Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite
Abstract
:1. Introduction
2. Results
2.1. Characterization of GO/ZTO/TO Composite
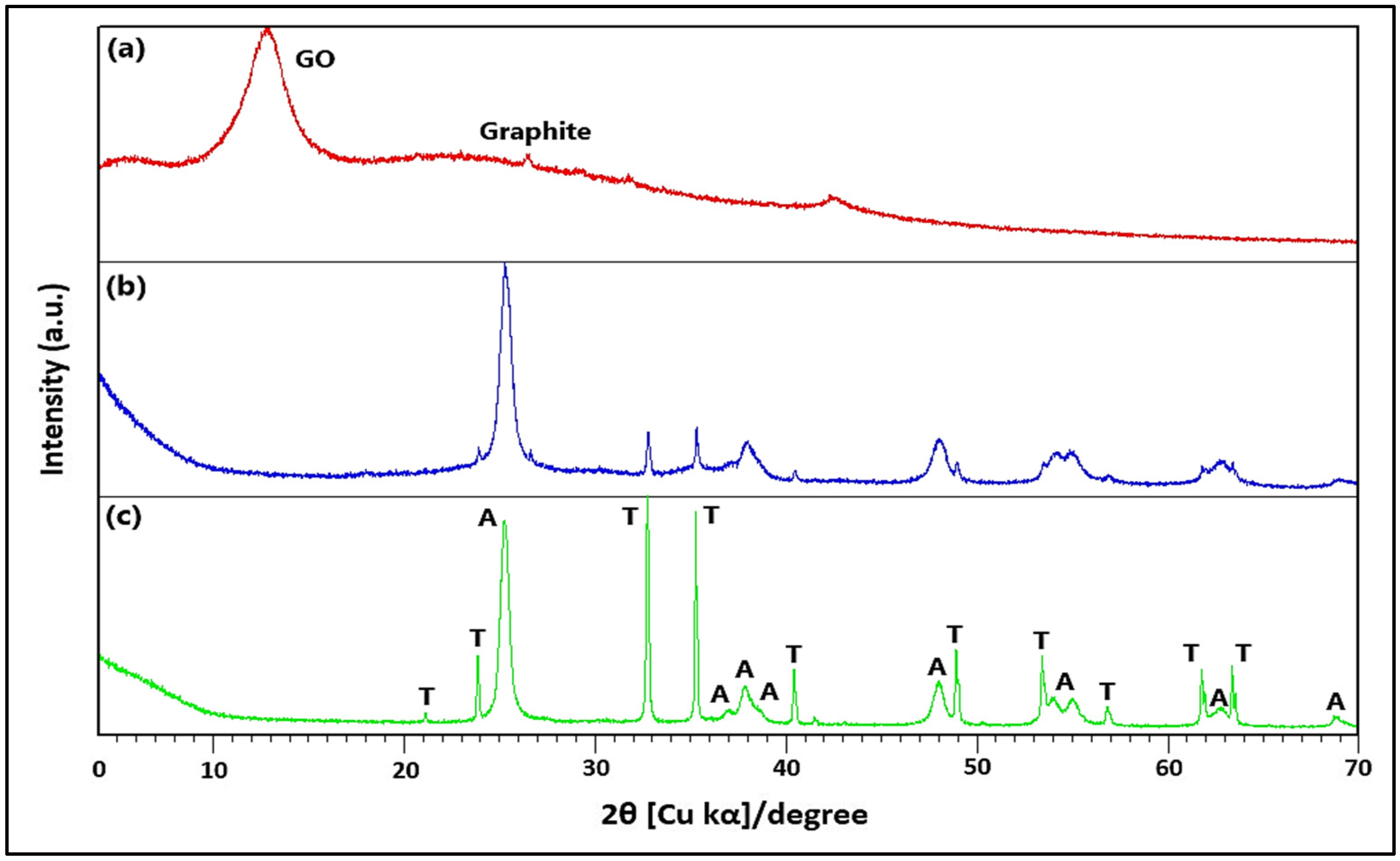
2.1.1. XRD and FTIR Analysis
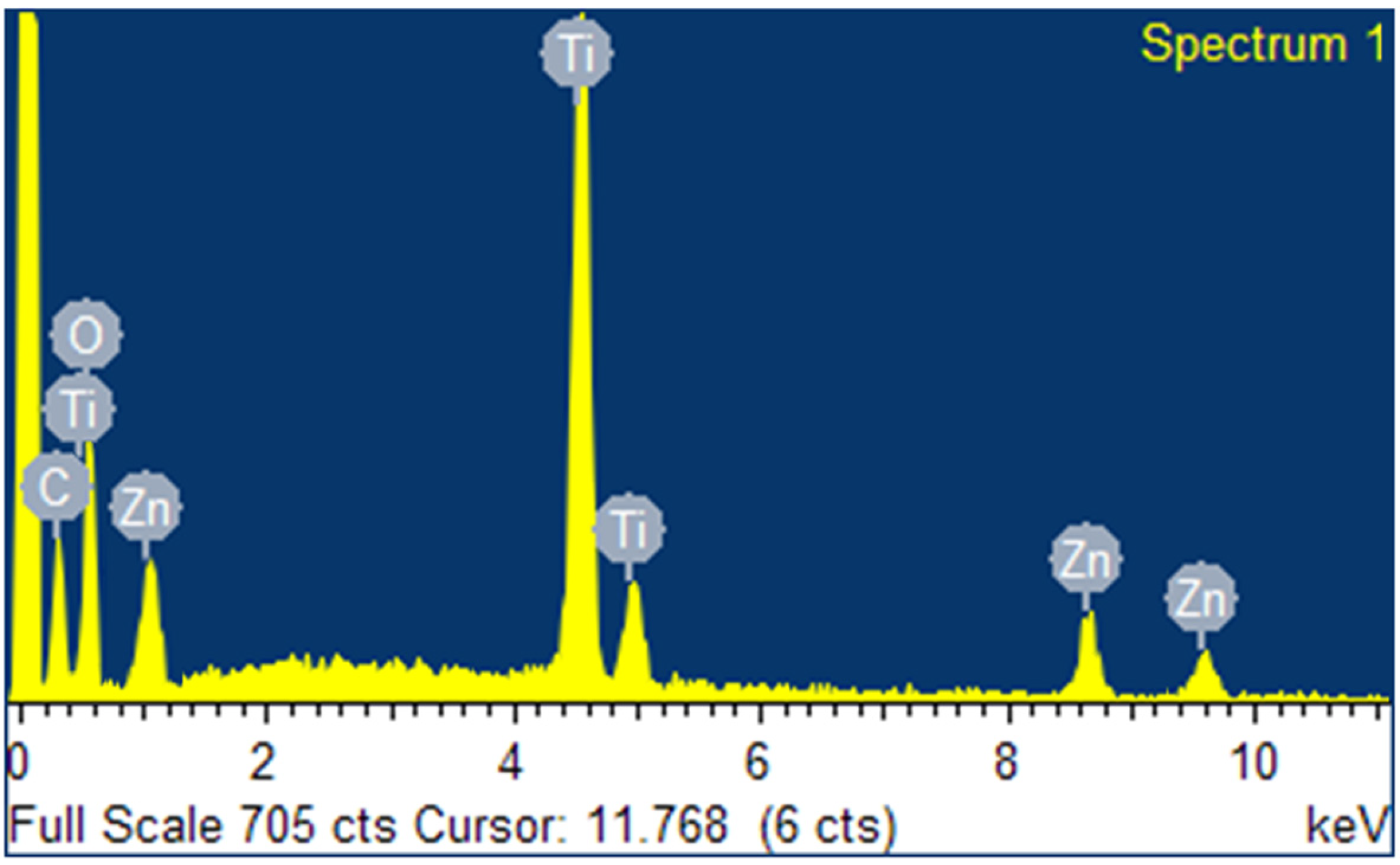
2.1.2. SEM and EDS Analysis
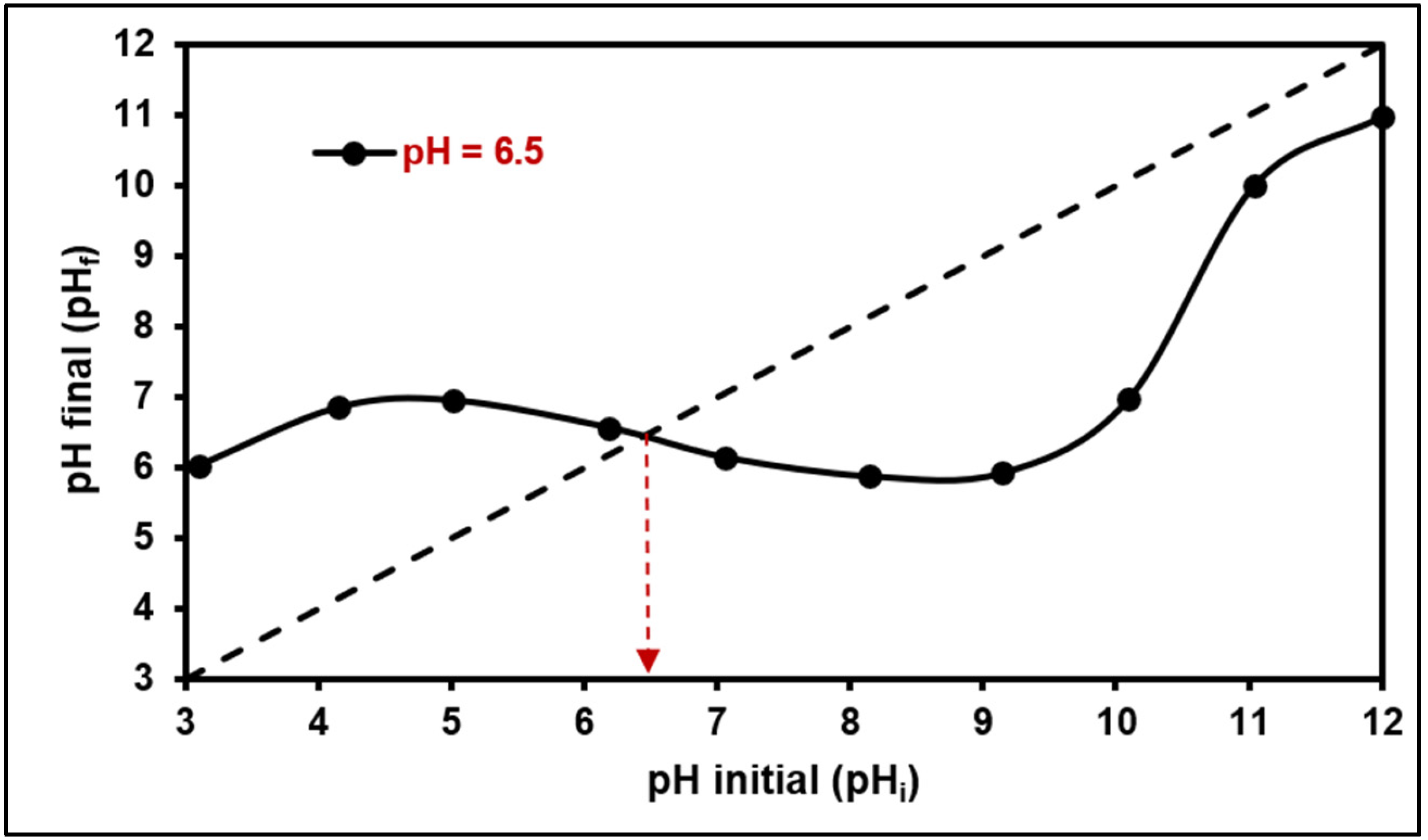
2.1.3. SSA and pHPZC Analysis
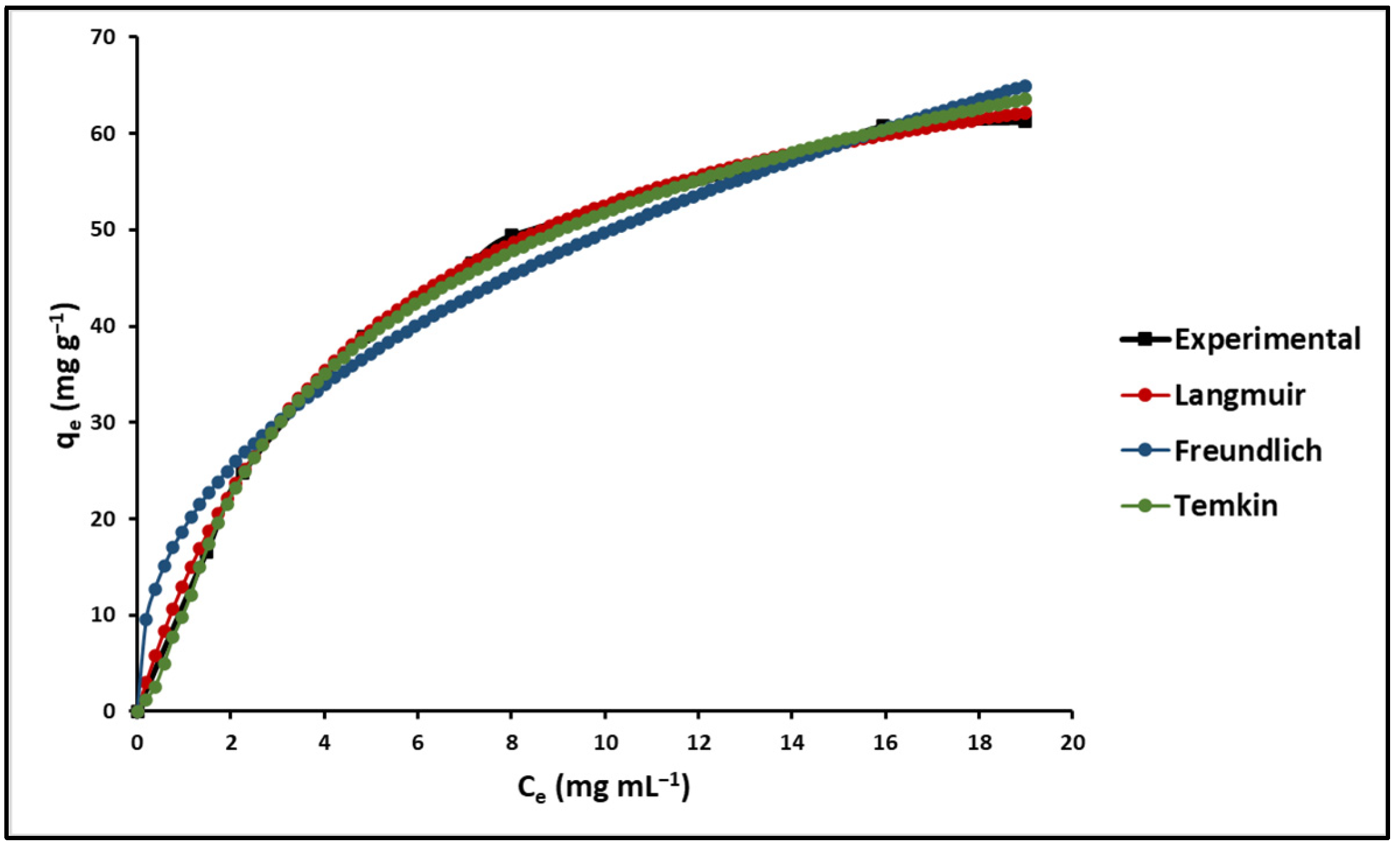
2.2. Adsorption Studies
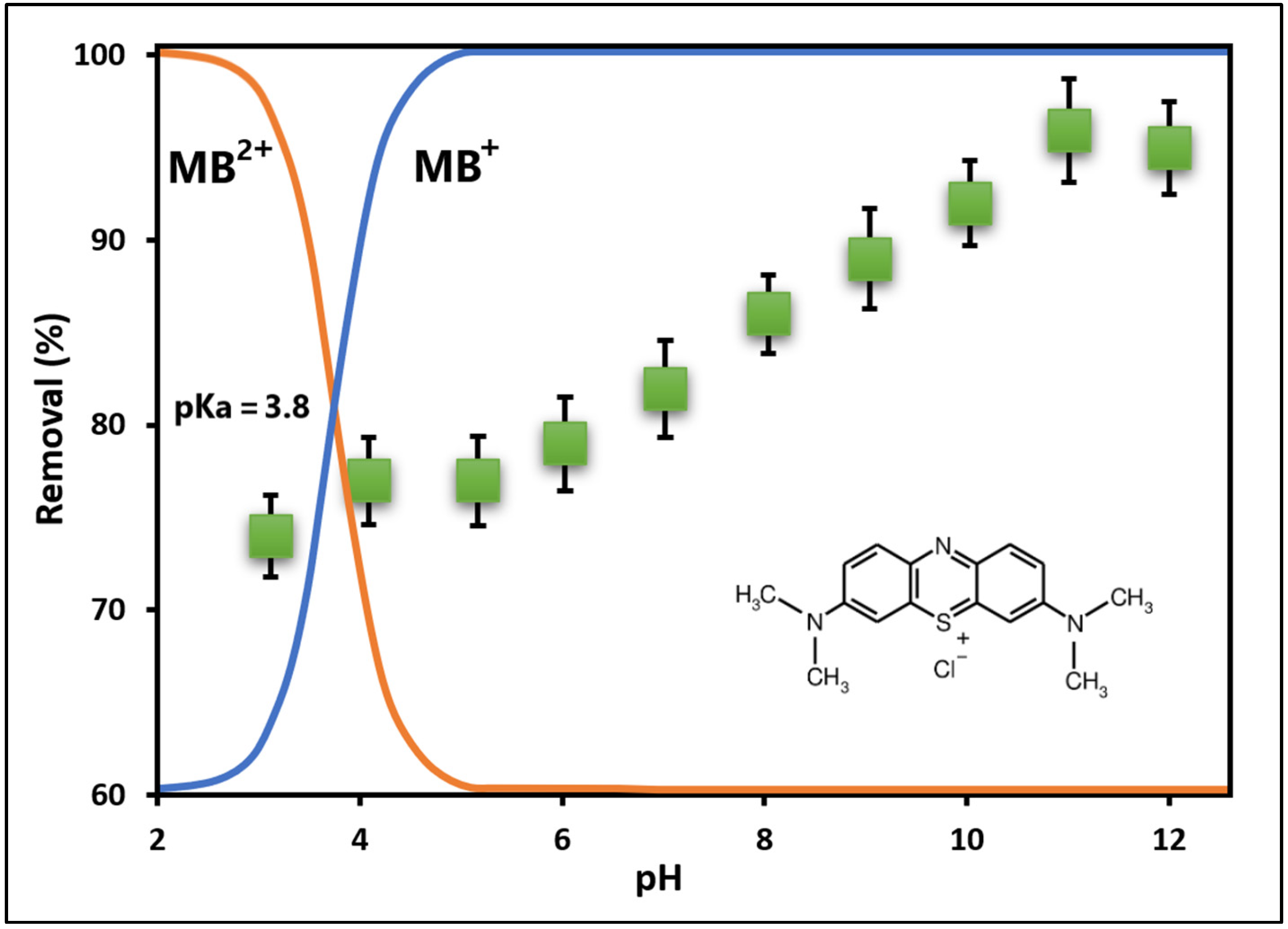
2.2.1. Effect of pH on MB Adsorption
2.2.2. Effect of MB Dosage
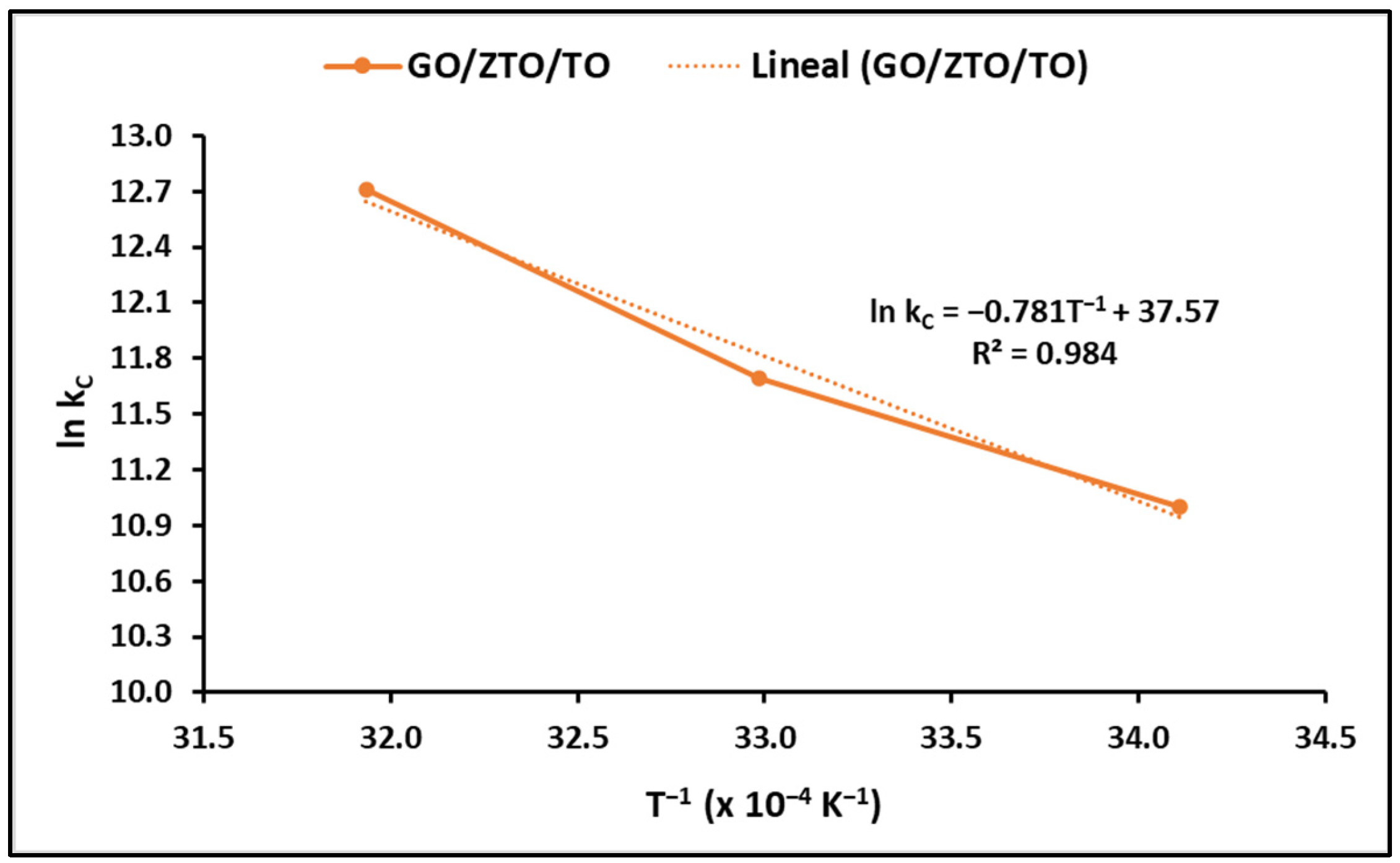
2.2.3. Effect of Temperature on MB Adsorption
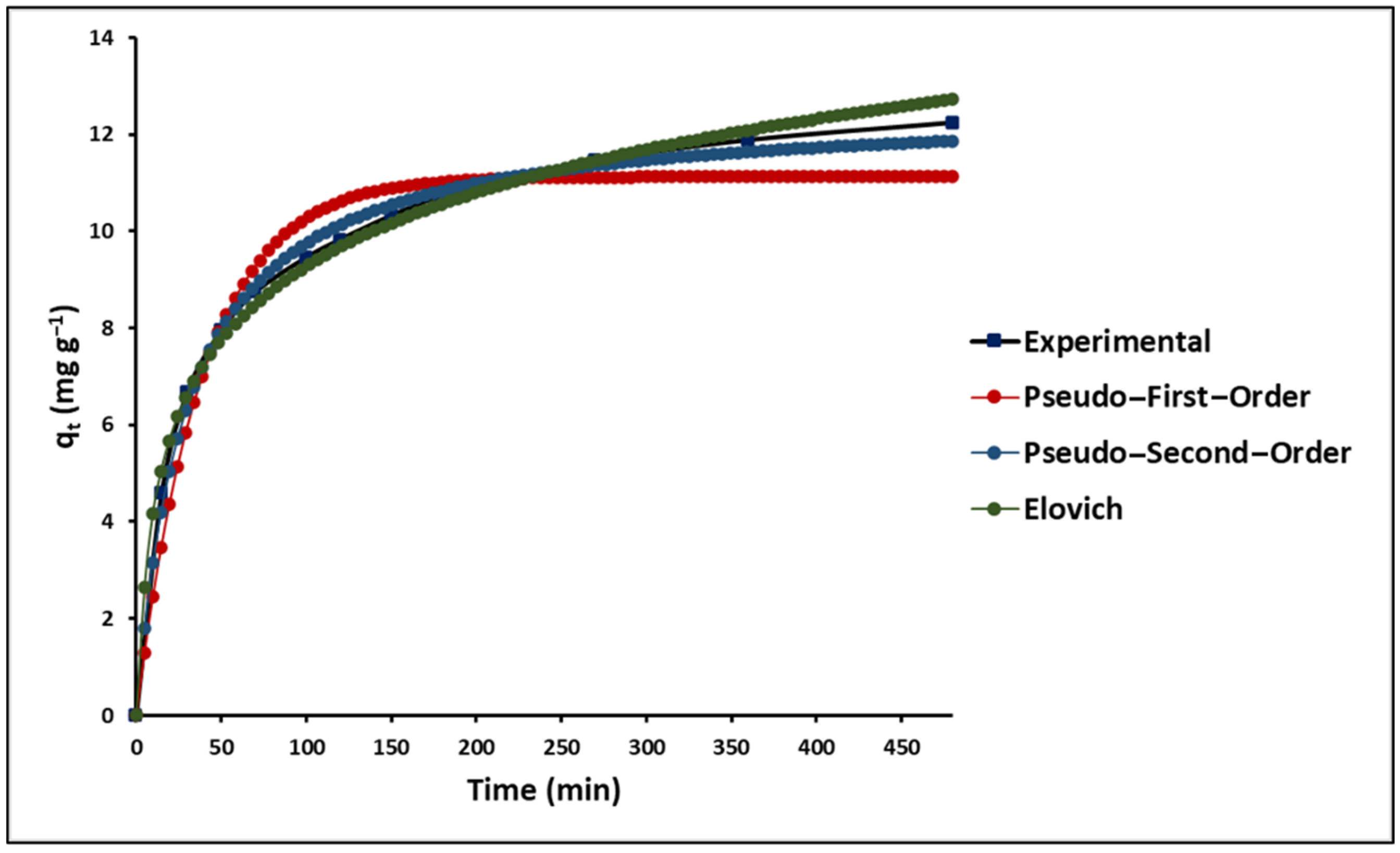
2.2.4. Effect of Contact Time on MB Adsorption
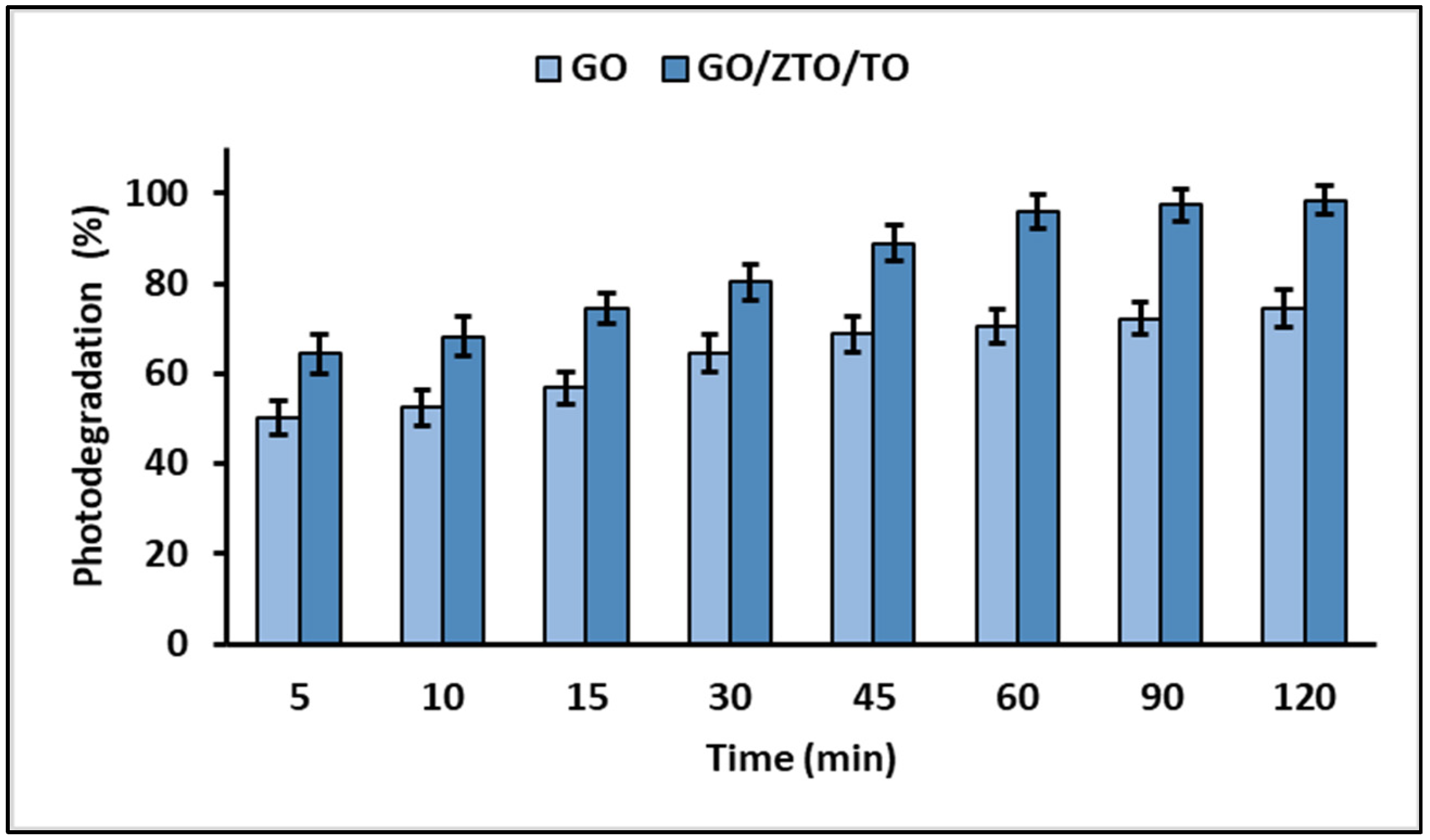
2.3. Photodegradation Studies
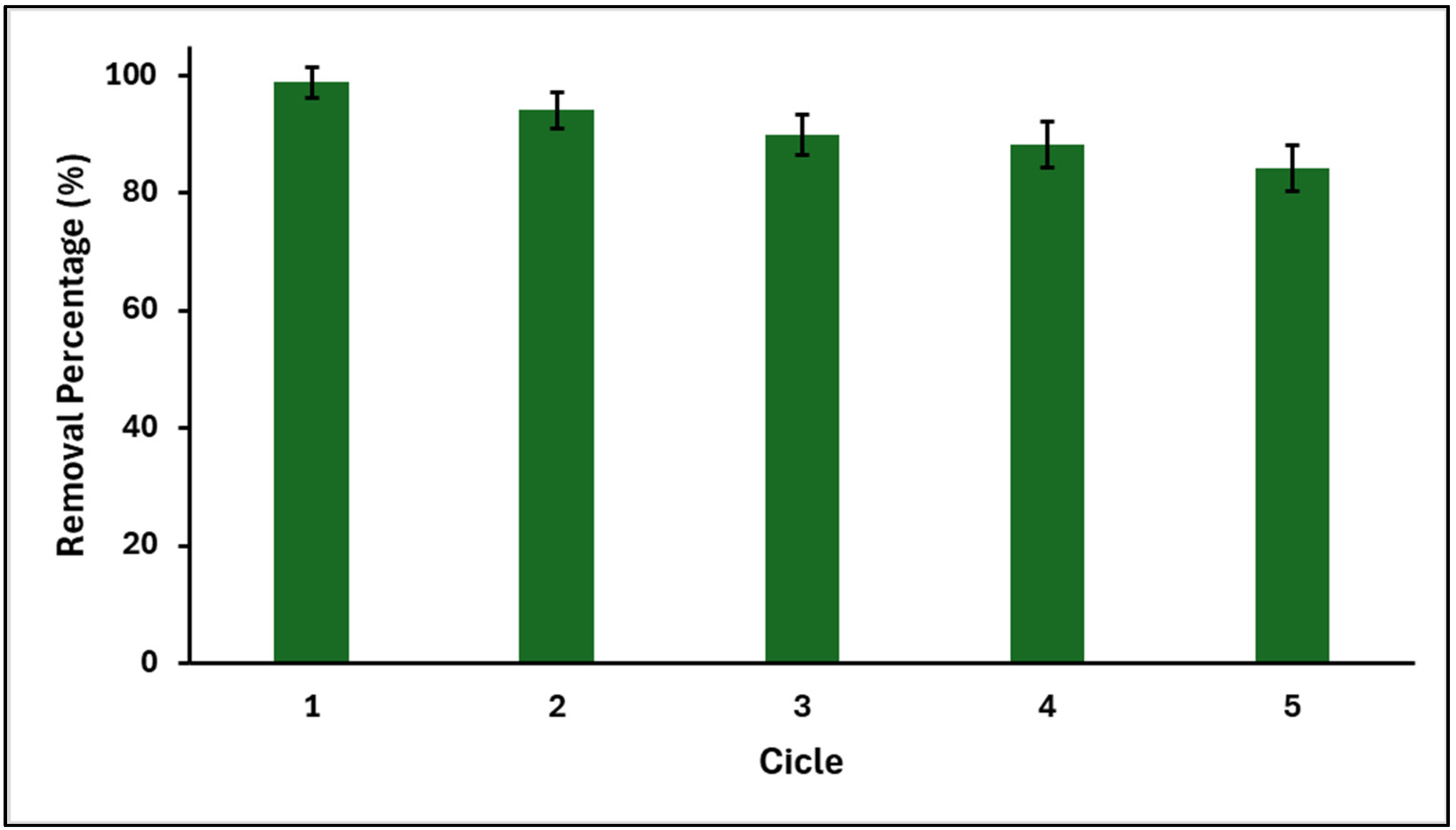
2.4. Reusability of GO/ZTO/TO for MB Removal
3. Discussion
3.1. Characterization of GO/ZTO/TO
3.2. Adsorption Studies
3.2.1. Effect of pH on MB Adsorption
3.2.2. Effect of Initial Concentration of MB
3.2.3. Effect of Temperature on MB Adsorption
3.2.4. Effect of Contact Time on MB Adsorption
3.3. Photodegradation Studies
3.4. Reusability of GO/ZTO/TO for MB Removal
4. Materials and Methods
4.1. Materials
4.2. Synthesis of GO/ZTO/TO Composite
4.3. Characterization of GO/ZTO/TO Composite
4.4. Adsorption Studies
aDSA
4.5. Photodegradation Studies
4.6. Reuse of GO/ZTO/TO Composite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmen, Z.; Daniel, S. Textile Organic Dyes—Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants Ten Years after the Stockholm Convention—Environmental and Analytical Update; IntechOpen: London, UK, 2012; ISBN 978-953-307-917-2. [Google Scholar]
- Ashrafi, M.; Arab Chamjangali, M.; Bagherian, G.; Goudarzi, N. Application of linear and non-linear methods for modeling removal efficiency of textile dyes from aqueous solutions using magnetic Fe3O4 impregnated onto walnut shell. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Alsukaibi, A.K. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Dutta, A.K.; Ghorai, U.K.; Chattopadhyay, K.K.; Banerjee, D. Removal of textile dyes by carbon nanotubes: A comparison between adsorption and UV assisted photocatalysis. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 99, 6–15. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Manappadan, Z.; Kumar, S.; Joshi, K.; Govindaraja, T.; Krishnamurty, S.; Selvaraj, K. Unravelling the distinct surface interactions of modified graphene nanostructures with methylene blue dye through experimental and computational approaches. J. Hazard. Mater. 2020, 388, 121755. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Schönbächler, M.; Fehr, M.A. Basics of Ion Exchange Chromatography for Selected Geological Applications. In Treatise on Geochemistry: Second Edition; Elsevier: Amsterdam, The Netherlands, 2013; Volume 15, pp. 123–146. ISBN 978-0-08098-300-4. [Google Scholar]
- Pronk, W.; Ding, A.; Morgenroth, E.; Derlon, N.; Desmond, P.; Burkhardt, M.; Wu, B.; Fane, A.G. Gravity-driven membrane filtration for water and wastewater treatment: A review. Water Res. 2019, 149, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, K. Physicochemical technologies for HRPs and risk control. In High-Risk Pollutants in Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–207. [Google Scholar] [CrossRef]
- Martín-Pozo, L.; Del Carmen Gómez-Regalado, M.; García-Córcoles, M.T.; Zafra-Gómez, A. Removal of quinolone antibiotics from wastewaters and sewage sludge. In Emerging Contaminants in the Environment: Challenges and Sustainable Practices; Elsevier: Amsterdam, The Netherlands, 2022; pp. 381–406. ISBN 978-0-32385-160-2. [Google Scholar]
- Moura, L.; Picão, R.C. Removal of antimicrobial resistance determinants from wastewater: A risk perspective on conventional and emerging technologies. In Emerging Contaminants in the Environment: Challenges and Sustainable Practices; Elsevier: Amsterdam, The Netherlands, 2022; pp. 603–642. ISBN 978-0-32385-160-2. [Google Scholar]
- Ayare, S.D.; Gogate, P.R. Degradation of Tricyclazole fungicide using combined oxidation strategies based on ultrasound, ultraviolet irradiation and microwave. Environ. Technol. Innov. 2022, 26, 102533. [Google Scholar] [CrossRef]
- Rabajczyk, A.; Zielecka, M.; Klapsa, W.; Dziechciarz, A. Self-cleaning coatings and surfaces of modern building materials for the removal of some air pollutants. Materials 2021, 14, 2161. [Google Scholar] [CrossRef]
- Abu Hasan, H.; Muhammad, M.H.; Ismail, N.I. A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng. 2020, 33, 101035. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater: The perspective role of nanomaterials and nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 978-0-12818-489-9. [Google Scholar]
- Liu, T.; Wang, Z.; Wang, X.; Yang, G.; Liu, Y. Adsorption-photocatalysis performance of polyaniline/dicarboxyl acid cellulose@graphene oxide for dye removal. Int. J. Biol. Macromol. 2021, 182, 492–501. [Google Scholar] [CrossRef]
- Minitha, C.R.; Lalitha, M.; Jeyachandran, Y.L.; Senthilkumar, L.; Rajendra Kumar, R.T. Adsorption behaviour of reduced graphene oxide towards cationic and anionic dyes: Co-action of electrostatic and π–π interactions. Mater. Chem. Phys. 2017, 194, 243–252. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Rajput, J.K.; Pathak, T.K.; Kumar, V.; Purohit, L.P. Synthesis of ZnO:TiO2 nanocomposites for photocatalyst application in visible light. Vacuum 2019, 160, 154–163. [Google Scholar] [CrossRef]
- Amaro-Medina, B.M.; Martinez-Luevanos, A.; Soria-Aguilar, M.; Sanchez-Castillo, M.A.; Estrada-Flores, S.; Carrillo-Pedroza, F.R. Efficiency of Adsorption and Photodegradation of Composite TiO2/Fe2O3 and Industrial Wastes in Cyanide Removal. Water 2022, 14, 3502. [Google Scholar] [CrossRef]
- Muthirulan, P.; Nirmala Devi, C.; Meenakshi Sundaram, M. Synchronous role of coupled adsorption and photocatalytic degradation on CAC–TiO2 composite generating excellent mineralization of alizarin cyanine green dye in aqueous solution. Arab. J. Chem. 2017, 10, S1477–S1483. [Google Scholar] [CrossRef]
- Rout, D.R.; Jena, H.M.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Graphene-based materials for effective adsorption of organic and inorganic pollutants: A critical and comprehensive review. Sci. Total Environ. 2023, 863, 160871. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Nissanka, B.; Kottegoda, N.; Jayasundara, D.R. Probing structural variations of graphene oxide and reduced graphene oxide using methylene blue adsorption method. J. Mater. Sci. 2020, 55, 1996–2005. [Google Scholar] [CrossRef]
- Wang, H.; Yi, L.; Huang, F.; Huang, Q.; Zhou, T. Facile synthesis of graphene nanosheets on wastewater sediments for high efficient adsorption of methylene blue. Sep. Purif. Technol. 2024, 337, 126366. [Google Scholar] [CrossRef]
- Ben Gouider Trabelsi, A.; Kusmartsev, F.V.; Kusmartseva, A.; Alkallas, F.H.; Alfaify, S.; Shkir, M. Raman Spectroscopy Imaging of Exceptional Electronic Properties in Epitaxial Graphene Grown on SiC. Nanomaterials 2020, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Ihsanullah; Sajid, M.; Saleh, T.A. Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J. Environ. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Rivera Tito, H.A.; Hernandez-Sosa, G.; Cucinotta, F.; Huang, X.; Quintana Caceda, M.E. Photoluminescent graphene oxide porous particles in solution under environmental conditions produced by hydrothermal treatment. Mater. Today Commun. 2019, 20, 100621. [Google Scholar] [CrossRef]
- Gascho, J.L.S.; Costa, S.F.; Recco, A.A.C.; Pezzin, S.H. Graphene oxide films obtained by vacuum filtration: X-ray diffraction evidence of crystalline reorganization. J. Nanomater. 2019, 2019, 5963148. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Carvalho, M.N.; Ghislandi, M.G.; Da Motta Sobrinho, M.A. Functionalized graphene-based materials as innovative adsorbents of organic pollutants: A concise overview. Braz. J. Chem. Eng. 2019, 36, 1–31. [Google Scholar] [CrossRef]
- Esteban-Arranz, A.; Compte-Tordesillas, D.; Muñoz-Andrés, V.; Pérez-Cadenas, M.; Guerrero-Ruiz, A. Effect of surface, structural and textural properties of graphenic materials over cooperative and synergetic adsorptions of two chloroaromatic compounds from aqueous solution. Catal. Today 2018, 301, 104–111. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Ménard-Moyon, C.; Bianco, A. Chemical Functionalization of Graphene Family Members. Phys. Sci. Rev. 2017, 2, 20160103. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Murali, S.; Zhu, Y.; Ruoff, R.S.; Bielawski, C.W. Reduction of graphite oxide using alcohols. J. Mater. Chem. 2011, 21, 3443–3447. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, G.; Liu, J.; Sun, X. Evaluation criteria for reduced graphene oxide. J. Phys. Chem. C 2011, 115, 11327–11335. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A. Synthesis, properties and applications of graphene doped with boron, nitrogen and other elements. Nano Today 2014, 9, 324–343. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, B.; Bao, Q.; Liu, Y. Controllable Synthesis of Doped Graphene and Its Applications. Small 2014, 10, 2975–2991. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507. [Google Scholar] [CrossRef]
- Luo, J.; Luo, X.; Gan, Y.; Xu, X.; Xu, B.; Liu, Z.; Ding, C.; Cui, Y.; Sun, C. Advantages of Bimetallic Organic Frameworks in the Adsorption, Catalysis and Detection for Water Contaminants. Nanomaterials 2023, 13, 2194. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, G.K.; Vijaya Kumara, A.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Justino, D.D.; Alves, M.O.; Galvão, B.R.L.; Santamaría, R.; De Sousa, F.B.; Ortega, P.F.R. The effects of functionalization on graphene oxide for organic dye adsorption: An experimental-theoretical study using electronic structure calculations and statistical mechanical modeling. J. Mol. Liq. 2023, 387, 122612. [Google Scholar] [CrossRef]
- Dayana Priyadharshini, S.; Manikandan, S.; Kiruthiga, R.; Rednam, U.; Babu, P.S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Graphene oxide-based nanomaterials for the treatment of pollutants in the aquatic environment: Recent trends and perspectives—A review. Environ. Pollut. 2022, 306, 119377. [Google Scholar] [CrossRef] [PubMed]
- Mandeep; Gulati, A.; Kakkar, R. Graphene-based adsorbents for water remediation by removal of organic pollutants: Theoretical and experimental insights. Chem. Eng. Res. Des. 2020, 153, 21–36. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; Ghislandi, M.G.; Carvalho, M.N.; da Motta Sobrinho, M.A. One step forward: How can functionalization enhance the adsorptive properties of graphene towards metallic ions and dyes? Environ. Res. 2020, 184, 109362. [Google Scholar] [CrossRef] [PubMed]
- Tee, W.T.; Loh, N.Y.L.; Lai, K.C.; Hiew, B.Y.Z.; Gan, S.; Lee, L.Y. Application of 3D heteroatom-doped graphene in adsorptive removal of water pollutants: Review on hydrothermal synthesis and its influencing factors. Sep. Purif. Technol. 2023, 320, 124072. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, Q.; Zhang, W.; Cui, S.; Yang, B.; Wang, Q.; Li, S.; Zhang, D. A high sensitivity dual-mode optical thermometry based on charge compensation in ZnTiO3:M (M = Eu3+, Mn4+) hexagonal prisms. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 274, 121101. [Google Scholar] [CrossRef] [PubMed]
- Mofokeng, S.J.; Noto, L.L.; Dhlamini, M.S. Photoluminescence properties of ZnTiO3:Eu3+ phosphor with enhanced red emission by Al3+ charge compensation. J. Lumin. 2020, 228, 117569. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Y.S.; Qin, Z.; Ke, S.H. First-principles investigation of the ferroelectric, piezoelectric and nonlinear optical properties of LiNbO3-type ZnTiO3. Sci. Rep. 2019, 9, 17632. [Google Scholar] [CrossRef] [PubMed]
- Djellabi, R.; Ordonez, M.F.; Conte, F.; Falletta, E.; Bianchi, C.L.; Rossetti, I. A Review of Advances in Multifunctional XTiO3 Perovskite-type Oxides as piezo-photocatalysts for Environmental Remediation and Energy Production. J. Hazard. Mater. 2021, 421, 126792. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, U.O.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis of ZnTiO3@TiO2 Heterostructure Nanomaterial as a Visible light Photocatalyst. ChemistrySelect 2019, 4, 6106–6112. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; Hernández, K.; González, S. Cu(C3H3N3S3)3 Adsorption onto ZnTiO3/TiO2 for Coordination-Complex Sensitized Photochemical Applications. Materials 2022, 15, 3252. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi Zalani, N.; Koozegar Kaleji, B.; Mazinani, B. Synthesis and characterisation of the mesoporous ZnO-TiO2 nanocomposite; Taguchi optimisation and photocatalytic methylene blue degradation under visible light. Mater. Technol. 2020, 35, 281–289. [Google Scholar] [CrossRef]
- Jose, M.; Elakiya, M.; Dhas, S.A.M.B. Structural and optical properties of nanosized ZnO/ZnTiO3 composite materials synthesized by a facile hydrothermal technique. J. Mater. Sci. Mater. Electron. 2017, 28, 13649–13658. [Google Scholar] [CrossRef]
- Chen, F.; Yu, C.; Wei, L.; Fan, Q.; Ma, F.; Zeng, J.; Yi, J.; Yang, K.; Ji, H. Fabrication and characterization of ZnTiO3/Zn2Ti3O8/ZnO ternary photocatalyst for synergetic removal of aqueous organic pollutants and Cr(VI) ions. Sci. Total Environ. 2020, 706, 136026. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, A.; Dan, S.; Hashemipour, H. Preparation and optimization of novel graphene oxide and adsorption isotherm study of methylene blue. Arab. J. Chem. 2021, 14, 103003. [Google Scholar] [CrossRef]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Xiao, K.; Yao, Z.; Wang, Y.; Huang, D.; Zhu, L.; Liu, F.; Tian, T. Hydrothermal synthesis of graphene-ZnTiO3 nanocomposites with enhanced photocatalytic activities. Res. Chem. Intermed. 2018, 44, 6621–6636. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the “Debye-Scherrer equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Mehrabi, M.; Javanbakht, V. Photocatalytic degradation of cationic and anionic dyes by a novel nanophotocatalyst of TiO2/ZnTiO3/αFe2O3 by ultraviolet light irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 9908–9919. [Google Scholar] [CrossRef]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N.; Zamanian, A. Synthesis of cubic and hexagonal ZnTiO3 as catalyst support in steam reforming of methanol: Study of physical and chemical properties of copper catalysts on the H2 and CO selectivity and coke formation. Int. J. Hydrogen Energy 2020, 45, 9484–9495. [Google Scholar] [CrossRef]
- Gabal, M.A.; Angari, Y.M.A. Zinc titanates nanopowders: Synthesis and characterization. Mater. Res. Express 2022, 9, 025010. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillai, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and photovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Tene, T.; Guevara, M.; Valarezo, A.; Salguero, O.; Arias Arias, F.; Arias, M.; Scarcello, A.; Caputi, L.S.; Vacacela Gomez, C. Drying-time study in graphene oxide. Nanomaterials 2021, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Nguyen, V.N. RGO/persulfate metal-free catalytic system for the degradation of tetracycline: Effect of reaction parameters. Mater. Res. Express 2020, 7, 075501. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; Luneau, D.; Baouab, M.H.V. Functionalization of graphene oxide sheets with magnetite nanoparticles for the adsorption of copper ions and investigation of its potential catalytic activity toward the homocoupling of alkynes under green conditions. J. Catal. 2020, 388, 91–103. [Google Scholar] [CrossRef]
- Tavakoli-Azar, T.; Mahjoub, A.R.; Sadjadi, M.S.; Farhadyar, N.; Sadr, M.H. Improving the photocatalytic performance of a perovskite ZnTiO3 through ZnTiO3@S nanocomposites for degradation of Crystal violet and Rhodamine B pollutants under sunlight. Inorg. Chem. Commun. 2020, 119, 108091. [Google Scholar] [CrossRef]
- Kunnamareddy, M.; Diravidamani, B.; Rajendran, R.; Singaram, B.; Varadharajan, K. Synthesis of silver and sulphur codoped TiO2 nanoparticles for photocatalytic degradation of methylene blue. J. Mater. Sci. Mater. Electron. 2018, 29, 18111–18119. [Google Scholar] [CrossRef]
- Jahan, N.; Roy, H.; Reaz, A.H.; Arshi, S.; Rahman, E.; Firoz, S.H.; Islam, M.S. A comparative study on sorption behavior of graphene oxide and reduced graphene oxide towards methylene blue. Case Stud. Chem. Environ. Eng. 2022, 6, 100239. [Google Scholar] [CrossRef]
- Dan, S.; Bagheri, H.; Shahidizadeh, A.; Hashemipour, H. Performance of graphene Oxide/SiO2 Nanocomposite-based: Antibacterial Activity, dye and heavy metal removal. Arab. J. Chem. 2023, 16, 104450. [Google Scholar] [CrossRef]
- Batzias, F.A.; Sidiras, D.K. Simulation of dye adsorption by beech sawdust as affected by pH. J. Hazard. Mater. 2007, 141, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Soltani, A.; Faramarzi, M.; Parsa, S.A.M. A review on adsorbent parameters for removal of dye products from industrial wastewater. Water Qual. Res. J. 2021, 56, 181–193. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Chen, C. Insights into key factors controlling GO stability in natural surface waters. J. Hazard. Mater. 2017, 335, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Khatib, K.; Lahmyed, L.; El Azhari, M. Synthesis, Characterization, and Application of Geopolymer/TiO2 Nanoparticles Composite for Efficient Removal of Cu(II) and Cd(II) Ions from Aqueous Media. Minerals 2022, 12, 1445. [Google Scholar] [CrossRef]
- Bhunia, K.; Chandra, M.; Kumar Sharma, S.; Pradhan, D.; Kim, S.J. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord. Chem. Rev. 2023, 478, 214956. [Google Scholar] [CrossRef]
- De Araujo, C.M.B.; De Assis Filho, R.B.; Baptisttella, A.M.S.; Do Nascimento, G.F.O.; Da Costa, G.R.B.; Carvalho, M.N.; Ghislandi, M.G.; Sobrinho, M.A.D.M. Systematic study of graphene oxide production using factorial design techniques and its application to the adsorptive removal of methylene blue dye in aqueous medium. Mater. Res. Express 2018, 5, 065042. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Cui, Y.; Dai, R.; Shan, Z.; Chen, H. Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chem. Eng. J. 2021, 405, 126953. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto White Pine (Pinus durangensis) sawdust: Effect of operating conditions. Sustain. Environ. Res. 2017, 27, 32–40. [Google Scholar] [CrossRef]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Tang, Z.; Bai, X.; Zhang, J.; Yu, L.; Cheng, H. Aggregation and resuspension of graphene oxide in simulated natural surface aquatic environments. Environ. Pollut. 2015, 205, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Badeenezhad, A.; Azhdarpoor, A.; Bahrami, S.; Yousefinejad, S. Removal of methylene blue dye from aqueous solutions by natural clinoptilolite and clinoptilolite modified by iron oxide nanoparticles. Mol. Simul. 2019, 45, 564–571. [Google Scholar] [CrossRef]
- An, F.; Liu, J.; Xu, Z.; Zheng, S. Efficient removal of three dyes using porous covalent triazine frameworks: Adsorption mechanism and role of pore distribution. Water Sci. Technol. 2020, 82, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, G.; Huan, Y.; Hao, C.; Chen, W. Acrylic acid functionalized graphene oxide: High-efficient removal of cationic dyes from wastewater and exploration on adsorption mechanism. Chemosphere 2020, 261, 127736. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.A.; Guevara, M.; Tene, T.; Angamarca, P.; Molina, R.; Valarezo, A.; Salguero, O.; Gomez, C.V.; Arias, M.; Caputi, L.S. The adsorption of methylene blue on eco-friendly reduced graphene oxide. Nanomaterials 2020, 10, 681. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Mercante, L.A.; Facure, M.H.M.; Locilento, D.A.; Sanfelice, R.C.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Solution blow spun PMMA nanofibers wrapped with reduced graphene oxide as an efficient dye adsorbent. New J. Chem. 2017, 41, 9087–9094. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Qi, Y.; Sun, W.; Men, Y. Preparation of κ-carrageenan/graphene oxide gel beads and their efficient adsorption for methylene blue. J. Colloid Interface Sci. 2017, 506, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, M.; Han, J.; Liu, K.; Liu, M.; Wu, Q. Syntheses of magnetic GO @ melamine formaldehyde resin for dyes adsorption. Mater. Res. Express 2019, 6, 086103. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Li, X.; Ning, J.; Zhou, Z.; Li, G. Efficient adsorption of methylene blue from aqueous solution by graphene oxide modified persimmon tannins. Mater. Sci. Eng. C 2020, 108, 110196. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene-carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Pourzare, K.; Farhadi, S.; Mansourpanah, Y. Graphene oxide/CO3O4 nanocomposite: Synthesis, characterization, and its adsorption capacity for the removal of organic dye pollutants from water. Acta Chim. Slov. 2017, 64, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Bulin, C. Facile fabrication of MgO/graphene oxide composite as an efficient adsorbent for rapid removal of aqueous organic dyes: Performance evaluation and mechanistic investigation. J. Phys. Chem. Solids 2021, 158, 110251. [Google Scholar] [CrossRef]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene oxide/Fe3O4/chitosan nanocomposite: A recoverable and recyclable adsorbent for organic dyes removal. Application to methylene blue. Mater. Res. Express 2017, 4, 035701. [Google Scholar] [CrossRef]
- Allouss, D.; Essamlali, Y.; Amadine, O.; Chakir, A.; Zahouily, M. Response surface methodology for optimization of methylene blue adsorption onto carboxymethyl cellulose-based hydrogel beads: Adsorption kinetics, isotherm, thermodynamics and reusability studies. RSC Adv. 2019, 9, 37858–37869. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.; Patel, B.; Singh, S.; Hirpara, D.; Rajeswari, V.D.; Kumar, S. A sustainable approach for the adsorption of methylene blue from an aqueous background: An adsorbent based on DES/CGS modified GO@ZrO2. RSC Sustain. 2023, 1, 2038–2057. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Zhang, T.; Zhu, J.; Deng, W.; He, D. Construction and Adsorption Performance Study of GO-CNT/Activated Carbon Composites for High Efficient Adsorption of Pollutants in Wastewater. Polymers 2022, 14, 4951. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward large-scale production of oxidized graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Fierro, X.; Cuenca, M.F. Novel Semiconductor Cu(C3H3N3S3)3/ZnTiO3/TiO2 for the Photoinactivation of E. coli and S. aureus under Solar Light. Nanomaterials 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Fierro, X.; Ramón, J.; Valarezo, E. Cyanide Removal by ZnTiO3/TiO2/H2O2/UVB System: A Theoretical-Experimental Approach. Int. J. Mol. Sci. 2023, 24, 16446. [Google Scholar] [CrossRef] [PubMed]
- Lamaiphan, N.; Sakaew, C.; Sricharoen, P.; Nuengmatcha, P.; Chanthai, S.; Limchoowong, N. Highly efficient ultrasonic-assisted preconcentration of trace amounts of Ag(I), Pb(II), and Cd(II) ions using 3-mercaptopropyl trimethoxysilane-functionalized graphene oxide–magnetic nanoparticles. J. Korean Ceram. Soc. 2021, 58, 314–329. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of point of zero charge of natural organic materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef] [PubMed]
- Benmessaoud, A.; Nibou, D.; Mekatel, E.H.; Amokrane, S. A Comparative Study of the Linear and Non-Linear Methods for Determination of the Optimum Equilibrium Isotherm for Adsorption of Pb2+ Ions onto Algerian Treated Clay. Iran. J. Chem. Chem. Eng. 2020, 39, 153–171. [Google Scholar] [CrossRef]
- Eke-emezie, N.; Etuk, B.R.; Akpan, O.P.; Chinweoke, O.C. Cyanide removal from cassava wastewater onto H3PO4 activated periwinkle shell carbon. Appl. Water Sci. 2022, 12, 157. [Google Scholar] [CrossRef]
- Pirmoradi, M.; Hashemian, S.; Shayesteh, M.R. Kinetics and thermodynamics of cyanide removal by ZnO@NiO nanocrystals. Trans. Nonferrous Met. Soc. China 2017, 27, 1394–1403. [Google Scholar] [CrossRef]
- Noroozi, R.; Al-Musawi, T.J.; Kazemian, H.; Kalhori, E.M.; Zarrabi, M. Removal of cyanide using surface-modified Linde Type-A zeolite nanoparticles as an efficient and eco-friendly material. J. Water Process Eng. 2018, 21, 44–51. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Ind. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X. The Unit Problem in the Thermodynamic Calculation of Adsorption Using the Langmuir Equation. Chem. Eng. Commun. 2014, 201, 1459–1467. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Medina, F. La-doped ZnTiO3/TiO2 nanocomposite supported on ecuadorian diatomaceous earth as a highly efficient photocatalyst driven by solar light. Molecules 2021, 26, 6232. [Google Scholar] [CrossRef] [PubMed]
- Bettoni, M.; Falcinelli, S.; Rol, C.; Rosi, M.; Sebastiani, G.V. Gas-Phase TiO2 Photosensitized Mineralization of Some VOCs: Mechanistic Suggestions through a Langmuir–Hinshelwood Kinetic Approach. Catalysts 2020, 11, 20. [Google Scholar] [CrossRef]
- Jaramillo-Fierro, X.; González, S.; Montesdeoca-Mendoza, F.; Medina, F. Structuring of ZnTiO3/TiO2 adsorbents for the removal of methylene blue, using zeolite precursor clays as natural additives. Nanomaterials 2021, 11, 898. [Google Scholar] [CrossRef] [PubMed]




| Denomination | Equation | Parameters | |
|---|---|---|---|
| Scherrer equation | (1) | D = Crystallite size (nm) λ = Wavelength of the X-ray beam (0.15406 nm) κ = Shape factor (0.89) θ = Bragg angle β = Full width at half peak height maximum (FWHM) of the X-ray diffraction peak | |
| Adsorbate adsorbed | (2) | C0 = Initial concentration (mg L−1) Ce = Equilibrium concentration (mg L−1) w = Mass of the adsorbent (g) v = Volume of the solution (L) | |
| Langmuir | (3) | qmax = Maximum monolayer adsorption (mg g−1) KL = Equilibrium Langmuir constant related to the adsorption energy (L mg−1) Ce = Concentration of adsorbate in solution at equilibrium (mg L−1) | |
| Freundlich | (4) | KF = Freundlich constant (L mg−1) 1/n = Adsorption intensity constant Note: For favorable adsorption, the value of n should be between 1 and 10 | |
| Temkin | (5) | qe = Adsorbate adsorbed per unit weight (mg g−1) at equilibrium A = Temkin isotherm constant (L g−1) Ce = Concentration of adsorbate in solution at equilibrium (mg L−1) B = Constant related to the heat adsorption | |
| Constant of heat adsorption | (6) | b = Temkin constant (J mol−1) T = Absolute temperature (K) R = Gas constant (8.314 J mol−1 K−1) | |
| Separation factor | (7) | KL = Equilibrium Langmuir constant related to the adsorption energy (L mg−1) Ce = Concentration of adsorbate in solution at equilibrium (mg L−1) Note: 0 < RL < 1, suitable adsorption, RL > 1 suitable adsorption, RL = 0 irreversible adsorption, RL = 1 linear adsorption. | |
| Gibbs free energy | (8) | ΔG0 = Gibbs free energy (kJ mol−1), ΔH0 = Enthalpy (kJ mol−1) ΔS0 = Entropy (kJ mol−1 K−1) | |
| Van’t Hoff equation | (9) | kC = Dimensionless parameter T = Absolute temperature (K) R = Universal gas constant (8.314 J mol−1 K−1) | |
| (10) | kL = Langmuir constant (L mg−1) Mw = Adsorbate weight (g mol−1) | ||
| Pseudo-first-order | (11) | k1 = Rate constant (min−1) qe = Adsorbate adsorbed per unit weight (mg g−1) at equilibrium qt = Adsorbate adsorbed per unit weight (mg g−1) at any time (t) | |
| Pseudo-second-order | (12) | k2 = Rate constant (g mg−1 min−1) qe = Adsorbate adsorbed per unit weight (mg g−1) at equilibrium qt = Adsorbate adsorbed per unit weight (mg g−1) at any time (t) | |
| Elovich | (13) | qt = Adsorbate adsorbed per unit weight (mg g−1) at any time (t) α = Constant related to chemisorption rate β = Constant which depicts the extent of surface coverage | |
| Intraparticle diffusion | (14) | k3 = Intraparticle diffusion rate constant (mg g−1 min−1/2) A = Constant indicating the width of the boundary layer (mg g−1). The larger the value of A, the greater the boundary layer effect. | |
| Particle diffusion | (15) | qe = Adsorbate adsorbed per unit weight (mg g−1) at equilibrium qt = Adsorbate adsorbed per unit weight (mg g−1) at any time (t) Cz = Ion concentration of the adsorbent (mg kg−1). Dp = Diffusion coefficient in the adsorbent phase (m2 min−1) r = Average radius of the adsorbent particles (1 × 10−7 m) t = Contact time (min) | |
| External film diffusion | (16) | qe = Adsorbate adsorbed per unit weight (mg g−1) at equilibrium qt = Adsorbate adsorbed per unit weight (mg g−1) at any time (t) Df = Diffusion in the film phase surrounding the adsorbent particles (m2 min−1) Cs = Ion concentration in the solution (mg L−1) h = Film thickness around the adsorbent particles (10−6 m in poorly stirred solutions) r = Average radius of the adsorbent particles (1 × 10−7 m) t = Contact time (min) | |
| Langmuir–Hinshelwood equation | (17) | k = Actual rate constant (min−1) K = Adsorption constant of the substrate on the nanoparticles C0 = Initial concentration of the substrate (mg L−1) Ct = Concentration at a specific time (mg L−1) kapp = Apparent rate constant (min−1) |
| Peak | Assignment | GO (cm−1) | GO/ZTO/TO (cm−1) | ZTO/TO (cm−1) |
|---|---|---|---|---|
| O-H | Stretching | Not prominent | 3400 | 3400 |
| C-H/TiO2 | Stretching | Not prominent | 2920 | 2920 |
| C=O | Stretching | 1720 | Not prominent | Not observed |
| C-O | Stretching/Carbonyl | ~1220–1300 | ~1220–1300 | ~1220–1300 |
| C=C | Stretching | 1620 | 1620 | Not observed |
| Ti-O | Metal–Oxygen bonds | Not observed | Not prominent | ~500 |
| Zn-O | Metal–Oxygen bonds | Not observed | Not prominent | ~500 |
| Peak | Assignment | GO/ZTO/TO (cm−1) | MB-GO/ZTO/TO (cm−1) |
|---|---|---|---|
| Ti-O | Metal–Oxygen bonds | Not prominent | ~500 |
| Zn-O | Metal–Oxygen bonds | Not prominent | ~500 |
| C-H | Bending vibrations | Not observed | 835 |
| S=O | Stretching vibrations | Not observed | 1060–1244 |
| C-O | Stretching/Carbonyl | 1220–1300 | 1220–1300 |
| C-N/N-H | Stretching and bending vibrations | Not observed | 1354–1444 |
| C=C | Stretching | 1620 | Not prominent |
| C=O | Stretching vibrations | 1720 | 1748 |
| C-H/O-H | Stretching vibrations | 3400–3600 | 3400–3600 |
| Isotherm Parameters | 293.15 K | 303.15 K | 313.15 K | |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 77.95 (±2.15) | 86.69 (±1.95) | 95.97 (±2.56) |
| KL (L mg−1) | 0.04 (±0.03) | 0.08 (±0.02) | 0.22 (±0.04) | |
| RL | 0.56 | 0.38 | 0.19 | |
| χ2 | 2.57 | 2.36 | 2.97 | |
| R2 | 0.98 | 0.97 | 0.99 | |
| Freundlich | KF (L mg−1) | 18.98 (±2.18) | 21.11 (±2.87) | 23.37 (±2.34) |
| N | 2.39 (±0.36) | 2.66 (±0.40) | 2.94 (±0.61) | |
| 1/n | 0.42 | 0.38 | 0.34 | |
| χ2 | 2.84 | 3.14 | 2.95 | |
| R2 | 0.94 | 0.93 | 0.95 | |
| Temkin | B | 18.35 (±1.26) | 20.41 (±1.78) | 22.59 (±1.97) |
| A | 1.68 (±0.29) | 1.87 (±0.37) | 2.07 (±0.35) | |
| χ2 | 2.62 | 3.08 | 2.91 | |
| R2 | 0.96 | 0.95 | 0.97 | |
| Temperature (K) | ln kC | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (kJ mol−1 K−1) |
|---|---|---|---|---|
| 293.15 | 11.00 | −26.81 | 64.90 | 0.31 |
| 303.15 | 11.70 | −29.28 | ||
| 313.15 | 12.71 | −33.08 |
| Kinetic Parameters | 293.15 K | |
|---|---|---|
| Pseudo-first-order | qmax (mg g−1) | 11.13 (±0.29) |
| k1 (L mg−1) | 0.03 (±2.92 × 10−3) | |
| χ2 | 0.50 | |
| R2 | 0.96 | |
| Pseudo-second-order | qmax (mg g−1) | 12.58 (±0.16) |
| k2 (L mg−1) | 2.74 × 10−3 (±1.89 × 10−4) | |
| χ2 | 0.06 | |
| R2 | 1.00 | |
| Elovich | α | 30.82 (±5.61) |
| β | 0.02 (±9.39 × 10−4) | |
| χ2 | 6.34 | |
| R2 | 0.98 | |
| Intraparticle diffusion | k (mg g−1 min−1/2) | 0.51 (±0.06) |
| A | 3.24 (±0.85) | |
| R2 | 0.82 | |
| External film diffusion | Df (m2 min−1) | 9.90 × 10−12 |
| R2 | 0.83 | |
| Internal pore diffusion | Dp (m2 min−1) | 7.24 × 10−18 |
| R2 | 0.91 | |
| Adsorbent | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|
| Reduced GO | 68 | [87] |
| Multi-wall Carbon Nanotube | 48 | [88] |
| PMMA-rGO | 699 | [89] |
| κ-Carrageenan/GO gel beads | 658 | [90] |
| Fe3O4/GO@MF | 418 | [91] |
| PT-GO | 257 | [92] |
| GO/calcium alginate | 182 | [93] |
| Graphene–carbon nanotube | 82 | [94] |
| Graphene nanosheet/Fe3O4 | 44 | [95] |
| GO/Co3O4 | 40 | [96] |
| MgO/GO | 172 | [97] |
| CS/Fe3O4/GO | 30 | [98] |
| CMC-Alg/GO | 45 | [99] |
| GO@ZrO2 | 23 | [100] |
| GO-CNT/AC | 175 | [101] |
| GO/ZnTiO3/TiO2 | 78 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaramillo-Fierro, X.; Cuenca, G. Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite. Int. J. Mol. Sci. 2024, 25, 4367. https://doi.org/10.3390/ijms25084367
Jaramillo-Fierro X, Cuenca G. Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite. International Journal of Molecular Sciences. 2024; 25(8):4367. https://doi.org/10.3390/ijms25084367
Chicago/Turabian StyleJaramillo-Fierro, Ximena, and Guisella Cuenca. 2024. "Enhancing Methylene Blue Removal through Adsorption and Photocatalysis—A Study on the GO/ZnTiO3/TiO2 Composite" International Journal of Molecular Sciences 25, no. 8: 4367. https://doi.org/10.3390/ijms25084367






