Fine Mapping of Five Grain Size QTLs Which Affect Grain Yield and Quality in Rice
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Variation and Correlation in the BC1F2 and BC1F2:3 Populations
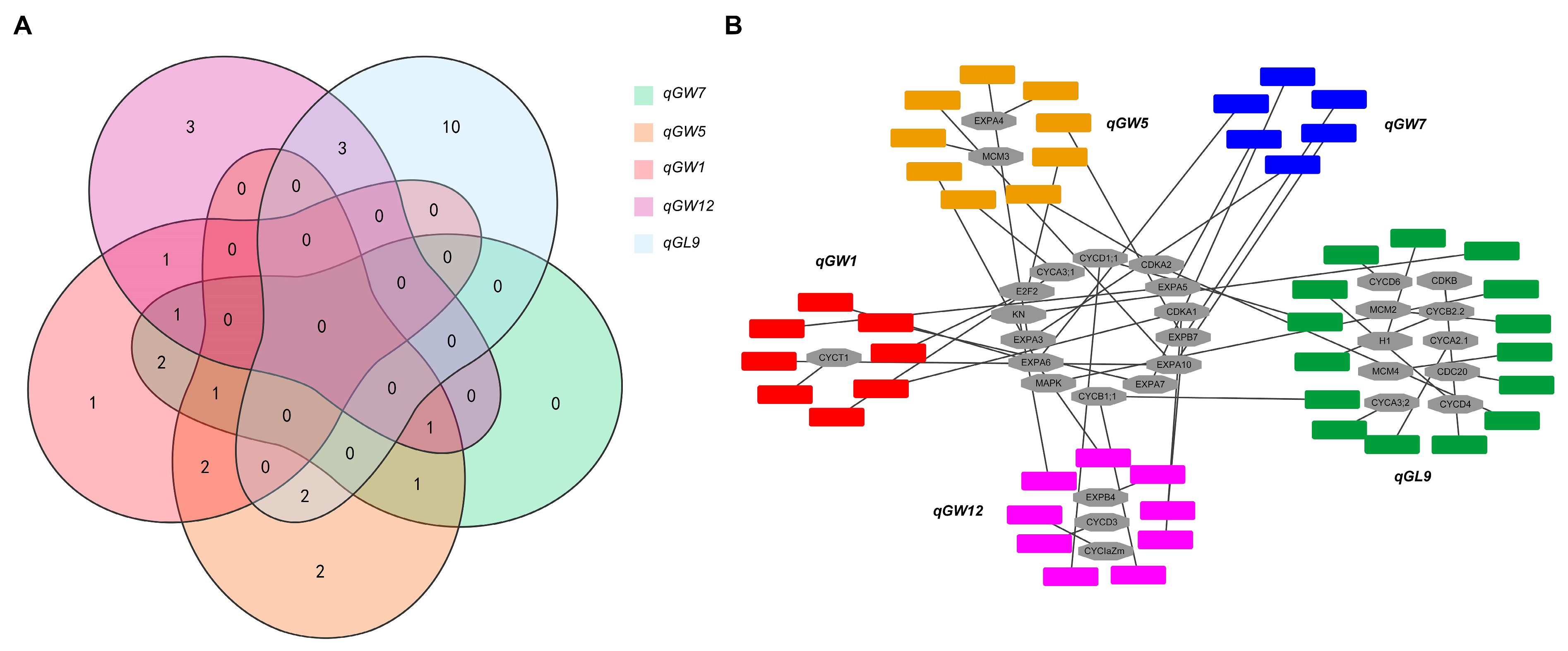
2.2. QTL Mapping and Verification
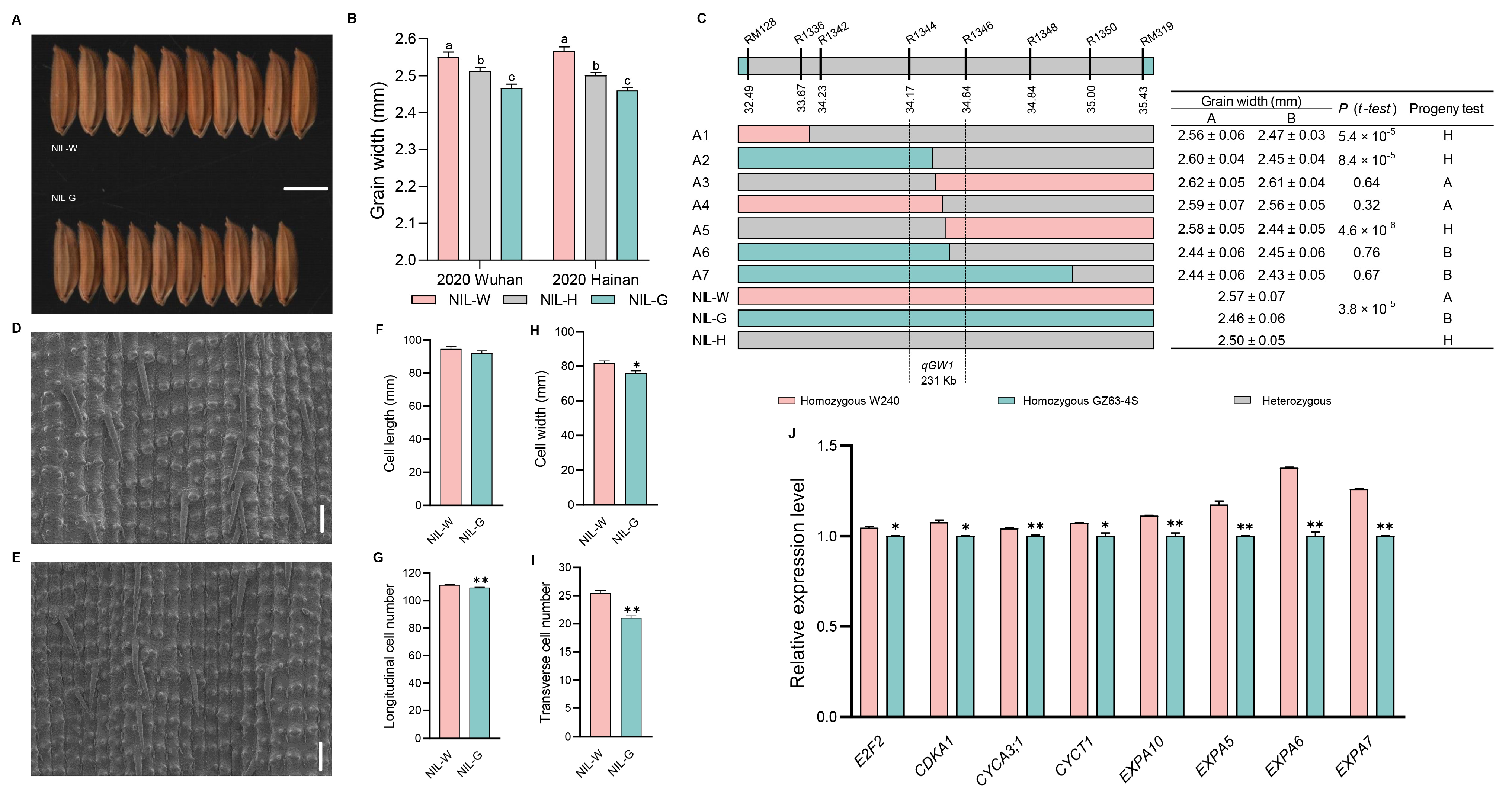
2.3. The Genetic Effect of qGW1
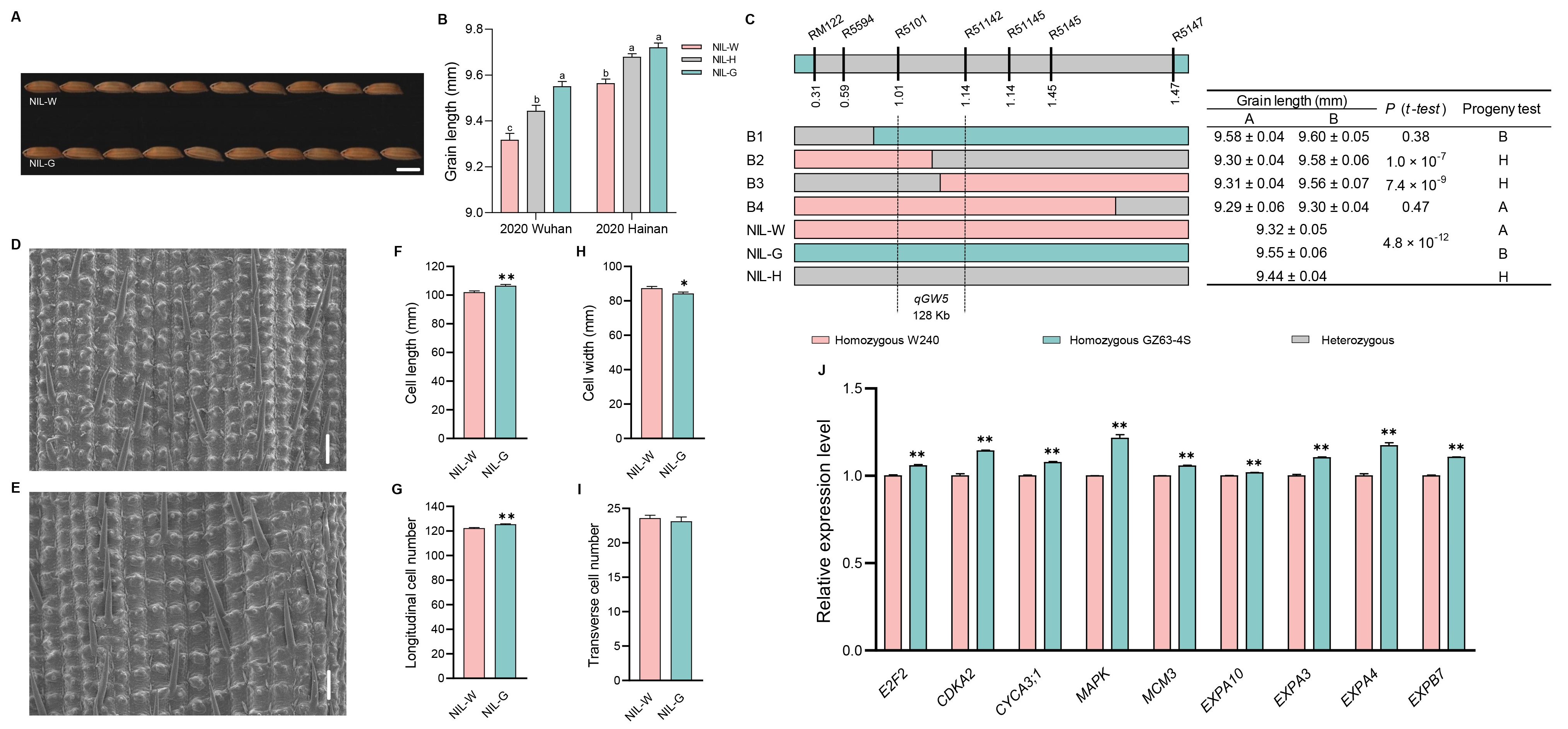
2.4. The Genetic Effect of qGW5
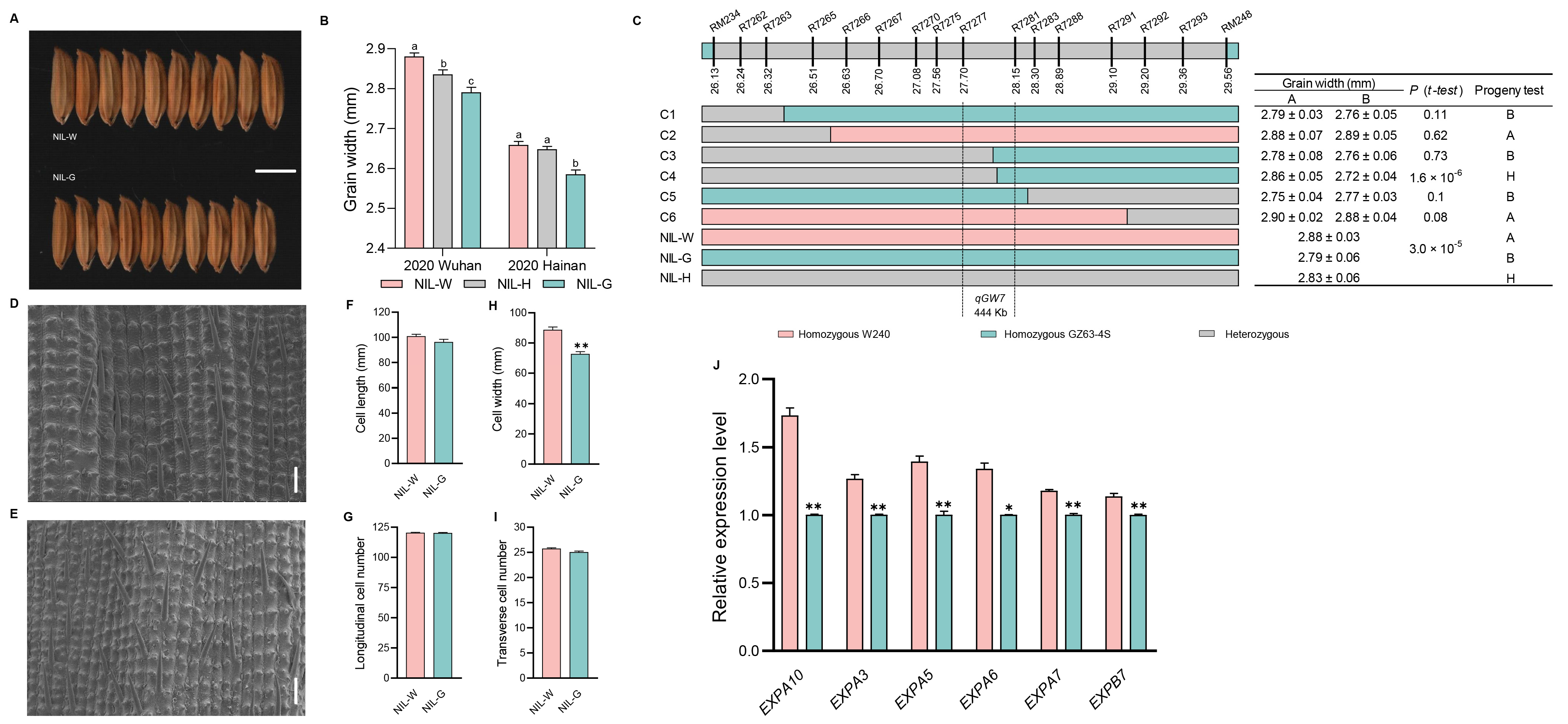
2.5. The Genetic Effect of qGW7
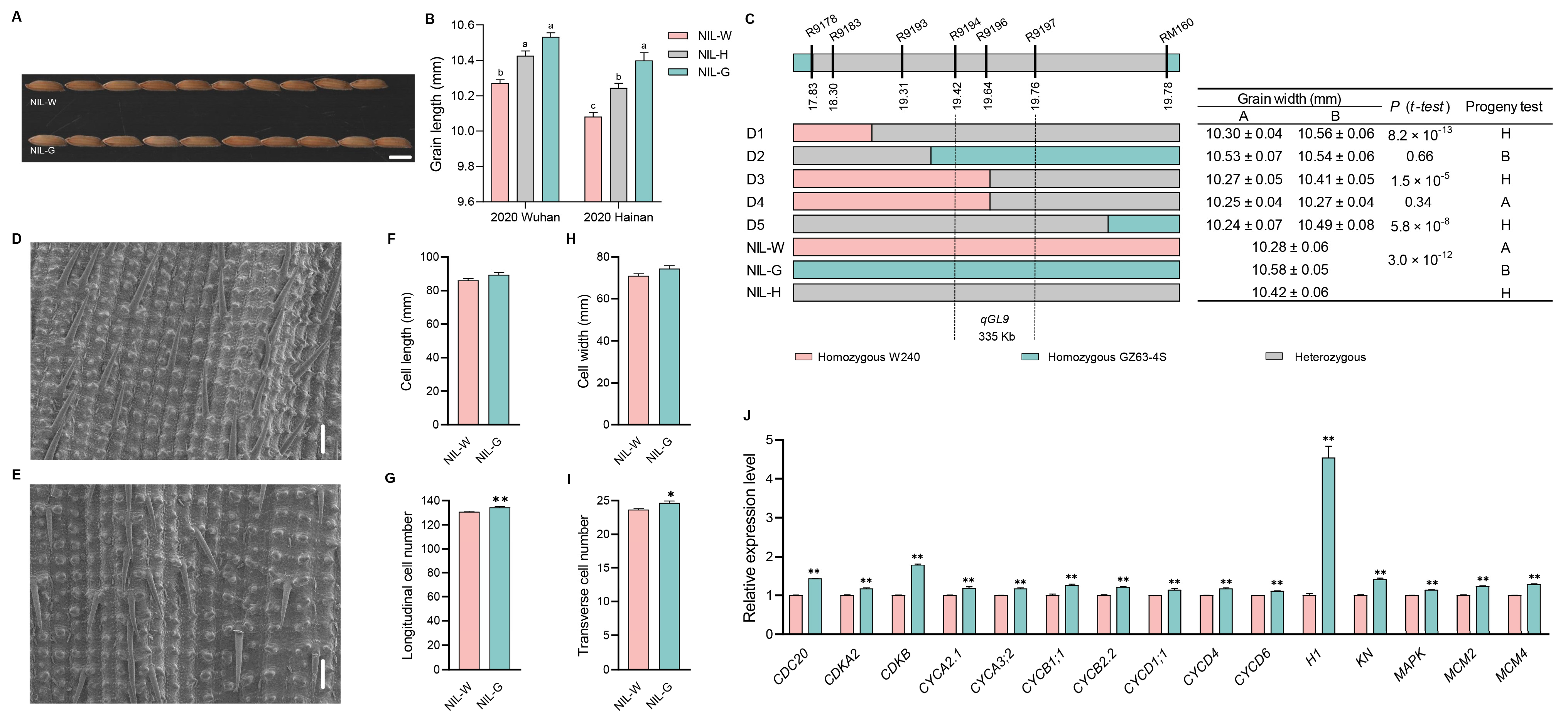
2.6. The Genetic Effect of qGL9
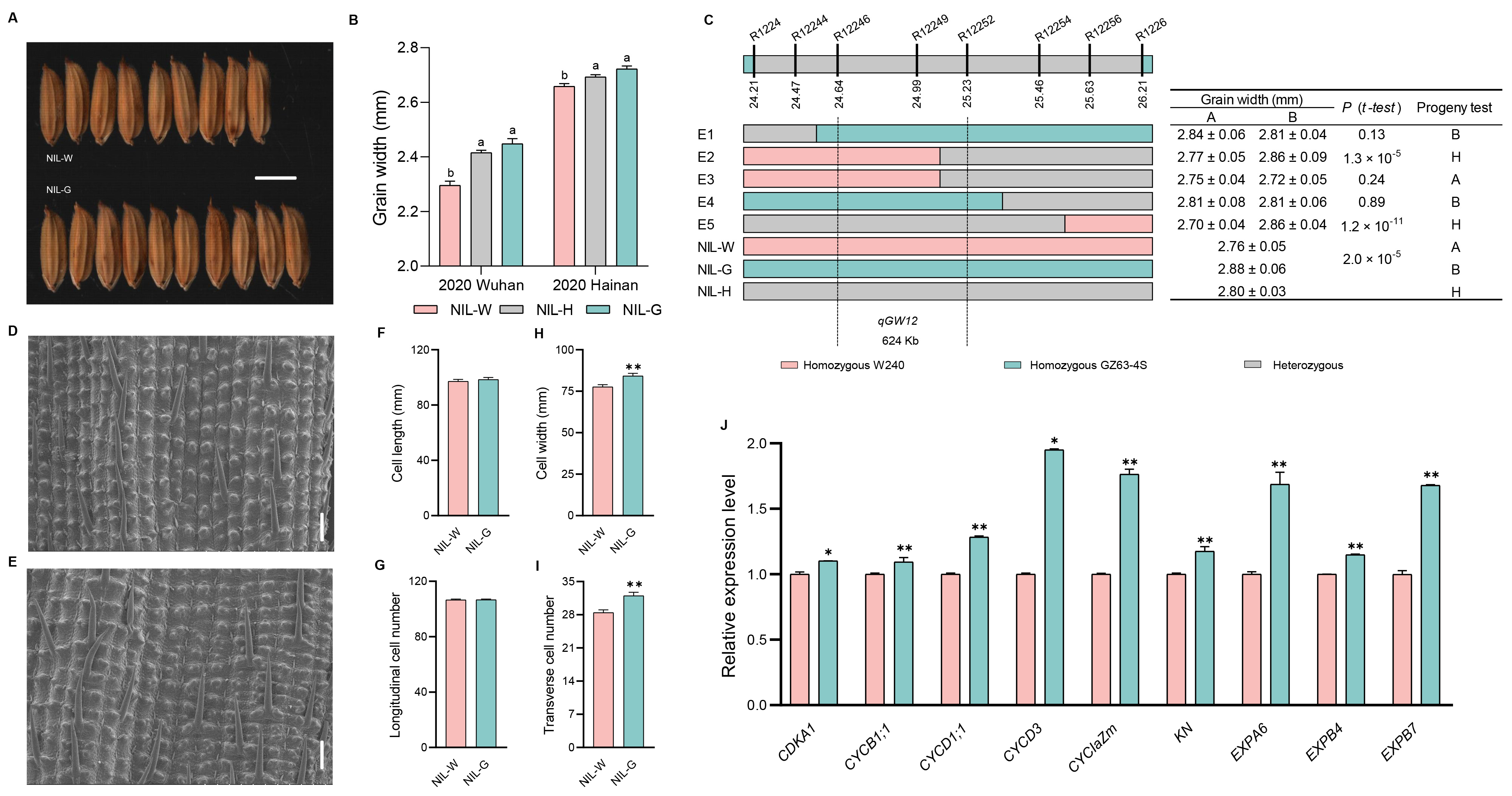
2.7. The Genetic Effect of qGW12
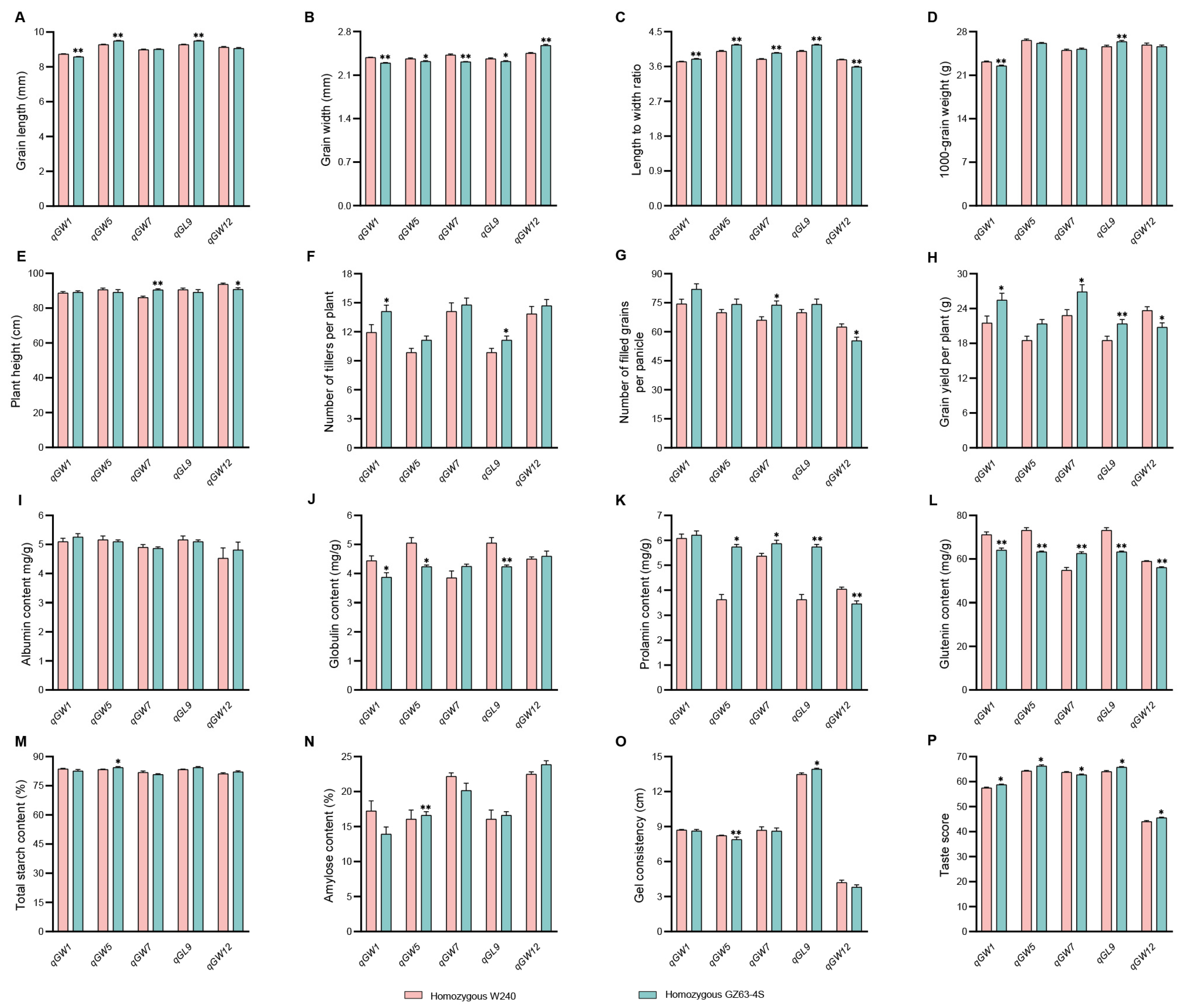
2.8. Investigation of Traits Related to Rice Yield and Quality in Five NILs
3. Discussion
3.1. QTL Mapping for Grain Size and Weight
3.2. Validation and Fine Mapping of the Five QTLs
3.3. Candidate Gene Analysis of the Five QTLs
3.4. Cytological Analysis of the Five QTLs
3.5. Potential Uses of the Five QTLs in Rice Breeding
4. Materials and Methods
4.1. Population Development and Field Experiment
4.2. Trait Measurement
4.3. Genetic Map Construction and QTL Mapping
4.4. Scanning Election Microscopy
4.5. RNA Extraction, Reverse Transcription, and qRT-PCR
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Rice Genome Sequencing Project; Sasaki, T. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, Q. Genetic and molecular bases of rice yield. Annu. Rev. Genet. 2010, 61, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Duan, P.; Li, Y. Control of grain size in rice. Plant Reprod. 2018, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Jiang, L.; Zheng, J.; Wang, T.; Wang, H.; Huang, Y.; Hong, Z. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci. 2013, 18, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, G.; Yang, Z.; He, G. Pyramiding QTL for yield-related traits and grain shape in rice using single-segment substitution lines. Indian J. Genet. Pl. Br. 2014, 74, 496–498. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y. Molecular, cellular and Yin-Yang regulation of grain size and number in rice. Mol. Breed. 2019, 39, 163. [Google Scholar] [CrossRef]
- Li, N.; Xu, R.; Li, Y. Molecular networks of seed size control in plants. Annu. Rev. Genet. 2019, 70, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ding, C.; Qian, Q. Molecular bases of rice grain size and quality for optimized productivity. Sci. Bull. 2023, 68, 314–350. [Google Scholar] [CrossRef]
- Fan, C.; Yu, S.; Wang, C.; Xing, Y. A causal C-A mutation in the second exon of GS3 highly associated with rice grain length and validated as a functional marker. Theor. Appl. Genet. 2009, 118, 465–472. [Google Scholar] [CrossRef]
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef]
- Mao, H.; Sun, S.; Yao, J.; Wang, C.; Yu, S.; Xu, C.; Li, X.; Zhang, Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Nati. Acad. Sci. 2010, 107, 19579–19584. [Google Scholar] [CrossRef] [PubMed]
- Takano-Kai, N.; Jiang, H.; Kubo, T.; Sweeney, M.; Matsumoto, T.; Kanamori, H.; Padhukasahasram, B.; Bustamante, C.; Yoshimura, A.; Doi, K.; et al. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 2009, 182, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, L.; Mao, H.; Shao, L.; Li, X.; Xiao, J.; Ouyang, Y.; Zhang, Q. A G-protein pathway determines grain size in rice. Nat. Commun. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Han, R.; Wu, K.; Zhang, J.; Ye, Y.; Wang, S.; Chen, J.; Pan, Y.; Li, Q.; Xu, X.; et al. G-protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 2018, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef]
- Choi, B.S.; Kim, Y.J.; Markkandan, K.; Koo, Y.J.; Song, J.T.; Seo, H.S. GW2 functions as an E3 ubiquitin ligase for rice expansin-like 1. Int. J. Mol. Sci. 2018, 19, 1904. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Wang, D.; Wu, Y.; Huang, K.; Duan, P.; Li, N.; Xu, R.; Zeng, D.; Dong, G.; Zhang, B.; et al. The GW2-WG1-OsbZIP47 pathway controls grain size and weight in rice. Mol. Plant 2021, 14, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Z.; Lin, J.; Guan, B.; Chen, J.; Zhang, Z.; Chen, F.; Jiang, L.; Zheng, J.; Wang, T.; et al. gw2.1, a new allele of GW2, improves grain weight and grain yield in rice. Plant Sci. 2022, 325, 111495. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Wang, D.; Duan, P.; Zhang, B.; Xu, R.; Li, N.; Li, Y. WIDE AND THICK GRAIN 1, which encodes an otubain-like protease with deubiquitination activity, influences grain size and shape in rice. Plant J. 2017, 91, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, K.; Qian, Q.; Liu, Q.; Li, Q.; Pan, Y.; Ye, Y.; Liu, X.; Wang, J.; Zhang, J.; et al. Non-canonical regulation of SPL transcription factors by a human OTUB1-like deubiquitinase defines a new plant type rice associated with higher grain yield. Cell Res. 2017, 27, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Rao, Y.; Zeng, D.; Yang, Y.; Xu, R.; Zhang, B.; Dong, G.; Qian, Q.; Li, Y. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2014, 77, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, K.; Dong, N.Q.; Shi, C.L.; Ye, W.; Gao, J.; Shan, J.; Lin, H. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell 2018, 30, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Lu, Z.; Shan, J.; Ye, W.; Dong, N.; Lin, H. ERECTA1 acts upstream of the OsMKKK10-OsMKK4-OsMPK6 cascade to control spikelet number by regulating cytokinin metabolism in rice. Plant Cell 2020, 32, 2763–2779. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hua, L.; Dong, S.; Chen, H.; Zhu, X.; Jiang, J.; Zhang, F.; Li, Y.; Fang, X.; Chen, F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015, 84, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Duan, P.; Yu, H.; Zhou, Z.; Zhang, B.; Wang, R.; Li, J.; Zhang, G.; Zhuang, S.; Lyu, J.; et al. Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol. Plant 2018, 11, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, X.; Zhou, W.; Ren, Y.; Wang, Z.; Liu, Z.; Tang, J.; Tong, H.; Fang, J.; Bu, Q. Transcription factor OsWRKY53 positively regulates brassinosteroid signaling and plant architecture. Plant Physiol. 2017, 175, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, J.; Huang, X.; Gong, H.; Luo, J.; Hou, Q.; Zhou, T.; Lu, T.; Zhu, J.; Shangguan, Y.; et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 2016, 48, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Ni, S.; Wang, J.; Zhang, B.; Xu, R.; Wang, Y.; Chen, H.; Zhu, X.; Li, Y. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat. Plants 2015, 2, 15203. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, Y.; Fang, Y.; Zeng, L.; Xu, J.; Yu, H.; Shi, Z.; Pan, J.; Zhang, D.; Kang, S.; et al. A rare allele of GS2 enhances grain size and grain yield in rice. Mol. Plant 2015, 8, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, Q.; Zhang, C.; Zhang, C.; Yang, Q.; Pan, L.; Ren, X.; Lu, J.; Gu, M.; Liu, Q. GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat. Commun. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hou, Q.; Si, L.; Huang, X.; Luo, J.; Lu, D.; Zhu, J.; Shangguan, Y.; Miao, J.; Xie, Y.; et al. The PLATZ transcription factor GL6 affects grain length and number in rice. Plant Physiol. 2019, 180, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xue, H. The rice PLATZ protein SHORT GRAIN6 determines grain size by regulating spikelet hull cell division. J. Integr. Plant Biol. 2020, 62, 847–864. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Xu, J.; Zeng, D.; Zhang, B.; Geng, M.; Zhang, G.; Huang, K.; Huang, L.; Xu, R.; Ge, S.; et al. Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol. Plant 2017, 10, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Zheng, X.; Wu, F.; Lin, Q.; Heng, Y.; Tian, P.; Cheng, Z.; Yu, X.; Zhou, K.; et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 2017, 3, 17043. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; He, H.; Zhang, S.; Sun, F.; Xin, X.; Wang, W.; Qian, X.; Yang, J.; Luo, X. A Kelch motif-containing serine/threonine protein phosphatase determines the large grain QTL trait in rice. J. Integr. Plant Biol. 2012, 54, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Lin, Y.; Song, X.; Shen, J.; Huang, W.; Shan, J.; Zhu, M.; Jiang, L.; Gao, J.; Lin, H. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, J.; Zhang, X.; Zhou, J.; Jiang, Z.; Huang, P.; Tang, Z.; Bao, Y.; Cheng, J.; Tang, H.; et al. Rice qGL3/OsPPKL1 functions with the GSK3/SHAGGY-like kinase OsGSK3 to modulate brassinosteroid signaling. Plant Cell 2019, 31, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Hirotsu, N.; Madoka, Y.; Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.; Onishi, A.; et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lu, S.J.; Wang, M.; He, H.; Sun, L.; Wang, H.; Liu, X.; Jiang, L.; Sun, J.; Xin, X.; et al. A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol. Plant 2018, 11, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhou, H.; Liu, R.; Dan, W.; Li, P.; Wu, B.; Chen, J.; Wang, L.; Gao, G.; Zhang, Q.; et al. GL3.3, a novel QTL encoding a GSK3/SHAGGY-like kinase, epistatically interacts with GS3 to produce extra-long grains in rice. Mol. Plant 2018, 11, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Ma, M.; Bai, C.; Huang, X.; Liu, J.; Fan, Y.; Song, X. TGW3, a major QTL that negatively modulates grain length and weight in rice. Mol. Plant 2018, 11, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, X.; Yu, C.; Lei, J.; Hu, P.; Wang, Z.; Li, M.; Zhu, C.; Liang, G. Correlation analysis of eating quality with quality characters of indica rice. J. Chin. Cereals Oils Assoc. 2012, 27, 1–5. [Google Scholar]
- Lin, F.; Salman, M.; Zhang, Z.; McCoy, A.; Li, W.; Magar, R.; Mitchell, D.; Zhao, M.; Gu, C.; Chilvers, M.; et al. Identification and molecular mapping of a major gene conferring resistance to Phytophthora sansomeana in soybean ‘Colfax’. Theor. Appl. Genet. 2024, 137, 55. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Liu, H.; Yang, L.; Ma, T.; Wang, J.; Zheng, H.; Liu, W.; Zou, D. BSA-Seq and fine linkage mapping for the identification of a novel locus (qPH9) for mature plant height in rice (Oryza sativa). Rice 2022, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Saito, T.; Negoro, S.; Yamaguchi, H.; Nunome, T.; Ohyama, A.; Fukuoka, H. Detailed mapping of a resistance locus against Fusarium wilt in cultivated eggplant (Solanum melongena). Theor. Appl. Genet. 2016, 129, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wu, J.; Wang, L.; Mantri, N.; Zhang, X.; Zhu, Z.; Wang, S. Mapping and genetic structure analysis of the anthracnose resistance locus Co-1HY in the common bean (Phaseolus vulgaris L.). PLoS ONE 2017, 12, e0169954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Wu, L.; Zhou, K.; Yang, J.; Miao, Y.; Hao, M.; Ning, S.; Yuan, Z.; Jiang, B.; et al. Mapping of a recessive gene for all-stage resistance to stripe rust in a wheat line derived from cultivated einkorn (Triticum monococcum L.). In Plant Disease; The American Phytopathological Society: Merida, Mexico, 2024. [Google Scholar] [CrossRef]
- Silva, A.; Montoya, M.; Quintero, C.; Cuasquer, J.; Tohme, J.; Graterol, E.; Cruz, M.; Lorieux, M. Genetic bases of resistance to the rice hoja blanca disease deciphered by a quantitative trait locus approach. G3-Genes Genom. Genet. 2023, 13, jkad223. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Liu, Y.; Chang, Z.; Liu, Z.; Ding, Y.; Ding, C. Cytokinin oxidase/dehydrogenase family genes exhibit functional divergence and overlap in rice growth and development, especially in control of tillering. J. Exp. Bot. 2022, 73, 3552–3568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, P.; Wang, L.; Sun, L.; Xia, D.; Luo, L.; Gao, G.; Zhang, Q.; He, Y. Genetic dissection of large grain shape in rice cultivar ‘Nanyangzhan’ and validation of a grain thickness QTL (qGT3.1) and a grain length QTL (qGL3.4). Mol. Breed. 2017, 37, 42. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, X.; Wang, Y.; Yang, Y.; Zhang, M.; Yu, H.; Xu, Q.; Wang, S.; Niu, X.; Wei, X. Validation of a QTL for grain size and weight using an introgression line from a cross between Oryza sativa and Oryza minuta. Rice 2021, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shang, L.; Ruan, B.; Zhang, A.; Yang, S.; Jiang, H.; Liu, C.; Hong, K.; Lin, H.; Gao, Z.; et al. Development of three sets of high-throughput genotyped rice chromosome segment substitution lines and QTL mapping for eleven traits. Rice 2019, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Inukai, Y.; Ueguchi-Tanaka, M.; Itoh, H.; Izawa, T.; Kobayashi, Y.; Hattori, T.; Miyao, A.; Hirochika, H.; Ashikari, M.; et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell 2004, 16, 33–44. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Guo, S.; Wang, Z.; Zhang, Z.; Fang, Z. MiR319-targeted OsTCP21 and OsGAmyb regulate tillering and grain yield in rice. J. Integr. Plant Biol. 2021, 63, 1260–1272. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Jin, F.; Ding, L.; Luo, J.; Nie, S.; Fang, Z. Research progress of MYB transcription factors in rice. J. Plant Genet. Res. 2023, 24, 917–926. [Google Scholar] [CrossRef]
- Li, G.; Xu, B.; Zhang, Y.; Xu, Y.; Khan, N.; Xie, J.; Sun, X.; Guo, H.; Wu, Z.; Wang, X.; et al. RGN1 controls grain number and shapes panicle architecture in rice. Plant Biotechnol. J. 2022, 20, 158–167. [Google Scholar] [CrossRef]
- Wang, F.; Han, T.; Song, Q.; Ye, W.; Song, X.; Chu, J.; Li, J.; Chen, Z.J. The rice circadian clock regulates tiller growth and panicle development through strigolactone signaling and sugar sensing. Plant Cell 2020, 32, 3124–3138. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; Tang, W.; Chen, X.; Lin, C.; Liu, Y.; Ye, Y.; Wu, W.; Duan, Y. LUX ARRHYTHMO interacts with ELF3a and ELF4a to coordinate vegetative growth and photoperiodic flowering in rice. Front. Plant Sci. 2022, 13, 853042. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, C.; Zhou, Y.; Xiong, M.; Wang, J.; Bai, H.; Lu, C.; Zhang, C.; Liu, Q.; Li, Q. OsbZIP09, a unique OsbZIP transcription factor of rice, promotes rather than suppresses seed germination by attenuating abscisic acid pathway. Rice Sci. 2021, 28, 358–367. [Google Scholar] [CrossRef]
- He, Y.; Li, L.; Shi, W.; Tan, J.; Luo, X.; Zheng, S.; Chen, W.; Li, J.; Zhuang, C.; Jiang, D. Florigen repression complexes involving rice CENTRORADIALIS2 regulate grain size. Plant Physiol. 2022, 190, 1260–1274. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Deng, H.; Li, T.; Sharma, S.; Yun, Q.; Li, Q.; Zhiquo, E.; Chen, C. OsbZIP76 interacts with OsNF-YBs and regulates endosperm cellularization in rice (Oryza sativa). J. Integr. Plant Biol. 2020, 62, 1983–1996. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Rai, R.; Zamzam, M.; Ahmad, O.; Peesapati, R.; Vijayraghavan, U. OsbZIP47 is an integrator for meristem regulators during rice plant growth and development. Front. Plant Sci. 2022, 13, 865928. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Xue, H. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ma, X.; Zhao, S.; Tang, Y.; Liu, F.; Gu, P.; Fu, Y.; Zhu, Z.; Cai, H.; Sun, C.; et al. The APETALA2-like transcription factor SUPERNUMERARY BRACT controls rice seed shattering and seed size. Plant Cell 2019, 31, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, F.; Zhang, Y.; Elesawi, I.; Xu, K.; Li, T.; Mei, H.; Liu, H.; Gao, N.; Chen, C.; et al. A novel rice grain size gene OsSNB was identified by genome-wide association study in natural population. PLOS Genet. 2019, 15, e1008191. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Jin, Y.; Wu, T.; Hu, L.; Zhang, Y.; Jiang, W.; Du, X. OsDREB2B, an AP2/ERF transcription factor, negatively regulates plant height by conferring GA metabolism in rice. Front. Plant Sci. 2022, 13, 1007811. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Hu, J.; Xu, Q.; Cui, Y.; Zhang, Y.; Zhou, T.; Rao, Y.; Xue, D.; Zeng, D.; Zhang, G.; et al. FZP determines grain size and sterile lemma fate in rice. J. Exp. Bot. 2018, 69, 4853–4866. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, W.; Fu, Y.; Shan, Z.; Xu, J.; Wang, P.; Kong, F.; Jin, J.; Yan, H.; Ge, X.; et al. Du13 encodes a C2H2 zinc-finger protein that regulates Wxb pre-mRNA splicing and microRNA biogenesis in rice endosperm. Plant Biotechnol. J. 2022, 20, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yu, H.; Xia, S.; Cui, Y.; Yu, X.; Liu, H.; Zeng, D.; Hu, J.; Zhang, Q.; Gao, Z.; et al. The C2H2 zinc-finger protein LACKING RUDIMENTARY GLUME 1 regulates spikelet development in rice. Sci. Bull. 2020, 65, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Liu, L.L.; Wang, C.; Gan, T.; Zhang, Z.; Wang, Y.; Wang, D.; Niu, M.; Long, W.; et al. Isolation and characterization of a spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice. Sci. Rep. 2017, 7, 41846. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, W.; Mohammadi, M.; He, Z.; She, Z.; Yan, M.; Shi, C.; Lin, L.; Wang, A.; Liu, J.; et al. OsDDM1b controls grain size by influencing cell cycling and regulating homeostasis and signaling of brassinosteroid in rice. Front. Plant Sci. 2022, 13, 873993. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qin, P.; Hu, L.; Zhan, S.; Wang, S.; Gao, P.; Li, J.; Jin, M.; Xu, Z.; Gao, Q.; et al. OsSPL18 controls grain weight and grain number in rice. J. Genet. Genom. 2019, 46, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, C.; Lin, H.; Wang, J.; Xue, H. Rice SPL12 coevolved with GW5 to determine grain shape. Sci. Bull. 2021, 66, 2353–2357. [Google Scholar] [CrossRef]
- Shi, C.; Ren, Y.; Liu, L.; Wang, F.; Zhang, H.; Tian, P.; Pan, T.; Wang, Y.; Jing, R.; Liu, T.; et al. Ubiquitin specific protease 15 has an important role in regulating grain width and size in rice. Plant Physiol. 2019, 180, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Hirano, H. Function and diversification of MADS-box genes in rice. Sci. World J. 2006, 6, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Ma, S.; Xiao, Z.; Li, F.; Wei, X.; Lin, S.; Wang, X.; Ji, Z.; Fu, Y.; Pan, J.; et al. Natural variations in grain length 10 (GL10) regulate rice grain size. J. Genet. Genom. 2022, 49, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, H.; Liu, J.; Wang, W.; Sun, J.; Gao, Q.; Zhang, Y.; Ma, D.; Wang, J.; Xu, Z.; et al. Loss of function of OsMADS34 leads to large sterile lemma and low grain yield in rice (Oryza sativa L.). Mol. Breed. 2016, 36, 147. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, N.; Xu, Z.; Xu, Q. Heterotrimeric G protein are involved in the regulation of multiple agronomic traits and stress tolerance in rice. BMC Plant Biology 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Lin, Q.; Zhou, C.; Ren, Y.; Liu, X.; Miao, R.; Jing, R.; Mou, C.; Nguyen, T.; Zhu, X.; et al. Small grain and semi-dwarf 3, a WRKY transcription factor, negatively regulates plant height and grain size by stabilizing SLR1 expression in rice. Plant Mol. Biol. 2020, 104, 429–450. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, S.; Chen, Y.; Mou, C.; Huang, Y.; Liu, X.; Ji, J.; Yu, J.; Hao, Q.; Yang, C.; et al. Decreased grain size1, a C3HC4-type RING protein, influences grain size in rice (Oryza sativa L.). Plant Mol. Biol. 2021, 105, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, W.; Deng, C.; Chen, X.; Gou, L.; Zhu, F.; Guo, W.; Zhang, J.; Wang, T. Flowering-Related RING Protein 1 (FRRP1) regulates flowering time and yield potential by affecting histone H2B monoubiquitination in rice (Oryza Sativa). PLoS ONE 2016, 11, e0150458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tan, J.; Chen, Y.; Sun, Z.; Yan, X.; Ouyang, J.; Li, S.; Wang, X. New fructokinase, OsFRK3, regulates starch accumulation and grain filling in rice. J. Agric. Food Chem. 2023, 71, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Yun, P.; Zhu, Y.; Wang, L.; Li, P.; Lou, G.; Xia, D.; Zhang, Q.; Xiao, J.; Li, X.; et al. Fine mapping of qTGW2b and qGL9, two minor QTL conferring grain size and weight in rice. Mol. Breed. 2022, 42, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Feng, Q.; Li, P.; Lou, G.; Chen, G.; Jiang, H.; Gao, G.; Zhang, Q.; Xiao, J.; Li, X.; et al. GLW7.1, a strong functional allele of Ghd7, enhances grain size in rice. Int. J. Mol. Sci. 2022, 23, 8715. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, W.; Wang, Y.; He, Q.; Shu, F.; Liu, H.; Wang, J.; Wang, J.; Yuan, L.; Deng, H. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J. Integr. Plant Biol. 2016, 58, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, M.; Yang, Y.; Li, J.; Zhu, L.; Jiang, D.; Dong, J.; Liu, Q.; Gu, L.; Zhou, L.; et al. RNase Z(S1) processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat. Commun. 2014, 5, 4884. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, Z.; Huang, C.; Duan, L.; Chen, G.; Jiang, N.; Fang, W.; Feng, H.; Xie, W.; Lian, X.; et al. Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat. Commun. 2014, 5, 5087. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.; Richard, O.; Bett, K.; Grimm, C.; Vinyard, B.; Webb, B.; McClung, A.; Barton, F.; Lyon, B.; Moldenhauer, K.; et al. Quality evaluation of U.S. medium-grain rice using a Japanese taste analyzer. Cereal Chem. 1996, 73, 290–294. [Google Scholar]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Panaud, O.; Chen, X.; McCouch, S. Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol. Gen. Genet. 1996, 252, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, S. Constructing Genetic Maps with MAPMAKER/EXP 3.0; Whitehead Institute: Cambridge, MA, USA, 1992. [Google Scholar]
- Silva Lda, C.; Wang, S.; Zeng, Z. Composite interval mapping and multiple interval mapping: Procedures and guidelines for using Windows QTL Cartographer. Methods Mol. Biol. 2012, 871, 75–119. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.; Wang, J.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
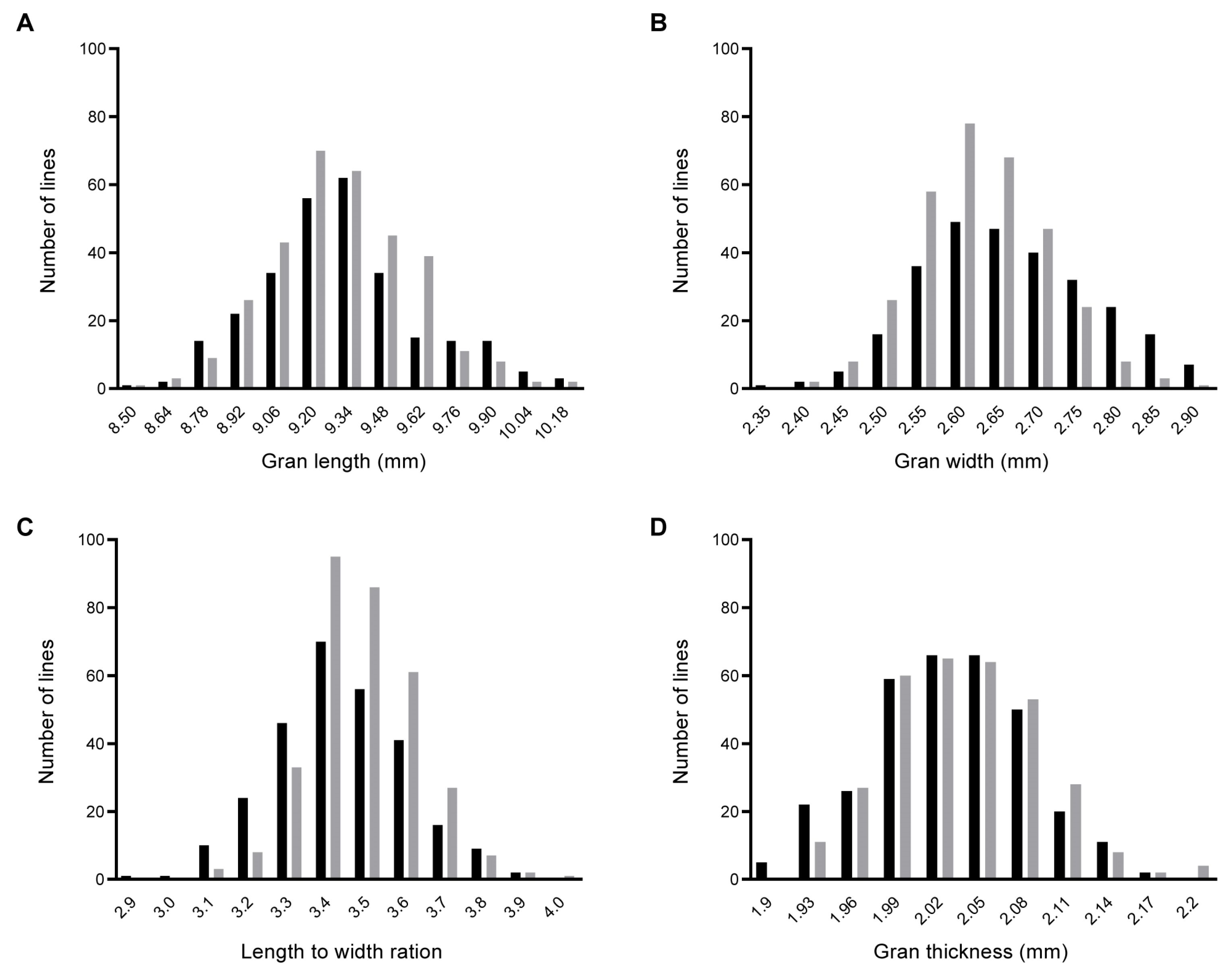
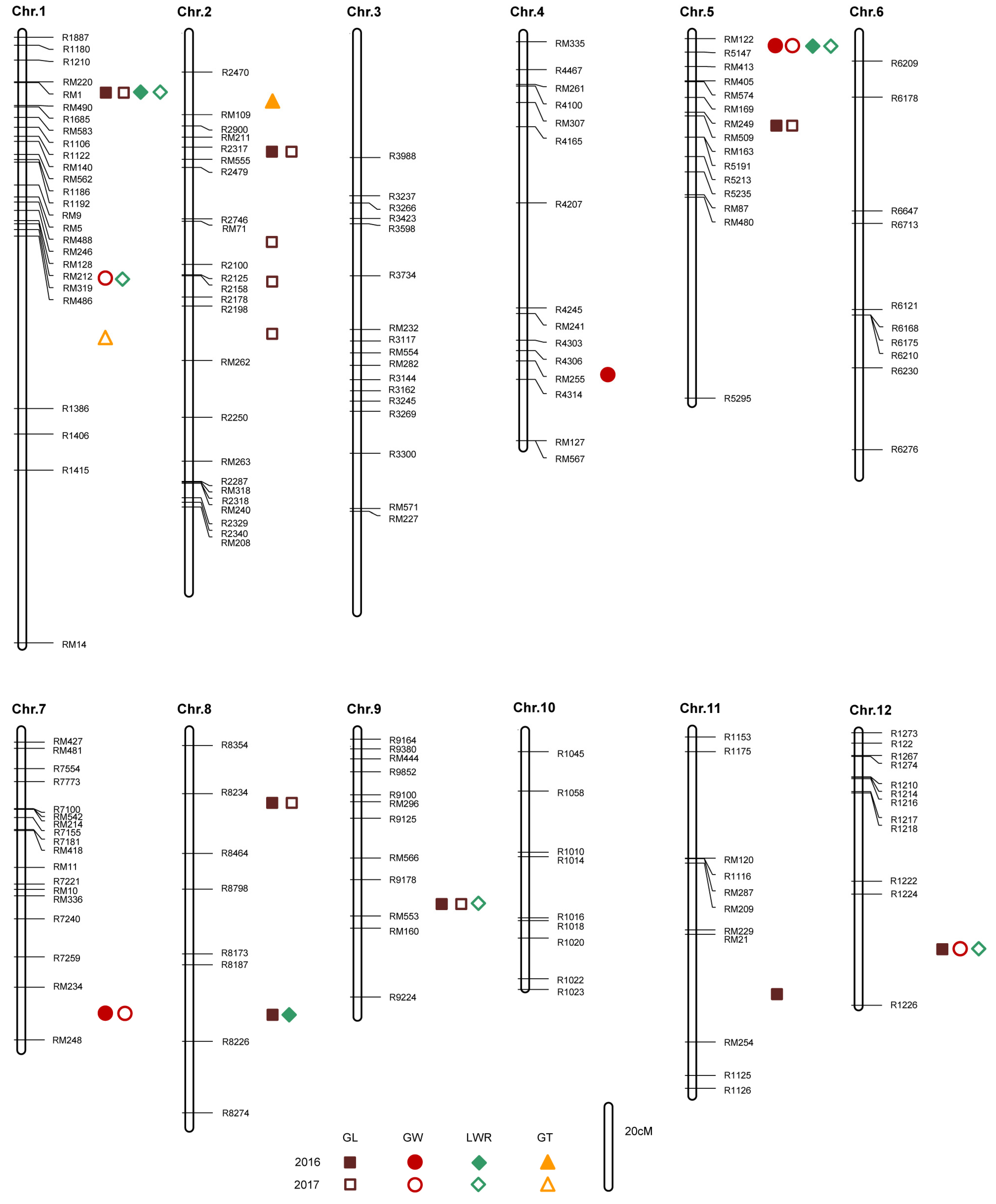
| QTL | Chromosome | Interval | 2016 | 2017 | Known Genes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOD | Add | PVE (%) | LOD | Add | PVE (%) | |||||
| qGL1 | 1 | RM220 | RM490 | 15.78 | 0.20 | 14.82 | 16.42 | 0.21 | 21.56 | OsCKX1 |
| qGL2.1 | 2 | RM211 | R2479 | 4.95 | −0.05 | 0.42 | 7.98 | −0.09 | 3.09 | |
| qGL2.2 | 2 | R2746 | R2100 | 2.60 | −0.21 | 14.04 | GW2 | |||
| qGL2.3 | 2 | R2100 | R2178 | 2.54 | −0.09 | 5.84 | ||||
| qGL2.4 | 2 | R2198 | RM262 | 3.62 | 0.07 | 8.92 | ||||
| qGL5 | 5 | RM169 | RM163 | 4.77 | −0.07 | 1.50 | 3.28 | −0.06 | 1.47 | |
| qGL8.1 | 8 | R8354 | R8464 | 2.73 | −0.05 | 0.24 | 4.00 | −0.06 | 1.81 | |
| qGL8.2 | 8 | R8173 | R8274 | 2.85 | −0.03 | 0.06 | ||||
| qGL9 | 9 | R9178 | RM160 | 5.66 | −0.13 | 11.87 | 9.07 | −0.15 | 12.26 | |
| qGL11 | 11 | RM21 | R1126 | 4.74 | −0.09 | 2.15 | ||||
| qGL12 | 12 | R1224 | R1226 | 4.46 | −0.06 | 6.04 | ||||
| qGW1 | 1 | RM128 | RM319 | 2.63 | 0.20 | 13.24 | ||||
| qGW4 | 4 | R4306 | R4314 | 4.82 | −0.04 | 5.71 | ||||
| qGW5 | 5 | RM122 | R5147 | 2.68 | 0.10 | 23.32 | 5.24 | 0.04 | 18.33 | |
| qGW7 | 7 | RM234 | RM248 | 4.15 | 0.04 | 6.27 | 2.68 | 0.07 | 15.01 | |
| qGW12 | 12 | R1224 | R1226 | 6.29 | −0.05 | 8.95 | ||||
| qLWR1.1 | 1 | RM220 | RM490 | 3.67 | 0.05 | 3.31 | 3.37 | 0.04 | 2.72 | OsCKX1 |
| qLWR1.2 | 1 | RM128 | RM319 | 2.81 | −0.03 | 1.71 | ||||
| qLWR5 | 5 | RM122 | R5147 | 3.23 | −0.05 | 4.37 | 2.52 | −0.04 | 3.10 | |
| qLWR8 | 8 | R8173 | R8274 | 2.78 | −0.02 | 0.16 | ||||
| qLWR9 | 9 | R9178 | RM160 | 5.93 | −0.04 | 2.99 | ||||
| qLWR12 | 12 | R1224 | R1226 | 4.02 | 0.04 | 8.81 | ||||
| qGT1 | 1 | RM319 | R1386 | 3.58 | −0.01 | 7.67 | ||||
| qGT2 | 2 | R2470 | RM211 | 7.20 | −0.03 | 21.23 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, H.; Liu, E.; Liu, R.; Alam, M.; Gao, H.; Gao, G.; Zhang, Q.; Li, Y.; Xiong, L.; et al. Fine Mapping of Five Grain Size QTLs Which Affect Grain Yield and Quality in Rice. Int. J. Mol. Sci. 2024, 25, 4149. https://doi.org/10.3390/ijms25084149
Zhou Y, Yang H, Liu E, Liu R, Alam M, Gao H, Gao G, Zhang Q, Li Y, Xiong L, et al. Fine Mapping of Five Grain Size QTLs Which Affect Grain Yield and Quality in Rice. International Journal of Molecular Sciences. 2024; 25(8):4149. https://doi.org/10.3390/ijms25084149
Chicago/Turabian StyleZhou, Yin, Hanyuan Yang, Enyu Liu, Rongjia Liu, Mufid Alam, Haozhou Gao, Guanjun Gao, Qinglu Zhang, Yanhua Li, Lizhong Xiong, and et al. 2024. "Fine Mapping of Five Grain Size QTLs Which Affect Grain Yield and Quality in Rice" International Journal of Molecular Sciences 25, no. 8: 4149. https://doi.org/10.3390/ijms25084149






