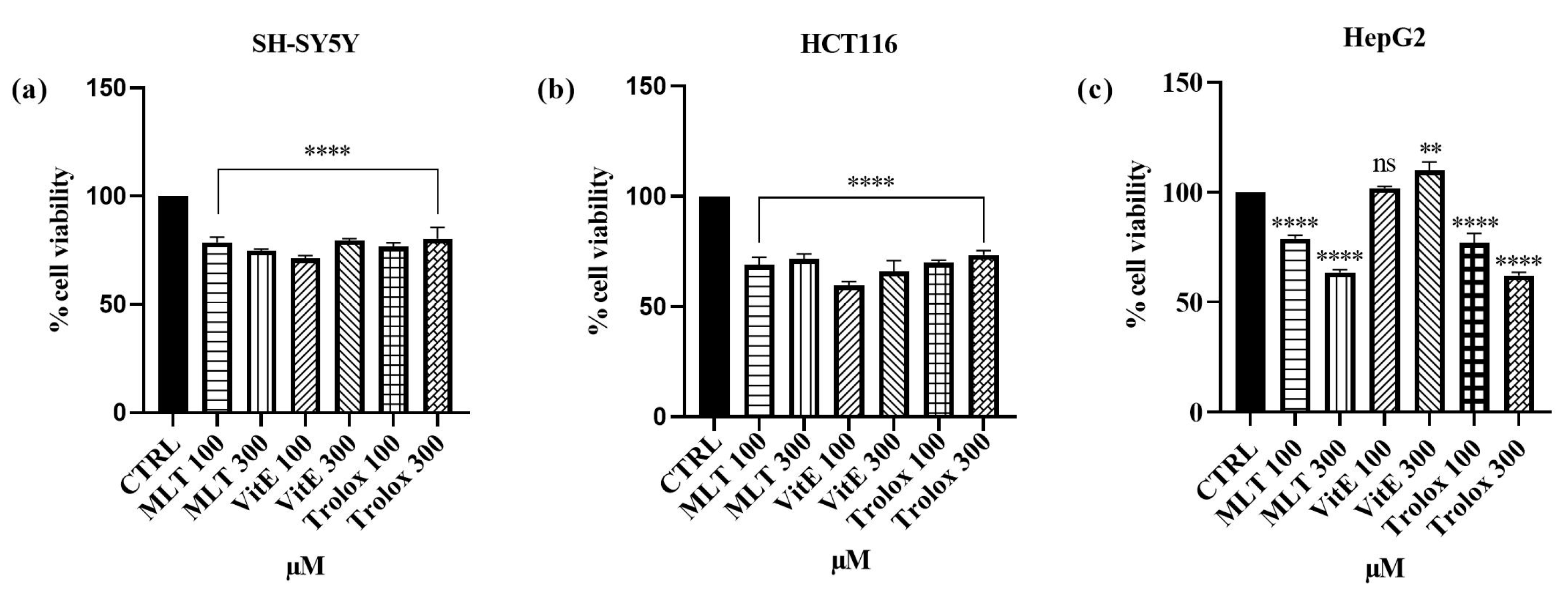
2.1. Effect Cd2+, MLT, Vit E, and Trolox on Cell Viability
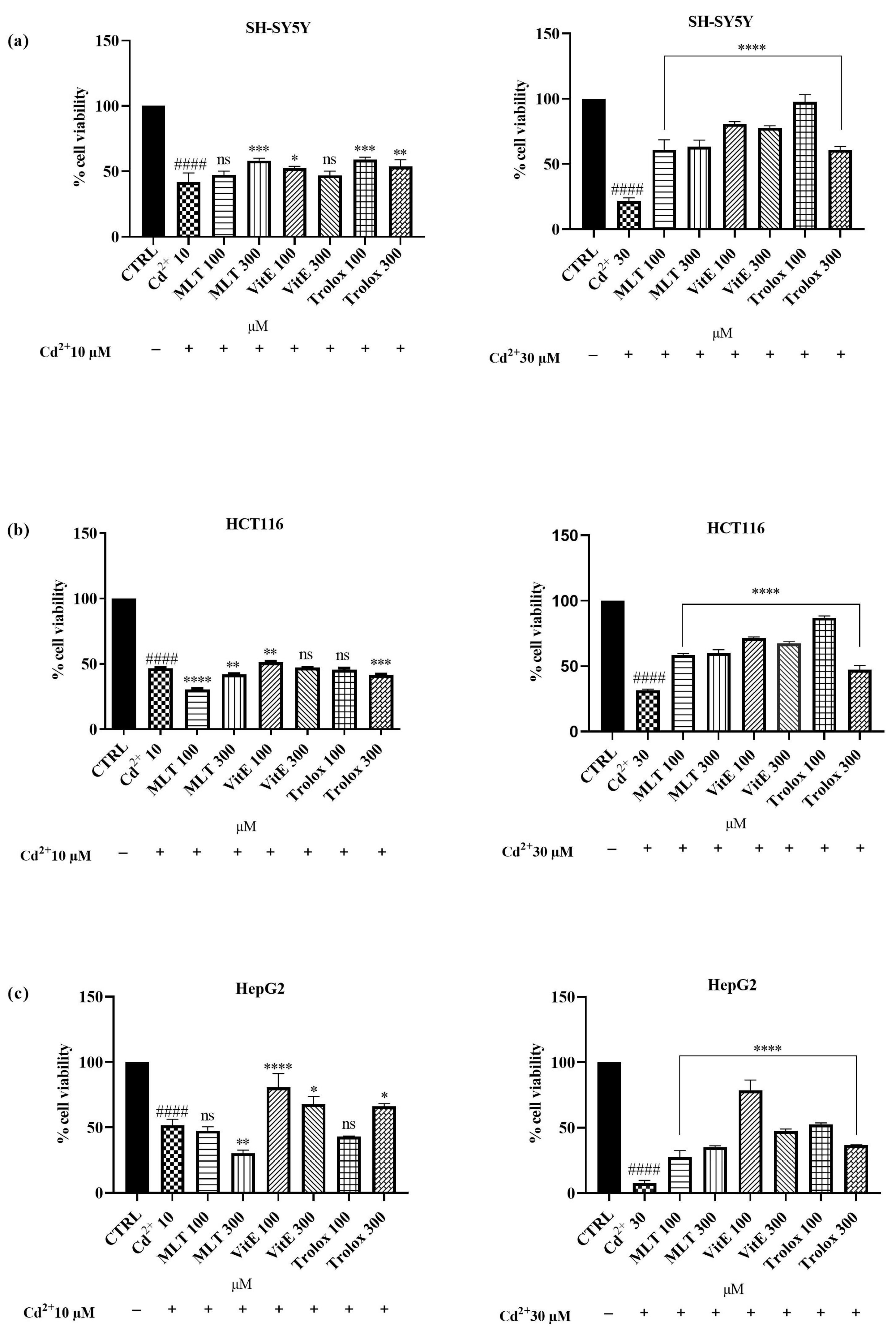
2.2. Effect of MLT, VitE, and Trolox on Cd2+-Induced Cytotoxicity
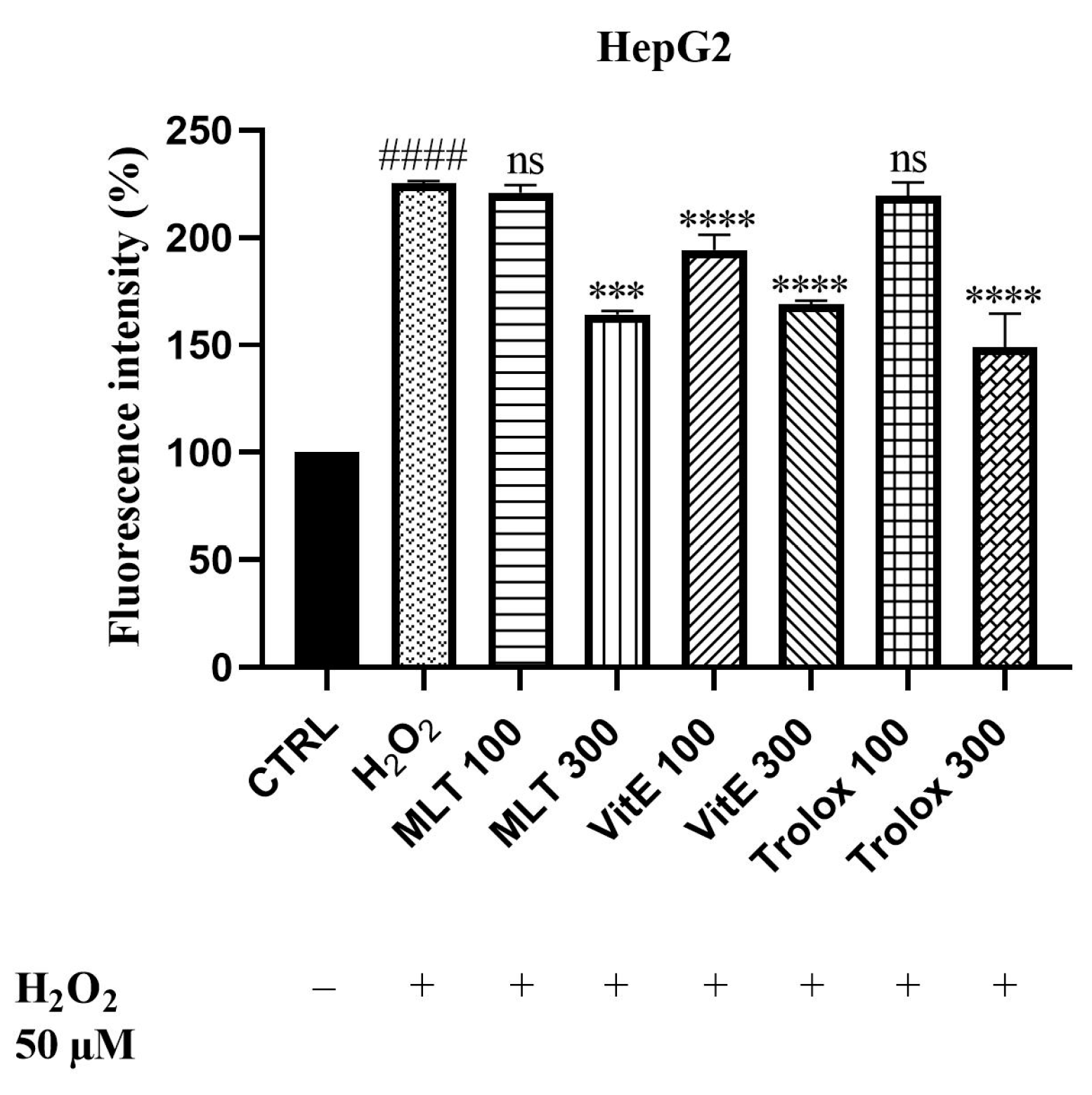
2.3. ROS Scavenging Effects of MLT, VitE, and Trolox against Cd2+- and H2O2-Induced Oxidative Stress
3. Discussion
3.1. Effect of Cd, MLT, VitE, and Trolox on Cell Viability
3.2. Effect of MLT, VitE, and Trolox on Cd-Induced Cytotoxicity
3.3. ROS Scavenging Effects of MLT, VitE, and Trolox on Cd-Induced Oxidative Stress
4. Materials and Methods
4.1. Cytotoxicity and Cytoprotective Assays In Vitro
4.1.1. Cell Culture
4.1.2. Preparation of Heavy Metal Solutions and Antioxidant Agents
4.1.3. Cytoprotective and Proliferative Effects
4.1.4. Cell Viability Evaluation by the Colorimetric MTT Assay
4.1.5. Measurement of Intracellular ROS Production
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Suhani, I.; Sahab, S.; Srivastava, V.; Singh, R.P. Impact of Cd pollution on food safety and human health. Curr. Opin. Toxicol. 2021, 27, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cd Exposure in the Population and Intervention Strategies Against Cd Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Storelli, A.; Quaglia, N.C.; Garofalo, R.; Meleleo, D.; Busco, A.; Storelli, M.M. Trace Metals in Pork Meat Products Marketed in Italy: Occurrence and Health Risk Characterization. Biol. Trace Elem. Res. 2020, 199, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Moghadamnia, A.A. Cd toxicity and treatment: An up-date. Caspian J. Intern. Med. 2017, 8, 135–145. [Google Scholar]
- Fujiwara, Y.; Lee, J.Y.; Banno, H.; Imai, S.; Tokumoto, M.; Hasegawa, T.; Seko, Y.; Nagase, H.; Satoh, M. Cadmium induces iron deficiency anemia through the suppression of iron transport in the duodenum. Toxicol. Lett. 2020, 332, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cd Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Jannetto, P.J.; Cowl, C.T. Elementary Overview of Heavy Metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.; Mohamad Noor, N.; Paiman, N.A.; Ahmad Zaidi, A.M.; Zainal Bakri, S.F. Heavy metals found in the breathing zone, toenails and lung function of welders working in an air-conditioned welding workplace. Int. J. Occup. Saf. Ergon. 2018, 24, 646–651. [Google Scholar] [CrossRef]
- Rana, S.V.S. Perspectives in Endocrine Toxicity of Heavy Metals—A Review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Lentini, P.; Zanoli, L.; Granata, A.; Signorelli, S.S.; Castellino, P.; Dell’Aquila, R. Kidney and heavy metals—The role of environmental exposure (Review). Mol. Med. Rep. 2017, 15, 3413–3419. [Google Scholar] [CrossRef]
- Lin, H.C.; Hao, W.M.; Chu, P.H. Cadmium and cardiovascular disease: An overview of pathophysiology, epidemiology, therapy, and predictive value. Rev. Port. Cardiol. 2021, 40, 611–617. [Google Scholar] [CrossRef]
- Salama, S.A.; Mohamadin, A.M.; Abdel-Bakky, M.S. Arctigenin alleviates cadmium-induced nephrotoxicity: Targeting endoplasmic reticulum stress, Nrf2 signaling, and the associated inflammatory response. Life Sci. 2021, 287, 120121. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between serum heavy metals and prostate cancer risk—A multiple metal analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef]
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T. Cancer Mortality in Residents of the Cadmium-Polluted Jinzu River Basin in Toyama, Japan. Toxics 2018, 6, 23. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and Cd on the immune system and cancer progression. J. Environ. Health Sci. 2020, 18, 335–343. [Google Scholar]
- Lu, J.; Zhou, Z.; Zheng, J.; Zhang, Z.; Lu, R.; Liu, H.; Shi, H.; Tu, Z. 2D-DIGE and MALDI TOF/TOF MS analysis reveal that small GTPase signaling pathways may play an important role in cadmium-induced colon cell malignant transformation. Toxicol. Appl. Pharmacol. 2015, 288, 106–113. [Google Scholar] [CrossRef]
- Forcella, M.; Lau, P.; Oldani, M.; Melchioretto, P.; Bogni, A.; Gribaldo, L.; Fusi, P.; Urani, C. Neuronal specific and non-specific responses to Cd possibly involved in neurodegeneration: A toxicogenomics study in a human neuronal cell model. Neurotoxicology 2020, 76, 162–173. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cd-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Cai, J.; Guan, H.; Jiao, X.; Yang, J.; Chen, X.; Zhang, H.; Zheng, Y.; Zhu, Y.; Liu, Q.; Zhang, Z. NLRP3 inflammasome mediated pyroptosis is involved in Cd exposure-induced neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop in swine. Toxicology 2021, 453, 152720. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala Gopalakrishnan, A. Molecular Mechanism of Heavy Metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cd)—Induced Hepatotoxicity—A Review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef] [PubMed]
- Unsal, V.; Dalkıran, T.; Çiçek, M.; Kölükçü, E. The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cd Toxicity: A Review. Adv. Pharm. Bull. 2020, 10, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Barbarossa, A.; Carocci, A.; Meleleo, D. Evaluation of the Potential Protective Effect of Ellagic Acid against Heavy Metal (Cadmium, Mercury, and Lead) Toxicity in SH-SY5Y Neuroblastoma Cells. Foods 2024, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Argentieri, M.P.; Alexa, E.; Meleleo, D.; Statti, G.; Avato, P.; Filomena Conforti, F.; Mallamaci, R. Antioxidant activity and protective effect of the outer scales hydroalcoholic extract of Allium cepa L. var. Tropea on toxicity damage induced by Cadmiumin Caco-2 cells. Food Chem. Toxicol. 2022, 170, 113495. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Lovece, A.; Lentini, G.; Duranti, A.; Lucini, V.; Pannacci, M.; Scaglione, F.; Franchini, C. Design, synthesis, and pharmacological effects of structurally simple ligands for MT1 and MT2 MLT receptors. Bioorg. Med. Chem. 2010, 18, 6496–6511. [Google Scholar] [CrossRef] [PubMed]
- Chrustek, A.; Olszewska-Słonina, D. Melatonin as a powerful antioxidant. Acta Pharm. 2020, 71, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Carrieri, A.; Carocci, A. Melatonin and Related Compounds as Antioxidants. Mini-Rev. Med. Chem. 2024, 24, 546–565. [Google Scholar] [CrossRef]
- Carocci, A.; Catalano, A.; Bruno, C.; Lovece, A.; Roselli, M.G.; Cavalluzzi, M.M.; De Santis, F.; De Palma, A.; Rusciano, M.R.; Illario, M.; et al. N-(Phenoxyalkyl) amides as MT1 and MT2 ligands: Antioxidant properties and inhibition of Ca2+/CaM-dependent kinase II. Bioorg. Med. Chem. 2013, 21, 847–851. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Wang, H.; Zhang, Q. Protective effect of MLT against chronic Cd-induced hepatotoxicity by suppressing oxidative stress, inflammation, and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 228, 112947. [Google Scholar] [CrossRef]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The protective effects of MLT against oxidative stress and inflammation induced by acute Cd exposure in mice testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Kechiche, S.; Venditti, M.; Knani, L.; Jabłońska, K.; Dzięgiel, P.; Messaoudi, I.; Reiter, R.J.; Minucci, S. First evidence of the protective role of MLT in counteracting Cd toxicity in the rat ovary via the mTOR pathway. Environ. Pollut. 2021, 270, 116056. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Said, N.S.; Hadhoud, K.M.; Nada, W.M.; El Tarhouny, S.A. Superoxide dismutase, glutathione peroxidase and vitamin E in patients with diabetic retinopathy. Life Sci. J. 2013, 10, 1851–1856. [Google Scholar]
- Harmankaya, A.; Özcan, A.; Dalginli, K.; Erdağ, D.; Dursun, Y.A.; Güngör, B. The Effect of trolox on Oxidative Stress Index and Nitric Oxide Levels. J. Inst. Sci. Technol. 2021, 11, 3262–3268. [Google Scholar] [CrossRef]
- Trela-Makowej, A.; Leśkiewicz, M.; Kruk, J.; Żądło, A.; Basta-Kaim, A.; Szymańska, R. Antioxidant and Neuroprotective Activity of Vitamin E Homologues: In Vitro Study. Metabolites 2022, 12, 608. [Google Scholar] [CrossRef]
- Pazdro, R.; Burgess, J.R. Differential effects of α-tocopherol and N-acetylcysteine on advanced glycation end product-induced oxidative damage and neurite degeneration in SH-SY5Y cells. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 550–556. [Google Scholar] [CrossRef]
- Ayed-Boussema, I.; Abassi, H.; Bouaziz, C.; Hlima, W.B.; Ayed, Y.; Bacha, H. Antioxidative and antigenotoxic effect of vitamin E against patulin cytotoxicity and genotoxicity in HepG2 cells. Environ. Toxicol. 2011, 28, 299–306. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R. Vitamin E research: Past, now and future. Free. Radic. Biol. Med. 2021, 177, 381–390. [Google Scholar] [CrossRef]
- Usuki, F.; Yasutake, A.; Umehara, F.; Tokunaga, H.; Matsumoto, M.; Eto, K.; Ishiura, S.; Higuchi, I. In vivo protection of a water-soluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci. Lett. 2001, 304, 199–203. [Google Scholar] [CrossRef]
- Fang, J.; Xie, S.; Chen, Z.; Wang, F.; Chen, K.; Zuo, Z.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Protective effect of vitamin e on Cd-induced renal oxidative damage and apoptosis in rats. Biol. Trace Elem. Res. 2021, 199, 4675–4687. [Google Scholar] [CrossRef] [PubMed]
- Poli, V.; Aparna, Y.; Madduru, R.; Motireddy, S.R. Protective effect of Vitamin C and E on enzymatic and antioxidant system in liver and kidney toxicity of Cd in rats. Appl. Food Res. 2022, 2, 100098. [Google Scholar] [CrossRef]
- Dehn, P.F.; White, C.M.; Conners, D.E.; Shipkey, G.; Cumbo, T.A. Characterization of the human hepatocellular carcinoma (HepG2) cell line as an in vitro model for cadmium toxicity studies. Vitr. Cell Dev. Biol. Anim. 2004, 40, 172–182. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, T.; Li, D.; Ma, M.; Liang, X.; Ma, Z.; Ye, Q.; Yang, H.; Li, M.; Qu, A.; et al. The synergistic effect of Angelica sinensis (Oliv.) Diels and Rehmannia glutinosa (Gaertn.) DC. on antioxidant activity and protective ability against cell injury. J. Food Biochem. 2022, 46, e14196. [Google Scholar] [CrossRef]
- Mallamaci, R.; Storelli, M.M.; Barbarossa, A.; Messina, G.; Valenzano, A.; Meleleo, D. Potential Protective Effects of Spirulina (Spirulina platensis) against In Vitro Toxicity Induced by Heavy Metals (Cadmium, Mercury, and Lead) on SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 17076. [Google Scholar] [CrossRef]
- Crowley-Weber, C.L.; Payne, C.M.; Gleason-Guzman, M.; Watts, G.S.; Futscher, B.; Waltmire, C.N.; Crowley, C.; Dvorakova, K.; Bernstein, C.; Craven, M.; et al. Development and molecular characterization of HCT-116 cell lines resistant to the tumor promoter and multiple stress-induced, deoxycholate. Carcinog. 2002, 23, 2063–2080. [Google Scholar] [CrossRef]
- Ravindran, G.; Chakrabarty, D.; Sarkar, A. Cell specific stress responses of cadmium-induced cytotoxicity. Anim. Cells Syst. 2017, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.; Storelli, A.; Mallamaci, R.; Storelli, M.M. Comparative study on trace metal accumulation in liver of Mediterranean deep-sea fish and their selenium/mercury molar ratios. Water Air Soil Pollut. 2017, 228, 211. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Ðukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef]
- Bertin, G.; Averbeck, D. Cadmium: Cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 2006, 88, 1549–1559. [Google Scholar] [CrossRef]
- Nordberg, M.; Nordberg, G.F. Metallothionein and Cadmium Toxicology-Historical Review and Commentary. Biomolecules 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol. Environ. Saf. 2020, 208, 111610. [Google Scholar] [CrossRef]
- Poliandri, A.H.; Cabilla, J.P.; Velardez, M.O.; Bodo, C.C.; Duvilanski, B.H. Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol. Appl. Pharmacol. 2003, 190, 17–24. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.; Manchester, L.; Yan, M.; El-Sawi, M.; Sainz, R.; Mayo, J.; Kohen, R.; Allegra, M.; Hardelan, R. Chemical and Physical Properties and Potential Mechanisms: Melatonin as a Broad-Spectrum Antioxidant and Free Radical Scavenger. Curr. Top. Med. Chem. 2005, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Ozkan, E.; Yaman, H.; Cakir, E.; Deniz, O.; Oztosun, M.; Gumus, S.; Akgul, E.O.; Agilli, M.; Cayci, T.; Kurt, Y.G.; et al. Plasma MLT and urinary 6-hydroxyMLT levels in patients with pulmonary tuberculosis. Inflammation 2012, 35, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Mannino, F.; Pallio, G.; Imbesi, C.; Scarfone, A.; Puzzolo, D.; Micali, A.; Freni, J.; Squadrito, F.; Bitto, A.; Minutoli, L.; et al. Beta-Caryophyllene, a Plant-Derived CB2 Receptor Agonist, Protects SH-SY5Y Cells from Cadmium-Induced Toxicity. Int. J. Mol. Sci. 2023, 24, 15487. [Google Scholar] [CrossRef]
- Souid, G.; Sfar, M.; Timoumi, R.; Romdhane, M.H.; Essefi, S.A.; Majdoub, H. Protective effect assessment of Moringa oleifera against cadmium-induced toxicity in HCT116 and HEK293 cell lines. Environ. Sci. Pollut. Res. 2020, 27, 23783–23792. [Google Scholar] [CrossRef]
- Urani, C.; Melchioretto, P.; Canevali, C.; Crosta, G. Cytotoxicity and induction of protective mechanisms in HepG2 cells exposed to cadmium. Toxicol. Vitr. 2005, 19, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Cordier, W.; Yousaf, M.; Nell, M.J.; Steenkamp, V. Underlying Mechanisms of Cytotoxicity in HepG2 Hepatocarcinoma Cells Exposed to Arsenic, Cadmium and Mercury Individually and in Combination. Toxicol. Vitr. 2021, 72, 105101. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.-H.; Tsai, C.-C.; Chen, T.-W.; Lee, C.-H.; Chang, W.-J.; Hsieh, M.-Y.; Li, T.-K. Activation of multiple proteolysis systems contributes to acute cadmium cytotoxicity. Mol. Cell. Biochem. 2022, 477, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-C.; Deng, Y.; Song, H.-X.; Fang, Y.-Y.; Gan, C.-L.; Lin, J.-J.; Luo, J.-J.; Zheng, X.-W.; Aschner, M.; Jiang, Y.-M. Protective Effects of Sodium Para-Aminosalicylic Acid on Lead and Cadmium Co-Exposure in SH-SY5Y Cells. Brain Sci. 2023, 13, 382. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. Iubmb Life 2019, 71, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Bonomini, M.; Bartolini, D.; Zatini, L.; Reboldi, G.; Marcantonini, G.; Gentile, G.; Sirolli, V.; Di Pietro, N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants 2022, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A. Antioxidative Activity of Vitamin E. In Vitamin E in Human Health; Humana Press: Totova, NJ, USA, 2019; pp. 19–30. [Google Scholar]
- Rana, V.S. Protection of metal toxicity by melatonin—Recent advances”. EC Pharm. Toxicol 2018, 6, 851–864. [Google Scholar]
- Amoroso, S.; Gioielli, A.; Cataldi, M.; Di Renzo, G.; Annunziato, L. In the neuronal cell line SH-SY5Y, oxidative stress-induced free radical overproduction causes cell death without any participation of intracellular Ca2+ increase. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 151–16068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mzoughi, Z.; Souid, G.; Timoumi, R.; Le Cerf, D.; Majdoub, H. Partial characterization of the edible Spinacia oleracea polysaccharides: Cytoprotective and antioxidant potentials against Cd induced toxicity in HCT116 and HEK293 cells. Int. J. Biol. Macromol. 2019, 136, 332–340. [Google Scholar] [CrossRef]
- Meleleo, D.; Piacciarelli, V. Effect of calcium ions on human calcitonin. Possible implications for bone resorption by osteoclasts. Biometals 2016, 29, 61–79. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamaci, R.; Barbarossa, A.; Carrieri, A.; Meleleo, D.; Carocci, A. Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines. Int. J. Mol. Sci. 2024, 25, 8055. https://doi.org/10.3390/ijms25158055
Mallamaci R, Barbarossa A, Carrieri A, Meleleo D, Carocci A. Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines. International Journal of Molecular Sciences. 2024; 25(15):8055. https://doi.org/10.3390/ijms25158055
Chicago/Turabian StyleMallamaci, Rosanna, Alexia Barbarossa, Antonio Carrieri, Daniela Meleleo, and Alessia Carocci. 2024. "Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines" International Journal of Molecular Sciences 25, no. 15: 8055. https://doi.org/10.3390/ijms25158055
APA StyleMallamaci, R., Barbarossa, A., Carrieri, A., Meleleo, D., & Carocci, A. (2024). Evaluation of the Potential Cytoprotective Effect of Melatonin in Comparison with Vitamin E and Trolox against Cd2+-Induced Toxicity in SH-SY5Y, HCT 116, and HepG2 Cell Lines. International Journal of Molecular Sciences, 25(15), 8055. https://doi.org/10.3390/ijms25158055







