CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics
Abstract
:1. Introduction
2. Results
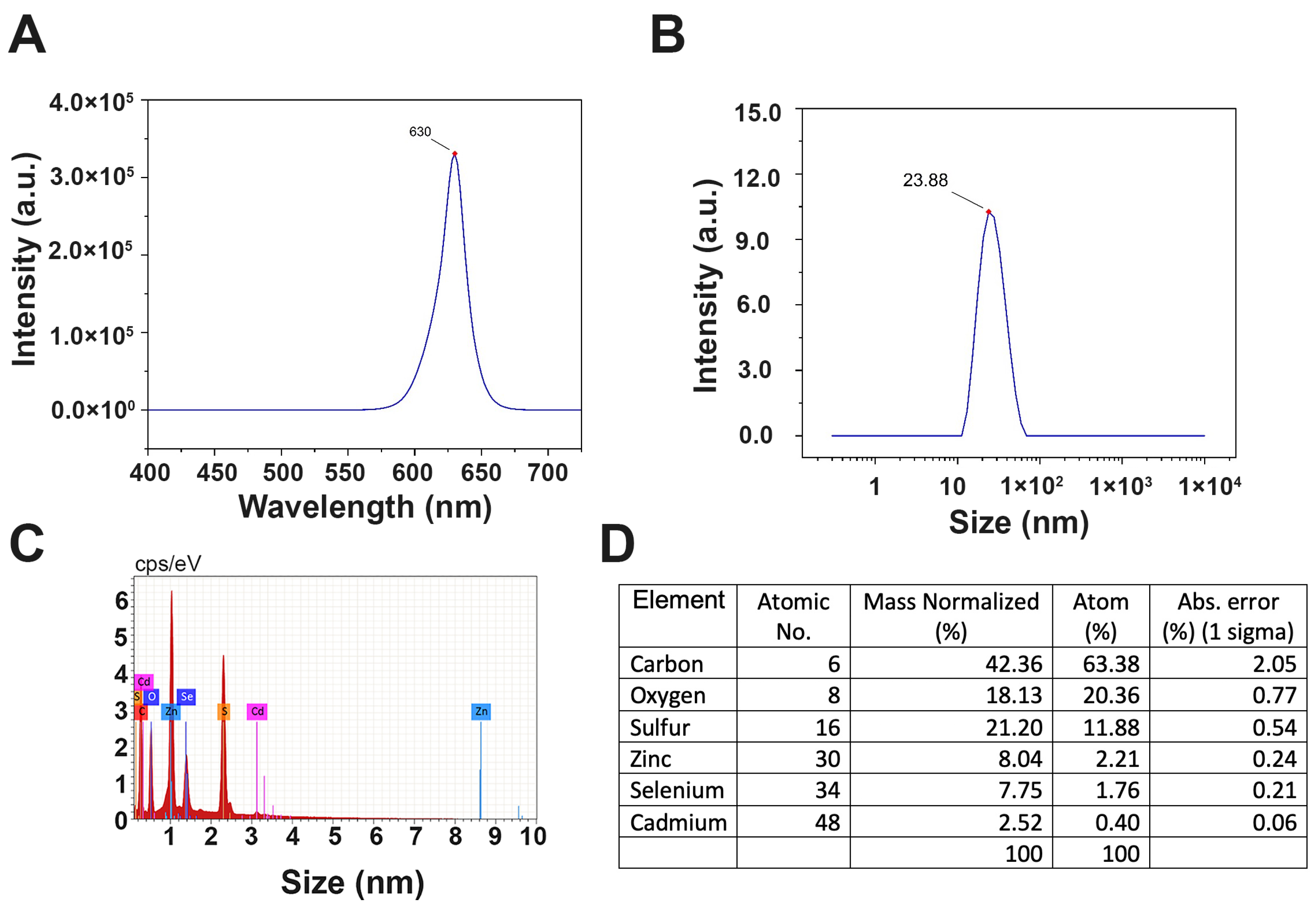
2.1. CdSe/ZnS QDs Characterization Using Fluorometer Emission, Dynamic Light Scattering (DLS), and Energy Dispersive X-ray Spectroscopy (EDS)
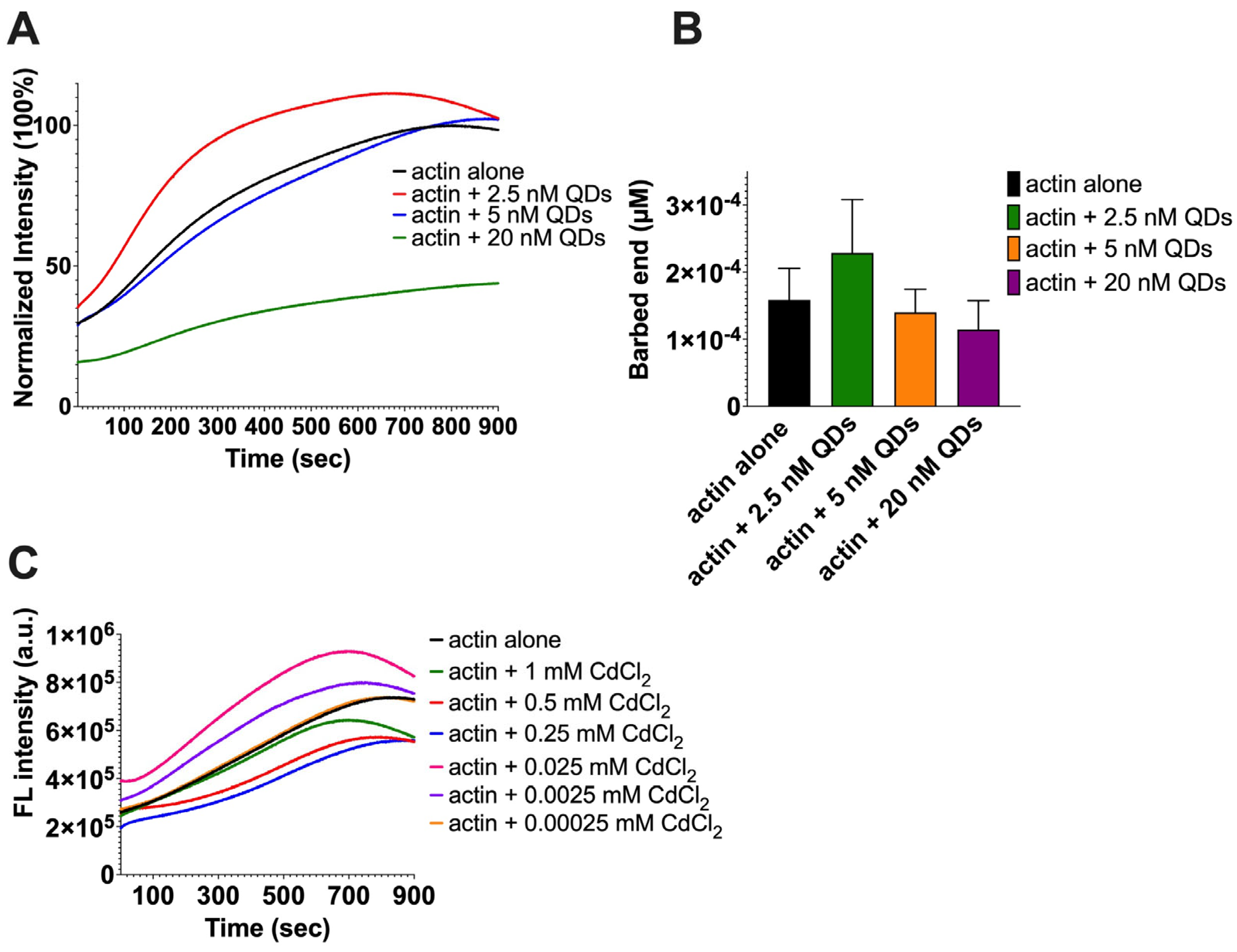
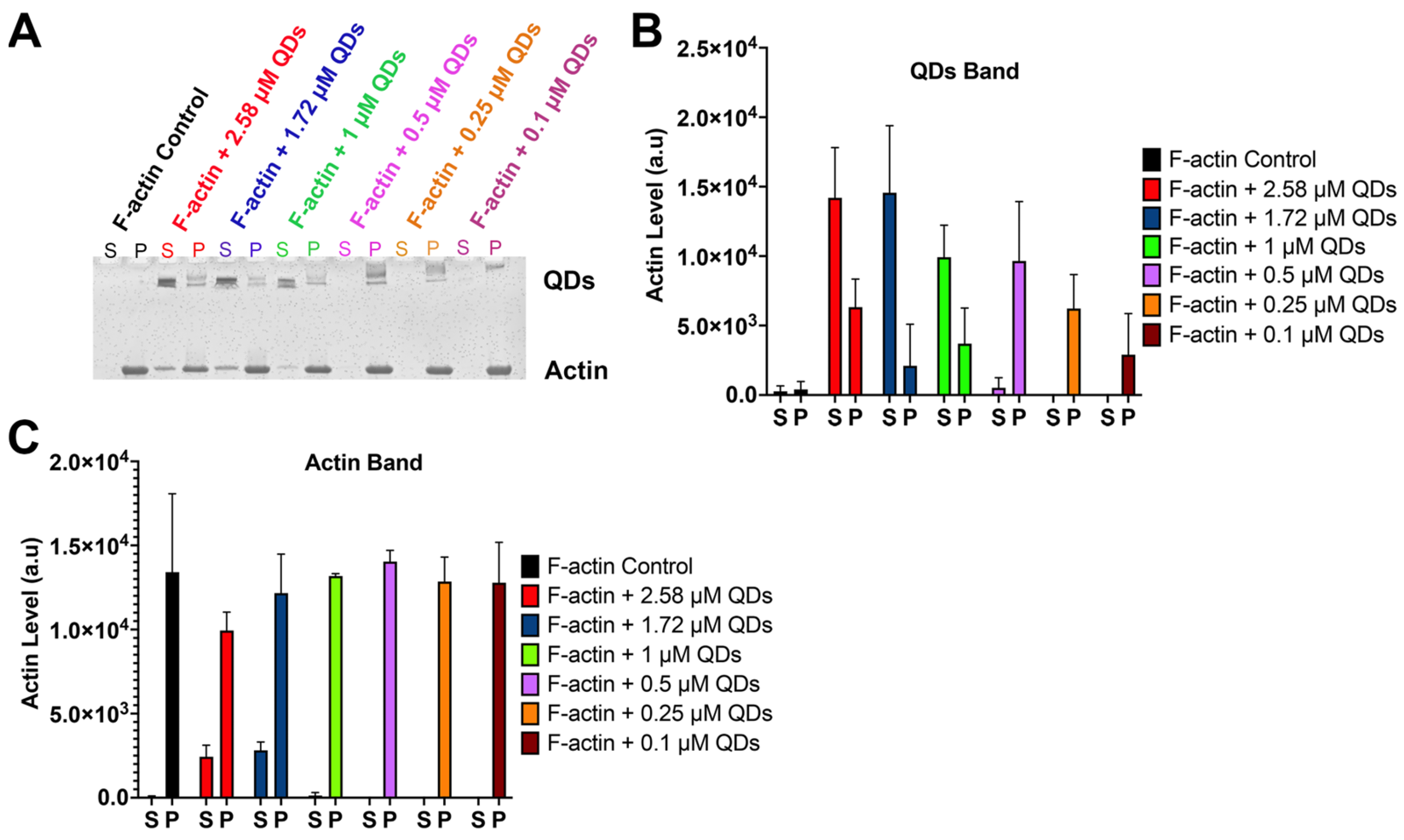
2.2. The Impact of CdSe/ZnS QDs on Actin Polymerization
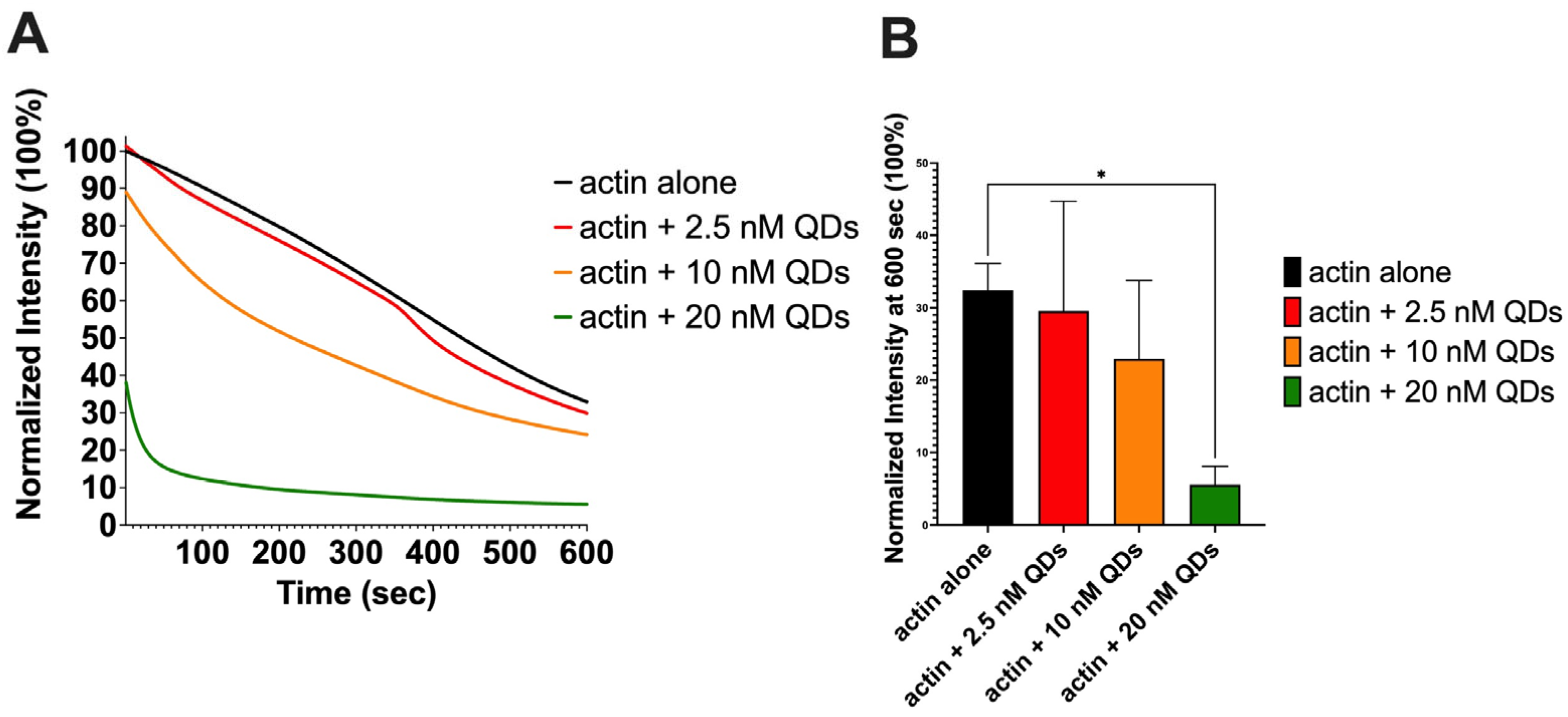
2.3. The Impact of CdSe/ZnS QDs on Actin Depolymerization
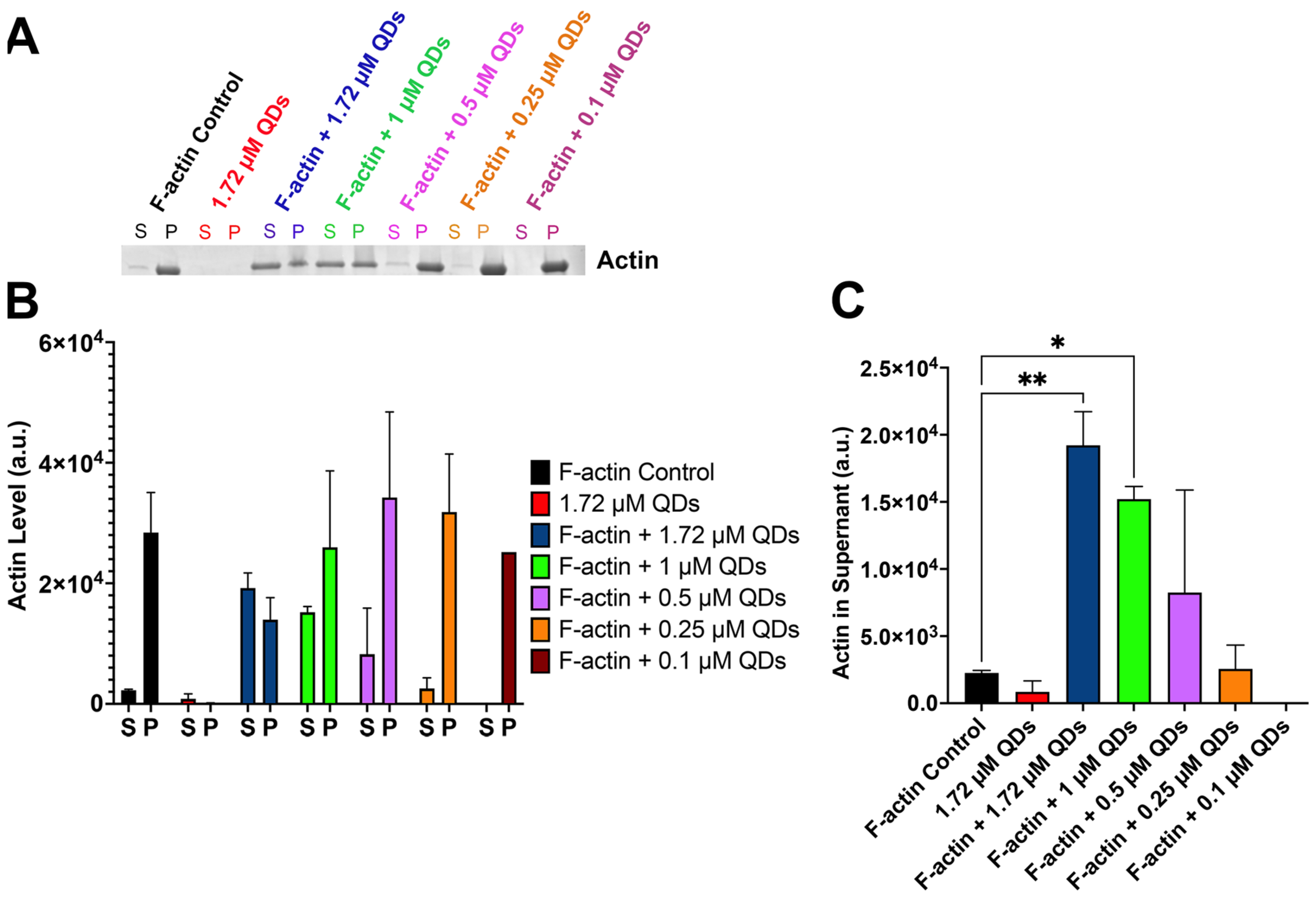
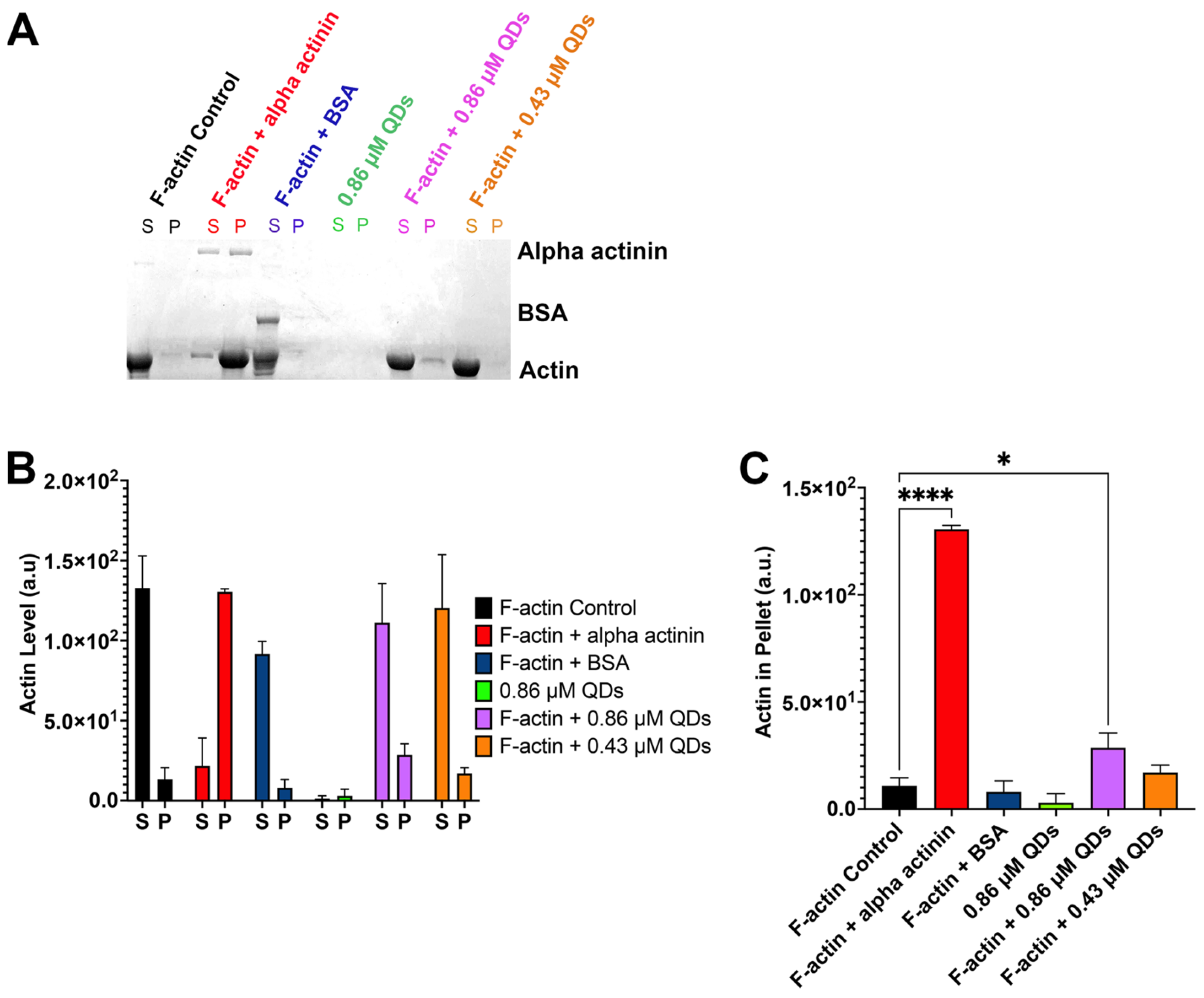
2.4. CdSe/ZnS QDs Can Bind to Filamentous Actin
2.5. CdSe/ZnS QDs Cause Actin Bundling
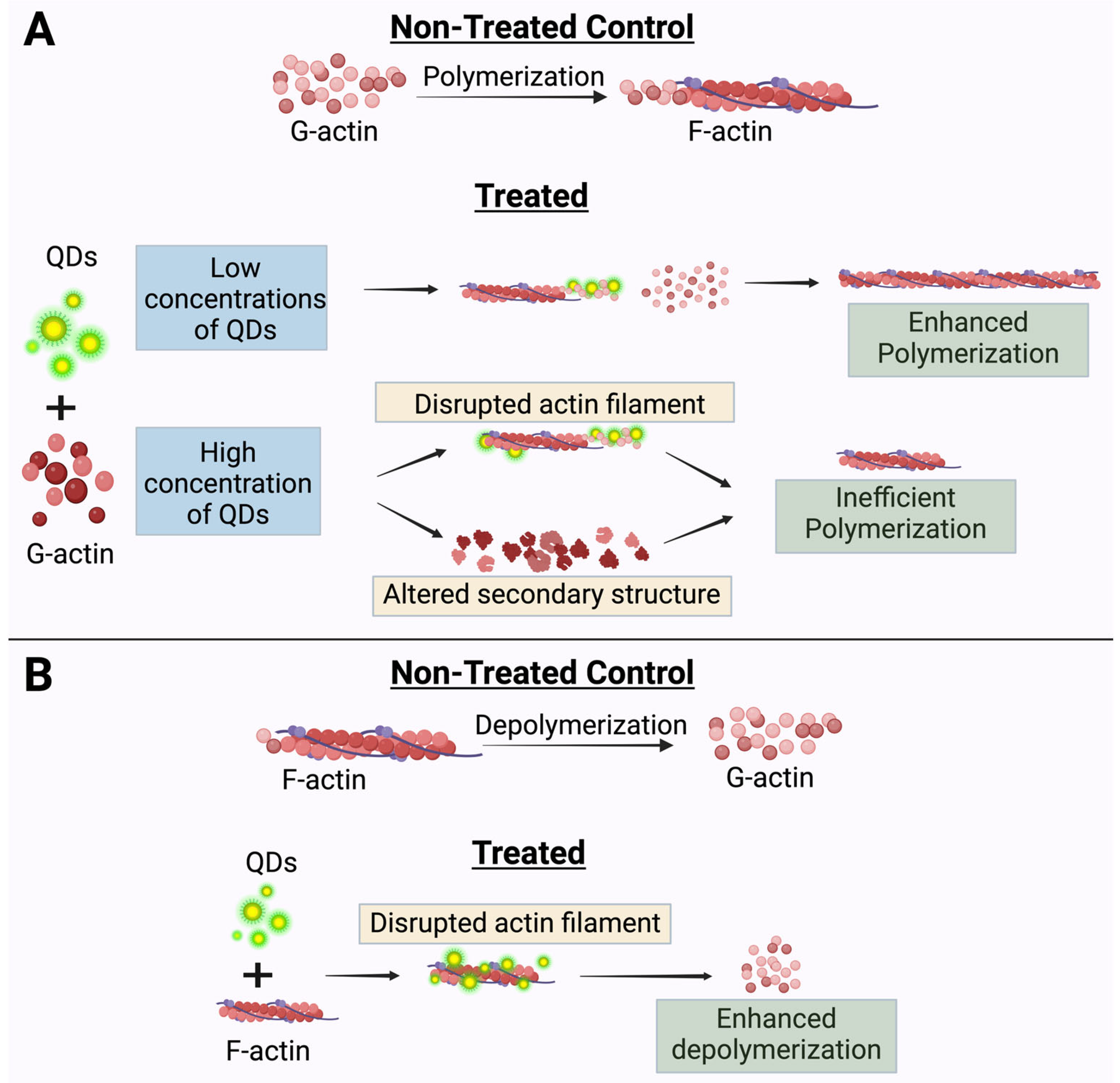
3. Discussion
4. Materials and Methods
4.1. QdSe/ZnS QDs Characterization
4.2. Actin Preparation
4.3. Assessment of Actin Polymerization by Spin-Down Assay
4.4. Assessment of Actin Polymerization by Fluorometer-Based Assay
4.5. Assessment of Actin Depolymerization by Fluorometer-Based Assay
4.6. Examine the Ability of QDs to Bind to F-Actin by Spin-Down Assay
4.7. Examine the Ability of QDs to Cause Actin Bundling by Spin-Down Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhang, R.; Li, J.; Sang, Y.; Tang, W.; Gil, P.R.; Liu, H. Fluorescent Graphene Quantum Dots as Traceable, PH-Sensitive Drug Delivery Systems. Int. J. Nanomed. 2015, 10, 6709–6724. [Google Scholar] [CrossRef]
- Massignani, M.; Canton, I.; Sun, T.; Hearnden, V.; MacNeil, S.; Blanazs, A.; Armes, S.P.; Lewis, A.; Battaglia, G. Enhanced Fluorescence Imaging of Live Cells by Effective Cytosolic Delivery of Probes. PLoS ONE 2010, 5, e10459. [Google Scholar] [CrossRef] [PubMed]
- Bagalkot, V.; Zhang, L.; Levy-Nissenbaum, E.; Jon, S.; Kantoff, P.W.; Langery, R.; Farokhzad, O.C. Quantum Dot-Aptamer Conjugates for Synchronous Cancer Imaging, Therapy, and Sensing of Drug Delivery Based on Bi-Fluorescence Resonance Energy Transfer. Nano Lett. 2007, 7, 3065–3070. [Google Scholar] [CrossRef] [PubMed]
- Gugula, K.; Szydlo, A.; Stegemann, L.; Strassert, C.A.; Bredol, M. Photobleaching-Resistant Ternary Quantum Dots Embedded in a Polymer-Coated Silica Matrix. J. Mater. Chem. C Mater. 2016, 4, 5263–5269. [Google Scholar] [CrossRef]
- Yu, C.; Xuan, T.; Chen, Y.; Zhao, Z.; Liu, X.; Lian, G.; Li, H. Gadolinium-Doped Carbon Dots with High Quantum Yield as an Effective Fluorescence and Magnetic Resonance Bimodal Imaging Probe. J. Alloys Compd. 2016, 688, 611–619. [Google Scholar] [CrossRef]
- Imran, A.; Jiang, J.; Eric, D.; Yousaf, M. Size and Shape Dependent Optical Properties of InAs Quantum Dots. In Proceedings of the International Conference on Optical Instruments and Technology 2017, Beijing, China, 28–30 October 2017; Volume 10622, pp. 54–62. [Google Scholar] [CrossRef]
- Tekle, C.; Van Deurs, B.; Sandvig, K.; Iversen, T.G. Cellular Trafficking of Quantum Dot-Ligand Bioconjugates and Their Induction of Changes in Normal Routing of Unconjugated Ligands. Nano Lett. 2008, 8, 1858–1865. [Google Scholar] [CrossRef]
- Abdul Ghani, S.F.; Wright, M.; Paramo, J.G.; Bottrill, M.; Green, M.; Long, N.; Thanou, M. Three Bisphosphonate Ligands Improve the Water Solubility of Quantum Dots. Faraday Discuss. 2014, 175, 153–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Schnoes, A.M.; Clapp, A.R. Dithiocarbamates as Capping Ligands for Water-Soluble Quantum Dots. ACS Appl. Mater. Interfaces 2010, 2, 3384–3395. [Google Scholar] [CrossRef]
- Tan, Y.; Jin, S.; Hamers, R.J. Photostability of Cdse Quantum Dots Functionalized with Aromatic Dithiocarbamate Ligands. ACS Appl. Mater. Interfaces 2013, 5, 12975–12983. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.J.; Jana, N.R.; Gao, S.; Patra, P.K.; Ying, J.Y. Surface-Ligand-Dependent Cellular Interaction, Subcellular Localization, and Cytotoxicity of Polymer-Coated Quantum Dots. Chem. Mater. 2010, 22, 2239–2247. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Chen, W.Q.; Li, X.H.; Liu, M.; He, X.H.; Liu, Y.; Jiang, F.L. Quantum Dots Meet Enzymes: Hydrophobicity of Surface Ligands and Size Do Matter. Langmuir 2022, 39, 3967–3978. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajaj, N.A.; Moquin, A.; Neibert, K.D.; Soliman, G.M.; Winnik, F.M.; Maysinger, D. Short Ligands Affect Modes of QD Uptake and Elimination in Human Cells. ACS Nano 2023, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Bottrill, M.; Green, M. Some Aspects of Quantum Dot Toxicity. Chem. Commun. 2011, 47, 7039–7050. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lai, L.; Tian, F.; Jiang, F.L.; Xiao, Q.; Li, Y.; Yu, Q.; Li, D.; Wang, J.; Zhang, Q.; et al. Toxicity of CdTe Quantum Dots on Yeast Saccharomyces Cerevisiae. Small 2012, 8, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Developmental Toxicity of CdTe QDs in Zebrafish Embryos and Larvae. J. Nanoparticle Res. 2013, 15, 1700. [Google Scholar] [CrossRef]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Horstmann, C.; Davenport, V.; Zhang, M.; Peters, A.; Kim, K. Transcriptome Profile Alterations with Carbon Nanotubes, Quantum Dots, and Silver Nanoparticles: A Review. Genes 2021, 12, 794. [Google Scholar] [CrossRef]
- Zhang, M.; Kim, D.S.; Patel, R.; Wu, Q.; Kim, K. Intracellular Trafficking and Distribution of Cd and InP Quantum Dots in HeLa and ML-1 Thyroid Cancer Cells. Nanomaterials 2022, 12, 1517. [Google Scholar] [CrossRef]
- Horstmann, C.; Kim, K. Comparing Transcriptome Profiles of Saccharomyces Cerevisiae Cells Exposed to Cadmium Selenide/Zinc Sulfide and Indium Phosphide/Zinc Sulfide. Genes 2021, 12, 428. [Google Scholar] [CrossRef]
- Horstmann, C.; Kim, D.S.; Campbell, C.; Kim, K. Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces Cerevisiae. Biomolecules 2019, 9, 653. [Google Scholar] [CrossRef]
- Hens, B.; Smothers, J.; Rizvanovic, H.; Patel, R.; Wu, Q.; Kim, K. The Future of Anticancer Drugs: A Cytotoxicity Assessment Study of CdSe/ZnS Quantum Dots. J. Nanotheranostics 2020, 1, 19–38. [Google Scholar] [CrossRef]
- Le, N.; Routh, J.; Kirk, C.; Wu, Q.; Patel, R.; Keyes, C.; Kim, K. Red CdSe/ZnS QDs’ Intracellular Trafficking and Its Impact on Yeast Polarization and Actin Filament. Cells 2023, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Kim, K. Current Advances in the Biomedical Applications of Quantum Dots: Promises and Challenges. Int. J. Mol. Sci. 2023, 24, 12682. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Chand, A.; Okafor, O.; Kim, K. The Impact of Cadmium Selenide Zinc Sulfide Quantum Dots on the Proteomic Profile of Saccharomyces Cerevisiae. Int. J. Mol. Sci. 2023, 24, 16332. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhong, H.; He, Z. Toxicity Evaluation of Cadmium-Containing Quantum Dots: A Review of Optimizing Physicochemical Properties to Diminish Toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111609. [Google Scholar] [CrossRef]
- Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Willmore, W.G. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol. Sci. 2015, 146, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, M. The Cytotoxicity of Core-Shell or Non-Shell Structure Quantum Dots and Reflection on Environmental Friendly: A Review. Environ. Res. 2021, 194, 110593. [Google Scholar] [CrossRef]
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core-Shell Quantum Dots: Properties and Applications. J. Alloys Compd. 2015, 636, 395–404. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Lai, L.; Li, D.W.; Li, R.; Xiang, C.; Jiang, F.L.; Sun, S.F.; Liu, Y. Toxicity of CdTe QDs with Different Sizes Targeted to HSA Investigated by Two Electrochemical Methods. Mol. Biol. Rep. 2013, 40, 1009–1019. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of Carbon Dots-Effect of Surface Functionalization on the Cell Viability, Reactive Oxygen Species Generation and Cell Cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Havrdová, M.; Urbančič, I.; Tománková, K.B.; Malina, L.; Poláková, K.; Štrancar, J.; Bourlinos, A.B. Intracellular Trafficking of Cationic Carbon Dots in Cancer Cell Lines MCF-7 and HeLa—Time Lapse Microscopy, Concentration-Dependent Uptake, Viability, DNA Damage, and Cell Cycle Profile. Int. J. Mol. Sci. 2022, 23, 1077. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.M. Quantum Dots in Cell Biology. J. Histochem. Cytochem. 2011, 59, 237. [Google Scholar] [CrossRef] [PubMed]
- Zdobnova, T.A.; Lebedenko, E.N.; Deyev, S.M. Quantum Dots for Molecular Diagnostics of Tumors. Acta Naturae 2011, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Chand, A.; Braun, E.; Keyes, C.; Wu, Q.; Kim, K. Interactions between Quantum Dots and G-Actin. Int. J. Mol. Sci. 2023, 24, 14760. [Google Scholar] [CrossRef]
- Joglekar, S.S.; Gholap, H.M.; Alegaonkar, P.S.; Kale, A.A. The Interactions between CdTe Quantum Dots and Proteins: Understanding Nano-Bio Interface. AIMS Mater. Sci. 2017, 4, 209–222. [Google Scholar] [CrossRef]
- Hu, Y.; Li, H.; Meng, P.; Li, K.; Xiong, Y.; Zhang, S.; Yang, Y.; Yin, A.; Huang, P. Interactions between CdTe Quantum Dots and Plasma Proteins: Kinetics, Thermodynamics and Molecular Structure Changes. Colloids Surf. B Biointerfaces 2020, 189, 110881. [Google Scholar] [CrossRef]
- Dominguez, R.; Holmes, K.C. Actin Structure and Function. Annu. Rev. Biophys. 2011, 40, 169. [Google Scholar] [CrossRef]
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin Assembly Mechanisms at a Glance. J. Cell Sci. 2017, 130, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Eskin, J.A.; Wendland, B. Actin and Endocytosis in Budding Yeast. Genetics 2014, 199, 315–358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Dominguez, R. Regulation of Actin Cytoskeleton Dynamics in Cells. Mol. Cells 2010, 29, 311. [Google Scholar] [CrossRef] [PubMed]
- Galletta, B.J.; Mooren, O.L.; Cooper, J.A. Actin Dynamics and Endocytosis in Yeast and Mammals. Curr. Opin. Biotechnol. 2010, 21, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Nickaeen, M.; Berro, J.; Pollard, T.D.; Slepchenko, B.M. Actin Assembly Produces Sufficient Forces for Endocytosis in Yeast. Mol. Biol. Cell 2019, 30, 2014–2024. [Google Scholar] [CrossRef] [PubMed]
- Moseley, J.B.; Goode, B.L. The Yeast Actin Cytoskeleton: From Cellular Function to Biochemical Mechanism. Microbiol. Mol. Biol. Rev. 2006, 70, 605–645. [Google Scholar] [CrossRef]
- Girao, H.; Geli, M.I.; Idrissi, F.Z. Actin in the Endocytic Pathway: From Yeast to Mammals. FEBS Lett. 2008, 582, 2112–2119. [Google Scholar] [CrossRef]
- Pruyne, D.; Bretscher, A. Polarization of Cell Growth in Yeast. II. The Role of the Cortical Actin Cytoskeleton. J. Cell Sci. 2000, 113, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Topf, A.; Franz, P.; Tsiavaliaris, G. Microscale Thermophoresis (MST) for Studying Actin Polymerization Kinetics. Biotechniques 2017, 63, 187–190. [Google Scholar] [CrossRef]
- Doolittle, L.K.; Rosen, M.K.; Padrick, S.B. Measurement and Analysis of in Vitro Actin Polymerization. Methods Mol. Biol. 2013, 1046, 273–293. [Google Scholar] [CrossRef]
- Shekhar, S.; Pernier, J.; Carlier, M.F. Regulators of Actin Filament Barbed Ends at a Glance. J. Cell Sci. 2016, 129, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Higgs, H.N.; Blanchoin, L.; Pollard, T.D. Influence of the C Terminus of Wiskott-Aldrich Syndrome Protein (WASp) and the Arp2/3 Complex on Actin Polymerization. Biochemistry 1999, 38, 15212–15222. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The Cytotoxicity of Cadmium-Based Quantum Dots. Biomaterials 2012, 33, 1238–1244. [Google Scholar] [CrossRef]
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-Containing Quantum Dots: Properties, Applications, and Toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733. [Google Scholar] [CrossRef] [PubMed]
- Rzigalinski, B.A.; Strobl, J.S. Cadmium-Containing Nanoparticles: Perspectives on Pharmacology and Toxicology of Quantum Dots. Toxicol. Appl. Pharmacol. 2009, 238, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Wu, S.B.; Wei, Y.H.; Chen, Y.C.; Tsai, M.H.; Ho, C.C.; Lin, S.Y.; Yang, C.S.; Lin, P. Cadmium-Based Quantum Dot Induced Autophagy Formation for Cell Survival via Oxidative Stress. Chem. Res. Toxicol. 2013, 26, 662–673. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Guallar, E.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Silbergeld, E.K.; Devereux, R.B.; Navas-Acien, A. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013, 24, 421–429. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Fang, C.; Wu, J.C.C.; Wu, C.J.; Dai, K.Y.; Chen, S.M. Neuroinflammation and Microglial Activation at Rostral Ventrolateral Medulla Underpin Cadmium-Induced Cardiovascular Dysregulation in Rats. J. Inflamm. Res. 2021, 14, 3863–3877. [Google Scholar] [CrossRef]
- Lin, H.C.; Hao, W.M.; Chu, P.H. Cadmium and Cardiovascular Disease: An Overview of Pathophysiology, Epidemiology, Therapy, and Predictive Value. Rev. Port. Cardiol. 2021, 40, 611–617. [Google Scholar] [CrossRef]
- James, K.A.; Meliker, J.R. Environmental Cadmium Exposure and Osteoporosis: A Review. Int. J. Public Health 2013, 58, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Lee, J.Y.; Tokumoto, M.; Satoh, M. Cadmium Renal Toxicity via Apoptotic Pathways. Biol. Pharm. Bull. 2012, 35, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Schwartz, G.G. Cadmium and Renal Cancer. Toxicol. Appl. Pharmacol. 2005, 207, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sung, G.H.; Lee, S.; Han, K.J.; Han, H.J. Serum Cadmium Is Associated with Hepatic Steatosis and Fibrosis: Korean National Health and Nutrition Examination Survey Data IV-VII. Medicine 2022, 101, e28559. [Google Scholar] [CrossRef] [PubMed]
- Goode, B.L.; Eskin, J.; Shekhar, S. Mechanisms of Actin Disassembly and Turnover. J. Cell Biol. 2023, 222, e202309021. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Kudryashov, D.S.; Reisler, E. Actin Bundles Dynamics and Architecture. Biomolecules 2023, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, N.; Park, J.; Kang, E.H. Regulation of Actin Bundle Mechanics and Structure by Intracellular Environmental Factors. Front. Phys. 2021, 9, 675885. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Structure and Organization of Actin Filaments. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Otto, J.J. Actin-Bundling Proteins. Curr. Opin. Cell Biol. 1994, 6, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, K.; Gu, W.; Dong, X.; Lei, R.; Chang, Y.; Bai, X.; Xia, S.; Zeng, L.; Zhang, J.; et al. Small Size Fullerenol Nanoparticles Suppress Lung Metastasis of Breast Cancer Cell by Disrupting Actin Dynamics. J. Nanobiotechnol. 2018, 16, 54. [Google Scholar] [CrossRef]
- Grady, M.E.; Parrish, E.; Caporizzo, M.A.; Seeger, S.C.; Composto, R.J.; Eckmann, D.M. Intracellular Nanoparticle Dynamics Affected by Cytoskeletal Integrity. Soft Matter 2017, 13, 1873. [Google Scholar] [CrossRef]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing Cytoskeleton by Engineered Nanomaterials for Enhanced Cancer Therapeutics. Bioact. Mater. 2023, 29, 50. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules and Actin Filaments: Dynamic Targets for Cancer Chemotherapy. Curr. Opin. Cell Biol. 1998, 10, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kravchuk, P.; Krishnaprasad, A.; Roy, T.; Kang, E.H. Graphene Enhances Actin Filament Assembly Kinetics and Modulates NIH-3T3 Fibroblast Cell Spreading. Int. J. Mol. Sci. 2022, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, Z.; Duan, G.; Wu, A.; Gu, Z.; Zhang, L.; Chen, C.; Chai, Z.; Ge, C.; Zhou, R. Graphene Oxide Nanosheets Retard Cellular Migration via Disruption of Actin Cytoskeleton. Small 2017, 13, 1602133. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.A.; Maftuch, M.; Kilawati, Y.; Risjani, Y. The Effect of Cadmium Exposure on the Cytoskeleton and Morphology of the Gill Chloride Cells in Juvenile Mosquito Fish (Gambusia Affinis). Egypt. J. Aquat. Res. 2019, 45, 337–343. [Google Scholar] [CrossRef]
- Apostolova, M.D.; Christova, T.; Templeton, D.M. Involvement of Gelsolin in Cadmium-Induced Disruption of the Mesangial Cell Cytoskeleton. Toxicol. Sci. 2006, 89, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Song, X.; Chen, L.; Hu, D.; Hua, L.; Cui, Y.; Liu, J.; An, Z.; Yin, Z.; Ning, H. Cadmium Induces Actin Cytoskeleton Alterations and Dysfunction in Neuro-2a Cells. Environ. Toxicol. 2019, 34, 469–475. [Google Scholar] [CrossRef] [PubMed]
- DalleDonne, I.; Milzani, A.; Colombo, R. Actin Assembly by Cadmium Ions. Biochim. Biophys. Acta 1997, 1357, 5–17. [Google Scholar] [CrossRef]
- Rogers, S.L.; Gelfand, V.I. Membrane Trafficking, Organelle Transport, and the Cytoskeleton. Curr. Opin. Cell Biol. 2000, 12, 57–62. [Google Scholar] [CrossRef]
- Henderson, C.A.; Gomez, C.G.; Novak, S.M.; Mi-Mi, L.; Gregorio, C.C. Overview of the Muscle Cytoskeleton. Compr. Physiol. 2017, 7, 891. [Google Scholar] [CrossRef]
- Kabaso, D.; Shlomovitz, R.; Schloen, K.; Stradal, T.; Gov, N.S. Theoretical Model for Cellular Shapes Driven by Protrusive and Adhesive Forces. PLoS Comput. Biol. 2011, 7, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, S.; Stringhi, R.; Marcello, E. Dendritic Spines in Alzheimer’s Disease: How the Actin Cytoskeleton Contributes to Synaptic Failure. Int. J. Mol. Sci. 2020, 21, 908. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, H.; Zhu, H.; Kakinen, A.; Wang, D.; Andrikopoulos, N.; Sun, Y.; Nandakumar, A.; Kwak, E.; Davis, T.P.; et al. Ultrasmall Molybdenum Disulfide Quantum Dots Cage Alzheimer’s Amyloid Beta to Restore Membrane Fluidity. ACS Appl. Mater. Interfaces 2021, 13, 29936–29948. [Google Scholar] [CrossRef]
- Nürnberg, A.; Kollmannsperger, A.; Grosse, R. Pharmacological Inhibition of Actin Assembly to Target Tumor Cell Motility. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 23–42. [Google Scholar] [CrossRef]
| Title | Author | Nanoparticle Type | Key Aspects | Citation |
|---|---|---|---|---|
| CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics. | Chand et al. | CdSe/ZnS QDs | QDs act in a biphasic manner, i.e., stimulate and inhibit polymerization, depending on their concentration. QDs also enhance depolymerization. | This study. |
| Interactions between Quantum Dots and G-Actin. | Le et al. | CdSe/ZnS QDs | QDs bind to G-actin and alter the secondary structure of actin. | [38] |
| Graphene Enhances Actin Filament Assembly Kinetics and Modulates NIH-3T3 Fibroblast Cell Spreading. | Park et al. | Graphene flakes | Graphene flakes increase actin filament elongation rate without hindering actin polymerization. | [75] |
| Graphene Oxide Nanosheets Retard Cellular Migration via Disruption of Actin Cytoskeleton. | Tian et al. | Graphene oxide nanosheets | GO bind to G-actin and alter the secondary structure. They also disrupt actin filaments. | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chand, A.; Le, N.; Kim, K. CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics. Int. J. Mol. Sci. 2024, 25, 4179. https://doi.org/10.3390/ijms25084179
Chand A, Le N, Kim K. CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics. International Journal of Molecular Sciences. 2024; 25(8):4179. https://doi.org/10.3390/ijms25084179
Chicago/Turabian StyleChand, Abhishu, Nhi Le, and Kyoungtae Kim. 2024. "CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics" International Journal of Molecular Sciences 25, no. 8: 4179. https://doi.org/10.3390/ijms25084179
APA StyleChand, A., Le, N., & Kim, K. (2024). CdSe/ZnS Quantum Dots’ Impact on In Vitro Actin Dynamics. International Journal of Molecular Sciences, 25(8), 4179. https://doi.org/10.3390/ijms25084179





