Scandium Ion-Promoted Electron-Transfer Disproportionation of 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) in Acetonitrile and Its Regeneration Induced by Water
Abstract
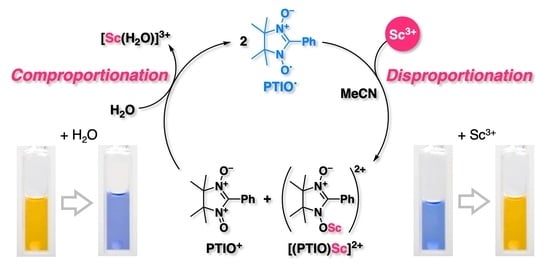
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Spectral and Kinetic Measurements
3.3. Electrochemical Measurements
3.4. EPR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al(OTf)3 | Aluminum triflate (OTf = OSO2CF3) |
| Bu4NClO4 | Tetra-n-butylammonium perchlorate |
| DPPH• | 2,2-Diphenyl-1-picrylhydrazyl |
| DPPH− | One-electron-reduced species of DPPH• |
| DPPH+ | One-electron-oxidized species of DPPH• |
| EPR | Electron paramagnetic resonance |
| EtOH | Ethanol |
| MeCN | Acetonitrile |
| MeOH | Methanol |
| •NO | Nitric oxide |
| PTIO• | 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide |
| PTIO− | One-electron-reduced species of PTIO• |
| PTIO+ | One-electron-oxidized species of PTIO• |
| PTIOH | Protonated form of PTIO− |
| SCE | Saturated calomel electrode |
| Sc(OTf)3 | Scandium triflate (OTf = OSO2CF3) |
| TEMPO | 2,2,6,6-Tetramethylpiperidyl-1-oxyl |
References
- Fukuzumi, S.; Ohkubo, K. Metal ion-coupled and decoupled electron transfer. Coord. Chem. Rev. 2010, 254, 372–385. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Maity, S.; Ghosh, S.; Gomila, R.M.; Frontera, A.; Ghosh, A. Role of redox-inactive metal ions in modulating the reduction potential of uranyl Schiff base complexes: Detailed experimental and theoretical studies. Inorg. Chem. 2022, 61, 7130–7142. [Google Scholar] [CrossRef]
- Mukai, K.; Nakamura, A.; Nagaoka, S.; Ouchi, A.; Azuma, N. Notable effects of the metal salts on the quenching reaction of singlet oxygen by α-tocopherol in ethanol solution. Bull. Chem. Soc. Jpn. 2015, 88, 1503–1510. [Google Scholar] [CrossRef]
- Mukai, K.; Kohno, Y.; Ouchi, A.; Nagaoka, S. Notable effect of metal salts on UV-vis absorption spectra of α-, β-, γ-, and δ-tocopheroxyl radicals in acetonitrile solution. The complex formation between tocopheroxyls and metal cations. J. Phys. Chem. B 2012, 116, 8930–8941. [Google Scholar] [CrossRef]
- Mukai, K.; Oi, M.; Ouchi, A.; Nagaoka, S. Kinetic study of the α-tocopherol-regeneration reaction of ubiquinol-10 in methanol and acetonitrile solutions: Notable effect of the alkali and alkaline earth metal salts on the reaction rates. J. Phys. Chem. B 2012, 116, 2615–2621. [Google Scholar] [CrossRef]
- Kohno, Y.; Fujii, M.; Matsuoka, C.; Hashimoto, H.; Ouchi, A.; Nagaoka, S.; Mukai, K. Notable effects of the metal salts on the formation and decay reactions of α-tocopheroxyl radical in acetonitrile solution. The complex formation between α-tocopheroxyl radical and metal cations. J. Phys. Chem. B 2011, 115, 9880–9888. [Google Scholar] [CrossRef]
- Ouchi, A.; Nagaoka, S.; Abe, K.; Mukai, K. Kinetic study of the aroxyl radical-scavenging reaction of α-tocopherol in methanol solution: Notable effect of the alkali and alkaline earth metal salts on the reaction rates. J. Phys. Chem. B 2009, 113, 13322–13331. [Google Scholar] [CrossRef]
- Ngueumaleu, Y.; Deutchoua, A.D.D.; Hanga, S.S.P.; Liendji, R.W.; Dedzo, G.K.; Ngameni, E. Probing the reactivity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) with metal cations and acids in acetonitrile by electrochemistry and UV-vis spectroscopy. Phys. Chem. Chem. Phys. 2023, 25, 5282–5290. [Google Scholar] [CrossRef]
- Deutchoua, A.D.D.; Ngueumaleu, Y.; Liendji, R.W.; Sonita, S.; Hanga, P.; Nguelo, B.B.; Dedzo, G.K.; Ngameni, E. Unusual reactivity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) with Fe3+ controlled by competing reactions. RSC Adv. 2024, 14, 1354–1359. [Google Scholar] [CrossRef]
- Yuasa, J.; Fukuzumi, S. Off-off-on switching of fluorescence and electron transfer depending on stepwise complex formation of a host ligand with guest metal ions. J. Am. Chem. Soc. 2008, 130, 566–575. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Yuasa, J.; Satoh, N.; Suenobu, T. Scandium ion-promoted photoinduced electron transfer from electron donors to acridine and pyrene. Essential role of scandium ion in photocatalytic oxygenation of hexamethylbenzene. J. Am. Chem. Soc. 2004, 126, 7585–7594. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Fujii, Y.; Suenobu, T. Metal ion-catalyzed cycloaddition vs. hydride transfer reactions of NADH analogues with p-benzoquinone. J. Am. Chem. Soc. 2001, 123, 10191–10199. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, G.; Zhang, D.; Zhu, D. A new tetrathiafulvalene–quinone–tetrathiafulvalene triad: Modulation of the intramolecular charge transfer by the electron-transfer process promoted by metal ions. J. Org. Chem. 2009, 74, 4375–4378. [Google Scholar] [CrossRef]
- Zhao, B.-T.; Peng, Q.-M.; Zhu, X.-M.; Yan, Z.-N.; Zhu, W.-M. Metal-promoted intermolecular electron transfer in tetrathiafulvalene–thiacalix[4]arene conjugates and tetrachlorobenzoquinone. J. Org. Chem. 2015, 80, 1052–1058. [Google Scholar] [CrossRef]
- Mühldorf, B.; Wolf, R. Photocatalytic benzylic C–H bond oxidation with a flavin scandium complex. Chem. Commun. 2015, 51, 8425–8428. [Google Scholar] [CrossRef]
- Sankaralingam, M.; Lee, Y.-M.; Pineda-Galvan, Y.; Karmalkar, D.G.; Seo, M.S.; Jeon, S.H.; Pushkar, Y.; Fukuzumi, S.; Nam, W. Redox reactivity of a mononuclear manganese-oxo complex binding calcium ion and other redox-inactive metal ions. J. Am. Chem. Soc. 2019, 141, 1324–1336. [Google Scholar] [CrossRef]
- Kojima, T.; Kobayashi, R.; Ishizuka, T.; Yamakawa, S.; Kotani, H.; Nakanishi, T.; Ohkubo, K.; Fukuzumi, S. Binding of scandium ions to metalloporphyrin–Flavin complexes for long-lived charge separation. Chem. Eur. J. 2014, 20, 15518–15532. [Google Scholar] [CrossRef]
- Kaur, S.; Bera, M.; Santra, A.; Munshi, S.; Sterbinsky, G.E.; Wu, T.; Moonshiram, D.; Paria, S. Effect of redox-inactive metal ion–nickel(III) interactions on the redox properties and proton-coupled electron transfer reactivity. Inorg. Chem. 2022, 61, 14252–14266. [Google Scholar] [CrossRef]
- Devi, T.; Lee, Y.-M.; Nam, W.; Fukuzumi, S. Metal ion-coupled electron-transfer reactions of metal-oxygen complexes. Coord. Chem. Rev. 2020, 410, 213219. [Google Scholar] [CrossRef]
- Liu, U.; Lau, T.-C. Activation of metal oxo and nitrido complexes by Lewis acids. J. Am. Chem. Soc. 2019, 141, 3755–3766. [Google Scholar] [CrossRef]
- Pfaff, F.F.; Kundu, S.; Risch, M.; Pandian, S.; Heims, F.; Pryjomska-Ray, I.; Haack, P.; Metzinger, R.; Bill, E.; Dau, H.; et al. An oxocobalt(IV) complex stabilized by Lewis acid interactions with scandium(III) ions. Angew. Chem. Int. Ed. 2011, 50, 1711–1715. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Quantitative evaluation of Lewis acidity of metal ions derived from the g values of ESR spectra of superoxide: Metal ion complexes in relation to the promoting effects in electron transfer reactions. Chem. Eur. J. 2000, 6, 4532–4535. [Google Scholar] [CrossRef]
- Nakanishi, I.; Kawashima, T.; Ohkubo, K.; Waki, T.; Uto, Y.; Kamada, T.; Ozawa, T.; Matsumoto, K.; Fukuzumi, S. Disproportionation of a 2,2-diphenyl-1-picrylhydrazyl radical as a model of reactive oxygen species catalysed by Lewis and/or Brønsted acids. Chem. Commun. 2014, 50, 814–816. [Google Scholar] [CrossRef]
- Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Ito, H.; Fukuzumi, S. Water-induced regeneration of a 2,2-diphenyl-1-picrylhydrazyl radical after its scandium ion-promoted electron-transfer disproportionation in an aprotic medium. Molecules 2023, 28, 5002. [Google Scholar] [CrossRef]
- Jiang, S.; Xie, Y.; Xie, Y.; Yu, L.-J.; Yan, X.; Zhao, F.-G.; Mudugamuwa, C.J.; Coote, M.L.; Jia, X.; Zhang, K. Lewis acid-induced reversible disproportionation o TEMPO enables aqueous aluminum radical batteries. J. Am. Chem. Soc. 2023, 145, 14519–14528. [Google Scholar] [CrossRef]
- Akaike, T.; Maeda, H. Quantitation of nitric oxide using 2-phenyl-4,4,5,5-tetramethylimizazoline-1-oxyl 3-oxide (PTIO). Methods Enzymol. 1996, 268, 211–221. [Google Scholar]
- Akaike, T.; Yoshida, M.; Miyamoto, Y.; Sato, K.; Kohno, M.; Sasamoto, K.; Miyazaki, K.; Ueda, S.; Maeda, H. Antagonistic action of imidazolineoxyl N-oxide against endothelium-derived relaxing factor/•NO through a radical reaction. Biochemistry 1993, 32, 827–832. [Google Scholar] [CrossRef]
- Sueishi, Y.; Hori, M.; Kita, M.; Kotake, Y. Nitric oxide (NO) scavenging capacity of natural antioxidants. Food Chem. 2011, 129, 866–870. [Google Scholar] [CrossRef]
- Fricker, S.P. Nitric oxide scavengers as a therapeutic approach to nitric oxide mediated disease. Expert Opin. Invesitig. Drugs 1999, 8, 1209–1222. [Google Scholar] [CrossRef]
- Kovaleva, V.D.; Uzdensky, A.B. Photodynamic therapy-induced nitric oxide production in neuronal and glial cells. J. Biomed. Opt. 2016, 21, 105005. [Google Scholar] [CrossRef]
- Perlikowski, D.; Lechowicz, K.; Pawłowicz, I.; Arasimonwicz-Jelonek, M.; Kosmala, A. Scavenging of nitric oxide up-regulates photosynthesis under drought in Festuca arundinacea and F. glaucescens but reduces their drought tolerance. Sci. Rep. 2022, 12, 6500. [Google Scholar] [CrossRef]
- Fu, J.; Chu, X.; Sun, Y.; Miao, Y.; Xu, Y.; Hu, T. Nitric oxide mediates 5-aminolevulinic acid-induced antioxidant defense in leaves of Elymus nutans griseb. exposed to chilling stress. PLoS ONE 2015, 10, e0130367. [Google Scholar] [CrossRef]
- Goldstein, S.; Russo, A.; Samuni, A. Reactions of PTIO and carboxy-PTIO with •NO, •NO2, and O2•−. J. Biol. Chem. 2003, 278, 50949–50955. [Google Scholar] [CrossRef]
- Joseph, J.; Kalyanaraman, B.; Hyde, J.S. Trapping of nitric oxide by nitronyl nitroxides: An electron spin resonance investigation. Biochem. Biophys. Res. Commun. 1994, 192, 926–934. [Google Scholar] [CrossRef]
- Duan, W.; Vemuri, R.S.; Milshtein, J.D.; Laramie, S.; Dmello, R.D.; Huang, J.; Zhang, L.; Hu, D.; Vijayakumar, M.; Wang, W.; et al. A symmetric organic-based nonaqueous redox flow battery and its state of charge diagnostic by FTIR. J. Mater. Chem. A 2016, 4, 5448–5456. [Google Scholar] [CrossRef]
- Tkacheva, A.; Sun, B.; Zhang, J.; Wang, G.; McDonagh, M. Nitronyl nitroxide-based redox mediators for Li-O2 batteries. J. Phys. Chem. C 2021, 125, 2824–2830. [Google Scholar] [CrossRef]
- Sinclair, N.S.; Poe, D.; Savinell, R.F.; Maginn, E.J.; Wainright, J.S. A nitroxide containing organic molecule in a deep eutectic solvent for flow battery applications. J. Electrochem. Soc. 2021, 168, 020527. [Google Scholar] [CrossRef]
- Zou, X.; Wu, Q.; Li, Z.; Zhang, S.; Dong, L.; Chen, Y.; Dai, Y.; Ji, C.; Liang, H.; Lin, X. Lactiplantibacillus plantarum A72, a strain with antioxidant properties, obtained through ARTP mutagenesis, affects Caenorhabditis elegans anti-aging. Foods 2024, 13, 924. [Google Scholar] [CrossRef]
- Li, X.; Han, W.; He, G.; Yang, J.; Li, J.; Ma, H.; Wang, S. Hydrogel-transformable antioxidant poly-γ-glutamic acid/polyethyleneimine hemostatic powder for efficient wound hemostasis. Gels 2024, 10, 68. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, H.; Xiao, Y.; Kou, X.; Chem, F.; Meng, Q.; Gao, W. A novel water-soluble polysaccharide from daylily (Hemerocallis citrina baroni): Isolation, structure analysis, and probiotics adhesion promotion effect. Food 2024, 13, 721. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, X.; Chen, B.; Liu, S.; Zeng, J. Linkage and stereochemistry characters of phenolic antioxidant product formation. J. Agric. Food Chem. 2023, 71, 5382–5390. [Google Scholar]
- Lu, H.; Wei, J.; Liu, K.; Li, Z.; Xu, T.; Yang, D.; Gao, Q.; Xiang, H.; Li, G.; Chen, Y. Radical-scavenging and subchondral bone-regenerating nanomedicine for osteoarthritis treatment. ACS Nano 2023, 17, 6131–6146. [Google Scholar] [CrossRef]
- Wang, J.; Xue, X.; Miao, X. Antioxidant effects of quercetin nanocrystals in nanosuspension against hydrogen peroxide-induced oxidative stress in a zebrafish model. Pharmaceuticals 2023, 16, 1209. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, Y.; Dong, Y.; Zhang, W.; Xia, F.; Bai, H.; Stevanovic, Z.D.; Li, H.; Shi, L. Effective improvement of the oxidative stability of Acer truncatum bunge seed oil, a new woody oil food resource, by rosemary extract. Antioxidants 2023, 12, 889. [Google Scholar] [CrossRef]
- Gong, J.; Liu, Q.; Cai, L.; Yang, Q.; Tong, Y.; Chen, X.; Kotha, S.; Mao, X.; He, W. Multimechanism collaborative superior antioxidant CDzymes to alleviate salt stress-induced oxidative damage in plant growth. ACS Sustain. Chem. Eng. 2023, 11, 4237–4247. [Google Scholar]
- Wang, Y.; Huo, L.; Jia, Q.Q.; Jin, L. Preparation and evaluation of antioxidant activity of citric acid-polyethylene glycol-tannin elastomers. Colloid Polym. Sci. 2023, 301, 1351–1363. [Google Scholar]
- Chen, B.; Li, X.; Ouyang, X.; Liu, J.; Liu, Y.; Chen, D. Comparison of ferroptosis-inhibitory mechanisms between ferrostatin-1 and dietary stilbene (piceatannol and astringin). Molecules 2021, 26, 1092. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Hua, Y.; Li, Z.; Chen, B.; Liu, A.; Lu, W.; Zhao, X.; Diao, Y.; Chen, D. Antioxidant product analysis of Hulu Tea (Tadehagi triquetrum). New J. Chem. 2021, 45, 20257–20265. [Google Scholar]
- Li, X.; Zeng, J.; Liu, Y.; Liang, M.; Liu, Q.; Li, Z.; Zhao, X.; Chen, D. Inhibitory effect and mechanism of action of quercetin and quercetin Diels-Alder anti-dimer on erastin-induced ferroptosis in bone marrow-derived mesenchymal stem cells. Antioxidants 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Shi, X.; Wang, J.; Wang, X.; Wang, Q.; Yu, D.; Ge, B.; Zhang, X.; Huang, F. Reactive oxygen species-induced aggregation of nanoqymes for neuron injury. ACS Appl. Mater. Interfaces 2020, 12, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Li, X.; Liu, J.; Liu, Y.; Xie, Y.; Du, Z.; Xie, H.; Chen, X.; Lu, W.; Chen, D. Structure-activity relationship and mechanism of four monostilbenes with respect to ferroptosis inhibition. RSC Adv. 2020, 10, 31171–31179. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chen, B.; Chen, Y.; Dai, W.; Li, Y.; Zhu, M. Covalent bridging of corilagin improves antiferroptosis activity: Comparison with 1,3,6-tri-O-galloyl-β-D-glucopyranose. ACS Med. Chem. Lett. 2020, 11, 2232–2237. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Zhao, X.; Chen, D. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) trapping activity and mechanisms of 16 phenolic xanthones. Molecules 2018, 23, 1692. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Xie, H.; HE, Y.; Zhong, D.; Chen, D. Antioxidant structure-activity relationship analysis of five dihydrochalcones. Molecules 2018, 23, 1162. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Xie, H.; Xie, Y.; Li, Y.; Zhao, X.; Jiang, X.; Chen, D. Antioxidant and cytoprotective effects of the di-O-caffeoylqunic acid family: The mechanism, structure–activity relationship, and conformational effect. Molecules 2018, 23, 222. [Google Scholar] [CrossRef]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, S.; Li, Z.; Li, J.; Xu, Z.; Liu, X.; Huang, X. Tyrosine residues initiated photopolymerization in living organisms. Nat. Commun. 2023, 14, 3598. [Google Scholar] [CrossRef]
- Samuni, U.; Samuni, Y.; Goldstein, S. On the distinction between nitroxyl and nitric oxide using nitronyl nitroxides. J. Am. Chem. Soc. 2010, 132, 8428–8432. [Google Scholar] [CrossRef]
- Israeli, A.; Pratt, M.; Oron, M.; Samuni, A.; Kohen, R.; Goldstein, S. Kinetics and mechanism of the comproportionation reaction between oxoammonium cation and hydroxylamine derived from cyclic nitroxides. Free Radic. Biol. Med. 2005, 38, 317–324. [Google Scholar] [CrossRef]
- Zhang, R.L.; Goldstein, S.; Samuni, A. Kinetics of superoxide-induced exchange among nitroxide antioxidants and their oxidized and reduced forms. Free Radic. Biol. Med. 1999, 26, 1245–1252. [Google Scholar] [CrossRef]
- Zhang, R.; Pinson, A.; Samuni, A. Both hydroxylamine and nitroxide protect cardiomyocytes from oxidative stress. Free Radic. Biol. Med. 1998, 24, 66–75. [Google Scholar] [CrossRef]
- Golubev, V.A.; Sen, V.D.; Kulyk, I.V.; Aleksandrov, A.L. Mechanism of the oxygen disproportionation of di-tert-alkylnitroxyl radicals. Izv. Akad. Nauk SSSR Ser. Khim. 1975, 10, 2235–2243. [Google Scholar]
- Zauche, T.H.; Espenson, J.H. Kinetics of oxidation and comproportionation reactions involving an oxoammonium ion, benzyl alcohol and methyltirioxorhenium(VII). Int. J. Chem. Kinet. 1999, 31, 381–385. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Dikalova, A.E.; Mason, R.P. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch. Biochem. Biophys. 2002, 402, 218–226. [Google Scholar] [CrossRef]
- Sen’, V.D.; Tikhonov, I.V.; Borodin, L.I.; Pliss, E.M.; Golubev, V.A.; Syroeshkin, M.A.; Rusakov, A.I. Kinetics and thermodynamics of reversible disproportionation–comproportionation in redox triad oxoammonium cations-nitroxyl radicals-hydroxylamines. J. Phys. Org. Chem. 2015, 28, 17–24. [Google Scholar] [CrossRef]
- Sen, V.D.; Golubev, V.A. Kinetics and mechanism for acid-catalyzed disproportionation of 2,2,6,6-tetramethylpiperidine-1-oxyl. J. Phys. Org. Chem. 2009, 22, 138–143. [Google Scholar] [CrossRef]
- Mann, C.K.; Barnes, K.K. Electrochemical Reactions in Nonaqueous Systems; Marcel Dekker Inc.: New York, NY, USA, 1990. [Google Scholar]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. Ser. B 1994, 104, 105–110. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoji, Y.; Terashima, Y.; Ohkubo, K.; Ito, H.; Maruyama, K.; Fukuzumi, S.; Nakanishi, I. Scandium Ion-Promoted Electron-Transfer Disproportionation of 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) in Acetonitrile and Its Regeneration Induced by Water. Int. J. Mol. Sci. 2024, 25, 4417. https://doi.org/10.3390/ijms25084417
Shoji Y, Terashima Y, Ohkubo K, Ito H, Maruyama K, Fukuzumi S, Nakanishi I. Scandium Ion-Promoted Electron-Transfer Disproportionation of 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) in Acetonitrile and Its Regeneration Induced by Water. International Journal of Molecular Sciences. 2024; 25(8):4417. https://doi.org/10.3390/ijms25084417
Chicago/Turabian StyleShoji, Yoshimi, Yuri Terashima, Kei Ohkubo, Hiromu Ito, Kouichi Maruyama, Shunichi Fukuzumi, and Ikuo Nakanishi. 2024. "Scandium Ion-Promoted Electron-Transfer Disproportionation of 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-Oxide (PTIO•) in Acetonitrile and Its Regeneration Induced by Water" International Journal of Molecular Sciences 25, no. 8: 4417. https://doi.org/10.3390/ijms25084417