Transport Properties in Multicomponent Systems Containing Cyclodextrins and Nickel Ions
Abstract
1. Introduction
2. Results
2.1. Diffusion Measurements
2.2. UV-Vis Spectroscopy Measurements
3. Discussion
3.1. Hydrolysis of Nickel Ion and Interactions between Cyclodextrins and Nickel Ion as Seen by Diffusion Coefficients Measurements
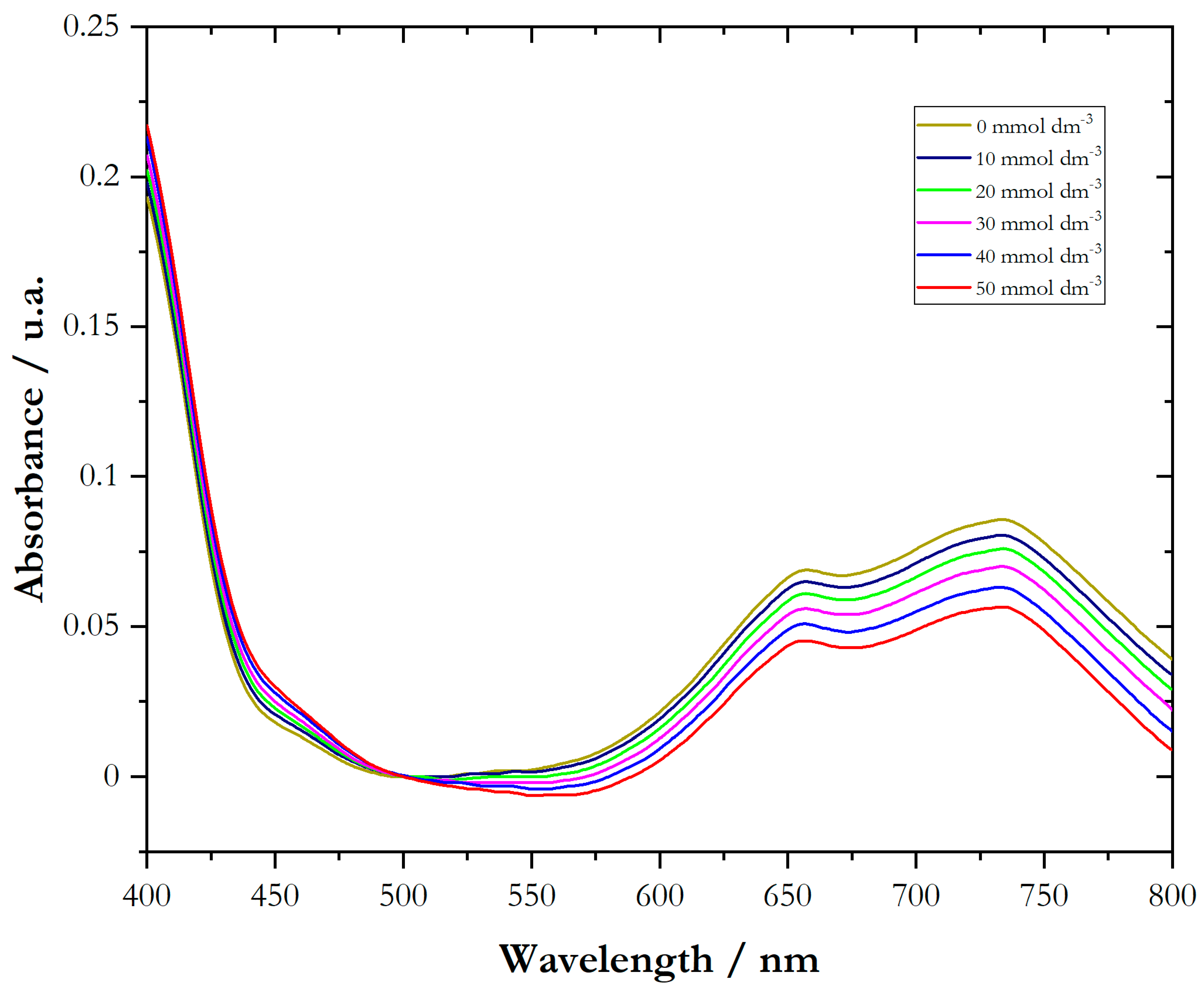
3.2. Interaction of NiCl2 and Cyclodextrins Analyzed Using UV-Vis Spectroscopy Measurements
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. pH Measurements
4.2.2. Taylor Dispersion Method
4.2.3. Ultraviolet-Visible Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellia, F.; La Mendola, D.; Pedone, C.; Rizzarelli, E.; Saviano, M.; Vecchio, G. Selectively Functionalized Cyclodextrins and Their Metal Complexes. Chem. Soc. Rev. 2009, 38, 2756. [Google Scholar] [CrossRef] [PubMed]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring Versatile Applications of Cyclodextrins: An Overview. Drug Deliv. 2016, 23, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Prochowicz, D.; Kornowicz, A.; Lewiński, J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. [Google Scholar] [CrossRef] [PubMed]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J. (Ed.) Phillip’s Science of Dental Materials, 11th ed.; Saunders: St. Louis, MO, USA, 2003; ISBN 0-7216-9387-3. [Google Scholar]
- Rao, S.B.; Chowdhary, R. Evaluation on the Corrosion of the Three Ni-Cr Alloys with Different Composition. Int. J. Dent. 2011, 2011, 397029. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Yilmaz, B.; Atilla, A.O.; Evis, Z. Nickel Titanium Alloys as Orthodontic Archwires: A Narrative Review. Eng. Sci. Technol. Int. J. 2022, 36, 101277. [Google Scholar] [CrossRef]
- Alipour, S.; Taromian, F.; Ghomi, E.R.; Zare, M.; Singh, S.; Ramakrishna, S. Nitinol: From Historical Milestones to Functional Properties and Biomedical Applications. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; Oliveira, F.; Cruz, H.V.; Sukotjo, C.; Mathew, M.T. Bio-Tribocorrosion in Dental Applications. In Bio-Tribocorrosion in Biomaterials and Medical Implants; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 223–249. ISBN 9780857095404. [Google Scholar]
- Mathew, M.T.; Srinivasa Pai, P.; Pourzal, R.; Fischer, A.; Wimmer, M.A. Significance of Tribocorrosion in Biomedical Applications: Overview and Current Status. Adv. Tribol. 2009, 2009, 250986. [Google Scholar] [CrossRef]
- Fróis, A.; Mendes, A.R.; Pereira, S.A.; Louro, C.S. Metal Release and Surface Degradation of Fixed Orthodontic Appliances during the Dental Levelling and Aligning Phase: A 12-Week Study. Coatings 2022, 12, 554. [Google Scholar] [CrossRef]
- Jafari, K.; Rahimzadeh, S.; Hekmatfar, S. Nickel Ion Release from Dental Alloys in Two Different Mouthwashes. J. Dent. Res. Dent. Clin. Dent. Prospect. 2019, 13, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Aluminum, Arsenic, Beryllium, Cadmium, Chromium, Cobalt, Copper, Iron, Lead, Mercury, Molybdenum, Nickel, Platinum, Thallium, Titanium, Vanadium, and Zinc: Molecular Aspects in Experimental Liver Injury. Int. J. Mol. Sci. 2022, 23, 12213. [Google Scholar] [CrossRef] [PubMed]
- Bellouard, M.; Gasser, M.; Lenglet, S.; Gilardi, F.; Bararpour, N.; Augsburger, M.; Thomas, A.; Alvarez, J.-C. Toxicity and Metabolomic Impact of Cobalt, Chromium, and Nickel Exposure on HepaRG Hepatocytes. Chem. Res. Toxicol. 2022, 35, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.R.; Abraham, A.P.; Murugesan, K.; Matsa, V. An Invitro Analysis of Elemental Release and Cytotoxicity of Recast Nickel–Chromium Dental Casting Alloys. J. Indian Prosthodont. Soc. 2011, 11, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rincic Mlinaric, M.; Durgo, K.; Katic, V.; Spalj, S. Cytotoxicity and Oxidative Stress Induced by Nickel and Titanium Ions from Dental Alloys on Cells of Gastrointestinal Tract. Toxicol. Appl. Pharmacol. 2019, 383, 114784. [Google Scholar] [CrossRef] [PubMed]
- Martín-Cameán, A.; Jos, Á.; Mellado-García, P.; Iglesias-Linares, A.; Solano, E.; Cameán, A.M. In Vitro and in Vivo Evidence of the Cytotoxic and Genotoxic Effects of Metal Ions Released by Orthodontic Appliances: A Review. Environ. Toxicol. Pharmacol. 2015, 40, 86–113. [Google Scholar] [CrossRef] [PubMed]
- Mulware, S.J. Trace Elements and Carcinogenicity: A Subject in Review. 3 Biotech 2013, 3, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Conte, M.P.; Fisher, J.; Gonçalves, L.S.; Dussart, C.; Llodra, J.C.; Bourgeois, D. COVID-19: A Recommendation to Examine the Effect of Mouthrinses with β-Cyclodextrin Combined with Citrox in Preventing Infection and Progression. J. Clin. Med. 2020, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Carrouel, F.; Gonçalves, L.S.; Conte, M.P.; Campus, G.; Fisher, J.; Fraticelli, L.; Gadea-Deschamps, E.; Ottolenghi, L.; Bourgeois, D. Antiviral Activity of Reagents in Mouth Rinses against SARS-CoV-2. J. Dent. Res. 2021, 100, 124–132. [Google Scholar] [CrossRef]
- Fangaia, S.I.G.; Cabral, A.M.T.D.P.V.; Nicolau, P.M.G.; Guerra, F.A.D.R.A.; Rodrigo, M.M.; Ribeiro, A.C.F.; Valente, A.J.M.; Esteso, M.A. Diffusion of Vanadium Ions in Artificial Saliva and Its Elimination from the Oral Cavity by Pharmacological Compounds Present in Mouthwashes. Biomolecules 2022, 12, 947. [Google Scholar] [CrossRef]
- Naureen, Z.; Capodicacasa, N.; Paolacci, S.; Anpilogov, K.; Dautaj, A.; Dhuli, K.; Camilleri, G.; Connelly, S.T.; Gasparetto, A.; Bertelli, M. Prevention of the Proliferation of Oral Pathogens Due to Prolonged Mask Use Based on α-Cyclodextrin and Hydroxytyrosol Mouthwash. Eur. Rev. Med. Pharmacol. Sci. 2021, 25 (Suppl. S1), 74–80. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins as Multi-Functional Ingredients in Dentistry. Pharmaceutics 2023, 15, 2251. [Google Scholar] [CrossRef] [PubMed]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-Based Host-Guest Supramolecular Nanoparticles for Delivery: From Design to Applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Trindade, A.C.V.; Fangaia, S.I.G.; Nicolau, P.M.G.; Messias, A.; Ribeiro, A.C.F.; Silva, D.S.A.; Valente, A.J.M.; Rodrigo, M.M.; Esteso, M.A. Transport Properties of Carbohydrates: Towards the Minimization Toxicological Risks of Cobalt and Chromium Ions. Processes 2023, 11, 1701. [Google Scholar] [CrossRef]
- Ji, J.; Cooper, W.C. Nickel Speciation in Aqueous Chloride Solutions. Electrochim. Acta 1996, 41, 1549–1560. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Veríssimo, L.V.M.M.; Gomes, J.C.S.; Santos, C.I.A.V.; Barros, M.C.F.; Lobo, V.M.M.; Sobral, A.J.F.N.; Esteso, M.A.; Leaist, D.G. Mutual Diffusion Coefficients in Systems Containing the Nickel Ion. Comptes Rendus Mécanique 2013, 341, 417–420. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Gomes, J.C.S.; Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A.; Leaist, D.G. Ternary Mutual Diffusion Coefficients of Aqueous NiCl2 + NaCl and NiCl2 + HCl Solutions at 298.15 K. J. Chem. Eng. Data 2011, 56, 4696–4699. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Valente, A.J.M.; Santos, C.I.A.V.; Prazeres, P.M.R.A.; Lobo, V.M.M.; Burrows, H.D.; Esteso, M.A.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B. Binary Mutual Diffusion Coefficients of Aqueous Solutions of α-Cyclodextrin, 2-Hydroxypropyl-α-Cyclodextrin, and 2-Hydroxypropyl-β-Cyclodextrin at Temperatures from (298.15 to 312.15) K. J. Chem. Eng. Data 2007, 52, 586–590. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Leaist, D.G.; Esteso, M.A.; Lobo, V.M.M.; Valente, A.J.M.; Santos, C.I.A.V.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B. Binary Mutual Diffusion Coefficients of Aqueous Solutions of β-Cyclodextrin at Temperatures from 298.15 to 312.15 K. J. Chem. Eng. Data 2006, 51, 1368–1371. [Google Scholar] [CrossRef]
- Aich, K.; Goswami, S.; Das, S.; Mukhopadhyay, C. Das A New ICT and CHEF Based Visible Light Excitable Fluorescent Probe Easily Detects in vivo Zn2+. RSC Adv. 2015, 5, 31189–31194. [Google Scholar] [CrossRef]
- Tyrrell, H.J.V.; Harris, K.R. Diffusion in Liquids: A Theoretical and Experimental Study; Butterworths: London, UK, 1984. [Google Scholar]
- Loh, W. A Técnica de Dispersão de Taylor Para Estudos de Difusão Em Líquidos e Suas Aplicações. Quim. Nova 1997, 20, 541–545. [Google Scholar] [CrossRef]
| Aqueous System | pH |
|---|---|
| NiCl2 | 6.08 |
| NiCl2/α-CD | 6.20 |
| NiCl2/β-CD | 5.98 |
| NiCl2/γ-CD | 6.14 |
| α-CD | 6.40 |
| β-CD | 6.00 |
| γ-CD | 5.95 |
| C1 (b) | C2 (b) | X1 (c) | D11 ± SD (d) | D12 ± SD (d) | D21 ± SD (d) | D22 ± SD (d) |
|---|---|---|---|---|---|---|
| NiCl2 (component 1) + α-CD (component 2) | ||||||
| 0.000 | 0.001 | 0.00 | 1.376 ± 0.015 | 0.069 ± 0.020 | −0.002 ± 0.005 | 0.376 ± 0.002 |
| 0.0005 | 0.0005 | 0.50 | 1.158 ± 0.001 | 0.001 ± 0.003 | 0.020 ± 0.006 | 0.369 ± 0.001 |
| 0.001 | 0.000 | 1.00 | 1.103 ± 0.001 | −0.169 ± 0.052 | 0.005 ± 0.002 | 0.393 ± 0.000 |
| NiCl2 (component 1) + β-CD (component 2) | ||||||
| 0.000 | 0.001 | 0.00 | 1.372 ± 0.036 | 0.009 ± 0.002 | −0.028 ± 0.005 | 0.361 ± 0.001 |
| 0.0005 | 0.0005 | 0.50 | 1.123 ± 0.011 | 0.086 ± 0.005 | 0.006 ± 0.001 | 0.324 ± 0.003 |
| 0.001 | 0.000 | 1.00 | 1.100 ± 0.001 | −0.135 ± 0.011 | 0.003 ± 0.001 | 0.369 ± 0.001 |
| NiCl2 (component 1) + γ-CD (component 2) | ||||||
| 0.000 | 0.001 | 0.00 | 1.312 ± 0.009 | 0.096 ± 0.014 | −0.017 ± 0.010 | 0.280 ± 0.011 |
| 0.0005 | 0.0005 | 0.50 | 1.192 ± 0.024 | −0.013 ± 0.002 | 0.009 ± 0.005 | 0.357 ± 0.003 |
| 0.001 | 0.000 | 1.00 | 1.106 ± 0.000 | −0.101 ± 0.021 | 0.003 ± 0.002 | 0.351 ± 0.000 |
| λ/nm | b1 (σ(b1))/dm3 mmol−1 | R2 (a) | ε/mol−1 dm3 cm−1 (b) | LOD/mmol dm−3 (c) | LOQ/mmol dm−3 (d) |
|---|---|---|---|---|---|
| 721 | (0.00200) ±(0.00002) | 0.9991 | 2.00 × 10−6 | 1.60 | 4.86 |
| Species | Ds (10−9 m2 s−1) |
|---|---|
| NiCl2 | 1.150 (a) |
| α-CD | 0.361 (a) |
| β-CD | 0.358 (a) |
| γ-CD | 0.239 (a) |
| NiCl2—α-CD | 0.367 (b) |
| NiCl2—β-CD | 0.354 (b) |
| NiCl2—γ-CD | 0.238 (b) |
| Aqueous System | D12/D22 |
|---|---|
| NiCl2—α-CD | −0.430 |
| NiCl2—β-CD | −0.366 |
| NiCl2—γ-CD | −0.288 |
| Chemical Name | Source | CAS Number | Mass Fraction Purity (a) |
|---|---|---|---|
| Nickel chloride hexahydrate | Panreac | 7791-13-1 | >0.98 |
| α-cyclodextrin (a) | Sigma-Aldrich (d) | 10016-20-3 | ≥0.98 |
| β-cyclodextrin (b) | Sigma-Aldrich (d) | 7585-39-9 | >0.97 |
| γ-cyclodextrin (c) | Sigma-Aldrich (d) | 17465-86-0 | ≥0.98 |
| H2O | Millipore-Q water (ρ = 1.82 × 105 Ω m at 298.15 K) | 7732–18-5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fangaia, S.I.G.; Silva, D.S.A.; Messias, A.; Nicolau, P.M.G.; Valente, A.J.M.; Rodrigo, M.M.; Ribeiro, A.C.F. Transport Properties in Multicomponent Systems Containing Cyclodextrins and Nickel Ions. Int. J. Mol. Sci. 2024, 25, 4328. https://doi.org/10.3390/ijms25084328
Fangaia SIG, Silva DSA, Messias A, Nicolau PMG, Valente AJM, Rodrigo MM, Ribeiro ACF. Transport Properties in Multicomponent Systems Containing Cyclodextrins and Nickel Ions. International Journal of Molecular Sciences. 2024; 25(8):4328. https://doi.org/10.3390/ijms25084328
Chicago/Turabian StyleFangaia, Sónia I. G., Daniela S. A. Silva, Ana Messias, Pedro M. G. Nicolau, Artur J. M. Valente, M. Melia Rodrigo, and Ana C. F. Ribeiro. 2024. "Transport Properties in Multicomponent Systems Containing Cyclodextrins and Nickel Ions" International Journal of Molecular Sciences 25, no. 8: 4328. https://doi.org/10.3390/ijms25084328
APA StyleFangaia, S. I. G., Silva, D. S. A., Messias, A., Nicolau, P. M. G., Valente, A. J. M., Rodrigo, M. M., & Ribeiro, A. C. F. (2024). Transport Properties in Multicomponent Systems Containing Cyclodextrins and Nickel Ions. International Journal of Molecular Sciences, 25(8), 4328. https://doi.org/10.3390/ijms25084328











