Preventive Effect of the Total Polyphenols from Nymphaea candida on Sepsis-Induced Acute Lung Injury in Mice via Gut Microbiota and NLRP3, TLR-4/NF-κB Pathway
Abstract
:1. Introduction
2. Results
2.1. Compound Analysis of NCTP
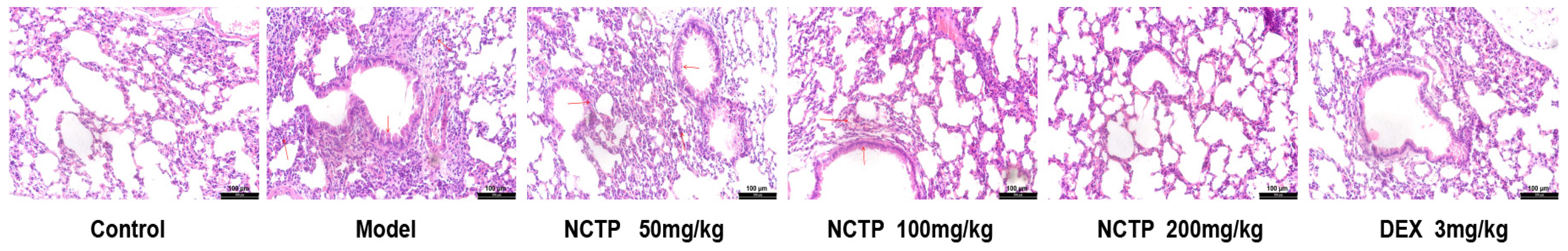
2.2. Protective Effect of NCTP on Lung Tissue in ALI Mice
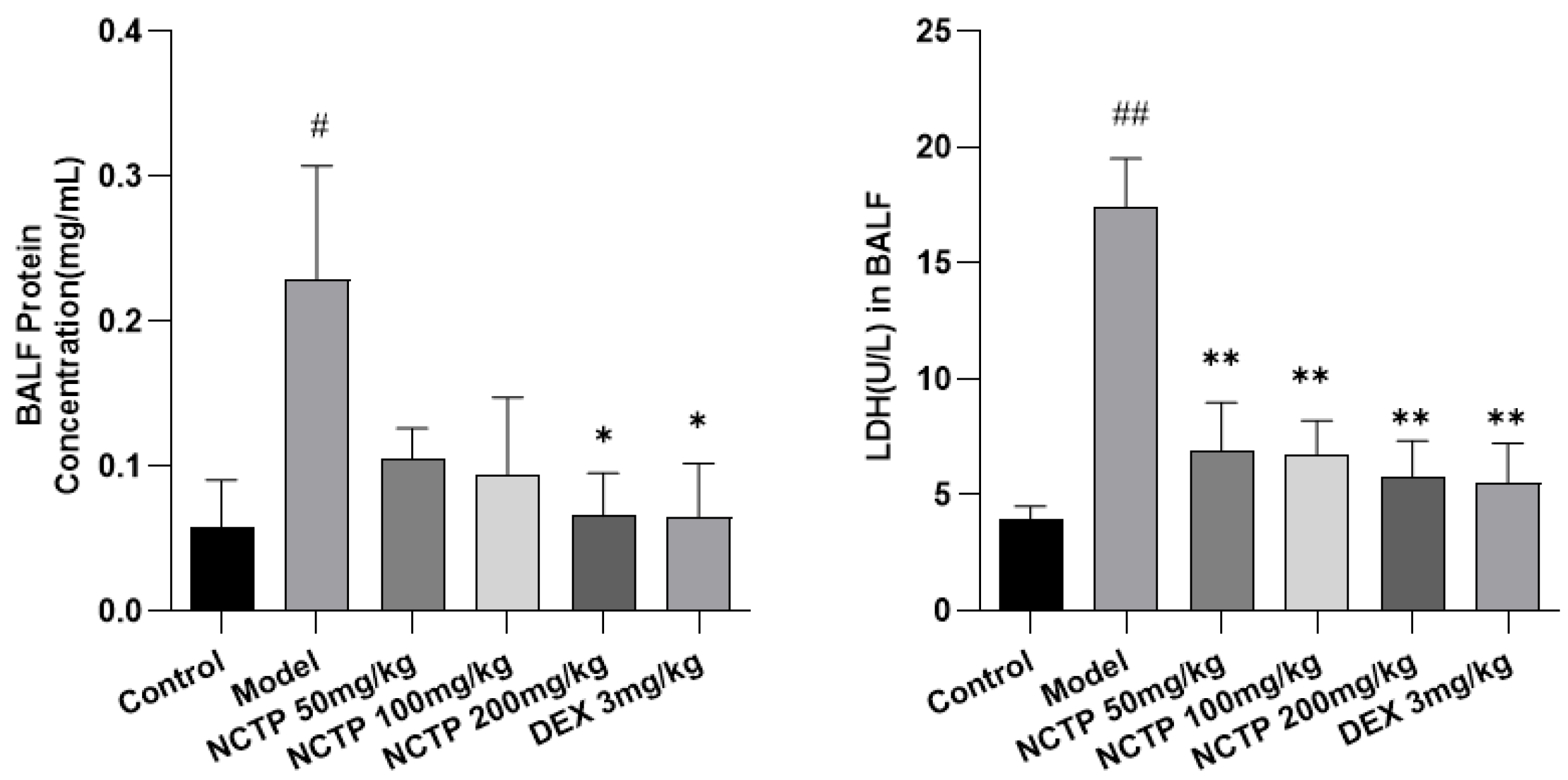
2.3. Effect of NCTP on BALF BCA and LDH Activities in ALI Mice
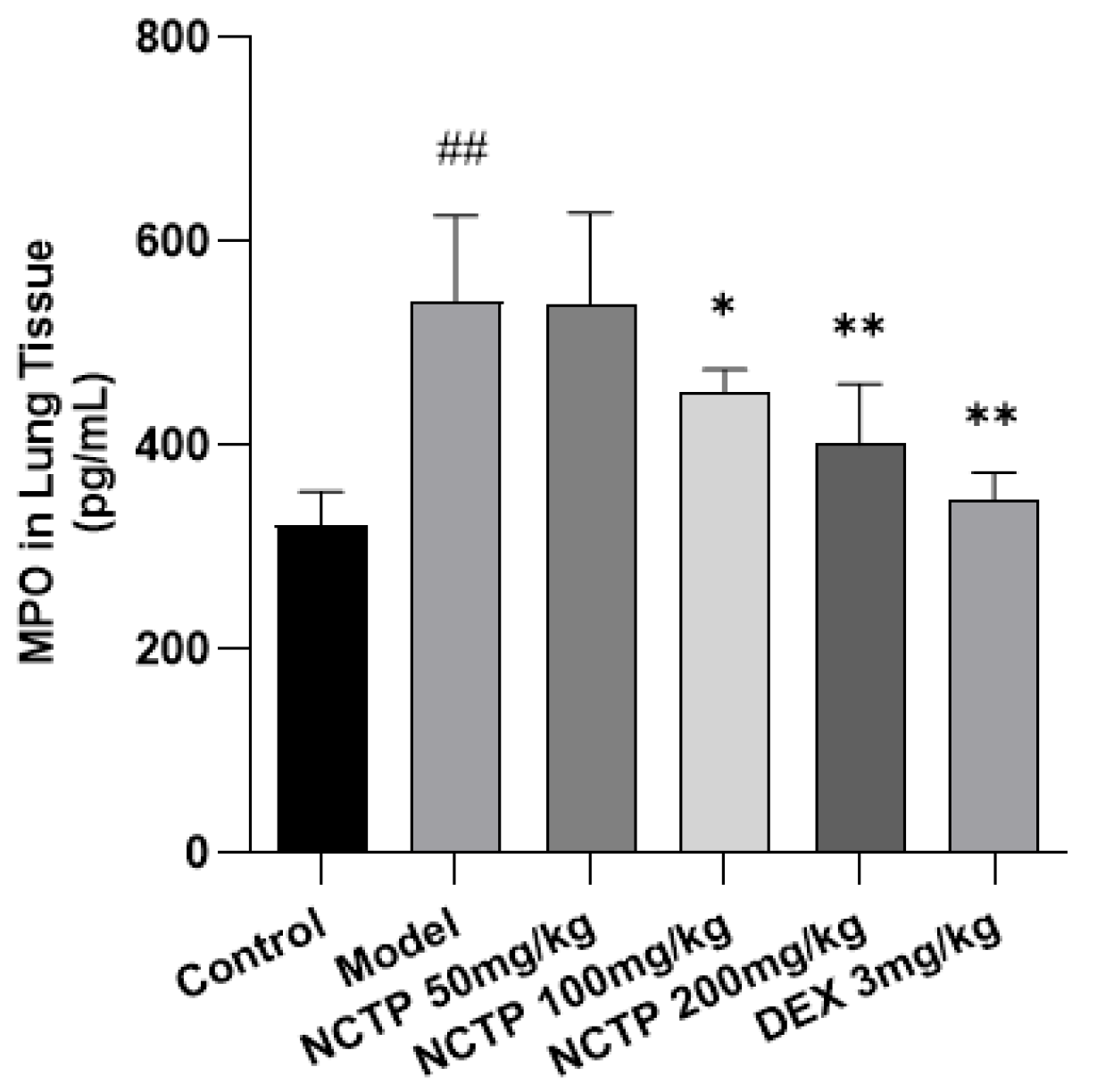
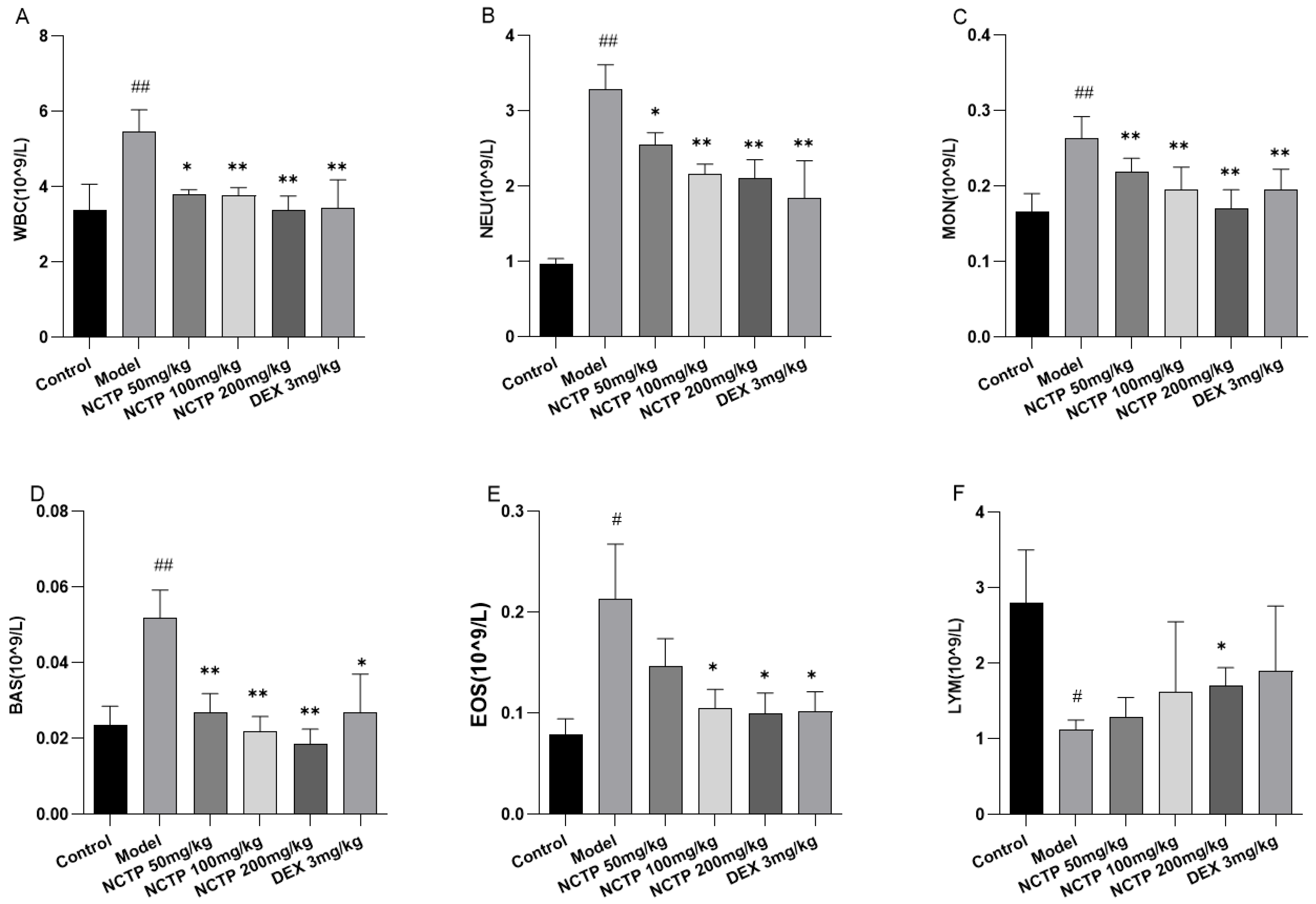
2.4. Effect of NCTP on Inflammatory Cells and Pro-Inflammatory Cytokines in ALI Mice
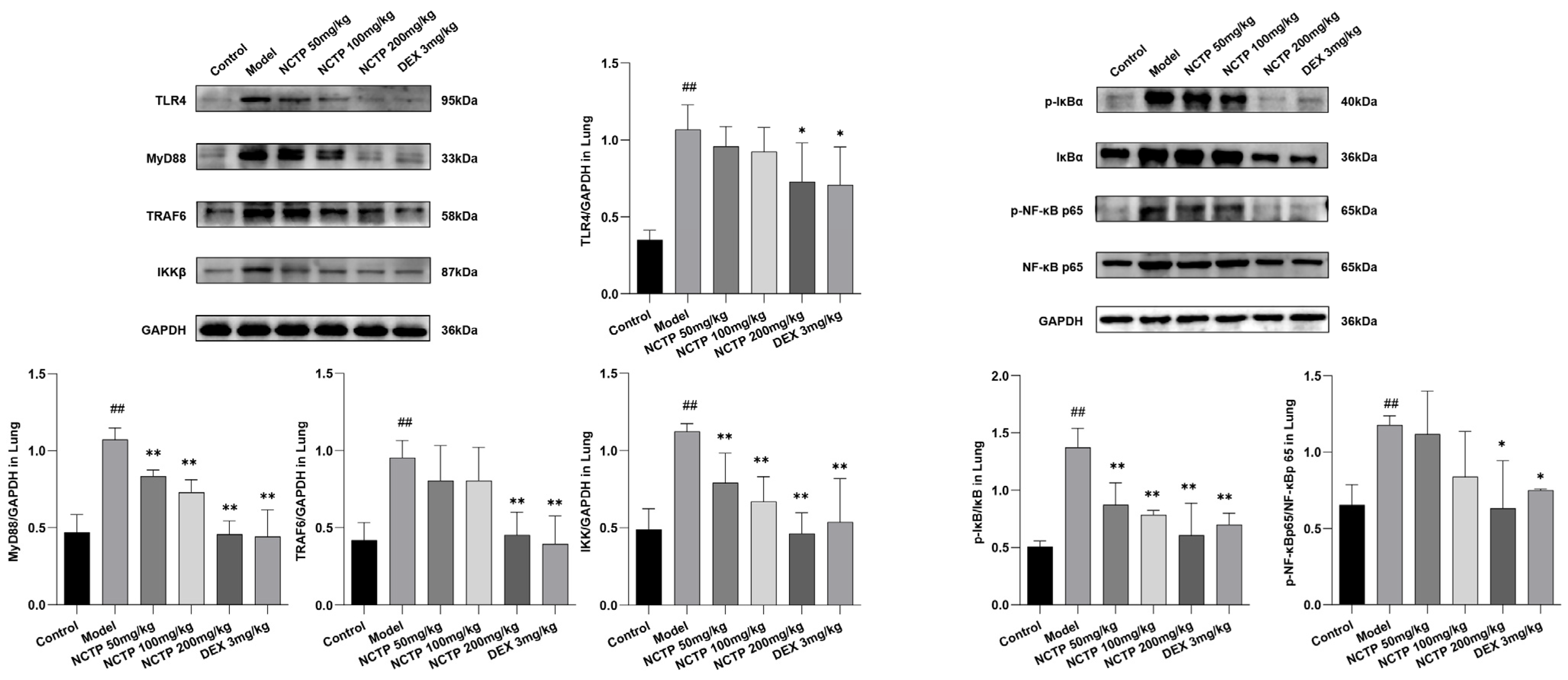
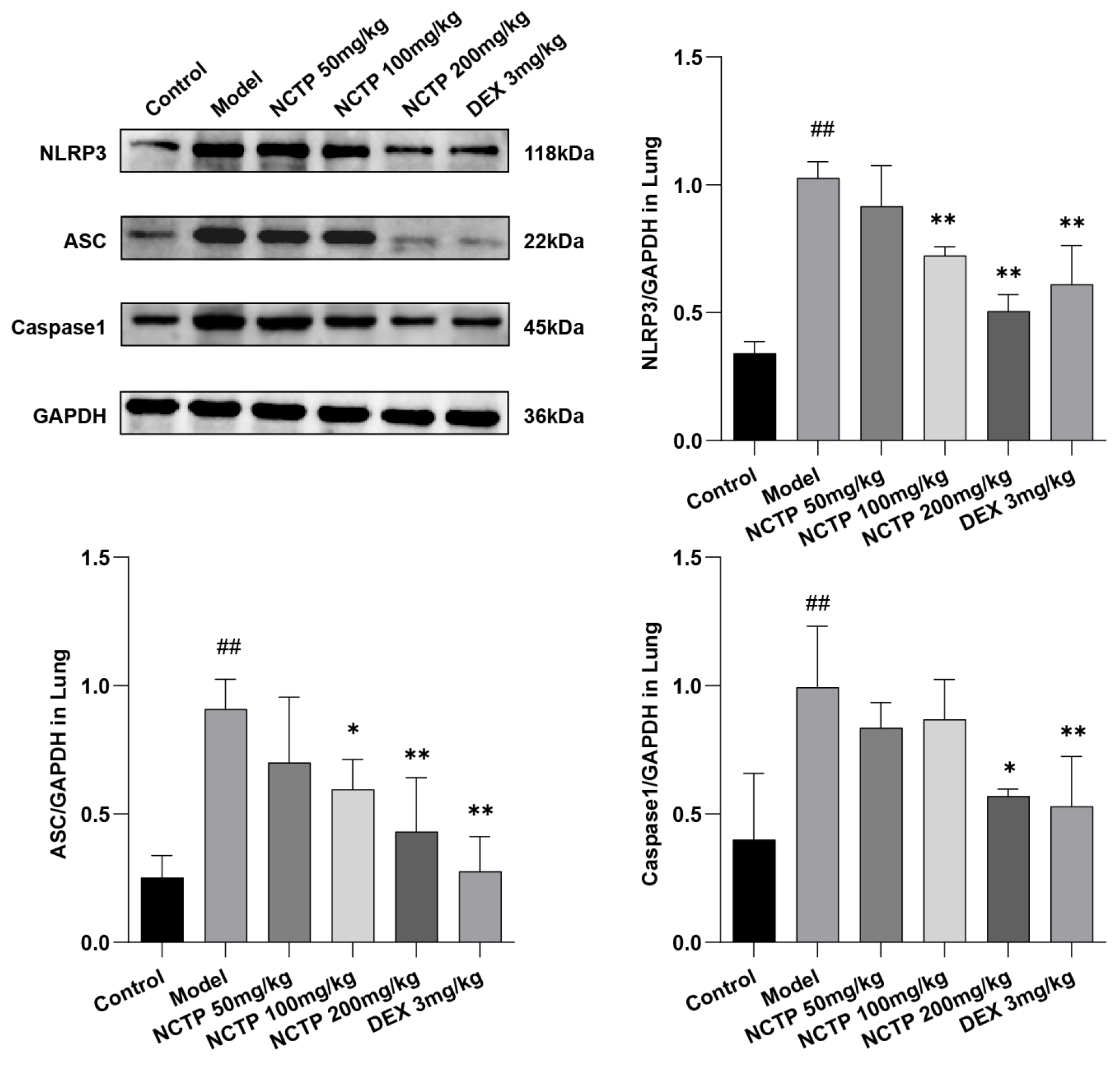
2.5. Effect of NCTP on NF-κB and NLRP3 Signaling Pathway Activation
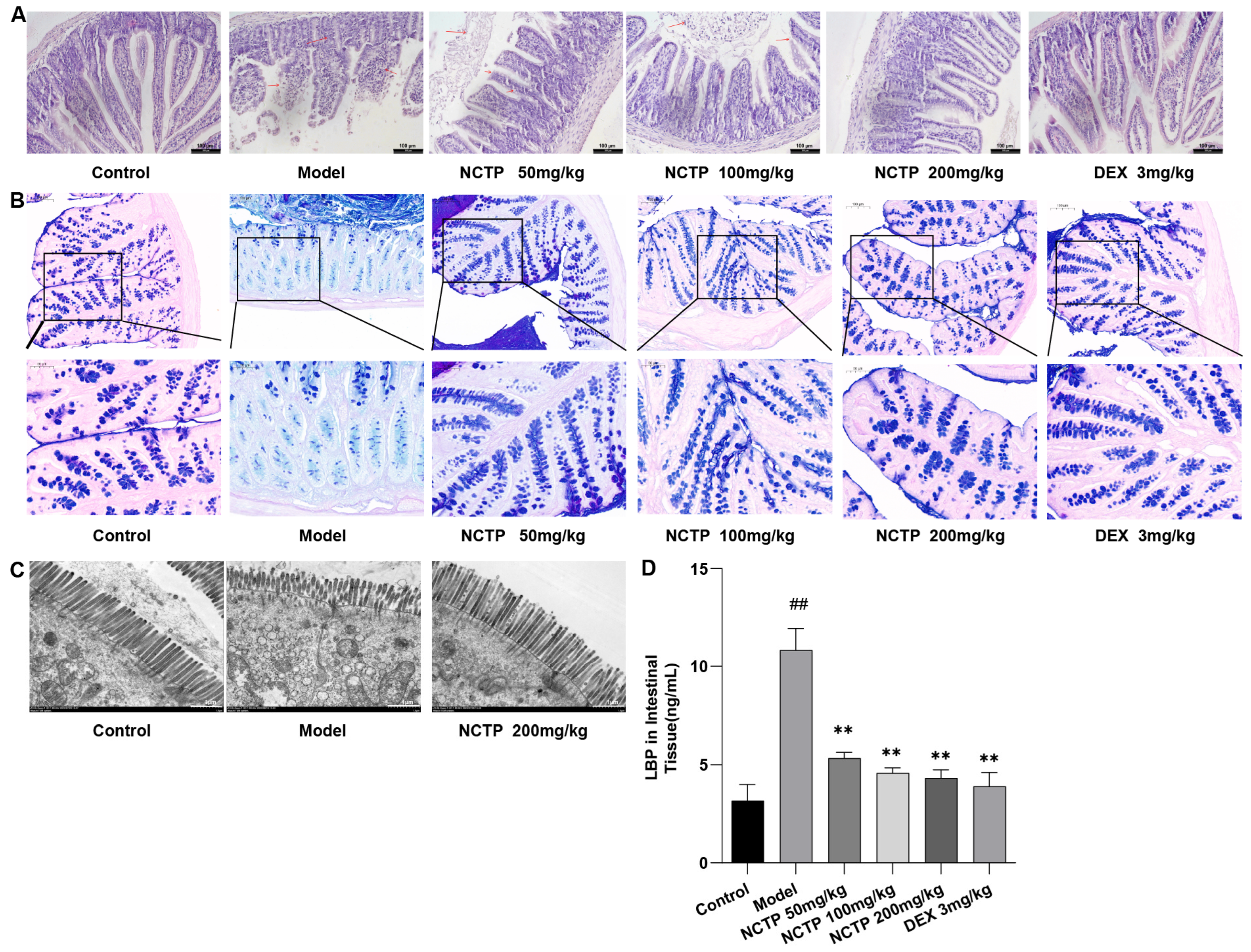
2.6. Protective Effect of NCTP on the Intestinal Mucosa
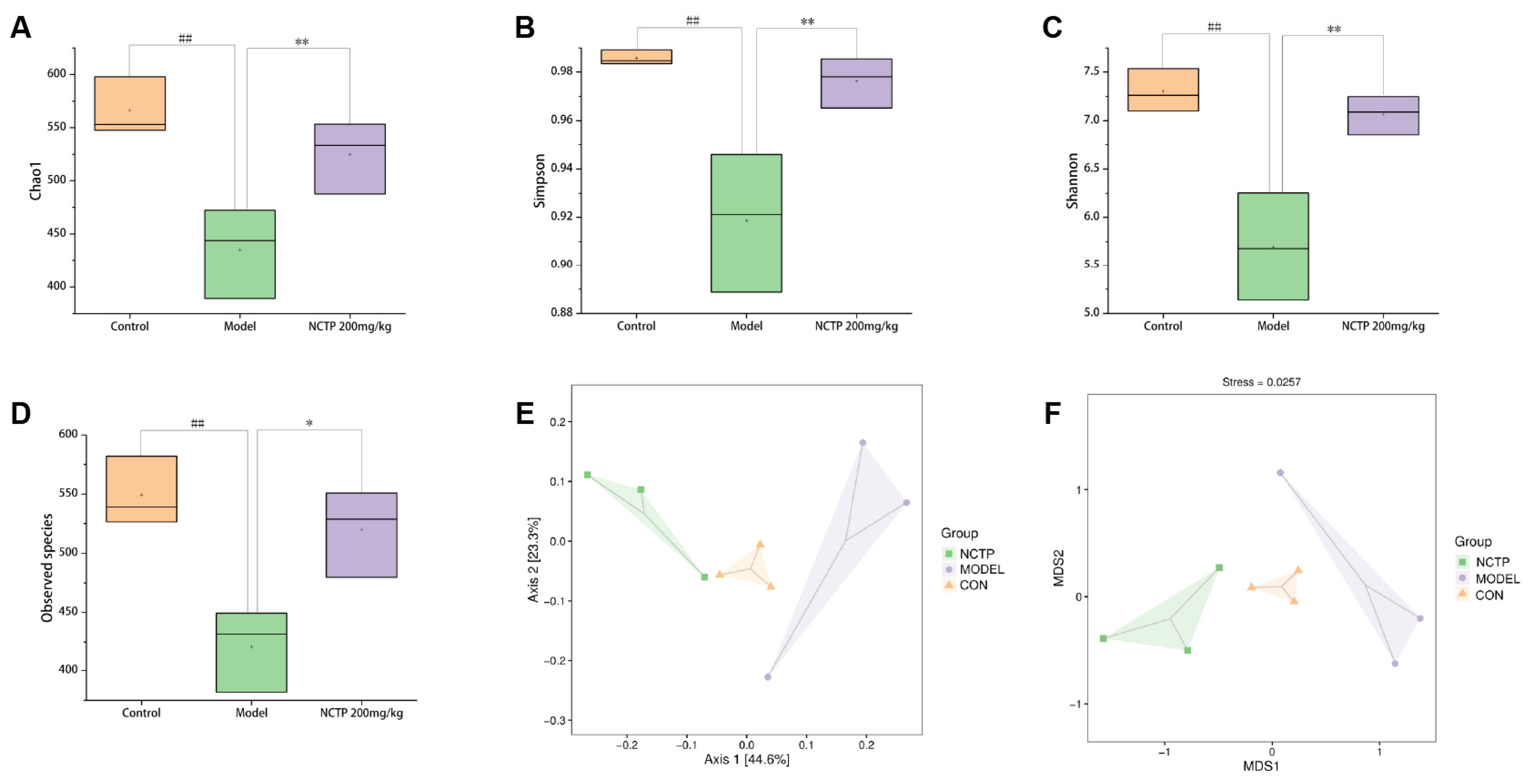
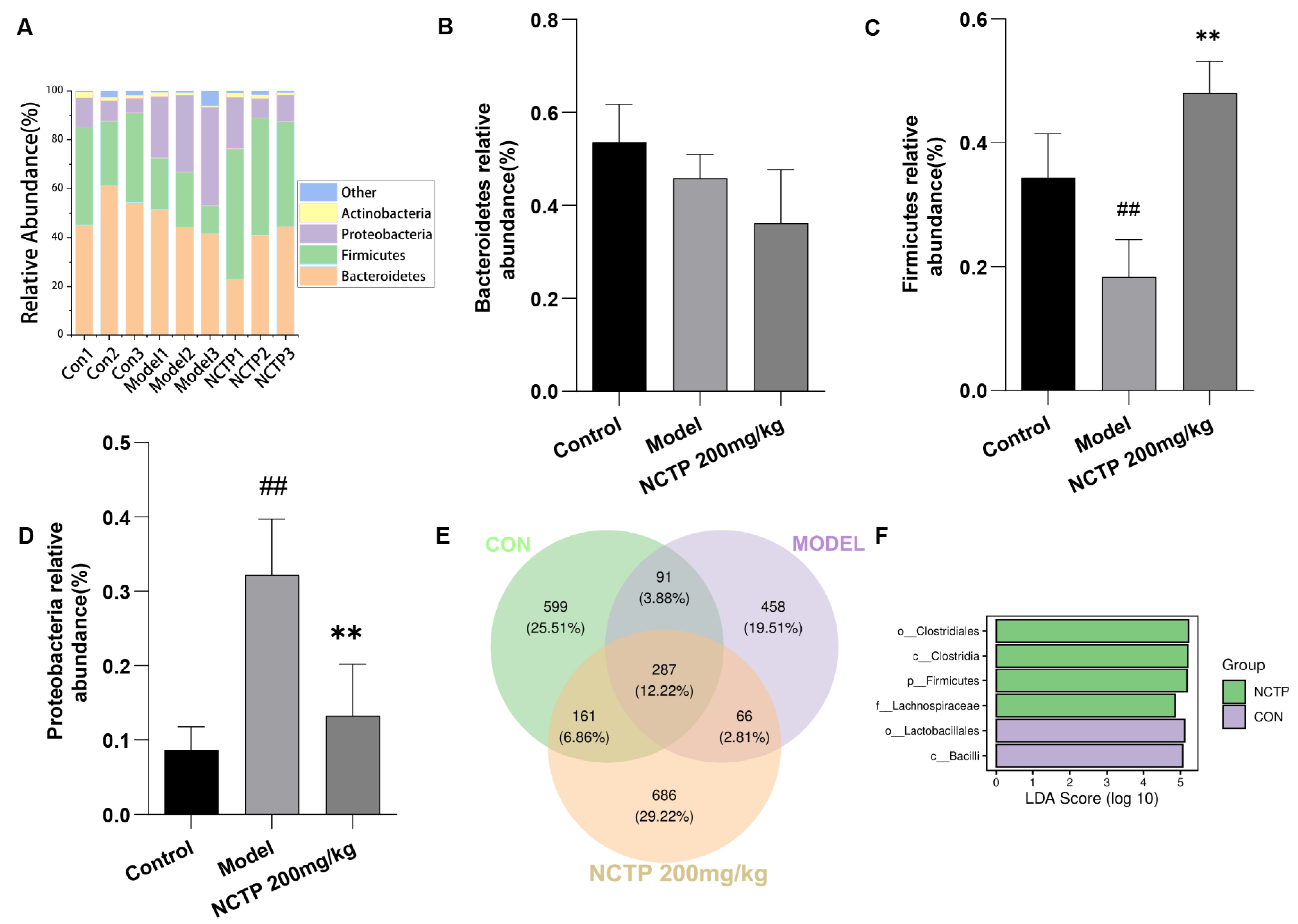
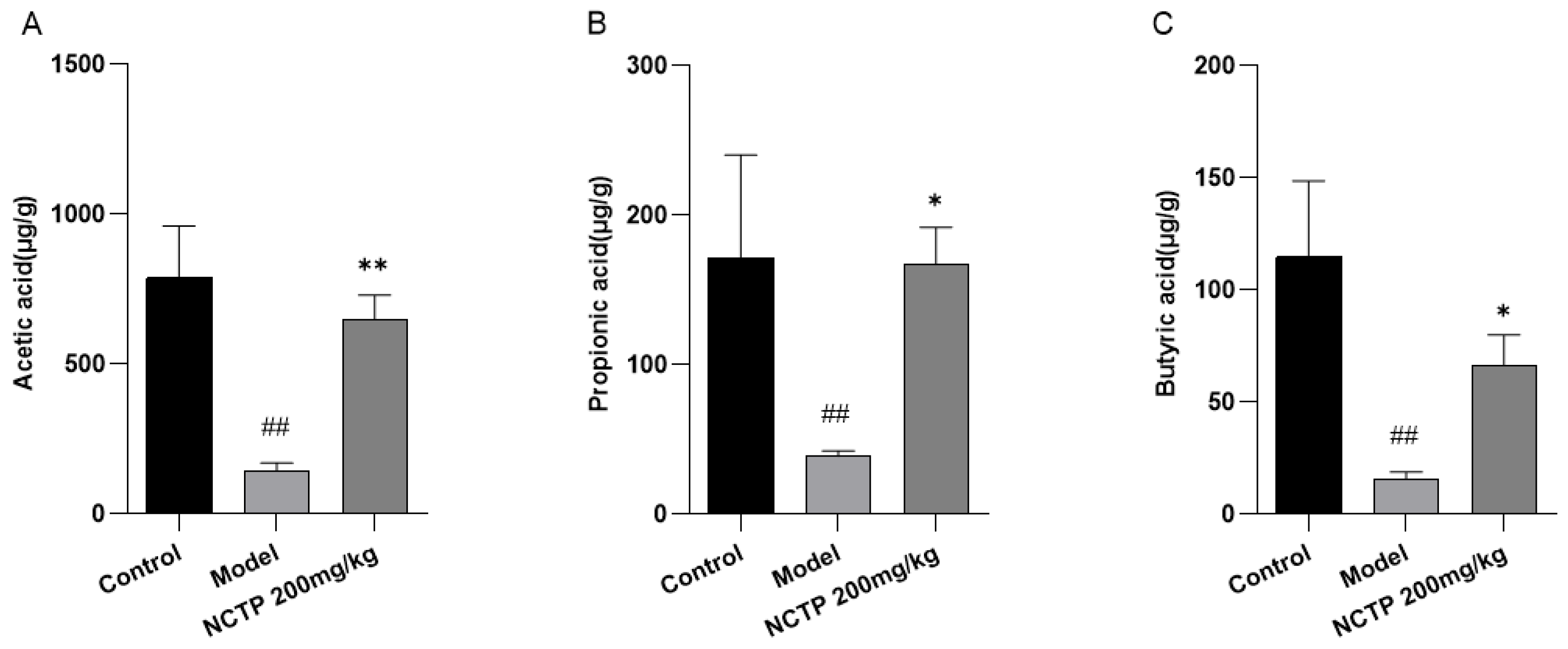
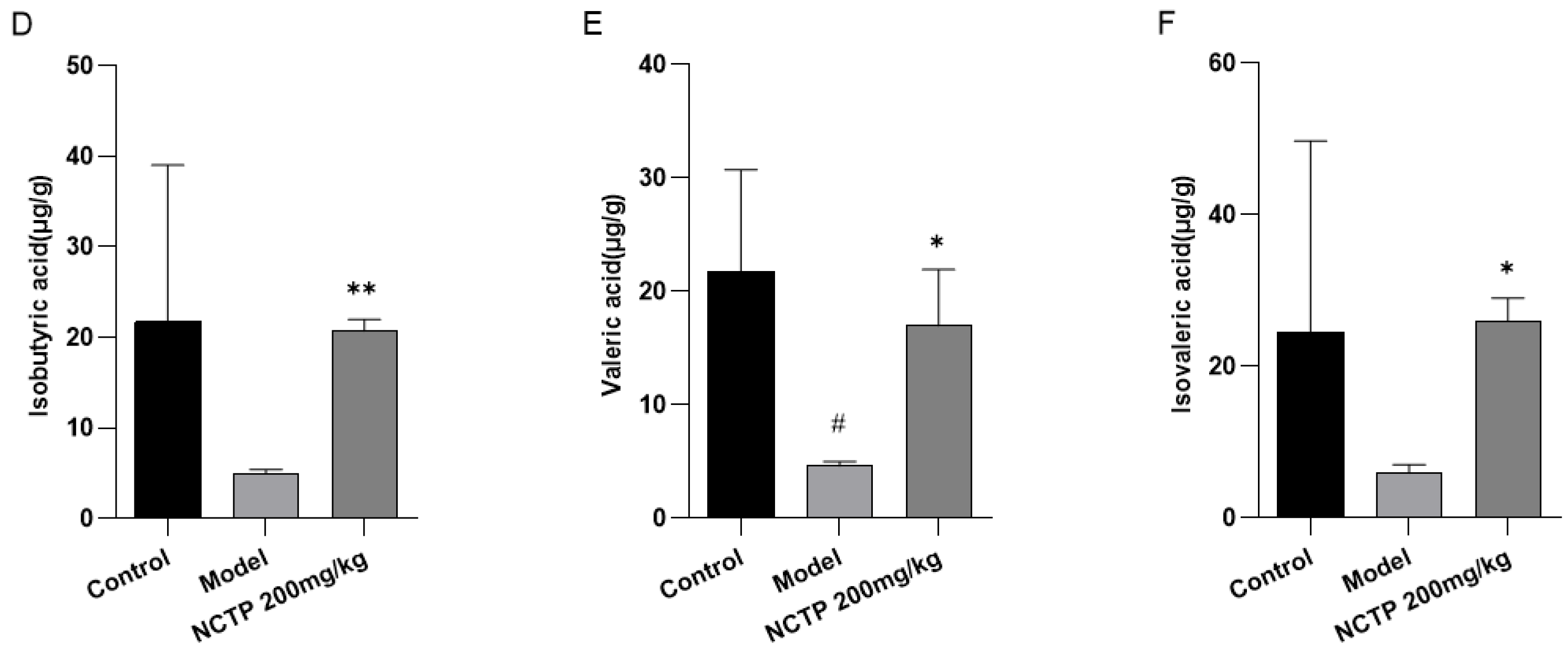
2.7. NCTP Reversed the Dysbiosis of Microbiota Composition and the Reduction in SCFA Secretion in ALI Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials and Preparation of NCTP
4.3. Compound Analysis of NCTP
4.4. Experimental Animals
4.5. Sepsis-Induced Acute Lung Injury (ALI) Model
4.6. Pathologic Analysis and Immunohistochemical Analysis
4.7. BALF Collection, Processing, and Determination of Protein and LDH Concentration
4.8. Measurement of Wet-to-Dry Ratio of the Lungs
4.9. Cell Count and Biochemical Assays
4.10. Determination of Lung Homogenate TNF-α, IL-1β, and IL-6
4.11. Analysis of Gut Microbiota
4.12. Fecal Short-Chain Fatty Acid (SCFA) Quantification Analysis
4.13. Western Blot
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Xu, D.; Liu, J.; Gu, L. Protective effect of sophocarpine on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2019, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef]
- Wang, N.; Geng, C.; Sun, H.; Wang, X.; Li, F.; Liu, X. Hesperetin ameliorates lipopolysaccharide-induced acute lung injury in mice through regulating the TLR4–MyD88–NF-κB signaling pathway. Arch. Pharmacal Res. 2019, 42, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Q.; Wang, D.C.; Ingbar, D.H.; Wang, X. Acute lung injury in patients with COVID-19 infection. Clin. Transl. Med. 2020, 10, 20–27. [Google Scholar] [CrossRef]
- Wang, Z.-F.; Yang, Y.-M.; Fan, H. Diagnostic value of miR-155 for acute lung injury/acute respiratory distress syndrome in patients with sepsis. J. Int. Med. Res. 2020, 48, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, P.; Yang, J.; Zhang, Z.; Wang, H.; Guo, Y.; Liu, M. The protective effect of the flavonoid fraction of Abutilon theophrasti Medic. leaves on LPS-induced acute lung injury in mice via the NF-κB and MAPK signalling pathways. Biomed. Pharmacother. 2019, 109, 1024–1031. [Google Scholar] [CrossRef]
- Huang, X.-T.; Liu, W.; Zhou, Y.; Hao, C.-X.; Zhou, Y.; Zhang, C.-Y.; Sun, C.-C.; Luo, Z.-Q.; Tang, S.-Y. Dihydroartemisinin attenuates lipopolysaccharide-induced acute lung injury in mice by suppressing NF-κB signaling in an Nrf2-dependent manner. Int. J. Mol. Med. 2019, 44, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, L.; Wu, H. Ferulic acid alleviates lipopolysaccharide-induced acute lung injury through inhibiting TLR4/NF-κB signaling pathway. J. Biochem. Mol. Toxicol. 2020, 35, e22664. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut–lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Gao, L.-L.; Ma, J.-M.; Fan, Y.-N.; Zhang, Y.-N.; Ge, R.; Tao, X.-J.; Zhang, M.-W.; Gao, Q.-H.; Yang, J.-J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chang, G.; Huang, J.; Wang, Y.; Ma, N.; Roy, A.-C.; Shen, X. Sodium Butyrate Inhibits the Inflammation of Lipopolysaccharide-Induced Acute Lung Injury in Mice by Regulating the Toll-Like Receptor 4/Nuclear Factor κB Signaling Pathway. J. Agric. Food Chem. 2019, 67, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Liu, J.; Yang, X.; Zhang, Y.; Huang, F. Sinomenine protects against E. coli-induced acute lung injury in mice through Nrf2-NF-κB pathway. Biomed. Pharmacother. 2018, 107, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.M. Chinese Materia Medica—Uyghur Medicine Volume; Shanghai Scientific & Technical Publishers: Shanghai, China, 1999. [Google Scholar]
- Zhao, J.; Tuersunmaimaiti, M.; Ji, T.; Liu, T.; Xu, F. Hepatoprotective activity of isostrictiniin from Nymphaea candida on Con A-induced acute liver injury in mice. Nat. Prod. Res. 2019, 35, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Hou, S.; Yin, H.; Mu, D.; Jiang, D.; Tian, F.; Li, J.J.; Huang, F. The analgesic and anti-inflammatory effects of zukamu granules, a traditional Chinese medical formulation. Pharm. Biol. 2019, 57, 729–735. [Google Scholar] [CrossRef]
- Yu, C.; Li, Y.; Li, Y.; Li, S.; Zeng, F.; Yu, J.; Ji, Z.; Li, K.; Zhai, H. A novel mechanism for regulating lung immune homeostasis: Zukamu granules alleviated acute lung injury in mice by inhibiting NLRP3 inflammasome activation and regulating Th17/Treg cytokine balance. J. Ethnopharmacol. 2024, 324, 117831. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Gu, M.; Li, Y.; Li, Y.; Ji, Z.; Li, K.; Wang, Y.; Zhai, H.; Wang, Y. A novel therapeutic approach for IPF: Based on the “Autophagy-Apoptosis” balance regulation of Zukamu Granules in alveolar macrophages. J. Ethnopharmacol. 2022, 297, 115568. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; You, S.; Liu, T.; Xu, F.; Ji, T.; Gu, Z. Hepatoprotective Effects of Nicotiflorin from Nymphaea candida against Concanavalin A-Induced and D-Galactosamine-Induced Liver Injury in Mice. Int. J. Mol. Sci. 2017, 18, 587. [Google Scholar] [CrossRef]
- Dong, H.j.; Guo, Y.; Liu, T.; Zhao, J. Preventive effect of isostrictiniin from Nymphaea candida on carbon tetrachloride-induced hepatic fibrosis in mice. Nat. Prod. Res. 2022, 37, 628–632. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, Z.; Xu, Y.; Li, C.; Zhao, J.; Liu, T. Anti-inflammatory, analgesic, antitussive and antipyretic activities of polyphenol-enriched fraction from Nymphaea candida. J. Ethnopharmacol. 2024, 324, 117789. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, W.-W.; Wu, R.-T.; Song, Y.-H.; Wu, W.-Y.; Yin, S.-H.; Li, W.-J.; Xie, M.-Y. Protective effect of Ganoderma atrum polysaccharides in acute lung injury rats and its metabolomics. Int. J. Biol. Macromol. 2020, 142, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.; Incani, A.; Serreli, G.; Porru, L.; Melis, M.P.; Tuberoso, C.I.G.; Rossin, D.; Biasi, F.; Deiana, M. Olive oil polyphenols reduce oxysterols -induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018, 17, 348–354. [Google Scholar] [CrossRef]
- He, Y.; Wen, Q.; Yao, F.; Xu, D.; Huang, Y.; Wang, J. Gut–lung axis: The microbial contributions and clinical implications. Crit. Rev. Microbiol. 2016, 43, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Jiang, T.; Li, P.; Zhao, J.; Dai, L.; Sun, D.; Liu, M.; An, L.; Jia, L.; Jing, X.; Wang, H.; et al. Long-chain polyunsaturated fatty acids improve airway pathological features and gut microbial imbalances in BALB/c mice with ovalbumin-induced asthma. J. Funct. Foods 2021, 81, 104465. [Google Scholar] [CrossRef]
- Li, N.; Dai, Z.; Wang, Z.; Deng, Z.; Zhang, J.; Pu, J.; Cao, W.; Pan, T.; Zhou, Y.; Yang, Z.; et al. Gut microbiota dysbiosis contributes to the development of chronic obstructive pulmonary disease. Respir. Res. 2021, 22, 274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, Y. Gut-Lung Crosstalk in Sepsis-Induced Acute Lung Injury. Front. Microbiol. 2021, 12, 779620. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, Y.-F.; Fan, G.-W.; Wang, X.-Y.; Xu, S.-Y.; Zhu, Y. Balancing Herbal Medicine and Functional Food for Prevention and Treatment of Cardiometabolic Diseases through Modulating Gut Microbiota. Front. Microbiol. 2017, 8, 270196. [Google Scholar] [CrossRef]
- Guo, C.-E.; Cui, Q.; Cheng, J.; Chen, J.; Zhao, Z.; Guo, R.; Dai, X.; Wei, Z.; Li, W. Probiotic-fermented Chinese dwarf cherry [Cerasus humilis (Bge.) Sok.] juice modulates the intestinal mucosal barrier and increases the abundance of Akkermansia in the gut in association with polyphenols. J. Funct. Foods 2021, 80, 104424. [Google Scholar] [CrossRef]
- Huppert, L.; Matthay, M.; Ware, L. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Tuerdi, B.; Zuo, L.; Ma, Y.; Wang, K. Downregulation of miR-155 attenuates sepsis-induced acute lung injury by targeting SIRT1. Int. J. Clin. Exp. Pathol. 2018, 11, 4483–4492. [Google Scholar] [PubMed]
- Wang, X.; Xu, T.; Jin, J.; Ting Gao, M.M.; Wan, B.; Gong, M.; Bai, L.; Lv, T.; Song, Y. Topotecan reduces sepsis-induced acute lung injury and decreases the inflammatory response via the inhibition of the NF-kappaB signaling pathway. Pulm. Circ. 2022, 12, e12070. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Feng, S.W.; Xu, G.H.; Chen, Y.Y.; Shi, Y.Y. Effects of total ginsenosides from Panax ginseng stems and leaves on gut microbiota and short-chain fatty acids metabolism in acute lung injury mice. Zhongguo Zhong Yao Za Zhi 2023, 48, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, W.; Zang, L.; Liu, F.; Yao, Q.; Zhao, J.; Zhi, W.; Niu, X. Fraxin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting the NF-kappaB and NLRP3 signalling pathways. Int. Immunopharmacol. 2019, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Su, Q.; Wang, H.; Guo, S.; Chen, Y.; Duan, J.; Wang, S. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. Int. Immunopharmacol. 2015, 27, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, Y.; Sun, H.; Xiong, Y.; Zhong, L.; Wu, Z.; Yang, M. An untargeted metabolomics approach to investigate the wine-processed mechanism of Scutellariae radix in acute lung injury. J. Ethnopharmacol. 2020, 253, 112665. [Google Scholar] [CrossRef]
- Jacobs, M.C.; Lankelma, J.M.; Wolff, N.S.; Hugenholtz, F.; de Vos, A.F.; van der Poll, T.; Wiersinga, W.J. Effect of antibiotic gut microbiota disruption on LPS-induced acute lung inflammation. PLoS ONE 2020, 15, e0241748. [Google Scholar] [CrossRef]
- Ju, M.; Liu, B.; He, H.; Gu, Z.; Liu, Y.; Su, Y.; Zhu, D.; Cang, J.; Luo, Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting in fl ammation and apoptosis through modulating TLR4/MyD88/NF-kappaB pathway. Cell Cycle 2018, 17, 2001–2018. [Google Scholar] [CrossRef]
- Gao, H.; Xiao, D.; Gao, L.; Li, X. MicroRNA-93 contributes to the suppression of lung inflammatory responses in LPS-induced acute lung injury in mice via the TLR4/MyD88/NF-kappaB signaling pathway. Int. J. Mol. Med. 2020, 46, 561–570. [Google Scholar] [CrossRef]
- Wang, X.; Deng, R.; Dong, J.; Huang, L.; Li, J.; Zhang, B. Eriodictyol ameliorates lipopolysaccharide-induced acute lung injury by suppressing the inflammatory COX-2/NLRP3/NF-kappaB pathway in mice. J. Biochem. Mol. Toxicol. 2020, 34, e22434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, W.; Gui, S. Procyanidin B2 protects rats from paraquat-induced acute lung injury by inhibiting NLRP3 inflammasome activation. Immunobiology 2018, 223, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Liu, Z.; Yu, L. Dexmedetomidine attenuates lipopolysaccharide induced acute lung injury by targeting NLRP3 via miR-381. J. Biochem. Mol. Toxicol. 2018, 32, e22211. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ni, L.; Zhang, X.; Zhang, J.; Abdel-Razek, O.; Wang, G. Surfactant Protein D Dampens Lung Injury by Suppressing NLRP3 Inflammasome Activation and NF-kappaB Signaling in Acute Pancreatitis. Shock 2019, 51, 557–568. [Google Scholar] [CrossRef]
- Chioma, O.S.; Mallott, E.K.; Chapman, A.; Van Amburg, J.C.; Wu, H.; Shah-Gandhi, B.; Dey, N.; Kirkland, M.E.; Blanca Piazuelo, M.; Johnson, J.; et al. Gut microbiota modulates lung fibrosis severity following acute lung injury in mice. Commun. Biol. 2022, 5, 1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Li, F.; Ma, S.; Zhao, L.; Ge, P.; Wen, H.; Zhang, Y.; Liu, X.; Luo, L.; et al. Mechanisms of Qingyi Decoction in Severe Acute Pancreatitis Associated Acute Lung Injury via Gut Microbiota: Targeting the Short-Chain Fatty Acids-Mediated AMPK/NF-kB/NLRP3 Pathway. Microbiol. Spectr. 2023, 11, e0366422. [Google Scholar] [CrossRef]
- Bai, R.-X.; Xu, Y.-Y.; Qin, G.; Chen, Y.-M.; Wang, H.-F.; Wang, M.; Du, S.-Y. Repression of TXNIP-NLRP3 axis restores intestinal barrier function via inhibition of myeloperoxidase activity and oxidative stress in nonalcoholic steatohepatitis. J. Cell Physiol. 2019, 234, 7524–7538. [Google Scholar] [CrossRef]
- Wang, Z.; Li, F.; Liu, J.; Luo, Y.; Guo, H.; Yang, Q.; Xu, C.; Ma, S.; Chen, H. Intestinal Microbiota—An Unmissable Bridge to Severe Acute Pancreatitis-Associated Acute Lung Injury. Front. Immunol. 2022, 13, 913178. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Liu, S.; Duan, L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Wu, Y.; Liu, X.; Wang, J.; Han, D. Ellagic Acid Alleviates Diquat-Induced Jejunum Oxidative Stress in C57BL/6 Mice through Activating Nrf2 Mediated Signaling Pathway. Nutrients 2022, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Rønning, S.B.; Voldvik, V.; Bergum, S.K.; Aaby, K.; Borge, G.I.A. Ellagic acid and urolithin A modulate the immune response in LPS-stimulated U937 monocytic cells and THP-1 differentiated macrophages. Food Funct. 2020, 11, 7946–7959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, P.; Tang, C.; Wang, Y.; Li, Y.; Zhang, H. Antinociceptive and anti-inflammatory activities of extract and two isolated flavonoids of Carthamus tinctorius L. J. Ethnopharmacol. 2014, 151, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhong, R.; Yang, Y.; Xia, T.; Wang, W.; Wang, Y.; Xing, N.; Luo, Y.; Li, S.; Shang, L.; et al. Systems pharmacology reveals the mechanism of activity of Ge-Gen-Qin-Lian decoction against LPS-induced acute lung injury: A novel strategy for exploring active components and effective mechanism of TCM formulae. Pharmacol. Res. 2020, 156, 104759. [Google Scholar] [CrossRef] [PubMed]
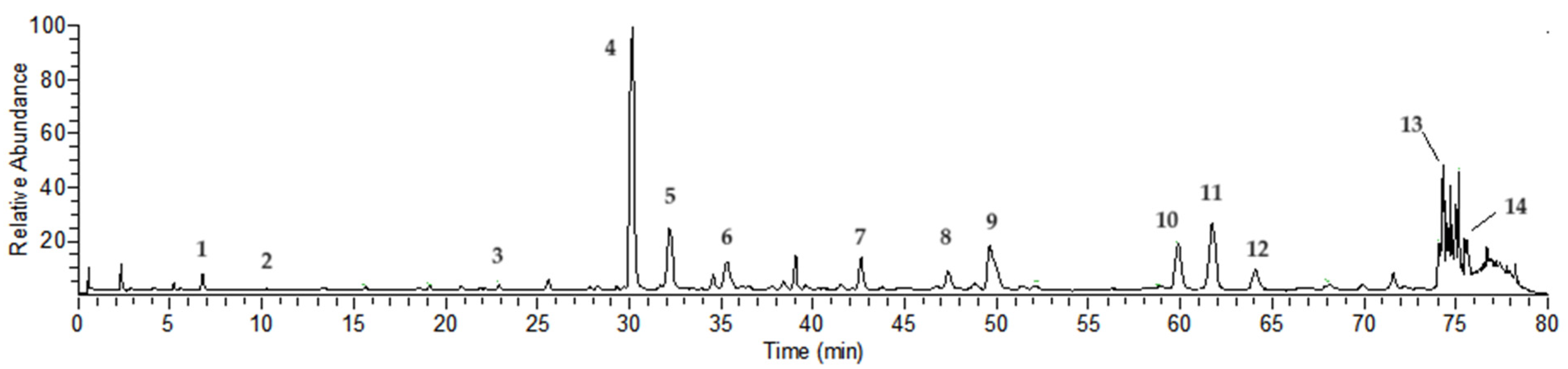



| Peak No | Rt (min) | [M-H]− | Error | MS/MS m/z (% of Base Peak) | Formula | Compound Identity |
|---|---|---|---|---|---|---|
| 1 a | 6.75 | 169.01385 | 4.17 | 125 (100) | C7H6O5 | Gallic acid |
| 2 | 10.29 | 483.07758 | 1.33 | 331 (20), 169 (100), 125 (80) | C20H20O14 | Digalloyl-glucoside |
| 3 | 21.87 | 635.08838 | 0.77 | 483 (40), 313 (20), 169 (100), 125 (80) | C27H24O18 | Trigalloyl-glucoside |
| 4 a | 30.19 | 633.07281 | 0.91 | 463 (20), 301 (100), 275 (20), 185 (10) | C27H22O18 | Isostrictiniin |
| 5 a | 32.15 | 197.04503 | 0.77 | 169 (100), 125 (50) | C9H10O5 | Gallic acid ethyl ester |
| 6 | 35.44 | 785.08282 | −0.48 | 633 (10), 301 (100), 275 (50), 257 (10), 169 (40), 125 (30) | C34H26O22 | Digalloyl-ellagitannoyl-glucoside |
| 7 | 42.54 | 787.09906 | 0.26 | 635 (20), 617 (70), 465 (40), 313 (30), 169 (100), 125 (60) | C34H28O22 | Tetragalloyl-glucoside |
| 8 | 47.28 | 433.04095 | 1.85 | 301 (100), 271 (5), 243 (5) | C19H13O12 | Quercetin-O-arabinoside |
| 9 a | 49.69 | 300.99802 | 0.41 | 283 (20), 229 (10), 185 (20) | C14H6O8 | Ellagic acid |
| 10 a | 59.92 | 939.10931 | −0.53 | 787 (20), 769 (100), 465 (10), 313 (10), 169 (90), 125 (80) | C41H32O26 | Pentagalloyl-glucoside |
| 11 a | 61.66 | 593.15015 | 0.08 | 285 (80), 255 (60), 227 (40), 151 (10) | C27H30O15 | Nicotiflorin |
| 12 a | 64.07 | 447.09293 | 1.65 | 285 (60), 255 (70), 227 (50), 151 (10) | C21H20O11 | Kaempferol O-glucoside |
| 13 | 74.66 | 447.09305 | 1.92 | 300 (100), 271 (70), 227 (50), 169 (100), 125 (20) | C21H20O11 | Ellagitannoyl-rhamnoside |
| 14 a | 75.47 | 285.03970 | 1.18 | 257 (10), 229 (10), 151 (10) | C15H10O6 | Kaempferol |
| Group | Pre-Modeling Weight (g) | Post-Modeling Weight (g) | W/D Ratio |
|---|---|---|---|
| Control | 24.72 ± 0.58 | 22. 54 ± 0.51 | 3.96 ± 0.46 |
| Model | 23.38 ± 1.32 | 20.48 ± 1.29 | 5.62 ± 0.42 ## |
| NCTP, 50 mg/kg | 23.08 ± 0.82 | 21.28 ± 0.66 | 4.37 ± 0.77 |
| NCTP, 100 mg/kg | 22.90 ± 0.69 | 20.76 ± 0.48 | 4.26 ± 0.66 * |
| NCTP, 200 mg/kg | 23.01 ± 1.25 | 21.14 ± 1.10 | 4.38 ± 0.33 ** |
| DEX, 3 mg/kg | 23.04 ± 1.56 | 21.22 ± 1.10 | 4.12 ± 0.30 ** |
| Group | n | The Degree of Lung Injury | Rank Mean | |||
|---|---|---|---|---|---|---|
| 0 | Ⅰ | Ⅱ | Ⅲ | |||
| Control | 6 | 6 | 0 | 0 | 0 | 9.50 |
| Model | 6 | 0 | 0 | 6 | 0 | 33.50 ## |
| NCTP, 50 mg/kg | 6 | 0 | 6 | 0 | 0 | 24.50 |
| NCTP, 100 mg/kg | 6 | 3 | 3 | 0 | 0 | 17.00 * |
| NCTP, 200 mg/kg | 6 | 4 | 2 | 0 | 0 | 14.50 ** |
| DEX, 3 mg/kg | 6 | 5 | 1 | 0 | 0 | 12.00 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Qi, X.; Xu, L.; Sun, Y.; Chen, Y.; Yao, Y.; Zhao, J. Preventive Effect of the Total Polyphenols from Nymphaea candida on Sepsis-Induced Acute Lung Injury in Mice via Gut Microbiota and NLRP3, TLR-4/NF-κB Pathway. Int. J. Mol. Sci. 2024, 25, 4276. https://doi.org/10.3390/ijms25084276
Li C, Qi X, Xu L, Sun Y, Chen Y, Yao Y, Zhao J. Preventive Effect of the Total Polyphenols from Nymphaea candida on Sepsis-Induced Acute Lung Injury in Mice via Gut Microbiota and NLRP3, TLR-4/NF-κB Pathway. International Journal of Molecular Sciences. 2024; 25(8):4276. https://doi.org/10.3390/ijms25084276
Chicago/Turabian StyleLi, Chenyang, Xinxin Qi, Lei Xu, Yuan Sun, Yan Chen, Yuhan Yao, and Jun Zhao. 2024. "Preventive Effect of the Total Polyphenols from Nymphaea candida on Sepsis-Induced Acute Lung Injury in Mice via Gut Microbiota and NLRP3, TLR-4/NF-κB Pathway" International Journal of Molecular Sciences 25, no. 8: 4276. https://doi.org/10.3390/ijms25084276





