Lack of Direct Effects of Neurotrophic Factors in an In Vitro Model of Neuroinflammation
Abstract
1. Introduction
2. Results
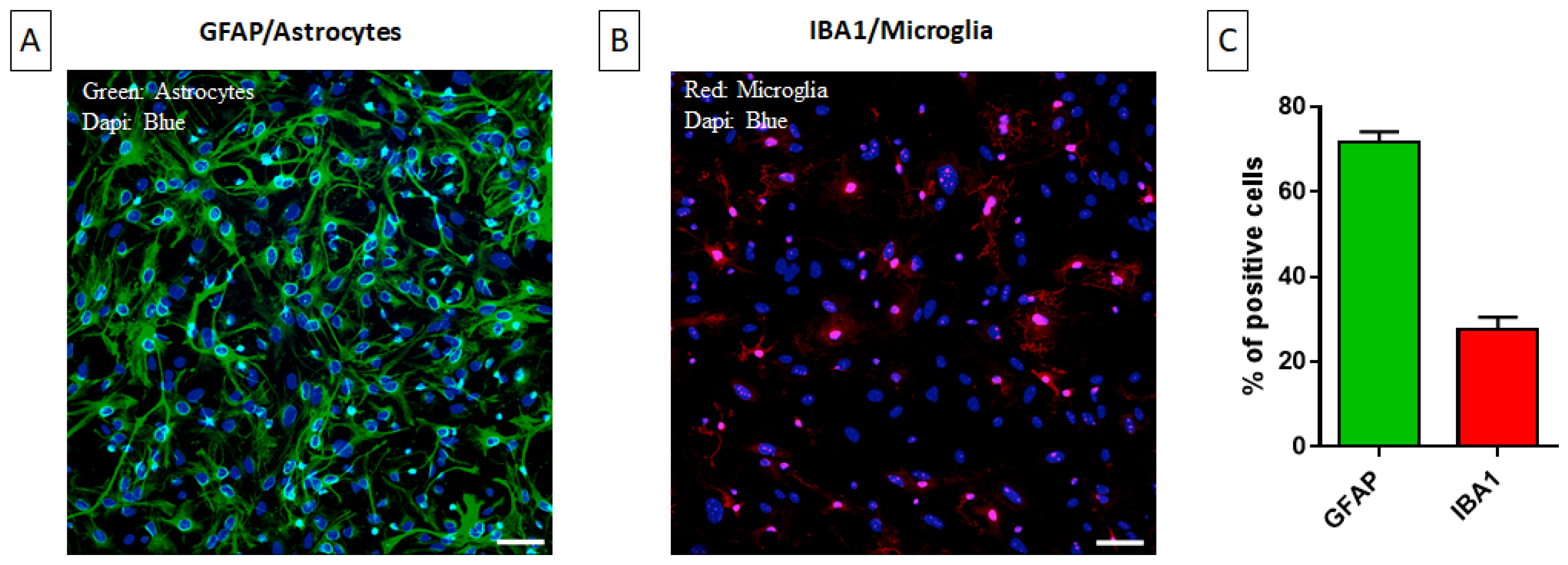
2.1. Characterization of the Primary Mixed Glial Culture
2.2. Setting up a Neuroinflammation In Vitro Model: Concentration- and Time-Dependent Effect of LPS on Cell Viability, Cytokine Level, and NO Production
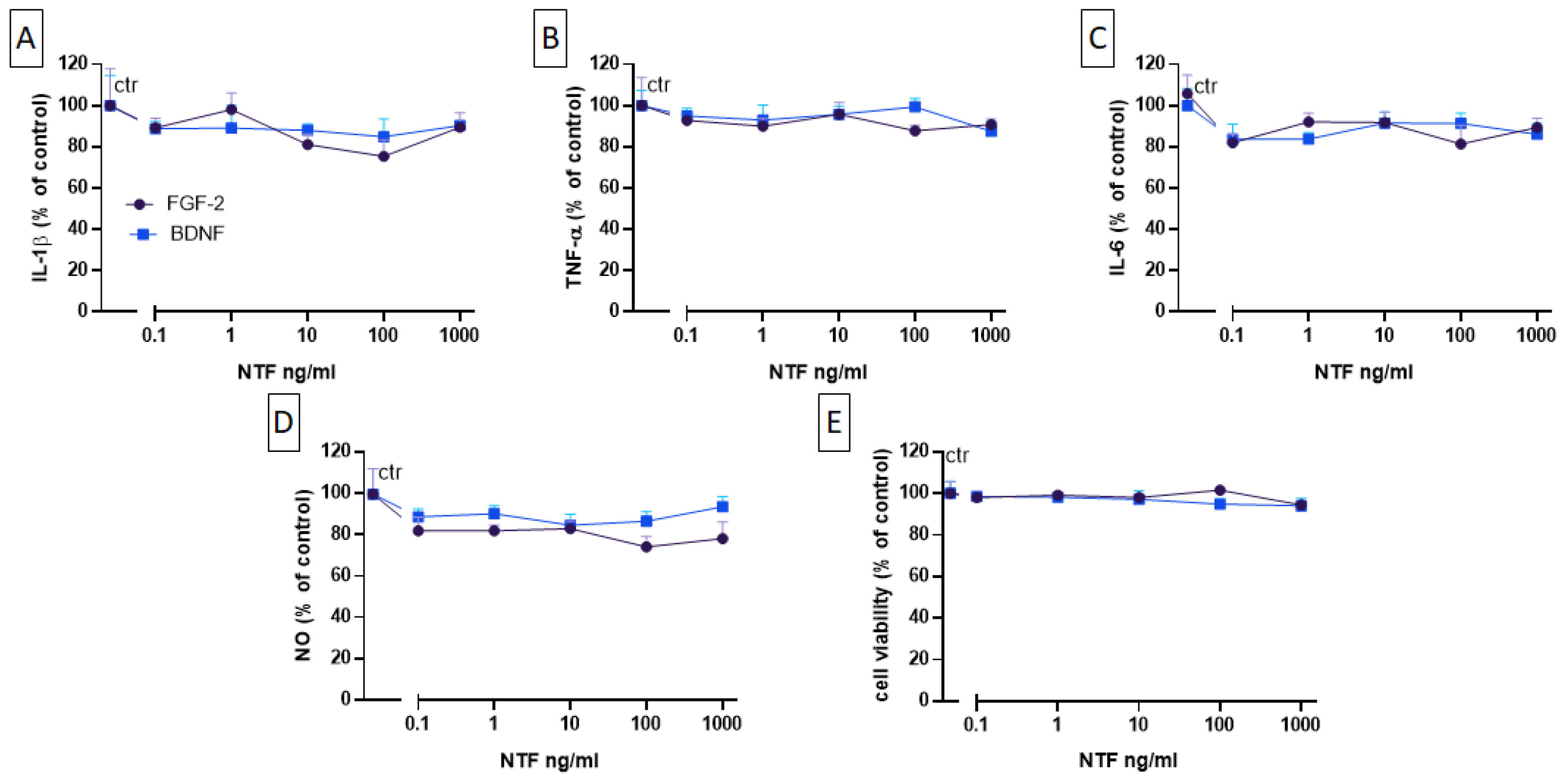
2.3. Effects of FGF-2 and BDNF on Cell Viability, Cytokine Level, and NO Production
2.4. Effects of FGF-2 and BDNF Co-Administration on Cell Viability, Cytokine Level, and NO Production
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Primary Mixed Glial Culture and Neuronal Culture
4.3. Treatments
4.4. Immunocytochemistry
4.5. MTT Assay
4.6. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Nitric Oxide Measurement
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation 2018, 15, 144. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Pracucci, E.; Pillai, V.; Lamers, D.; Parra, R.; Landi, S. Neuroinflammation: A signature or a cause of epilepsy? Int. J. Mol. Sci. 2021, 22, 6981. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Zucchini, S. Are the neurotrophic factors a suitable therapeutic target for the prevention of epileptogenesis? Epilepsia 2010, 51 (Suppl. S3), 48–51. [Google Scholar] [CrossRef] [PubMed]
- Schinder, A.F.; Poo, M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000, 23, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Simonato, M.; Tongiorgi, E.; Kokaia, M. Angels and demons: Neurotrophic factors and epilepsy. Trends Pharmacol. Sci. 2006, 27, 631–638. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, B.; Marconi, P.; Zucchini, S.; Berto, E.; Binaschi, A.; Bozac, A.; Buzzi, A.; Mazzuferi, M.; Magri, E.; Navarro Mora, G.; et al. Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proc. Natl. Acad. Sci. USA 2009, 106, 7191–7196. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, R.; Zucchini, S.; Paradiso, B.; Rodi, D.; Merigo, F.; Navarro Mora, G.; Osculati, F.; Berto, E.; Marconi, P.; Marzola, A.; et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J. Neuroinflammation 2010, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.; Hudson, P.; Wilson, B.; Haddon, L.; Hong, J.S. Influence of neurons on lipopolysaccharide-stimulated production of nitric oxide and tumor necrosis factor-alpha by cultured glia. Brain Res. 2000, 853, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Dollé, J.-P.; Rezvan, A.; Allen, F.D.; Lazarovici, P.; Lelkes, P.I. Nerve growth factor-induced migration of endothelial cells. J. Pharmacol. Exp. Ther. 2005, 315, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive astrocytes in neurodegenerative diseases. Aging Dis. 2019, 10, 664–675. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk between astrocytes and microglia: An overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Solà, C.; Casal, C.; Tusell, J.M.; Serratosa, J. Astrocytes enhance lipopolysaccharide-induced nitric oxide production by microglial cells. Eur. J. Neurosci. 2002, 16, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, P.; Forni, P.E.; Carbone, E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur. J. Neurosci. 2000, 12, 4017–4032. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Shindel, A.W.; Fandel, T.M.; Bella, A.J.; Lin, C.-S.; Lue, T.F. Neurotrophic effects of brain-derived neurotrophic factor and vascular endothelial growth factor in major pelvic ganglia of young and aged rats. BJU Int. 2010, 105, 114–120. [Google Scholar] [CrossRef]
- Hollborn, M.; Jahn, K.; Limb, G.A.; Kohen, L.; Wiedemann, P.; Bringmann, A. Characterization of the basic fibroblast growth factor-evoked proliferation of the human Müller cell line, MIO-M1. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Andres, R.H.; Fukuhara, T.; Bieri, G.; Hasegawa-Moriyama, M.; He, Y.; Guzman, R.; Wyss-Coray, T. Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci. 2012, 15, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Bettegazzi, B.; Bellani, S.; Cattaneo, S.; Codazzi, F.; Grohovaz, F.; Zacchetti, D. Gα13 Contributes to LPS-Induced Morphological Alterations and Affects Migration of Microglia. Mol. Neurobiol. 2021, 58, 6397–6414. [Google Scholar] [CrossRef] [PubMed]
- Barres, B.A. New roles for glia. J. Neurosci. 1991, 11, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, C.; Di Liddo, R.; Facci, L.; Bertalot, T.; Conconi, M.T.; Zusso, M.; Skaper, S.D.; Giusti, P. Ligand engagement of Toll-like receptors regulates their expression in cortical microglia and astrocytes. J. Neuroinflammation 2015, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, Z.; Ren, W.; Jiang, W. Acute lipopolysaccharide exposure facilitates epileptiform activity via enhanced excitatory synaptic transmission and neuronal excitability in vitro. Neuropsychiatr. Dis. Treat. 2014, 10, 1489–1495. [Google Scholar] [CrossRef]
- Patrizio, M.; Levi, G. Glutamate production by cultured microglia: Differences between rat and mouse, enhancement by lipopolysaccharide and lack effect of HIV coat protein gp120 and depolarizing agents. Neurosci. Lett. 1994, 178, 184–189. [Google Scholar] [CrossRef]
- Wang, Y.S.; White, T.D. The bacterial endotoxin lipopolysaccharide causes rapid inappropriate excitation in rat cortex. J. Neurochem. 1999, 72, 652–660. [Google Scholar] [CrossRef]
- Mahfoz, A.M.; Shahzad, N. Neuroinflammation impact in epileptogenesis and new treatment strategy. Behav. Pharmacol. 2019, 30, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-R.; Liu, J.-C.; Bao, J.-S.; Bai, Q.-Q.; Wang, G.-Q. Interaction of microglia and astrocytes in the neurovascular unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Yin, R.; Zhao, S.; Qiu, C. Brain-derived neurotrophic factor fused with a collagen-binding domain inhibits neuroinflammation and promotes neurological recovery of traumatic brain injury mice via TrkB signalling. J. Pharm. Pharmacol. 2020, 72, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-J.; Shi, M.; Xiao, L.-J.; Li, L.; Zou, L.-H.; Li, C.-Y.; Zhang, Q.-J.; Zhou, L.-F.; Ji, X.-C.; Huang, H.; et al. Inhibitive Effects of FGF2/FGFR1 Pathway on Astrocyte-Mediated Inflammation in vivo and in vitro After Infrasound Exposure. Front. Neurosci. 2018, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, E.; Dreier, J.P.; Ivens, S.; Bechmann, I.; Tomkins, O.; Heinemann, U.; Friedman, A. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004, 24, 7829–7836. [Google Scholar] [CrossRef] [PubMed]
- Ivens, S.; Kaufer, D.; Flores, L.P.; Bechmann, I.; Zumsteg, D.; Tomkins, O.; Seiffert, E.; Heinemann, U.; Friedman, A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 2007, 130, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-promoting effect of blood-brain barrier disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Iñigo, P.M.; Mestre, L.; Hernangómez, M.; Borrell, J.; Guaza, C. An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: A beginners approach. Protoc. Exch. 2011. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Dong, H.; Zhang, S.; Sun, J.; Qian, Y. Lithium Ameliorates LPS-Induced Astrocytes Activation Partly via Inhibition of Toll-Like Receptor 4 Expression. Cell Physiol. Biochem. 2016, 38, 714–725. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, N.; Ruzza, C.; Falcicchia, C.; Guarino, A.; Soukupova, M.; Asth, L.; Aleotti, V.; Bettegazzi, B.; Simonato, M.; Zucchini, S. Lack of Direct Effects of Neurotrophic Factors in an In Vitro Model of Neuroinflammation. Int. J. Mol. Sci. 2024, 25, 4160. https://doi.org/10.3390/ijms25084160
Aziz N, Ruzza C, Falcicchia C, Guarino A, Soukupova M, Asth L, Aleotti V, Bettegazzi B, Simonato M, Zucchini S. Lack of Direct Effects of Neurotrophic Factors in an In Vitro Model of Neuroinflammation. International Journal of Molecular Sciences. 2024; 25(8):4160. https://doi.org/10.3390/ijms25084160
Chicago/Turabian StyleAziz, Nimra, Chiara Ruzza, Chiara Falcicchia, Annunziata Guarino, Marie Soukupova, Laila Asth, Valentina Aleotti, Barbara Bettegazzi, Michele Simonato, and Silvia Zucchini. 2024. "Lack of Direct Effects of Neurotrophic Factors in an In Vitro Model of Neuroinflammation" International Journal of Molecular Sciences 25, no. 8: 4160. https://doi.org/10.3390/ijms25084160
APA StyleAziz, N., Ruzza, C., Falcicchia, C., Guarino, A., Soukupova, M., Asth, L., Aleotti, V., Bettegazzi, B., Simonato, M., & Zucchini, S. (2024). Lack of Direct Effects of Neurotrophic Factors in an In Vitro Model of Neuroinflammation. International Journal of Molecular Sciences, 25(8), 4160. https://doi.org/10.3390/ijms25084160






