Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance
Abstract
:1. Introduction
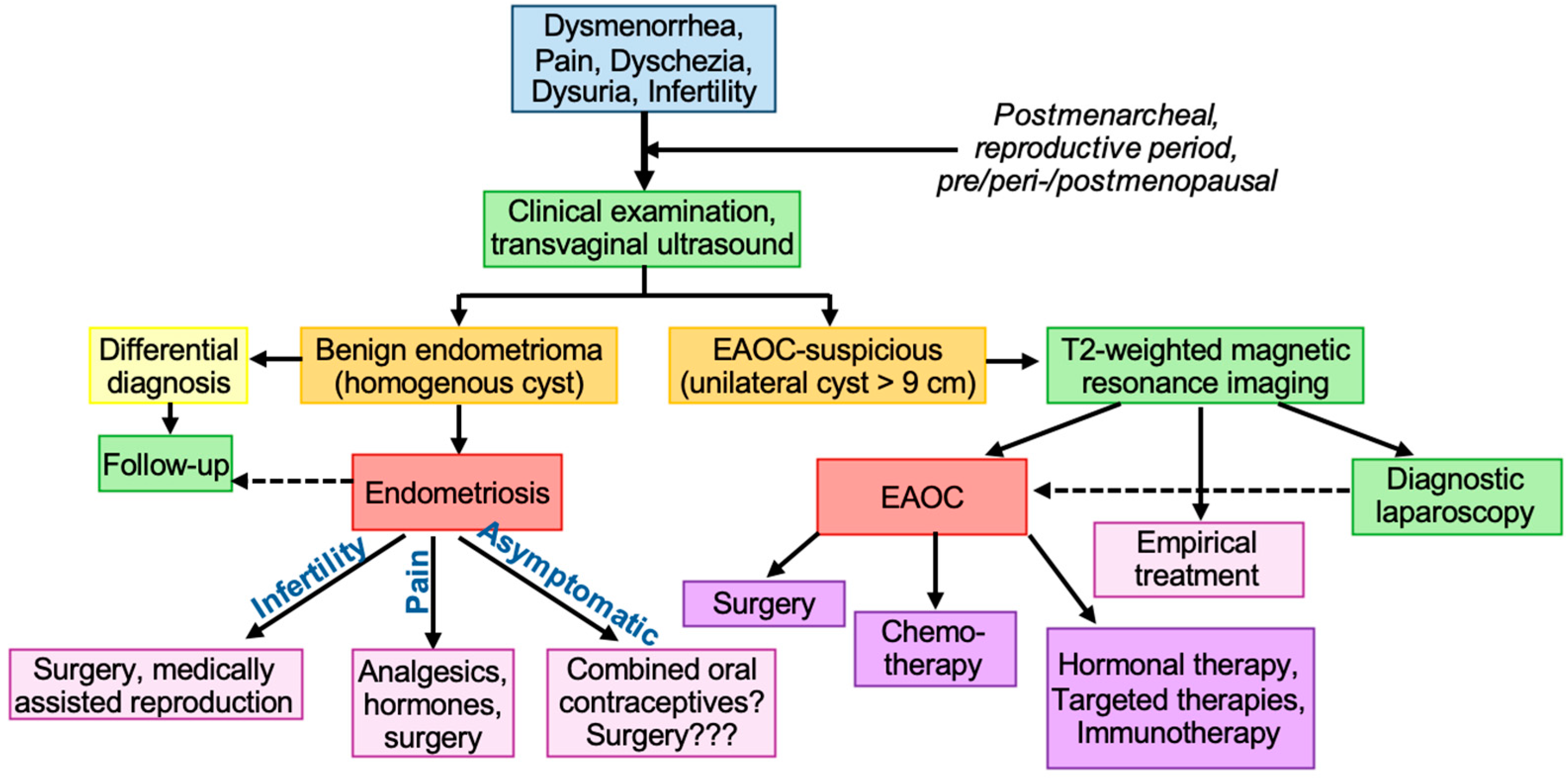
1.1. Diagnosis
1.2. Risk Factors
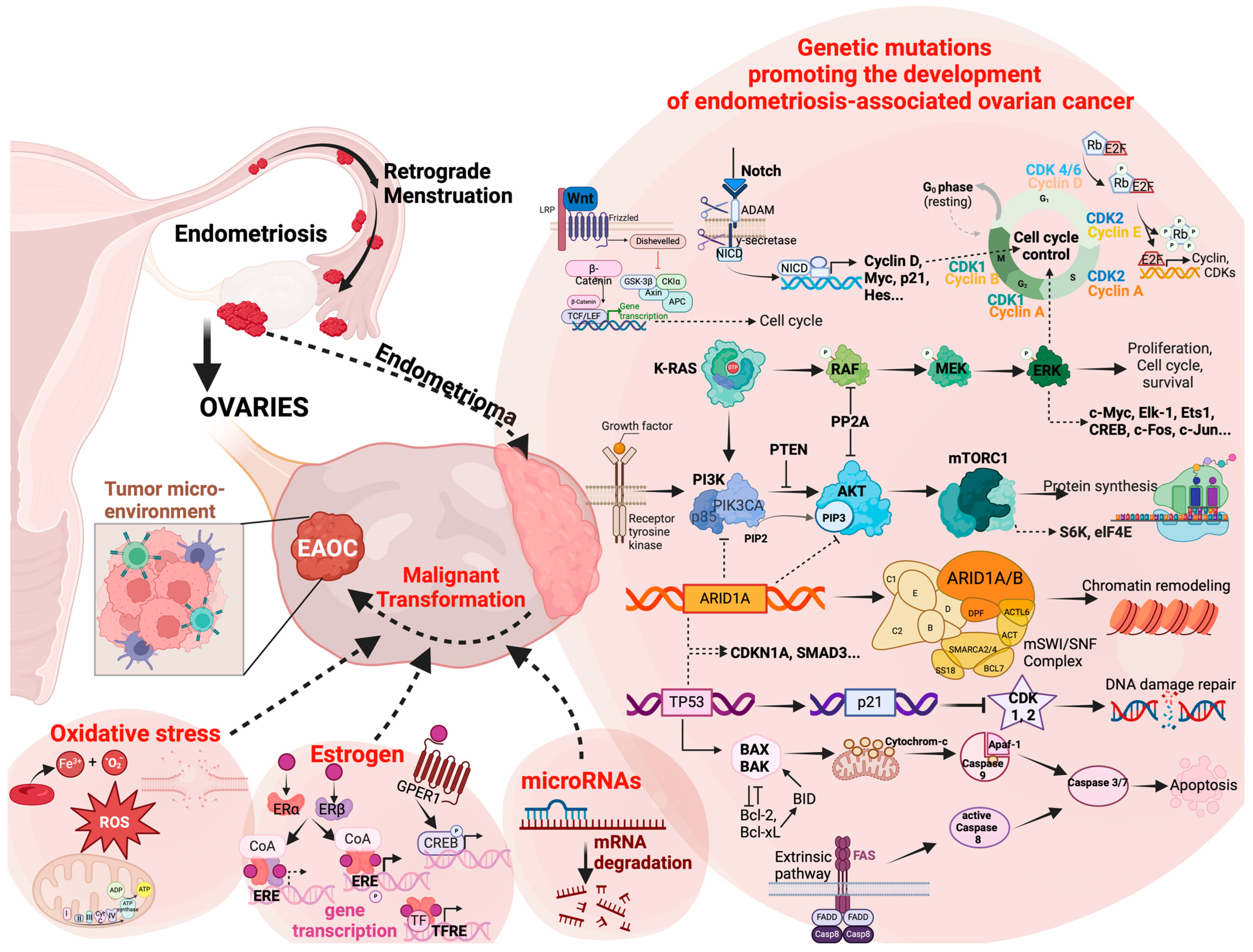
2. Molecular Pathologies
2.1. Genetic Mutations
2.1.1. Chromatin Remodeling
2.1.2. PI3K/AKT/mTOR Pathway
2.1.3. Other Genetic Alterations
2.2. Epigenetic Reprogramming
2.3. The Tumor Microenvironment
2.3.1. Estrogen Concentration
2.3.2. microRNAs
2.3.3. Oxidative Stress
2.3.4. Inflammation
2.3.5. Nutrient Availability
2.4. The Complex and Heterogenous Nature of Cancer
3. Therapeutic Strategies
3.1. Endometriosis
3.2. Prevention of Cancer in Patients with Endometriosis
3.3. Treatment of Diagnosed Endometriosis-Associated Ovarian Cancer
3.3.1. Surgical Treatment
3.3.2. Chemotherapy
3.3.3. Hormonal Therapies
3.3.4. Targeted Therapies
3.3.5. Immunotherapy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Buis, C.C.; van Leeuwen, F.E.; Mooij, T.M.; Burger, C.W.; OMEGA_ProjectGroup. Increased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosis. Hum. Reprod. 2013, 28, 3358–3369. [Google Scholar] [CrossRef] [PubMed]
- Al-Badawi, I.A.; Abu-Zaid, A.; Alomar, O.; Alsabban, M.; Alsehaimi, S.O.; Alqarni, S.M.S.; Alabdrabalamir, S.N.; Baradwan, S.; Al Baalharith, M.; AlOdaini, A.A.; et al. Association between Endometriosis and the Risk of Ovarian, Endometrial, Cervical, and Breast Cancer: A Population-Based Study from the U.S. National Inpatient Sample 2016–2019. Curr. Oncol. 2024, 31, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Ñiguez Sevilla, I.; Machado Linde, F.; Marín Sánchez, M.D.P.; Arense, J.J.; Torroba, A.; Nieto Díaz, A.; Sánchez Ferrer, M.L. Prognostic importance of atypical endometriosis with architectural hyperplasia versus cytologic atypia in endometriosis-associated ovarian cancer. J. Gynecol. Oncol. 2019, 30, e63. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J. Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg. 1925, 10, 1–72. [Google Scholar] [CrossRef]
- Scott, R. Malignant changes in endometriosis. Obstet. Gynecol. 1953, 2, 283–289. Available online: https://journals.lww.com/greenjournal/citation/1953/09000/malignant_changes_in_endometriosis.8.aspx (accessed on 8 April 2024).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, L.N.; Hartwell, D.; Heidemann, C.H.; Jochumsen, K.M. The relation between endometriosis and ovarian cancer—A review. Acta Obstet. Gynecol. Scand. 2014, 93, 20–31. [Google Scholar] [CrossRef]
- Bergamini, A.; Mangili, G.; Ambrosi, A.; Taccagni, G.; Rabaiotti, E.; Bocciolone, L.; Candotti, G.; Cioffi, R.; Pella, F.; Sabetta, G.; et al. Endometriosis-Related Ovarian Cancers: Evidence for a Dichotomy in the Histogenesis of the Two Associated Histotypes. Diagnostics 2023, 13, 1425. [Google Scholar] [CrossRef]
- Samartzis, E.P.; Labidi-Galy, S.I.; Moschetta, M.; Uccello, M.; Kalaitzopoulos, D.R.; Perez-Fidalgo, J.A.; Boussios, S. Endometriosis-associated ovarian carcinomas: Insights into pathogenesis, diagnostics, and therapeutic targets-a narrative review. Ann. Transl. Med. 2020, 8, 1712. [Google Scholar] [CrossRef] [PubMed]
- Chiaffarino, F.; Cipriani, S.; Ricci, E.; Esposito, G.; Parazzini, F.; Vercellini, P. Histologic Subtypes in Endometriosis-Associated Ovarian Cancer and Ovarian Cancer Arising in Endometriosis: A Systematic Review and Meta-Analysis. Reprod. Sci. 2024; advance online publication. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Webster, P.; Kumbang, J.; Kennedy, S.H.; Zondervan, K.T. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil. Steril. 2012, 98, 702–712.e6. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.M.; Holman, C.D.; Aboagye-Sarfo, P.; Finn, J.C.; Preen, D.B.; Hart, R. In vitro fertilization, endometriosis, nulliparity and ovarian cancer risk. Gynecol. Oncol. 2013, 128, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bandini, V.; Viganò, P.; Di Stefano, G.; Merli, C.E.M.; Somigliana, E. Proposal for targeted, neo-evolutionary-oriented, secondary prevention of early-onset endometriosis and adenomyosis. Part I: Pathogenic aspects. Hum. Reprod. 2024, 39, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Torng, L.P. Clinical implication for endometriosis associated with ovarian cancer. Gynecol. Minim. Invasive Ther. 2017, 6, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B. Endometriosis and ovarian cancer: Thoughts on shared pathophysiology. Am. J. Obstet. Gynecol. 2003, 189, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Townsend, M.K.; Wentzensen, N.; Trabert, B.; White, E.; Arslan, A.A.; Weiderpass, E.; Buring, J.E.; Clendenen, T.V.; Giles, G.G.; et al. Reproductive and Hormonal Factors and Risk of Ovarian Cancer by Tumor Dominance: Results from the Ovarian Cancer Cohort Consortium (OC3). Cancer Epidemiol. Biomark. Prev. 2020, 29, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Eskenazi, B.; Consonni, D.; Somigliana, E.; Parazzini, F.; Abbiati, A.; Fedele, L. Oral contraceptives and risk of endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2011, 17, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liang, Z.; Liu, X.; Zhang, Q.; Li, S. The Association between Endometriosis, Tubal Ligation, Hysterectomy and Epithelial Ovarian Cancer: Meta-Analyses. Int. J. Environ. Res. Public Health 2016, 13, 1138. [Google Scholar] [CrossRef]
- Throwba, H.P.; Unnikrishnan, L.; Pangath, M.; Vasudevan, K.; Jayaraman, S.L.M.; Iyaswamy, A.; Palaniyandi, K.; Gnanasampanthapandian, D. The epigenetic correlation among ovarian cancer, endometriosis and PCOS: A review. Crit. Rev. Oncol. Hematol. 2022, 180, 103852. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Holt, V.L. Recreational physical activity and endometrioma risk. Am. J. Epidemiol. 2003, 158, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mørch, L.S.; Løkkegaard, E.; Andreasen, A.H.; Krüger-Kjaer, S.; Lidegaard, O. Hormone therapy and ovarian cancer. JAMA 2009, 302, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.S.; Qu, Z.; Lv, P.P.; Huang, H.F. Pediatric and adult obesity concerns in female health: A Mendelian randomization study. Endocrine 2022, 75, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, M.; Zdun, D.; Owczarek, A.J.; Skrzypulec-Plinta, V.; Olszanecka-Glinianowicz, M. Evaluation of adipokines concentrations in plasma, peritoneal, and endometrioma fluids in women operated on for ovarian endometriosis. Front. Endocrinol. 2023, 14, 1218980. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M.; Green, A.C.; Whiteman, D.C.; Sadeghi, S.; Kolahdooz, F.; Webb, P.M. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2007, 43, 690–709. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Kotani, Y.; Shiro, R.; Takaya, H.; Nakai, H.; Matsumura, N. Endometriosis-associated ovarian cancer occurs early during follow-up of endometrial cysts. Int. J. Clin. Oncol. 2020, 25, 51–58. [Google Scholar] [CrossRef]
- Worley, M.J.; Welch, W.R.; Berkowitz, R.S.; Ng, S.W. Endometriosis-associated ovarian cancer: A review of pathogenesis. Int. J. Mol. Sci. 2013, 14, 5367–5379. [Google Scholar] [CrossRef] [PubMed]
- Rier, S.E.; Martin, D.C.; Bowman, R.E.; Dmowski, W.P.; Becker, J.L. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1993, 21, 433–441. [Google Scholar] [CrossRef]
- Cummings, A.M.; Metcalf, J.L.; Birnbaum, L. Promotion of endometriosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats and mice: Time-dose dependence and species comparison. Toxicol. Appl. Pharmacol. 1996, 138, 131–139. [Google Scholar] [CrossRef]
- Huang, P.C.; Tsai, E.M.; Li, W.F.; Liao, P.C.; Chung, M.C.; Wang, Y.H.; Wang, S.L. Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Hum. Reprod. 2010, 25, 986–994. [Google Scholar] [CrossRef]
- L’Espérance, K.; Grundy, A.; Abrahamowicz, M.; Arseneau, J.; Gilbert, L.; Gotlieb, W.H.; Provencher, D.; Koushik, A. Alcohol intake and the risk of epithelial ovarian cancer. Cancer Causes Control CCC 2023, 34, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, S.; Du, F.; Zhang, K.; Shen, Y. Association of smoking, alcohol, and coffee consumption with the risk of ovarian cancer and prognosis: A mendelian randomization study. BMC Cancer 2023, 23, 256. [Google Scholar] [CrossRef]
- Sung, S.; Hong, Y.; Kim, B.G.; Choi, J.Y.; Kim, J.W.; Park, S.Y.; Kim, J.H.; Kim, Y.M.; Lee, J.M.; Kim, T.J.; et al. Stratifying the risk of ovarian cancer incidence by histologic subtypes in the Korean Epithelial Ovarian Cancer Study (Ko-EVE). Cancer Med. 2023, 12, 8742–8753. [Google Scholar] [CrossRef] [PubMed]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. EclinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc1931779/ (accessed on 8 April 2024).
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 1984, 64, 151–154. Available online: https://journals.lww.com/greenjournal/abstract/1984/08000/retrograde_menstruation_in_healthy_women_and_in.1.aspx (accessed on 8 April 2024).
- Marí-Alexandre, J.; Carcelén, A.P.; Agababyan, C.; Moreno-Manuel, A.; García-Oms, J.; Calabuig-Fariñas, S.; Gilabert-Estellés, J. Interplay Between MicroRNAs and Oxidative Stress in Ovarian Conditions with a Focus on Ovarian Cancer and Endometriosis. Int. J. Mol. Sci. 2019, 20, 5322. [Google Scholar] [CrossRef] [PubMed]
- Poole, E.M.; Lin, W.T.; Kvaskoff, M.; De Vivo, I.; Terry, K.L.; Missmer, S.A. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer Causes Control CCC 2017, 28, 437–445. [Google Scholar] [CrossRef]
- Körner, M.; Burckhardt, E.; Mazzucchelli, L. Higher frequency of chromosomal aberrations in ovarian endometriosis compared to extragonadal endometriosis: A possible link to endometrioid adenocarcinoma. Mod. Pathol. 2006, 19, 1615–1623. [Google Scholar] [CrossRef]
- Okuda, T.; Otsuka, J.; Sekizawa, A.; Saito, H.; Makino, R.; Kushima, M.; Farina, A.; Kuwano, Y.; Okai, T. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol. Oncol. 2003, 88, 318–325. [Google Scholar] [CrossRef]
- Amemiya, S.; Sekizawa, A.; Otsuka, J.; Tachikawa, T.; Saito, H.; Okai, T. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int. J. Gynaecol. Obstet. 2004, 86, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, J.; Okuda, T.; Sekizawa, A.; Amemiya, S.; Saito, H.; Okai, T.; Kushima, M.; Tachikawa, T. K-ras mutation may promote carcinogenesis of endometriosis leading to ovarian clear cell carcinoma. Med. Electron Microsc. 2004, 37, 188–192. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Yachida, N.; Yoshihara, K.; Yamaguchi, M.; Suda, K.; Tamura, R.; Enomoto, T. How Does Endometriosis Lead to Ovarian Cancer? The Molecular Mechanism of Endometriosis-Associated Ovarian Cancer Development. Cancers 2021, 13, 1439. [Google Scholar] [CrossRef]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef]
- Manchana, T.; Phoolcharoen, N.; Tantbirojn, P. BRCA mutation in high grade epithelial ovarian cancers. Gynecol. Oncol. Rep. 2019, 29, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, K.C.; Hennessy, B.T.; Leung, S.; Wang, Y.; Ju, Z.; McGahren, M.; Kalloger, S.E.; Finlayson, S.; Stemke-Hale, K.; Lu, Y.; et al. A functional proteogenomic analysis of endometrioid and clear cell carcinomas using reverse phase protein array and mutation analysis: Protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation. BMC Cancer 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, J.H.; Kim, S.J.; Kwon, S.J.; Kwon, J. A cooperative activation loop among SWI/SNF, gamma-H2AX and H3 acetylation for DNA double-strand break repair. EMBO J. 2010, 29, 1434–1445. [Google Scholar] [CrossRef]
- Chene, G.; Ouellet, V.; Rahimi, K.; Barres, V.; Caceres, K.; Meunier, L.; Cyr, L.; DeLadurantaye, M.; Provencher, D.; Mes Masson, A.M. DNA damage signaling and apoptosis in preinvasive tubal lesions of ovarian carcinoma. Int. J. Gynecol. Cancer 2015, 25, 761–769. [Google Scholar] [CrossRef]
- Guan, B.; Wang, T.L.; Shih, I. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011, 71, 6718–6727. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Mao, T.L.; Jones, S.; Veras, E.; Ayhan, A.; Wang, T.L.; Glas, R.; Slamon, D.; Velculescu, V.E.; Kuman, R.J.; et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am. J. Pathol. 2009, 174, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Iwaya, K.; Tamai, S.; Matsubara, O. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J. Pathol. 2011, 225, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tsuda, H.; Takano, M.; Tamai, S.; Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 2012, 25, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Lin, M.C.; Huang, W.C.; Chiang, Y.C.; Kuo, K.T. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations and ZNF217 amplification in ovarian clear cell carcinoma. Mod. Pathol. 2014, 27, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Rogers-Broadway, K.R.; Kumar, J.; Sisu, C.; Wander, G.; Mazey, E.; Jeyaneethi, J.; Pados, G.; Tsolakidis, D.; Klonos, E.; Grunt, T.; et al. Differential expression of mTOR components in endometriosis and ovarian cancer: Effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 stoichiometry. Int. J. Mol. Med. 2019, 43, 47–56. [Google Scholar] [CrossRef]
- Shibuya, Y.; Tokunaga, H.; Saito, S.; Shimokawa, K.; Katsuoka, F.; Bin, L.; Kojima, K.; Nagasaki, M.; Yamamoto, M.; Yaegashi, N.; et al. Identification of somatic genetic alterations in ovarian clear cell carcinoma with next generation sequencing. Genes Chromosomes Cancer 2018, 57, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, S.A.; Arguello, D.; Ramos, P.; Mahdi, H.; ElNaggar, A.; Winer, I.; Holloway, R.; Krivak, T.; Jones, N.; Turner, V.G.; et al. Molecular portraits of clear cell ovarian and endometrial carcinoma with comparison to clear cell renal cell carcinoma. Gynecol. Oncol. 2023, 169, 164–171. [Google Scholar] [CrossRef]
- Jones, S.; Wang, T.L.; Shih, I.; Mao, T.L.; Nakayama, K.; Roden, R.; Glas, R.; Slamon, D.; Diaz, L.A.; Vogelstein, B.; et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 2010, 330, 228–231. [Google Scholar] [CrossRef]
- Wera, S.; Hemmings, B.A. Serine/threonine protein phosphatases. Biochem. J. 1995, 311 Pt 1, 17–29. [Google Scholar] [CrossRef]
- Aziz, A.U.R.; Farid, S.; Qin, K.; Wang, H.; Liu, B. PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer. Biomolecules 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Driva, T.S.; Schatz, C.; Haybaeck, J. Endometriosis-Associated Ovarian Carcinomas: How PI3K/AKT/mTOR Pathway Affects Their Pathogenesis. Biomolecules 2023, 13, 1253. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Hoshiai, H. Common genetic changes between endometriosis and ovarian cancer. Gynecol. Obstet. Investig. 2000, 50 (Suppl. S1), 39–43. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Tsunoda, H.; Nishida, M.; Morishita, Y.; Takimoto, Y.; Kubo, T.; Noguchi, M. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: Possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000, 60, 7052–7056. Available online: https://aacrjournals.org/cancerres/article/60/24/7052/506957/Loss-of-Heterozygosity-on-10q23-3-and-Mutation-of (accessed on 8 April 2024). [PubMed]
- Martini, M.; Ciccarone, M.; Garganese, G.; Maggiore, C.; Evangelista, A.; Rahimi, S.; Zannoni, G.; Vittori, G.; Larocca, L.M. Possible involvement of hMLH1, p16(INK4a) and PTEN in the malignant transformation of endometriosis. Int. J. Cancer 2002, 102, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Banz, C.; Ungethuem, U.; Kuban, R.J.; Diedrich, K.; Lengyel, E.; Hornung, D. The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertil. Steril. 2010, 94, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Er, T.K.; Su, Y.F.; Wu, C.C.; Chen, C.C.; Wang, J.; Hsieh, T.H.; Herreros-Villanueva, M.; Chen, W.T.; Chen, Y.T.; Liu, T.C.; et al. Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. J. Mol. Med. 2016, 94, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Schüring, A.N.; Dahlhues, B.; Korte, A.; Kiesel, L.; Titze, U.; Heitkötter, B.; Ruckert, C.; Götte, M. The endometrial stem cell markers notch-1 and numb are associated with endometriosis. Reprod. Biomed. Online 2018, 36, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Strauß, T.; Greve, B.; Gabriel, M.; Achmad, N.; Schwan, D.; Espinoza-Sanchez, N.A.; Laganà, A.S.; Kiesel, L.; Poutanen, M.; Götte, M.; et al. Impact of Musashi-1 and Musashi-2 Double Knockdown on Notch Signaling and the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2022, 23, 2851. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef]
- Ren, F.; Wang, D.; Jiang, Y.; Ren, F. Epigenetic inactivation of hMLH1 in the malignant transformation of ovarian endometriosis. Arch. Gynecol. Obstet. 2012, 285, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Wang, D.B.; Li, T.; Chen, Y.H.; Li, Y. Identification of differentially methylated genes in the malignant transformation of ovarian endometriosis. J. Ovarian Res. 2014, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ren, F.; Wang, D.; Li, Y.; Liu, K.; Liu, S.; Chen, P. RUNX3 is inactivated by promoter hypermethylation in malignant transformation of ovarian endometriosis. Oncol. Rep. 2014, 32, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lavery, S.; Gillmer, M. Malignant transformation of residual endometriosis in women on unopposed oestrogen hormone replacement therapy. BJOG Int. J. Obstet. Gynaecol. 2001, 108, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Epidemiological Studies of Ovarian Cancer; Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Huang, Z.Y.; Yu, K.; Li, J.; Fu, X.W.; Deng, S.L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. 2022, 13, 827032. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Piastowska-Ciesielska, A.W. Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 14673. [Google Scholar] [CrossRef]
- Fujimoto, J.; Alam, S.M.; Jahan, I.; Sato, E.; Sakaguchi, H.; Tamaya, T. Clinical implication of estrogen-related receptor (ERR) expression in ovarian cancers. J. Steroid Biochem. Mol. Biol. 2007, 104, 301–304. [Google Scholar] [CrossRef]
- Brandenberger, A.W.; Tee, M.K.; Jaffe, R.B. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: Down-regulation of ER-beta in neoplastic tissues. J. Clin. Endocrinol. Metab. 1998, 83, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Rey, J.M.; Nirde, P.; Roger, P.; Gastaldi, M.; Laffargue, F.; Rochefort, H.; Maudelonde, T. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998, 58, 5367–5373. Available online: https://aacrjournals.org/cancerres/article/58/23/5367/504774/Differential-Expression-of-Estrogen-Receptor-and (accessed on 8 April 2024).
- Rutherford, T.; Brown, W.D.; Sapi, E.; Aschkenazi, S.; Muñoz, A.; Mor, G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet. Gynecol. 2000, 96, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Bardin, A.; Boulle, N.; Lazennec, G.; Vignon, F.; Pujol, P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr.-Relat. Cancer 2004, 11, 537–551. [Google Scholar] [CrossRef]
- Bossard, C.; Busson, M.; Vindrieux, D.; Gaudin, F.; Machelon, V.; Brigitte, M.; Jacquard, C.; Pillon, A.; Balaguer, P.; Balabanian, K.; et al. Potential role of estrogen receptor beta as a tumor suppressor of epithelial ovarian cancer. PLoS ONE 2012, 7, e44787. [Google Scholar] [CrossRef]
- Trukhacheva, E.; Lin, Z.; Reierstad, S.; Cheng, Y.H.; Milad, M.; Bulun, S.E. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 615–622. [Google Scholar] [CrossRef]
- Andersen, C.L.; Boisen, M.M.; Sikora, M.J.; Ma, T.; Tseng, G.; Suryawanshi, S.; Vlad, A.; Elishaev, E.; Edwards, R.P.; Oesterreich, S. The Evolution of Estrogen Receptor Signaling in the Progression of Endometriosis to Endometriosis-Associated Ovarian Cancer. Horm. Cancer 2018, 9, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Tarumi, Y.; Fujii, M.; Ogawa, K.; Maeda, E.; Tanaka, Y.; Okimura, H.; Kataoka, H.; Takaoka, O.; Ito, F.; et al. Low-Nutrient Environment-Induced Changes in Inflammation, Cell Proliferation, and PGC-1α Expression in Stromal Cells with Ovarian Endometriosis. Reprod. Sci. 2023, 30, 1094–1102. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. Integrating modern approaches to pathogenetic concepts of malignant transformation of endometriosis. Oncol. Rep. 2019, 41, 1729–1738. [Google Scholar] [CrossRef]
- Shin, S.; Chung, Y.J.; Moon, S.W.; Choi, E.J.; Kim, M.R.; Chung, Y.J.; Lee, S.H. Single-cell profiling identifies distinct hormonal, immunologic, and inflammatory signatures of endometriosis-constituting cells. J. Pathol. 2023, 261, 323–334. [Google Scholar] [CrossRef]
- Liu, G.; Sun, P.; Dong, B.; Sehouli, J. Key regulator of cellular metabolism, estrogen-related receptor α, a new therapeutic target in endocrine-related gynecological tumor. Cancer Manag. Res. 2018, 10, 6887–6895. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Cheung, L.W.; Wong, A.S.; Leung, P.C. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol. Endocrinol. 2008, 22, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y.; Zheng, Y. Antitumor effects of aconitine in A2780 cells via estrogen receptor β mediated apoptosis, DNA damage and migration. Mol. Med. Rep. 2020, 22, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- Kozieł, M.J.; Habrowska-Górczyńska, D.E.; Urbanek, K.A.; Domińska, K.; Piastowska-Ciesielska, A.W.; Kowalska, K. Estrogen receptor α mediates alternariol-induced apoptosis and modulation of the invasiveness of ovarian cancer cells. Toxicol. Lett. 2023, 386, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, C.; Li, Y.; Zhou, M.; Wang, H.; Liu, J.; Chen, P. Oestrogen up-regulates DNMT1 and leads to the hypermethylation of RUNX3 in the malignant transformation of ovarian endometriosis. Reprod. Biomed. Online 2022, 44, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Vlad, A.M.; Lin, H.M.; Mantia-Smaldone, G.; Laskey, R.; Lee, M.; Lin, Y.; Donnellan, N.; Klein-Patel, M.; Lee, T.; et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2013, 19, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of novel genes coding for small expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef]
- Lee, R.C.; Ambros, V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef]
- Resnick, K.E.; Alder, H.; Hagan, J.P.; Richardson, D.L.; Croce, C.M.; Cohn, D.E. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol. Oncol. 2009, 112, 55–59. [Google Scholar] [CrossRef]
- Filigheddu, N.; Gregnanin, I.; Porporato, P.E.; Surico, D.; Perego, B.; Galli, L.; Patrignani, C.; Graziani, A.; Surico, N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J. Biomed. Biotechnol. 2010, 2010, 369549. [Google Scholar] [CrossRef]
- Teague, E.M.O.; VanderHoek, K.H.; VanderHoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- SiufiNeto, J.; Kho, R.M.; Siufi, D.F.; Baracat, E.C.; Anderson, K.S.; Abrão, M.S. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J. Minim. Invasive Gynecol. 2014, 21, 55–63. [Google Scholar] [CrossRef] [PubMed]
- vanJaarsveld, M.T.; Helleman, J.; Berns, E.M.; Wiemer, E.A. MicroRNAs in ovarian cancer biology and therapy resistance. Int. J. Biochem. Cell Biol. 2010, 42, 1282–1290. [Google Scholar] [CrossRef]
- Büssing, I.; Slack, F.J.; Grosshans, H. let-7 microRNAs in development, stem cells and cancer. Trends Mol. Med. 2008, 14, 400–409. [Google Scholar] [CrossRef]
- Liu, Y.N.; Yin, J.J.; Abou-Kheir, W.; Hynes, P.G.; Casey, O.M.; Fang, L.; Yi, M.; Stephens, R.M.; Seng, V.; Sheppard-Tillman, H.; et al. MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 2013, 32, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Zabala, M.; Cho, R.W.; Lobo, N.; Dalerba, P.; Qian, D.; Diehn, M.; Liu, H.; Panula, S.P.; Chiao, E.; et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009, 138, 592–603. [Google Scholar] [CrossRef]
- Olson, P.L.J.; Zhang, H.; Shai, A.; Chun, M.G.; Wang, Y.; Libutti, S.K.; Nakakura, E.K.; Golub, T.R.; Hanahan, D. MicroRNA dynamics in the stages of tumorigenesis correlate with hallmark capabilities of cancer. Genes Dev. 2009, 23, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J.C.; Martino, V.; Reinbold, R.; Schäfer, S.D.; Kiesel, L.; Starzinski-Powitz, A.; Schüring, A.N.; Kemper, B.; Greve, B.; Götte, M. microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod. Biomed. Online 2016, 32, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Stejskalová, A.; Fincke, V.; Nowak, M.; Schmidt, Y.; Borrmann, K.; vonWahlde, M.K.; Schäfer, S.D.; Kiesel, L.; Greve, B.; Götte, M. Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci. Rep. 2021, 11, 4115. [Google Scholar] [CrossRef] [PubMed]
- Szubert, M.; Nowak-Glück, A.; Domańska-Senderowska, D.; Szymańska, B.; Sowa, P.; Rycerz, A.; Wilczyński, J.R. miRNA Expression Profiles in Ovarian Endometriosis and Two Types of Ovarian Cancer-Endometriosis-Associated Ovarian Cancer and High-Grade Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 17470. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.C.; Liu, C.J.; Tu, H.F.; Chung, Y.T.; Yang, C.C.; Kao, S.Y.; Chang, K.W.; Lin, S.C. miR-31 targets ARID1A and enhances the oncogenicity and stemness of head and neck squamous cell carcinoma. Oncotarget 2016, 7, 57254–57267. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Tsai, M.M.; Hung, P.S.; Kao, S.Y.; Liu, T.Y.; Wu, K.J.; Chiou, S.H.; Lin, S.C.; Chang, K.W. miR-31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010, 70, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Muharam, R.; RahmalaFebri, R.; Mutia, K.; Iffanolida, P.A.; Maidarti, M.; Wiweko, B.; Hestiantoro, A. Down-Regulation of miR-93 Negatively Correlates with Overexpression of VEGFA and MMP3 in Endometriosis: A Cross-Sectional Study. Int. J. Fertil. Steril. 2023, 17, 28–33. [Google Scholar] [CrossRef] [PubMed]
- DelCarmen, M.G.; SmithSehdev, A.E.; Fader, A.N.; Zahurak, M.L.; Richardson, M.; Fruehauf, J.P.; Montz, F.J.; Bristow, R.E. Endometriosis-associated ovarian carcinoma: Differential expression of vascular endothelial growth factor and estrogen/progesterone receptors. Cancer 2003, 98, 1658–1663. [Google Scholar] [CrossRef]
- Fontana, L.; Pelosi, E.; Greco, P.; Racanicchi, S.; Testa, U.; Liuzzi, F.; Croce, C.M.; Brunetti, E.; Grignani, F.; Peschle, C. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat. Cell Biol. 2007, 9, 775–787. [Google Scholar] [CrossRef]
- Ramón, L.A.; Braza-Boïls, A.; Gilabert-Estellés, J.; Gilabert, J.; España, F.; Chirivella, M.; Estellés, A. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum. Reprod. 2011, 26, 1082–1090. [Google Scholar] [CrossRef]
- Winarto, H.; Tan, M.I.; Sadikin, M.; Wanandi, S.I. ARID1A Expression is Down-Regulated by Oxidative Stress in Endometriosis and Endometriosis-Associated Ovarian Cancer. Transl. Oncogenomics 2017, 9, 1177272716689818. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Yamaguchi, K.; Matsumura, N.; Baba, T.; Konishi, I. Ovarian cancer in endometriosis: Molecular biology, pathology, and clinical management. Int. J. Clin. Oncol. 2009, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Rockfield, S.; Raffel, J.; Mehta, R.; Rehman, N.; Nanjundan, M. Iron overload and altered iron metabolism in ovarian cancer. Biol. Chem. 2017, 398, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Huang, X.; Elishaev, E.; Budiu, R.A.; Zhang, L.; Kim, S.; Donnellan, N.; Mantia-Smaldone, G.; Ma, T.; Tseng, G.; et al. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin. Cancer Res. 2014, 20, 6163–6174. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Wang, M.L.; Lu, K.H.; Yang, Y.P.; Juang, C.M.; Wang, P.H.; Hsu, R.J.; Yu, M.H.; Chang, C.C. Integrating the dysregulated inflammasome-based molecular functionome in the malignant transformation of endometriosis-associated ovarian carcinoma. Oncotarget 2017, 9, 3704–3726. [Google Scholar] [CrossRef] [PubMed]
- Linder, A.; Westbom-Fremer, S.; Mateoiu, C.; OlssonWidjaja, A.; Österlund, T.; Veerla, S.; Ståhlberg, A.; Ulfenborg, B.; Hedenfalk, I.; Sundfeldt, K. Genomic alterations in ovarian endometriosis and subsequently diagnosed ovarian carcinoma. Hum. Reprod. 2024; deae043, advance online publication. [Google Scholar] [CrossRef]
- De Berardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Shigetomi, H.; Imanaka, S. Nonhormonal therapy for endometriosis based on energy metabolism regulation. Reprod. Fertil. 2021, 2, C42–C57. [Google Scholar] [CrossRef]
- Kobayashi, H. Recent advances in understanding the metabolic plasticity of ovarian cancer: A systematic review. Heliyon 2022, 8, e11487. [Google Scholar] [CrossRef]
- Nantasupha, C.; Thonusin, C.; Charoenkwan, K.; Chattipakorn, S.; Chattipakorn, N. Metabolic reprogramming in epithelial ovarian cancer. Am. J. Transl. Res. 2021, 13, 9950–9973. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc8507042/ (accessed on 8 April 2024). [PubMed]
- Wu, Y.; Zhang, X.; Wang, Z.; Zheng, W.; Cao, H.; Shen, W. Targeting oxidative phosphorylation as an approach for the treatment of ovarian cancer. Front. Oncol. 2022, 12, 971479. [Google Scholar] [CrossRef] [PubMed]
- Dar, S.; Chhina, J.; Mert, I.; Chitale, D.; Buekers, T.; Kaur, H.; Giri, S.; Munkarah, A.; Rattan, R. Bioenergetic Adaptations in Chemoresistant Ovarian Cancer Cells. Sci. Rep. 2017, 7, 8760. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Kreuzaler, P.; Panina, Y.; Segal, J.; Yuneva, M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol. Metab. 2020, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Wu, Y. Tumor metabolism rewiring in epithelial ovarian cancer. J. Ovarian Res. 2023, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhao, G.; Orsulic, S.; Matei, D. Metabolic dependencies and targets in ovarian cancer. Pharmacol. Ther. 2023, 245, 108413. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, S.; Chen, L.; Wang, J. Analysis of Clinical Characteristics and Prognostic Factors Related to EMs Correlation in Ovarian Cancer Patients. Altern. Ther. Health Med. 2024; AT9934, advance online publication. Available online: http://alternative-therapies.com/oa/index.html?fid=9934(accessed on 8 April 2024).
- Vercellini, P.; Viganò, P.; Buggio, L.; Makieva, S.; Scarfone, G.; Cribiù, F.M.; Parazzini, F.; Somigliana, E. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Younis, J. Should Endometriosis-Associated Ovarian Cancer Alter the Management of Women with an Intact Endometrioma in the Reproductive Age? Reprod. Med. 2023, 4, 100–105. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Tang, Z.; Li, X.; Song, T. Differentiation between endometriosis-associated ovarian cancers and non-endometriosis-associated ovarian cancers based on magnetic resonance imaging. Br. J. Radiol. 2021, 94, 20201441. [Google Scholar] [CrossRef]
- Thomsen, L.H.; Schnack, T.H.; Buchardi, K.; Hummelshoj, L.; Missmer, S.A.; Forman, A.; Blaakaer, J. Risk factors of epithelial ovarian carcinomas among women with endometriosis: A systematic review. Acta Obstet. Gynecol. Scand. 2017, 96, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz-Hanege, B.; Güler Çekıç, S.; Ata, B. Endometrioma and ovarian reserve: Effects of endometriomata per se and its surgical treatment on the ovarian reserve. Facts Views Vis. ObGyn 2019, 11, 151–157. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6897522/ (accessed on 8 April 2024). [PubMed]
- Parker, W.H.; Broder, M.S.; Chang, E.; Feskanich, D.; Farquhar, C.; Liu, Z.; Shoupe, D.; Berek, J.S.; Hankinson, S.; Manson, J.E. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet. Gynecol. 2009, 113, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Meczekalski, B.; Podfigurna-Stopa, A.; Genazzani, A.R. Hypoestrogenism in young women and its influence on bone mass density. Gynecol. Endocrinol. 2010, 26, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bandini, V.; Viganò, P.; Ambruoso, D.; Cetera, G.E.; Somigliana, E. Proposal for targeted, neo-evolutionary-oriented secondary prevention of early-onset endometriosis and adenomyosis. Part II: Medical interventions. Hum. Reprod. 2024, 39, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Westhoff, C.; Kher, U.; Korver, T. Pooled analysis of two randomized, open-label studies comparing the effects of nomegestrol acetate/17β-estradiol and drospirenone/ethinyl estradiol on bleeding patterns in healthy women. Contraception 2017, 95, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Modugno, F.; Ness, R.B.; Allen, G.O.; Schildkraut, J.M.; Davis, F.G.; Goodman, M.T. Oral contraceptive use, reproductive history, and risk of epithelial ovarian cancer in women with and without endometriosis. Am. J. Obstet. Gynecol. 2004, 191, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Rodriguez, T.; Negri, E.; La Vecchia, C. Global trends and predictions in ovarian cancer mortality. Ann. Oncol. 2016, 27, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Fielding, S.; Lidegaard, Ø.; Mørch, L.S.; Skovlund, C.W.; Hannaford, P.C. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: Prospective, nationwide cohort study. BMJ 2018, 362, k3609. [Google Scholar] [CrossRef]
- Dalmartello, M.; La Vecchia, C.; Bertuccio, P.; Boffetta, P.; Levi, F.; Negri, E.; Malvezzi, M. European cancer mortality predictions for the year 2022 with focus on ovarian cancer. Ann. Oncol. 2022, 33, 330–339. [Google Scholar] [CrossRef]
- Murumägi, A.; Ungureanu, D.; Khan, S.; Arjama, M.; Välimäki, K.; Ianevski, A.; Ianevski, P.; Bergström, R.; Dini, A.; Kanerva, A.; et al. Drug response profiles in patient-derived cancer cells across histological subtypes of ovarian cancer: Real-time therapy tailoring for a patient with low-grade serous carcinoma. Br. J. Cancer 2023, 128, 678–690. [Google Scholar] [CrossRef]
- Gil-Martin, M.; Pardo, B.; Barretina-Ginesta, M.P. Rare ovarian tumours. Other treatments for ovarian cancer. Eur. J. Cancer Suppl. 2020, 15, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, C.; Wang, D.; Zhu, Y.; Chen, P. Correlation of clinicopathological and prognostic characteristics between endometriosis-associated and primary ovarian cancer. BMC Cancer 2023, 23, 1210. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; van der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Sananpanichkul, P.; Muangtan, S.; Suknikhom, W.; Bhamarapravatana, K.; Suwannarurk, K. Does Endometriosis Hinder Successful Ovarian Debulking Surgery? Asian Pac. J. Cancer Prev. APJCP 2018, 19, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Erzen, M.; Rakar, S.; Klancnik, B.; Syrjänen, K. Endometriosis-associated ovarian carcinoma (EAOC): An entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol. Oncol. 2001, 83, 100–108. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Y.; Zhang, X.; Wang, L.; Wu, W.; Wu, M.; Meng, C.; Liu, G. Endometriosis-associated ovarian cancer is a single entity with distinct clinicopathological characteristics. Cancer Biol. Ther. 2019, 20, 1029–1034. [Google Scholar] [CrossRef]
- Trimbos, B.; Timmers, P.; Pecorelli, S.; Coens, C.; Ven, K.; van der Burg, M.; Casado, A. Surgical staging and treatment of early ovarian cancer: Long-term analysis from a randomized trial. J. Natl. Cancer Inst. 2010, 102, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Trimbos, J.B.; Vergote, I.; Bolis, G.; Vermorken, J.B.; Mangioni, C.; Madronal, C.; Franchi, M.; Tateo, S.; Zanetta, G.; Scarfone, G.; et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J. Natl. Cancer Inst. 2003, 95, 113–125. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 191–226. [Google Scholar] [CrossRef]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: Pathology and molecular biology and early, advanced and recurrent disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.; Zekri, J.; Wong, H.; Walking, J.; Green, J.A. Platinum-based adjuvant chemotherapy for early-stage epithelial ovarian cancer: Single or combination chemotherapy? BJOG Int. J. Obstet. Gynaecol. 2010, 117, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.; Brady, M.F.; Young, R.C.; Lage, J.; Walker, J.L.; Look, K.Y.; Rose, G.S.; Spirtos, N.M.; Gynecologic Oncology Group. Randomized phase III trial of three versus six cycles of adjuvant carboplatin and paclitaxel in early stage epithelial ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2006, 102, 432–439. [Google Scholar] [CrossRef] [PubMed]
- ICON-Collaborators. ICON2: Randomised trial of single-agent carboplatin against three-drug combination of CAP (cyclophosphamide, doxorubicin, and cisplatin) in women with ovarian cancer. Lancet 1998, 352, 1571–1576. [Google Scholar] [CrossRef]
- International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: The ICON3 randomised trial. Lancet 2002, 360, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F.M.; Braly, P.S.; Brady, M.F.; Sutton, G.; Niemann, T.H.; Lentz, S.L.; Alvarez, R.D.; Kucera, P.R.; Small, J.M. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: A gynecologic oncology group study. J. Clin. Oncol. 2000, 18, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Borella, F.; Fucina, S.; Mangherini, L.; Cosma, S.; Carosso, A.R.; Cusato, J.; Cassoni, P.; Bertero, L.; Katsaros, D.; Benedetto, C. Hormone Receptors and Epithelial Ovarian Cancer: Recent Advances in Biology and Treatment Options. Biomedicines 2023, 11, 2157. [Google Scholar] [CrossRef] [PubMed]
- Hollis, R.L.; Stanley, B.; Iida, Y.; Thomson, J.; Churchman, M.; Rye, T.; Mackean, M.; Nussey, F.; Gourley, C.; Herrington, C.S. Hormone receptor expression patterns define clinically meaningful subgroups of endometrioid ovarian carcinoma. Gynecol. Oncol. 2019, 155, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Burges, A.; Brüning, A.; Dannenmann, C.; Blankenstein, T.; Jeschke, U.; Shabani, N.; Friese, K.; Mylonas, I. Prognostic significance of estrogen receptor alpha and beta expression in human serous carcinomas of the ovary. Arch. Gynecol. Obstet. 2010, 281, 511–517. [Google Scholar] [CrossRef]
- Halon, A.; Materna, V.; Drag-Zalesinska, M.; Nowak-Markwitz, E.; Gansukh, T.; Donizy, P.; Spaczynski, M.; Zabel, M.; Dietel, M.; Lage, H.; et al. Estrogen receptor alpha expression in ovarian cancer predicts longer overall survival. Pathol. Oncol. Res. POR 2011, 17, 511–518. [Google Scholar] [CrossRef]
- Schüler-Toprak, S.; Weber, F.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Expression of estrogen-related receptors in ovarian cancer and impact on survival. J. Cancer Res. Clin. Oncol. 2021, 147, 2555–2567. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet. Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef]
- Langdon, S.P.; Herrington, C.S.; Hollis, R.L.; Gourley, C. Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer. Cancers 2020, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, T.; Alotaibi, F.; Kyeremateng, J.; Halaweish, H.; Kasten, A.; Iram, S.; Halaweish, F. Triazole-estradiol analogs: A potential cancer therapeutic targeting ovarian and colorectal cancer. Steroids 2022, 177, 108950. [Google Scholar] [CrossRef]
- Ono, M.; Miyamoto, T.; Asaka, R.; Uchikawa, J.; Ando, H.; Tanaka, Y.; Shinagawa, M.; Yokokawa, Y.; Asaka, S.; Wang, T.L.; et al. Establishment of a novel model of endometriosis-associated ovarian cancer by transplanting uterine tissue from Arid1a/Pten knockout mice. Sci. Rep. 2023, 13, 8348. [Google Scholar] [CrossRef]
- Wuyung, P.E.; Rahadiati, F.B.; Tjahjadi, H.; Salinah, S.; Kusmardi, K.; Kodariah, R.; Wiweko, B. Histopathology and ARID1A Expression in Endometriosis-Associated Ovarian Carcinoma (EAOC) Carcinogenesis Model with Endometrial Autoimplantation and DMBA Induction. Asian Pac. J. Cancer Prev. APJCP 2021, 22, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.D.C.R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; Baumann, K.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Hirschl, N.; Leveque, W.; Granitto, J.; Sammarco, V.; Fontillas, M.; Penson, R.T. PARP Inhibitors: Strategic Use and Optimal Management in Ovarian Cancer. Cancers 2024, 16, 932. [Google Scholar] [CrossRef] [PubMed]
- Jain, R. A new target for tumor therapy. N. Engl. J. Med. 2009, 360, 2669–2671. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Hardesty, M.M.; Krivak, T.C.; Wright, G.S.; Hamilton, E.; Fleming, E.L.; Belotte, J.; Keeton, E.K.; Wang, P.; Gupta, D.; Clements, A.; et al. OVARIO phase II trial of combination niraparib plus bevacizumab maintenance therapy in advanced ovarian cancer following first-line platinum-based chemotherapy with bevacizumab. Gynecol. Oncol. 2022, 166, 219–229. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, J.; Ganguly, S.; Biswas, B.; Bhaumik, J. First-line Rucaparib Plus Bevacizumab Maintenance Completed One-Year in Germline BRCA1-Mutated Advanced Ovarian Cancer. Cureus 2022, 14, e32493. [Google Scholar] [CrossRef]
- Li, J.; Yue, H.; Li, W.; Zhu, G.; Zhu, T.; Chen, R.; Lu, X. Bevacizumab confers significant improvements in survival for ovarian cancer patients with low miR-25 expression and high miR-142 expression. J. Ovarian Res. 2021, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, A.R.; Kristensen, G.; Embleton, A.; Adusei, C.; Barretina-Ginesta, M.P.; Beale, P.; Helland, Å. Evaluation of Prognostic and Predictive Significance of Circulating MicroRNAs in Ovarian Cancer Patients. Dis. Markers 2017, 2017, 3098542. [Google Scholar] [CrossRef]
- Hartman, J.L.; Garvik, B.; Hartwell, L. Principles for the buffering of genetic variation. Science 2001, 291, 1001–1004. [Google Scholar] [CrossRef]
- Kaelin, W.G. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 2005, 5, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, S.; Cheng, S.; Yang, J.; Wang, Y. Clinical application of PARP inhibitors in ovarian cancer: From molecular mechanisms to the current status. J. Ovarian Res. 2023, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Leconte, M.; Nicco, C.; Ngô, C.; Chéreau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.H.; Brady, W.E.; O’Malley, D.; Fujiwara, K.; Yonemori, K.; Bonebrake, A.; Secord, A.A.; Stephan, J.M.; Walker, J.L.; Nam, J.H.; et al. A phase II evaluation of temsirolimus with carboplatin and paclitaxel followed by temsirolimus consolidation in clear cell ovarian cancer: An NRG oncology trial. Gynecol. Oncol. 2022, 167, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Nero, C.; Romito, I.; Spadola, S.; Romito, A.; Turco, L.C.; Cosentino, F.; DeNinno, M.; Catena, U.; De Cicco Nardone, A.; Moroni, R.; et al. Infiltrating T lymphocytes and programmed cell death protein-1/programmed death-ligand 1 expression in endometriosis-associated ovarian cancer. Fertil. Steril. 2022, 117, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Chen, Y.; Yang, Q. Significance of tumor mutation burden combined with immune infiltrates in the progression and prognosis of ovarian cancer. Cancer Cell Int. 2020, 20, 373. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Hsu, C.Y.; Wu, C.W.; Wang, S.H.; Yeh, C.H.; Cheng, K.H.; Tsai, E.M. Vorinostat decrease M2 macrophage polarization through ARID1A6488delG/HDAC6/IL-10 signaling pathway in endometriosis-associated ovarian carcinoma. Biomed. Pharmacother. 2023, 161, 114500. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbuch, S.C.; Lüß, A.-M.; Eltrop, S.; Götte, M.; Kiesel, L. Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance. Int. J. Mol. Sci. 2024, 25, 4306. https://doi.org/10.3390/ijms25084306
Steinbuch SC, Lüß A-M, Eltrop S, Götte M, Kiesel L. Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance. International Journal of Molecular Sciences. 2024; 25(8):4306. https://doi.org/10.3390/ijms25084306
Chicago/Turabian StyleSteinbuch, Sophie Charlotte, Anne-Marie Lüß, Stephanie Eltrop, Martin Götte, and Ludwig Kiesel. 2024. "Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance" International Journal of Molecular Sciences 25, no. 8: 4306. https://doi.org/10.3390/ijms25084306
APA StyleSteinbuch, S. C., Lüß, A.-M., Eltrop, S., Götte, M., & Kiesel, L. (2024). Endometriosis-Associated Ovarian Cancer: From Molecular Pathologies to Clinical Relevance. International Journal of Molecular Sciences, 25(8), 4306. https://doi.org/10.3390/ijms25084306







