Shigella Vaccines: The Continuing Unmet Challenge
Abstract
1. Introduction
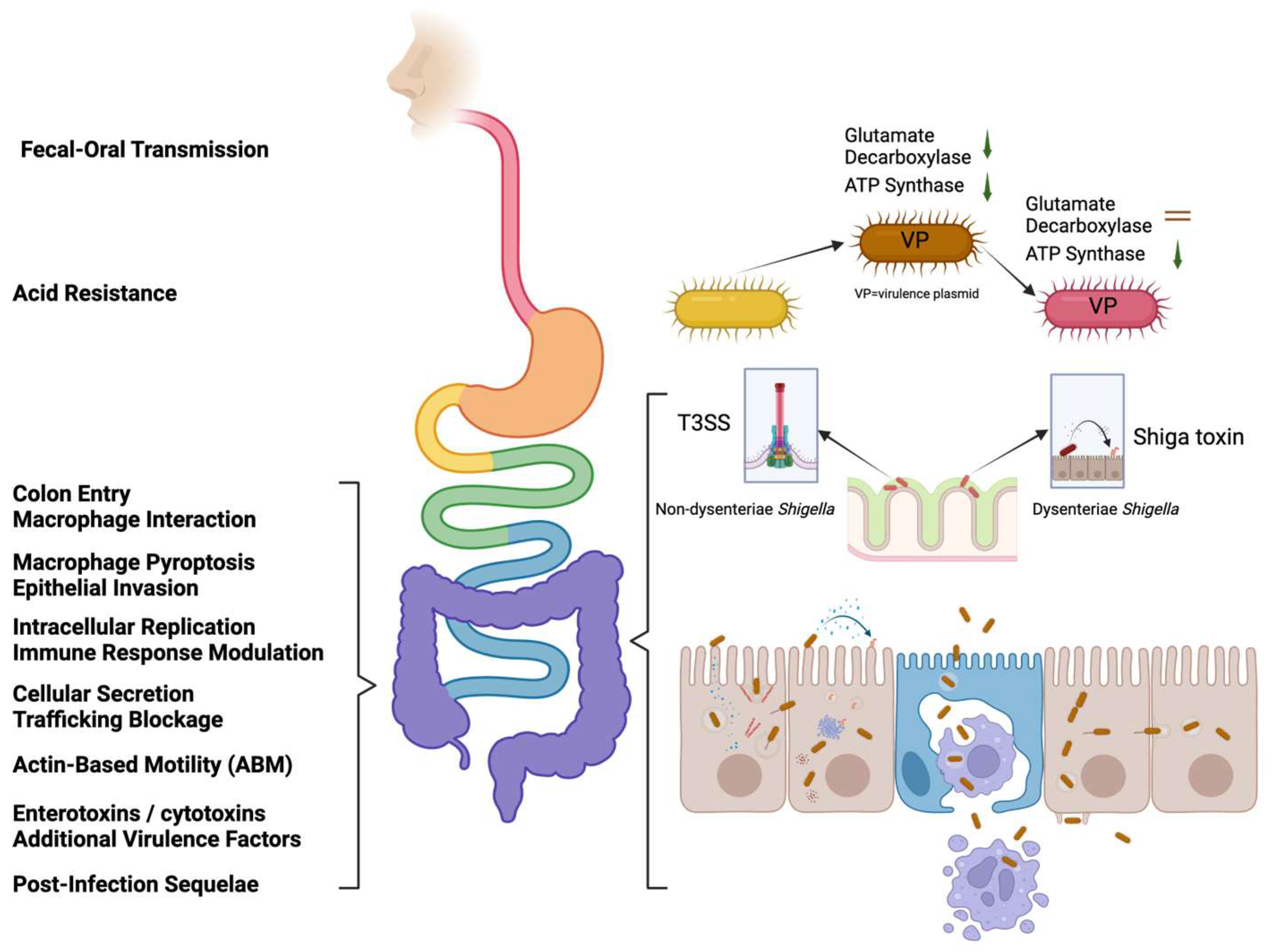
2. Shigella Pathogenesis
3. Challenges Faced in Treating Shigellosis: Antimicrobial Resistance
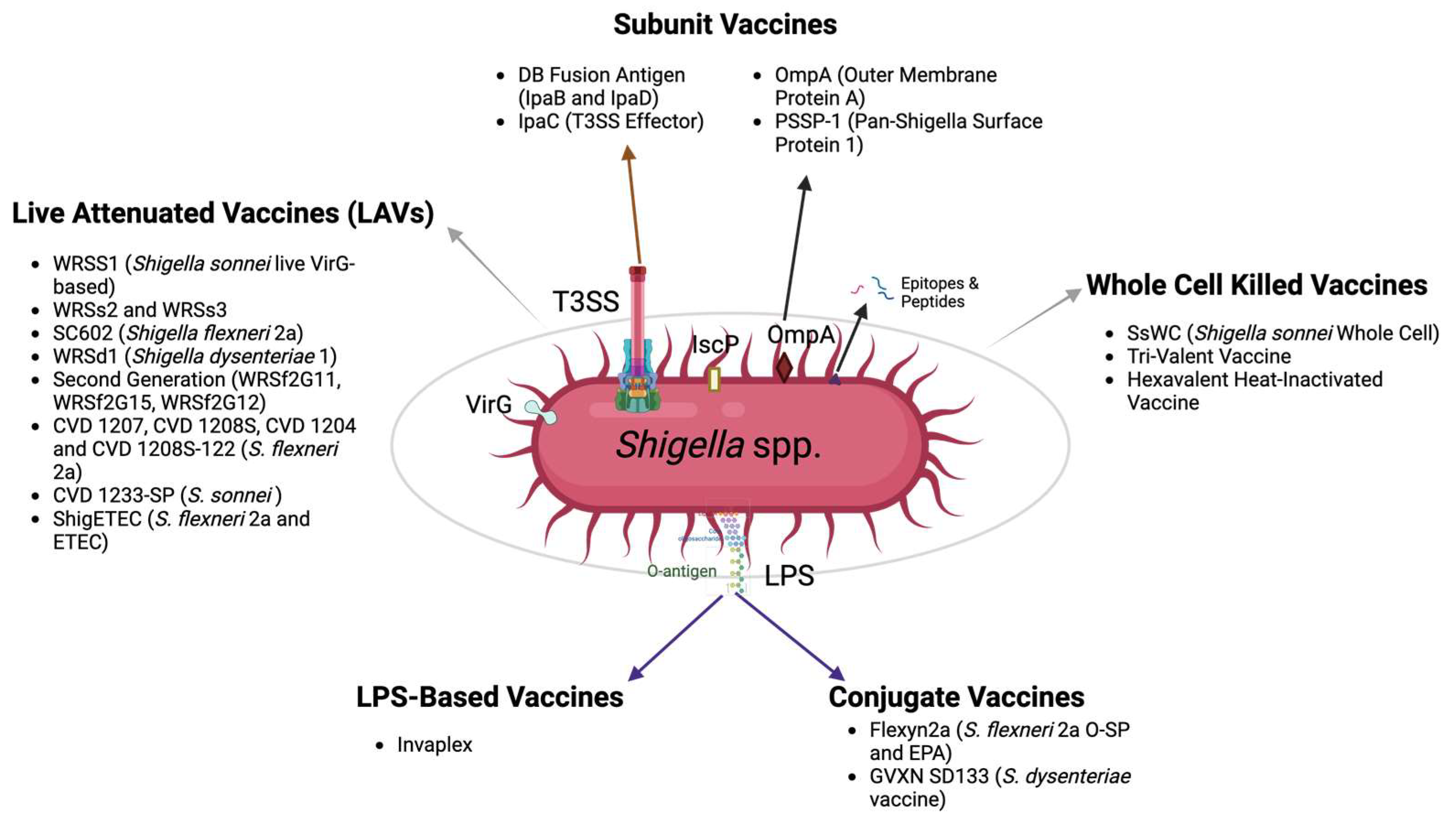
4. The Status of Shigella Vaccines in Development
4.1. Whole, Killed Cells
4.2. Shigella Live Attenuated Vaccines (LAVs)
4.3. LPS-Based Vaccines
4.4. Conjugate Vaccines
4.5. Subunit Vaccines
4.5.1. Vaccines Targeting the T3SS
4.5.2. Other Subunit Vaccine Targets
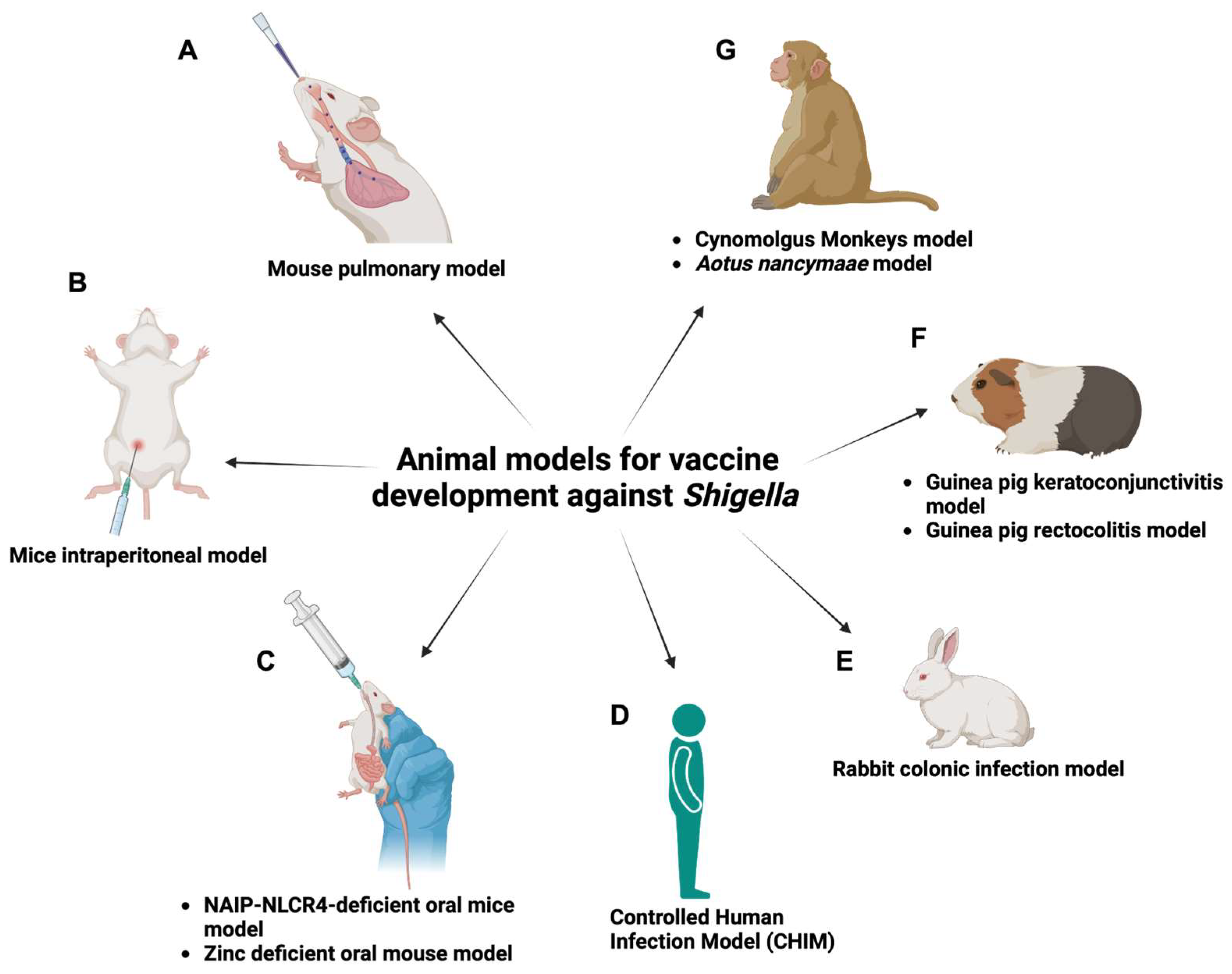
5. The Important Role of Animal Models in Assessing Shigella Vaccines
5.1. Mouse Pulmonary Model
5.2. Mouse Intraperitoneal Model
5.3. NAIP-NLCR4-Deficient Oral Mouse Model
5.4. Zinc-Deficient Oral Mouse Model
5.5. Mouse Intracolonic Model
5.6. Mouse Xenotransplant Model
5.7. Guinea Pig Rectocolitis Model
5.8. Guinea Pig Keratoconjunctivitis Model
5.9. Rabbit Colonic Infection Model
5.10. Cynomolgus Monkey Model
5.11. Aotus Nancymaae Model
5.12. Controlled Human Infection Model (CHIM)
6. The Future of Developing a Shigella Vaccine
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (Ed.) WHO Preferred Product Characteristics for Vaccines against Shigella; World Health Organization: Geneva, Switzerland, 2021; 26p. [Google Scholar]
- Tacconelli, E.M.N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Hale, T.L.; Keusch, G.T. Shigella. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Mani, S.; Wierzba, T.; Walker, R.I. Status of vaccine research and development for Shigella. Vaccine 2016, 34, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Libby, T.E.; Delawalla, M.L.; Al-Shimari, F.; MacLennan, C.A.; Vannice, K.S.; Pavlinac, P.B. Consequences of Shigella infection in young children: A systematic review. Int. J. Infect. Dis. 2023, 129, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Lutfy, C.; Cookson, S.T.; Talley, L.; Rochat, R. Malnourished children in refugee camps and lack of connection with services after US resettlement. J. Immigr. Minor. Health 2013, 16, 1016–1622. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella Isolates From the Global Enteric Multicenter Study Inform Vaccine Development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Bassal, R.; Goren, S.; Rouach, T.; Taran, D.; Schemberg, B.; Peled, N.; Keness, Y.; Ken-Dror, S.; Vasilev, V.; et al. Recent trends in the epidemiology of shigellosis in Israel. Epidemiol. Infect. 2014, 142, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Korin, H.; Bassal, R.; Markovich, M.P.; Sivan, Y.; Goren, S.; Muhsen, K. Burden and risk factors of Shigella sonnei shigellosis among children aged 0–59 months in hyperendemic communities in Israel. Int. J. Infect. Dis. 2019, 82, 117–123. [Google Scholar] [CrossRef]
- Behar, A.; Baker, K.S.; Bassal, R.; Ezernitchi, A.; Valinsky, L.; Thomson, N.R.; Cohen, D. Microevolution and Patterns of Transmission of Shigella sonnei within Cyclic Outbreaks Shigellosis, Israel. Emerg. Infect. Dis. 2018, 24, 1335–1339. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.-L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2020, 21, 546–558. [Google Scholar] [CrossRef]
- Cohen, D.; Ashkenazi, S.; Schneerson, R.; Farzam, N.; Bialik, A.; Meron-Sudai, S.; Asato, V.; Goren, S.; Baran, T.Z.; Muhsen, K.; et al. Threshold protective levels of serum IgG to Shigella lipopolysaccharide: Re-analysis of Shigella vaccine trials data. Clin. Microbiol. Infect. 2022, 29, 366–371. [Google Scholar] [CrossRef]
- A MacLennan, C.; Talaat, K.R.; Kaminski, R.W.; Cohen, D.; Riddle, M.S.; Giersing, B.K. Critical Needs in Advancing Shigella Vaccines for Global Health. J. Infect. Dis. 2021, 225, 1500–1503. [Google Scholar] [CrossRef]
- Miti, S.; Chilyabanyama, O.N.; Chisenga, C.C.; Chibuye, M.; Bosomprah, S.; Mumba, C.; Chitondo, S.; Siziya, S.; Cohen, D.; Chilengi, R.; et al. Sensitivity and predictive value of dysentery in diagnosing shigellosis among under five children in Zambia. PLoS ONE 2023, 18, e0279012. [Google Scholar] [CrossRef]
- Karimi-Yazdi, M.; Ghalavand, Z.; Shabani, M.; Houri, H.; Sadredinamin, M.; Taheri, M.; Eslami, G. High Rates of Antimicrobial Resistance and Virulence Gene Distribution Among Shigella spp. Isolated from Pediatric Patients in Tehran, Iran. Infect. Drug Resist. 2020, 13, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.S.; Mosavat, A.; Youssefi, M.; Jamehdar, S.A.; Ghazvini, K.; Safdari, H.; Amini, Y.; Farsiani, H. High prevalence of blaCMY AmpC beta-lactamase in ESBL co-producing Escherichia coli and Klebsiella spp. clinical isolates in the northeast of Iran. J. Glob. Antimicrob. Resist. 2020, 22, 477–482. [Google Scholar] [CrossRef]
- Sheikh, A.F.; Moosavian, M.; Abdi, M.; Heidary, M.; Shahi, F.; Jomehzadeh, N.; Seyed-Mohammadi, S.; Saki, M.; Khoshnood, S. Prevalence and antimicrobial resistance of Shigella species isolated from diarrheal patients in Ahvaz, southwest Iran. Infect. Drug Resist. 2019, 12, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Abtahi, H.; van Belkum, A.; Ghaznavi-Rad, E. Multidrug-resistant Shigella infection in pediatric patients with diarrhea from central Iran. Infect. Drug Resist. 2019, 12, 1535–1544. [Google Scholar] [CrossRef]
- Martin, D.J.; White, B.K.; Rossman, M.G. Reactive Arthritis After Shigella Gastroenteritis in American Military in Afghanistan. Am. J. Clin. Oncol. 2012, 18, 257–258. [Google Scholar] [CrossRef]
- Gharpure, R.; A Marsh, Z.; Tack, D.M.; A Collier, S.; Strysko, J.; Ray, L.; Payne, D.C.; Garcia-Williams, A.G. Disparities in Incidence and Severity of Shigella Infections Among Children—Foodborne Diseases Active Surveillance Network (FoodNet), 2009-2018. J. Pediatr. Infect. Dis. Soc. 2021, 10, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Mattison, C.P.; Calderwood, L.E.; Marsh, Z.A.; Wikswo, M.E.; Balachandran, N.; Kambhampati, A.K.; Gleason, M.E.; Lawinger, H.; Mirza, S.A. Childcare and School Acute Gastroenteritis Outbreaks: 2009–2020. Pediatrics 2022, 150. [Google Scholar] [CrossRef]
- Gaufin, T.; Blumenthal, J.; Ramirez-Sanchez, C.; Mehta, S.; Pride, D.T.; Fierer, J.; Jenks, J.D. Antimicrobial-Resistant Shigella spp. in San Diego, California, USA, 2017–2020. Emerg. Infect. Dis. 2022, 28, 1110–1116. [Google Scholar] [CrossRef]
- Mo, Y.; Fang, W.; Li, H.; Chen, J.; Hu, X.; Wang, B.; Feng, Z.; Shi, H.; He, Y.; Huang, D.; et al. Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China. Vaccines 2021, 10, 33. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Zuraina, N.M.N.N.; Hajissa, K.; Ilias, M.I.; Singh, K.K.B.; Deris, Z.Z. Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1653. [Google Scholar] [CrossRef]
- Moreno-Mingorance, A.; Mir-Cros, A.; Goterris, L.; Rodriguez-Garrido, V.; Sulleiro, E.; Barberà, M.J.; Alberny, M.; Hoyos-Mallecot, Y.; Descalzo, V.; Bravo, A.; et al. Increasing trend of antimicrobial resistance in Shigella associated with MSM transmission in Barcelona, 2020–21: Outbreak of XRD Shigella sonnei and dissemination of ESBL-producing Shigella flexneri. J. Antimicrob. Chemother. 2023, 78, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Tosisa, W.; Mihret, A.; Ararsa, A.; Eguale, T.; Abebe, T. Prevalence and antimicrobial susceptibility of Salmonella and Shigella species isolated from diarrheic children in Ambo town. BMC Pediatr. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, C.A.; Grow, S.; Ma, L.-F.; Steele, A.D. The Shigella Vaccines Pipeline. Vaccines 2022, 10, 1376. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yang, J.; Liu, H.; Cui, Y.; Xu, H.; Wang, R.; Liu, X.; Feng, E.; Wang, D.; Pan, C.; et al. Role of the virulence plasmid in acid resistance of Shigella flexneri. Sci. Rep. 2017, 7, 46465. [Google Scholar] [CrossRef] [PubMed]
- Buchrieser, C.; Glaser, P.; Rusniok, C.; Nedjari, H.; D’Hauteville, H.; Kunst, F.; Sansonetti, P.; Parsot, C. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 2000, 38, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.A.; Bhagwat, M. Comparative analysis of transcriptional regulatory elements of glutamate-dependent acid-resistance systems of Shigella flexneri and Escherichia coli O157:H7. FEMS Microbiol. Lett. 2004, 234, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Pennacchietti, E.; D’Alonzo, C.; Freddi, L.; Occhialini, A.; De Biase, D. The Glutaminase-Dependent Acid Resistance System: Qualitative and Quantitative Assays and Analysis of Its Distribution in Enteric Bacteria. Front. Microbiol. 2018, 9, 2869. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.B.; Estrada-García, T. Shigella: A Highly Virulent and Elusive Pathogen. Curr. Trop. Med. Rep. 2014, 1, 81–87. [Google Scholar] [CrossRef]
- Van Nhieu, G.T.; Caron, E.; Hall, A.; Sansonetti, P.J. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 1999, 18, 3249–3262. [Google Scholar] [CrossRef]
- Terry, C.M.; Picking, W.L.; Birket, S.E.; Flentie, K.; Hoffman, B.M.; Barker, J.R.; Picking, W.D. The C-terminus of IpaC is required for effector activities related to Shigella invasion of host cells. Microb. Pathog. 2008, 45, 282–289. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, H.; Park, Y.H. Molecular Mechanisms of Host Cytoskeletal Rearrangements by Shigella Invasins. Int. J. Mol. Sci. 2014, 15, 18253–18266. [Google Scholar] [CrossRef] [PubMed]
- Ashida, H.; Mimuro, H.; Sasakawa, C. Shigella Manipulates Host Immune Responses by Delivering Effector Proteins with Specific Roles. Front. Immunol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Ferrari, M.L.; Malardé, V.; Grassart, A.; Salavessa, L.; Nigro, G.; Descorps-Declere, S.; Rohde, J.R.; Schnupf, P.; Masson, V.; Arras, G.; et al. Shigella promotes major alteration of gut epithelial physiology and tissue invasion by shutting off host intracellular transport. Proc. Natl. Acad. Sci. USA 2019, 116, 13582–13591. [Google Scholar] [CrossRef] [PubMed]
- Zumsteg, A.B.; Goosmann, C.; Brinkmann, V.; Morona, R.; Zychlinsky, A. IcsA Is a Shigella flexneri Adhesin Regulated by the Type III Secretion System and Required for Pathogenesis. Cell Host Microbe 2014, 15, 435–445. [Google Scholar] [CrossRef]
- Shere, K.D.; Sallustio, S.; Manessis, A.; D’Aversa, T.G.; Goldberg, M.B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 1997, 25, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Wing, H.J.; Yan, A.W.; Goldman, S.R.; Goldberg, M.B. Regulation of IcsP, the outer membrane protease of the Shigella actin tail assembly protein IcsA, by virulence plasmid regulators VirF and VirB. J. Bacteriol. 2004, 186, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Kayath, C.A.; Hussey, S.; El Hajjami, N.; Nagra, K.; Philpott, D.; Allaoui, A. Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 2010, 12, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Noriega, F.R.; Liao, F.M.; Wang, W.; Levine, M.M. Effect of shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut 1997, 40, 505–511. [Google Scholar] [CrossRef]
- Joseph, A.; Cointe, A.; Kurkdjian, P.M.; Rafat, C.; Hertig, A. Shiga Toxin-Associated Hemolytic Uremic Syndrome: A Narrative Review. Toxins 2020, 12, 67. [Google Scholar] [CrossRef]
- Mattock, E.; Blocker, A.J. How Do the Virulence Factors of Work Together to Cause Disease? Front. Cell Infect. Microbiol. 2017, 7, 249163. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.D.; Lampel, K.A.; Strockbine, N.A.; Fernandez, R.E.; Melton-Celsa, A.R.; Maurelli, A.T. Clinical isolates of Shiga toxin 1a-producing Shigella flexneri with an epidemiological link to recent travel to Hispañiola. Emerg. Infect. Dis. 2014, 20, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Puzari, M.; Sharma, M.; Chetia, P. Emergence of antibiotic resistant Shigella species: A matter of concern. J. Infect. Public Heal. 2018, 11, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Mahbubur, R.; Shoma, S.; Rashid, H.; El Arifeen, S.; Baqui, A.; Siddique, A.; Nair, G.; Sack, D. Increasing Spectrum in Antimicrobial Resistance of Shigella Isolates in Bangladesh: Resistance to Azithromycin and Ceftriaxone and Decreased Susceptibility to Ciprofloxacin. J. Health Popul. Nutr. 2007, 25, 158–167. [Google Scholar]
- Taneja, N.; Mewara, A. Shigellosis: Epidemiology in India. Indian J. Med Res. 2016, 143, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 Is a General Sensor of Peptidoglycan through Muramyl Dipeptide (MDP) Detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, A.; Dar, M.A.; Kaur, R.J.; Charan, J.; Iskandar, K.; Haque, M.; Murti, K.; Ravichandiran, V.; Dhingra, S. Menace of antimicrobial resistance in LMICs: Current surveillance practices and control measures to tackle hostility. J. Infect. Public Heal. 2021, 15, 172–181. [Google Scholar] [CrossRef]
- Fontaine, O. Antibiotics in the Management of Shigellosis in Children: What Role for the Quinolones? Clin. Infect. Dis. 1989, 11, S1145–S1150. [Google Scholar] [CrossRef]
- Williams, P.C.M.; Berkley, J.A. Guidelines for the treatment of dysentery (shigellosis): A systematic review of the evidence. Ann. Trop. Paediatr. 2018, 38, S50–S65. [Google Scholar] [CrossRef]
- Arumugham, V.B.; Gujarathi, R.; Cascella, M. Third-Generation Cephalosporins; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Salleh, M.Z.; Singh, K.K.B.; Deris, Z.Z. Structural Insights into Substrate Binding and Antibiotic Inhibition of Enterobacterial Penicillin-Binding Protein 6. Life 2022, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet. Genom. 2017, 27, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Choia, H.; Navarathnaa, D.H.; Harstona, B.L.; Hwanga, M.; Coronaa, B.; Juana, M.R.S.; Jinadathaa, C. Case of Extensively Drug-Resistant Shigella sonnei Infection, United States. Emerg. Infect. Dis. 2023, 29, 1708–1711. [Google Scholar] [CrossRef]
- Charles, H.; Prochazka, M.; Thorley, K.; Crewdson, A.; Greig, D.R.; Jenkins, C.; Painset, A.; Fifer, H.; Browning, L.; Cabrey, P.; et al. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021–22: A descriptive epidemiological study. Lancet Infect. Dis. 2022, 22, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef]
- Herrera, C.M.; Schmitt, J.S.; Chowdhry, E.I.; Riddle, M.S. From Kiyoshi Shiga to Present-Day Shigella Vaccines: A Historical Narrative Review. Vaccines 2022, 10, 645. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Koldijk, M.; Schuitemaker, H. Inactivated Viral Vaccines. In Vaccine Analysis: Strategies, Principles, and Control; Springer: Berlin/Heidelberg, Germany, 2014; pp. 45–80. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- McKenzie, R.; Walker, R.I.; Nabors, G.S.; Van De Verg, L.L.; Carpenter, C.; Gomes, G.; Forbes, E.; Tian, J.H.; Yang, H.H.; Pace, J.L.; et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: Preclinical studies and a Phase I trial. Vaccine 2006, 24, 3735–3745. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Wu, M.; Turbyfill, K.R.; Clarkson, K.; Tai, B.; Bourgeois, A.L.; Van De Verg, L.L.; Walker, R.I.; Oaks, E.V. Development and Preclinical Evaluation of a Trivalent, Formalin-Inactivated Shigella Whole-Cell Vaccine. Clin. Vaccine Immunol. 2014, 21, 366–382. [Google Scholar] [CrossRef]
- Barman, S.; Koley, H.; Ramamurthy, T.; Chakrabarti, M.K.; Shinoda, S.; Nair, G.B.; Takeda, Y. Protective immunity by oral immunization with heat-killed Shigella strains in a guinea pig colitis model. Microbiol. Immunol. 2013, 57, 762–771. [Google Scholar] [CrossRef]
- Nag, D.; Sinha, R.; Mitra, S.; Barman, S.; Takeda, Y.; Shinoda, S.; Chakrabarti, M.; Koley, H. Heat killed multi-serotype Shigella immunogens induced humoral immunity and protection against heterologous challenge in rabbit model. Immunobiology 2015, 220, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Raqib, R.; Sarker, P.; Zaman, K.; Alam, N.H.; Wierzba, T.F.; Maier, N. A phase I trial of WRSS1, a Shigella sonnei live oral vaccine in Bangladeshi adults and children. Hum. Vacc. Immunother. 2019, 15, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.B.; Venkatesan, M.M. Construction of a stable attenuated Shigella sonnei DeltavirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect. Immun. 1998, 66, 4572–4576. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Taylor, D.N.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; Nataro, J.P. Phase I evaluation of delta virG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 2002, 70, 2016–2021. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orr, N.; Katz, D.E.; Atsmon, J.; Radu, P.; Yavzori, M.; Halperin, T.; Sela, T.; Kayouf, R.; Klein, Z.; Ambar, R.; et al. Community-Based Safety, Immunogenicity, and Transmissibility Study of the Shigella sonnei WRSS1 Vaccine in Israeli Volunteers. Infect. Immun. 2005, 73, 8027–8032. [Google Scholar] [CrossRef]
- Collins, T.A.; Barnoy, S.; Baqar, S.; Ranallo, R.T.; Nemelka, K.W.; Venkatesan, M.M. Safety and colonization of two novel VirG(IcsA)-based live Shigella sonnei vaccine strains in rhesus macaques (Macaca mulatta). Comp. Med. 2008, 58, 88–94. [Google Scholar]
- Barnoy, S.; Jeong, K.I.; Helm, R.F.; Suvarnapunya, A.E.; Ranallo, R.T.; Tzipori, S.; Venkatesan, M.M. Characterization of WRSs2 and WRSs3, new second-generation virG(icsA)-based Shigella sonnei vaccine candidates with the potential for reduced reactogenicity. Vaccine 2010, 28, 1642–1654. [Google Scholar] [CrossRef]
- Barnoy, S.; Baqar, S.; Kaminski, R.; Collins, T.; Nemelka, K.; Hale, T.; Ranallo, R.; Venkatesan, M. Shigella sonnei vaccine candidates WRSs2 and WRSs3 are as immunogenic as WRSS1, a clinically tested vaccine candidate, in a primate model of infection. Vaccine 2011, 29, 6371–6378. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Baqar, S.; Alexander, W.; Dickey, M.; McNeal, M.; El-Khorazaty, J.; Baughman, H.; Hoeper, A.; Barnoy, S.; Suvarnapunya, A.E.; et al. A Phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine 2018, 36, 4880–4889. [Google Scholar] [CrossRef]
- Barzu, S.; Fontaine, A.; Sansonetti, P.; Phalipon, A. Induction of a local anti-IpaC antibody response in mice by use of a Shigella flexneri 2a vaccine candidate: Implications for use of IpaC as a protein carrier. Infect. Immun. 1996, 64, 1190–1196. [Google Scholar] [CrossRef]
- Coster, T.S.; Hoge, C.W.; VanDeVerg, L.L.; Hartman, A.B.; Oaks, E.V.; Venkatesan, M.M.; Cohen, D.; Robin, G.; Fontaine-Thompson, A.; Sansonetti, P.J.; et al. Vaccination against Shigellosis with Attenuated Shigella flexneri 2a Strain SC602. Infect. Immun. 1999, 67, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.E.; Coster, T.S.; Wolf, M.K.; Trespalacios, F.C.; Cohen, D.; Robins, G.; Hartman, A.B.; Venkatesan, M.M.; Taylor, D.N.; Hale, T.L. Two Studies Evaluating the Safety and Immunogenicity of a Live, Attenuated Shigella flexneri 2a Vaccine (SC602) and Excretion of Vaccine Organisms in North American Volunteers. Infect. Immun. 2004, 72, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Lee, S.-N.; Chang, S.-Y.; Ko, H.-J.; Ryu, S.; Kweon, M.-N. A Mouse Model of Shigellosis by Intraperitoneal Infection. J. Infect. Dis. 2013, 209, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Bonny, T.; Azmuda, N.; Khan, S.; Birkeland, N.; Rahman, M. Virulence of Environmental Stenotrophomonas maltophilia Serologically Cross-reacting with Shigella-specific Antisera. Pak. J. Biol. Sci. 2010, 13, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, D.; Liang, F.; Fu, L.; Guo, C. HPV16L1-attenuated Shigella recombinant vaccine induced strong vaginal and systemic immune responses in guinea pig model. Hum. Vaccines Immunother. 2014, 10, 3491–3498. [Google Scholar] [CrossRef] [PubMed]
- Sadorge, C.; Ndiaye, A.; Beveridge, N.; Frazer, S.; Giemza, R.; Jolly, N.; Johnson, J.; Liddy, H.; Cosgrove, C.A.; Allavena, P.; et al. Phase 1 clinical trial of live attenuated Shigella dysenteriae type-1 ΔicsA Δent Δfep ΔstxA:HgR oral vaccine SC599 in healthy human adult volunteers. Vaccine 2008, 26, 978–987. [Google Scholar] [CrossRef]
- Launay, O.; Sadorge, C.; Jolly, N.; Poirier, B.; Béchet, S.; van der Vliet, D.; Seffer, V.; Fenner, N.; Dowling, K.; Giemza, R.; et al. Safety and immunogenicity of SC599, an oral live attenuated Shigella dysenteriae type-1 vaccine in healthy volunteers: Results of a Phase 2, randomized, double-blind placebo-controlled trial. Vaccine 2009, 27, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.M.; Hartman, A.B.; Newland, J.W.; Ivanova, V.S.; Hale, T.L.; McDonough, M.; Butterton, J. Construction, characterization, and animal testing of WRSd1, a Shigella dysenteriae 1 vaccine. Infect. Immun. 2002, 70, 2950–2958. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R.; Venkatesan, M.M.; Wolf, M.K.; Islam, D.; Grahek, S.; Jones, A.M.; Bloom, A.; Taylor, D.N.; Hale, T.L.; Bourgeois, A.L. Safety and immunogenicity of WRSd1, a live attenuated Shigella dysenteriae type 1 vaccine candidate. Vaccine 2008, 26, 3291–3296. [Google Scholar] [CrossRef]
- Sarker, P.; Mily, A.; Ara, A.; Haque, F.; Maier, N.; Wierzba, T.F.; I Walker, R.; Venkatesan, M.M.; Raqib, R. Functional Antibodies and Innate Immune Responses to WRSS1, a Live Oral Shigella sonnei Vaccine Candidate, in Bangladeshi Adults and Children. J. Infect. Dis. 2021, 224, S829–S839. [Google Scholar] [CrossRef]
- Ranallo, R.T.; Thakkar, S.; Chen, Q.; Venkatesan, M.M. Immunogenicity and characterization of WRSF2G11: A second generation live attenuated Shigella flexneri 2a vaccine strain. Vaccine 2007, 25, 2269–2278. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, R.T.; Fonseka, S.; Boren, T.L.; Bedford, L.A.; Kaminski, R.W.; Thakkar, S.; Venkatesan, M.M. Two live attenuated Shigella flexneri 2a strains WRSf2G12 and WRSf2G15: A new combination of gene deletions for 2nd generation live attenuated vaccine candidates. Vaccine 2012, 30, 5159–5171. [Google Scholar] [CrossRef] [PubMed]
- Ranallo, R.; Kaminski, R.; Baqar, S.; Dutta, M.; Lugo-Roman, L.; Boren, T.; Barnoy, S.; Venkatesan, M. Oral administration of live Shigella vaccine candidates in rhesus monkeys show no evidence of competition for colonization and immunogenicity between different serotypes. Vaccine 2014, 32, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Simon, J.K.; Pasetti, M.F.; Sztein, M.B.; Wooden, S.L.; Livio, S. Safety and immunogenicity of CVD 1208S, a live, oral DeltaguaBA Deltasen Deltaset Shigella flexneri 2a vaccine grown on animal-free media. Hum. Vaccines 2007, 3, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kotloff, K.L.; Pasetti, M.F.; Barry, E.M.; Nataro, J.P.; Wasserman, S.S.; Sztein, M.B.; Picking, W.D.; Levine, M.M. Deletion in the Shigella Enterotoxin Genes Further Attenuates Shigella flexneri 2a Bearing Guanine Auxotrophy in a Phase 1 Trial of CVD 1204 and CVD 1208. J. Infect. Dis. 2004, 190, 1745–1754. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Altboum, Z.; Barry, E.M.; Losonsky, G.; Galen, J.E.; Levine, M.M. Attenuated Shigella flexneri 2a Delta guaBA strain CVD 1204 expressing enterotoxigenic Escherichia coli (ETEC) CS2 and CS3 fimbriae as a live mucosal vaccine against Shigella and ETEC infection. Infect. Immun. 2001, 69, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.M.; Wang, J.; Wu, T.; Davis, T.; Levine, M.M. Immunogenicity of multivalent Shigella-ETEC candidate vaccine strains in a guinea pig model. Vaccine 2006, 24, 3727–3734. [Google Scholar] [CrossRef]
- Medeiros, P.H.Q.S.; Bolick, D.T.; Ledwaba, S.E.; Kolling, G.L.; Costa, D.V.S.; Oriá, R.B.; Lima, A.M.; Barry, E.M.; Guerrant, R.L. A bivalent vaccine confers immunogenicity and protection against Shigella flexneri and enterotoxigenic Escherichia coli infections in mice. npj Vaccines 2020, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pilla, G.; Wu, T.; Grassel, C.; Moon, J.; Foulke-Abel, J.; Tang, C.M.; Barry, E.M. Evaluation of a Live Attenuated S. sonnei Vaccine Strain in the Human Enteroid Model. Pathogens 2021, 10, 1079. [Google Scholar] [CrossRef]
- Harutyunyan, S.; Neuhauser, I.; Mayer, A.; Aichinger, M.; Szijártó, V.; Nagy, G.; Nagy, E.; Girardi, P.; Malinoski, F.J.; Henics, T. Characterization of ShigETEC, a Novel Live Attenuated Combined Vaccine against Shigellae and ETEC. Vaccines 2020, 8, 689. [Google Scholar] [CrossRef]
- Girardi, P.; Harutyunyan, S.; Neuhauser, I.; Glaninger, K.; Korda, O.; Nagy, G.; Nagy, E.; Szijártó, V.; Pall, D.; Szarka, K.; et al. Evaluation of the Safety, Tolerability and Immunogenicity of ShigETEC, an Oral Live Attenuated Shigella-ETEC Vaccine in Placebo-Controlled Randomized Phase 1 Trial. Vaccines 2022, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, Q.; Wang, S.; Curtiss, R.; Kong, Q. Regulated Delayed Shigella flexneri 2a O-antigen Synthesis in Live Recombinant Salmonella enterica Serovar Typhimurium Induces Comparable Levels of Protective Immune Responses with Constitutive Antigen Synthesis System. Theranostics 2019, 9, 3565–3579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, H.; Bian, X.; Wang, S.; Kong, Q. Live attenuated Salmonella Typhimurium with monophosphoryl lipid A retains ability to induce T-cell and humoral immune responses against heterologous polysaccharide of Shigella flexneri 2a. Int. J. Med Microbiol. 2020, 310, 151427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sang, S.L.; Guan, Q.; Tao, H.X.; Wang, Y.C.; Liu, C.J. Oral Administration of a 2aT32-Based Vaccine Expressing UreB-HspA Fusion Antigen with and Without Parenteral rUreB-HspA Boost Confers Protection Against in Mice Model. Front. Immunol. 2022, 13, 894206. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, K.A.; Frenck, R.W., Jr.; Dickey, M.; Suvarnapunya, A.E.; Chandrasekaran, L.; Weerts, H.P.; Heaney, C.D.; McNeal, M.; Detizio, K.; Parker, S.; et al. Immune Response Characterization after Controlled Infection with Lyophilized Shigella sonnei 53G. mSphere 2020, 5, e00988-19. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Hartman, A.B.; Oaks, E.V. Isolation and Characterization of a Shigella flexneri Invasin Complex Subunit Vaccine. Infect. Immun. 2000, 68, 6624–6632. [Google Scholar] [CrossRef] [PubMed]
- Turbyfill, K.R.; Clarkson, K.A.; Vortherms, A.R.; Oaks, E.V.; Kaminski, R.W. Assembly, Biochemical Characterization, Immunogenicity, Adjuvanticity, and Efficacy of Shigella Artificial Invaplex. mSphere 2018, 3, e00583-17. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Clarkson, K.A.; Oaks, E.V.; Zurawski, D.V.; Vortherms, A.R.; Kaminski, R.W. Development of the Shigella flexneri 2a, 3a, 6, and S. sonnei artificial Invaplex (InvaplexAR) vaccines. mSphere 2023, 8, e0007323. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, C.; Clarkson, K.A.; Turbyfill, K.R.; Alcala, A.N.; Gutierrez, R.; Riddle, M.S.; Lee, T.; Paolino, K.; Weerts, H.P.; Lynen, A.; et al. GMP manufacture of Shigella flexneri 2a Artificial Invaplex (InvaplexAR) and evaluation in a Phase 1 Open-label, dose escalating study administered intranasally to healthy, adult volunteers. Vaccine 2023, 41, 6261–6271. [Google Scholar] [CrossRef]
- Ledov, V.A.; Golovina, M.E.; Markina, A.A.; Knirel, Y.A.; L’Vov, V.L.; Kovalchuk, A.L.; Aparin, P.G. Highly homogenous tri-acylated S-LPS acts as a novel clinically applicable vaccine against Shigella flexneri 2a infection. Vaccine 2019, 37, 1062–1072. [Google Scholar] [CrossRef]
- Ledov, V.A.; Golovina, M.E.; Alkhazova, B.I.; Lvov, V.L.; Kovalchuk, A.L.; Aparin, P.G. A Pentavalent LPS-Based Vaccine Candidate Is Safe and Immunogenic in Animal Models. Vaccines 2023, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Qasim, M.; Wrage, M.; Nüse, B.; Mattner, J. Shigella Outer Membrane Vesicles as Promising Targets for Vaccination. Int. J. Mol. Sci. 2022, 23, 994. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, U.; Halder, P.; Howlader, D.R.; Banerjee, S.; Maiti, S.; Dutta, S.; Koley, H. A tetravalent Shigella outer membrane vesicles based candidate vaccine offered cross-protection against all the serogroups of Shigella in adult mice. Microbes Infect. 2023, 25, 105100. [Google Scholar] [CrossRef] [PubMed]
- Passwell, J.H.; Harlev, E.; Ashkenazi, S.; Chu, C.; Miron, D.; Ramon, R.; Farzan, N.; Shiloach, J.; Bryla, D.A.; Majadly, F.; et al. Safety and Immunogenicity of Improved Shigella O-Specific Polysaccharide-Protein Conjugate Vaccines in Adults in Israel. Infect. Immun. 2001, 69, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Passwell, J.H.; Ashkenazi, S.; Robbins, J.B.; Schneerson, R.; Grp, I.S.S. Safety and immunogenicity of experimental Shigella conjugate vaccine in toddlers. Pediatr. Res. 2002, 51, 282. [Google Scholar]
- Passwell, J.H.; Ashkenazi, S.; Harlev, E.; Miron, D.; Ramon, R.; Farzam, N. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPA(succ) conjugate vaccines in one- to four-year-old children. Pediatr. Infect. Dis. J. 2003, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Passwell, J.H.; Ashkenazi, S.; Banet-Levi, Y.; Ramon-Saraf, R.; Farzam, N.; Lerner-Geva, L.; Even-Nir, H.; Yerushalmi, B.; Chu, C.; Shiloach, J.; et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine 2010, 28, 2231–2235. [Google Scholar] [CrossRef] [PubMed]
- Raqib, R.; Venkatesan, M. Shigella conjugate vaccine efficacy trial in controlled human model and potential immune correlates of protection. EBioMedicine 2021, 66, 103343. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S.; Kaminski, R.W.; Di Paolo, C.; Porter, C.K.; Gutierrez, R.L.; Clarkson, K.A.; Weerts, H.E.; Duplessis, C.; Castellano, A.; Alaimo, C.; et al. Safety and Immunogenicity of a Candidate Bioconjugate Vaccine against Shigella flexneri 2a Administered to Healthy Adults: A Single-Blind, Randomized Phase I Study. Clin. Vaccine Immunol. 2016, 23, 908–917. [Google Scholar] [CrossRef]
- Ravenscroft, N.; Braun, M.; Schneider, J.; Dreyer, A.M.; Wetter, M.; Haeuptle, M.A.; Kemmler, S.; Steffen, M.; Sirena, D.; Herwig, S.; et al. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 2019, 29, 669–680. [Google Scholar] [CrossRef]
- Ravenscroft, N.; A Haeuptle, M.; Kowarik, M.; Fernandez, F.S.; Carranza, P.; Brunner, A.; Steffen, M.; Wetter, M.; Keller, S.; Ruch, C.; et al. Purification and characterization of a Shigella conjugate vaccine, produced by glycoengineering Escherichia coli. Glycobiology 2015, 26, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Odundo, E.A.; Weerts, H.P.; Musila, L.; Ogonda, L.; Dreyer, A.M.; Schneider, J.; Carranza, P.; Kaminski, R.W. Immunization of Rabbits with a Quadrivalent Shigella Bioconjugate Vaccine Induces Functional Antibodies Reactive with Shigella Isolates from Kenya. mSphere 2022, 7, e0102021. [Google Scholar] [CrossRef] [PubMed]
- Hatz, C.F.; Bally, B.; Rohrer, S.; Steffen, R.; Kramme, S.; Siegrist, C.-A.; Wacker, M.; Alaimo, C.; Fonck, V.G. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella dysenteriae type 1 administered to healthy adults: A single blind, partially randomized Phase I study. Vaccine 2015, 33, 4594–4601. [Google Scholar] [CrossRef] [PubMed]
- Farzam, N.; Ramon-Saraf, R.; Banet-Levi, Y.; Lerner-Geva, L.; Ashkenazi, S.; Kubler-Kielb, J.; Vinogradov, E.; Robbins, J.B.; Schneerson, R. Vaccination with Shigella flexneri 2a conjugate induces type 2a and cross-reactive type 6 antibodies in humans but not in mice. Vaccine 2017, 35, 4990–4996. [Google Scholar] [CrossRef] [PubMed]
- Laird, R.M.; Ma, Z.; Dorabawila, N.; Pequegnat, B.; Omari, E.; Liu, Y.; Maue, A.C.; Poole, S.T.; Maciel, M.; Satish, K.; et al. Evaluation of a conjugate vaccine platform against enterotoxigenic Escherichia coli (ETEC), Campylobacter jejuni and Shigella. Vaccine 2018, 36, 6695–6702. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Mandlik, A.; Charles, R.C.; Verma, S.; Calderwood, S.B.; Leung, D.T.; Biswas, R.; Islam, K.; Kamruzzaman, M.; Chowdhury, F.; et al. Development of Shigella conjugate vaccines targeting Shigella flexneri 2a and S. flexneri 3a using a simple platform-approach conjugation by squaric acid chemistry. Vaccine 2023, 41, 4967–4977. [Google Scholar] [CrossRef] [PubMed]
- Bélot, F.; Guerreiro, C.; Baleux, F.; Mulard, L.A. Synthesis of Two Linear PADRE Conjugates Bearing a Deca- or Pentadecasaccharide B Epitope as Potential Synthetic Vaccines against Shigella flexneri Serotype 2a Infection. Chem.–A Eur. J. 2005, 11, 1625–1635. [Google Scholar] [CrossRef]
- Phalipon, A.; Tanguy, M.; Grandjean, C.; Guerreiro, C.; Bélot, F.; Cohen, D.; Sansonetti, P.J.; Mulard, L.A. A Synthetic Carbohydrate-Protein Conjugate Vaccine Candidate against Shigella flexneri 2a Infection. J. Immunol. 2009, 182, 2241–2247. [Google Scholar] [CrossRef]
- van der Put, R.M.F.; Kim, T.H.; Guerreiro, C.; Thouron, F.; Hoogerhout, P.; Sansonetti, P.J.; Westdijk, J.; Stork, M.; Phalipon, A.; Mulard, L.A. A Synthetic Carbohydrate Conjugate Vaccine Candidate against Shigellosis: Improved Bioconjugation and Impact of Alum on Immunogenicity. Bioconjugate Chem. 2016, 27, 883–892. [Google Scholar] [CrossRef]
- Desalegn, G.; Kapoor, N.; Pill-Pepe, L.; Bautista, L.; Yin, L.; Ndungo, E.; Oaks, E.V.; Fairman, J.; Pasetti, M.F. A Novel Shigella O-Polysaccharide–IpaB Conjugate Vaccine Elicits Robust Antibody Responses and Confers Protection against Multiple Shigella Serotypes. mSphere 2023, 8, e0001923. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Tóth, I. Micro- and Nanotechnology in Vaccine Development; Elsevier: Amsterdam, The Netherlands; William Andrew: Boston, MA, USA, 2017; Volume XVIII, 441p. [Google Scholar]
- Martinez-Becerra, F.J.; Kissmann, J.M.; Diaz-McNair, J.; Choudhari, S.P.; Quick, A.M.; Mellado-Sanchez, G.; Clements, J.D.; Pasetti, M.F.; Picking, W.L. Broadly Protective Shigella Vaccine Based on Type III Secretion Apparatus Proteins. Infect. Immun. 2012, 80, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Picking, W.L.; Tzipori, S. The immune response of two microbial antigens delivered intradermally, sublingually, or the combination thereof. Microbes Infect. 2014, 16, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Becerra, F.J.; Chen, X.; Dickenson, N.E.; Choudhari, S.P.; Harrison, K.; Clements, J.D.; Picking, W.D.; Van De Verg, L.L.; Walker, R.I.; Picking, W.L. Characterization of a Novel Fusion Protein from IpaB and IpaD of Shigella spp. and Its Potential as a Pan-Shigella Vaccine. Infect. Immun. 2013, 81, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Choudhari, S.P.; Martinez-Becerra, F.J.; Kim, J.H.; Dickenson, N.E.; Toth, R.T.; Joshi, S.B.; Greenwood, J.C.; Clements, J.D.; Picking, W.D.; et al. Impact of Detergent on Biophysical Properties and Immune Response of the IpaDB Fusion Protein, a Candidate Subunit Vaccine against Shigella Species. Infect. Immun. 2015, 83, 292–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anam, K.; Endharti, A.T.; Poeranto, S.; Prawiro, S.R. Peptide Sequence of Pili Subunit Protein 49.8 kDa Shigella flexneri as Antigenic Epitope for Shigellosis Vaccine Development. Turk. J. Pharm. Sci. 2022, 19, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Valli, E.; Harriett, A.J.; Nowakowska, M.K.; Baudier, R.L.; Provosty, W.B.; McSween, Z.; Lawson, L.B.; Nakanishi, Y.; Norton, E.B. LTA1 is a safe, intranasal enterotoxin-based adjuvant that improves vaccine protection against influenza in young, old and B-cell-depleted (μMT) mice. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Howlader, D.R.; Das, S.; Dietz, Z.K.; Nagel, A.C.; Whittier, S.K.; Picking, W.D.; Picking, W.L. The L-DBF vaccine cross protects mice against different Shigella serotypes after prior exposure to the pathogen. Microbiol. Spectr. 2023, 11, e0006223. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Das, S.; Howlader, D.R.; Jain, A.; Hu, G.; Dietz, Z.K.; Zheng, Q.; Ratnakaram, S.S.K.; Whittier, S.K.; Varisco, D.J.; et al. Impact of the TLR4 agonist BECC438 on a novel vaccine formulation against Shigella spp. Front. Immunol. 2023, 14, 1194912. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.M. A novel protein-based subunit Shigella vaccine candidate. Immunol. Cell Biol. 2015, 93, 603–604. [Google Scholar] [CrossRef]
- Heine, S.J.; Franco-Mahecha, O.L.; Chen, X.; Choudhari, S.; Blackwelder, W.C.; van Roosmalen, M.L.; Leenhouts, K.; Picking, W.L.; Pasetti, M.F. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol. Cell Biol. 2015, 93, 641–652. [Google Scholar] [CrossRef]
- Felegary, A.; Nazarian, S.; Kordbacheh, E.; Fathi, J.; Minae, M.E. An approach to chimeric subunit immunogen provides efficient protection against toxicity, type III and type v secretion systems of Shigella. Int. Immunopharmacol. 2021, 100, 108132. [Google Scholar] [CrossRef] [PubMed]
- Malaei, F.; Hesaraki, M.; Saadati, M.; Ahdi, A.M.; Sadraeian, M.; Honari, H.; Nazarian, S. Immunogenicity of a new recombinant IpaC from Shigella dysenteriae type I in guinea pig as a vaccine candidate. Iran J. Immunol. 2013, 10, 110–117. [Google Scholar] [PubMed]
- Khalouie, F.; Mousavi, S.L.; Nazarian, S.; Amani, J.; Pourfarzam, P. Immunogenic evaluation of chimeric recombinant protein against ETEC, EHEC and Shigella. Mol. Biol. Res. Commun. 2017, 6, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Baruah, N.; Ahamad, N.; Maiti, S.; Howlader, D.R.; Bhaumik, U.; Patil, V.V.; Chakrabarti, M.K.; Koley, H.; Katti, D.S. Development of a Self-Adjuvanting, Cross-Protective, Stable Intranasal Recombinant Vaccine for Shigellosis. ACS Infect. Dis. 2021, 7, 3182–3196. [Google Scholar] [CrossRef] [PubMed]
- Pore, D.; Chakrabarti, M.K. Outer membrane protein A (OmpA) from Shigella flexneri 2a: A promising subunit vaccine candidate. Vaccine 2013, 31, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Scribano, D.; Damico, R.; Ambrosi, C.; Superti, F.; Marazzato, M.; Conte, M.P.; Longhi, C.; Palamara, A.T.; Zagaglia, C.; Nicoletti, M. The Shigella flexneri OmpA amino acid residues 188 EVQ 190 are essential for the interaction with the virulence factor PhoN2. Biochem. Biophys. Rep. 2016, 8, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Pompili, M.; Scribano, D.; Zagaglia, C.; Ripa, S.; Nicoletti, M. Outer Membrane Protein A (OmpA): A New Player in Shigella flexneri Protrusion Formation and Inter-Cellular Spreading. PLoS ONE 2012, 7, e49625. [Google Scholar] [CrossRef] [PubMed]
- Pastor, Y.; Camacho, A.I.; Zúñiga-Ripa, A.; Merchán, A.; Rosas, P.; Irache, J.M.; Gamazo, C. Towards a subunit vaccine from a Shigella flexneri ΔtolR mutant. Vaccine 2018, 36, 7509–7519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Rho, S.; Kim, S.H.; Kim, H.; Song, H.J.; Kim, E.J.; Kim, R.Y.; Kim, E.H.; Sinha, A.; Dey, A.; et al. Shigella Outer Membrane Protein PSSP-1 Is Broadly Protective against Shigella Infection. Clin. Vaccine Immunol. 2015, 22, 381–388. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.-H.; Kim, H.; Rho, S.; Shin, Y.K.; Song, M.; Walker, R.; Czerkinsky, C.; Kim, D.W.; Kim, J.-O. Cross-Protective Shigella Whole-Cell Vaccine With a Truncated O-Polysaccharide Chain. Front. Microbiol. 2018, 9, 2609. [Google Scholar] [CrossRef]
- Baseer, S.; Ahmad, S.; Ranaghan, K.E.; Azam, S.S. Towards a peptide-based vaccine against Shigella sonnei: A subtractive reverse vaccinology based approach. Biologicals 2017, 50, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Oany, A.R.; Pervin, T.; Mia, M.; Hossain, M.; Shahnaij, M.; Mahmud, S.; Kibria, K.M.K. Vaccinomics Approach for Designing Potential Peptide Vaccine by Targeting Shigella spp. Serine Protease Autotransporter Subfamily Protein SigA. J. Immunol. Res. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- León, Y.; Zapata, L.; Salas-Burgos, A.; Oñate, A. In silico design of a vaccine candidate based on autotransporters and HSP against the causal agent of shigellosis, Shigella flexneri. Mol. Immunol. 2020, 121, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Anam, K.; Endharti, A.T.; Poeranto, S.; Sujuti, H.; Hidayati, D.Y.N.; Prawiro, S.R. Shigella flexneri vaccine development: Oral administration of peptides derived from the 49.8 kDa pili protein subunit activates the intestinal immune response in mice. Veter-World 2022, 15, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hidayati, D.Y.N.; Rully, S.; Amalia, A.; Widyani, E.L.; Indraswari, G.; Prasetya, A.; Soraya, M.; Winarsih, S.; Prawiro, S.R. Cross Protectivity Analysis of 49.8 kDa Pili Subunits of S. flexneri against Vibrio cholerae Infection. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shipley, S.T.; Panda, A.; Khan, A.Q.; Kriel, E.H.; Maciel, M.; Livio, S.; Nataro, J.P.; Levine, M.M.; Sztein, M.B.; DeTolla, L.J. A challenge model for Shigella dysenteriae 1 in cynomolgus monkeys (Macaca fascicularis). Comp. Med. 2010, 60, 54–61. [Google Scholar]
- Rabbani, G.H.; Albert, M.J.; Rahman, H.; Islam, M.; Mahalanabis, D.; Kabir, I.; Alam, K.; Ansaruzzaman, M. Development of an improved animal model of shigellosis in the adult rabbit by colonic infection with Shigella flexneri 2a. Infect. Immun. 1995, 63, 4350–4357. [Google Scholar] [CrossRef]
- Alphonse, N.; Odendall, C. Animal models of shigellosis: A historical overview. Curr. Opin. Immunol. 2023, 85, 102399. [Google Scholar] [CrossRef] [PubMed]
- van de Verg, L.L.; Mallett, C.P.; Collins, H.H.; Larsen, T.; Hammack, C.; Hale, T.L. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect. Immun. 1995, 63, 1947–1954. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Roncaioli, J.L.; A Turcotte, E.; Goers, L.; A Chavez, R.; Lee, A.Y.; Lesser, C.F.; Rauch, I.; E Vance, R. NAIP–NLRC4-deficient mice are susceptible to shigellosis. eLife 2020, 9. [Google Scholar] [CrossRef]
- Roncaioli, J.L.; Babirye, J.P.; A Chavez, R.; Liu, F.L.; A Turcotte, E.; Lee, A.Y.; Lesser, C.F.; E Vance, R. A hierarchy of cell death pathways confers layered resistance to shigellosis in mice. eLife 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, P.H.Q.S.; Ledwaba, S.E.; Bolick, D.T.; Giallourou, N.; Yum, L.K.; Costa, D.V.S.; Oriá, R.B.; Barry, E.M.; Swann, J.R.; Lima, A.Â.M.; et al. A murine model of diarrhea, growth impairment and metabolic disturbances with Shigella flexneri infection and the role of zinc deficiency. Gut Microbes 2019, 1–16. [Google Scholar] [CrossRef]
- Singer, M.; Sansonetti, P.J. IL-8 Is a Key Chemokine Regulating Neutrophil Recruitment in a New Mouse Model of Shigella-Induced Colitis. J. Immunol. 2004, 173, 4197–4206. [Google Scholar] [CrossRef]
- Seydel, K.B.; Li, E.; E Swanson, P.; Stanley, S.L. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect. Immun. 1997, 65, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Sperandio, B.; Regnault, B.; Guo, J.; Zhang, Z.; Stanley, S.L.; Sansonetti, P.J.; Pédron, T. Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J. Exp. Med. 2008, 205, 1121–1132. [Google Scholar] [CrossRef]
- Shim, D.-H.; Suzuki, T.; Chang, S.-Y.; Park, S.-M.; Sansonetti, P.J.; Sasakawa, C.; Kweon, M.-N. New Animal Model of Shigellosis in the Guinea Pig: Its Usefulness for Protective Efficacy Studies. J. Immunol. 2007, 178, 2476–2482. [Google Scholar] [CrossRef]
- Wenzel, H.; Kaminski, R.W.; Clarkson, K.A.; Maciel, M.; Smith, M.A.; Zhang, W.; Oaks, E.V. Improving chances for successful clinical outcomes with better preclinical models. Vaccine 2017, 35, 6798–6802. [Google Scholar] [CrossRef]
- Sereny, B. Experimental shigella keratoconjunctivitis; a preliminary report. Acta Microbiol. Acad. Sci. Hung. 1955, 2, 293–296. [Google Scholar] [PubMed]
- Gregory, M.; Kaminski, R.W.; Lugo-Roman, L.A.; Galvez Carrillo, H.; Tilley, D.H.; Baldeviano, C.; Simons, M.P.; Reynolds, N.D.; Ranallo, R.T.; Suvarnapunya, A.E.; et al. Development of an Aotus nancymaae Model for Shigella Vaccine Immunogenicity and Efficacy Studies. Infect. Immun. 2014, 82, 2027–2036. [Google Scholar] [CrossRef]
- Frenck, R.W.; Dickey, M.; Suvarnapunya, A.E.; Chandrasekaran, L.; Kaminski, R.W.; Clarkson, K.A.; McNeal, M.; Lynen, A.; Parker, S.; Hoeper, A.; et al. Establishment of a Controlled Human Infection Model with a Lyophilized Strain of Shigella sonnei 53G. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Song, Y.-J.; Cheong, H.-K.; Ki, M.; Shin, J.-Y.; Hwang, S.-S.; Park, M.; Ki, M.; Lim, J. The Epidemiological Influence of Climatic Factors on Shigellosis Incidence Rates in Korea. Int. J. Environ. Res. Public Health 2018, 15, 2209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lin, C.-Y.; Chen, K.-T. Epidemiologic features of shigellosis and associated climatic factors in Taiwan. Medicine 2019, 98, e16928. [Google Scholar] [CrossRef] [PubMed]
- Aiman, S.; Ahmad, A.; Khan, A.; Ali, Y.; Malik, A.; Alkholief, M.; Akhtar, S.; Khan, R.S.; Li, C.; Jalil, F. Vaccinomics-aided next-generation novel multi-epitope-based vaccine engineering against multidrug resistant Shigella Sonnei: Immunoinformatics and chemoinformatics approaches. PLoS ONE 2023, 18, e0289773. [Google Scholar] [CrossRef] [PubMed]
- van der Put, R.M.F.; Smitsman, C.; de Haan, A.; Hamzink, M.; Timmermans, H.; Uittenbogaard, J.; Westdijk, J.; Stork, M.; Ophorst, O.; Thouron, F.; et al. The First-in-Human Synthetic Glycan-Based Conjugate Vaccine Candidate against Shigella. ACS Central Sci. 2022, 8, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Giersing, B.K.; Isbrucker, R.; Kaslow, D.C.; Cavaleri, M.; Baylor, N.; Maiga, D.; Pavlinac, P.B.; Riddle, M.S.; Kang, G.; Maclennan, C.A. Clinical and regulatory development strategies for vaccines intended for children younger than 5 years in low-income and middle-income countries. Lancet Glob. Health 2023, 11, E1819–E1826. [Google Scholar] [CrossRef]

| Vaccine Type | Development Stage |
|---|---|
| Whole-cell formalin-inactivated vaccine (SsWC) | Phase 1 human trial |
| Trivalent vaccine using whole-killed S. flexneri 2a, S. sonnei, and S. flexneri 3a | Preclinical |
| Hexavalent heat-inactivated vaccine covering multiple Shigella strains | Preclinical |
| S. sonnei live virG knockout vaccine candidate, WRSs1 | Phase 1 human trials completed |
| S. sonnei live attenuated vaccine candidates, WRSs2 and WRSs3 | Phase 1 trials completed |
| S. flexneri 2a SC602 | Phase 1 trials ongoing |
| S. dysenteriae 1 SC599 | Phase 1 trials completed |
| S. dysenteriae 1 live attenuated vaccine candidate, WRSd1 | Preclinical |
| Second-generation live attenuated vaccine candidates (e.g., WRSf2G11, WRSf2G12, WRSf2G15) | Preclinical |
| S. flexneri 2a live attenuated vaccine candidate, CVD 1207 | Phase 1 trials ongoing |
| S. flexneri CVD 1208S and CVD 1204 | Phase 1 trials ongoing |
| Combined Shigella-ETEC vaccine candidate, CVD 1208S-122 | Preclinical |
| Live attenuated S. sonnei vaccine strain, CVD 1233-SP | Preclinical |
| ShigETEC (live attenuated combined vaccine targeting S. flexneri 2a and ETEC) | Phase 1 clinical trials ongoing |
| Salmonella vaccine system for delivering S. flexneri 2a (Sf2a) O-antigen | Preclinical |
| RASV-delivered Sf2a O-antigen vaccine | Preclinical |
| Oral Shigella 2aT32-based vaccine expressing a fusion antigen of UreB-HspA | Preclinical |
| nvaplex (Shigella LPS combined with T3SS proteins IpaB and IpaC) | Phase 1 studies completed |
| Invaplex AR (second-generation artificial Invaplex product) | Phase 1 studies completed |
| Ac3-S-LPS (tri-acylated lipid A variant) | Phase 1 studies completed |
| PLVF (pentavalent LPS candidate vaccine) | Preclinical |
| Needle-free delivery systems for OMVs derived from S. flexneri | Preclinical |
| Flexyn2a (S. flexneri 2a O-SP conjugated with EPA) | Phase 1 studies completed |
| Sd1-EPA and Sf2a-EPA (bioconjugates) | Phase 1 studies ongoing |
| GVXN SD133 (S. dysenteriae vaccine featuring Shigella O1 LPS and EPA) | Phase 1 studies completed |
| Shigella 4V (quadrivalent bioconjugate vaccine) | Preclinical |
| SCV (multivalent Shigella conjugate vaccine) | Preclinical |
| Shigella O-polysaccharide (OPS)-IpaB conjugate vaccine | Preclinical |
| Various subunit vaccines targeting T3SS proteins (e.g., IpaB, IpaD, IpaC) | Varying stages of preclinical and clinical development |
| Subunit vaccines targeting other Shigella proteins (e.g., OmpA, PSSP-1) | Varying stages of preclinical and clinical development |
| Peptide-based vaccines derived from subunit proteins (e.g., peptides from 49.8 kDa pili protein subunit) | Preclinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.; Das, S.; Howlader, D.R.; Picking, W.D.; Picking, W.L. Shigella Vaccines: The Continuing Unmet Challenge. Int. J. Mol. Sci. 2024, 25, 4329. https://doi.org/10.3390/ijms25084329
Lu T, Das S, Howlader DR, Picking WD, Picking WL. Shigella Vaccines: The Continuing Unmet Challenge. International Journal of Molecular Sciences. 2024; 25(8):4329. https://doi.org/10.3390/ijms25084329
Chicago/Turabian StyleLu, Ti, Sayan Das, Debaki R. Howlader, William D. Picking, and Wendy L. Picking. 2024. "Shigella Vaccines: The Continuing Unmet Challenge" International Journal of Molecular Sciences 25, no. 8: 4329. https://doi.org/10.3390/ijms25084329
APA StyleLu, T., Das, S., Howlader, D. R., Picking, W. D., & Picking, W. L. (2024). Shigella Vaccines: The Continuing Unmet Challenge. International Journal of Molecular Sciences, 25(8), 4329. https://doi.org/10.3390/ijms25084329






