Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner
Abstract
:1. Introduction
2. Results
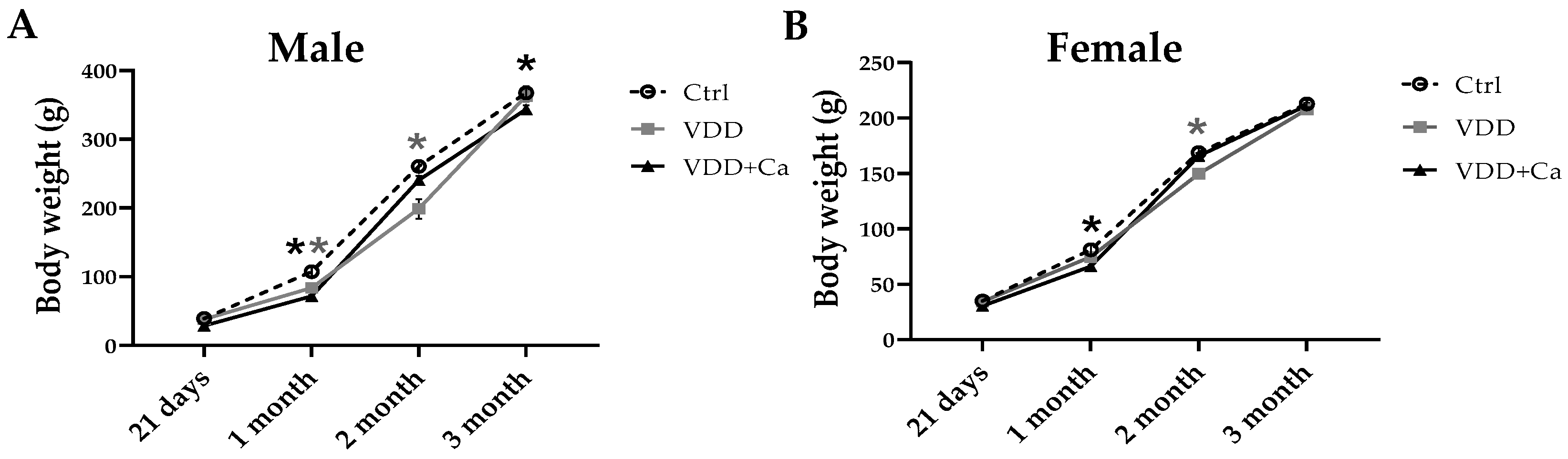
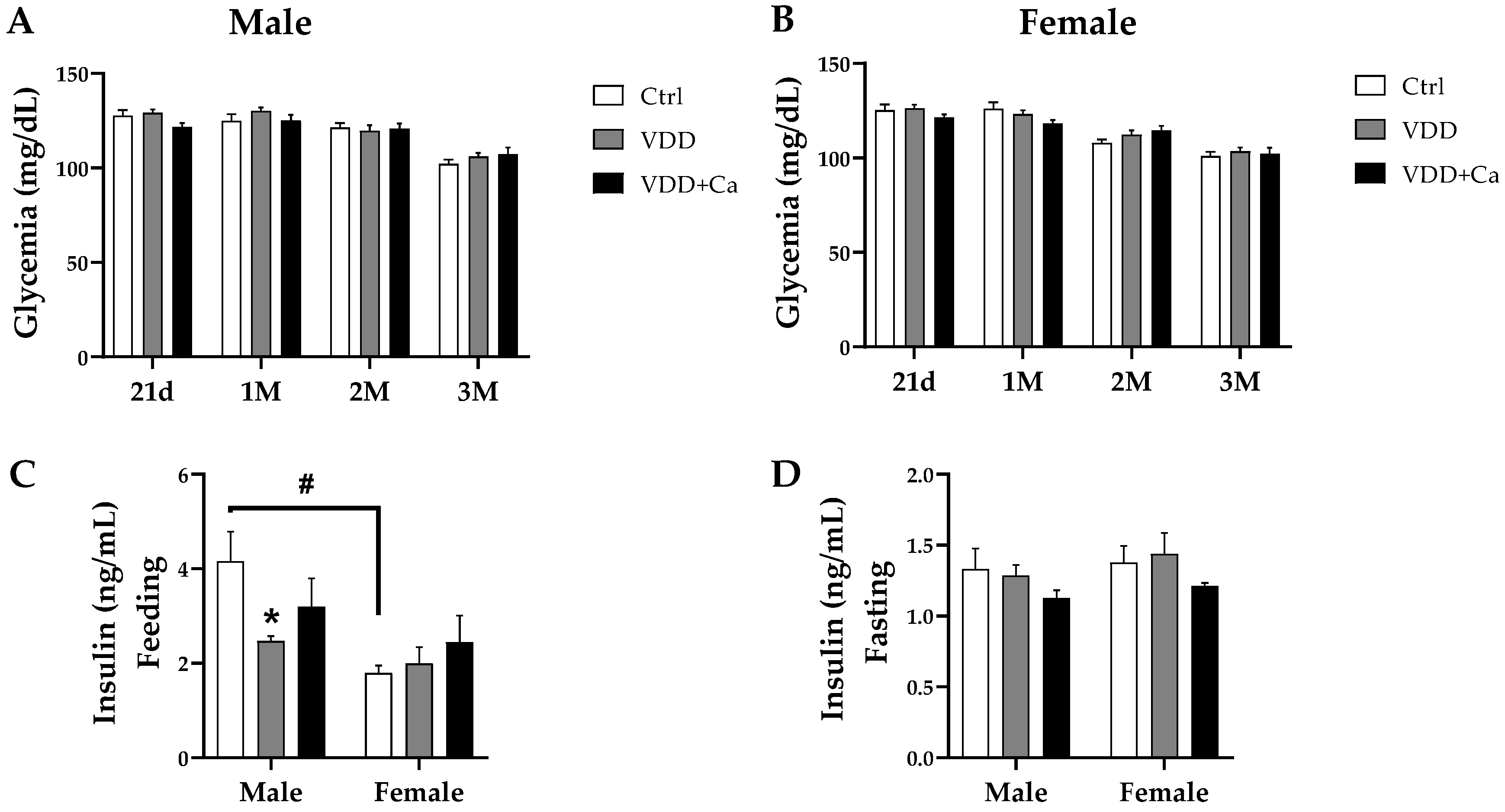
2.1. Metabolites, Hormones, and Growth in Dams and Offspring
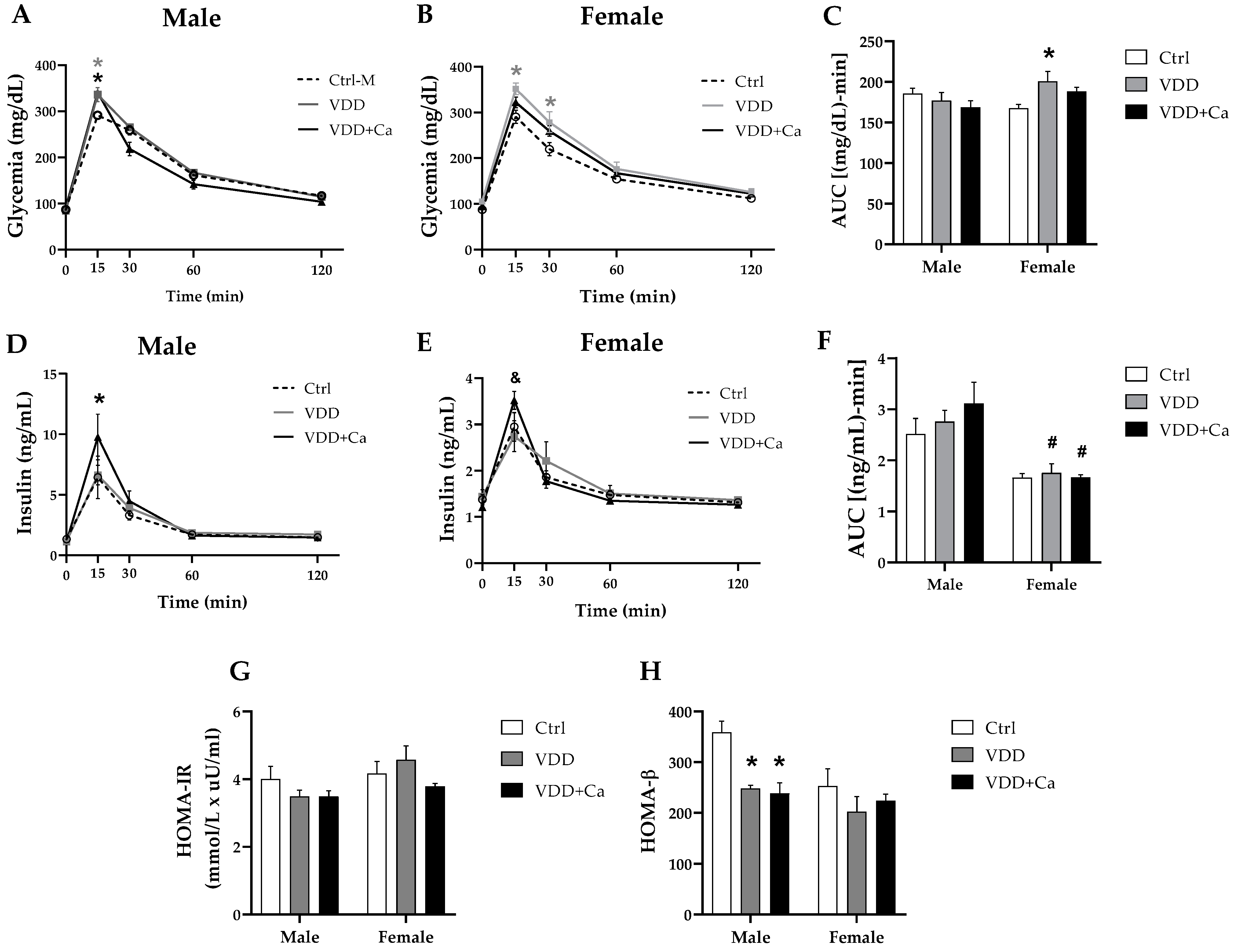
2.2. Glycemic Homeostasis and Insulinemia
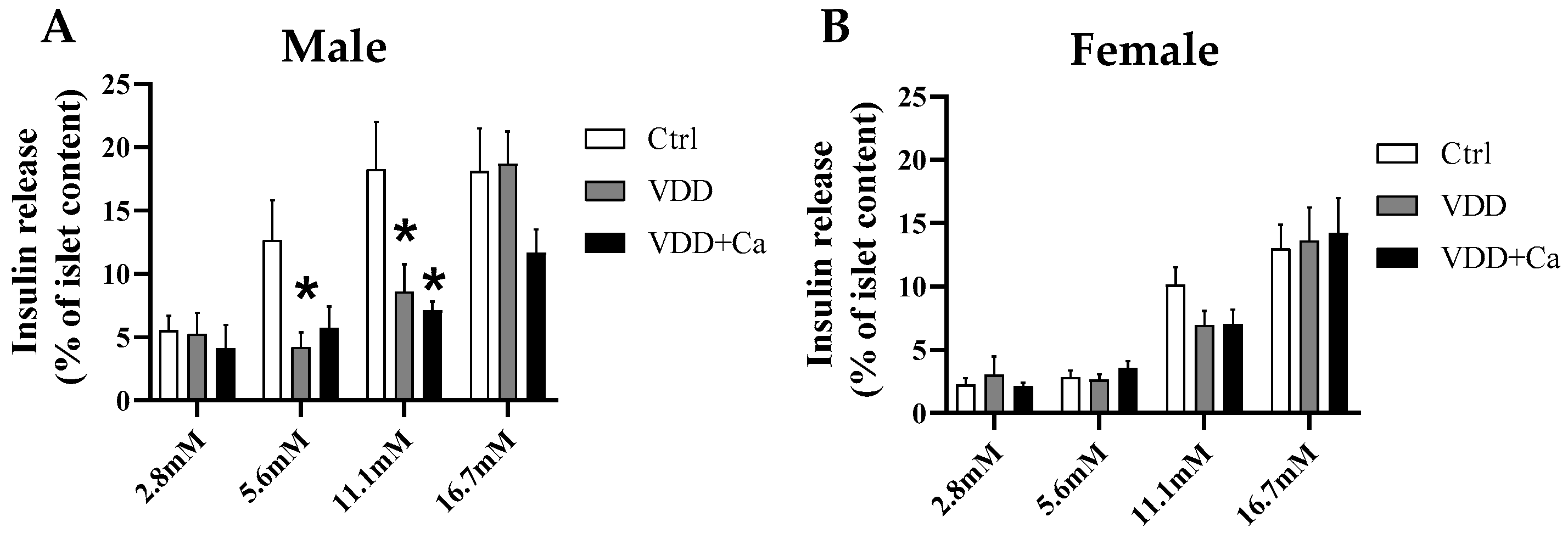
2.3. Pancreatic Islet Secretory Function
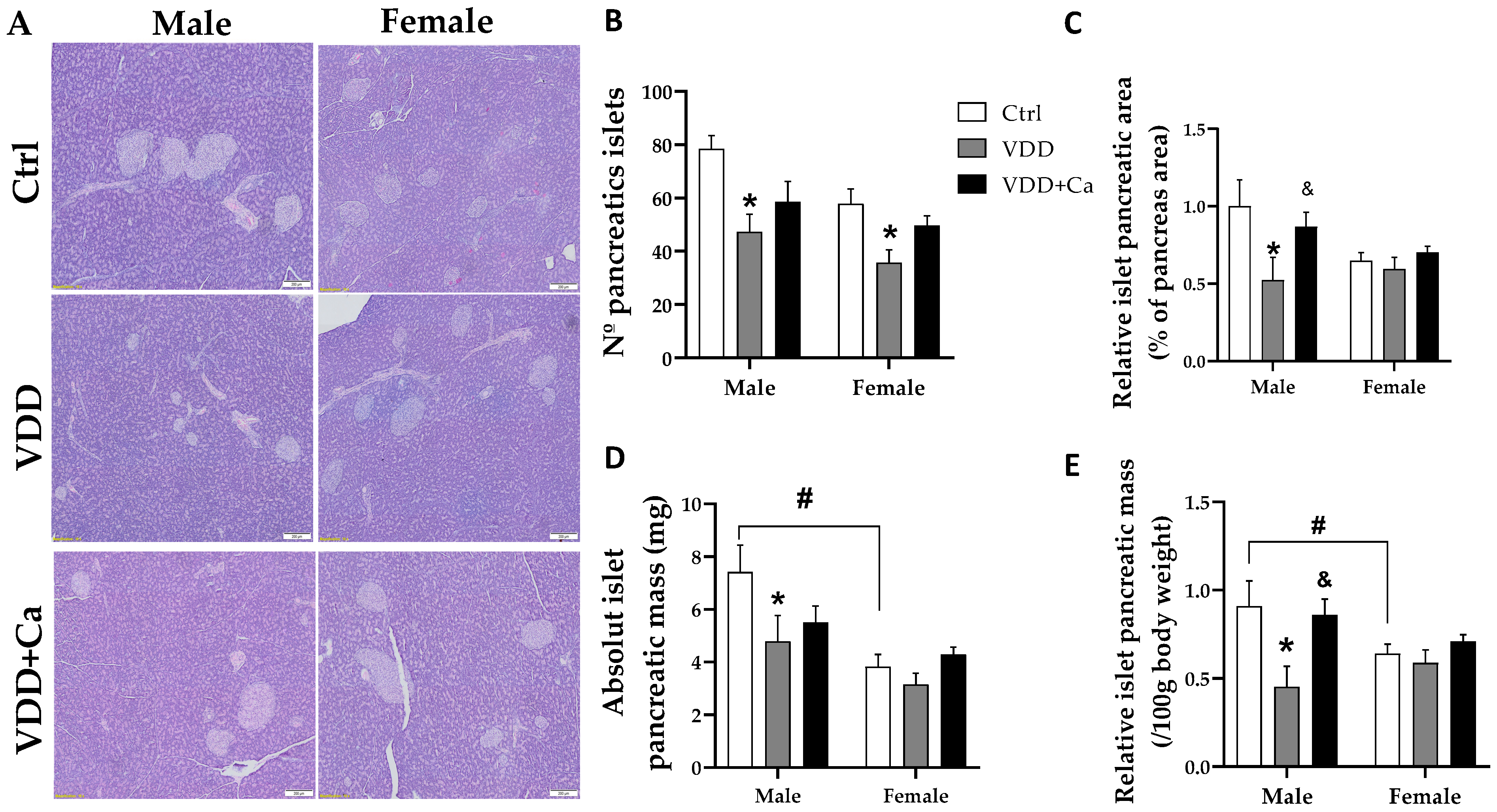
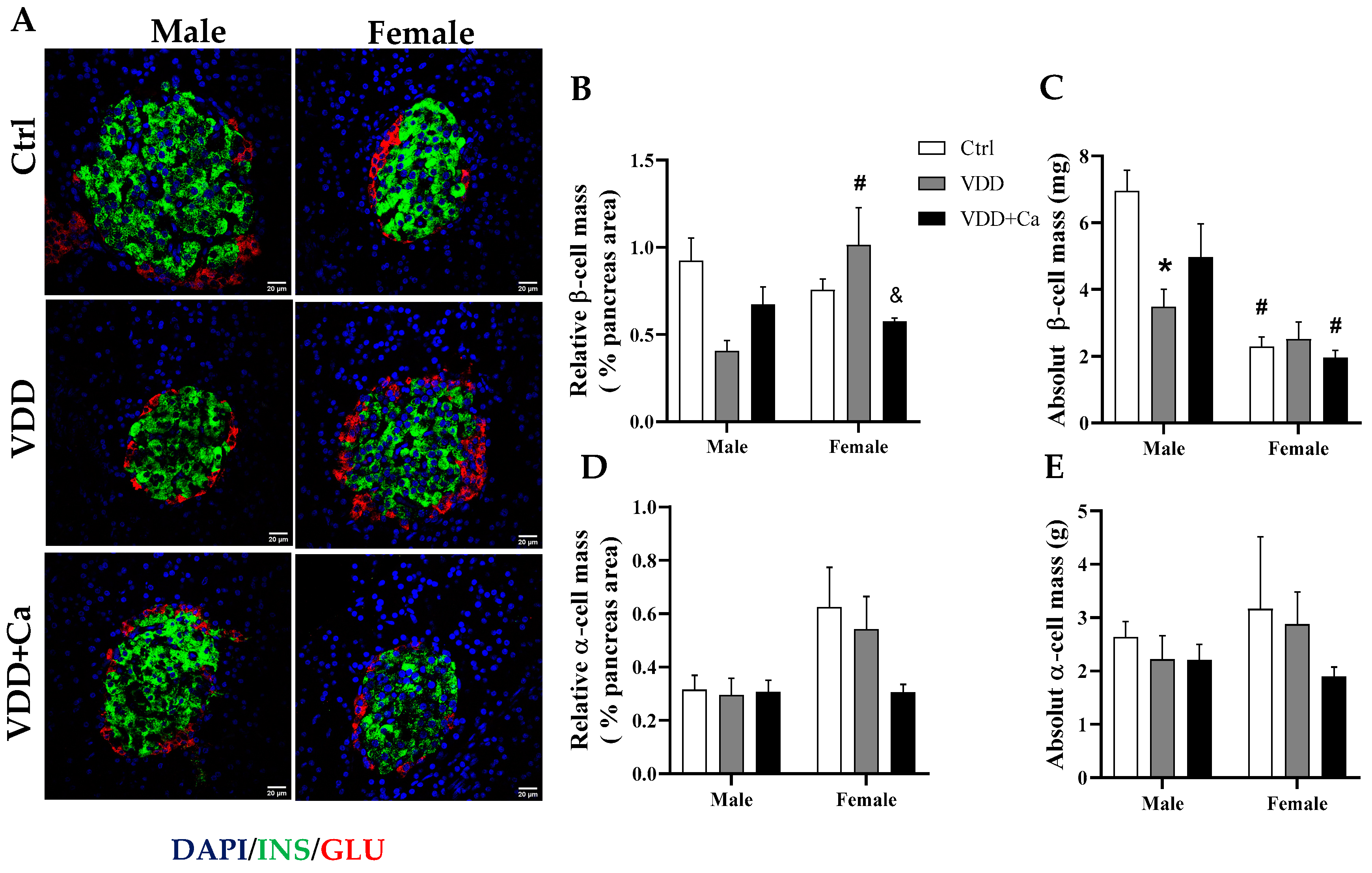
2.4. Pancreatic Islet and β/α Cell Morphology
2.5. Expression of Genes Related to the Development and Function of β Cells
2.6. Methylation Analysis by Methylation-Sensitive Enzymatic Restriction (MSER) Associated with RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Glucose Tolerance Test and Insulin Tolerance Test
4.3. Dosage of Metabolites and Hormones
4.4. Pancreatic Islets
4.5. Insulin Content and Static Secretion
4.6. Histology and Microscopy
4.7. Quantitative PCR
4.8. Genomic DNA Extraction
4.9. Methylation-Sensitive Enzymatic Restriction Associated with RT-qPCR
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nutrient (g/kg) | AIN93G (Control Diet) | AIN93G-Vitamin D3 - (VDD Diet) | AIN93G-Vitamin D3 + Ca + P (VDD Diet) |
|---|---|---|---|
| Corn starch | 397.50 | 397.50 | 397.50 |
| Casein | 200.00 | 200.00 | 200.00 |
| Starch dextrinated | 132.00 | 132.00 | 132.00 |
| Sucrose | 100.00 | 100.00 | 100.00 |
| Soy bean oil | 70 | 70 | 70 |
| L-Cystine | 3.00 | 3.00 | 3.00 |
| Choline | 2.50 | 2.50 | 2.50 |
| Mineral mix | 35.00 | 35.00 | 35.00 |
| Vitamin mix | 10.00 | 10.00 | 10.00 |
| Fibre | 50.00 | 50.00 | 50.00 |
| Vitamin D3 | 0.25 | 0.00 | 0.00 |
| Calcium carbonate | 0.00 | 0.00 | 35.00 |
| Sodium phosphate dibasic anhydrous | 0.00 | 0.00 | 21.50 |
| Lactose | 0.00 | 0.00 | 200.00 |
References
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef]
- Barker, D.J.; Gluckman, P.D.; Godfrey, K.M.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Cherif, H.; Reusens, B.; Dahri, S.; Remacle, C. A Protein-Restricted Diet during Pregnancy Alters in Vitro Insulin Secretion from Islets of Fetal Wistar Rats. J. Nutr. 2001, 131, 1555–1559. [Google Scholar] [CrossRef]
- Ideraabdullah, F.Y.; Belenchia, A.M.; Rosenfeld, C.S.; Kullman, S.W.; Knuth, M.; Mahapatra, D.; Bereman, M.; Levin, E.D.; Peterson, C.A. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J. Endocrinol. 2019, 241, R65–R80. [Google Scholar] [CrossRef] [PubMed]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low maternal vitamin D status in pregnancy increases the risk of childhood obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, R.E.; Simpson, R.U. Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am. J. Physiol. 1987, 253 Pt 1, E675–E683. [Google Scholar] [CrossRef]
- Zhang, H.; Chu, X.; Huang, Y.; Li, G.; Wang, Y.; Li, Y.; Sun, C. Maternal vitamin D deficiency during pregnancy results in insulin resistance in rat offspring, which is associated with inflammation and Iκbα methylation. Diabetologia 2014, 57, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, X.; Wang, Y.; Mao, Z.; Wang, C.; Ba, Y.; Li, W. Maternal transmission disequilibrium of rs2248359 in type 2 diabetes mellitus families and its association with vitamin D level in offspring. Sci. Rep. 2018, 8, 1345. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Altieri, B.; Grant, W.B.; Della Casa, S.; Orio, F.; Pontecorvi, A.; Colao, A.; Sarno, G.; Muscogiuri, G. Vitamin D and pancreas: The role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit. Rev. Food Sci. Nutr. 2017, 57, 3472–3488. [Google Scholar] [CrossRef]
- Hummel, D.; Aggarwal, A.; Borka, K.; Bajna, E.; Kállay, E.; Horváth, H.C. The vitamin D system is deregulated in pancreatic diseases. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.F.; Squires, P.E.; Hewison, M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Maia-Ceciliano, T.C.; Barreto-Vianna, A.R.C.; Barbosa-da-Silva, S.; Aguila, M.B.; Faria, T.S.; Mandarim-de-Lacerda, C.A. Maternal vitamin D-restricted diet has consequences in the formation of pancreatic islet/insulin-signaling in the adult offspring of mice. Endocrine 2016, 54, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Reis, N.G.; Assis, A.P.; Lautherbach, N.; Gonçalves, D.A.; Silveira, W.A.; Morgan, H.J.; Valentim, R.R.; Almeida, L.F.; Heck, L.C.; Zanon, N.M.; et al. Maternal vitamin D deficiency affects the morphology and function of glycolytic muscle in adult offspring rats. J. Cachexia Sarcopenia Muscle 2022, 13, 2175–2187. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.R.; Crowther, N.J.; Desai, M.; Hales, C.N.; Ozanne, S.E. Altered adipocyte properties in the offspring of protein malnourished rats. Br. J. Nutr. 1997, 78, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.J.; Dorling, M.W.; Pawlak, D.B.; Ozanne, S.E.; Hales, C.N. Diabetes in Old Male Offspring of Rat Dams Fed a Reduced Protein Diet. Int. J. Exp. Diabetes Res. 2001, 2, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Petrik, J.; Reusens, B.; Arany, E.; Remacle, C.; Coelho, C.; Hoet, J.J.; Hill, D.J. A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 1999, 140, 4861–4873. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.T.; Dayeh, T.A.; Volkov, P.A.; Kirkpatrick, C.L.; Malmgren, S.; Jing, X.; Renström, E.; Wollheim, C.B.; Nitert, M.D.; Ling, C. Increased DNA methylation and decreased expression of glut-1 in pancreatic islets from patients with type 2 diabetes. Mol. Endocrinol. 2012, 26, 1203–1212. [Google Scholar] [CrossRef]
- Arantes, V.C.; Reis, M.A.B.; Leite, A.R.; Carneiro, E.M.; Boschero, A.C.; Teixeira, V.P.A.; Latorraca, M.Q.; Yamada, A.T. Expression of PDX-1 is reduced in pancreatic islets from pups of rat dams fed a low protein diet during gestation and lactation. J. Nutr. 2002, 132, 3030–3035. [Google Scholar] [CrossRef]
- Sosa-Larios, T.C.; Cerbón, M.A.; Morimoto, S. Epigenetic Alterations Caused by Nutritional Stress During Fetal Programming of the Endocrine Pancreas. Arch. Med. Res. 2015, 46, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Stoffers, D.A.; Nicholls, R.D.; Simmons, R.A. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J. Clin. Investig. 2008, 118, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Larios, T.C.; Ortega-Márquez, A.L.; Rodríguez-Aguilera, J.R.; Vázquez-Martínez, E.R.; Domínguez-López, A.; Morimoto, S. A low-protein maternal diet during gestation affects the expression of key pancreatic β-cell genes and the methylation status of the regulatory region of the MafA gene in the offspring of Wistar rats. Front. Vet. Sci. 2023, 10, 1138564. [Google Scholar] [CrossRef] [PubMed]
- Gesina, E.; Tronche, F.; Herrera, P.; Duchene, B.; Tales, W.; Czernichow, P.; Breant, B. Dissecting the Role of Glucocorticoids on Pancreas Development. Diabetes 2004, 53, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, B.; Lesage, J.; Czernichow, P.; Dupouy, J.P.; Bréant, B. Glucocorticoids impair fetal β-cell development in rats. Am. J. Physiol.-Endocrinol. Metab. 2001, 281, E592–E599. [Google Scholar] [CrossRef] [PubMed]
- Tesic, D.; Hawes, J.E.; Zosky, G.R.; Wyrwoll, C.S. Vitamin D Deficiency in BALB/c Mouse Pregnancy Increases Placental Transfer of Glucocorticoids. Endocrinology 2015, 156, 3673–3679. [Google Scholar] [CrossRef] [PubMed]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.-J.; Mauvais-Jarvis, F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor α protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Sex-Specific Placental Responses in Fetal Development. Endocrinology 2015, 156, 3422–3434. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; García-Quiroz, J.; López-Marure, R.; González-Curiel, I.; Rivas-Santiago, B.; Olivares, A.; Avila, E.; Barrera, D.; Halhali, A.; Caldiño, F.; et al. Evidence of sexual dimorphism in placental vitamin D metabolism: Testosterone inhibits calcitriol-dependent cathelicidin expression. J. Steroid Biochem. Mol. Biol. 2016, 163, 173–182. [Google Scholar] [CrossRef]
- Durlo, F.V.; Castro, M.; Elias, L.L.K.; Antunes-Rodrigues, J. Interaction of prolactin, ANPergic, oxytocinergic and adrenal systems in response to extracellular volume expansion in rats. Exp. Physiol. 2004, 89, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Rafacho, A.; Giozzet, V.A.G.; Boschero, A.C.; Bosqueiro, J.R. Functional Alterations in Endocrine Pancreas of Rats with Different Degrees of Dexamethasone-Induced Insulin Resistance. Pancreas 2008, 36, 284. [Google Scholar] [CrossRef] [PubMed]
- Taschetto, A.P.; Zimath, P.L.; Silvério, R.; Dos Santos, C.; Boschero, A.C.; Dos Santos, G.J.; Rafacho, A. Reduced insulin sensitivity and increased β/α cell mass is associated with reduced hepatic insulin-degrading enzyme activity in pregnant rats. Life Sci. 2021, 277, 119509. [Google Scholar] [CrossRef]
- Gomes, M.V.; Gomes, C.C.; Pinto, W., Jr.; Ramos, E.S. Methylation pattern at the KvDMR in a child with Beckwith-Wiedemann syndrome conceived by ICSI. Am. J. Med. Genet. A 2007, 143, 625–629. [Google Scholar] [CrossRef] [PubMed]


| M-Ctrl | M-VDD | M-VDD + Ca | F-Ctrl | F-VDD | F-VDD + Ca | |
|---|---|---|---|---|---|---|
| Calcidiol (ng/mL) | 29 ± 0.9 | 5 ± 0.3 * | 6 ± 0.5 * | 28 ± 0.8 | 5 ± 0.4 * | 5 ± 0.2 * |
| Calcium (mg/dL) | 8 ± 0.6 | 6.8 ± 0.1 * | 9.2 ± 0.2 | 8 ± 0.3 | 6.8 ± 0.2 * | 8 ± 0.2 |
| Insulin (ng/mL) | 2 ± 0.1 | 0.8 ± 0.1 * | 0.5 ± 0.1 * | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.1 ± 0.2 |
| M-Ctrl | M-VDD | M-VDD + Ca | F-Ctrl | F-VDD | F-VDD + Ca | |
|---|---|---|---|---|---|---|
| Calcidiol (ng/mL) | 48.4 ± 1.5 | 43.0 ± 1.3 | 44.9 ± 1.4 | 48.1 ± 1.1 | 51.5 ± 1.1 | 53.6 ± 1.1 |
| Calcium (mg/dL) | 9.4 ± 0.5 | 9.4 ± 0.5 | 9.7 ± 0.6 | 9.4 ± 0.5 | 9.5 ± 0.8 | 9.5 ± 0.5 |
| Phosphorus (mg/dL) | 6.3 ± 0.9 | 6.3 ± 1.1 | 6.4 ± 1.0 | 5.3 ± 0.7 | 6.0 ± 0.9 | 4.9 ± 0.8 |
| Corticosterone (µg/dL) | 11.5 ± 1.7 | 13.3 ± 2.2 | 6.9 ± 2.1 | 20.5 ± 2.7 | 17.8 ± 2.7 | 18.3 ± 2.2 |
| Glucagon (ng/mL) | 0.26 ± 0.05 | 0.22 ± 0.06 | 0.33 ± 0.05 | 0.26 ± 0.04 | 0.27 ± 0.03 | 0.25 ± 0.04 |
| Retroperitoneal WAT (g/100 g BW) | 1.17 ± 0.19 | 1.06 ± 0.11 | 1.67 ± 0.11 | 0.70 ± 0.11 | 0.84 ± 0.09 | 0.71 ± 0.06 # |
| Epididymal WAT (g/100 g BW) | 1.07 ± 0.12 | 0.95 ± 0.07 | 1.11 ± 0.05 | - | - | - |
| Periovarian WAT (g/100 g BW) | - | - | - | 0.4 ± 0.1 | 0.44 ± 0.03 | 0.51 ± 0.03 |
| EDL (mg/100 g BW) | 43 ± 1.3 | 46 ± 0.2 | 44 ± 0.9 | 46 ± 3.3 | 46 ± 2.4 | 45 ± 1.5 |
| Soleus (mg/100 g BW) | 36 ± 2.9 | 37 ± 1.5 | 35 ± 1.4 | 36 ± 3.3 | 42 ± 0.5 | 39 ± 1.2 |
| Average CT | n | Met% | ||
|---|---|---|---|---|
| Male | U Ctrl | 27.2 ± 0.6 | ||
| D Ctrl | 32.0 ± 1.1 | 4.8 | 0.04 | |
| U VDD | 26.8 ± 0.3 | |||
| D VDD | 32.8 ± 0.4 | 6 | 0.01 | |
| U VDD + Ca | 26.2 ± 1.1 | |||
| D VDD + Ca | 32.3 ± 1.3 | 6.1 | 0.01 | |
| Female | U Ctrl | 26.7 ± 1.2 | ||
| D Ctrl | 32.4 ± 1.0 | 5.7 | 0.03 | |
| U VDD | 27.2 ± 1.2 | |||
| D VDD | 33.2 ± 0.8 | 6 | 0.03 | |
| U VDD + Ca | 26.1 ± 1.2 | |||
| D VDD + Ca | 32 ± 1 | 5.9 | 0.02 |
| Gene | Reverse | Reverse |
|---|---|---|
| Rat Ins1 | CCATCAGCAAGCAGGTCAT | TGTGTAGAAGAAACCACGTTCC |
| Rat Ins2 | TCTTCTACACACCCATGTCCC | GGTGCAGCACTGATCCAC |
| Rat Pdx1 | CCGCGTTCATCTCCCTTT | TGCCCACTGGCTTTTCCA |
| Rat SLC2A2 | GTCCAGAAAGCCCCAGATACC | TGCCCCTTAGTCTTTTCAAGCT |
| Rat β-actin | TGAAGTGTGACGTTGACATCC | ACAGTGAGGCCAGGATAGAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schavinski, A.Z.; Reis, N.G.; Morgan, H.J.N.; Assis, A.P.; Moro, M.L.; Valentim, R.R.; Seni-Silva, A.C.; Ramos, E.S.; Kettelhut, I.C.; Navegantes, L.C.C. Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner. Int. J. Mol. Sci. 2024, 25, 4136. https://doi.org/10.3390/ijms25084136
Schavinski AZ, Reis NG, Morgan HJN, Assis AP, Moro ML, Valentim RR, Seni-Silva AC, Ramos ES, Kettelhut IC, Navegantes LCC. Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner. International Journal of Molecular Sciences. 2024; 25(8):4136. https://doi.org/10.3390/ijms25084136
Chicago/Turabian StyleSchavinski, Aline Z., Natany G. Reis, Henrique J. N. Morgan, Ana Paula Assis, Matheus L. Moro, Rafael R. Valentim, Ana Carolina Seni-Silva, Ester S. Ramos, Isis C. Kettelhut, and Luiz C. C. Navegantes. 2024. "Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner" International Journal of Molecular Sciences 25, no. 8: 4136. https://doi.org/10.3390/ijms25084136
APA StyleSchavinski, A. Z., Reis, N. G., Morgan, H. J. N., Assis, A. P., Moro, M. L., Valentim, R. R., Seni-Silva, A. C., Ramos, E. S., Kettelhut, I. C., & Navegantes, L. C. C. (2024). Maternal Vitamin D Deficiency Impairs the Development of β Cells in Offspring Rats in a Sex-Dependent Manner. International Journal of Molecular Sciences, 25(8), 4136. https://doi.org/10.3390/ijms25084136







