Dental Stem Cells and Lipopolysaccharides: A Concise Review
Abstract
1. Introduction
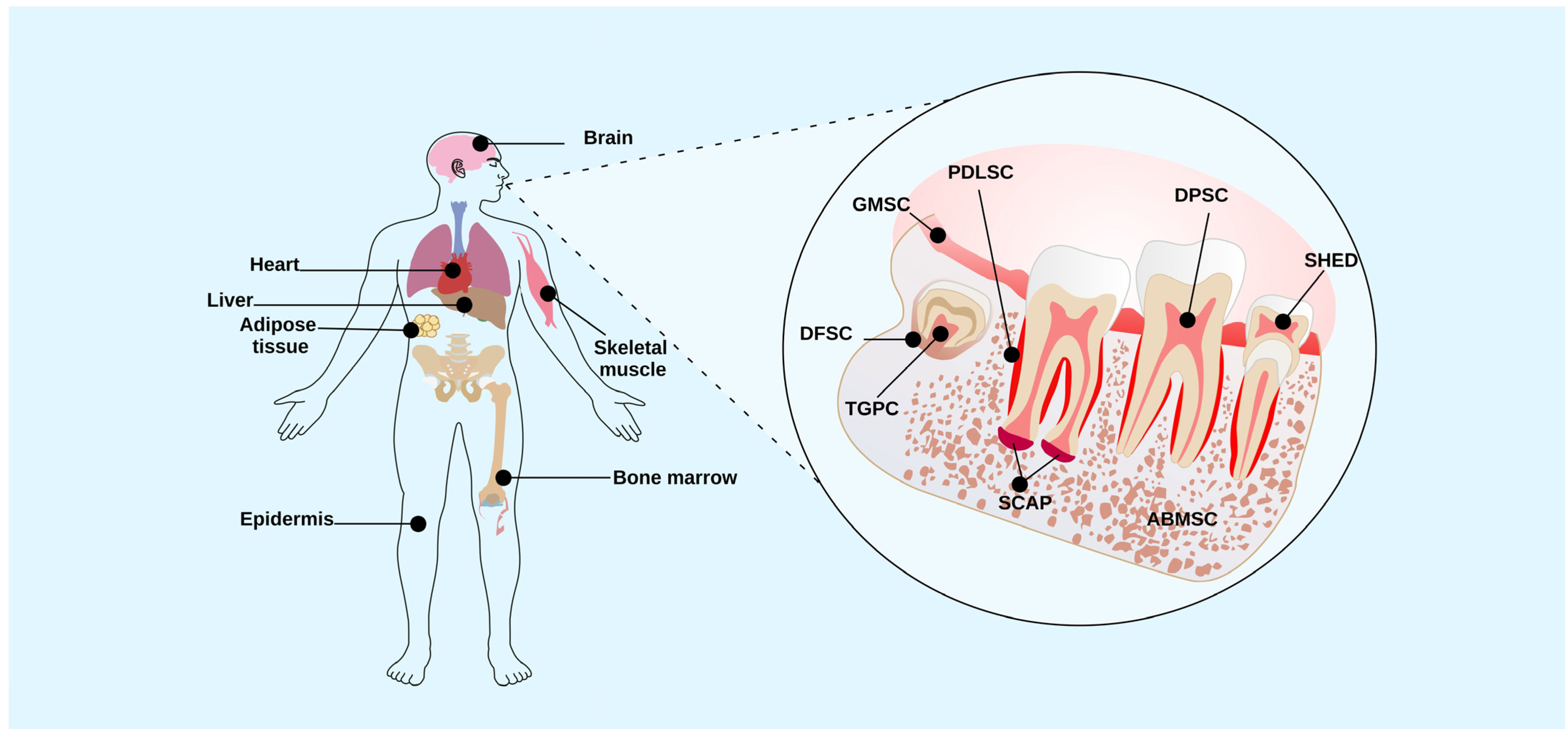
2. Dental Tissue Stem Cells
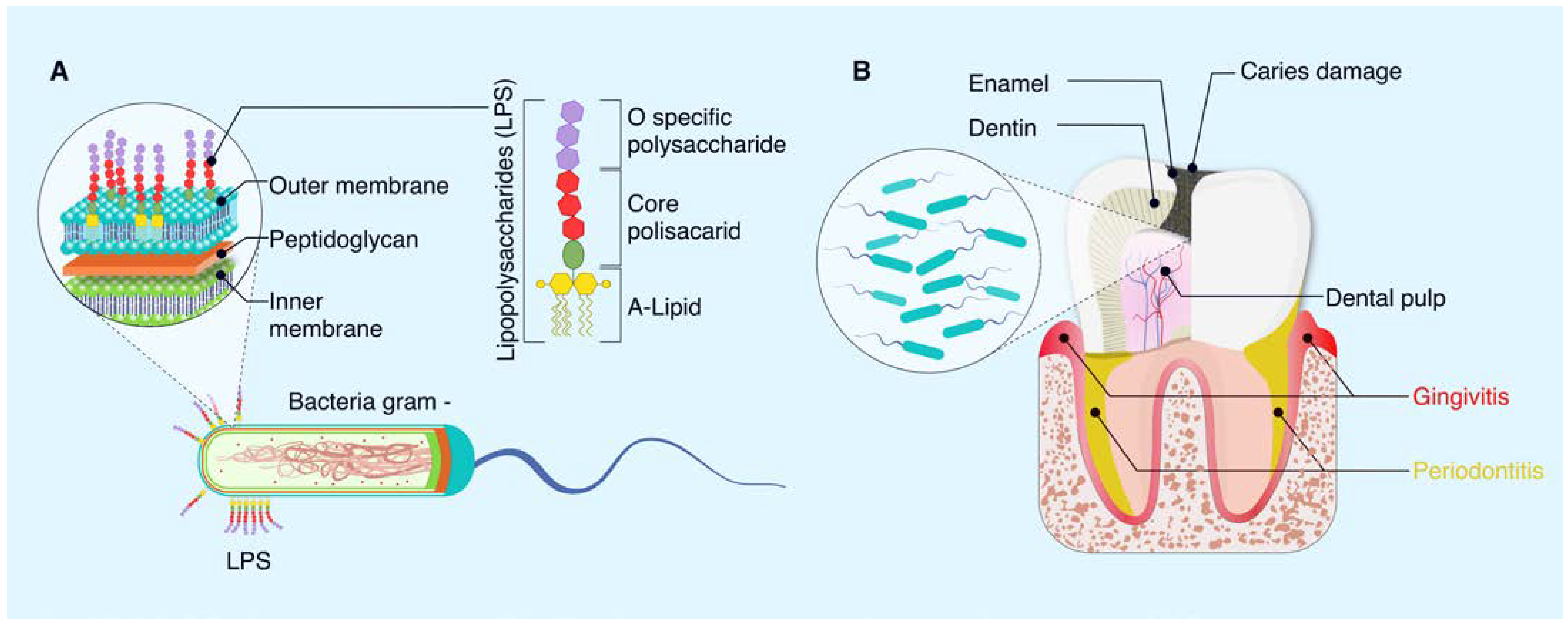
3. Lipopolysaccharides as a Component of Gram-Negative Bacteria
3.1. Structure of LPS Bacteria
3.2. Lipopolysaccharides on Oral Diseases
4. Effect of Bacterial LPS on the Biological Characteristics of Dental Stem Cells
Influence of LPS on Cell Viability and Cellular Differentiation
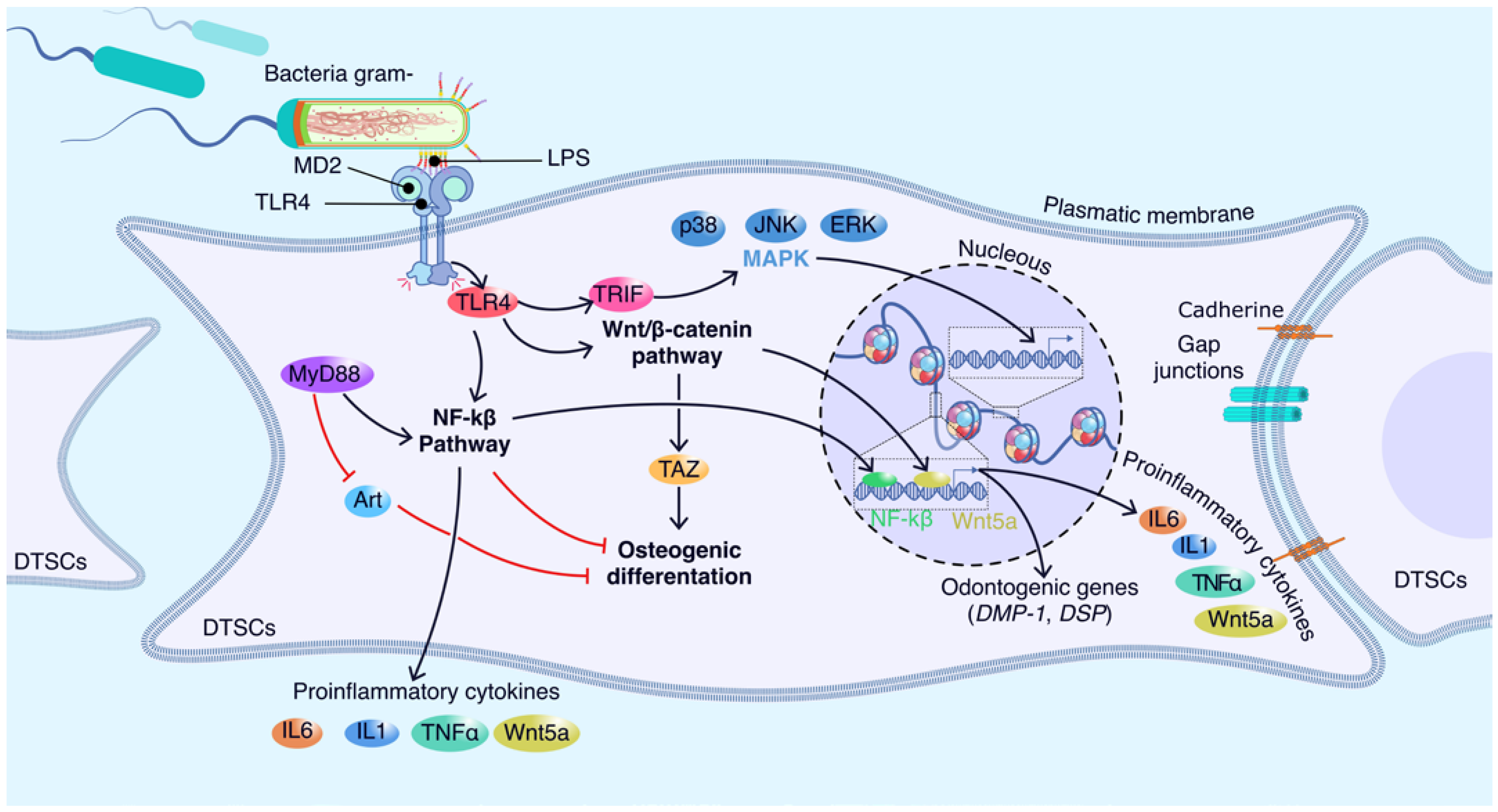
5. Molecular Players Involved in the Interaction between Dental Stem Cells and LPS
5.1. Molecular Sensors of LPS in Dental Stem Cells
5.2. Emerging Players in DTSCs-LPS
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The oralome and its dysbiosis: New insights into oral microbiome-host interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The oral microbiota: Community composition, influencing factors, pathogenesis, and interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Mercado-Rubio, M.D.; Pérez-Argueta, E.; Zepeda-Pedreguera, A.; Aguilar-Ayala, F.J.; Peñaloza-Cuevas, R.; Kú-González, A.; Rojas-Herrera, R.A.; Rodas-Junco, B.A.; Nic-Can, G.I. Similar features, different behaviors: A comparative in vitro study of the adipogenic potential of stem cells from human follicle, dental pulp, and periodontal ligament. J. Pers. Med. 2021, 11, 738. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Chen, S.; Jiang, S.; Lei, H.; Cai, Z.; Huang, X. Different expression patterns of inflammatory cytokines induced by lipopolysaccharides from Escherichia coli or Porphyromonas gingivalis in human dental pulp stem cells. BMC Oral Health 2022, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Bindal, P.; Ramasamy, T.S.; Kasim, N.H.A.; Gnanasegaran, N.; Chai, W.L. Immune responses of human dental pulp stem cells in lipopolysaccharide-induced microenvironment. Cell Biol. Int. 2018, 42, 832–840. [Google Scholar] [CrossRef]
- Jung, J.; Woo, S.; Kim, W.; Lee, B.; Nör, J.; Min, K.; Choi, C.; Koh, J.; Lee, K.; Hwang, Y. Simvastatin inhibits the expression of inflammatory cytokines and cell adhesion molecules induced by LPS in human dental pulp cells. Int. Endod. J. 2017, 50, 377–386. [Google Scholar] [CrossRef]
- Qiao, S.; Luo, Q.; Zhao, Y.; Zhang, X.C.; Huang, Y. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature 2014, 511, 108–111. [Google Scholar] [CrossRef]
- Richter, W.; Vogel, V.; Howe, J.; Steiniger, F.; Brauser, A.; Koch, M.H.; Roessle, M.; Gutsmann, T.; Garidel, P.; Mäntele, W. Morphology, size distribution, and aggregate structure of lipopolysaccharide and lipid A dispersions from enterobacterial origin. Innate Immun. 2011, 17, 427–438. [Google Scholar] [CrossRef]
- Caroff, M.; Karibian, D. Structure of bacterial lipopolysaccharides. Carbohydr. Res. 2003, 338, 2431–2447. [Google Scholar] [CrossRef]
- Jerala, R. Structural biology of the LPS recognition. Int. J. Med. Microbiol. 2007, 297, 353–363. [Google Scholar] [CrossRef]
- Dixon, D.; Darveau, R. Lipopolysaccharide heterogeneity: Innate host responses to bacterial modification of lipid a structure. J. Dental Res. 2005, 84, 584–595. [Google Scholar] [CrossRef]
- Gao, Y.; You, X.; Liu, Y.; Gao, F.; Zhang, Y.; Yang, J.; Yang, C. Induction of autophagy protects human dental pulp cells from lipopolysaccharide-induced pyroptotic cell death. Exp. Ther. Med. 2020, 19, 2202–2210. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Kwack, K.H.; Lee, H.-W. Clinical potential of dental pulp stem cells in pulp regeneration: Current endodontic progress and future perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Li, G.; Yu, J.; Fang, F.; Qiu, W. Dental-derived mesenchymal stem cell sheets: A prospective tissue engineering for regenerative medicine. Stem Cell Res. Ther. 2022, 13, 38. [Google Scholar] [CrossRef]
- Baru, O.; Nutu, A.; Braicu, C.; Cismaru, C.A.; Berindan-Neagoe, I.; Buduru, S.; Badea, M. Angiogenesis in regenerative dentistry: Are we far enough for therapy? Int. J. Mol. Sci. 2021, 22, 929. [Google Scholar] [CrossRef]
- Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate gram-negative bacteria evasion of host innate immunity. Front. Immunol. 2013, 4, 109. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; d’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef]
- Herath, T.D.; Wang, Y.; Seneviratne, C.J.; Darveau, R.P.; Wang, C.-Y.; Jin, L. The expression and regulation of matrix metalloproteinase-3 is critically modulated by Porphyromonas gingivalis lipopolysaccharide with heterogeneous lipid A structures in human gingival fibroblasts. BMC Microbiol. 2013, 13, 73. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent advances in lipopolysaccharide recognition systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Gorman, A.; Golovanov, A.P. Lipopolysaccharide structure and the phenomenon of low endotoxin recovery. Eur. J. Pharm. Biopharm. 2022, 180, 289–307. [Google Scholar] [CrossRef]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Hashim, D.; Cionca, N.; Combescure, C.; Mombelli, A. The diagnosis of peri-implantitis: A systematic review on the predictive value of bleeding on probing. Clin. Oral Implants Res. 2018, 29, 276–293. [Google Scholar] [CrossRef]
- Dahlen, G.; Basic, A.; Bylund, J. Importance of virulence factors for the persistence of oral bacteria in the inflamed gingival crevice and in the pathogenesis of periodontal disease. J. Clin. Med. 2019, 8, 1339. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Hegde, R.; Awan, K. Effects of periodontal disease on systemic health. Dis. Month 2019, 65, 185–192. [Google Scholar] [CrossRef]
- Bozkurt, S.B.; Hakki, S.S.; Hakki, E.E.; Durak, Y.; Kantarci, A. Porphyromonas gingivalis lipopolysaccharide induces a pro-inflammatory human gingival fibroblast phenotype. Inflammation 2017, 40, 144–153. [Google Scholar] [CrossRef]
- Ding, P.H.; Jin, L. The role of lipopolysaccharide-binding protein in innate immunity: A revisit and its relevance to oral/periodontal health. J. Periodontal Res. 2014, 49, 1–9. [Google Scholar] [CrossRef]
- Shaddox, L.; Gonçalves, P.; Vovk, A.; Allin, N.; Huang, H.; Hou, W.; Aukhil, I.; Wallet, S. LPS-induced inflammatory response after therapy of aggressive periodontitis. J. Dental Res. 2013, 92, 702–708. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Meyle, J.; Dommisch, H.; Groeger, S.; Giacaman, R.A.; Costalonga, M.; Herzberg, M. The innate host response in caries and periodontitis. J. Clin. Periodontol. 2017, 44, 1215–1225. [Google Scholar] [CrossRef]
- Priyamvara, A.; Dey, A.K.; Bandyopadhyay, D.; Katikineni, V.; Zaghlol, R.; Basyal, B.; Barssoum, K.; Amarin, R.; Bhatt, D.L.; Lavie, C.J. Periodontal inflammation and the risk of cardiovascular disease. Curr. Atheroscler. Rep. 2020, 22, 28. [Google Scholar] [CrossRef]
- Lorenz, W.; Buhrmann, C.; Mobasheri, A.; Lueders, C.; Shakibaei, M. Bacterial lipopolysaccharides form procollagen-endotoxin complexes that trigger cartilage inflammation and degeneration: Implications for the development of rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R111. [Google Scholar] [CrossRef]
- Caccese, R.G.; Zimmerman, J.L.; Carlson, R.P. Bacterial lipopolysaccharide potentiates type II collagen-induced arthritis in mice. Mediat. Inflamm. 1992, 1, 273–279. [Google Scholar] [CrossRef]
- Huang, Z.; Stabler, T.; Pei, F.; Kraus, V.B. Both systemic and local lipopolysaccharide (LPS) burden are associated with knee OA severity and inflammation. Osteoarthr. Cartil. 2016, 24, 1769–1775. [Google Scholar] [CrossRef]
- Koziel, J.; Potempa, J. Pros and cons of causative association between periodontitis and rheumatoid arthritis. Periodontology 2000 2022, 89, 83–98. [Google Scholar] [CrossRef]
- Barutta, F.; Bellini, S.; Durazzo, M.; Gruden, G. Novel insight into the mechanisms of the bidirectional relationship between diabetes and periodontitis. Biomedicines 2022, 10, 178. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamashita, M.; Oda, M.; Tjendana Tjhin, V.; Inagawa, H.; Soma, G.-I. Oral administration of lipopolysaccharide enhances insulin signaling-related factors in the KK/Ay mouse model of type 2 diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 4619. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ren, W.; Liu, J.; Yang, P.; Chen, Z.; Zhang, Q.; Yang, F. Lipopolysaccharide-induced suppression of periodontal ligament cell proliferation and apoptosis are strengthened under high glucose conditions. Arch. Oral Biol. 2017, 79, 70–76. [Google Scholar] [CrossRef]
- Kim, D.; Rho, J.; Woo, B.; Joo, J.; Lee, J.; Song, J.; Lee, J.; Park, H. Periodontal pathogens modulate lipid flux via fatty acid binding protein 4. J. Dental Res. 2019, 98, 1511–1520. [Google Scholar] [CrossRef]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease—Plausible mechanisms. Periodontology 2000 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martínez, M.S.; Chacín, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C. Microbiota and diabetes mellitus: Role of lipid mediators. Nutrients 2020, 12, 3039. [Google Scholar] [CrossRef]
- Heinbockel, L.; Weindl, G.; Martinez-de-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of lipopolysaccharide-and lipoprotein-induced inflammation by antitoxin peptide Pep19-2.5. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef]
- Stinghen, A.; Gonçalves, S.; Bucharles, S.; Branco, F.; Gruber, B.; Hauser, A.; Pecoits-Filho, R. Sevelamer decreases systemic inflammation in parallel to a reduction in endotoxemia. Blood Purif. 2010, 29, 352–356. [Google Scholar] [CrossRef]
- Sattari, M.; Masoudnia, M.; Mashayekhi, K.; Hashemi, S.M.; Khannazer, N.; Sattari, S.; Mohammadian Haftcheshmeh, S.; Momtazi-Borojeni, A.A. Evaluating the effect of LPS from periodontal pathogenic bacteria on the expression of senescence-related genes in human dental pulp stem cells. J. Cell. Mol. Med. 2022, 26, 5647–5656. [Google Scholar] [CrossRef]
- Lei, S.; Liu, X.-M.; Liu, Y.; Bi, J.; Zhu, S.; Chen, X. Lipopolysaccharide downregulates the osteo-/odontogenic differentiation of stem cells from apical papilla by inducing autophagy. J. Endod. 2020, 46, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-C.; Wu, J.-H.; Chen, S.-Y.; Hsu, Y.-T.; Yeung, S.-Y.; Pan, Y.-H.; Jeng, J.-H. Inducing cyclooxygenase-2 expression, prostaglandin E2 and prostaglandin F2α production of human dental pulp cells by activation of toll-like receptor-3, mitogen-activated protein kinase kinase/extracellular signal-regulated kinase and p38 signaling. J. Dental Sci. 2024, 19, 1190–1199. [Google Scholar] [CrossRef]
- Brodzikowska, A.; Ciechanowska, M.; Kopka, M.; Stachura, A.; Włodarski, P.K. Role of Lipopolysaccharide, Derived from Various Bacterial Species, in Pulpitis—A Systematic Review. Biomolecules 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- MENDES SOARES, I.P.; Anselmi, C.; Pires, M.L.B.A.; Ribeiro, R.A.d.O.; Leite, M.L.; Soares, D.G.; de Souza Costa, C.A.; Hebling, J. Chronic exposure to lipopolysaccharides as an in vitro model to simulate the impaired odontogenic potential of dental pulp cells under pulpitis conditions. J. Appl. Oral Sci. 2023, 31, e20230032. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Eidt, A.; Wölflick, M.; Lindner, S.; Schweikl, H.; Hiller, K.A.; Buchalla, W.; Galler, K. Interactive effects of LPS and dentine matrix proteins on human dental pulp stem cells. Int. Endod. J. 2018, 51, 877–888. [Google Scholar] [CrossRef]
- Firouzi, N.; Yavari, H.R.; Rahimi, S.; Roshangar, L.; Chitsazha, R.; Amini, M. Concentrated Growth Factors Combined with Lipopolysaccharide Stimulate the In Vitro Regenerative and Osteogenic Activities of Human Dental Pulp Stem Cells by Balancing Inflammation. Int. J. Dent. 2022, 2022, 2316666. [Google Scholar] [CrossRef]
- Azaryan, E.; Karbasi, S.; Saharkhiz, M.; Hanafi-Bojd, M.Y.; Zarban, A.; Emadian Razavi, F.; Naseri, M. Effect of HM-Exos on the migration and inflammatory response of LPS-exposed dental pulp stem cells. BMC Oral Health 2023, 23, 95. [Google Scholar] [CrossRef]
- Rothermund, K.; Calabrese, T.C.; Syed-Picard, F.N. Differential Effects of Escherichia coli–Versus Porphyromonas gingivalis–derived Lipopolysaccharides on Dental Pulp Stem Cell Differentiation in Scaffold-free Engineered Tissues. J. Endod. 2022, 48, 1378–1386.e2. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, W.; Xiong, Y.; Zhang, Y.; Zhang, D.; Xu, X. Effects of rutin on the oxidative stress, proliferation and osteogenic differentiation of periodontal ligament stem cells in LPS-induced inflammatory environment and the underlying mechanism. J. Mol. Histol. 2020, 51, 161–171. [Google Scholar] [CrossRef]
- Jin, S.; Jiang, H.; Sun, Y.; Li, F.; Xia, J.; Li, Y.; Zheng, J.; Qin, Y. Osteogenic differentiation of periodontal membrane stem cells in inflammatory environments. Open Life Sci. 2022, 17, 1240–1248. [Google Scholar] [CrossRef]
- Lertchirakarn, V.; Aguilar, P. Effects of lipopolysaccharide on the proliferation and osteogenic differentiation of stem cells from the apical papilla. J. Endod. 2017, 43, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, C.; Bucchi, A.; Martínez-Rodríguez, P. Biological properties of stem cells from the apical papilla exposed to lipopolysaccharides: An in vitro study. Arch. Oral Biol. 2024, 159, 105876. [Google Scholar] [CrossRef] [PubMed]
- Ramenzoni, L.L.; Russo, G.; Moccia, M.D.; Attin, T.; Schmidlin, P.R. Periodontal bacterial supernatants modify differentiation, migration and inflammatory cytokine expression in human periodontal ligament stem cells. PLoS ONE 2019, 14, e0219181. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.d.S.; Vilela, H.d.S.; Rahhal, J.G.; Bueno, N.P.; Lima, F.S.; Nogueira, F.N.; Sipert, C.R. Exposure to lipopolysaccharide and calcium silicate-based materials affects the behavior of dental pulp cells. Braz. Dental J. 2022, 33, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, N.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Pathogenesis of important virulence factors of Porphyromonas gingivalis via toll-like receptors. Front. Cell. Infec. Microbiol. 2019, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O. Toll-like receptors and dental mesenchymal stromal cells. Front. Oral Health 2021, 2, 648901. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Avalos, A.M.; Ploegh, H.L. Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 2012, 12, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Irfan, M.; Hossain, M.A.; Shin, S.; George, A.; Chung, S. LPS-induced inflammation potentiates dental pulp stem cell odontogenic differentiation through C5aR and p38. Connect. Tissue Res. 2023, 64, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, Y.; Zhan, X.; Cui, L.; Xu, S.; Ma, D.; Yue, J.; Wu, B.; Gao, J. TLR4 activation by lipopolysaccharide and Streptococcus mutans induces differential regulation of proliferation and migration in human dental pulp stem cells. J. Endod. 2014, 40, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Z.; Zeng, W.-N.; Zeng, Q.; Zhang, X. The role of toll-like receptors in orchestrating osteogenic differentiation of mesenchymal stromal cells and osteoimmunology. Front. Cell Dev. Biol. 2023, 11, 1277686. [Google Scholar] [CrossRef]
- Yu, B.; Li, Q.; Zhou, M. LPS-induced upregulation of the TLR4 signaling pathway inhibits osteogenic differentiation of human periodontal ligament stem cells under inflammatory conditions. Int. J. Mol. Med. 2019, 43, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lin, X.; Zhang, L.; Hu, X. Effects of nuclear factor-κB signaling pathway on periodontal ligament stem cells under lipopolysaccharide-induced inflammation. Bioengineered 2022, 13, 7951–7961. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Z.; Zhou, Z.; Zhang, Y.; Zhu, Q.; Wei, K.; Lin, Y.; Cooper, P.R.; Smith, A.J.; Yu, Q. Lipopolysaccharide enhances Wnt5a expression through toll-like receptor 4, myeloid differentiating factor 88, phosphatidylinositol 3-OH kinase/AKT and nuclear factor kappa B pathways in human dental pulp stem cells. J. Endod. 2014, 40, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Feng, G.; Xing, J.; Shen, B.; Tan, W.; Huang, D.; Lu, X.; Tao, T.; Zhang, J.; Li, L. Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell. Tissue Res. 2014, 356, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Kobayashi, Y.; Kearney, M.; Shimizu, E. Epigenetic therapeutics in dental pulp treatment: Hopes, challenges and concerns for the development of next-generation biomaterials. Bioact. Mater. 2023, 27, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Jiao, Y.; Larsson, L.; Almeida, L.; Garaicoa-Pazmino, C.; Le, J.; Squarize, C.; Inohara, N.; Giannobile, W.V.; Castilho, R. Epigenetic modifications of histones in periodontal disease. J. Dental Res. 2016, 95, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Yamada, S. Epigenetics in susceptibility, progression, and diagnosis of periodontitis. Jpn. Dent. Sci. Rev. 2022, 58, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas gingivalis lipopolysaccharide stimulation in human periodontal ligament stem cells: Role of epigenetic modifications to the inflammation. Eur. J. Histochem. EJH 2017, 61, 2826. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, F.; Shao, L.; Huang, P.; Xu, Y. LncRNA TUG1 mediates lipopolysaccharide-induced proliferative inhibition and apoptosis of human periodontal ligament cells by sponging miR-132. Acta Biochim. Biophys. Sin. 2019, 51, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Carrillo, J.L.; Vázquez-Alcaraz, S.J.; Vargas-Barbosa, J.M.; Ramos-Gracia, L.G.; Alvarez-Barreto, I.; Medina-Quiroz, A.; Díaz-Huerta, K.K. The role of microRNAs in pulp inflammation. Cells 2021, 10, 2142. [Google Scholar] [CrossRef]
- Santonocito, S.; Polizzi, A.; Palazzo, G.; Isola, G. The emerging role of microRNA in periodontitis: Pathophysiology, clinical potential and future molecular perspectives. Int. J. Mol. Sci. 2021, 22, 5456. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Li, C.; Sun, W.; Wu, J.; Xiao, F. Long non-coding RNA TUG1 participates in LPS-induced periodontitis by regulating miR-498/RORA pathway. Oral Dis. 2021, 27, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Feng, S.; Dong, F. Maternally-expressed gene 3 (meg3)/mir-143-3p regulates injury to periodontal ligament cells by mediating the akt/inhibitory κb kinase (ikk) pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922486-1–e922486-11. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Planello, A.C.; Marques, M.R.; De Carvalho, D.D.; Line, S.R.P. High-throughput DNA analysis shows the importance of methylation in the control of immune inflammatory gene transcription in chronic periodontitis. Clin. Epigenetics 2014, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Li, Q.; Cai, L.; Zhan, M.; Xu, Q. The effect of DNA methylation on the miRNA expression pattern in lipopolysaccharide-induced inflammatory responses in human dental pulp cells. Mol. Immunol. 2019, 111, 11–18. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Dang, R.; Feng, C.; Xiao, R.; Hua, Y.; Wang, W.; Jia, Z.; Liu, D. Epigenetic modifier trichostatin A enhanced osteogenic differentiation of mesenchymal stem cells by inhibiting NF-κB (p65) DNA binding and promoted periodontal repair in rats. J. Cell. Physiol. 2020, 235, 9691–9701. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodas-Junco, B.A.; Hernández-Solís, S.E.; Serralta-Interian, A.A.; Rueda-Gordillo, F. Dental Stem Cells and Lipopolysaccharides: A Concise Review. Int. J. Mol. Sci. 2024, 25, 4338. https://doi.org/10.3390/ijms25084338
Rodas-Junco BA, Hernández-Solís SE, Serralta-Interian AA, Rueda-Gordillo F. Dental Stem Cells and Lipopolysaccharides: A Concise Review. International Journal of Molecular Sciences. 2024; 25(8):4338. https://doi.org/10.3390/ijms25084338
Chicago/Turabian StyleRodas-Junco, Beatriz A., Sandra E. Hernández-Solís, Angelica A. Serralta-Interian, and Florencio Rueda-Gordillo. 2024. "Dental Stem Cells and Lipopolysaccharides: A Concise Review" International Journal of Molecular Sciences 25, no. 8: 4338. https://doi.org/10.3390/ijms25084338
APA StyleRodas-Junco, B. A., Hernández-Solís, S. E., Serralta-Interian, A. A., & Rueda-Gordillo, F. (2024). Dental Stem Cells and Lipopolysaccharides: A Concise Review. International Journal of Molecular Sciences, 25(8), 4338. https://doi.org/10.3390/ijms25084338





