Adsorption of Nitrogen Dioxide on Nitrogen-Enriched Activated Carbons
Abstract
:1. Introduction
2. Results and Discussion
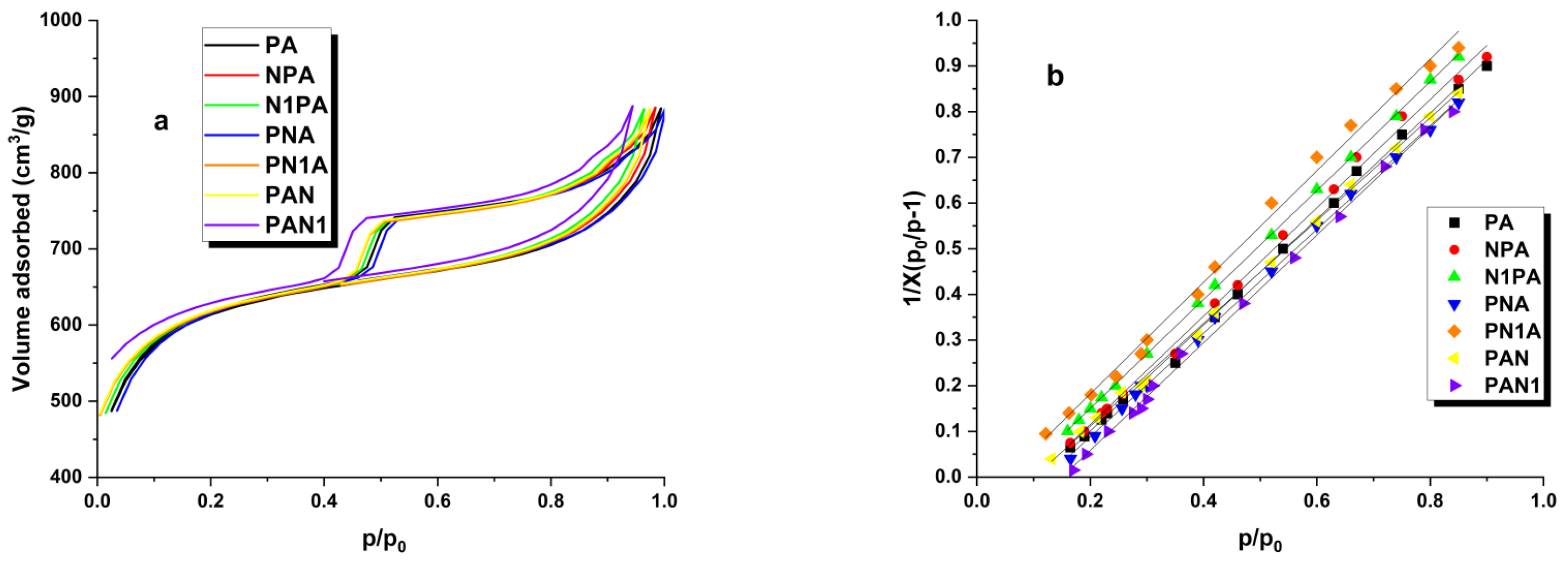
2.1. Characterisation of the Starting Material
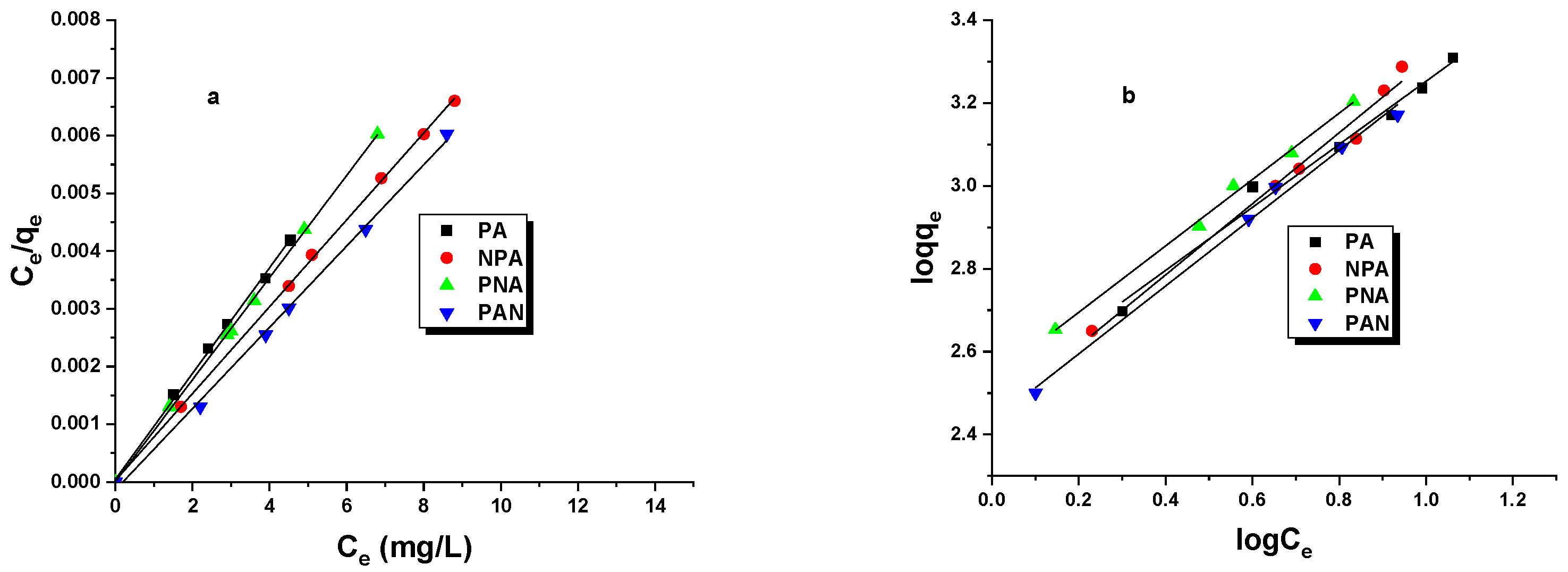
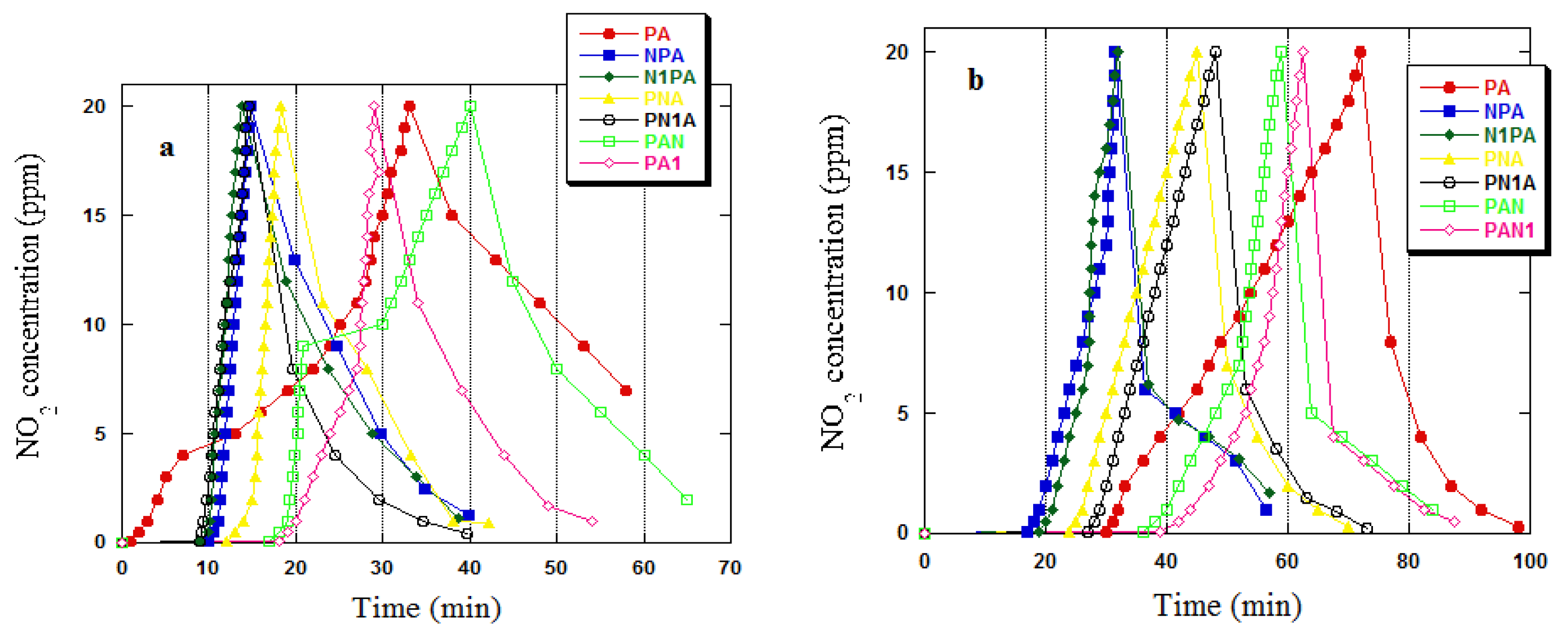
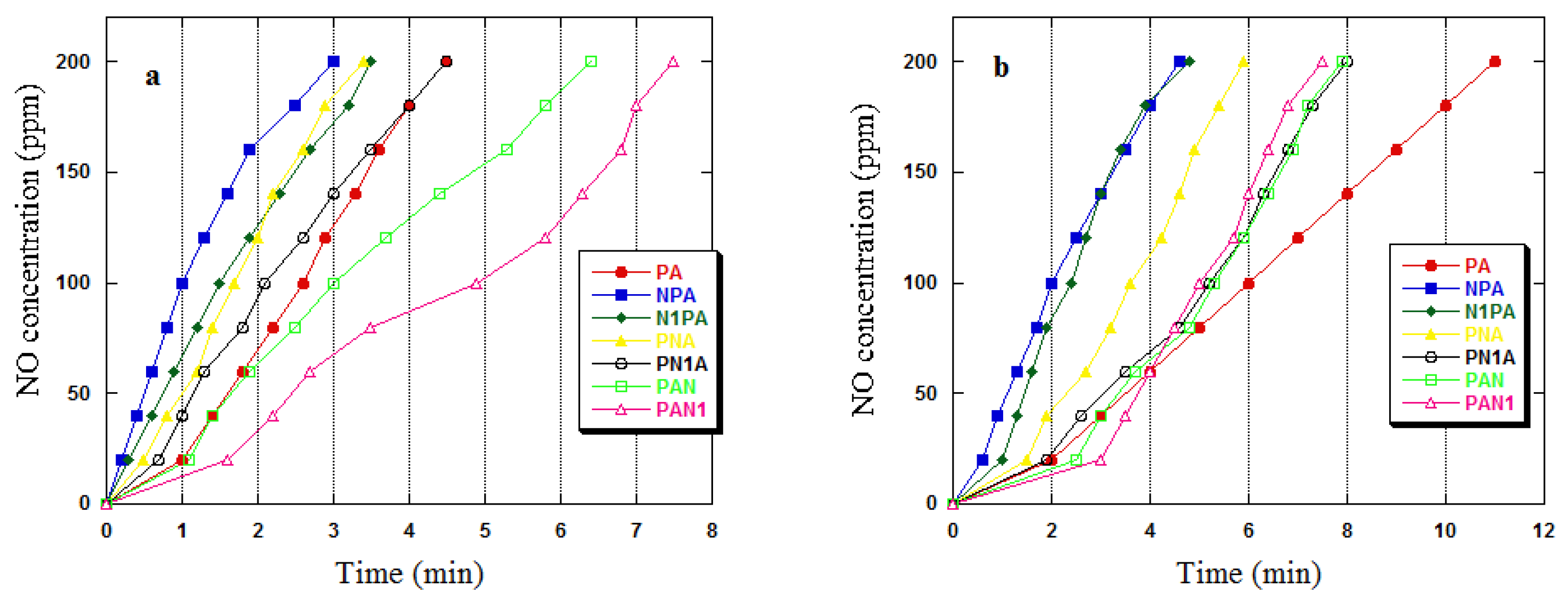
2.2. Adsorption Study Iodine Number/NO2
3. Materials and Methods
3.1. Materials and Activated Carbon Preparation
3.2. Adsorbents Characteristic
3.3. Adsorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Numee, P.; Sangtawesin, T.; Yilmaz, M.; Kanjana, K. Activated carbon derived from radiation-processed durian shell for energy storage application. Carbon Resour. Convers. 2023, 7, 100192. [Google Scholar] [CrossRef]
- Guclu, C.; Alper, K.; Erdem, M.; Tekin, K.; Karagoz, S. Activated carbons from co-carbonization of waste truck tires and spent tea leaves. Sustain. Chem. Pharm. 2021, 21, 100410. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gómez-Avilés, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belve, C. Adsorption of emerging pollutants on lignin-based activated carbon: Analysis of adsorption mechanism via characterization, kinetics and equilibrium studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Shukla, V.; Panchal, D.; Prakash, O.; Mondal, P.; Hiwrale, I.; Dhodapkar, R.S.; Pal, S. Magnetically engineered sulfurized peat-based activated carbon for remediation of emerging pharmaceutical contaminants. Bioresour. Technol. 2023, 369, 128399. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, A.R.; Chinnadurai, D.; Cho, I.; Bak, J.-S.; Prabaka, K. Bio-waste wood-derived porous activated carbon with tuned microporosity for high performance supercapacitors. J. Energy Storage 2022, 52, 104928. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Pietrzak, R.; Jurewicz, K.; Nowicki, P.; Babeł, K.; Wachowska, H. Nitrogen-enriched bituminous coal-based active carbons as materials for supercapacitors. Fuel 2010, 89, 3457–3467. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Ayala-Cortés, A.; Arias, D.M.; Longoria, A.; Cuentas-Gallegos, A.K.; Sebastian, P.J.; Okoye, P.U. Advances in activated carbon modification, surface heteroatom configuration, reactor strategies, and regeneration methods for enhanced wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 105626. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Yang, J. Biomass-derived activated carbon materials with plentiful heteroatoms for high-performance electrochemical capacitor electrodes. J. Energy Chem. 2016, 25, 35–40. [Google Scholar] [CrossRef]
- Shao, Y.; Zha, Z.; Wang, H. Heteroatom-doped porous carbon-supported single-atom catalysts for electrocatalytic energy conversion. J. Energy Chem. 2021, 63, 54–73. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.-J. Self-activated, urea modified microporous carbon cryogels for high-performance CO2 capture and separation. Carbon 2022, 192, 14–29. [Google Scholar] [CrossRef]
- Starck, J.; Burg, P.; Muller, S.; Bimer, J.; Furdin, G.; Fioux, P.; VixGuterl, C.; Begin, D.; Faure, P.; Azambre, B. The influence of demineralisation and ammoxidation on the adsorption properties of an activated carbon prepared from a Polish lignite. Carbon 2006, 44, 2549–2557. [Google Scholar] [CrossRef]
- Mahaninia, M.H.; Mansooreh Soleimani, T.K.; Rahimian, P.; Esfandiari, A. Pre-oxidation effect on ammoxidation of activated carbon and its influence on Cu(II) adsorption. Desalin. Water Treat. 2016, 57, 4597–4605. [Google Scholar] [CrossRef]
- Langhammer, D.; Kullgren, J.; Österlund, L. Adsorption and Oxidation of NO2 on Anatase TiO2: Concerted Nitrate Interaction and Photon-Stimulated Reaction. ACS Catal. 2022, 12, 10472–10481. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Loh, H.; Andiappan, M.; Bristow, A.D. Photocarrier Recombination Dynamics in Highly Scattering Cu2O Nanocatalyst Clusters. J. Phys. Chem. C 2024, 128, 2003–2011. [Google Scholar] [CrossRef]
- Choi, H.-J.; Kwon, S.-H.; Lee, W.-S.; Im, K.-G.; Kim, T.-H.; Noh, B.-R.; Park, S.; Oh, S.; Kim, K.-K. Ultraviolet Photoactivated Room Temperature NO2 Gas Sensor of ZnO Hemitubes and Nanotubes Covered with TiO2 Nanoparticles. Nanomaterials 2020, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Y.; Zhang, G.; Guo, C.; Zhang, X.; Li, B.; Zhou, X.; Zheng, M.; Xu, Y.; Gao, S.; et al. Simple synthesis of Cu/Cu2O/CuO composites with dual adsorption capacity for conductometric NO2 detection. Sens. Actuators B Chem. 2024, 399, 134820. [Google Scholar] [CrossRef]
- Mohammadparast, F.; Ramakrishnan, S.B.; Khatri, N.; Tirumala, R.T.A.; Tan, S.; Kalkan, A.K.; Andiappan, M. Cuprous Oxide Cubic Particles with Strong and Tunable Mie Resonances for Use as Nanoantennas. ACS Appl. Nano Mater. 2020, 3, 6806–6815. [Google Scholar] [CrossRef]
- Gyawali, S.; Tirumala, R.T.A.; Andiappan, M.; Bristow, A.D. Size- and Shape-Dependent Charge-Carrier Dynamics in Sub-micron Cuprous Oxide Nanoparticles. In Frontiers in Optics + Laser Science 2022 (FIO, LS); Technical Digest Series; Optica Publishing Group: Washington, DC, USA, 2022; p. JTu4A.86. [Google Scholar]
- Gyawali, S.; Tirumala, R.T.A.; Andiappan, M.; Bristow, A.D. Carrier dynamics in cuprous oxide-based nanoparticles and heterojunctions. In Proceedings of the SPIE 12884, Ultrafast Phenomena and Nanophotonics XXVIII, San Francisco, CA, USA, 8 March 2024; p. 128840F. [Google Scholar]
- Levasseur, B.; Petit, C.; Bandosz, T.J. Reactive Adsorption of NO2 on Copper-Based Metal−Organic Framework and Graphite Oxide/Metal−Organic Framework Composites. ACS Appl. Mater. Interfaces 2010, 2, 3606–3613. [Google Scholar] [CrossRef]
- Sun, M.; Hanif, A.; Wang, T.; Gu, Q.; Shang, J. Ambient temperature NO2 removal by reversible NO2 adsorption on copper-based metal-organic frameworks (MOFs)-derived nanoporous adsorbents. Sep. Purif. Technol. 2023, 314, 123563. [Google Scholar] [CrossRef]
- Dada, A.O.; Inyinbor, A.A.; Tokula, B.E.; Bello, O.S.; Pal, U. Preparation and characterization of rice husk activated carbon-supported zinc oxide nanocomposite (RHAC-ZnO-NC). Heliyon 2022, 8, e10167. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, T.E.; Odunoye, B.O.; Elehinafe, F.B.; Obanla, O.R.; Odunlami, O.A. Production of activated carbon from sawdust and its efficiency in the treatment of sewage water. Heliyon 2021, 7, e05960. [Google Scholar] [CrossRef] [PubMed]
- Yirdaw, G.; Dessie, A.; Birhan, T.A. Optimization of process variables to prepare activated carbon from Noug (Guizotiaabyssinicacass) stalk using response surface methodology. Heliyon 2023, 9, e17254. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasekar, N.; Baskar, R. Adsorption of Basic Magenta II onto H2SO4 activated immature Gossypium hirsutum seeds: Kinetics, isotherms, mass transfer, thermodynamics and process design. Arab. J. Chem. 2019, 12, 1322–1337. [Google Scholar] [CrossRef]
- Reddy, Y.S.; Rotte, N.K.; Hussain, S.; Srikanth, V.V.S.S.; Chandra, M.R. Sustainable mesoporous graphitic activated carbon as biosorbent for efficient adsorption of acidic and basic dyes from wastewater: Equilibrium, kinetics and thermodynamic studies. J. Hazard. Mater. Adv. 2023, 9, 100214. [Google Scholar] [CrossRef]
- NdjientcheuYossa, L.M.; Ouiminga, S.K.; Sidibe, S.S.; Ouedraogo, I.W.K. Synthesis of a cleaner potassium hydroxide-activated carbon from baobab seeds hulls and investigation of adsorption mechanisms for diuron: Chemical activation as alternative route for preparation of activated carbon from baobab seeds hulls and adsorption of diuron. Sci. Afr. 2020, 9, e00476. [Google Scholar]
- Pietrzak, R. XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 2009, 88, 1871–1877. [Google Scholar] [CrossRef]
- Asnawia, T.M.; Husina, H.; Adisalamuna, A.; Rinaldia, W.; Zakia, M.; Hasfita, F. Activated Carbons from Palm Kernels Shells Prepared by Physical and Chemical Activation for Copper Removal from Aqueous Solution. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012096. [Google Scholar] [CrossRef]
- Nowicki, P.; Kuszyńska, I.; Przepiórski, J.; Pietrzak, R. The effect of chemical activation method on properties of activated carbons obtained from pine cones. Cent. Eur. J. Chem. 2013, 11, 78–85. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Limousy, L.; Bader, N.; Ouederni, A.; Bennici, S. Factors Influencing NO2 Adsorption/Reduction on Microporous Activated Carbon: Porosity vs. Surface Chemistry. Materials 2018, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Belala, Z.; Belhachemi, M.; Jeguirim, M. Activated Carbon Prepared from Date Pits for the Retention of NO2 at Low Temperature. Int. J. Chem. React. Eng. 2014, 12, 717–726. [Google Scholar] [CrossRef]
- Belhachemi, M.; Jeguirim, M.; Limousy, L.; Addoun, F. Comparison of NO2 removal using date pits activated carbon and modified commercialized activated carbon via different preparation methods: Effect of porosity and surface chemistry. Chem. Eng. J. 2014, 253, 121–129. [Google Scholar] [CrossRef]
- Pietrzak, R. Sawdust pellets from coniferous species as adsorbents for NO2 removal. Bioresour. Technol. 2010, 101, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Yen, Y.-J.; Tseng, Y.-H.; Chung, S.-H. Module-Designed Carbon-Coated Separators for High-Loading, High-Sulfur-Utilization Cathodes in Lithium–Sulfur Batteries. Molecules 2022, 27, 228. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak-Razna, J.; Nowicki, P.; Pietrzak, R. Characterization and application of bio-activated carbons prepared by direct activation of hay with the use of microwave radiation. Powder Technol. 2017, 319, 302–312. [Google Scholar] [CrossRef]
- Han, P.; Chung, S.-H.; Manthiram, A. Pyrrolic-Type Nitrogen-Doped Hierarchical Macro/Mesoporous Carbon as a Bifunctional Host for High-Performance Thick Cathodes for Lithium-Sulfur Batteries. Small 2019, 15, 1900690. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Wang, X.; Chen, J.; Fan, M.; Shi, Z.; Liu, J.; Xu, L.; Zang, Y. Construction of hydroxyl-functionalized hyper-crosslinked networks from polyimide for highly efficient iodine adsorption. iScience 2024, 27, 108993. [Google Scholar] [CrossRef]

| Adsorbent | Ash | Moisture | Volatile Compounds |
|---|---|---|---|
| Precursor | 2.5 | 0.9 | 19.4 |
| Precursordem | 0.7 | 0.0 | 17.7 |
| Adsorbent | Cdaf | Hdaf | Ndaf | Sdaf | Odaf * | Ash |
|---|---|---|---|---|---|---|
| Precursor | 88.4 | 4.7 | 1.4 | 0.3 | 5.2 | 2.5 |
| Precursordem | 90.6 | 4.5 | 1.1 | 0.2 | 3.6 | 0.7 |
| PrecursordemN | 84.2 | 4.0 | 6.2 | 0.2 | 5.4 | 0.6 |
| PrecursordemN1 | 82.7 | 3.7 | 8.3 | 0.2 | 5.1 | 0.6 |
| PrecursordemNP | 85.7 | 3.7 | 6.0 | 0.2 | 4.4 | 0.8 |
| PrecursordemN1P | 83.3 | 3.5 | 8.1 | 0.2 | 4.9 | 0.9 |
| NPA | 95.2 | 0.5 | 0.2 | 0.0 | 4.1 | 1.0 |
| N1PA | 96.8 | 0.3 | 0.1 | 0.0 | 2.8 | 1.1 |
| P | 91.0 | 3.5 | 0.4 | 0.3 | 4.8 | 0.9 |
| PN | 88.2 | 3.2 | 2.5 | 0.3 | 5.8 | 0.7 |
| PN1 | 85.9 | 3.0 | 3.6 | 0.3 | 7.2 | 0.7 |
| PNA | 94.4 | 0.9 | 0.5 | 0.0 | 4.2 | 0.9 |
| PN1A | 93.2 | 0.7 | 0.4 | 0.0 | 5.7 | 0.8 |
| PA | 96.6 | 0.5 | 0.1 | 0.0 | 2.8 | 0.8 |
| PAN | 94.9 | 0.4 | 1.3 | 0.0 | 3.4 | 1.1 |
| PAN1 | 94.5 | 0.3 | 1.4 | 0.0 | 3.8 | 1.0 |
| Adsorbent | Surface Area (m2/g) | Micropore Area (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Vmes (cm3/g) | V<1 nm (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|---|---|
| PA | 2207 | 2199 | 1.18 | 1.14 | 0.13 | 0.009 | 2.19 |
| NPA | 2221 | 2177 | 1.21 | 1.17 | 0.18 | 0.007 | 2.27 |
| N1PA | 2185 | 2143 | 1.22 | 1.16 | 0.16 | 0.003 | 2.28 |
| PNA | 1832 | 1800 | 1.11 | 1.01 | 0.18 | 0.004 | 2.35 |
| PN1A | 1799 | 1766 | 1.07 | 0.99 | 0.12 | 0.005 | 2.31 |
| PAN | 2155 | 2132 | 1.14 | 1.07 | 0.15 | 0.003 | 2.10 |
| PAN1 | 2153 | 2128 | 1.12 | 1.08 | 0.17 | 0.001 | 2.09 |
| Adsorbent | Total Content of Groups (mmol/g) | Acidic Groups (mmol/g) | Basic Groups (mmol/g) |
|---|---|---|---|
| PA | 1.74 | 1.03 | 0.71 |
| NPA | 1.45 | 0.66 | 0.79 |
| N1PA | 1.38 | 0.57 | 0.81 |
| PNA | 1.32 | 0.54 | 0.78 |
| PN1A | 1.33 | 0.51 | 0.82 |
| PAN | 1.58 | 0.59 | 0.99 |
| PAN1 | 1.66 | 0.61 | 1.05 |
| Adsorbent/Material | Active Agent | Iodine Number (mg/g) | References |
|---|---|---|---|
| PA | NaOH | 2045 | This study |
| NPA | 1699 | ||
| N1PA | 1721 | ||
| PNA | 1924 | ||
| PN1A | 1934 | ||
| PAN | 2026 | ||
| PAN1 | 2029 | ||
| Rice husk | H3PO4 | 660.4 | [24] |
| Sawdust | H3PO4 | 1628.95 | [25] |
| Noug stalk | H3PO4 | 576 | [26] |
| Immature G. hirsutum seeds | H2SO4 | 510 | [27] |
| Peltophorum pterocarpum Leaves | H3PO4 + H2SO4 | 1508.76 | [28] |
| Baobab seed hulls | KOH | 1854.2 | [29] |
| Bituminous coal | KOH | 1736–2095 | [30] |
| Palm kernel shells | NaOH | 1062.8 | [31] |
| Pine cones | NaOH | 913 | [32] |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| R2 | qmax [mg/g] | KL [L/mg] | R2 | KF [mg/g(L/mg)1/n] | 1/n | |
| PA | 0.989 | 2060 | 0.0011 | 0.936 | 1154.52 | 0.238 |
| NPA | 0.990 | 1941 | 0.0001 | 0.974 | 1040.88 | 0.285 |
| PNA | 0.991 | 2000 | 0.0004 | 0.946 | 981.97 | 0.320 |
| PAN | 0.999 | 2000 | 0.0500 | 0.903 | 1872.08 | 0.052 |
| Adsorbent | Dry Conditions | Wet Conditions | ||
|---|---|---|---|---|
| [mg/g] | [mg/cm3] | [mg/g] | [mg/cm3] | |
| PA | 22.8 | 6.9 | 69.3 | 24.8 |
| NPA | 10.9 | 1.9 | 16.9 | 3.9 |
| N1PA | 10.3 | 1.7 | 17.4 | 3.7 |
| PNA | 11.9 | 2.3 | 28.0 | 6.9 |
| PN1A | 10.8 | 2.1 | 29.8 | 6.8 |
| PAN | 23.0 | 6.8 | 73.3 | 27.4 |
| PAN1 | 23.5 | 6.7 | 75.0 | 27.5 |
| Adsorbent/Material | Active Agent | NO2 [mg/g] | References |
|---|---|---|---|
| PA | NaOH | 69.3 | This study |
| NPA | 16.9 | ||
| N1PA | 17.4 | ||
| PNA | 28.0 | ||
| PN1A | 29.8 | ||
| PAN | 73.3 | ||
| PAN1 | 75.0 | ||
| activated carbon (olive stones) | H3PO4 | 64.5 | [33] |
| activated carbon (olive stones) | NaOH | 79.1 | [33] |
| activated carbon (date stones) | CO2 | 106.9 | [34] |
| NORIT GAC 1240 | steam | 127 | [35] |
| modified GAC | (NH4)2S2O8, N2 | 136 | [36] |
| carbonaceous adsorbent (pine sawdust pellets) | N2 | 45.3 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Cielecka-Piontek, J.; Yilmaz, S.; Pietrzak, R. Adsorption of Nitrogen Dioxide on Nitrogen-Enriched Activated Carbons. Int. J. Mol. Sci. 2024, 25, 4421. https://doi.org/10.3390/ijms25084421
Bazan-Wozniak A, Nosal-Wiercińska A, Cielecka-Piontek J, Yilmaz S, Pietrzak R. Adsorption of Nitrogen Dioxide on Nitrogen-Enriched Activated Carbons. International Journal of Molecular Sciences. 2024; 25(8):4421. https://doi.org/10.3390/ijms25084421
Chicago/Turabian StyleBazan-Wozniak, Aleksandra, Agnieszka Nosal-Wiercińska, Judyta Cielecka-Piontek, Selehattin Yilmaz, and Robert Pietrzak. 2024. "Adsorption of Nitrogen Dioxide on Nitrogen-Enriched Activated Carbons" International Journal of Molecular Sciences 25, no. 8: 4421. https://doi.org/10.3390/ijms25084421





