Abstract
Energy metabolism plays a pivotal role in the pathogenesis of endometriosis. For the initial stages of the disease in adolescents, this aspect remains unexplored. The objective of this paper was to analyze the association of cellular and endosomal profiles of markers of glycolysis, mitochondrial biogenesis, apoptosis, autophagy and estrogen signaling in peritoneal endometriosis (PE) in adolescents. We included 60 girls aged 13–17 years in a case–control study: 45 with laparoscopically confirmed PE (main group) and 15 with paramesonephric cysts (comparison group). Samples of plasma and peritoneal fluid exosomes, endometrioid foci and non-affected peritoneum were tested for estrogen receptor (Erα/β), hexokinase (Hex2), pyruvate dehydrogenase kinase (PDK1), glucose transporter (Glut1), monocarboxylate transporters (MCT1 and MCT2), optic atrophy 1 (OPA1, mitochondrial fusion protein), dynamin-related protein 1 (DRP1, mitochondrial fission protein), Bax, Bcl2, Beclin1, Bnip3, P38 mitogen-activated protein kinase (MAPK), hypoxia-inducible factor 1 (Hif-1α), mitochondrial voltage-dependent anion channel (VDAC) and transforming growth factor (TGFβ) proteins as markers of estrogen signaling, glycolysis rates, mitochondrial biogenesis and damage, apoptosis and autophagy (Western-Blot and PCR). The analysis identified higher levels of molecules associated with proliferation (ERβ), glycolysis (MCT2, PDK1, Glut1, Hex2, TGFβ and Hif-1α), mitochondrial biogenesis (OPA1, DRP1) and autophagy (P38, Beclin1 and Bnip3) and decreased levels of apoptosis markers (Bcl2/Bax) in endometrioid foci compared to non-affected peritoneum and that in the comparison group (p < 0.05). Patients with PE had altered profiles of ERβ in plasma and peritoneal fluid exosomes and higher levels of Glut1, MCT2 and Bnip3 in plasma exosomes (p < 0.05). The results of the differential expression profiles indicate microenvironment modification, mitochondrial biogenesis, estrogen reception activation and glycolytic switch along with apoptosis suppression in peritoneal endometrioid foci already in adolescents.
1. Introduction
Endometriosis (EMS) is a multifactorial and complex pathology associated with the presence of endometrial-like tissue (glands and stroma) in locations outside the uterus. The first symptoms of EMS may develop from disease manifestation in children and adolescents [,]; meanwhile, a diagnostic delay of 7–10 years could impair the clinical outcome and determine the progression of the disease [,]. EMS is considered to be one of the leading causes of secondary dysmenorrhea and chronic pelvic pain in adolescents, including menstrual pain, non-cyclic pelvic pain, dysuria, dyschesia and dyspareunia in sexually active girls []. Despite the high incidence of EMS in young patients, the onset of pathogenetic changes in adolescents with the initial forms of the disease remains poorly elucidated in the literature [,].
Different forms of EMS involve specific combinations of hereditary and environmental factors that favor hyperestrogenic and hypoxic local milieus outside the uterus [,,,] and enhance the capacity of endometrioid cells to colonize ectopic locations in the peritoneum [,,]. Endometrioid lesions sustain themselves by promoting immunological changes, metabolic reprogramming and activating neoangio- and neurogenesis at the site of engraftment, similar to neoplastic lesions [,,]. The demand for pathogenetic therapies in endometriosis necessitates clinical research on juvenile cohorts.
Endometrioid lesions tend to replenish their usable energy by activating aerobic glycolysis pathways, similarly with cancers, supporting pathogenesis (steroid synthesis, proliferation signaling, etc.) []. Increased expression of hypoxia-inducible factor 1α (HIF1α), pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase (LDHA) in adult patients with endometriosis suggests metabolic reprogramming patterns similar to those of cancers [,,,]. The metabolic switch is also indicated by high peritoneal levels of lactate and TGF-β1 in adult patients with endometriosis []. The increased extracellular lactate levels can stimulate angiogenesis, immune suppression and expansion of ectopic foci similar to cancer dissemination [,].
A fundamental concept of endometriosis should therefore account for the metabolic switch from mitochondrial respiration to glycolysis. Investigation of this aspect has, so far, been confined to experimental models and/or advanced stages of the disease in adult patients, without matching evidence for juvenile cases.
In this study, we analyze the association of cellular and exosomal profiles of molecular markers of glycolysis, mitochondrial biogenesis, apoptosis, autophagy and estrogen signaling in peritoneal endometriosis in adolescents.
2. Results
2.1. Study Population
All patients of the main group were diagnosed with endometriosis according to the revised American Society for Reproductive Medicine (rASRM) score, classified as stage I (22.2%, 10/45, the rASRM score 3.5 ± 1.4 OR P1-2, O 0/0, T0/0, B1/0 C0 by #Enzian(s) score), stage II (57.7%, 26/45 the rASRM score 11.9 ± 2.8 or P2, O 0-1/0, T0/0, B1/2, C0 by #Enzian(s)) or stage III (20.0%, 9/45, the rASRM score 23.5 ± 7.8 or P2-3, B1/2 by #Enzian(s)). In three cases, stage III of the disease was complicated by adenomyomas (O2-3/0) with tubo-ovarian adhesions (T1/0).
The body mass indexes of patients in both groups were similar (20.5 ± 3.7 vs. 20.3 ± 5.8 kg/m2; p = 0.54).
Patients with EMS were significantly younger at menarche (11.8 ± 2.5 vs. 12.5 ± 1.2 years in the other group; p < 0.001) and self-reported irregular (44.4%, 20/45 vs. 14.3%, 5/35; p = 0.004) and heavy menses (33.3%, 15/45 vs. 2.8%, 1/35; p < 0.001). Almost all patients with PE had severe dysmenorrhea and/or chronic pelvic pains resistant to NSAIDs and antispasmodics (95.6%, 43/45 vs. none in the other group; p < 0.001, χ2-test), and 24.4%, 11/45 of them reported blood spotting mid-cycle or close to menstruation date (p = 0.002, χ2-test).
General blood tests, biochemical/hormone profiles and blood coagulation parameters were similar between the groups, except higher prolactin levels in patients with endometriosis (465.6 ± 299.4 vs. 255.4 ± 114.9; p < 0.001). Leukocyte counts (6.5 ± 2.4 vs. 7.6 ± 2.5; p = 0.5), erythrocyte sedimentation rates (2.6 ± 1.1 vs. 2.0 ± 0.6; p = 0.7), C-reactive protein (1.0 ± 0.9 vs. 2.0 ± 1.6; p = 0.1), Ca-125 (25.9 ± 25.1 vs. 13.1 ± 8.3; p = 0.2), fibrinogen (25.9 ± 25.1 vs. 13.1 ± 8.3; p = 0.2) and iron (Fe2+) (20.8 ± 9.6 vs. 19.9 ± 8.5; p = 0.8) levels in the two groups were similar.
2.2. Estrogen Receptor Isoforms
Considering the estrogen-dependent nature of the disease, estrogen receptor isoform representation was comparatively assessed in blood, peritoneal fluid and peritoneal tissues.
Exosomal levels of ERβ in the peripheral blood and peritoneal fluid of patients with EMS were significantly higher than in the other group (Figure 1 and Figure S1A–C).
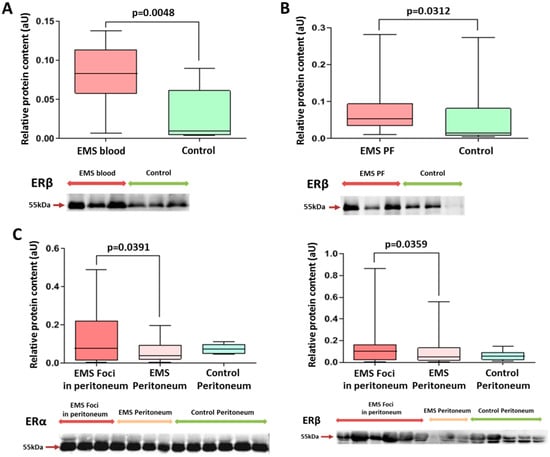
Figure 1.
Comparative analysis of estrogen receptor levels in exosomes and in tissues of patients in the studied groups. Levels of exosomal ERβ in peripheral blood (A) and peritoneal fluid (B) in patients with endometriosis were significantly higher than those in the comparison group. (C) ERβ and ERα protein levels (left and right panels, respectively) in the peritoneal tissues of patients with endometriosis were higher in endometrioid foci vs. intact peritoneum. Data of relative protein level quantification are presented as boxes with median, interquartile range and min–max values. Representative Western blots are presented on panels below graphs. See also corresponding Ponceau-stained images in the Figure S1A–C of Supplemental Materials, original Western Blot in Figure S7.
Tissue expression levels of ERβ and ERα were increased, respectively, by 1.5- and 3-fold in endometrioid foci compared with intact peritoneum (Figure 1).
It is interesting to note that a higher level of ERβ in endometriotic lesions was a significant factor in more severe forms (grade 3–4 according to rARMS) of peritoneal endometriosis in adolescents (Chi2 = 6.42; p = 0.011).
On the other hand, the level of ERα in the endometrioid lesion turned out to be a significant factor in milder forms (grade 1–2 according to rARMS) of peritoneal endometriosis in adolescents (Chi2 = 4.13; p = 0.042).
At the same time, levels of the major estrogen receptor isoform, ERα, in exosomes of peripheral blood and peritoneal fluid were similar between the groups.
2.3. Markers of Glycolysis and Mitochondrial Biogenesis
Glycolysis is the plausible mode of energy production via endometrioid heterotopias associated with pathogenesis. Expression levels for the key effectors of glycolysis, from the uptake of glucose by membrane transporters of the GLUT family (notably GLUT1) and its conversion (phosphorylation) to glucose-6-phosphate by hexokinase Hex2, were assessed via Western blot analysis.
GLUT1 expression was increased in both the EMS foci and the intact peritoneum biopsies of the main group by 3- and 4-fold, respectively, compared to the intact peritoneum biopsies of the other group (Figure 2).

Figure 2.
Estimation of energy metabolism markers level in peritoneal tissues. (A) Expression of Glut1 in peritoneal tissue biopsies according to RT-PCR data (see Section 4 for details). (B) Western blot analysis for glycolysis markers Glut1, Hex2, Hif1α, MCT1, MCT2, PDK1 and also VDAC1 and TGFβ. Data of relative protein level quantification and gene expression are presented as boxes with median, interquartile range and min–max values. Representative corresponding Western blots are presented below graphs on panels. See also Ponceau-stained images in Figure S2 of Supplemental Materials, original Western Blot in Figure S7.
Hex2 expression was increased by 2-fold in EMS foci vs. the intact peritoneum of the main group, indicating intensification of the first and limiting the enzymatic step of glycolysis within the heterotopias (Figure 2 and Figure S2).
The analysis revealed increased expression of pyruvate dehydrogenase kinase PDK1 protein in the peritoneal foci of patients with EMS compared to the intact peritoneal tissues of both groups (Figure 2 and Figure S2). The enhanced phosphorylation of the E1 subunit of pyruvate dehydrogenase PDH by PDK1 limits pyruvate conversion to acetyl-CoA, thereby disconnecting glycolysis from the tricarboxylic acid cycle in mitochondria and reinforcing pyruvate conversion to lactate, according to our data, specifically in EMS foci.
Facile reduction of supraphysiological lactate concentrations is crucial for homeostasis and glycolysis running. Expression of lactate transporter protein MCT1 in the tissue biopsies of patients with EMS (in the foci and in intact peritoneum) was significantly higher than in the peritoneal biopsies of the comparison group, and a similar trend was observed for MCT2 (Figure 2). The identified differences prove the activation of glycolytic pathways in endometriotic lesions and the beginning of metabolic reprogramming in peritoneal cells in other compartments of the abdominal cavity outside the lesions in patients of the main group. It is noteworthy that a higher level of MCT2 in endometriotic lesions turned out to be a significant factor behind more severe forms (grade 3–4 according to rARMS) of peritoneal endometriosis in adolescents (Chi2 = 6.42; p = 0.011).
TGFβ signaling has been implicated in a positive feedback loop with glycolytic switch and associated cell reprogramming. Expression of TGFβ protein was increased in the peritoneal foci of patients with EMS compared to the intact peritoneal tissues of both groups (Figure 2 and Figure S2). Positive correlations were revealed between the level of TGF-β and the level of ER-β (r = 0.90; p = 0.037), as well as ER-α (r = 0.75; p = 0.019), MCT2 (r = 0.66; p = 0.049) in endometriotic lesions and MCT2 in the intact peritoneum (r = 0.85; p = 0.003) in patients in the main group (r = 0.90; p = 0.037).
Interestingly, the Hif-1α protein level was also significantly higher in EMS foci than in the peritoneal biopsies of the comparison group.
A similar trend was identified for glucose transporter GLUT1 mRNA over-represented in the peritoneal biopsies of the main group vs. the comparison group (Figure 2A).
A 1.5-fold enrichment of blood exosomes with GLUT1 in patients with EMS compared with the other group (Figure 3 and Figure S3) may indicate activation of glucose intake by cells at a systemic level. Decreased levels of MCT2 protein in the blood exosomes of patients with EMS was noticed as well (Figure 3). Higher levels of MCT2 in blood exosomes turned out to be a significant signifier of more severe forms (grade 3–4 according to rARMS) of peritoneal EMS in adolescents (Chi2 = 5.03; p = 0.025).

Figure 3.
Comparative analysis of glucose and lactate transporter proteins in blood exosomes of patients of studied groups. Data of relative protein level quantification are presented as boxes with median, interquartile range and min–max values. Representative Western blots are presented below corresponding graphs on panels. See also Ponceau-stained images in Figure S3 of Supplemental Materials, original Western Blot in Figure S7.
A 3-fold decrease in expression of DRP1 protein involved in mitochondrial fission was observed in the peritoneal tissue biopsies of patients with EMS compared with the other group, which is consistent with corresponding PCR data for DRP1 mRNA in the same biopsies (Figure 4A,B and Figure S4A), indirectly indicating a decreased role of oxidative phosphorylation in mitochondria associated with the disease. Notably, blood exosomes of patients with EMS were significantly enriched in DRP1 compared with the other group (Figure 4C and Figure S4B).

Figure 4.
Mitochondrial fission and fusion markers DRP1 and OPA1 levels are changed in patients with EMS. Reliable decrease in DRP1 transcripts (A) and Drp1 protein (B) levels in the foci biopsies of patients with EMS and increase in blood exosomes (C). (D) Relative expression of OPA1 protein increased for EMS patients compared to the intact peritoneum biopsies of patients of the comparison group. Relative data of protein level quantification and gene expression are presented as boxes with median, interquartile range and min–max values. Representative Western blots are presented below graphs. See also corresponding Ponceau-stained images in Figure S4A,B of Supplemental Materials, original Western Blot in Figure S7.
A 4-fold increase in OPA1 mitochondrial fusion protein expression in peritoneal biopsies, specifically in EMS (Figure 4D), may indicate the disease-associated stabilization of mitochondrial cristae and enhanced apoptosis resistance within the peritoneum.
2.4. Apoptosis and Autophagy Markers
According to PCR data, the Bcl-2/Bax ratio in the peritoneal biopsies of patients with EMS was increased 3-fold compared with the other group, indicating enhanced resistance to apoptosis in heterotopias associated with peritoneal dissemination (Figure 5A). Decreased expression of pro-apoptotic Bax and increased expression of anti-apoptotic Bcl-2 in both the foci and the intact peritoneum of patients with EMS vs. the comparison group was demonstrated (Figure 5A), which is consistent with the Western blot data revealing similar trends at the protein level, i.e., a significantly higher expression of Bax within endometrioid foci compared to the intact peritoneum (Figure 5B and Figure S5).
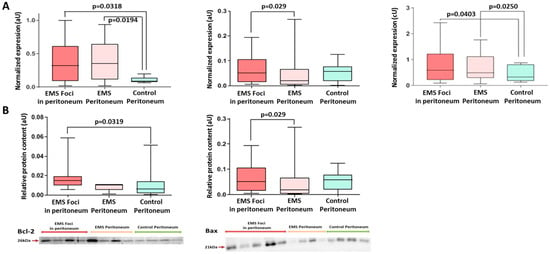
Figure 5.
Expression of apoptotic markers in biopsies of peritoneal tissues. (A) Increased expression of the anti-apoptotic Bcl2 and decreased expression of pro-apoptotic Bax in the foci vs. intact peritoneum of both groups. Bcl-2/Bax transcripts ratio is reliably high in EMS foci vs. intact peritoneum of the comparison group. (B) Levels of Bcl2 and Bax proteins in the peritoneal tissues. Data of relative protein level and gene expression are presented as boxes with median, interquartile range and min–max values. Representative Western blots are presented below graphs. See corresponding Ponceau-stained images in Figure S5 of Supplemental Materials, original Western Blot in Figure S7.
A mini-panel of autophagy markers included p38 MAPK proteins known to inhibit autophagy and beclin1 and BNIP3 proteins directly involved in autophagosome formation. Expression of p38 was decreased 2-fold in the peritoneal biopsies of patients with EMS (both in the foci and in the intact peritoneum) vs. the comparison group, and the opposite trend was demonstrated for beclin1 and BNIP3 (Figure 6A and Figure S6A). These results indirectly indicate an increase in autophagy rates probably reflecting the altered mitochondrial homeostasis. A 2-fold decrease in BNIP3 protein expression in blood exosomes of patients with EMS compared with the other group was also evident (Figure 6B and Figure S6B).

Figure 6.
Levels of autophagy markers in peritoneal tissue biopsies and blood exosomes of patients with EMS. (A) Decreased level of p38 protein and increased level of Beclin1 protein in EMS foci. (B) Decreased exosomal level of BNIP3 in the blood of patients with EMS vs. comparison group. Data of relative proteins level quantification are presented as boxes with median, interquartile range and min–max values. Representative Western blots are presented below graphs. See corresponding Ponceau-stained images in Figure S6A,B of Supplemental Materials, original Western Blot in Figure S7.
3. Discussion
The pathogenesis of EMS is estrogen-dependent and involves the locally increased estrogen synthesis combined to progesterone resistance [,]. The high proliferative capacity of endometrioid heterotopias has been demonstrated using experimental models and in adult patients [,,,]. The lesions hyperexpress estrogen receptors (ER) dominated by the ERβ isoform known to support proliferation and suppress apoptosis [].
The altered estrogen receptor profiles can be rate-limiting for the pathogenesis and accentuate the inflammatory component in EMS. The paracrine milieus dominated by pro-inflammatory cytokines IL-1, IL-18 and TNF-α [,] favor the recruitment of macrophages that produce neuro- and angiogenic factors at the site of engraftment [,,,,]. ERβ also triggers the expression of PGC-1α and promotes the overexpression of antioxidant mitochondrial protein (SOD2) and Bcl-2, thereby suppressing the oxidative damage-induced apoptosis []. The local hyperestrogenic milieus and enhanced estrogen sensitivity are considered essential for the oxidative stress resistance and engraftment of endometrium fragments stranded in the peritoneal cavity. The increased content of ERβ in exosomal fractions of the blood and peritoneal fluid characteristic of PE patients indicates a systemic scale of the altered estrogen signaling [,].
Endometrioid heterotopias actively modify the peritoneal microenvironments to promote engraftment. It is possible that endometrial lineages in patients with EMS and predisposed individuals become specifically reprogrammed while still in the uterus, prior to the retrograde menstrual transfer and colonization of the peritoneum [,,]. The emergence of the so-called “pre-metastatic” cell niche in eutopic endometrium may be a prerequisite for peritoneal EMS, which would explain the limited incidence of the disease despite the rather physiological occurrence of retrograde menstruation.
Proliferation of endometrial cells at ectopic locations is energy-consuming and stressful. Estrogens are known to enhance cell survival by boosting ATP synthesis and mitochondrial DNA repair and to relieve oxidative stress by suppressing reactive oxygen species production [,,]. Our analysis reveals increased expression of glycolysis-associated genes, including the transporter-encoding GLUT1 and MCT2 and the glycolytic enzyme-encoding HK2 and PDK1. Membrane transporters GLUT1 and MCT2 provide kinetic support to glycolysis by ensuring, respectively, the influx of glucose and the efflux of lactate. Hexokinase (HK2) converts glucose to glucose-6-phosphate and pyruvate dehydrogenase kinase (PDK1) interferes with pyruvate conversion to acetyl-CoA, thereby inhibiting mitochondrial respiration. Similarly altered expression of glycolysis-associated genes in endometrioid heterotopias was reported for adult patients [,,]. The increased expression of GLUT1, MCT2 and PDK1 in heterotopias and the surrounding intact peritoneum starting from the early stages of the disease may indicate a microenvironmental remodeling throughout the peritoneum, probably regulated by exosome-mediated signaling accompanied by high GLUT1 and low MCT levels in the exosomal fraction.
Lactate dehydrogenase LDHA converts pyruvate to lactate, thereby decoupling glycolysis from mitochondrial oxidation. Increased expression of LDHA in the endometrioid heterotopias of adult patients showing an activation of glycolysis [] is consistent with our findings. Subsequent clearance of lactate from cells via MCT transporters acidifies the microenvironment and promotes its TGFβ/SNAIL- and ADAM10/17-mediated remodeling; it also favors angiogenesis and thus the expansion of the foci [,]. TGFβ has been shown to stimulate glycolysis in murine pulmonary fibroblasts and those of patients with idiopathic pulmonary fibrosis. TGFβ expression has been positively correlated with the expression of HK2 in adults with EMS []. Our data reveal a similar association with increased levels of both TGFβ and HK2 in the foci. High local levels of TGFβ and Hif-1α accompanied by lactate accumulation can support cell proliferation and invasion while promoting local immune tolerance to colonization by the foci [,].
We further analyzed the dynamics of proteins involved in mitochondrial fission and fusion (respectively, DRP1 and dynamin-related GTPase OPA1) and GRP75 implicated in steroidogenesis. Under optimal physiological conditions, mitochondria ensure cell respiration and dominate the energy metabolism. Mitochondria also control the balance of reactive oxygen species and are instrumental for apoptosis. In healthy endometrium, mitochondrial pools undergo continuous fission and fusion, amounting to a dynamic equilibrium essential for energy homeostasis. Mitochondrial fission helps discard an excess of accumulated protons in order to avoid oxidative stress and adjust to reduced energy demands.
Impaired mitochondrial fission interferes with electron transfer regulation and may result in oxidative stress. Reduced expression of the mitochondrial fission protein DRP1 in the foci and surrounding peritoneum indirectly indicates a decline in mitochondrial respiration and reduced means for its proper control associated with the disease. A concomitant increase in OPA1 protein expression suggests enhanced mitochondrial fusion, accommodating the cell-to-glycolytic metabolic switch and promoting apoptosis resistance. Mouse studies reveal OPA1-mediated stabilization of mitochondrial cristae, which inhibits remodeling of the cristae and facilitates the release of cytochrome C that triggers apoptosis [].
These data on mitochondrial protein expression at the initial stages of peritoneal EMS in adolescents are unique but difficult to interpret unambiguously due to the scarcity of reports on DRP1 biology and their potential role in EMS []. We observed significantly increased blood exosomal levels of DRP1 in peritoneal EMS in adolescents. The enhanced mitochondrial reticulum fragmentation may reflect pathogenic changes []. The trend is significant, makes sense biologically and deserves further investigation.
High rates of cell proliferation in endometrioid heterotopias are accompanied by reduced rates of apoptosis—programmed cell death regulated by multiple intersecting molecular networks. In contrast with necrosis, the process is “clean” of inflammation around the dying cell as plasma membrane integrity is preserved. The cell progressively shrinks and dissipates into apoptotic bodies subsequently eliminated by macrophages. Apoptosis is a normal component of the cyclic remodeling processes in eutopic endometrium. The rates of spontaneous apoptosis in healthy endometrium reach maximum during the secretory and early proliferative phases of the menstrual cycle. In patients with endometriosis, spontaneous apoptosis in the endometrium is inhibited and the cyclic dynamics are lost, indicating the increased viability of endometrial cells [,,]. Here, we demonstrate increased expression of anti-apoptotic factor Bcl-2 and an increase in Bcl-2/Bax expression ratio associated with peritoneal EMS in adolescents. More specifically, the PCR tests revealed increased Bcl-2 and decreased Bax mRNA levels in the foci and also in the surrounding intact peritoneum compared to the other group. Altered Bcl-2 levels are characteristic of various cancers, and the upregulation of Bcl-2 in endometriosis has been demonstrated by Istrate-Ofiţeru et al. (2018) [].
Exosomal fractions isolated from physiological media (peritoneal and follicular fluids, blood) significantly differ between patients with endometriosis and healthy donors [], particularly in terms of loads and the composition of regulatory factors involved in angiogenesis, neurogenesis, immune dysfunction, inflammation and invasion [,]. Although exosomes derived from endometrial cells have been identified in both uterine and peritoneal fluids [,], it is difficult to accurately trace their origin; the vesicles can be derived from eutopic endometrium, stranded endometrium fragments or endometrioid heterotopias, and even from macrophages scavenging the endometrial debris. In any event, the endometrial exosomes may exert a conditioning effect on peritoneal microenvironments, enhancing their receptivity to stranded endometrium fragments, suppressing immunological reactivity and ultimately facilitating engraftment []. Exosomal fractions are increasingly considered as a basis for diagnostics and monitoring in various focal disorders as they often provide a proteomic and regulomic footprint of poorly accessible lesions. Here, we identify altered exosomal levels of ERβ, MCT2, GLUT1, DRP1 and Bnip3 associated with peritoneal EMS in adolescents.
The data we obtained are schematically presented in Figure 7.

Figure 7.
Activation of aerobic glycolysis and mitochondrial biogenesis under the conditions of increased estrogen reception in the pathogenesis of peritoneal endometriosis in adolescents. Expression levels for the key effectors of glycolysis, from the uptake of glucose by membrane transporters of the GLUT family (notably GLUT1) and its phosphorylation to glucose-6-phosphate by hexokinase (Hex2), are increased. The enhanced phosphorylation of pyruvate dehydrogenase (PDH) by its kinase (PDK1) limits pyruvate conversion to acetyl-CoA, thereby disconnecting glycolysis from tricarboxylic acid cycle (TCA) in mitochondria and reinforcing pyruvate conversion to lactate by lactate dehydrogenase A (LDHA). Lactate transport out of the cell through its transporters MCT is also increased. At the same time, a decrease in the activity of oxidative phosphorylation (TCA) in mitochondria is associated with less electron leakage and reactive oxygen species (ROS) formation and, accordingly, with the control of oxidative stress. Mitochondrial biogenesis is also increased, along with stabilization of mitochondrial cristae and enhanced apoptosis resistance (fusion marker OPA1 and fission marker DRP1). Contacts with EPR (glucose-regulated protein (GRP75) facilitate mitochondria-associated ER membrane (MAM) formation) and Ca2+ influx (VDAC) reinforce activation of cholesterol synthesis and steroidogenesis pathways. The implementation of ER-β signals leads to changes (p38 MAPK kinase cascade) in the expression of nuclear (p53) and mitochondrial genes, particularly those responsible for protection against apoptosis (the ratio of Bcl2 to Bax increases) and autophagy activation (Beclin, Bnip). HIF-1α and TGFβ signaling has a positive feedback loop with glycolytic switch and associated cell reprogramming.
Overall, we demonstrate EMS-associated metabolic reprogramming in adolescent patients; the signs include altered exosomal profiles of signaling molecules in peritoneal fluid and notably in the blood. The respiration-to-glycolysis switch is observed as starting from early stages of the disease. The switch is estrogen-dependent, linked to mitochondrial biogenesis and positively associated with apoptosis suppression and autophagy.
4. Materials and Methods
The case–control study enrolled 60 post-menarchal girls, 15–17 years old, with confirmed diagnoses of peritoneal endometriosis (PE) (n =45, main group) and paramesonephric cysts (n = 15, comparison group). All participants underwent inpatient treatment in 2020–2022 in the Pediatric and Adolescent Gynecology Department at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology, Moscow, Russia.
The inclusion criteria in the main group we as follows: age of patients from menarche to 17 years inclusive; the confirmed diagnosis of peritoneal endometriosis; absence of other drug administration (psychotropic and any hormonal drugs, including combined oral contraceptives) for at least 3 months preceding the study; informed consent of the patient or her legal representative for participation in the research.
The indications for laparoscopy in the main group were persistent moderate/severe dysmenorrhea or/and chronic pelvic pain, resistant to symptomatic therapy with NSAIDs for at least 3 months preceding the study, with suspicion on PE according to MRI.
The exclusion criteria for the main group were as follows: age over 18; aggravated chronic or acute diseases (infectious, endocrine, oncological, etc.); mental conditions; pelvic tumors; absence of dysmenorrhea and/or chronic pelvic pain; hereditary syndromes and congenital malformations associated with menstrual outflow obstruction; lack of informed consent.
The comparison group consisted of 15 adolescent girls of the same age (16.0 (15.0–17.0)).
The inclusion criteria in the comparison group were as follows: regular moderate periods (within 24–38 days, lasting 4–7 days, without complains on pain); no other gynecological pathology except of paramesonephric cyst (4 cm ≤ diameter of the cyst ≤ 6 cm); no endocrine/somatic pathology; absence of routine drug administration for at least 3 months preceding the study; informed consent of the patient or her legal representative for participation in the research study.
The exclusion criteria were mostly the same as with the main group: age over 18; an aggravation of chronic or acute somatic and/or infectious disease; mental illnesses; endocrine or gynecological disorders; other tumors of the pelvic organs (except of paramesonephric cyst or diameter of paramesonephric cyst more than 6 cm); oncological diseases; dysmenorrhea and/or chronic pelvic pain; inherited syndromes and congenital malformations; lack of informed consent of the patient or her legal representative for participation in the research study. The indication for laparoscopy in the comparison group was an ultrasound or MRI sign of paramesonephric cyst (4 cm ≤ diameter of the cyst ≤ 6 cm).
4.1. Laparoscopy
Laparoscopy reports included surgical diagnosis; endometriosis stage according to the revised American Society for Reproductive Medicine (rASRM) criteria and revised Enzian Classification; and localization, type, color, size and area of the lesions. Histological reports included macro- and microscopic descriptions of the biopsies according to the current standards.
The peritoneal tissue and fluid samples were collected during laparoscopic intervention. In patients of the comparison group, a biopsy of the peritoneum was performed in the right lateral peritoneal recess area. In patients of the main group, tissues of endometrioid peritoneal foci were collected mostly at Douglas space and uterosacral ligaments; intact peritoneum was also collected in the right lateral peritoneal recess. The biological samples were immediately cryopreserved in liquid nitrogen, stored at −80 °C and thawed immediately before the analysis
4.2. Protein Expression
Exosomal fractions of peripheral blood and peritoneal fluid in conjunction with peritoneum biopsies (endometrioid lesion and intact peritoneum in the main group and intact peritoneum in the comparison group) were analyzed for estrogen receptor (Erα/β), hexokinase (Hex2), pyruvate dehydrogenase kinase (PDK1), glucose transporter (Glut1), monocarboxylate transporters (MCT1 and MCT2), optic atrophy 1 (OPA1, mitochondrial fusion protein), dynamin-related protein 1 (DRP1, mitochondrial fission protein), Bax, Bcl2, Beclin1, Bnip3, P38 mitogen-activated protein kinase (MAPK), hypoxia-inducible factor 1 (Hif-1α), mitochondrial voltage-dependent anion channel (VDAC) and transforming growth factor (TGFβ) proteins as markers of estrogen signaling, glycolysis rates, mitochondrial biogenesis and damage, apoptosis and autophagy.
4.3. Biological Sample Processing
The blood was collected from the antecubital vein into an EDTA-containing tube via the established protocol. The peritoneum tissue biopsies (endometrioid lesion and intact peritoneum in the main group and intact peritoneum in the comparison group) were ground into powder in liquid nitrogen and halved. One portion of each sample was partly homogenized in a glass Potter homogenizer (glass–Teflon, clearance 20 µm) in RIPA lysis solution (sc-24948; Santa Cruz Biotechnology, Dallas, TX, USA) at a 10:1 ratio (v/w). The remaining portion of the tissue powder was used to purify total RNA using QIAzol® lysis reagent at a 10:1 ratio (v/w). The total RNA and total protein samples were quantified using a NanoPhotometer (Implen, Munich, Germany) at 260/240 and 280/260 nm, respectively.
4.4. Exosome Isolation
Exosomes were isolated from blood plasma or peritoneal fluid via double centrifugation for 70 min at 100,000× g and 4 °C in a Beckman Coulter Optima XPN-100 ultracentrifuge (Beckman Coulter, Inc., Brea, CA, USA). The protein concentration in the exosomal fraction obtained via ultracentrifugation was assessed spectrophotometrically (NanoPhotometer, Implen), which made it possible to normalize the application of 20–30 μg of protein to the lanes for electrophoretic separation.
4.5. Western Blot Assay
Total proteins from each biological sample were separated via SDS-PAGE according to Laemmli [] and electrotransferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA); the membranes were stained for total protein in 0.3% PONCEAU S solution. The proteins were visualized, staining with ChemiDoc (Bio-Rad), before incubation with primary antibodies. We used Ponceau reversible staining of our blots to normalize the amount of protein of interest to the total protein level in the blot lanes.
After pre-blocking in 5% NFDM/TBST for 1 h, the membranes were incubated overnight at 4 °C in a Shaker-Rocker MR-12 (Biosan, Riga, Latvia) with monoclonal primary antibodies to Bax—ab263897; TGF-β—ab179695; PDK1—ab207450; OPA1—ab157457; MCT1—ab85021; MCT2—ab81262; Hex2—ab104836; GLUT1—ab128033; Erα—ab75635; Erβ—ab3576; DRP1—ab184274; Beclin1—ab62557; Hif-1α—ab216842; VDAC1—ab154856; Hexokinase 1 (Hex1)—ab150423; all antibodies—Abcam (Cambridge, UK), P38–9212 (Cell Signaling Technology, Danvers, MA, USA), Bcl-2—13-8800 (Thermo Fisher Scientific, Waltham, MA, USA). The membranes were subsequently washed in TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (polyclonal goat anti-mouse ab6789; Abcam). The signal was developed using Clarity Western ECL Substrate kits (Bio-Rad Laboratories, Hercules, CA, USA).
4.6. Real-Time Polymerase Chain Reaction Assay
Gene expression levels were assessed via reverse transcription real-time polymerase chain reaction (PCR) with transcript-specific primers (Table 1).

Table 1.
Primers used in PCR assay.
Reverse transcription reactions were set up using MMLV RT Kit (Evrogen, Moscow, Russia) in accordance with the kit manual.
PCRs were run in a DT-96 Real-Time Cycler (DNA-Technology, Moscow, Russia); a 95 °C 5 min primary denaturation step followed by 45 cycles of 95 °C 10 s, 60 °C 20 s and 67 °C 20 s). The curves were analyzed by in-built software and the QGene 4.3.3 software (QIAGEN LLC, Germantown, MD, USA) using the 2−ΔΔCT method to quantify gene expression with GAPDH as a reference.
Statistical data analysis was performed using Statistica 12 software (StatSoft Inc., Tulsa, OK, USA). Categorical variables were compared via χ2 test. The distribution normalities were challenged via Shapiro–Wilk test. Non-normally distributed variables were described via median (Me) and interquartile range values and compared using the Mann–Whitney U-test. The variables in the dependent samples were estimated using the Wilcoxon signed rank test and χ2 McNemar’s test for dependent proportions; the trends were considered significant at p < 0.05. The results in the graphs are normalized to total protein in GraphPad Prism 6.0 and presented as median, 25–75% quartiles and min–max. Correlations were estimated using Pearson’s correlation coefficient (for normally distributed data) or Spearman’s rank correlation method (non-parametric). The influence of risk factors was assessed via logistic regression using adjusted odds ratio (OR) values with 95% confidence intervals (CI). The influences of categorical factors and quantitative variables were estimated via factorial ANOVA and multiple logistic regression methods, respectively.
5. Conclusions
- The data indicate higher exosomal levels of glycolysis and mitochondrial biogenesis markers (GLUT1, MCT2 and DRP1) and estrogen receptors (Erα and ERβ) in the blood and peritoneal fluid in adolescent patients with peritoneal endometriosis, suggesting systemically altered modes of energy metabolism and estrogen signaling from early stages of the disease.
- The significantly altered cellular expression levels of glycolysis markers (HEX2, GLUT1, PDK1, MCT1, MCT2, TGF-β and Hif-1α), mitochondrial biogenesis markers (DRP1, OPA1) and autophagy/apoptosis markers (p38, beclin1, Bcl-2 and Bax), accompanied by high levels of ERβ in endometrioid heterotopias and the surrounding intact peritoneum, indicate early metabolic reprogramming of the lesions in peritoneal endometriosis already at the manifestation of the disease in adolescents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25084238/s1.
Author Contributions
E.P.K. devised the project, the main conceptual ideas, analyzed the data and results; M.Y.V., M.V.M., L.A.M. and K.O.S. performed Western-blot investigation and observation; E.V.U. and T.K.F. supported proof outline and supervision; V.D.C. and L.V.A. contributed to clinical and surgical intervention; E.P.K. and K.O.S. wrote the original draft and revised the manuscript; K.O.S. and N.N.S. performed the sample preparation and PCR analysis; E.P.K., M.V.M. and K.O.S. contributed to statistical data and graphs analysis; G.T.S. developed the theoretical framework, supported funding acquisition and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out with the financial support of the state assignment of the Ministry of Health: “The role of energy metabolism and immune defense disorders in the development of various forms of endometriosis, the development of personalized therapy and the forecast of its effectiveness in the early reproductive period (from menarche to 18 years)” 18-A21.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee for Biomedical Research at the National Medical Research Center for Obstetrics, Gynecology and Perinatology, named after Academician V.I. Kulakov Ministry of Healthcare of the Russian Federation, Moscow, Russia (Project identification code No. 9, 22 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects (or their legal representatives in cases where the girls was under 15 years old) involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because the database contains the personal data of patients.
Acknowledgments
We thank Usman N.Yu. for help with translating this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- DiVasta, A.D.; Vitonis, A.F.; Laufer, M.R.; Missmer, S.A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am. J. Obstet. Gynecol. 2018, 218, 324.e1–324.e11. [Google Scholar] [CrossRef]
- Hirsch, M.; Dhillon-Smith, R.; Cutner, A.S.; Yap, M.; Creighton, S.M. The Prevalence of Endometriosis in Adolescents with Pelvic Pain: A Systematic Review. J. Pediatr. Adolesc. Gynecol. 2020, 33, 623–630. [Google Scholar] [CrossRef]
- Yeung, P.; Gupta, S.; Gieg, S. Endometriosis in Adolescents: A Systematic Review. J. Endometr. Pelvic Pain Disord. 2017, 9, 17–29. [Google Scholar] [CrossRef]
- Ding, D.; Wang, X.; Chen, Y.; Benagiano, G.; Liu, X.; Guo, S.-W. Evidence in Support for the Progressive Nature of Ovarian Endometriomas. J. Clin. Endocrinol. Metab. 2020, 105, 2189–2202. [Google Scholar] [CrossRef] [PubMed]
- Khashchenko, E.P.; Uvarova, E.V.; Chuprynin, V.D.; Pustynnikova, M.Y.; Fatkhudinov, T.K.; Elchaninov, A.V.; Gardanova, Z.R.; Ivanets, T.Y.; Vysokikh, M.Y.; Sukhikh, G.T. Pelvic Pain, Mental Health and Quality of Life in Adolescents with Endometriosis after Surgery and Dienogest Treatment. J. Clin. Med. 2023, 12, 2400. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 1522. [Google Scholar] [CrossRef]
- Simpson, C.N.; Lomiguen, C.M.; Chin, J. Combating Diagnostic Delay of Endometriosis in Adolescents via Educational Awareness: A Systematic Review. Cureus 2021, 13, e15143. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Lenk, E.E.; Lebovic, D.I.; Shu, Y.; Yu, J.; Taylor, R.N. Pathogenesis of endometriosis: Interaction between Endocrine and inflammatory pathways. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 50–60. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.D.; Bertschi, D.; Bersinger, N.A.; Mueller, M.D. Inflammation and nerve fiber interaction in endometriotic pain. Trends Endocrinol. Metab. 2015, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, H.; Wu, J.; Liu, D.; Yao, S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Sikora, J.; Smycz-Kubańska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation—The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77, e12622. [Google Scholar] [CrossRef]
- Johan, M.Z.; Ingman, W.V.; Robertson, S.A.; Hull, M.L. Macrophages infiltrating endometriosis-like lesions exhibit progressive phenotype changes in a heterologous mouse model. J. Reprod. Immunol. 2019, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Horne, A.W.; Greaves, E. Endometriosis-Associated Macrophages: Origin, Phenotype, and Function. Front. Endocrinol. 2020, 11, 7. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.J.; Kim, M.J.; Lee, S.J.; Kwon, H.; Lee, J.H. Novel Medicine for Endometriosis and Its Therapeutic Effect in a Mouse Model. Biomedicines 2020, 8, 619. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kimura, M.; Maruyama, S.; Nagayasu, M.; Imanaka, S. Revisiting estrogen-dependent signaling pathways in endometriosis: Potential targets for non-hormonal therapeutics. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, H.; Lee, S.; Youn, H.; Youn, B. Role of Metabolic Reprogramming in Epithelial–Mesenchymal Transition (EMT). Int. J. Mol. Sci. 2019, 20, 2042. [Google Scholar] [CrossRef]
- Young, V.J.; Brown, J.K.; Maybin, J.; Saunders, P.T.K.; Duncan, W.C.; Horne, A.W. Transforming Growth Factor-β Induced Warburg-Like Metabolic Reprogramming May Underpin the Development of Peritoneal Endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, 3450–3459. [Google Scholar] [CrossRef] [PubMed]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A Metabolic Key Player in Cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Wilson, R.B.; Archid, R.; Reymond, M.A. Reprogramming of Mesothelial-Mesenchymal Transition in Chronic Peritoneal Diseases by Estrogen Receptor Modulation and TGF-β1 Inhibition. Int. J. Mol. Sci. 2020, 21, 4158. [Google Scholar] [CrossRef]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Istrate-Ofiţeru, A.M.; Pirici, D.; Niculescu, M.; Berceanu, C.; Berceanu, S.; Voicu, N.L.; Piringă, G.D.; Roşu, G.C.; Iovan, L.; Căpitănescu, R.G.; et al. Clinical, morphological and immunohistochemical survey in different types of endometriosis. Rom. J. Morphol. Embryol. 2018, 59, 1133–1153. [Google Scholar]
- Chen, C.; Zhou, Y.; Hu, C.; Wang, Y.; Yan, Z.; Li, Z.; Wu, R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019, 136, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Shigetomi, H.; Imanaka, S. Nonhormonal therapy for endometriosis based on energy metabolism regulation. Reprod. Fertil. 2021, 2, C42–C57. [Google Scholar] [CrossRef]
- Shen, H.-H.; Zhang, T.; Yang, H.-L.; Lai, Z.-Z.; Zhou, W.-J.; Mei, J.; Shi, J.-W.; Zhu, R.; Xu, F.-Y.; Li, D.-J.; et al. Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis. Theranostics 2021, 11, 3512–3526. [Google Scholar] [CrossRef]
- Slabe, N.; Meden-Vrtovec, H.; Verdenik, I.; Kosir-Pogacnik, R.; Ihan, A. Cytotoxic T-Cells in Peripheral Blood in Women with Endometriosis. Geburtshilfe Frauenheilkd 2013, 73, 1042–1048. [Google Scholar] [CrossRef]
- Takebayashi, A.; Kimura, F.; Kishi, Y.; Ishida, M.; Takahashi, A.; Yamanaka, A.; Wu, D.; Zheng, L.; Takahashi, K.; Suginami, H.; et al. Subpopulations of Macrophages within Eutopic Endometrium of Endometriosis Patients. Am. J. Reprod. Immunol. 2015, 73, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Chantalat, E.; Valera, M.-C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Yu, J.; Francisco, A.M.; Patel, B.G.; Cline, J.M.; Zou, E.; Berga, S.L.; Taylor, R.N. IL-1β Stimulates Brain-Derived Neurotrophic Factor Production in Eutopic Endometriosis Stromal Cell Cultures. Am. J. Pathol. 2018, 188, 2281–2292. [Google Scholar] [CrossRef]
- Liao, T.-L.; Lee, Y.-C.; Tzeng, C.-R.; Wang, Y.-P.; Chang, H.-Y.; Lin, Y.-F.; Kao, S.-H. Mitochondrial translocation of estrogen receptor β affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free. Radic. Biol. Med. 2019, 134, 359–373. [Google Scholar] [CrossRef]
- Freger, S.; Leonardi, M.; Foster, W. Exosomes and their cargo are important regulators of cell function in endometriosis. Reprod. Biomed. Online 2021, 43, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nicholes, K.; Shih, I.-M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Ahmad, S.F.; Carter, R.; Simitsidellis, I.; Greaves, E.; Hogg, C.; Morton, N.M.; Saunders, P.T.K. Repurposing dichloroacetate for the treatment of women with endometriosis. Proc. Natl. Acad. Sci. USA 2019, 116, 25389–25391. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Qiao, P.; Fu, R.; Wang, Y.; Lu, J.; Ling, X.; Liu, L.; Sun, Y.; Ren, C.; Yu, Z. Phosphorylation of PFKFB4 by PIM2 promotes anaerobic glycolysis and cell proliferation in endometriosis. Cell Death Dis. 2022, 13, 790. [Google Scholar] [CrossRef]
- Wang, Y.; Xiu, J.; Yang, T.; Ren, C.; Yu, Z. HSF1 promotes endometriosis development and glycolysis by up-regulating PFKFB3 expression. Reprod. Biol. Endocrinol. 2021, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Imai, H.; Koumura, T.; Kobayashi, T.; Nakagawa, Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000, 351, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, X.; Ni, C.; Dai, Y.; Guo, Y. Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol. Med. Rep. 2018, 17, 7797–7806. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, X.; Lv, Y. HMGB1 Mediated Inflammation and Autophagy Contribute to Endometriosis. Front. Endocrinol. 2021, 12, 616696. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-J.; Lin, X.-J.; Zheng, T.-T.; Tang, X.-Y.; Zhang, Y.; Hua, K.-Q. The Exosomal Long Noncoding RNA aHIF is Upregulated in Serum from Patients With Endometriosis and Promotes Angiogenesis in Endometriosis. Reprod. Sci. 2019, 26, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Gao, H.; Shao, W.; Wang, J.; Li, M.; Liu, S. The Extracellular Vesicle–Macrophage Regulatory Axis: A Novel Pathogenesis for Endometriosis. Biomolecules 2023, 13, 1376. [Google Scholar] [CrossRef]
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial Exosomes/Microvesicles in the Uterine Microenvironment: A New Paradigm for Embryo-Endometrial Cross Talk at Implantation. PLoS ONE 2013, 8, e58502. [Google Scholar] [CrossRef] [PubMed]
- Nazri, H.M.; Imran, M.; Fischer, R.; Heilig, R.; Manek, S.; Dragovic, R.A.; Kessler, B.M.; Zondervan, K.T.; Tapmeier, T.T.; Becker, C.M. Characterization of exosomes in peritoneal fluid of endometriosis patients. Fertil. Steril. 2020, 113, 364–373.e2. [Google Scholar] [CrossRef] [PubMed]
- Soltani-Fard, E.; Asadi, M.; Taghvimi, S.; Vafadar, A.; Vosough, P.; Tajbakhsh, A.; Savardashtaki, A. Exosomal microRNAs and long noncoding RNAs: As novel biomarkers for endometriosis. Cell Tissue Res. 2023, 394, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).