Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model
Abstract
1. Introduction
2. Results
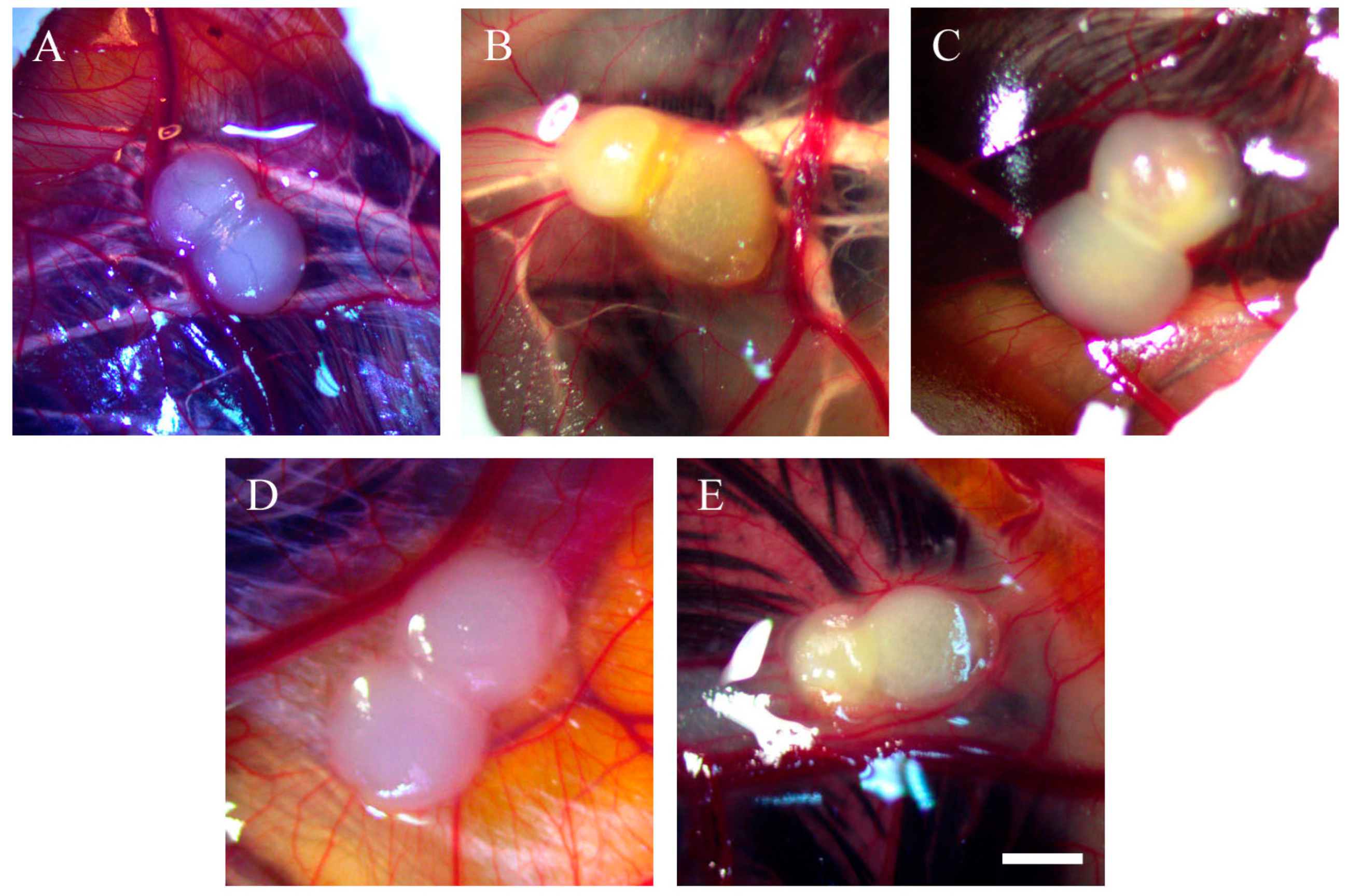
2.1. Morphology of Tumour Implants Grown on CAMs
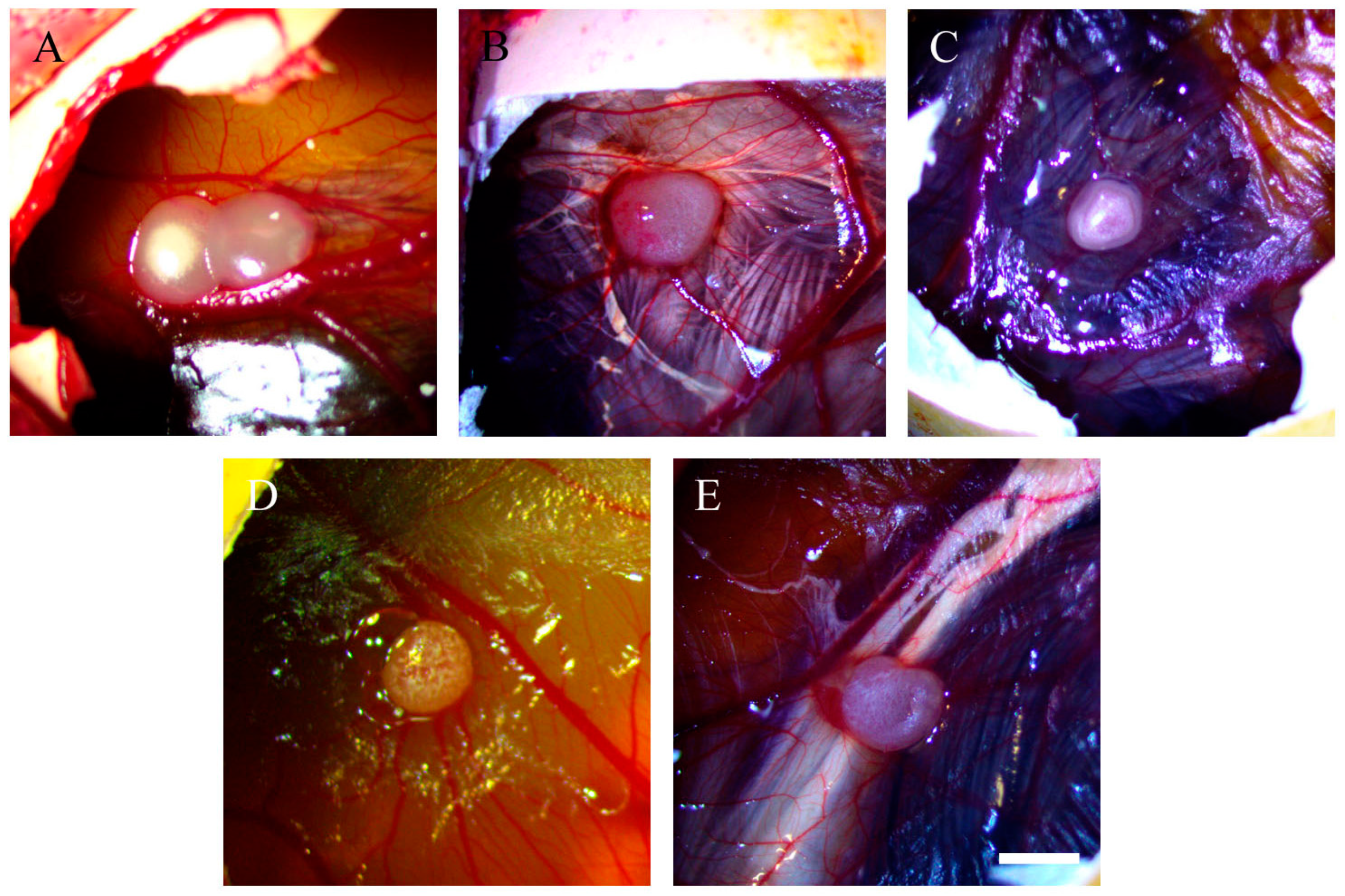
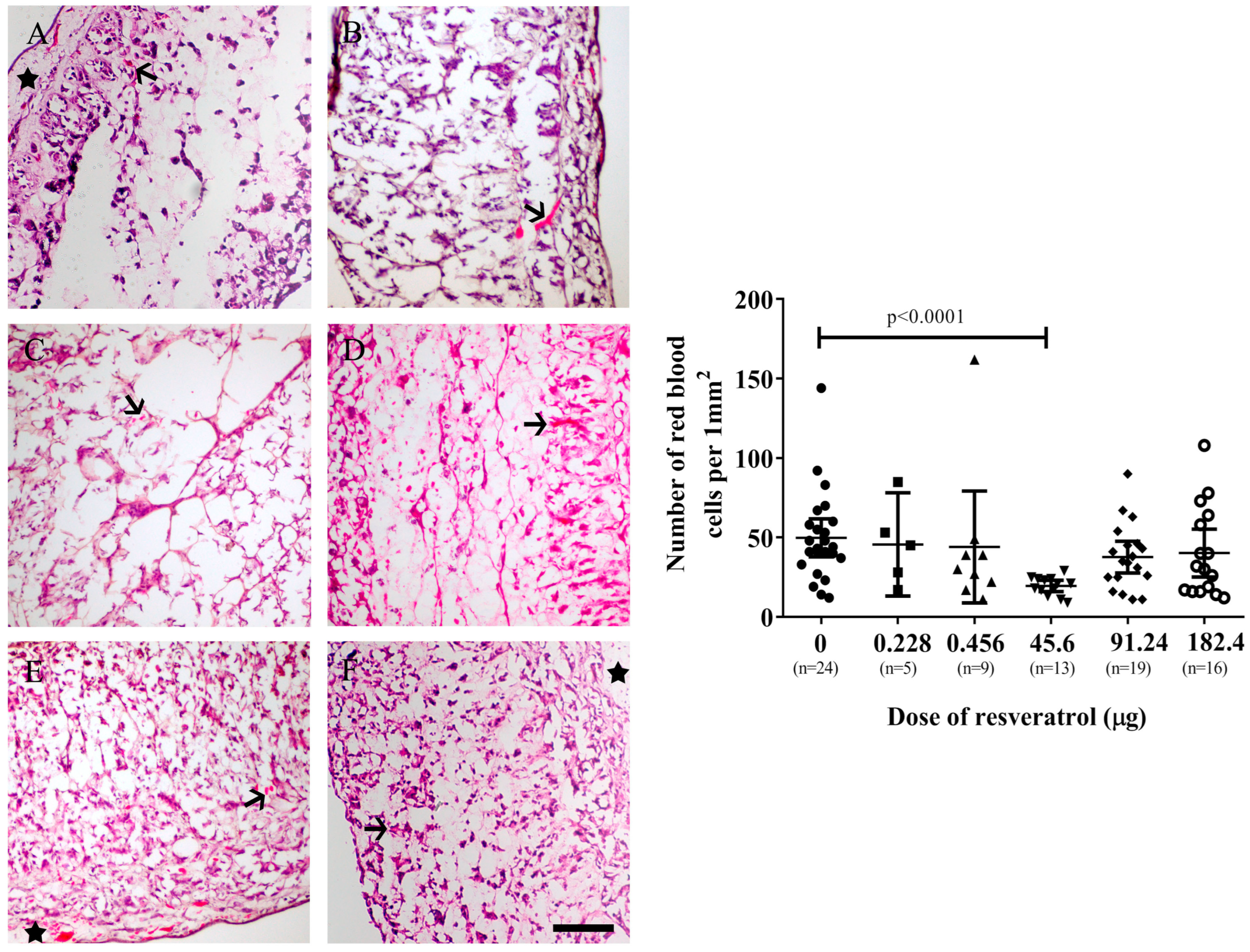
2.2. Resveratrol Affects Vascularisation in CAM Tumour Implants
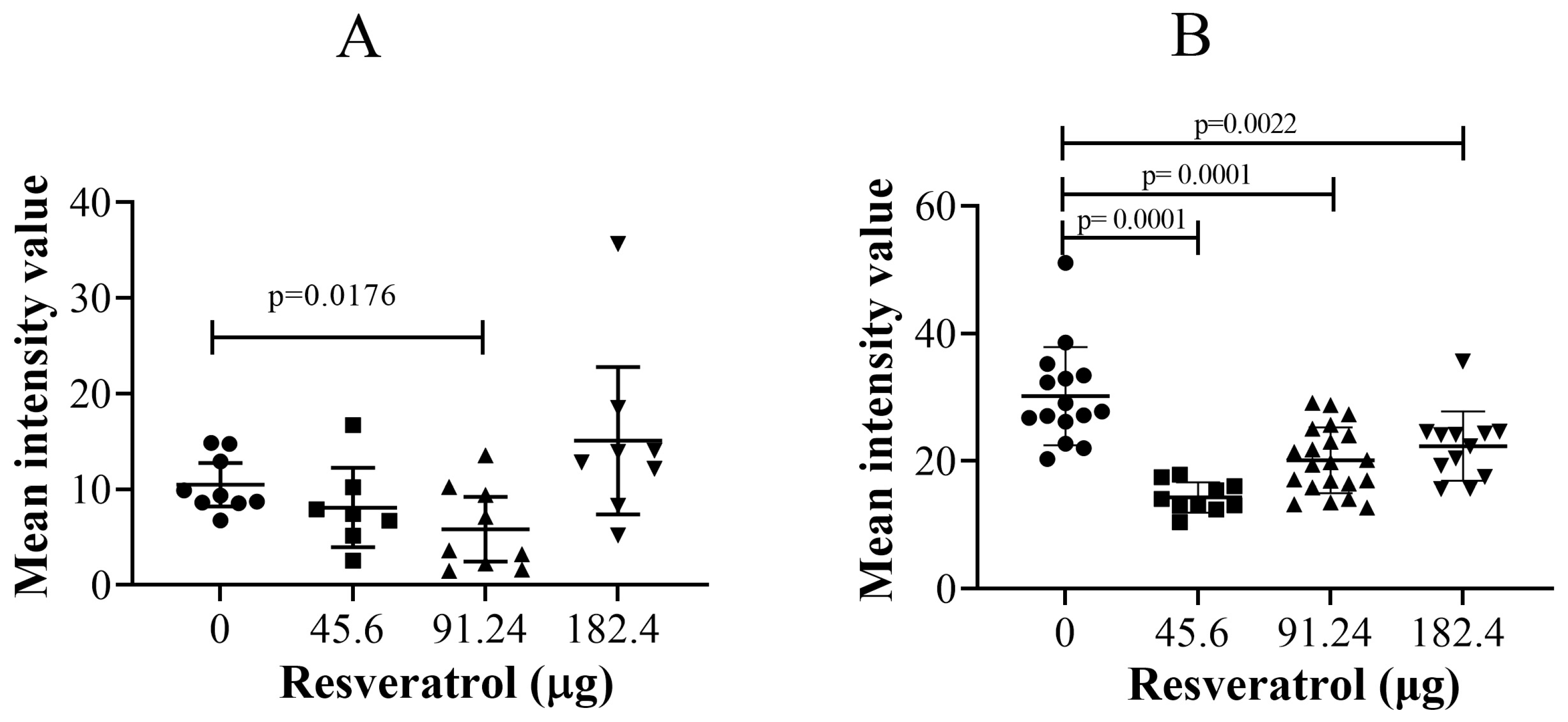
2.3. Resveratrol Did Not Affect Immunohistochemistry Levels of Vascular Endothelial Growth Factor (VEGF) or Ki67 in Tumour Implants
2.4. Resveratrol at Selective Dose Affected pNF-κB Activation and Cancer Cell Invasion in the Histological Sections of Tumour Implants
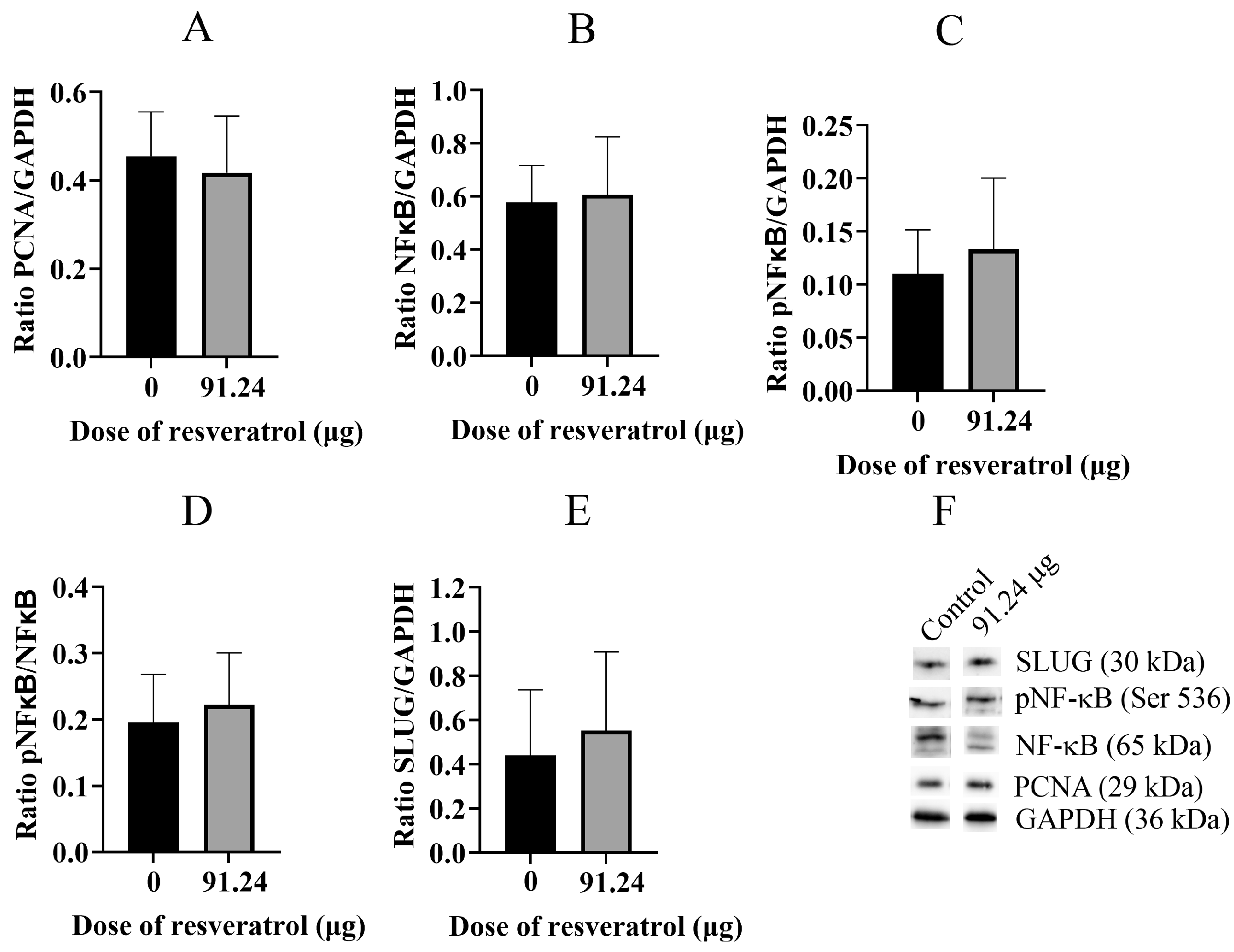
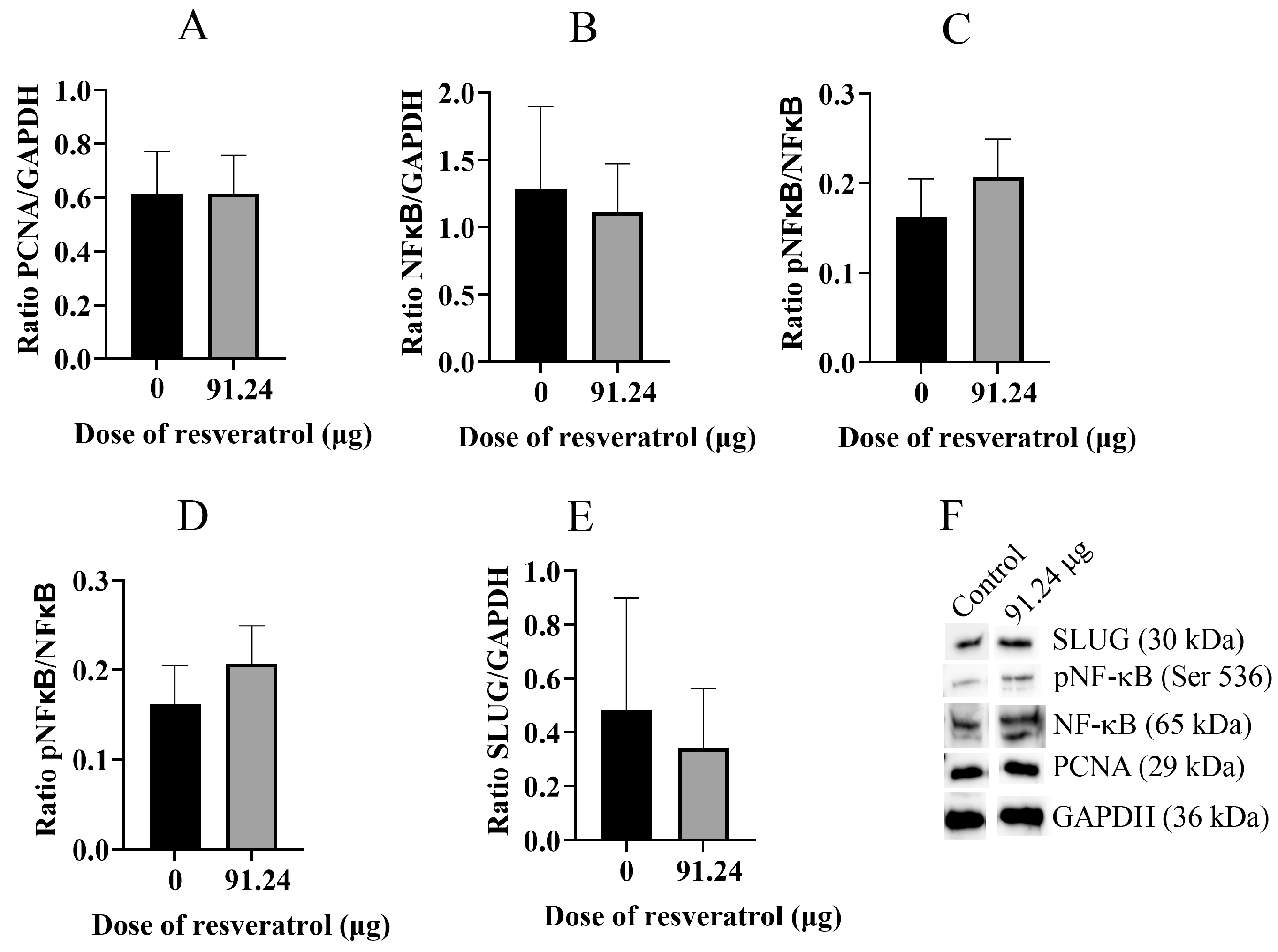
2.5. Resveratrol Does Not Change Total Protein Level of PCNA, NF-κB, pNF-κB, and SLUG
2.6. Immunohistological Fluorescent Staining of SLUG Protein: Resveratrol Significantly Reduced SLUG Proteins in Cancer Cells in the CAM Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Three-Dimensional (3D), Chicken Embryo Model and Resveratrol Treatment
4.3. Collections of Tumour CAMs and Histological and Immunohistological Analysis
4.4. Protein Analysis with Western Blot
4.5. Imaging Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-dependency of resveratrol in providing health benefits. Former. Nonlinearity Biol. Toxicol. Med. 2010, 8, 478–500. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.H.G.; Abd Elmageed, Z.Y.; Zhou, J.; Gaur, R.L.; Nguyen, L.; Azam, G.A.; Braley, P.; Rao, P.N.; Fathi, I.M.; Ouhtit, A. Synergistic action of dietary phyto-antioxidants on survival and proliferation of ovarian cancer cells. Gynecol. Oncol. 2008, 110, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Jeonga, K.J.; Choa, K.H.; Panupinthub, N.; Kimc, H.; Kanga, J.; Parka, C.G.; Millsb, G.B.; Leea, H.Y. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: Inhibition by resveratrol. Mol. Oncol. 2013, 7, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jeong, K.J.; Lee, J.; Yoon, D.S.; Choi, W.S.; Kim, Y.K.; Han, J.W.; Kim, Y.M.; Kim, B.K.; Lee, H.Y. Hypoxia enhances LPA-induced HIF-1a and VEGF expression: Their inhibition by resveratrol. Cancer Lett. 2007, 258, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Simeone, P.; Toraldo, D.; Boccio, P.D.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol downregulates Akt/GSK and ERK signalling pathways in OVCAR-3 ovarian cancer cells. Mol. BioSystems 2012, 8, 1078–1087. [Google Scholar] [CrossRef]
- Björklund, M.; Roos, J.; Gogvadze, V.; Shoshan, M. Resveratrol induces SIRT1- and energy–stress-independent inhibition of tumor cell regrowth after low-dose platinum treatment. Cancer Chemother. Pharmacol. 2011, 68, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Choi, B.Y.; Kundu, J.K.; Shin, Y.K.; Na, H.; Surh, Y. Resveratrol Suppresses Growth of Human Ovarian Cancer Cells in Culture and in a Murine Xenograft Model: Eukaryotic Elongation Factor 1A2 as a Potential Target. Cancer Res. 2009, 69, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, S.; Wolter, F.; Stein, J.M. Molecular mechanisms of the chemopreventive effects of resveratrol and its analogs in carcinogenesis. Mol. Nutr. Food Res. 2005, 49, 452–461. [Google Scholar] [CrossRef]
- Stakleff, K.S.; Sloan, T.; Blanco, D.; Marcanthony, S.; Booth, T.D.; Bishayee, A. Resveratrol Exerts Differential Effects in Vitro and in Vivo against Ovarian Cancer Cells. Asian Pac. J. Cancer Prev. 2012, 13, 1333–1340. [Google Scholar] [CrossRef]
- Osmond, G.W.; Masko, E.M.; Tyler, D.S.; Freedland, S.J.; Pizzo, S. In vitro and in vivo evaluation of resveratrol and 3,5-dihydroxy-4′-acetoxy-trans-stilbene in the treatment of human prostate carcinoma and melanoma. J. Surg. Res. 2013, 179, E141–E148. [Google Scholar] [CrossRef]
- Wang, T.T.Y.; Hudson, T.S.; Wang, T.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; et al. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Chapter Two: Chick Embryo Chorioallantoic Membrane Models to Quantify Angiogenesis Induced by Inflammatory and Tumor Cells or Purified Effector Molecules. Methods Enzym. 2008, 444, 21–41. [Google Scholar] [CrossRef]
- Durupt, F.; Koppers-Lalic, D.; Balme, B.; Budel, L.; Terrier, O.; Lina, B.; Thomas, L.; Hoeben, R.C.; Rosa-Calatrava, M. The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses. Cancer Gene Ther. 2012, 19, 58–68. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Makanya, A.N.; Dimova, I.; Koller, T.; Styp-Rekowska, B.; Djonov, V. Dynamics of the Developing Chick Chorioallantoic Membrane Assessed by Stereology, Allometry, Immunohistochemistry and Molecular Analysis. PLoS ONE 2016, 11, e0152821. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, S.; Feng, Y.; Zhang, J.; Du, Y.; Zhang, J.; Ongeval, C.V.; Ni, Y.; Li, Y. Utilisation of Chick Embryo Chorioallantoic Membrane as a Model Platform for Imaging-Navigated Biomedical Research. Cells 2021, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Lokman, N.A.; Elder, A.S.F.; Ricciardelli, C.; Oehler, M.K. Chick Chorioallantoic Membrane (CAM) Assay as an In Vivo Model to Study the Effect of Newly Identified Molecules on Ovarian Cancer Invasion and Metastasis. Int. J. Mol. Sci. 2012, 13, 9959–9970. [Google Scholar] [CrossRef] [PubMed]
- Vu, B.T.; Shahin, S.A.; Croissant, J.; Fatieiev, Y.; Matsumoto, K.; Doan, T.L.; Yik, T.; Simargi, S.; Conteras, A.; Ratliff, L.; et al. Chick chorioallantoic membrane assay as an in vivo model to study the effect of nanoparticle-based anticancer drugs in ovarian cancer. Sci. Rep. 2018, 8, 8524. [Google Scholar] [CrossRef] [PubMed]
- Tino, A.B.; Chitcholtan, K.; Sykes, P.H.; Garrill, A. Resveratrol and acetyl-resveratrol modulate activity of VEGF and IL-8 in ovarian cancer cell aggregates via attenuation of the NF-κB protein. J. Ovarian Res. 2016, 9, 84. [Google Scholar] [CrossRef]
- Ritch, S.J.; Telleria, C.M. The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination. Front. Endocrinol. 2022, 13, 886533. [Google Scholar] [CrossRef]
- Khan, A.A.; Dace, D.S.; Ryazanov, A.G.; Kelly, J.; Apte, R.S. Resveratrol Regulates Pathologic Angiogenesis by a Eukaryotic Elongation Factor-2 Kinase-Regulated Pathway. Am. J. Pathol. 2010, 177, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, L.; Shi, J.; Hou, X.; Zhang, H.; Zhang, X.; An, Q.; Fan, F. Resveratrol inhibits VEGF-induced angiogenesis in human endothelial cells associated with suppression of aerobic glycolysis via modulation of PKM2 nuclear translocation. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Dias, P.F.; Berti, F.V.; Siqueira, J.M., Jr.; Maraschin, M.; Gagliardi, A.R.; Ribeiro-do-Valle, R.M. Trans-Resveratrol Inhibits Early Blood Vessel Formation (Vasculogenesis) without Impairment of Embryonic Growth. J. Pharmacol. Sci. 2008, 107, 118–127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hogg, S.J.; Chitcholtan, K.; Hassan, W.; Sykes, P.H.; Garrill, A. Resveratrol, Acetyl-Resveratrol, and Polydatin Exhibit Antigrowth Activity against 3D Cell Aggregates of the SKOV-3 and OVCAR-8 Ovarian Cancer Cell Lines. Obstet. Gynecol. Int. 2016, 2015, 279591. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A. The effects of resveratrol on the expression of VEGF, TGF-β, and MMP-9 in endometrial stromal cells of women with endometriosis. Sci. Rep. 2021, 11, 6054. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmian, M.; Amidi, F.; Moini, A.; Kashani, L.; Salahi, E.; Danaii-mehrabad, S.; Nashtaei, M.S.; Mojtahedi, M.F.; Esfandyari, S.; Sobhani, A. A randomized exploratory trial to assess the effects of resveratrol on VEGF and TNF-α 2 expression in endometriosis women. J. Repro Immun. 2021, 143, 103248. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz-Mohammadi, R.; Shidfar, F.; Khodaverdi, S.; Arablou, T.; Heidari, S.; Rashidi, N.; Delbandi, A. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J. Cell. Mol. Med. 2021, 25, 1116–1127. [Google Scholar] [CrossRef]
- Menicacci, B.; Margheri, F.; Laurenzana, A.; Chillà, A.; Rosso, M.D.; Giovannelli, L.; Fibbi, G.; Mocali, A. Chronic Resveratrol Treatment Reduces the Proangiogenic Effect of Human Fibroblast “Senescent-Associated Secretory Phenotype” on Endothelial Colony-Forming Cells: The Role of IL8. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 625–633. [Google Scholar] [CrossRef]
- Zagotta, I.; Dimova, E.Y.; Debatin, K.; Wabitsch, M.; Kietzmann, T.; Fischer-Posovszky, P. Obesity and inflammation: Reduced cytokine expression due to resveratrol in a human in vitro model of inflamed adipose tissue. Front. Pharmacol. 2015, 6, 79. [Google Scholar] [CrossRef]
- Thiel, G.; Ulrich, M.; Mukaida, N.; Rösslera, O.G. Resveratrol stimulation induces interleukin-8 gene transcription via NF-κB. Pharmacol. Res. 2018, 134, 238–245. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P.; Calabrese, V. Resveratrol commonly displays hormesis: Occurrence and biomedical significance. Hum. Exp. Toxicol. 2010, 29, 980–1015. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, B.; Mukherjee, S.; Das, D.K. Hormetic response of resveratrol against cardioprotection. Exp. Clin. Cardiol. 2010, 15, e134–e138. [Google Scholar] [PubMed]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Theofanous, D.; Britton, R.G.; Aburido, G.; Pepper, C.; Undru, S.S.; Howells, L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024, 25, 747. [Google Scholar] [CrossRef]
- Ramírez-Garza, S.L.; Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Storniolo, C.E.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients 2018, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Wu, M.; Li, H.; Liu, J.; Lin, L. Efficacy and safety of intraperitoneally administered resveratrol against rat orthotopic ovarian cancers. Cancer Manag. Res. 2019, 11, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Li, Z.; Liu, R.; Liu, S.; Guo, M.; Zhang, M.; Wu, H.; Huang, L. Effects of resveratrol on HIF-1α/VEGF pathway and apoptosis in vitrified duck ovary transplantation. Theriogenology 2023, 210, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kiamehr, P.; Shahidi, M.; Samii, A.; Zaker, F. Dual Effects of Resveratrol on the Expression and Secretion of Angiogenic Factors. Int. J. Mol. Cell. Med. 2022, 11, 16–30. [Google Scholar] [CrossRef]
- Simão, F.; Pagnussat, A.S.; Seo, J.H.; Navaratna, D.; Leung, W.; Lok, J.; Guo, S.; Waeber, C.; Salbego, C.G.; Lo, E.H. Pro-Angiogenic Effects of Resveratrol in Brain Endothelial Cells: Nitric Oxide-Mediated Regulation of Vascular Endothelial Growth Factor and Metalloproteinases. J. Cereb. Blood Flow Metab. 2012, 32, 884–895. [Google Scholar] [CrossRef]
- Kaga, S.; Zhan, L.; Matsumoto, M.; Maulik, N. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J. Mol. Cell. Cardiol. 2005, 39, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Dosiera, C.R.; Erdmana, C.P.; Parkb, J.H.; Schwartza, Z.; Boyana, B.D.; Guldberga, R.E. Resveratrol effect on osteogenic differentiation of rat and human adipose derived stem cells in a 3-D culture environment. J. Mech. Behav. Biomed. Mater. 2012, 11, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Ruhe, C.; Derikito, M.G.; Böse, G.; Sauer, H.; Wartenberg, M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett. 2007, 250, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.K.; Yang, J.W.; Kang, K.W. Bifunctional effect of resveratrol on the expression of ErbB2 in human breast cancer cell. Cancer Lett. 2006, 242, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Provinciali, M.; Re, F.; Donnini, A.; Orlando, F.; Bartozzi, B.; Stasio, G.D.; Smorlesi, A. Effect of Resveratrol on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Int. J. Cancer 2005, 115, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Klink, J.C.; Tewari, A.K.; Masko, E.M.; Antonelli, J.; Febbo, P.G.; Cohen, P.; Dewhirst, M.W.; Pizzo, S.V.; Freedland, S.J. Resveratrol Worsens Survival in SCID Mice with Prostate Cancer Xenografts in a Cell-Line Specific Manner, through Paradoxical Effects on Oncogenic Pathways. Prostate 2013, 73, 754–762. [Google Scholar] [CrossRef]
- Castillo-Pichardo, L.; Cubano, L.A.; Dharmawardhane, S. Dietary grape polyphenol resveratrol increases mammary tumor growth and metastasis in immunocompromised mice. BMC Complement. Altern. Med. 2013, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Brockmueller, A.; Mueller, A.; Shayan, P.; Shakibaei, M. β1-Integrin plays a major role in resveratrol-mediated anti-invasion effects in the CRC microenvironment. Front. Pharmacol. 2022, 13, 978625. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-1-Induced Epithelial-Mesenchymal Transition. Molecules 2019, 24, 1131. [Google Scholar] [CrossRef]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213. [Google Scholar] [CrossRef]
- Lin, C.; Lee, C.; Shih, Y.; Lin, T.; Wang, S.; Lin, Y.; Shih, C. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic. Biol. Med. 2012, 52, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chan, G.K.; Duan, R.; Wang, H.; Kong, X.; Dong, T.T.; Tsim, K. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced Angiogenesis: A Therapy in Treating Colorectal Cancer. Cancers 2019, 11, 1828. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitcholtan, K.; Singh, M.; Tino, A.; Garrill, A.; Sykes, P. Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model. Int. J. Mol. Sci. 2024, 25, 4374. https://doi.org/10.3390/ijms25084374
Chitcholtan K, Singh M, Tino A, Garrill A, Sykes P. Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model. International Journal of Molecular Sciences. 2024; 25(8):4374. https://doi.org/10.3390/ijms25084374
Chicago/Turabian StyleChitcholtan, Kenny, Melanie Singh, Alex Tino, Ashley Garrill, and Peter Sykes. 2024. "Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model" International Journal of Molecular Sciences 25, no. 8: 4374. https://doi.org/10.3390/ijms25084374
APA StyleChitcholtan, K., Singh, M., Tino, A., Garrill, A., & Sykes, P. (2024). Effects of Resveratrol on In Vivo Ovarian Cancer Cells Implanted on the Chorioallantoic Membrane (CAM) of a Chicken Embryo Model. International Journal of Molecular Sciences, 25(8), 4374. https://doi.org/10.3390/ijms25084374






