The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors
Abstract
1. Introduction
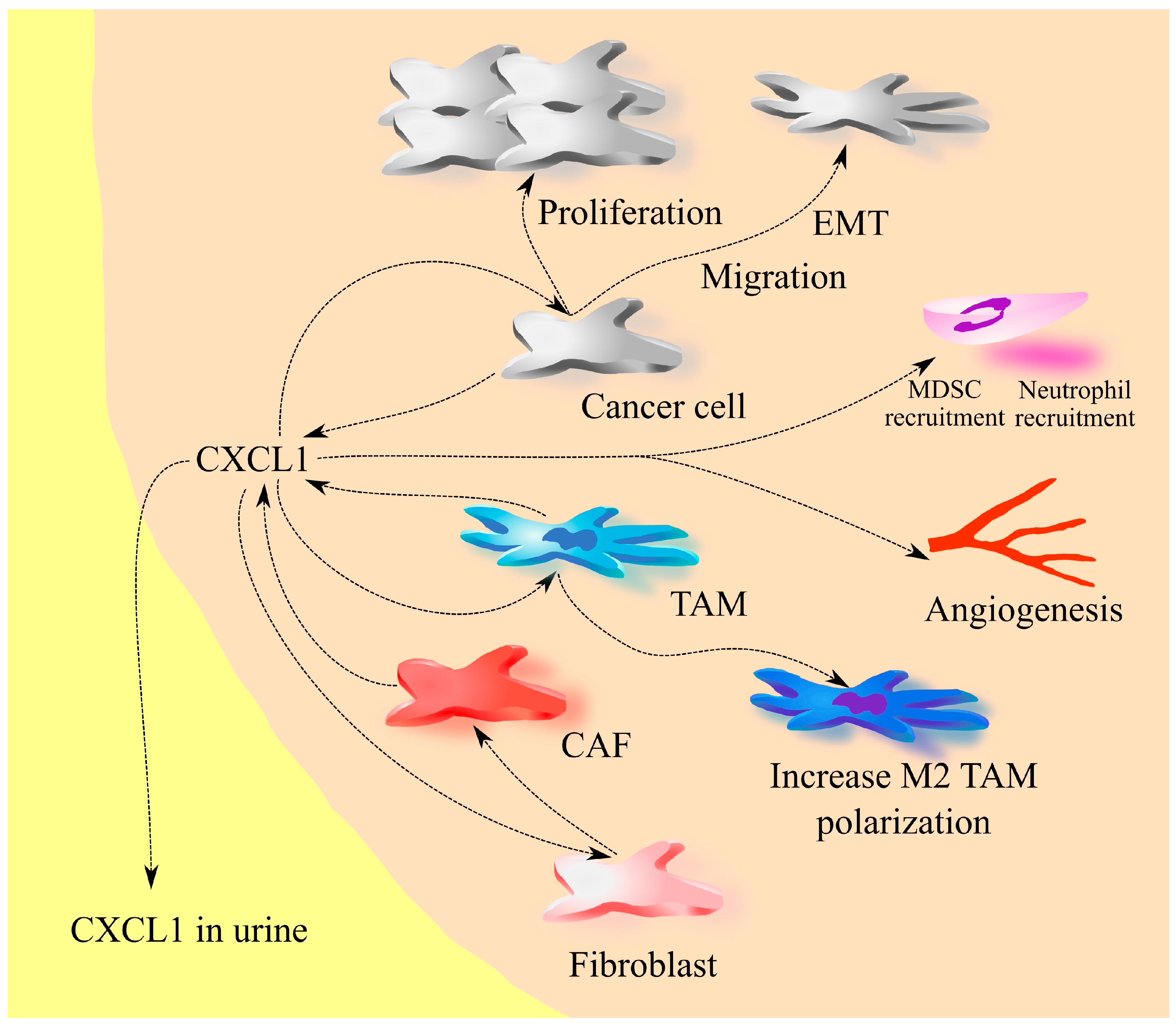
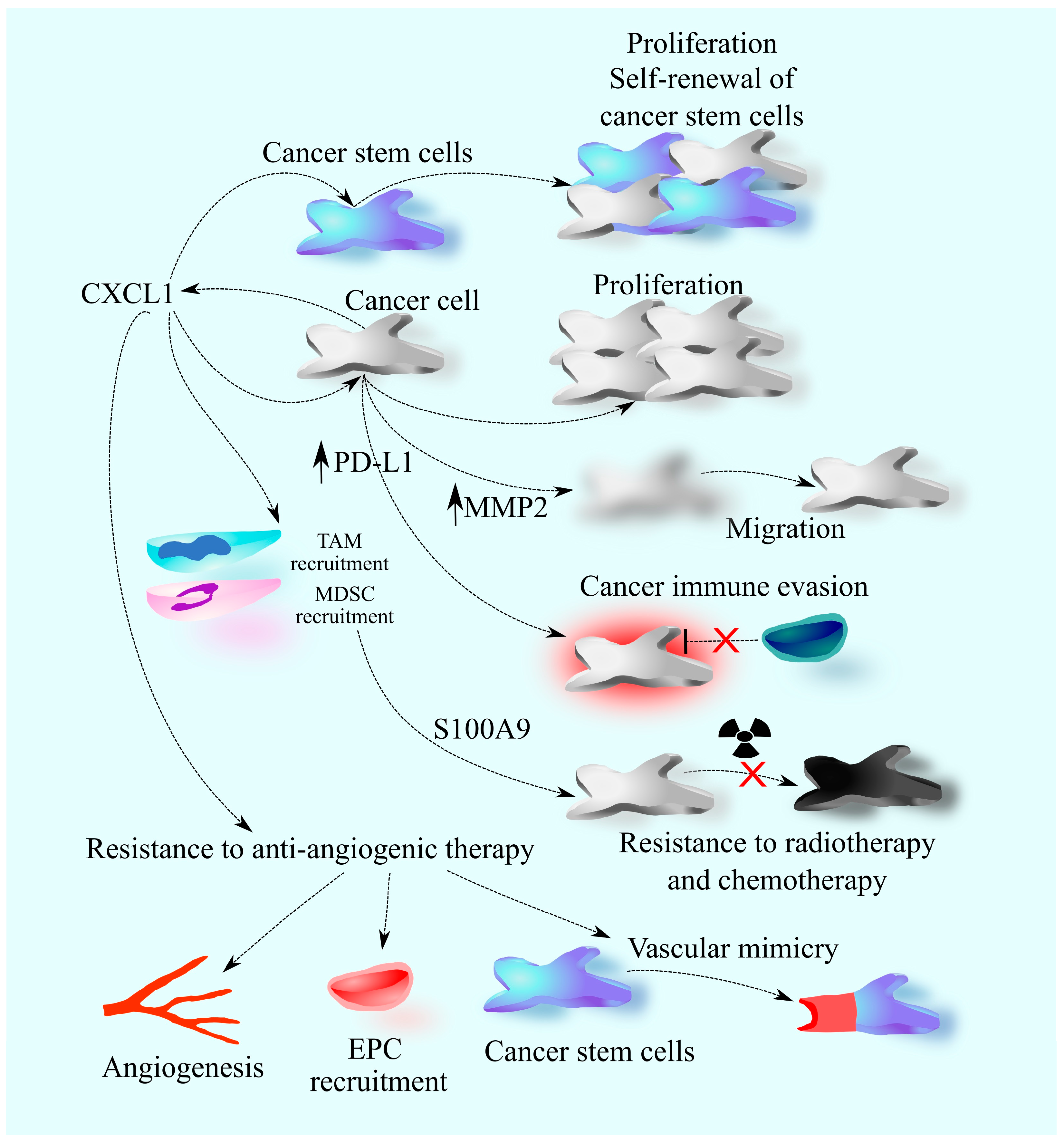
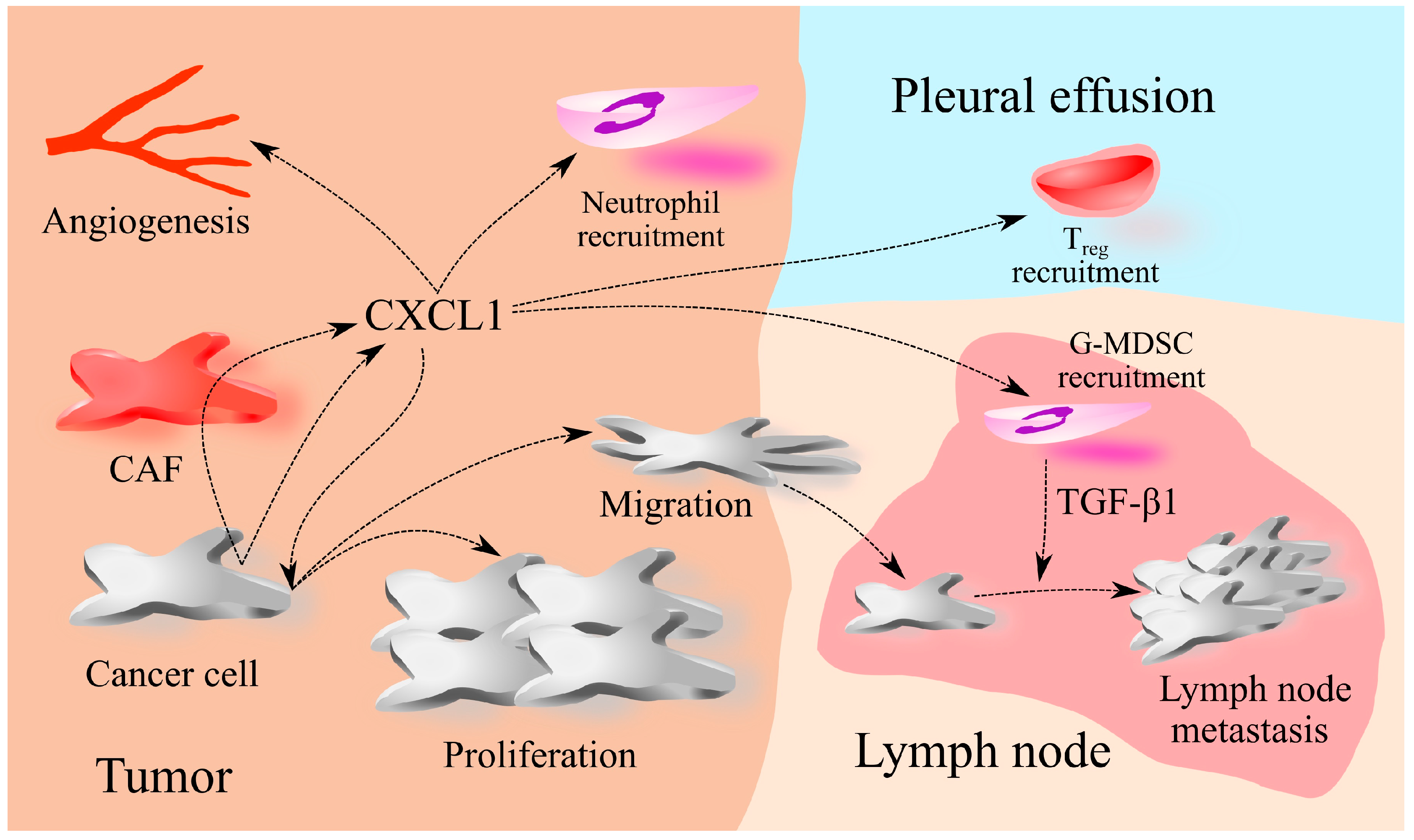
2. The Involvement of CXCL1 in Cancers: A Universal Model
3. Bladder Cancer
4. Primary Brain Tumors: Glioblastoma
5. Hemangioendothelioma
6. Hematolymphoid Tumors
6.1. Acute Myeloid Leukemia
6.2. Chronic Myeloid Leukemia
6.3. Acute Lymphocytic Leukemia
6.4. Multiple Myeloma
7. Kaposi’s Sarcoma
8. Lung Cancer
9. Osteosarcoma
10. Renal Cancer
11. Rhabdomyosarcoma
12. Skin Cancer
12.1. Malignant Melanoma
12.2. Non-Melanoma Skin Cancer
13. Thyroid Cancer
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, P.; Seitz, M.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Both Interleukin-8 Receptors Independently Mediate Chemotaxis. Jurkat Cells Transfected with IL-8R1 or IL-8R2 Migrate in Response to IL-8, GRO Alpha and NAP-2. FEBS Lett. 1994, 341, 187–192. [Google Scholar] [CrossRef]
- Damaj, B.B.; McColl, S.R.; Mahana, W.; Crouch, M.F.; Naccache, P.H. Physical Association of Gi2alpha with Interleukin-8 Receptors. J. Biol. Chem. 1996, 271, 12783–12789. [Google Scholar] [CrossRef]
- Kuwano, Y.; Adler, M.; Zhang, H.; Groisman, A.; Ley, K. Gαi2 and Gαi3 Differentially Regulate Arrest from Flow and Chemotaxis in Mouse Neutrophils. J. Immunol. 2016, 196, 3828–3833. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Farooq, S.M.; Castelvetere, M.P.; Hou, Y.; Gao, J.-L.; Navarro, J.V.; Oupicky, D.; Sun, F.; Li, C. A Chemokine Receptor CXCR2 Macromolecular Complex Regulates Neutrophil Functions in Inflammatory Diseases. J. Biol. Chem. 2012, 287, 5744–5755. [Google Scholar] [CrossRef] [PubMed]
- Raman, D.; Neel, N.F.; Sai, J.; Mernaugh, R.L.; Ham, A.-J.L.; Richmond, A.J. Characterization of Chemokine Receptor CXCR2 Interacting Proteins Using a Proteomics Approach to Define the CXCR2 “Chemosynapse”. Methods Enzymol. 2009, 460, 315–330. [Google Scholar] [CrossRef]
- Devalaraja, M.N.; Wang, D.Z.; Ballard, D.W.; Richmond, A. Elevated Constitutive IkappaB Kinase Activity and IkappaB-Alpha Phosphorylation in Hs294T Melanoma Cells Lead to Increased Basal MGSA/GRO-Alpha Transcription. Cancer Res. 1999, 59, 1372–1377. [Google Scholar] [PubMed]
- Wu, Z.; Neufeld, H.; Torlakovic, E.; Xiao, W. Uev1A-Ubc13 Promotes Colorectal Cancer Metastasis through Regulating CXCL1 Expression via NF-κB Activation. Oncotarget 2018, 9, 15952–15967. [Google Scholar] [CrossRef]
- Wood, L.D.; Farmer, A.A.; Richmond, A. HMGI(Y) and Sp1 in Addition to NF-Kappa B Regulate Transcription of the MGSA/GRO Alpha Gene. Nucleic Acids Res. 1995, 23, 4210–4219. [Google Scholar] [CrossRef]
- Lo, H.; Lai, T.; Li, C.; Wu, W. TNF-α Induces CXCL1 Chemokine Expression and Release in Human Vascular Endothelial Cells In Vitro via Two Distinct Signaling Pathways. Acta Pharmacol. Sin. 2014, 35, 339–350. [Google Scholar] [CrossRef]
- Yan, W.; Chen, X. Identification of GRO1 as a Critical Determinant for Mutant P53 Gain of Function. J. Biol. Chem. 2009, 284, 12178–12187. [Google Scholar] [CrossRef] [PubMed]
- Botton, T.; Puissant, A.; Cheli, Y.; Tomic, T.; Giuliano, S.; Fajas, L.; Deckert, M.; Ortonne, J.-P.; Bertolotto, C.; Tartare-Deckert, S.; et al. Ciglitazone Negatively Regulates CXCL1 Signaling through MITF to Suppress Melanoma Growth. Cell Death Differ. 2011, 18, 109–121. [Google Scholar] [CrossRef]
- Herjan, T.; Hong, L.; Bubenik, J.; Bulek, K.; Qian, W.; Liu, C.; Li, X.; Chen, X.; Yang, H.; Ouyang, S.; et al. IL-17-Receptor-Associated Adaptor Act1 Directly Stabilizes mRNAs to Mediate IL-17 Inflammatory Signaling. Nat. Immunol. 2018, 19, 354–365. [Google Scholar] [CrossRef]
- Kronski, E.; Fiori, M.E.; Barbieri, O.; Astigiano, S.; Mirisola, V.; Killian, P.H.; Bruno, A.; Pagani, A.; Rovera, F.; Pfeffer, U.; et al. miR181b Is Induced by the Chemopreventive Polyphenol Curcumin and Inhibits Breast Cancer Metastasis via Down-Regulation of the Inflammatory Cytokines CXCL1 and -2. Mol. Oncol. 2014, 8, 581–595. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Barczak, K.; Łagocka, R.; Chlubek, D.; Baranowska-Bosiacka, I. The Clinical Significance and Role of CXCL1 Chemokine in Gastrointestinal Cancers. Cells 2023, 12, 1406. [Google Scholar] [CrossRef]
- Korbecki, J.; Bosiacki, M.; Barczak, K.; Łagocka, R.; Brodowska, A.; Chlubek, D.; Baranowska-Bosiacka, I. Involvement in Tumorigenesis and Clinical Significance of CXCL1 in Reproductive Cancers: Breast Cancer, Cervical Cancer, Endometrial Cancer, Ovarian Cancer and Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 7262. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rosen, D.G.; Liu, G.; Yang, F.; Guo, X.; Xiao, X.; Xue, F.; Mercado-Uribe, I.; Huang, J.; Lin, S.-H.; et al. CXCR2 Promotes Ovarian Cancer Growth through Dysregulated Cell Cycle, Diminished Apoptosis, and Enhanced Angiogenesis. Clin. Cancer Res. 2010, 16, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Sugimoto, A.; Maruo, K.; Tsujio, G.; Sera, T.; Kushiyama, S.; Nishimura, S.; Kuroda, K.; Togano, S.; Eguchi, S.; et al. CXCR2 Signaling Might Have a Tumor-Suppressive Role in Patients with Cholangiocarcinoma. PLoS ONE 2022, 17, e0266027. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Chen, Y.-J.; Chang, W.-A.; Jian, S.-F.; Fan, H.-L.; Wang, J.-Y.; Kuo, P.-L. Interaction between Tumor-Associated Dendritic Cells and Colon Cancer Cells Contributes to Tumor Progression via CXCL1. Int. J. Mol. Sci. 2018, 19, 2427. [Google Scholar] [CrossRef]
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.; Di, G.; Yang, G. CXCR2 Promotes Breast Cancer Metastasis and Chemoresistance via Suppression of AKT1 and Activation of COX2. Cancer Lett. 2018, 412, 69–80. [Google Scholar] [CrossRef]
- Nywening, T.M.; Belt, B.A.; Cullinan, D.R.; Panni, R.Z.; Han, B.J.; Sanford, D.E.; Jacobs, R.C.; Ye, J.; Patel, A.A.; Gillanders, W.E.; et al. Targeting Both Tumour-Associated CXCR2+ Neutrophils and CCR2+ Macrophages Disrupts Myeloid Recruitment and Improves Chemotherapeutic Responses in Pancreatic Ductal Adenocarcinoma. Gut 2018, 67, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Wang, D.; Daikoku, T.; Sun, H.; Dey, S.K.; Dubois, R.N. CXCR2-Expressing Myeloid-Derived Suppressor Cells Are Essential to Promote Colitis-Associated Tumorigenesis. Cancer Cell 2013, 24, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shi, H.; Sun, Y.; Shang, C.; Luan, T.; Wang, D.; Ba, X.; Zeng, X. CXCR2 Expression on Granulocyte and Macrophage Progenitors under Tumor Conditions Contributes to Mo-MDSC Generation via SAP18/ERK/STAT3. Cell Death Dis. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Bigorgne, A.E.; John, B.; Ebrahimkhani, M.R.; Shimizu-Albergine, M.; Campbell, J.S.; Crispe, I.N. TLR4-Dependent Secretion by Hepatic Stellate Cells of the Neutrophil-Chemoattractant CXCL1 Mediates Liver Response to Gut Microbiota. PLoS ONE 2016, 11, e0151063. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Müller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic Acute-Phase Proteins Control Innate Immune Responses during Infection by Promoting Myeloid-Derived Suppressor Cell Function. J. Exp. Med. 2010, 207, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- MacCarthy-Morrogh, L.; Martin, P. The Hallmarks of Cancer Are Also the Hallmarks of Wound Healing. Sci. Signal 2020, 13, eaay8690. [Google Scholar] [CrossRef]
- Cai, L.; Xu, S.; Piao, C.; Qiu, S.; Li, H.; Du, J. Adiponectin Induces CXCL1 Secretion from Cancer Cells and Promotes Tumor Angiogenesis by Inducing Stromal Fibroblast Senescence. Mol. Carcinog. 2016, 55, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.-U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder Cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef]
- Miyake, M.; Lawton, A.; Goodison, S.; Urquidi, V.; Gomes-Giacoia, E.; Zhang, G.; Ross, S.; Kim, J.; Rosser, C.J. Chemokine (C-X-C) Ligand 1 (CXCL1) Protein Expression Is Increased in Aggressive Bladder Cancers. BMC Cancer 2013, 13, 322. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Q.; Zhang, L.; Chen, J.; Zhang, X. Exploration of Prognostic Biomarkers and Therapeutic Targets in the Microenvironment of Bladder Cancer Based on CXC Chemokines. Math. Biosci. Eng. 2021, 18, 6262–6287. [Google Scholar] [CrossRef]
- Mandelli, G.E.; Missale, F.; Bresciani, D.; Gatta, L.B.; Scapini, P.; Caveggion, E.; Roca, E.; Bugatti, M.; Monti, M.; Cristinelli, L.; et al. Tumor Infiltrating Neutrophils Are Enriched in Basal-Type Urothelial Bladder Cancer. Cells 2020, 9, 291. [Google Scholar] [CrossRef]
- Burnier, A.; Shimizu, Y.; Dai, Y.; Nakashima, M.; Matsui, Y.; Ogawa, O.; Rosser, C.J.; Furuya, H. CXCL1 Is Elevated in the Urine of Bladder Cancer Patients. Springerplus 2015, 4, 610. [Google Scholar] [CrossRef]
- Nakashima, M.; Matsui, Y.; Kobayashi, T.; Saito, R.; Hatahira, S.; Kawakami, K.; Nakamura, E.; Nishiyama, H.; Ogawa, O. Urine CXCL1 as a Biomarker for Tumor Detection and Outcome Prediction in Bladder Cancer. Cancer Biomark. 2015, 15, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Soukup, V.; Kalousová, M.; Capoun, O.; Sobotka, R.; Breyl, Z.; Pešl, M.; Zima, T.; Hanuš, T. Panel of Urinary Diagnostic Markers for Non-Invasive Detection of Primary and Recurrent Urothelial Urinary Bladder Carcinoma. Urol. Int. 2015, 95, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H.; Matsui, Y.; Ito, M.; Watanabe, J.; Takahashi, T.; Nishizawa, K.; Nishiyama, H.; Kamoto, T.; Mikami, Y.; Tanaka, Y.; et al. Secreted CXCL1 Is a Potential Mediator and Marker of the Tumor Invasion of Bladder Cancer. Clin. Cancer Res. 2008, 14, 2579–2587. [Google Scholar] [CrossRef]
- Miyake, M.; Hori, S.; Morizawa, Y.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Shimada, K.; et al. CXCL1-Mediated Interaction of Cancer Cells with Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia 2016, 18, 636–646. [Google Scholar] [CrossRef]
- Miyake, M.; Furuya, H.; Onishi, S.; Hokutan, K.; Anai, S.; Chan, O.; Shi, S.; Fujimoto, K.; Goodison, S.; Cai, W.; et al. Monoclonal Antibody against CXCL1 (HL2401) as a Novel Agent in Suppressing IL6 Expression and Tumoral Growth. Theranostics 2019, 9, 853–867. [Google Scholar] [CrossRef]
- Chen, L.; Pan, X.-W.; Huang, H.; Gao, Y.; Yang, Q.-W.; Wang, L.-H.; Cui, X.-G.; Xu, D.-F. Epithelial-Mesenchymal Transition Induced by GRO-α-CXCR2 Promotes Bladder Cancer Recurrence after Intravesical Chemotherapy. Oncotarget 2017, 8, 45274–45285. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Wang, T.-Y.; Hsu, C.-L.; Lin, W.-C.; Chen, J.-Y.; Li, J.-H.; Pu, Y.-S.; Cheng, A.-L.; Cheng, J.C.-H.; Su, S.-F. Selective Inhibition of HDAC6 Promotes Bladder Cancer Radiosensitization and Mitigates the Radiation-Induced CXCL1 Signalling. Br. J. Cancer 2023, 128, 1753–1764. [Google Scholar] [CrossRef]
- Takeyama, Y.; Kato, M.; Tamada, S.; Azuma, Y.; Shimizu, Y.; Iguchi, T.; Yamasaki, T.; Gi, M.; Wanibuchi, H.; Nakatani, T. Myeloid-Derived Suppressor Cells Are Essential Partners for Immune Checkpoint Inhibitors in the Treatment of Cisplatin-Resistant Bladder Cancer. Cancer Lett. 2020, 479, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, M.; Chen, G.; Wang, W.; Zhang, P.; Yue, Y.; Guan, Z.; Wang, X.; Fan, J. Bladder Cancer Cells Interact with Vascular Endothelial Cells Triggering EGFR Signals to Promote Tumor Progression. Int. J. Oncol. 2019, 54, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, iv1–iv96. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, S.; DeDeo, M.R.; Free, J.; Rosenfeld, S.S.; Quinones-Hinojosa, A.; Paulus, A.; Manna, A.; Manochakian, R.; Chanan-Khan, A.A.; Ailawadhi, S. Survival Trends in Glioblastoma and Association with Treating Facility Volume. J. Clin. Neurosci. 2019, 68, 271–274. [Google Scholar] [CrossRef]
- Seifert, M.; Garbe, M.; Friedrich, B.; Mittelbronn, M.; Klink, B. Comparative Transcriptomics Reveals Similarities and Differences between Astrocytoma Grades. BMC Cancer 2015, 15, 952. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Singh, A.; Sharma, K.C.; Saxena, S. Gene Expression Profiling of Chemokines and Their Receptors in Low and High Grade Astrocytoma. Asian Pac. J. Cancer Prev. 2017, 18, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Cohen, M.; Prayson, R.; Ransohoff, R.M.; Tabrizi, N.; Miller, R.H. Constitutive Expression of Growth-Related Oncogene and Its Receptor in Oligodendrogliomas. Neurosurgery 2001, 48, 864–873; discussion 873–874. [Google Scholar] [CrossRef]
- Alafate, W.; Li, X.; Zuo, J.; Zhang, H.; Xiang, J.; Wu, W.; Xie, W.; Bai, X.; Wang, M.; Wang, J. Elevation of CXCL1 Indicates Poor Prognosis and Radioresistance by Inducing Mesenchymal Transition in Glioblastoma. CNS Neurosci. Ther. 2020, 26, 475–485. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Q.; Kong, L.-Y.; Wang, J.; Yan, J.; Xia, X.; Jia, Z.; Heimberger, A.B.; Li, S. Regulation of Tumor Immune Suppression and Cancer Cell Survival by CXCL1/2 Elevation in Glioblastoma Multiforme. Sci. Adv. 2021, 7, eabc2511. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Deng, H.; Zhou, Y.; Ye, Y.; Zhao, S.; Liang, S.; Cai, S.; Lin, J.; Tang, Y.; Wu, Y. Expression and Clinical Significance of CXC Chemokines in the Glioblastoma Microenvironment. Life Sci. 2020, 261, 118486. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, D.; Hooper, D.C.; Roberts, A.L.; Harshyne, L.A.; Nagurney, M.; Curtis, M.T. Potential Role of CSF Cytokine Profiles in Discriminating Infectious from Non-Infectious CNS Disorders. PLoS ONE 2018, 13, e0205501. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, N.; Zhao, S.; Liu, H.; Wang, X.; Yang, M.; Wang, S.; Li, Y.; Liu, Z.; Teng, L. AKIP1 Promotes Glioblastoma Viability, Mobility and Chemoradiation Resistance via Regulating CXCL1 and CXCL8 Mediated NF-κB and AKT Pathways. Am. J. Cancer Res. 2021, 11, 1185–1205. [Google Scholar] [PubMed]
- Fang, J.; Chen, X.; Wang, S.; Xie, T.; Du, X.; Liu, H.; Wang, S.; Li, X.; Chen, J.; Zhang, B.; et al. The Expression of P2X₇ Receptors in EPCs and Their Potential Role in the Targeting of EPCs to Brain Gliomas. Cancer Biol. Ther. 2015, 16, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Pattarozzi, A.; Corsaro, A.; Barbieri, F.; Daga, A.; Bosio, A.; Gatti, M.; Pisaturo, V.; Sirito, R.; Florio, T. Different Effects of Human Umbilical Cord Mesenchymal Stem Cells on Glioblastoma Stem Cells by Direct Cell Interaction or Via Released Soluble Factors. Front. Cell Neurosci. 2017, 11, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, J.; Liu, Q.; Bell, R.; Muruve, D.A.; Forsyth, P.; Arcellana-Panlilio, M.; Robbins, S.; Yong, V.W. The Chemokine GRO-Alpha (CXCL1) Confers Increased Tumorigenicity to Glioma Cells. Carcinogenesis 2005, 26, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, J.H.; Lee, J.K.; Choi, S.J.; Kim, J.-S.; Jeun, S.-S.; Oh, W.; Yang, Y.S.; Chang, J.W. Overexpression of CXC Chemokine Receptors Is Required for the Superior Glioma-Tracking Property of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2009, 18, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Brennenstuhl, H.; Armento, A.; Braczysnki, A.K.; Mittelbronn, M.; Naumann, U. IκBζ, an Atypical Member of the Inhibitor of Nuclear Factor Kappa B Family, Is Induced by γ-Irradiation in Glioma Cells, Regulating Cytokine Secretion and Associated with Poor Prognosis. Int. J. Oncol. 2015, 47, 1971–1980. [Google Scholar] [CrossRef]
- McDonald, J.T.; Gao, X.; Steber, C.; Lee Breed, J.; Pollock, C.; Ma, L.; Hlatky, L. Host Mediated Inflammatory Influence on Glioblastoma Multiforme Recurrence Following High-Dose Ionizing Radiation. PLoS ONE 2017, 12, e0178155. [Google Scholar] [CrossRef]
- Jin, L.; Tao, H.; Karachi, A.; Long, Y.; Hou, A.Y.; Na, M.; Dyson, K.A.; Grippin, A.J.; Deleyrolle, L.P.; Zhang, W.; et al. CXCR1- or CXCR2-Modified CAR T Cells Co-Opt IL-8 for Maximal Antitumor Efficacy in Solid Tumors. Nat. Commun. 2019, 10, 4016. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, H.; Zhang, W.; Yu, J.; Zhang, X.; Wu, R.; Niu, M.; Liu, X.; Yu, R. Csnk1a1 Inhibition Modulates the Inflammatory Secretome and Enhances Response to Radiotherapy in Glioma. J. Cell Mol. Med. 2021, 25, 7395–7406. [Google Scholar] [CrossRef] [PubMed]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D. The Functional Role of the ELR Motif in CXC Chemokine-Mediated Angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef] [PubMed]
- Addison, C.L.; Daniel, T.O.; Burdick, M.D.; Liu, H.; Ehlert, J.E.; Xue, Y.Y.; Buechi, L.; Walz, A.; Richmond, A.; Strieter, R.M. The CXC Chemokine Receptor 2, CXCR2, Is the Putative Receptor for ELR+ CXC Chemokine-Induced Angiogenic Activity. J. Immunol. 2000, 165, 5269–5277. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, G.; Liu, W.; Xu, W.; Li, H.; Piao, S.; Sui, Y.; Feng, W. CXCL1 Stimulates Decidual Angiogenesis via the VEGF-A Pathway during the First Trimester of Pregnancy. Mol. Cell Biochem. 2021, 476, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Urquidi, V.; Gomes Giacoia, E.; Rosser, C.J. Expression of CXCL1 in Human Endothelial Cells Induces Angiogenesis through the CXCR2 Receptor and the ERK1/2 and EGF Pathways. Lab. Investig. 2013, 93, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Angara, K.; Borin, T.F.; Rashid, M.H.; Lebedyeva, I.; Ara, R.; Lin, P.-C.; Iskander, A.; Bollag, R.J.; Achyut, B.R.; Arbab, A.S. CXCR2-Expressing Tumor Cells Drive Vascular Mimicry in Antiangiogenic Therapy-Resistant Glioblastoma. Neoplasia 2018, 20, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Requena, L.; Kutzner, H. Hemangioendothelioma. Semin. Diagn. Pathol. 2013, 30, 29–44. [Google Scholar] [CrossRef]
- Guo, L.; Song, N.; He, T.; Qi, F.; Zheng, S.; Xu, X.-G.; Fu, Y.; Chen, H.-D.; Luo, Y. Endostatin Inhibits the Tumorigenesis of Hemangioendothelioma via Downregulation of CXCL1. Mol. Carcinog. 2015, 54, 1340–1353. [Google Scholar] [CrossRef]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Gibson, B.; Smith, F.O. Acute Myeloid Leukemia. Hematol. Oncol. Clin. N. Am. 2010, 24, 35–63. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, B.; Hassanshahi, G.; Mousavi, Z.; Ahmadi, Z.; Khorramdelazad, H.; Moradabadi, A.; Shafiepoor, M.; Fatehi, A. CXCL1, CXCL10 and CXCL12 Chemokines Are Variously Expressed in Acute Myeloid Leukemia Patients Prior and Post Bone Marrow Transplantation. Asian Pac. J. Cancer Prev. 2021, 22, 3377–3384. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An Update to the Integrated Cancer Data Analysis Platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Horacek, J.M.; Kupsa, T.; Vasatova, M.; Jebavy, L.; Zak, P. Biochip Array Technology and Evaluation of Serum Levels of Multiple Cytokines and Adhesion Molecules in Patients with Newly Diagnosed Acute Myeloid Leukemia. Exp. Oncol. 2014, 36, 50–51. [Google Scholar] [PubMed]
- Lu, C.; Zhu, J.; Chen, X.; Hu, Y.; Xie, W.; Yao, J.; Huang, S. Risk Stratification in Acute Myeloid Leukemia Using CXCR Gene Signatures: A Bioinformatics Analysis. Front. Oncol. 2020, 10, 584766. [Google Scholar] [CrossRef]
- Tang, W.; Li, Z.; Li, X.; Huo, Z. High CXCR2 Expression Predicts Poor Prognosis in Adult Patients with Acute Myeloid Leukemia. Ther. Adv. Hematol. 2020, 11, 2040620720958586. [Google Scholar] [CrossRef]
- Bruserud, Ø.; Ryningen, A.; Olsnes, A.M.; Stordrange, L.; Øyan, A.M.; Kalland, K.H.; Gjertsen, B.T. Subclassification of Patients with Acute Myelogenous Leukemia Based on Chemokine Responsiveness and Constitutive Chemokine Release by Their Leukemic Cells. Haematologica 2007, 92, 332–341. [Google Scholar] [CrossRef]
- Hao, X.; Gu, M.; Sun, J.; Cong, L. A-Kinase Interacting Protein 1 Might Serve as a Novel Biomarker for Worse Prognosis through the Interaction of Chemokine (C-X-C Motif) Ligand 1/Chemokine (C-X-C Motif) Ligand 2 in Acute Myeloid Leukemia. J. Clin. Lab. Anal. 2020, 34, e23052. [Google Scholar] [CrossRef]
- Hatfield, K.J.; Bedringsaas, S.L.; Ryningen, A.; Gjertsen, B.T.; Bruserud, O. Hypoxia Increases HIF-1α Expression and Constitutive Cytokine Release by Primary Human Acute Myeloid Leukaemia Cells. Eur. Cytokine Netw. 2010, 21, 154–164. [Google Scholar] [PubMed]
- Padró, T.; Ruiz, S.; Bieker, R.; Bürger, H.; Steins, M.; Kienast, J.; Büchner, T.; Berdel, W.E.; Mesters, R.M. Increased Angiogenesis in the Bone Marrow of Patients with Acute Myeloid Leukemia. Blood 2000, 95, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, D.B.; Varela, V.A.; Datoguia, T.S.; Caraciolo, V.B.; Lopes, G.H.; Pereira, W.O. The Bone Marrow Microenvironment Mechanisms in Acute Myeloid Leukemia. Front. Cell Dev. Biol. 2021, 9, 764698. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.H.M.; Ailles, L.E.; Dylla, S.J.; Muijtjens, M.; Jones, C.; Zehnder, J.L.; Gotlib, J.; Li, K.; Manz, M.G.; Keating, A.; et al. Granulocyte-Macrophage Progenitors as Candidate Leukemic Stem Cells in Blast-Crisis CML. N. Engl. J. Med. 2004, 351, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Stuart, S.A.; Ikawa, T.; Jiang, Y.; Banno, A.; Hunton, I.C.; Young, D.J.; Naoe, T.; Murre, C.; Jamieson, C.H.M.; et al. BCR-ABL-Transformed GMP as Myeloid Leukemic Stem Cells. Proc. Natl. Acad. Sci. USA 2008, 105, 17967–17972. [Google Scholar] [CrossRef] [PubMed]
- Hehlmann, R.; Hochhaus, A.; Baccarani, M. European LeukemiaNet Chronic Myeloid Leukaemia. Lancet 2007, 370, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Onciu, M. Acute Lymphoblastic Leukemia. Hematol. Oncol. Clin. N. Am. 2009, 23, 655–674. [Google Scholar] [CrossRef] [PubMed]
- Neuendorff, N.R.; Burmeister, T.; Dörken, B.; Westermann, J. BCR-ABL-Positive Acute Myeloid Leukemia: A New Entity? Analysis of Clinical and Molecular Features. Ann. Hematol. 2016, 95, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Li, H.; Choi, K.; Hueneman, K.; He, J.; Welner, R.S.; Starczynowski, D.T.; Bhatia, R. TNF-α-Induced Alterations in Stromal Progenitors Enhance Leukemic Stem Cell Growth via CXCR2 Signaling. Cell Rep. 2021, 36, 109386. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, S.-J.; Kang, K.-W.; Lee, B.-H.; Park, Y.; Kim, B.-S. CXCR2, a Novel Target to Overcome Tyrosine Kinase Inhibitor Resistance in Chronic Myelogenous Leukemia Cells. Biochem. Pharmacol. 2021, 190, 114658. [Google Scholar] [CrossRef]
- Khandany, B.K.; Hassanshahi, G.; Khorramdelazad, H.; Balali, Z.; Shamsizadeh, A.; Arababadi, M.K.; Ostadebrahimi, H.; Fatehi, A.; Rezazadeh, M.; Ahmadi, Z.; et al. Evaluation of Circulating Concentrations of CXCL1 (Gro-α), CXCL10 (IP-10) and CXCL12 (SDF-1) in ALL Patients Prior and Post Bone Marrow Transplantation. Pathol. Res. Pract. 2012, 208, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Tang, Y.; He, F.; Zhang, Y.; Cheng, A.; Gan, R.; Wu, Y. Screening and Functional Analysis of Differentially Expressed Genes in EBV-Transformed Lymphoblasts. Virol. J. 2012, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Weischendorff, S.; De Pietri, S.; Rathe, M.; Frandsen, T.L.; Hasle, H.; Nielsen, C.H.; Moser, C.; Müller, K. Markers of Neutrophil Chemotaxis for Identification of Blood Stream Infections in Children with Acute Lymphoblastic Leukemia Undergoing Induction Treatment. Eur. J. Haematol. 2023, 110, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Burdon, P.C.E.; Bridger, G.; Gutierrez-Ramos, J.C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return Following Senescence. Immunity 2003, 19, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Corcione, A.; Arduino, N.; Ferretti, E.; Pistorio, A.; Spinelli, M.; Ottonello, L.; Dallegri, F.; Basso, G.; Pistoia, V. Chemokine Receptor Expression and Function in Childhood Acute Lymphoblastic Leukemia of B-Lineage. Leuk. Res. 2006, 30, 365–372. [Google Scholar] [CrossRef]
- De Vasconcellos, J.F.; Laranjeira, A.B.A.; Leal, P.C.; Bhasin, M.K.; Zenatti, P.P.; Nunes, R.J.; Yunes, R.A.; Nowill, A.E.; Libermann, T.A.; Zerbini, L.F.; et al. SB225002 Induces Cell Death and Cell Cycle Arrest in Acute Lymphoblastic Leukemia Cells through the Activation of GLIPR1. PLoS ONE 2015, 10, e0134783. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.E.; Koyama, M.; Sowa, Y.; Elokely, K.M.; Yoshida, T.; Kim, B.-Y.; Sakai, T. Molecular Mechanisms of the Antitumor Activity of SB225002: A Novel Microtubule Inhibitor. Biochem. Pharmacol. 2013, 85, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.E.; Sakai, T. Molecular Insights into the Microtubules Depolymerizing Activity of the IL-8 Receptor B Antagonist SB225002. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3726–3734. [Google Scholar] [CrossRef] [PubMed]
- Van de Donk, N.W.C.J.; Pawlyn, C.; Yong, K.L. Multiple Myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Pappa, C.A.; Tsirakis, G.; Kanellou, P.; Kaparou, M.; Stratinaki, M.; Xekalou, A.; Alegakis, A.; Boula, A.; Stathopoulos, E.N.; Alexandrakis, M.G. Monitoring Serum Levels ELR+ CXC Chemokines and the Relationship between Microvessel Density and Angiogenic Growth Factors in Multiple Myeloma. Cytokine 2011, 56, 616–620. [Google Scholar] [CrossRef]
- Kline, M.; Donovan, K.; Wellik, L.; Lust, C.; Jin, W.; Moon-Tasson, L.; Xiong, Y.; Witzig, T.E.; Kumar, S.; Rajkumar, S.V.; et al. Cytokine and Chemokine Profiles in Multiple Myeloma; Significance of Stromal Interaction and Correlation of IL-8 Production with Disease Progression. Leuk. Res. 2007, 31, 591–598. [Google Scholar] [CrossRef]
- Yang, Q.; Li, K.; Li, X.; Liu, J. Identification of Key Genes and Pathways in Myeloma Side Population Cells by Bioinformatics Analysis. Int. J. Med. Sci. 2020, 17, 2063–2076. [Google Scholar] [CrossRef]
- Garcia-Gomez, A.; De Las Rivas, J.; Ocio, E.M.; Díaz-Rodríguez, E.; Montero, J.C.; Martín, M.; Blanco, J.F.; Sanchez-Guijo, F.M.; Pandiella, A.; San Miguel, J.F.; et al. Transcriptomic Profile Induced in Bone Marrow Mesenchymal Stromal Cells after Interaction with Multiple Myeloma Cells: Implications in Myeloma Progression and Myeloma Bone Disease. Oncotarget 2014, 5, 8284–8305. [Google Scholar] [CrossRef]
- Zahedi, S.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P. NF-Kβ Activation in U266 Cells on Mesenchymal Stem Cells. Adv. Pharm. Bull. 2016, 6, 415–422. [Google Scholar] [CrossRef]
- De Veirman, K.; Wang, J.; Xu, S.; Leleu, X.; Himpe, E.; Maes, K.; De Bruyne, E.; Van Valckenborgh, E.; Vanderkerken, K.; Menu, E.; et al. Induction of miR-146a by Multiple Myeloma Cells in Mesenchymal Stromal Cells Stimulates Their pro-Tumoral Activity. Cancer Lett. 2016, 377, 17–24. [Google Scholar] [CrossRef]
- Pappa, C.A.; Tsirakis, G.; Devetzoglou, M.; Zafeiri, M.; Vyzoukaki, R.; Androvitsanea, A.; Xekalou, A.; Sfiridaki, K.; Alexandrakis, M.G. Bone Marrow Mast Cell Density Correlates with Serum Levels of VEGF and CXC Chemokines ENA-78 and GRO-α in Multiple Myeloma. Tumour Biol. 2014, 35, 5647–5651. [Google Scholar] [CrossRef]
- Iftode, N.; Rădulescu, M.A.; Aramă, Ș.S.; Aramă, V. Update on Kaposi Sarcoma-Associated Herpesvirus (KSHV or HHV8)—Review. Rom. J. Intern. Med. 2020, 58, 199–208. [Google Scholar] [CrossRef]
- Li, S.; Bai, L.; Dong, J.; Sun, R.; Lan, K. Kaposi’s Sarcoma-Associated Herpesvirus: Epidemiology and Molecular Biology. Adv. Exp. Med. Biol. 2017, 1018, 91–127. [Google Scholar] [CrossRef]
- Luo, Q.; Satcher Johnson, A.; Hall, H.I.; Cahoon, E.K.; Shiels, M. Kaposi Sarcoma Rates Among Persons Living with Human Immunodeficiency Virus in the United States: 2008–2016. Clin. Infect. Dis. 2021, 73, e2226–e2233. [Google Scholar] [CrossRef] [PubMed]
- García-Astudillo, L.A.; Leyva-Cobián, F. Human Herpesvirus-8 Infection and Kaposi’s Sarcoma after Liver and Kidney Transplantation in Different Geographical Areas of Spain. Transpl. Immunol. 2006, 17, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Gorsane, I.; Bacha, M.M.; Abderrahim, E.; Amri, N.; Hajri, M.; Ounissi, M.; Harzallah, A.; El Younsi, F.; Hedri, H.; Ben Abdallah, T. Post Kidney Transplantation Kaposi’s Sarcoma: The Experience of a Mediterranean North African Center. Clin. Transplant. 2016, 30, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.K.; Linet, M.S.; Clarke, C.A.; Pawlish, K.S.; Engels, E.A.; Pfeiffer, R.M. Risk of Kaposi Sarcoma after Solid Organ Transplantation in the United States. Int. J. Cancer 2018, 143, 2741–2748. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Liu, J.; Bock, P.J.; Schols, D.; Coffey, M.J.; Strieter, R.M.; Polverini, P.J.; Markovitz, D.M. Interleukin-8 and Growth-Regulated Oncogene Alpha Mediate Angiogenesis in Kaposi’s Sarcoma. J. Virol. 2002, 76, 11570–11583. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Lee, J.; Kang, S.-K.; Wirth, D.; Yoo, S.-M.; Park, C.; Lee, M.-S. CXCL1 Confers a Survival Advantage in Kaposi’s Sarcoma-Associated Herpesvirus-Infected Human Endothelial Cells through STAT3 Phosphorylation. J. Med. Virol. 2023, 95, 5192. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jia, X.; Shen, C.; Zhang, M.; Xu, J.; Shang, Y.; Zhu, K.; Hu, M.; Yan, Q.; Qin, D.; et al. A KSHV microRNA Enhances Viral Latency and Induces Angiogenesis by Targeting GRK2 to Activate the CXCR2/AKT Pathway. Oncotarget 2016, 7, 32286–32305. [Google Scholar] [CrossRef] [PubMed]
- Gershengorn, M.C.; Geras-Raaka, E.; Varma, A.; Clark-Lewis, I. Chemokines Activate Kaposi’s Sarcoma-Associated Herpesvirus G Protein-Coupled Receptor in Mammalian Cells in Culture. J. Clin. Investig. 1998, 102, 1469–1472. [Google Scholar] [CrossRef]
- Smit, M.J.; Verzijl, D.; Casarosa, P.; Navis, M.; Timmerman, H.; Leurs, R. Kaposi’s Sarcoma-Associated Herpesvirus-Encoded G Protein-Coupled Receptor ORF74 Constitutively Activates P44/P42 MAPK and Akt via G(i) and Phospholipase C-Dependent Signaling Pathways. J. Virol. 2002, 76, 1744–1752. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Y.; Xiao, J.; Zhou, M.; Zhou, W.; Feng, H. The Unsulfated Extracellular N-Terminus of vGPCR Reduces the Tumorigenicity of hGRO-α in Nude Mice. Sci. China Life Sci. 2013, 56, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Verzijl, D.; Fitzsimons, C.P.; Van Dijk, M.; Stewart, J.P.; Timmerman, H.; Smit, M.J.; Leurs, R. Differential Activation of Murine Herpesvirus 68- and Kaposi’s Sarcoma-Associated Herpesvirus-Encoded ORF74 G Protein-Coupled Receptors by Human and Murine Chemokines. J. Virol. 2004, 78, 3343–3351. [Google Scholar] [CrossRef]
- Couty, J.-P.; Lupu-Meiri, M.; Oron, Y.; Gershengorn, M.C. Kaposi’s Sarcoma-Associated Herpesvirus-G Protein-Coupled Receptor-Expressing Endothelial Cells Exhibit Reduced Migration and Stimulated Chemotaxis by Chemokine Inverse Agonists. J. Pharmacol. Exp. Ther. 2009, 329, 1142–1147. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.S.; Licaj, I.; Braaten, T.; Lund, E.; Gram, I.T. The Fraction of Lung Cancer Attributable to Smoking in the Norwegian Women and Cancer (NOWAC) Study. Br. J. Cancer 2021, 124, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Najafi, F.; Dobson, A. Meta-Analysis of Studies of Passive Smoking and Lung Cancer: Effects of Study Type and Continent. Int. J. Epidemiol. 2007, 36, 1048–1059. [Google Scholar] [CrossRef]
- Lipsett, M.; Campleman, S. Occupational Exposure to Diesel Exhaust and Lung Cancer: A Meta-Analysis. Am. J. Public. Health 1999, 89, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D.; Wei, H.; Sapkota, A.; Choudhury, I.; Bruce, N.; Smith, K.R.; Rothman, N.; Lan, Q. Household Coal Use and Lung Cancer: Systematic Review and Meta-Analysis of Case-Control Studies, with an Emphasis on Geographic Variation. Int. J. Epidemiol. 2011, 40, 719–728. [Google Scholar] [CrossRef]
- Yu, S.; Yi, M.; Xu, L.; Qin, S.; Li, A.; Wu, K. CXCL1 as an Unfavorable Prognosis Factor Negatively Regulated by DACH1 in Non-Small Cell Lung Cancer. Front. Oncol. 2019, 9, 1515. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, H.; Wang, Z.; Zhang, G. The Inflammatory CXC Chemokines, GROαhigh, IP-10low, and MIGlow, in Tumor Microenvironment Can Be Used as New Indicators for Non-Small Cell Lung Cancer Progression. Immunol. Investig. 2017, 46, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Unver, N. Identification of the Dominant Angiogenic CXCL Class Chemokines Associated with Non-Small Cell Lung Cancer via Bioinformatics Tools. Med. Oncol. 2021, 38, 68. [Google Scholar] [CrossRef]
- Baird, A.-M.; Gray, S.G.; O’Byrne, K.J. Epigenetics Underpinning the Regulation of the CXC (ELR+) Chemokines in Non-Small Cell Lung Cancer. PLoS ONE 2011, 6, e14593. [Google Scholar] [CrossRef]
- Kowalczuk, O.; Burzykowski, T.; Niklinska, W.E.; Kozlowski, M.; Chyczewski, L.; Niklinski, J. CXCL5 as a Potential Novel Prognostic Factor in Early Stage Non-Small Cell Lung Cancer: Results of a Study of Expression Levels of 23 Genes. Tumour Biol. 2014, 35, 4619–4628. [Google Scholar] [CrossRef]
- Spaks, A.; Jaunalksne, I.; Spaka, I.; Chudasama, D.; Pirtnieks, A.; Krievins, D. Diagnostic Value of Circulating CXC Chemokines in Non-Small Cell Lung Cancer. Anticancer. Res. 2015, 35, 6979–6983. [Google Scholar] [PubMed]
- Chen, W.; Xu, X.; Bai, L.; Padilla, M.T.; Gott, K.M.; Leng, S.; Tellez, C.S.; Wilder, J.A.; Belinsky, S.A.; Scott, B.R.; et al. Low-Dose Gamma-Irradiation Inhibits IL-6 Secretion from Human Lung Fibroblasts That Promotes Bronchial Epithelial Cell Transformation by Cigarette-Smoke Carcinogen. Carcinogenesis 2012, 33, 1368–1374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ovrevik, J.; Låg, M.; Holme, J.A.; Schwarze, P.E.; Refsnes, M. Cytokine and Chemokine Expression Patterns in Lung Epithelial Cells Exposed to Components Characteristic of Particulate Air Pollution. Toxicology 2009, 259, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Bai, F.; Inoue, K.; Takayama, K.; Pei, X.H.; Harada, T.; Izumi, M.; Kimotsuki, K.; Tokiwa, H.; Hara, N. Polychlorinated Biphenyls Promote 1-Nitropyrene-Induced Lung Tumorigenesis without the Induction of K-Ras Gene Mutation in A/J Mice. Teratog. Carcinog. Mutagen. 2001, 21, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, N.; Sakao, Y.; Hayashi, S.; Hadden, W.A.; Harmon, C.L.; Miller, E.J. Alpha-Chemokine Growth Factors for Adenocarcinomas; a Synthetic Peptide Inhibitor for Alpha-Chemokines Inhibits the Growth of Adenocarcinoma Cell Lines. J. Cancer Res. Clin. Oncol. 2000, 126, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Roybal, J.; Chaerkady, R.; Zhang, W.; Choi, K.; Alvarez, C.A.; Tran, H.; Creighton, C.J.; Yan, S.; Strieter, R.M.; et al. Identification of Secreted Proteins That Mediate Cell-Cell Interactions in an In Vitro Model of the Lung Cancer Microenvironment. Cancer Res. 2008, 68, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-M.; Shieh, J.-M.; Chen, C.-L.; Tsou, C.-J.; Wu, W.-B. Vascular Endothelial Growth Factor Induces CXCL1 Chemokine Release via JNK and PI-3K-Dependent Pathways in Human Lung Carcinoma Epithelial Cells. Int. J. Mol. Sci. 2013, 14, 10090–10106. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Lang, X.P. Correlation Between MMP-7 and bFGF Expressions in Non-Small Cell Lung Cancer Tissue and Clinicopathologic Features. Cell Biochem. Biophys. 2015, 73, 427–432. [Google Scholar] [CrossRef]
- Numasaki, M.; Watanabe, M.; Suzuki, T.; Takahashi, H.; Nakamura, A.; McAllister, F.; Hishinuma, T.; Goto, J.; Lotze, M.T.; Kolls, J.K.; et al. IL-17 Enhances the Net Angiogenic Activity and In Vivo Growth of Human Non-Small Cell Lung Cancer in SCID Mice through Promoting CXCR-2-Dependent Angiogenesis. J. Immunol. 2005, 175, 6177–6189. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, M.; Hu, Z.; Ma, Y.; Qi, W.; Zhang, Y.; Li, Y.; Yu, M.; Wang, H.; Mo, W. Thrombin Is a Therapeutic Target for Non-Small-Cell Lung Cancer to Inhibit Vasculogenic Mimicry Formation. Signal Transduct. Target. Ther. 2020, 5, 117. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.-M.; Han, R.; Yu, Y.; Deng, S.-H.; Liu, T.; Zhang, T.; Xu, Y. Low-Dose Radiation Promotes Invasion and Migration of A549 Cells by Activating the CXCL1/NF-κB Signaling Pathway. Onco Targets Ther. 2020, 13, 3619–3629. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Jones, D.M.; Horzempa, C.; Prasad, A.; McKeown-Longo, P.J. The First Type III Domain of Fibronectin Is Associated with the Expression of Cytokines within the Lung Tumor Microenvironment. J. Cancer 2011, 2, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Tao, L.; Shen, S.; Chen, L. Hypoxia Induced CCL28 Promotes Angiogenesis in Lung Adenocarcinoma by Targeting CCR3 on Endothelial Cells. Sci. Rep. 2016, 6, 27152. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Xu, Y.; Tang, R.; Ren, J.; Shen, S.; Chen, Y.; Liu, B.; Hou, Y.; Wang, T. miR141-CXCL1-CXCR2 Signaling-Induced Treg Recruitment Regulates Metastases and Survival of Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2014, 13, 3152–3162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, X.; Xu, B.; Deng, H.; Chen, L.; Jiang, J. Histone Methyltransferase SETD2 Inhibits Tumor Growth via Suppressing CXCL1-Mediated Activation of Cell Cycle in Lung Adenocarcinoma. Aging 2020, 12, 25189–25206. [Google Scholar] [CrossRef] [PubMed]
- Neote, K.; Mak, J.Y.; Kolakowski, L.F.; Schall, T.J. Functional and Biochemical Analysis of the Cloned Duffy Antigen: Identity with the Red Blood Cell Chemokine Receptor. Blood 1994, 84, 44–52. [Google Scholar] [CrossRef]
- Rudisch, A.; Dewhurst, M.R.; Horga, L.G.; Kramer, N.; Harrer, N.; Dong, M.; van der Kuip, H.; Wernitznig, A.; Bernthaler, A.; Dolznig, H.; et al. High EMT Signature Score of Invasive Non-Small Cell Lung Cancer (NSCLC) Cells Correlates with NFκB Driven Colony-Stimulating Factor 2 (CSF2/GM-CSF) Secretion by Neighboring Stromal Fibroblasts. PLoS ONE 2015, 10, e0124283. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-A.; Tsai, M.-J.; Hung, J.-Y.; Wu, K.-L.; Tsai, Y.-M.; Huang, Y.-C.; Chang, C.-Y.; Tsai, P.-H.; Hsu, Y.-L. miR-150-5p-Containing Extracellular Vesicles Are a New Immunoregulator That Favor the Progression of Lung Cancer in Hypoxic Microenvironments by Altering the Phenotype of NK Cells. Cancers 2021, 13, 6252. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, A.; Campbell, D.; Hannum, C.; Yssel, H.; Franz-Bacon, K.; McClanahan, T.; Kitamura, T.; Nicholl, J.; Sutherland, G.R.; Lanier, L.L.; et al. DNAM-1, a Novel Adhesion Molecule Involved in the Cytolytic Function of T Lymphocytes. Immunity 1996, 4, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Li, L.; Li, J.-C.; Yang, H.; Zhang, X.; Liu, L.-L.; Li, Y.; Zeng, T.-T.; Zhu, Y.-H.; Li, X.-D.; Li, Y.; et al. Expansion of Cancer Stem Cell Pool Initiates Lung Cancer Recurrence before Angiogenesis. Proc. Natl. Acad. Sci. USA 2018, 115, E8948–E8957. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-G.; Deng, J.; Xu, W.-J.; Chen, J.-Y.; Sun, J.; Deng, H. Histidine Decarboxylase-Expressing PMN-MDSC-Derived TGF-Β1 Promotes the Epithelial-Mesenchymal Transition of Metastatic Lung Adenocarcinoma. Int. J. Clin. Exp. Pathol. 2020, 13, 1361–1371. [Google Scholar] [PubMed]
- Yuan, M.; Zhu, H.; Xu, J.; Zheng, Y.; Cao, X.; Liu, Q. Tumor-Derived CXCL1 Promotes Lung Cancer Growth via Recruitment of Tumor-Associated Neutrophils. J. Immunol. Res. 2016, 2016, 6530410. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Yao, Y.; Chen, Z. An Inter-Correlation among Chemokine (C-X-C Motif) Ligand (CXCL) 1, CXCL2 and CXCL8, and Their Diversified Potential as Biomarkers for Tumor Features and Survival Profiles in Non-Small Cell Lung Cancer Patients. Transl. Cancer Res. 2021, 10, 748–758. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 21 (Suppl. S7), vii320–vii325. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Chiang, Y.-C.; Yu, P.-A.; Peng, K.-T.; Chi, M.-C.; Lee, M.-H.; Fang, M.-L.; Lee, K.-H.; Hsu, L.-F.; Liu, J.-F. A Role of CXCL1 Drives Osteosarcoma Lung Metastasis via VCAM-1 Production. Front. Oncol. 2021, 11, 735277. [Google Scholar] [CrossRef] [PubMed]
- Tieken, C.; Verboom, M.C.; Ruf, W.; Gelderblom, H.; Bovée, J.V.M.G.; Reitsma, P.H.; Cleton-Jansen, A.-M.; Versteeg, H.H. Tissue Factor Associates with Survival and Regulates Tumour Progression in Osteosarcoma. Thromb. Haemost. 2016, 115, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Ucci, A.; Cappariello, A.; Ponzetti, M.; Tennant, F.; Loftus, A.E.P.; Shefferd, K.; Maurizi, A.; Delle Monache, S.; Teti, A.; Rucci, N. Anti-Osteoblastogenic, pro-Inflammatory and pro-Angiogenic Effect of Extracellular Vesicles Isolated from the Human Osteosarcoma Cell Line MNNG/HOS. Bone 2021, 153, 116130. [Google Scholar] [CrossRef]
- Avnet, S.; Di Pompo, G.; Chano, T.; Errani, C.; Ibrahim-Hashim, A.; Gillies, R.J.; Donati, D.M.; Baldini, N. Cancer-Associated Mesenchymal Stroma Fosters the Stemness of Osteosarcoma Cells in Response to Intratumoral Acidosis via NF-κB Activation. Int. J. Cancer 2017, 140, 1331–1345. [Google Scholar] [CrossRef]
- Chao, C.-C.; Lee, C.-W.; Chang, T.-M.; Chen, P.-C.; Liu, J.-F. CXCL1/CXCR2 Paracrine Axis Contributes to Lung Metastasis in Osteosarcoma. Cancers 2020, 12, 459. [Google Scholar] [CrossRef]
- Rini, B.I.; Campbell, S.C.; Escudier, B. Renal Cell Carcinoma. Lancet 2009, 373, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-Alpha to the von Hippel-Lindau Ubiquitylation Complex by O2-Regulated Prolyl Hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Favazza, L.; Chitale, D.A.; Barod, R.; Rogers, C.G.; Kalyana-Sundaram, S.; Palanisamy, N.; Gupta, N.S.; Williamson, S.R. Renal Cell Tumors with Clear Cell Histology and Intact VHL and Chromosome 3p: A Histological Review of Tumors from the Cancer Genome Atlas Database. Mod. Pathol. 2017, 30, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiao, F.; Sugimoto, H.; Li, B.; McAndrews, K.M.; Kalluri, R. Acute Kidney Injury Instigates Malignant Renal Cell Carcinoma via CXCR2 in Mice with Inactivated Trp53 and Pten in Proximal Tubular Kidney Epithelial Cells. Cancer Res. 2021, 81, 2690–2702. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Sun, S.; Li, Y.; Li, X.; Li, Z.; Liang, H. Identification of Therapeutic Targets and Prognostic Biomarkers Among CXC Chemokines in the Renal Cell Carcinoma Microenvironment. Front. Oncol. 2019, 9, 1555. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Burdick, M.D.; Reckamp, K.; Pantuck, A.; Figlin, R.A.; Strieter, R.M. The Role of CXCR2/CXCR2 Ligand Biological Axis in Renal Cell Carcinoma. J. Immunol. 2005, 175, 5351–5357. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.; Dong, Z.; Peng, W.; Jiang, W.; Huang, G.; Liu, G.; Ye, Z.; Wang, Y.; Xu, Z.; Fu, J.; et al. Ubiquitin-Specific Peptidase 53 Inhibits the Occurrence and Development of Clear Cell Renal Cell Carcinoma through NF-κB Pathway Inactivation. Cancer Med. 2021, 10, 3674–3688. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, C.S.; Rayman, P.A.; Pavicic, P.G.; Kim, J.S.; Wei, W.; Polefko, A.; Wallace, W.; Rini, B.I.; Morris-Stiff, G.; Allende, D.S.; et al. Mediators of Inflammation-Driven Expansion, Trafficking, and Function of Tumor-Infiltrating MDSCs. Cancer Immunol. Res. 2019, 7, 1687–1699. [Google Scholar] [CrossRef]
- Baechle, J.J.; Hanna, D.N.; Sekhar, K.R.; Rathmell, J.C.; Rathmell, W.K.; Baregamian, N. Multiplatform Computational Analysis of Mast Cells in Adrenocortical Carcinoma Tumor Microenvironment. Surgery 2022, 171, 111–118. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, W.; Gan, Z.; Abudurexiti, A.; Hu, X.; Sang, W. Identification of Biomarkers of Clear Cell Renal Cell Carcinoma by Bioinformatics Analysis. Medicine 2020, 99, e20470. [Google Scholar] [CrossRef]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Highfill, S.L.; Cui, Y.; Giles, A.J.; Smith, J.P.; Zhang, H.; Morse, E.; Kaplan, R.N.; Mackall, C.L. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci. Transl. Med. 2014, 6, 237ra67. [Google Scholar] [CrossRef] [PubMed]
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Desmond, R.A.; Soong, S. Epidemiology of Malignant Melanoma. Surg. Clin. N. Am. 2003, 83, 1–29. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, M.; He, Y.; Peng, J.; Zhang, X.; Wang, C.; Xia, X.; Song, W. CXC Chemokines as Therapeutic Targets and Prognostic Biomarkers in Skin Cutaneous Melanoma Microenvironment. Front. Oncol. 2021, 11, 619003. [Google Scholar] [CrossRef]
- Haqq, C.; Nosrati, M.; Sudilovsky, D.; Crothers, J.; Khodabakhsh, D.; Pulliam, B.L.; Federman, S.; Miller, J.R.; Allen, R.E.; Singer, M.I.; et al. The Gene Expression Signatures of Melanoma Progression. Proc. Natl. Acad. Sci. USA 2005, 102, 6092–6097. [Google Scholar] [CrossRef]
- Bordoni, R.; Thomas, G.; Richmond, A. Growth Factor Modulation of Melanoma Growth Stimulatory Activity mRNA Expression in Human Malignant Melanoma Cells Correlates with Cell Growth. J. Cell Biochem. 1989, 39, 421–428. [Google Scholar] [CrossRef]
- Sapoznik, S.; Ortenberg, R.; Galore-Haskel, G.; Kozlovski, S.; Levy, D.; Avivi, C.; Barshack, I.; Cohen, C.J.; Besser, M.J.; Schachter, J.; et al. CXCR1 as a Novel Target for Directing Reactive T Cells toward Melanoma: Implications for Adoptive Cell Transfer Immunotherapy. Cancer Immunol. Immunother. 2012, 61, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Lawson, D.H.; Nixon, D.W.; Chawla, R.K. Characterization of Autostimulatory and Transforming Growth Factors from Human Melanoma Cells. Cancer Res. 1985, 45, 6390–6394. [Google Scholar]
- Richmond, A.; Thomas, H.G. Melanoma Growth Stimulatory Activity: Isolation from Human Melanoma Tumors and Characterization of Tissue Distribution. J. Cell Biochem. 1988, 36, 185–198. [Google Scholar] [CrossRef]
- Rodeck, U.; Melber, K.; Kath, R.; Menssen, H.D.; Varello, M.; Atkinson, B.; Herlyn, M. Constitutive Expression of Multiple Growth Factor Genes by Melanoma Cells but Not Normal Melanocytes. J. Investig. Dermatol. 1991, 97, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Brown, K.; Landi, M.T.; Ghiorzo, P.; Badenas, C.; Xu, M.; Hayward, N.K.; Calista, D.; Landi, G.; Bruno, W.; et al. Duplication of CXC Chemokine Genes on Chromosome 4q13 in a Melanoma-Prone Family. Pigment. Cell Melanoma Res. 2012, 25, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Oka, M.; Edamatsu, H.; Kunisada, M.; Hu, L.; Takenaka, N.; Sakaguchi, M.; Kataoka, T.; Nishigori, C. Phospholipase Cɛ Has a Crucial Role in Ultraviolet B-Induced Neutrophil-Associated Skin Inflammation by Regulating the Expression of CXCL1/KC. Lab. Investig. 2011, 91, 711–718. [Google Scholar] [CrossRef]
- Shattuck, R.L.; Wood, L.D.; Jaffe, G.J.; Richmond, A. MGSA/GRO Transcription Is Differentially Regulated in Normal Retinal Pigment Epithelial and Melanoma Cells. Mol. Cell Biol. 1994, 14, 791–802. [Google Scholar] [CrossRef]
- Dhawan, P.; Richmond, A. A Novel NF-Kappa B-Inducing Kinase-MAPK Signaling Pathway UUp-Regulates NF-Kappa B Activity in Melanoma Cells. J. Biol. Chem. 2002, 277, 7920–7928. [Google Scholar] [CrossRef]
- Wang, D.; Richmond, A. Nuclear Factor-Kappa B Activation by the CXC Chemokine Melanoma Growth-Stimulatory Activity/Growth-Regulated Protein Involves the MEKK1/P38 Mitogen-Activated Protein Kinase Pathway. J. Biol. Chem. 2001, 276, 3650–3659. [Google Scholar] [CrossRef] [PubMed]
- Mangahas, C.R.; dela Cruz, G.V.; Friedman-Jiménez, G.; Jamal, S. Endothelin-1 Induces CXCL1 and CXCL8 Secretion in Human Melanoma Cells. J. Investig. Dermatol. 2005, 125, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Bardi, G.T.; Al-Rayan, N.; Richie, J.L.; Yaddanapudi, K.; Hood, J.L. Detection of Inflammation-Related Melanoma Small Extracellular Vesicle (sEV) mRNA Content Using Primary Melanocyte sEVs as a Reference. Int. J. Mol. Sci. 2019, 20, 1235. [Google Scholar] [CrossRef]
- Tang, H.; Zhou, X.; Zhao, X.; Luo, X.; Luo, T.; Chen, Y.; Liang, W.; Jiang, E.; Liu, K.; Shao, Z.; et al. HSP90/IKK-Rich Small Extracellular Vesicles Activate Pro-Angiogenic Melanoma-Associated Fibroblasts via the NF-κB/CXCL1 Axis. Cancer Sci. 2022, 113, 1168–1181. [Google Scholar] [CrossRef]
- Wang, D.; Yang, W.; Du, J.; Devalaraja, M.N.; Liang, P.; Matsumoto, K.; Tsubakimoto, K.; Endo, T.; Richmond, A. MGSA/GRO-Mediated Melanocyte Transformation Involves Induction of Ras Expression. Oncogene 2000, 19, 4647–4659. [Google Scholar] [CrossRef]
- Norgauer, J.; Metzner, B.; Schraufstätter, I. Expression and Growth-Promoting Function of the IL-8 Receptor Beta in Human Melanoma Cells. J. Immunol. 1996, 156, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Woodward, J.K.L.; Elshaw, S.R.; Murray, A.K.; Nichols, C.E.; Cross, N.; Laws, D.; Rennie, I.G.; Sisley, K. Stimulation and Inhibition of Uveal Melanoma Invasion by HGF, GRO, IL-1alpha and TGF-Beta. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3144–3152. [Google Scholar]
- Hatano, T.; Yashiro, M.; Fujikawa, H.; Motomura, H. C-X-C Motif Ligand 1 (CXCL1) from Melanoma Cells down-Regulates the Invasion of Their Metastatic Melanoma Cells. Oncotarget 2018, 9, 31090–31097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haghnegahdar, H.; Du, J.; Wang, D.; Strieter, R.M.; Burdick, M.D.; Nanney, L.B.; Cardwell, N.; Luan, J.; Shattuck-Brandt, R.; Richmond, A. The Tumorigenic and Angiogenic Effects of MGSA/GRO Proteins in Melanoma. J. Leukoc. Biol. 2000, 67, 53–62. [Google Scholar] [CrossRef]
- Fimmel, S.; Devermann, L.; Herrmann, A.; Zouboulis, C. GRO-Alpha: A Potential Marker for Cancer and Aging Silenced by RNA Interference. Ann. N. Y. Acad. Sci. 2007, 1119, 176–189. [Google Scholar] [CrossRef]
- Gallagher, P.G.; Bao, Y.; Prorock, A.; Zigrino, P.; Nischt, R.; Politi, V.; Mauch, C.; Dragulev, B.; Fox, J.W. Gene Expression Profiling Reveals Cross-Talk between Melanoma and Fibroblasts: Implications for Host-Tumor Interactions in Metastasis. Cancer Res. 2005, 65, 4134–4146. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Simiczyjew, A.; Dratkiewicz, E.; Pietraszek-Gremplewicz, K.; Majkowski, M.; Kot, M.; Ziętek, M.; Matkowski, R.; Nowak, D. Melanoma Cells with Diverse Invasive Potential Differentially Induce the Activation of Normal Human Fibroblasts. Cell Commun. Signal 2022, 20, 63. [Google Scholar] [CrossRef]
- Kodet, O.; Lacina, L.; Krejčí, E.; Dvořánková, B.; Grim, M.; Štork, J.; Kodetová, D.; Vlček, Č.; Šáchová, J.; Kolář, M.; et al. Melanoma Cells Influence the Differentiation Pattern of Human Epidermal Keratinocytes. Mol. Cancer 2015, 14, 1. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Z.; Wang, L.; Tian, G.; Tian, J.; Yang, Z.; Cao, G.; Zhou, H.; Zhao, L.; Wu, Z.; et al. Critical Role of Myeloid-Derived Suppressor Cells in Tumor-Induced Liver Immune Suppression through Inhibition of NKT Cell Function. Front. Immunol. 2017, 8, 129. [Google Scholar] [CrossRef]
- Singh, S.; Sadanandam, A.; Nannuru, K.C.; Varney, M.L.; Mayer-Ezell, R.; Bond, R.; Singh, R.K. Small-Molecule Antagonists for CXCR2 and CXCR1 Inhibit Human Melanoma Growth by Decreasing Tumor Cell Proliferation, Survival, and Angiogenesis. Clin. Cancer Res. 2009, 15, 2380–2386. [Google Scholar] [CrossRef]
- Shang, F.-M.; Li, J. A Small-Molecule Antagonist of CXCR1 and CXCR2 Inhibits Cell Proliferation, Migration and Invasion in Melanoma via PI3K/AKT Pathway. Med. Clin. 2019, 152, 425–430. [Google Scholar] [CrossRef]
- Kemp, D.M.; Pidich, A.; Larijani, M.; Jonas, R.; Lash, E.; Sato, T.; Terai, M.; De Pizzol, M.; Allegretti, M.; Igoucheva, O.; et al. Ladarixin, a Dual CXCR1/2 Inhibitor, Attenuates Experimental Melanomas Harboring Different Molecular Defects by Affecting Malignant Cells and Tumor Microenvironment. Oncotarget 2017, 8, 14428–14442. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, L.; Meng, L.; Shou, C. Topoisomerase Inhibitors Promote Cancer Cell Motility via ROS-Mediated Activation of JAK2-STAT1-CXCL1 Pathway. J. Exp. Clin. Cancer Res. 2019, 38, 370. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Muranaka, H.; Tanabe, Y.; Takahashi, C.; Matsugo, S.; Mukaida, N. Involvement of Prokineticin 2-Expressing Neutrophil Infiltration in 5-Fluorouracil-Induced Aggravation of Breast Cancer Metastasis to Lung. Mol. Cancer Ther. 2018, 17, 1515–1525. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Y.; Qi, F.; Jia, L.; Lu, X.; He, T.; Fu, Y.; Li, L.; Luo, Y. Specific Chemotherapeutic Agents Induce Metastatic Behaviour through Stromal- and Tumour-Derived Cytokine and Angiogenic Factor Signalling. J. Pathol. 2015, 237, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Middleman, B.R.; Friedman, M.; Lawson, D.H.; DeRose, P.B.; Cohen, C. Melanoma Growth Stimulatory Activity in Primary Malignant Melanoma: Prognostic Significance. Mod. Pathol. 2002, 15, 532–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brandt, M.G.; Moore, C.C. Nonmelanoma Skin Cancer. Facial Plast. Surg. Clin. N. Am. 2019, 27, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, Regional and National Incidence, Mortality and Disability-Adjusted Life-Years of Skin Cancers and Trend Analysis from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A Systematic Review of Worldwide Incidence of Nonmelanoma Skin Cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Schaper-Gerhardt, K.; Hansel, A.; Walter, A.; Grimmelmann, I.; Gutzmer, R. Sirolimus Diminishes the Expression of GRO-α (CXCL-1)/CXCR2 Axis in Human Keratinocytes and Cutaneous Squamous Cell Carcinoma Cells. J. Dermatol. Sci. 2021, 104, 30–38. [Google Scholar] [CrossRef]
- Tettelbach, W.; Nanney, L.; Ellis, D.; King, L.; Richmond, A. Localization of MGSA/GRO Protein in Cutaneous Lesions. J. Cutan. Pathol. 1993, 20, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Xiao, T.; Li, J.; Guo, A.; Lei, L.; Jin, C.; Long, Q.; Su, J.; Yin, M.; et al. CD147 Mediates Epidermal Malignant Transformation through the RSK2/AP-1 Pathway. J. Exp. Clin. Cancer Res. 2022, 41, 246. [Google Scholar] [CrossRef] [PubMed]
- Metzner, B.; Hofmann, C.; Heinemann, C.; Zimpfer, U.; Schraufstätter, I.; Schöpf, E.; Norgauer, J. Overexpression of CXC-Chemokines and CXC-Chemokine Receptor Type II Constitute an Autocrine Growth Mechanism in the Epidermoid Carcinoma Cells KB and A431. Oncol. Rep. 1999, 6, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Szabo, P.; Valach, J.; Smetana, K.; Dvořánková, B. Comparative Analysis of IL-8 and CXCL-1 Production by Normal and Cancer Stromal Fibroblasts. Folia Biol. 2013, 59, 134–137. [Google Scholar]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid Cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef] [PubMed]
- Liotti, F.; Collina, F.; Pone, E.; La Sala, L.; Franco, R.; Prevete, N.; Melillo, R.M. Interleukin-8, but Not the Related Chemokine CXCL1, Sustains an Autocrine Circuit Necessary for the Properties and Functions of Thyroid Cancer Stem Cells. Stem Cells 2017, 35, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Aust, G.; Steinert, M.; Boltze, C.; Kiessling, S.; Simchen, C. GRO-Alpha in Normal and Pathological Thyroid Tissues and Its Regulation in Thyroid-Derived Cells. J. Endocrinol. 2001, 170, 513–520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Melillo, R.M.; Guarino, V.; Avilla, E.; Galdiero, M.R.; Liotti, F.; Prevete, N.; Rossi, F.W.; Basolo, F.; Ugolini, C.; de Paulis, A.; et al. Mast Cells Have a Protumorigenic Role in Human Thyroid Cancer. Oncogene 2010, 29, 6203–6215. [Google Scholar] [CrossRef]
- Schulten, H.-J.; Hussein, D.; Al-Adwani, F.; Karim, S.; Al-Maghrabi, J.; Al-Sharif, M.; Jamal, A.; Bakhashab, S.; Weaver, J.; Al-Ghamdi, F.; et al. Microarray Expression Profiling Identifies Genes, Including Cytokines, and Biofunctions, as Diapedesis, Associated with a Brain Metastasis from a Papillary Thyroid Carcinoma. Am. J. Cancer Res. 2016, 6, 2140–2161. [Google Scholar]
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Bladder cancer | No effect | 402 | OS, DFS, GEPIA database | [31,43] |
| Bladder cancer | Decreased survival | No data | OS, GEO database | [31] |
| Bladder cancer | Decreased survival | 155 | Progression-free survival | [37] |
| Bladder cancer | Decreased survival | 201 | RFS, urine CXCL1 | [34] |
| Bladder cancer | Decreased survival | 40 | OS | [40] |
| Bladder cancer | Decreased survival | 142 | OS | [30] |
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Brain tumor: glioma | Decreased survival | 138 | OS, REMBRANDT database | [59] |
| Brain tumor: lower-grade glioma | Decreased survival | 514 | OS, GEPIA dataset | [43] |
| Brain tumor: glioblastoma | Decreased survival | No data | DFS, TCGA database, comparison of tumors with low expression of CXCL1 and CXCL2 with those with high expression of CXCL1 and CXCL2 | [51] |
| Brain tumor: glioblastoma | Decreased survival | 91 | OS | [50] |
| Brain tumor: glioblastoma | Decreased survival | 160 | OS, GEPIA dataset | [43] |
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Haematolymphoid tumors: AML | Decreased survival | 132 | OS from TCGA | [78] |
| Haematolymphoid tumors: AML | Decreased survival | 160 | mononuclear cells from bone marrow OS, EFS | [80] |
| Haematolymphoid tumors: AML | Decreased survival | 54 | lowest quartile vs. highest quartile, OS, GEPIA dataset | [43] |
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Lung cancer: NSCLC | Decreased survival | 232 | OS, DFS | [154] |
| Lung cancer: NSCLC | Decreased survival | 28 | PFS | [127] |
| Lung cancer: NSCLC | Decreased survival | 865 | OS, Kaplan–Meier plotter database | [128] |
| Lung cancer: NSCLC | Decreased survival | Meta-analysis | OS, ale nie PFS Meta-analysis of GEO databases | [126] |
| Lung cancer: NSCLC | No effect | 109 | OS, DFS, Only I- and II-stage patients | [130] |
| Lung cancer: lung adenocarcinoma | Decreased survival | Meta-analysis | OS, PFS Meta-analysis of GEO databases | [126] |
| Lung cancer: lung adenocarcinoma | Decreased survival | 71 | OS | [126] |
| Lung cancer: lung adenocarcinoma | No effect | 478 | OS, DFS, GEPIA dataset | [43] |
| Lung cancer: lung squamous cell carcinoma | Decreased survival | Meta-analysis | OS, but not PFS Meta-analysis of GEO databases | [126] |
| Lung cancer: lung squamous cell carcinoma | No effect | 482 | OS, DFS, GEPIA dataset | [43] |
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Renal cell carcinoma | Decreased survival | 516 | OS, DFS GEPIA dataset | [43,165,170] |
| Renal cell carcinoma | Decreased survival | 24 | OS | [168] |
| Type of Cancer | Impact on Survival at High CXCL1 Expression | Group Size | Notes | References |
|---|---|---|---|---|
| Malignant melanoma | No effect | 37 | OS, DFS | [206] |
| Malignant melanoma | No effect | 458 | OS, DFS GEPIA dataset | [43,175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korbecki, J.; Bosiacki, M.; Szatkowska, I.; Kupnicka, P.; Chlubek, D.; Baranowska-Bosiacka, I. The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors. Int. J. Mol. Sci. 2024, 25, 4365. https://doi.org/10.3390/ijms25084365
Korbecki J, Bosiacki M, Szatkowska I, Kupnicka P, Chlubek D, Baranowska-Bosiacka I. The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors. International Journal of Molecular Sciences. 2024; 25(8):4365. https://doi.org/10.3390/ijms25084365
Chicago/Turabian StyleKorbecki, Jan, Mateusz Bosiacki, Iwona Szatkowska, Patrycja Kupnicka, Dariusz Chlubek, and Irena Baranowska-Bosiacka. 2024. "The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors" International Journal of Molecular Sciences 25, no. 8: 4365. https://doi.org/10.3390/ijms25084365
APA StyleKorbecki, J., Bosiacki, M., Szatkowska, I., Kupnicka, P., Chlubek, D., & Baranowska-Bosiacka, I. (2024). The Clinical Significance and Involvement in Molecular Cancer Processes of Chemokine CXCL1 in Selected Tumors. International Journal of Molecular Sciences, 25(8), 4365. https://doi.org/10.3390/ijms25084365






