Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats
Abstract
1. Introduction
2. Results
2.1. Glycated Hemoglobin
2.2. Fasting Plasma Glucose, Insulin, and HOMA-IR
2.3. Oral Glucose Tolerance Test (OGTT)
2.4. Four-Limb Grip Strength Test
2.5. Malondialdehyde (MDA) Measurement
2.6. Correlation between HOMA-IR, Hb1Ac, Plasma Glucose, MDA Concentration, and Grip Strength
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Induction of Prediabetes
4.3. Experimental Design
4.4. Oral Glucose Tolerance (OGT) Response
4.5. Four-Limb Grip Strength Test
4.6. Tissue and Blood Collection
4.7. HOMA-IR Index
4.8. Biochemical Analysis
4.9. Statistical Analysis
5. Limitations and Future Recommendations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| AREC | Animal Research Ethics Committee |
| BRU | Biomedical Research Unit |
| CSA | cross-sectional area |
| ELISA | enzyme-linked immunosorbent assay |
| FBG | fasting blood glucose |
| FFA | free fatty acid |
| HbA1c | glycated hemoglobin |
| HFHC | high-fat high-carbohydrate |
| HOMA-IR | homeostatic model of insulin resistance |
| IFG | impaired fasting glucose |
| IGF | impaired Glucose tolerance |
| IMAT | inter- or intramuscular adipose tissue |
| MDA | Malondialdehyde |
| NAD(P)H | nicotinamide adenine dinucleotide phosphate |
| NPD | non-prediabetic |
| OGT | oral glucose tolerance |
| OGTT | oral glucose tolerance test |
| PD | Prediabetes |
| ROS | reactive oxygen species |
| T2DM | type 2 diabetes mellitus |
| UKZN | University of KwaZulu-Natal |
| VL | vastus lateralis |
References
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr Physiol. 2020, 10, 785–809. [Google Scholar]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J. Intern. Med. 2016, 31, 643. [Google Scholar] [CrossRef] [PubMed]
- Bazgir, B.; Fathi, R.; Rezazadeh Valojerdi, M.; Mozdziak, P.; Asgari, A. Satellite Cells Contribution to Exercise Mediated Muscle Hypertrophy and Repair. Cell J. 2017, 18, 473–484. [Google Scholar]
- Snijders, T.; Nederveen, J.P.; McKay, B.R.; Joanisse, S.; Verdijk, L.B.; van Loon, L.J.C.; Parise, G. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 2015, 6, 283. [Google Scholar] [CrossRef]
- Maltin, C.A.; Delday, M.; Sinclair, K.; Steven, J.; Sneddon, A. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction 2001, 122, 359–374. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Huang, P. The effect of type 2 diabetes mellitus and obesity on muscle progenitor cell function. Stem Cell Res. Ther. 2019, 10, 103. [Google Scholar] [CrossRef]
- D’Souza, D.M.; Al-Sajee, D.; Hawke, T.J. Diabetic myopathy: Impact of diabetes mellitus on skeletal muscle progenitor cells. Front. Physiol. 2013, 4, 379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.-H.; Cheng, M.; Koh, T.J. Impaired muscle regeneration in ob/ob and db/db mice. Sci. World J. 2011, 11, 1525–1535. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Lee, I.H.; Modi, S.; Wang, X.; Du, J.; Mitch, W.E. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes 2010, 59, 1312–1320. [Google Scholar] [CrossRef]
- Takada, Y.; Hanaoka, T.; Imagita, H.; Yasui, T.; Takeshita, D.; Abe, M.; Kawata, S.; Yamakami, T.; Okada, K.; Washio, H.; et al. Long-term wheel-running prevents reduction of grip strength in type 2 diabetic rats. Physiol. Rep. 2021, 9, e15046. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yan, J.; Zhu, H.; Zhang, Z.; Jiang, Y.; Zhang, X.; Wu, Y.; Lu, J.; Cun, H.; He, B. Low thigh muscle strength in relation to myosteatosis in patients with type 2 diabetes mellitus. Sci. Rep. 2023, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Di Pino, A.; Scicali, R.; Calanna, S.; Urbano, F.; Mantegna, C.; Rabuazzo, A.M.; Purrello, F.; Piro, S. Cardiovascular risk profile in subjects with prediabetes and new-onset type 2 diabetes identified by HbA1c according to American diabetes association criteria. Diabetes Care 2014, 37, 1447–1453. [Google Scholar] [CrossRef][Green Version]
- Bansal, N. Prediabetes diagnosis and treatment: A review. World J. Diabetes 2015, 6, 296–303. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006; pp. 1–50. [Google Scholar]
- Hostalek, U. Global epidemiology of prediabetes—Present and future perspectives. Clin. Diabetes Endocrinol. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.K.; Monu, J.U.V.; Doumanian, J. Diabetic Myopathy: MRI Patterns and Current Trends. Am. J. Roentgenol. 2010, 195, 198–204. [Google Scholar] [CrossRef]
- Kapur, S.; Brunet, J.A.; McKendry, R.J. Diabetic muscle infarction: Case report and review. J. Rheumatol. 2004, 31, 190–194. [Google Scholar]
- Horton, W.B.; Taylor, J.S.; Ragland, T.J.; Subauste, A.R. Diabetic muscle infarction: A systematic review. BMJ Open Diabetes Res. Care 2015, 3, e000082. [Google Scholar] [CrossRef]
- Ijzerman, T.H.; Schaper, N.C.; Melai, T.; Meijer, K.; Willems, P.J.B.; Savelberg, H.H.C.M. Lower extremity muscle strength is reduced in people with type 2 diabetes, with and without polyneuropathy, and is associated with impaired mobility and reduced quality of life. Diabetes Res. Clin. Pract. 2012, 95, 345–351. [Google Scholar] [CrossRef]
- Khathi, A.; Luvuno, M.; Mabandla, M. Voluntary Ingestion of a High-Fat high-carbohydrate diet: A model for prediabetes. PONTE Int. Sci. Res. J. 2018, 74, 120–140. [Google Scholar] [CrossRef]
- Bergman, M. Pathophysiology of prediabetes and treatment implications for the prevention of type 2 diabetes mellitus. Endocrine 2012, 43, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Stump, C.S.; Henriksen, E.J.; Wei, Y.; Sowers, J.R. The metabolic syndrome: Role of skeletal muscle metabolism. Ann. Med. 2006, 38, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Jansson, P.A. Endothelial dysfunction in insulin resistance and type 2 diabetes. J. Intern. Med. 2007, 262, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Gamede, M.; Mabuza, L.; Ngubane, P.; Khathi, A. The Effects of Plant-Derived Oleanolic Acid on Selected Parameters of Glucose Homeostasis in a Diet-Induced Prediabetic Rat Model. Molecules 2018, 23, 794. [Google Scholar] [CrossRef]
- Luvuno, M.; Khathi, A.; Mabandla, M.V. Diet-induced prediabetes: Effects of exercise treatment on risk factors for cardiovascular complications. Nutr. Metab. 2021, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Nielsen, S.; Mogensen, C.E.; Jakobsen, J. Muscle strength in type 2 diabetes. Diabetes 2004, 53, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Alrabiah, M.; Al-Aali, K.A.; Al-Sowygh, Z.H.; Binmahfooz, A.M.; Mokeem, S.A.; Abduljabbar, T. Association of advanced glycation end products with peri-implant inflammation in prediabetes and type 2 diabetes mellitus patients. Clin. Implant. Dent. Relat. Res. 2018, 20, 535–540. [Google Scholar] [CrossRef]
- Grundy, S.M. Prediabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 2012, 59, 635–643. [Google Scholar] [CrossRef]
- Tankova, T.; Chakarova, N.; Dakovska, L.; Atanassova, I. Assessment of HbA1c as a diagnostic tool in diabetes and prediabetes. Acta Diabetol. 2012, 49, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011, 95, 327–339, vii–viii. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.Y.; Ko, S.-H.; Kwon, H.-S.; Kim, N.H.; Kim, J.H.; Kim, C.S.; Song, K.-H.; Won, J.C.; Lim, S.; Choi, S.H.; et al. Prevalence of Diabetes and Prediabetes according to Fasting Plasma Glucose and HbA1c. Diabetes Metab. J. 2013, 37, 349–357. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; DeFronzo, R.A. Pathophysiology of prediabetes. Curr. Diab. Rep. 2009, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kabadi, U.M. Major Pathophysiology in Prediabetes and Type 2 Diabetes: Decreased Insulin in Lean and Insulin Resistance in Obese. J. Endocr. Soc. 2017, 1, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Bacopoulou, F.; Peppa, M. Prediabetes and Adolescence—Trends, Causes, Effects, and Screening. US Endocrinol. 2016, 12, 94–98. [Google Scholar] [CrossRef][Green Version]
- Heianza, Y.; Arase, Y.; Fujihara, K.; Tsuji, H.; Saito, K.; Hsieh, S.D.; Kodama, S.; Shimano, H.; Yamada, N.; Hara, S.; et al. Screening for prediabetes to predict future diabetes using various cut-off points for HbA(1c) and impaired fasting glucose: The Toranomon Hospital Health Management Center Study 4 (TOPICS 4). Diabet. Med. 2012, 29, e279–e285. [Google Scholar] [CrossRef] [PubMed]
- Mosili, P.; Mkhize, B.C.; Ngubane, P.; Sibiya, N.; Khathi, A. The dysregulation of the hypothalamic-pituitary-adrenal axis in diet-induced prediabetic male Sprague Dawley rats. Nutr. Metab. 2020, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Grzelkowska-Kowalczyk, K.; Wieteska-Skrzeczyńska, W.; Grabiec, K.; Tokarska, J. High glucose-mediated alterations of mechanisms important in myogenesis of mouse C2C12 myoblasts. Cell Biol. Int. 2013, 37, 29–35. [Google Scholar] [CrossRef]
- Sayer, A.A.; Dennison, E.M.; Syddall, H.E.; Gilbody, H.J.; Phillips, D.I.; Cooper, C. Type 2 diabetes, muscle strength, and impaired physical function: The tip of the iceberg? Diabetes Care 2005, 28, 2541–2543. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.S.; Pedersen, T.H.; Nielsen, O.B. Skeletal muscle dysfunction in the db/db mouse model of type 2 diabetes. Muscle Nerve 2016, 54, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Li, S.; Mao, J.; Zhou, W. Prediabetes is associated with loss of appendicular skeletal muscle mass and sarcopenia. Front. Nutr. 2023, 10, 1109824. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Ostler, J.E.; Maurya, S.K.; Dials, J.; Roof, S.R.; Devor, S.T.; Ziolo, M.T.; Periasamy, M.; Eshima, H.; Tamura, Y.; Kakehi, S.; et al. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E592–E605. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.A.; Snow, L.M.; Lowe, D.A.; Serfass, R.C.; Thompson, L.V. Effects of endurance exercise-training on single-fiber contractile properties of insulin-treated streptozotocin-induced diabetic rats. J. Appl. Physiol. 2005, 99, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Ayala, J.E.; Lee-Young, R.S.; Zhang, Z.; James, F.D.; Neufer, P.D.; Pozzi, A.; Zutter, M.M.; Wasserman, D.H. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin α2β1 in mice. Diabetes 2011, 60, 416–426. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; De Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Su, C.Y.; Payne, M.; Strauss, A.W.; Dillmann, W.H. Selective reduction of creatine kinase subunit mRNAs in striated muscle of diabetic rats. Am. J. Physiol.-Endocrinol. Metab. 1992, 263, E310–E316. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F.; Stephenson, D.G.; Kemp, J.G.; Stephenson, G.M.M. Ca2+-activation characteristics of single fibres from chemically skinned rat muscle incubated with glucose-6-phosphate. Pflügers Arch.-Eur. J. Physiol. 2000, 439, 845–852. [Google Scholar] [CrossRef]
- Ramamurthyb, B.; Höökp, P.; Jones, A.D.; Larsson, L. Changes in myosin structure and function in response to glycation. FASEB J. 2001, 15, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Ikeda, Y.; Nakao, S.; Ito, K.; Ishida, K.; Suehiro, T.; Hashimoto, K. Muscle Strength is a Marker of Insulin Resistance in Patients with Type 2 Diabetes: A Pilot Study. Endocr. J. 2007, 54, 791–796. [Google Scholar] [CrossRef]
- Guillet, C.; Boirie, Y. Insulin resistance: A contributing factor to age-related muscle mass loss? Diabetes Metab. 2005, 31, 5S20–5S26. [Google Scholar] [CrossRef] [PubMed]
- Halvatsiotis, P.; Short, K.R.; Bigelow, M.; Nair, K.S. Synthesis rate of muscle proteins, muscle functions, and amino acid kinetics in type 2 diabetes. Diabetes 2002, 51, 2395–2404. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J. Diabetes Investig. 2015, 6, 623–624. [Google Scholar] [CrossRef]
- Chen, T.H.; Koh, K.Y.; Lin, K.M.; Chou, C.K. Mitochondrial Dysfunction as an Underlying Cause of Skeletal Muscle Disorders. Int. J. Mol. Sci. 2022, 23, 12926. [Google Scholar] [CrossRef]
- Kaikini, A.A.; Kanchan, D.M.; Nerurkar, U.N.; Sathaye, S. Targeting Mitochondrial Dysfunction for the Treatment of Diabetic Complications: Pharmacological Interventions through Natural Products. Pharmacogn. Rev. 2017, 11, 128–135. [Google Scholar]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Kelley, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef]
- Bonnard, C.; Durand, A.; Peyrol, S.; Chanseaume, E.; Chauvin, M.-A.; Morio, B.; Vidal, H.; Rieusset, J. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J. Clin. Investig. 2008, 118, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P. The role of uncoupling protein 3 in fatty acid metabolism: Protection against lipotoxicity? Proc. Nutr. Soc. 2004, 63, 287–292. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.-I.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.J.; Oates, P.J.; Hammes, H.-P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Schrauwen, P. High-fat diet, muscular lipotoxicity and insulin resistance. Proc. Nutr. Soc. 2007, 66, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; Machann, J.; Stefan, N.; Thamer, C.; Häring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Intermuscular adipose tissue (IMAT): Association with other adipose tissue compartments and insulin sensitivity. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2009, 29, 1340–1345. [Google Scholar] [CrossRef]
- Scott, D.; Shore-Lorenti, C.; McMillan, L.B.; Mesinovic, J.; Clark, R.A.; Hayes, A.; Sanders, K.M.; Duque, G.; Ebeling, P.R. Calf muscle density is independently associated with physical function in overweight and obese older adults. J. Musculoskelet. Neuronal Interact. 2018, 18, 9. [Google Scholar] [PubMed]
- Marcus, R.L.; Addison, O.; Dibble, L.E.; Foreman, K.B.; Morrell, G.; LaStayo, P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J. Aging Res. 2012, 2012, 629637. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Thaete, F.L.; Kelley, D.E. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 885–892. [Google Scholar] [CrossRef]
- Chi, S.-W.; Hodgson, J.; Chen, J.-S.; Edgerton, V.R.; Shin, D.D.; Roiz, R.A.; Sinha, S. Finite element modeling reveals complex strain mechanics in the aponeuroses of contracting skeletal muscle. J. Biomech. 2010, 43, 1243–1250. [Google Scholar] [CrossRef]
- ragstrup, T.; Kjaer, M.; Mackey, A. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand. J. Med. Sci. Sports 2011, 21, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Perreault, L.; Bergman, B.C.; Hunerdosse, D.M.; Eckel, R.H. Altered intramuscular lipid metabolism relates to diminished insulin action in men, but not women, in progression to diabetes. Obesity 2010, 18, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2014, 38 (Suppl. 1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Khoo, H.E. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000, 67, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Geloneze, B.; Vasques, A.C.J.; Stabe, C.F.C.; Pareja, J.C.; de Lima Rosado, L.E.F.P.; De Queiroz, E.C.; Tambascia, M.A. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. Endocrinol. Metabol. 2009, 53, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Queensland Brain Institute. LAB_071 Grip Strength Tests for Rodents 2021. Available online: https://www.uq.edu.au/research/files/64526/LAB_071%20Grip%20Strength%20tests%20for%20Rodents.pdf (accessed on 15 March 2024).
- Bonetto, A.; Andersson, D.; Waning, D. Assessment of muscle mass and strength in mice. BoneKEy Rep. 2015, 4, 732. [Google Scholar] [CrossRef]
- Diabetes, T. Endocrinology and Metabolism. Homa Calculator. Available online: https://www2.dtu.ox.ac.uk/homacalculator/download.php (accessed on 15 March 2024).
- Moodley, K.; Joseph, K.; Naidoo, Y.; Islam, S.; Mackraj, I. Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement. Altern. Med. 2015, 15, 408. [Google Scholar] [CrossRef]
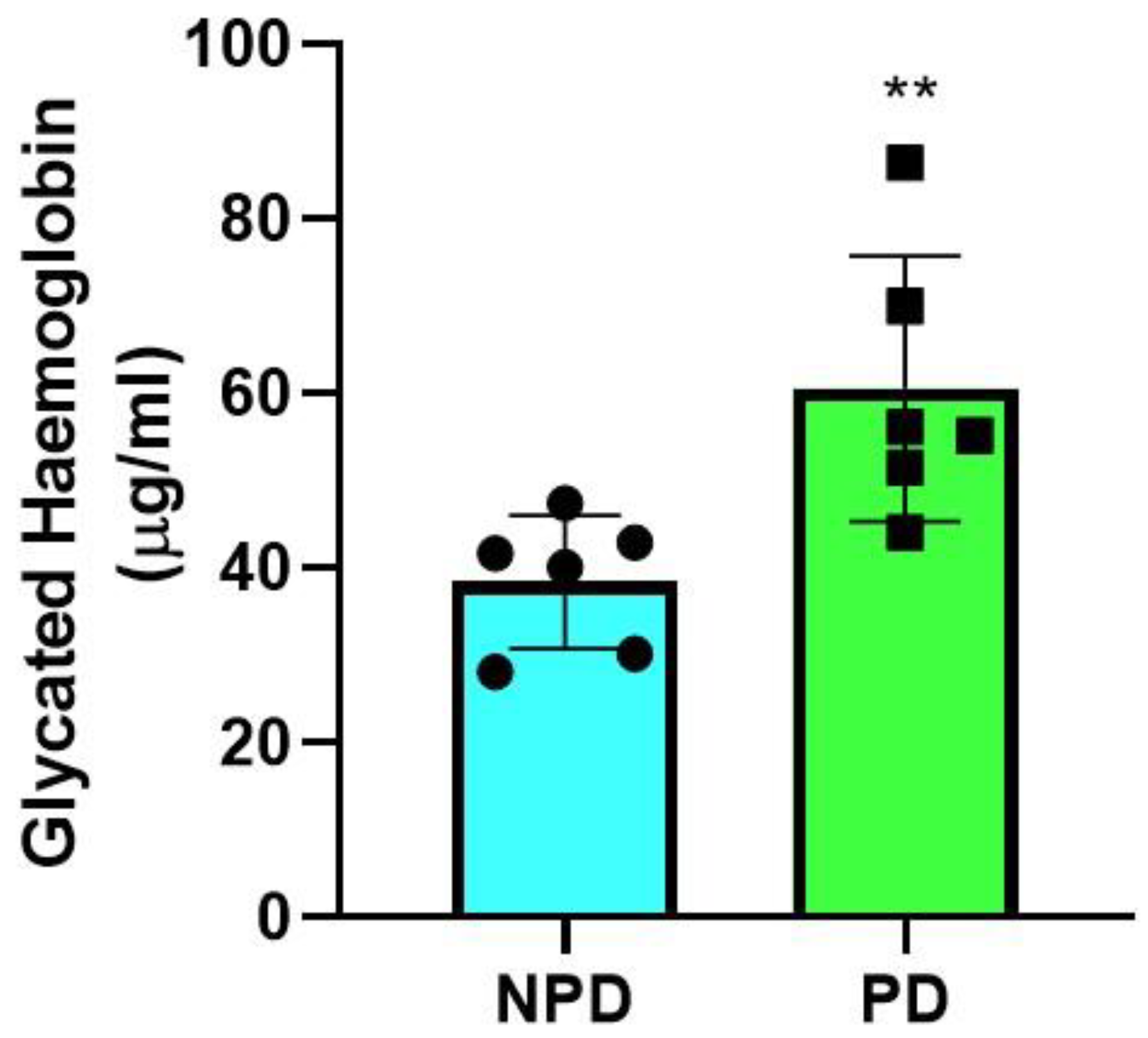
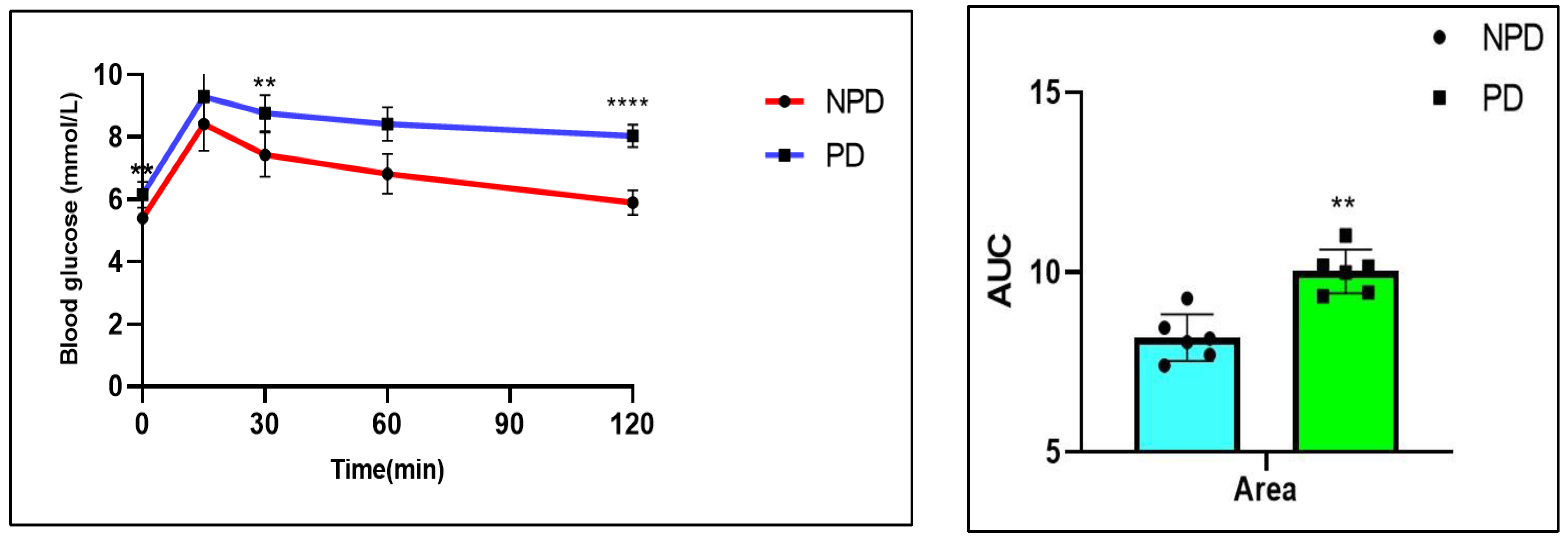

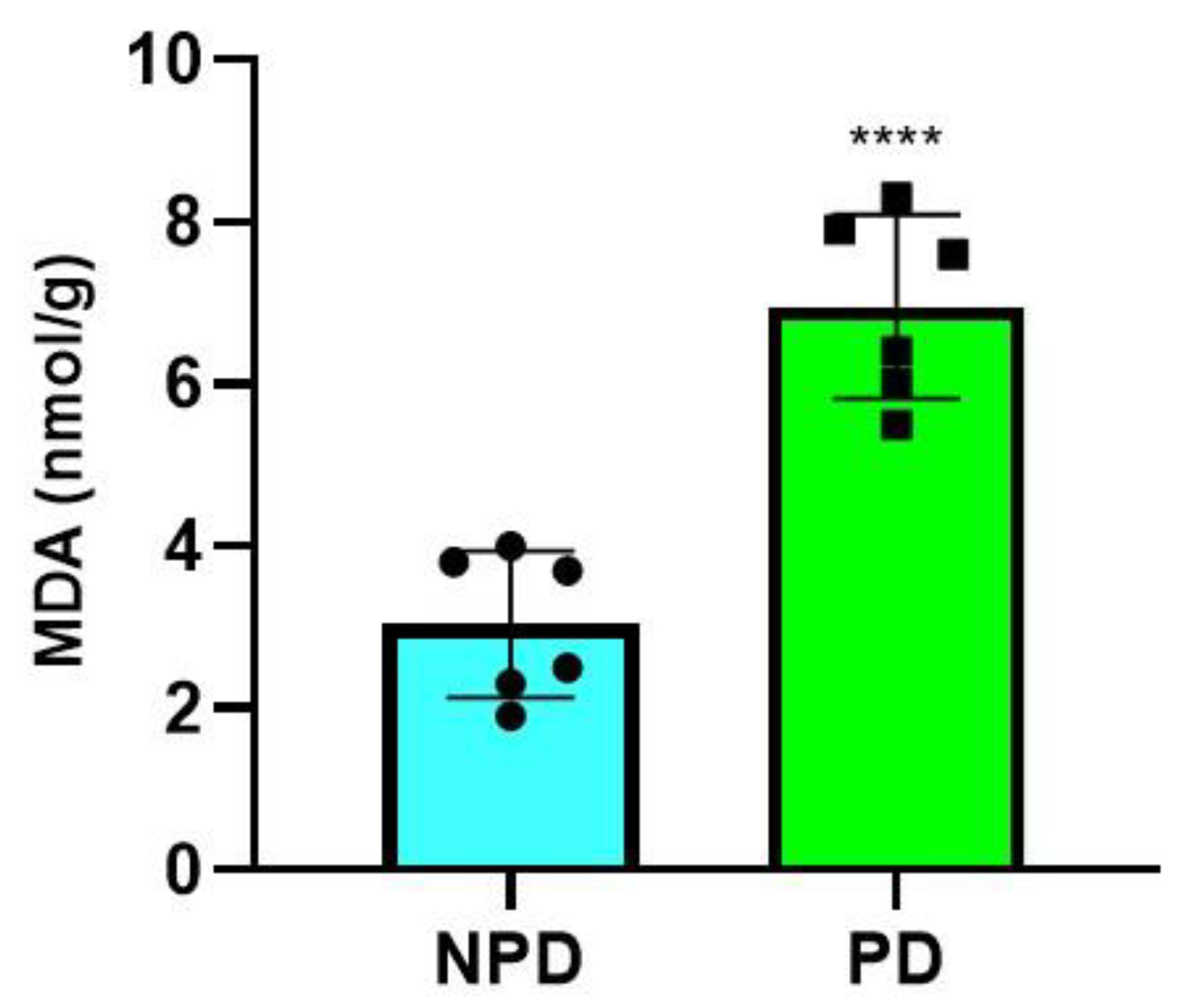

| Groups (n = 6) | Plasma Glucose (mmol/L) | Plasma Insulin (pmol/L) | HOMA-IR Values |
|---|---|---|---|
| NPD | 5.4 ± 0.08 | 30.42 ± 3.26 | 0.59 ± 0.06 |
| PD | 6.15 ± 0.17 ** | 322.80 ± 17.64 **** | 6.16 ± 0.40 **** |
| Metabolic Parameters | Reverse Hang Test Minimum Holding Impulse | |
|---|---|---|
| (g × s) | ||
| HbA1c | NPD | r = 0.33 |
| p = 0.67 | ||
| PD | r = −0.94 | |
| p = 0.005 ** | ||
| HOMA-IR | NPD | r = 0.10 |
| p = 0.85 | ||
| PD | r = −0.98 | |
| p = 0.02 * | ||
| Plasma glucose | NPD | r = 0.04 |
| p = 0.46 | ||
| PD | r = −0.86 | |
| p = 0.03 * | ||
| MDA | NPD | r = 0.24 |
| p = 0.65 | ||
| PD | r = −0.86 | |
| p = 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, M.; Khathi, A. Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats. Int. J. Mol. Sci. 2024, 25, 4076. https://doi.org/10.3390/ijms25074076
Dlamini M, Khathi A. Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats. International Journal of Molecular Sciences. 2024; 25(7):4076. https://doi.org/10.3390/ijms25074076
Chicago/Turabian StyleDlamini, Mandlakazi, and Andile Khathi. 2024. "Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats" International Journal of Molecular Sciences 25, no. 7: 4076. https://doi.org/10.3390/ijms25074076
APA StyleDlamini, M., & Khathi, A. (2024). Investigating the Effects of Diet-Induced Prediabetes on Skeletal Muscle Strength in Male Sprague Dawley Rats. International Journal of Molecular Sciences, 25(7), 4076. https://doi.org/10.3390/ijms25074076





