Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague–Dawley Rats
Abstract
1. Introduction
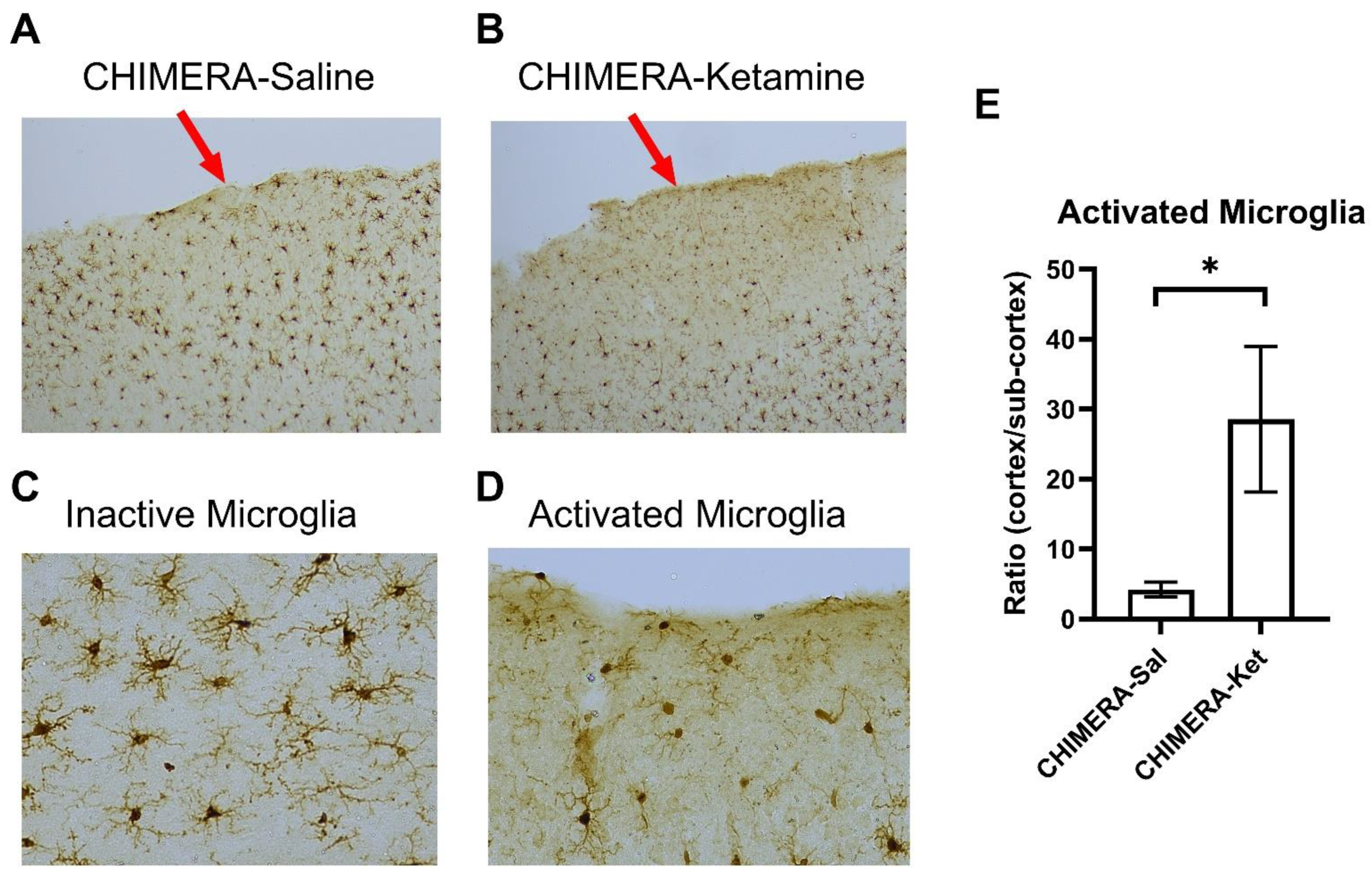
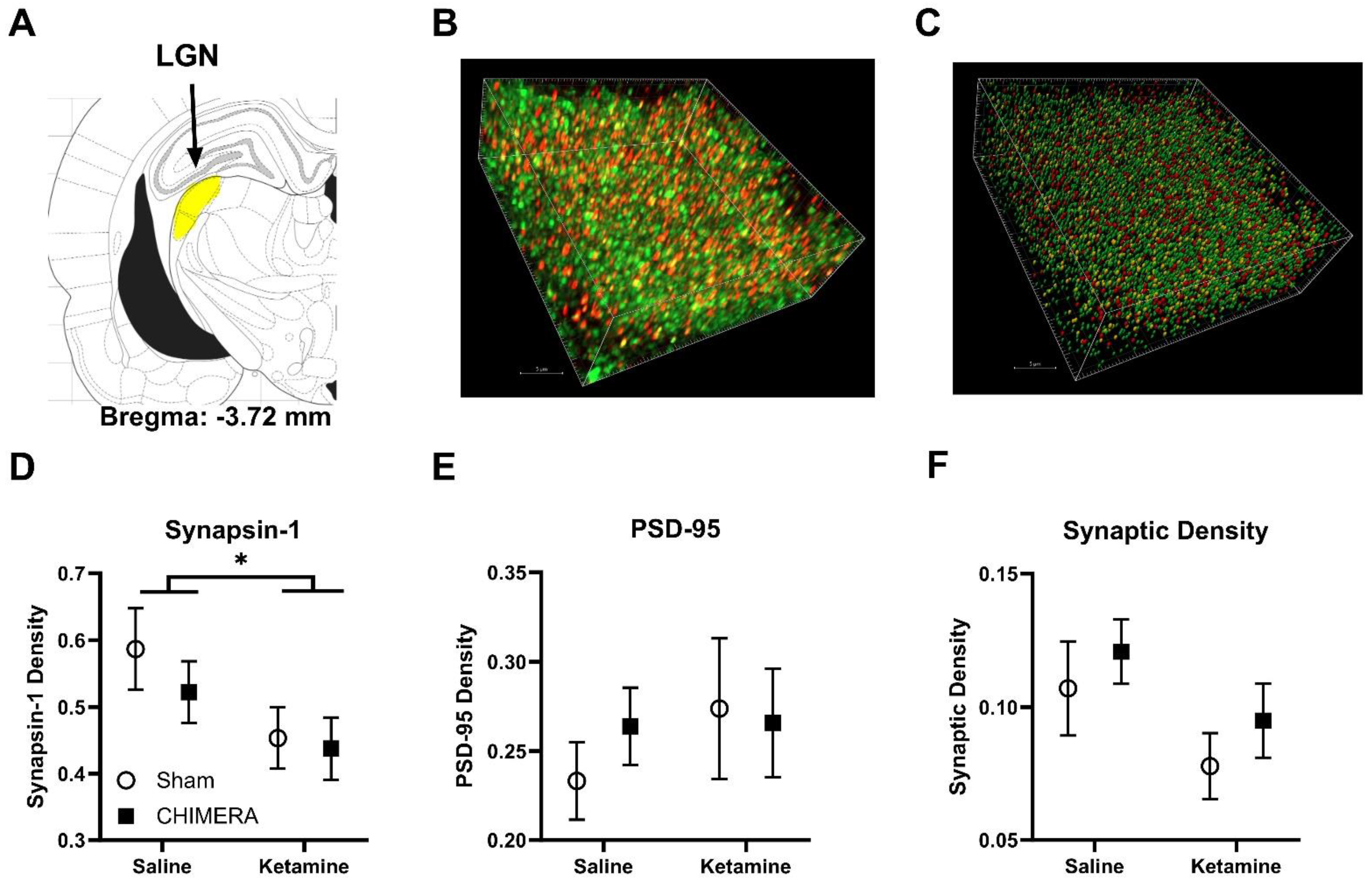
2. Results
3. Discussion
4. Methods
4.1. Animals
4.2. CHIMERA and Ketamine Infusion
4.3. Brain Tissue Collection
4.4. Silver Staining
4.5. Iba-1 Immunohistochemistry
4.6. Synapsin and PSD-95 Immunohistochemistry
4.7. SEQUIN
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Report to Congress on Traumatic Brain Injury Epidemiology and Rehabilitation; CDC: Atlanta, GA, USA, 2015. [Google Scholar]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS ONE 2017, 12, e0174847. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, C.; Verville, L.; Stubbs, J.L.; Yu, H.; Hincapie, C.A.; Cassidy, J.D.; Wong, J.J.; Shearer, H.M.; Connell, G.; Southerst, D.; et al. Post-Concussion Symptoms and Disability in Adults with Mild Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2023, 40, 1045–1059. [Google Scholar] [CrossRef]
- Va/DoD. VA/DoD Clinical Practice Guideline for the Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury; Va/DoD: Washington, DC, USA, 2021. [Google Scholar]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.B.; Leite-Morris, K.A.; Wang, L.; Rumbika, K.K.; Heinrichs, S.C.; Zeng, X.; Wu, L.; Arena, D.T.; Teng, Y.D. Pathophysiological Bases of Comorbidity: Traumatic Brain Injury and Post-Traumatic Stress Disorder. J. Neurotrauma 2018, 35, 210–225. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H.F.; Boese, M.; Berman, R.Y.; Radford, K.D.; Choi, K.H. Effects of a Subanesthetic Ketamine Infusion on Inflammatory and Behavioral Outcomes after Closed Head Injury in Rats. Bioengineering 2023, 10, 941. [Google Scholar] [CrossRef]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Q.; Ye, Y.; Chen, F.; Han, W.C.; Sun, J.M.; Lu, X.; Guo, R.; Cao, K.; Zheng, M.J.; Liao, L.C. Posttraumatic administration of a sub-anesthetic dose of ketamine exerts neuroprotection via attenuating inflammation and autophagy. Neuroscience 2017, 343, 30–38. [Google Scholar] [CrossRef]
- CDC. Surveillance Report of Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014; CDC: Atlanta, GA, USA, 2019. [Google Scholar]
- Namjoshi, D.R.; Cheng, W.H.; McInnes, K.A.; Martens, K.M.; Carr, M.; Wilkinson, A.; Fan, J.; Robert, J.; Hayat, A.; Cripton, P.A.; et al. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): A novel, surgery-free model of traumatic brain injury. Mol. Neurodegener. 2014, 9, 55. [Google Scholar] [CrossRef]
- Kleiven, S. Why Most Traumatic Brain Injuries are Not Caused by Linear Acceleration but Skull Fractures are. Front. Bioeng. Biotechnol. 2013, 1, 15. [Google Scholar] [CrossRef]
- Malik, S.; Alnaji, O.; Malik, M.; Gambale, T.; Farrokhyar, F.; Rathbone, M.P. Inflammatory cytokines associated with mild traumatic brain injury and clinical outcomes: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1123407. [Google Scholar] [CrossRef]
- Narayana, P.A. White matter changes in patients with mild traumatic brain injury: MRI perspective. Concussion 2017, 2, CNC35. [Google Scholar] [CrossRef]
- Smith, C.; Gentleman, S.M.; Leclercq, P.D.; Murray, L.S.; Griffin, W.S.; Graham, D.I.; Nicoll, J.A. The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 2013, 39, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; Cheng, W.H.; Bashir, A.; Wilkinson, A.; Stukas, S.; Martens, K.M.; Whyte, T.; Abebe, Z.A.; McInnes, K.A.; Cripton, P.A.; et al. Defining the biomechanical and biological threshold of murine mild traumatic brain injury using CHIMERA (Closed Head Impact Model of Engineered Rotational Acceleration). Exp. Neurol. 2017, 292, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Tucker, L.B.; Fu, A.H.; McCabe, J.T. Hippocampal-Dependent Cognitive Dysfunction following Repeated Diffuse Rotational Brain Injury in Male and Female Mice. J. Neurotrauma 2021, 38, 1585–1606. [Google Scholar] [CrossRef] [PubMed]
- Zietlow, J.; Berns, K.; Jenkins, D.; Zietlow, S. Prehospital Use of Ketamine: Effectiveness in Critically Ill and Injured Patients. Mil. Med. 2019, 184, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Reede, K.; Bartholomew, R.; Nielsen, D.; Ahmeti, M.; Zreik, K. Ketamine in Trauma: A Literature Review and Administration Guidelines. Cureus 2023, 15, e48099. [Google Scholar] [CrossRef] [PubMed]
- Eikermann, M.; Grosse-Sundrup, M.; Zaremba, S.; Henry, M.E.; Bittner, E.A.; Hoffmann, U.; Chamberlin, N.L. Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology 2012, 116, 35–46. [Google Scholar] [CrossRef]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; Witztum, J.; Shaver, D.C.; Rosenthal, D.L.; Alway, E.J.; et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019, 364, eaat8078. [Google Scholar] [CrossRef] [PubMed]
- Sala, N.; Paoli, C.; Bonifacino, T.; Mingardi, J.; Schiavon, E.; La Via, L.; Milanese, M.; Tornese, P.; Datusalia, A.K.; Rosa, J.; et al. Acute Ketamine Facilitates Fear Memory Extinction in a Rat Model of PTSD Along With Restoring Glutamatergic Alterations and Dendritic Atrophy in the Prefrontal Cortex. Front. Pharmacol. 2022, 13, 759626. [Google Scholar] [CrossRef]
- Ardalan, M.; Wegener, G.; Polsinelli, B.; Madsen, T.M.; Nyengaard, J.R. Neurovascular plasticity of the hippocampus one week after a single dose of ketamine in genetic rat model of depression. Hippocampus 2016, 26, 1414–1423. [Google Scholar] [CrossRef]
- Liu, W.X.; Wang, J.; Xie, Z.M.; Xu, N.; Zhang, G.F.; Jia, M.; Zhou, Z.Q.; Hashimoto, K.; Yang, J.J. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016, 233, 405–415. [Google Scholar] [CrossRef]
- Radford, K.D.; Spencer, H.F.; Zhang, M.; Berman, R.Y.; Girasek, Q.L.; Choi, K.H. Association between intravenous ketamine-induced stress hormone levels and long-term fear memory renewal in Sprague-Dawley rats. Behav. Brain Res. 2020, 378, 112259. [Google Scholar] [CrossRef]
- Radford, K.D.; Park, T.Y.; Jaiswal, S.; Pan, H.; Knutsen, A.; Zhang, M.; Driscoll, M.; Osborne-Smith, L.A.; Dardzinski, B.J.; Choi, K.H. Enhanced fear memories and brain glucose metabolism ((18)F-FDG-PET) following sub-anesthetic intravenous ketamine infusion in Sprague-Dawley rats. Transl. Psychiatry 2018, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and Neuroinflammation: Crucial Pathological Mechanisms in Traumatic Brain Injury-Induced Neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275, 316–327. [Google Scholar] [CrossRef]
- Broussard, J.I.; Acion, L.; De Jesus-Cortes, H.; Yin, T.; Britt, J.K.; Salas, R.; Costa-Mattioli, M.; Robertson, C.; Pieper, A.A.; Arciniegas, D.B.; et al. Repeated mild traumatic brain injury produces neuroinflammation, anxiety-like behaviour and impaired spatial memory in mice. Brain Inj. 2018, 32, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Walter, S.A.; Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 1999, 57, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Shukla, D.K.; Armstrong, R.C.; Marion, C.M.; Radomski, K.L.; Selwyn, R.G.; Dardzinski, B.J. Repetitive Model of Mild Traumatic Brain Injury Produces Cortical Abnormalities Detectable by Magnetic Resonance Diffusion Imaging, Histopathology, and Behavior. J. Neurotrauma 2017, 34, 1364–1381. [Google Scholar] [CrossRef]
- Patel, R.K.; Prasad, N.; Kuwar, R.; Haldar, D.; Abdul-Muneer, P.M. Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury. Brain Behav. Immun. 2017, 64, 244–258. [Google Scholar] [CrossRef]
- McAteer, K.M.; Corrigan, F.; Thornton, E.; Turner, R.J.; Vink, R. Short and Long Term Behavioral and Pathological Changes in a Novel Rodent Model of Repetitive Mild Traumatic Brain Injury. PLoS ONE 2016, 11, e0160220. [Google Scholar] [CrossRef]
- Zhang, N.; Yao, L.; Wang, P.; Liu, Z. Immunoregulation and antidepressant effect of ketamine. Transl. Neurosci. 2021, 12, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013, 246, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wilde, E.A.; Li, X.; Hunter, J.V.; Narayana, P.A.; Hasan, K.; Biekman, B.; Swank, P.; Robertson, C.; Miller, E.; McCauley, S.R.; et al. Loss of Consciousness Is Related to White Matter Injury in Mild Traumatic Brain Injury. J. Neurotrauma 2016, 33, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Sorg, S.F.; Delano-Wood, L.; Luc, N.; Schiehser, D.M.; Hanson, K.L.; Nation, D.A.; Lanni, E.; Jak, A.J.; Lu, K.; Meloy, M.J.; et al. White matter integrity in veterans with mild traumatic brain injury: Associations with executive function and loss of consciousness. J. Head. Trauma. Rehabil. 2014, 29, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Hayes, J.P.; Lafleche, G.; Salat, D.H.; Verfaellie, M. White matter abnormalities are associated with overall cognitive status in blast-related mTBI. Brain Imaging Behav. 2017, 11, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Hayes, J.P.; Lafleche, G.; Salat, D.H.; Verfaellie, M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Hum. Brain Mapp. 2016, 37, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Roine, T.; Hirvonen, J.; Kurki, T.; Posti, J.P.; Katila, A.J.; Takala, R.S.K.; Tallus, J.; Maanpaa, H.R.; Frantzen, J.; et al. Alterations in Microstructure and Local Fiber Orientation of White Matter Are Associated with Outcome after Mild Traumatic Brain Injury. J. Neurotrauma 2020, 37, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Gazdzinski, L.M.; Mellerup, M.; Wang, T.; Adel, S.A.A.; Lerch, J.P.; Sled, J.G.; Nieman, B.J.; Wheeler, A.L. White Matter Changes Caused by Mild Traumatic Brain Injury in Mice Evaluated Using Neurite Orientation Dispersion and Density Imaging. J. Neurotrauma 2020, 37, 1818–1828. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Chen, H.; Kim, H.Y. Multiple Mild Traumatic Brain Injuries Lead to Visual Dysfunction in a Mouse Model. J. Neurotrauma 2020, 37, 286–294. [Google Scholar] [CrossRef]
- Chen, H.; Kevala, K.; Aflaki, E.; Marugan, J.; Kim, H.Y. GPR110 ligands reduce chronic optic tract gliosis and visual deficit following repetitive mild traumatic brain injury in mice. J. Neuroinflamm. 2021, 18, 157. [Google Scholar] [CrossRef]
- De Moraes, C.G. Anatomy of the visual pathways. J. Glaucoma 2013, 22 (Suppl. 5), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Evanson, N.K.; Guilhaume-Correa, F.; Herman, J.P.; Goodman, M.D. Optic tract injury after closed head traumatic brain injury in mice: A model of indirect traumatic optic neuropathy. PLoS ONE 2018, 13, e0197346. [Google Scholar] [CrossRef] [PubMed]
- Hetzer, S.M.; Shalosky, E.M.; Torrens, J.N.; Evanson, N.K. Chronic Histological Outcomes of Indirect Traumatic Optic Neuropathy in Adolescent Mice: Persistent Degeneration and Temporally Regulated Glial Responses. Cells 2021, 10, 3343. [Google Scholar] [CrossRef]
- Sharma, S.; Chitranshi, N.; Wall, R.V.; Basavarajappa, D.; Gupta, V.; Mirzaei, M.; Graham, S.L.; Klistorner, A.; You, Y. Trans-synaptic degeneration in the visual pathway: Neural connectivity, pathophysiology, and clinical implications in neurodegenerative disorders. Surv. Ophthalmol. 2022, 67, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Eyolfson, E.; Carr, T.; Fraunberger, E.; Khan, A.; Clark, I.; Mychasiuk, R.; Lohman, A.W. Repeated mild traumatic brain injuries in mice cause age- and sex-specific alterations in dendritic spine density. Exp. Neurol. 2022, 357, 114172. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 2011, 70, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, H.; Zeng, J.; Pluimer, B.; Dong, S.; Xie, X.; Guo, X.; Ge, T.; Liang, X.; Feng, S.; et al. Mild traumatic brain injury induces microvascular injury and accelerates Alzheimer-like pathogenesis in mice. Acta Neuropathol. Commun. 2021, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Pilipovic, K.; Rajic Bumber, J.; Dolenec, P.; Grzeta, N.; Jankovic, T.; Kriz, J.; Zupan, G. Long-Term Effects of Repetitive Mild Traumatic Injury on the Visual System in Wild-Type and TDP-43 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 6584. [Google Scholar] [CrossRef] [PubMed]
- Reitz, S.J.; Sauerbeck, A.D.; Kummer, T.T. SEQUIN: An imaging and analysis platform for quantification and characterization of synaptic structures in mouse. STAR Protoc. 2021, 2, 100268. [Google Scholar] [CrossRef]
- Sauerbeck, A.D.; Gangolli, M.; Reitz, S.J.; Salyards, M.H.; Kim, S.H.; Hemingway, C.; Gratuze, M.; Makkapati, T.; Kerschensteiner, M.; Holtzman, D.M.; et al. SEQUIN Multiscale Imaging of Mammalian Central Synapses Reveals Loss of Synaptic Connectivity Resulting from Diffuse Traumatic Brain Injury. Neuron 2020, 107, 257–273.e5. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Mierzwa, A.J.; Kijpaisalratana, N.; Tang, H.; Wang, Y.; Song, S.K.; Selwyn, R.; Armstrong, R.C. Oligodendrocyte lineage and subventricular zone response to traumatic axonal injury in the corpus callosum. J. Neuropathol. Exp. Neurol. 2013, 72, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- DeWalt, G.J.; Mahajan, B.; Foster, A.R.; Thompson, L.D.E.; Marttini, A.A.; Schmidt, E.V.; Mansuri, S.; D’Souza, D.; Patel, S.B.; Tenenbaum, M.; et al. Region-specific alterations in astrocyte and microglia morphology following exposure to blasts in the mouse hippocampus. Neurosci. Lett. 2018, 664, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglial response to brain injury: A brief synopsis. Toxicol. Pathol. 2000, 28, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhao, L.; Wang, Y.; Guo, Q.; An, Q.; Geng, J.; Zhang, C.; Guo, Z. Ketamine Regulates the Autophagy Flux and Polarization of Microglia through the HMGB1-RAGE Axis and Exerts Antidepressant Effects in Mice. J. Neuropathol. Exp. Neurol. 2022, 81, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Shimazawa, M.; Inokuchi, Y.; Fukumitsu, H.; Furukawa, S.; Araie, M.; Hara, H. Degenerative alterations in the visual pathway after NMDA-induced retinal damage in mice. Brain Res. 2008, 1212, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, C.; Liang, H.F.; Barsamian, B.; Sun, S.W. Amyloid-beta induced retrograde axonal degeneration in a mouse tauopathy model. Neuroimage 2019, 189, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Weyand, T.G. The multifunctional lateral geniculate nucleus. Rev. Neurosci. 2016, 27, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, A.O.; Zheng, C.; Reddi, S.; Teng, S.L.; Berger, D.; Adler, D.; Sullivan, P.; Thakker-Varia, S.; Alder, J. APOE4 genetic polymorphism results in impaired recovery in a repeated mild traumatic brain injury model and treatment with Bryostatin-1 improves outcomes. Sci. Rep. 2020, 10, 19919. [Google Scholar] [CrossRef] [PubMed]
- McLendon, L.A.; Kralik, S.F.; Grayson, P.A.; Golomb, M.R. The Controversial Second Impact Syndrome: A Review of the Literature. Pediatr. Neurol. 2016, 62, 9–17. [Google Scholar] [CrossRef]
- Marietta, M.P.; White, P.F.; Pudwill, C.R.; Way, W.L.; Trevor, A.J. Biodisposition of ketamine in the rat: Self-induction of metabolism. J. Pharmacol. Exp. Ther. 1976, 196, 536–544. [Google Scholar] [CrossRef]
- Radford, K.D.; Park, T.Y.; Lee, B.H.; Moran, S.; Osborne, L.A.; Choi, K.H. Dose-response characteristics of intravenous ketamine on dissociative stereotypy, locomotion, sensorimotor gating, and nociception in male Sprague-Dawley rats. Pharmacol. Biochem. Behav. 2017, 153, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Phoumthipphavong, V.; Barthas, F.; Hassett, S.; Kwan, A.C. Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Knott, G.W.; Holtmaat, A.; Wilbrecht, L.; Welker, E.; Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nat. Neurosci. 2006, 9, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Thelen, C.; Flaherty, E.; Saurine, J.; Sens, J.; Mohamed, S.; Pitychoutis, P.M. Sex Differences in the Temporal Neuromolecular and Synaptogenic Effects of the Rapid-acting Antidepressant Drug Ketamine in the Mouse Brain. Neuroscience 2019, 398, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Radford, K.D.; Berman, R.Y.; Zhang, M.; Wu, T.J.; Choi, K.H. Sex-related differences in intravenous ketamine effects on dissociative stereotypy and antinociception in male and female rats. Pharmacol. Biochem. Behav. 2020, 199, 173042. [Google Scholar] [CrossRef]
- Carrier, N.; Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013, 70, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kabbaj, M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol. Psychiatry 2016, 80, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Tebianian, M.; Tofigh, N.; Taheri, R.S.; Mousavi, S.A.; Naseri, A.; Ahmadi, A.; Munawar, N.; Shahpasand, K. Active immunotherapy against pathogenic Cis pT231-tau suppresses neurodegeneration in traumatic brain injury mouse models. Neuropeptides 2022, 96, 102285. [Google Scholar] [CrossRef] [PubMed]
- Korotzer, A.R.; Pike, C.J.; Cotman, C.W. beta-Amyloid peptides induce degeneration of cultured rat microglia. Brain Res. 1993, 624, 121–125. [Google Scholar] [CrossRef]
- Toshimitsu, M.; Kamei, Y.; Ichinose, M.; Seyama, T.; Imada, S.; Iriyama, T.; Fujii, T. Atomoxetine, a selective norepinephrine reuptake inhibitor, improves short-term histological outcomes after hypoxic-ischemic brain injury in the neonatal male rat. Int. J. Dev. Neurosci. 2018, 70, 34–45. [Google Scholar] [CrossRef]
- Lin, C.T.; Lecca, D.; Yang, L.Y.; Luo, W.; Scerba, M.T.; Tweedie, D.; Huang, P.S.; Jung, Y.J.; Kim, D.S.; Yang, C.H.; et al. 3,6′-dithiopomalidomide reduces neural loss, inflammation, behavioral deficits in brain injury and microglial activation. eLife 2020, 9, e54726. [Google Scholar] [CrossRef] [PubMed]
- Doorn, K.J.; Goudriaan, A.; Blits-Huizinga, C.; Bol, J.G.; Rozemuller, A.J.; Hoogland, P.V.; Lucassen, P.J.; Drukarch, B.; van de Berg, W.D.; van Dam, A.M. Increased amoeboid microglial density in the olfactory bulb of Parkinson’s and Alzheimer’s patients. Brain Pathol. 2014, 24, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, A.E.; Salm, A.K. Increased morphological diversity of microglia in the activated hypothalamic supraoptic nucleus. J. Neurosci. 2003, 23, 7759–7766. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C.R. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boese, M.; Berman, R.Y.; Qiu, J.; Spencer, H.F.; Radford, K.D.; Choi, K.H. Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague–Dawley Rats. Int. J. Mol. Sci. 2024, 25, 4287. https://doi.org/10.3390/ijms25084287
Boese M, Berman RY, Qiu J, Spencer HF, Radford KD, Choi KH. Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague–Dawley Rats. International Journal of Molecular Sciences. 2024; 25(8):4287. https://doi.org/10.3390/ijms25084287
Chicago/Turabian StyleBoese, Martin, Rina Y. Berman, Jennifer Qiu, Haley F. Spencer, Kennett D. Radford, and Kwang H. Choi. 2024. "Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague–Dawley Rats" International Journal of Molecular Sciences 25, no. 8: 4287. https://doi.org/10.3390/ijms25084287
APA StyleBoese, M., Berman, R. Y., Qiu, J., Spencer, H. F., Radford, K. D., & Choi, K. H. (2024). Effects of Mild Closed-Head Injury and Subanesthetic Ketamine Infusion on Microglia, Axonal Injury, and Synaptic Density in Sprague–Dawley Rats. International Journal of Molecular Sciences, 25(8), 4287. https://doi.org/10.3390/ijms25084287





