Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents
Abstract
:1. Introduction
2. Molecular Pathways Involved in Macular Neovascularization
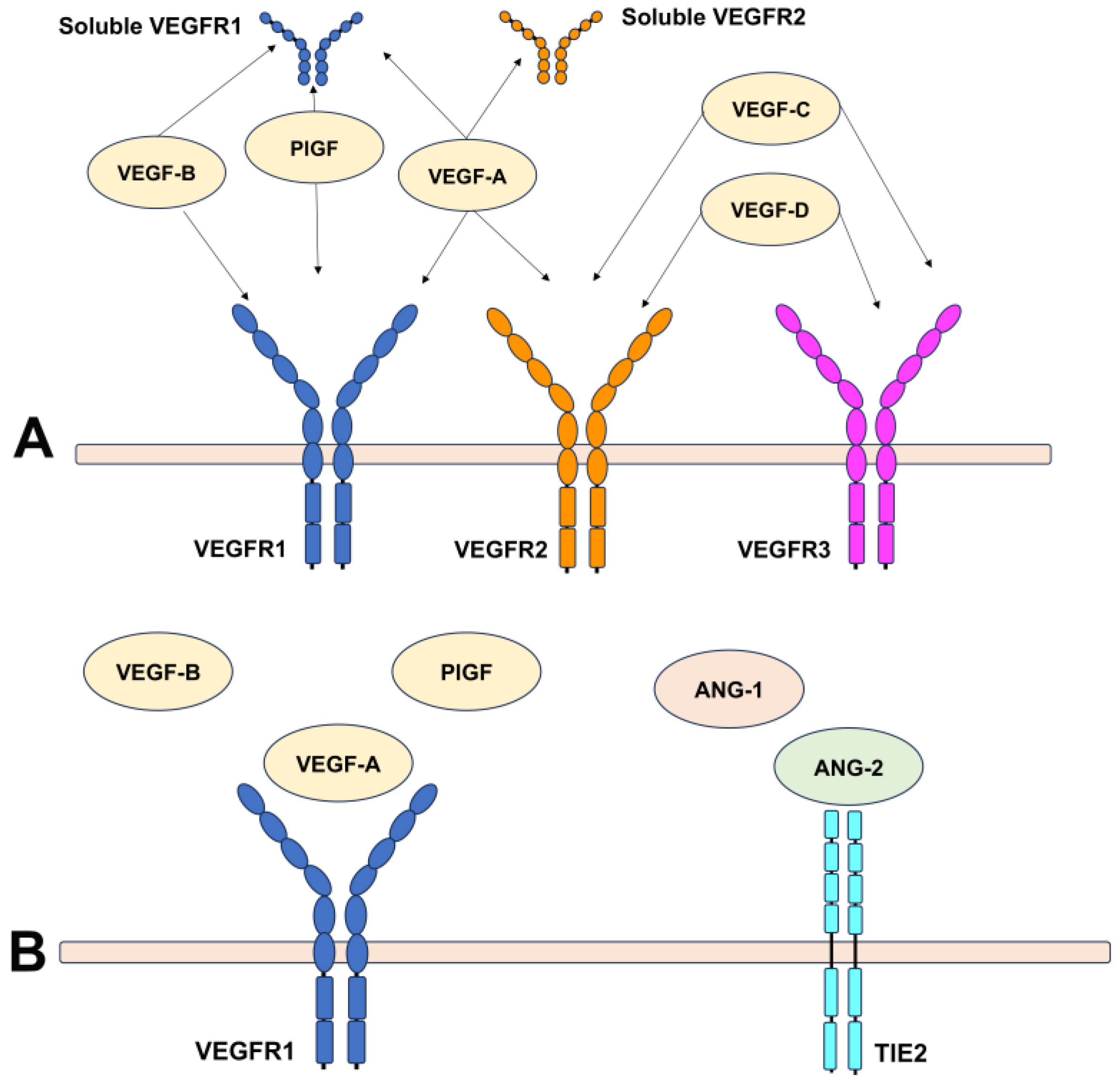
2.1. An Overview of Angiogenic Factors Involved in Angiogenesis
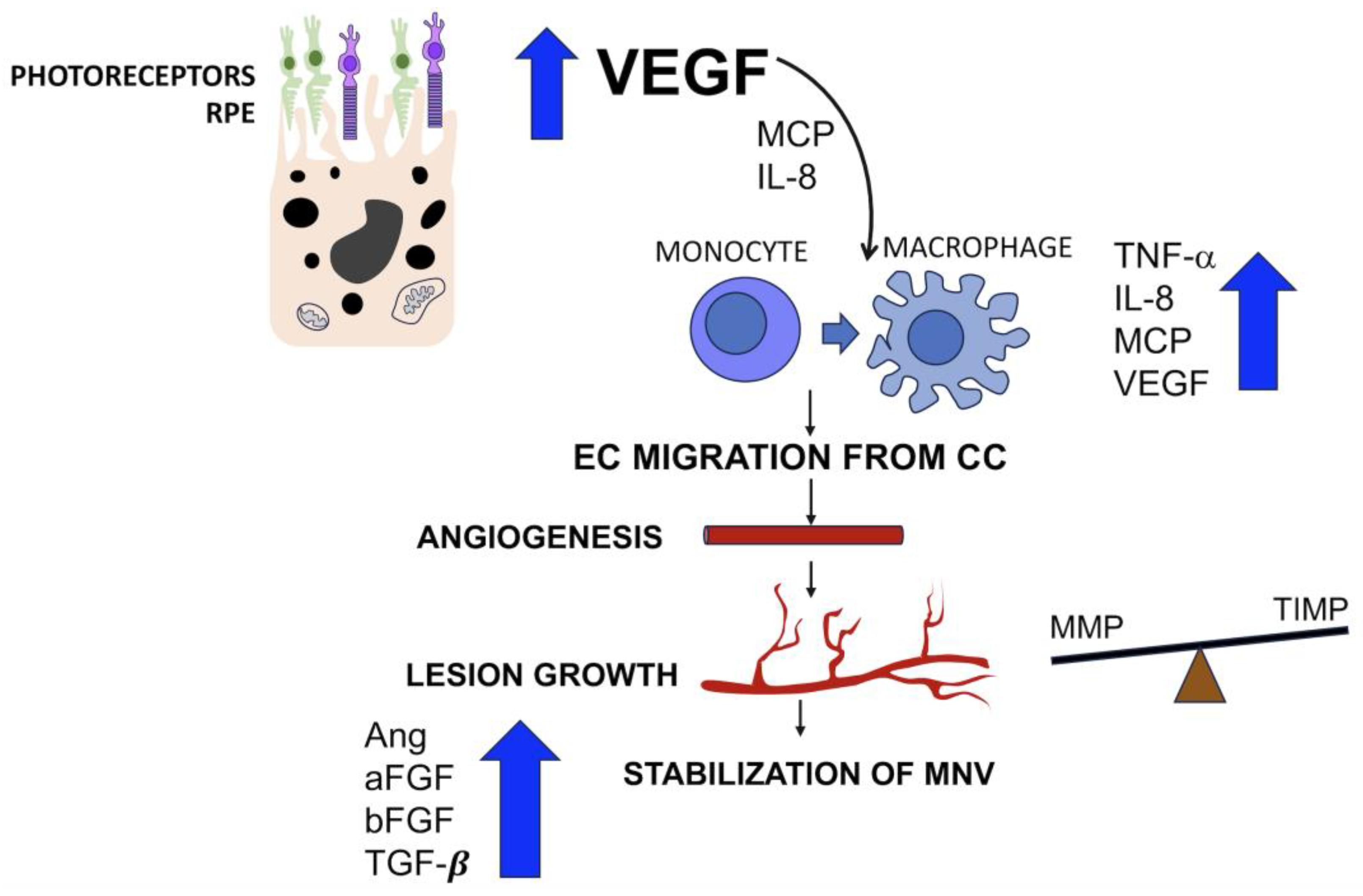
2.2. Mediators Isolated in Neovascular Membranes Secondary to AMD
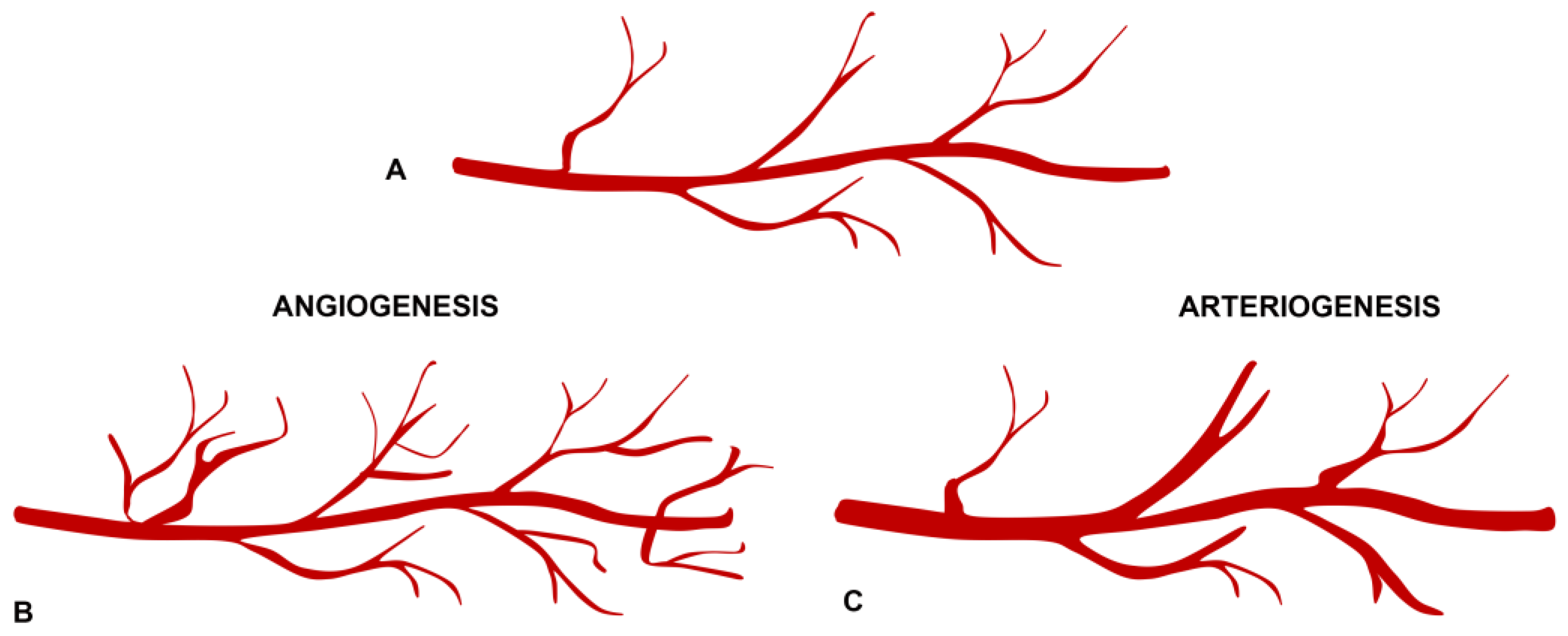
2.3. Angiogenesis and Arteriogenesis in the Pathogenesis of Neovascular Membranes
3. Current Anti-VEGF Therapies for MNV
3.1. Bevacizumab
3.2. Ranibizumab
3.3. Aflibercept
3.4. Brolucizumab
3.5. Faricimab
4. Strategies to Achieve Sustained Disease Control in AMD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Zweifel, S.A.; Engelbert, M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010, 30, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Fragiotta, S.; Sarraf, D.; Sadda, S.R.; Freund, K.B.; Parravano, M.; Corradetti, G.; Cabral, D.; Capuano, V.; Miere, A.; et al. Towards a better understanding of non-exudative choroidal and macular neovascularization. Prog. Retin. Eye Res. 2022, 92, 101113. [Google Scholar] [CrossRef]
- Aiello, L.P. Targeting intraocular neovascularization and edema–one drop at a time. N. Engl. J. Med. 2008, 359, 967–969. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2014, 8, CD005139. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; Group, A.S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M. Antiangiogenic approaches to age-related macular degeneration today. Ophthalmology 2009, 116, S15–S23. [Google Scholar] [CrossRef]
- Parikh, R.; Ross, J.S.; Sangaralingham, L.R.; Adelman, R.A.; Shah, N.D.; Barkmeier, A.J. Trends of Anti-Vascular Endothelial Growth Factor Use in Ophthalmology Among Privately Insured and Medicare Advantage Patients. Ophthalmology 2017, 124, 352–358. [Google Scholar] [CrossRef]
- Low, A.; Faridi, A.; Bhavsar, K.V.; Cockerham, G.C.; Freeman, M.; Fu, R.; Paynter, R.; Kondo, K.; Kansagara, D. Comparative effectiveness and harms of intravitreal antivascular endothelial growth factor agents for three retinal conditions: A systematic review and meta-analysis. Br. J. Ophthalmol. 2019, 103, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Pirakitikulr, N.; Chhablani, J.; Sakurada, Y.; Singh, R.P.; Modi, Y.S. A Multinational Comparison of Anti-Vascular Endothelial Growth Factor Use: The United States, the United Kingdom, and Asia-Pacific. Ophthalmol. Retin. 2019, 3, 16–26. [Google Scholar] [CrossRef]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin, D.f.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef]
- Group, C.R.; Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef]
- Guymer, R.H.; Markey, C.M.; McAllister, I.L.; Gillies, M.C.; Hunyor, A.P.; Arnold, J.J.; Investigators, F. Tolerating Subretinal Fluid in Neovascular Age-Related Macular Degeneration Treated with Ranibizumab Using a Treat-and-Extend Regimen: FLUID Study 24-Month Results. Ophthalmology 2019, 126, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Korobelnik, J.F.; Devenyi, R.; Framme, C.; Galic, J.; Herbert, E.; Hoerauf, H.; Lanzetta, P.; Michels, S.; Mitchell, P.; et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: A Literature Review and Consensus Recommendations. Retina 2015, 35, 1489–1506. [Google Scholar] [CrossRef]
- Gillies, M.C.; Hunyor, A.P.; Arnold, J.J.; Guymer, R.H.; Wolf, S.; Ng, P.; Pecheur, F.L.; McAllister, I.L. Effect of Ranibizumab and Aflibercept on Best-Corrected Visual Acuity in Treat-and-Extend for Neovascular Age-Related Macular Degeneration: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.H.; Downey, L.; Devonport, H.; Gale, R.P.; Kotagiri, A.; Mahmood, S.; Mehta, H.; Narendran, N.; Patel, P.J.; Parmar, N.; et al. Recommendations by a UK expert panel on an aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration. Eye 2020, 34, 1825–1834. [Google Scholar] [CrossRef]
- Khachigian, L.M.; Liew, G.; Teo, K.Y.C.; Wong, T.Y.; Mitchell, P. Emerging therapeutic strategies for unmet need in neovascular age-related macular degeneration. J. Transl. Med. 2023, 21, 133. [Google Scholar] [CrossRef]
- Hallare, J.; Gerriets, V. Half Life. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Bansal, M.; Lenis, T.L.; Iafe, N.A.; Sadda, S.R.; Bonini Filho, M.A.; De Carlo, T.E.; Waheed, N.K.; Duker, J.S.; Sarraf, D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2015, 160, 739–748.e2. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F. Optical Coherence Tomography Angiography Signs of Vascular Abnormalization With Antiangiogenic Therapy for Choroidal Neovascularization. Am. J. Ophthalmol. 2015, 160, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Pipp, F.; Boehm, S.; Cai, W.J.; Adili, F.; Ziegler, B.; Karanovic, G.; Ritter, R.; Balzer, J.; Scheler, C.; Schaper, W.; et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Tzima, E.; Irani-Tehrani, M.; Kiosses, W.B.; Dejana, E.; Schultz, D.A.; Engelhardt, B.; Cao, G.; DeLisser, H.; Schwartz, M.A. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 2005, 437, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Schierling, W.; Troidl, K.; Troidl, C.; Schmitz-Rixen, T.; Schaper, W.; Eitenmüller, I.K. The role of angiogenic growth factors in arteriogenesis. J. Vasc. Res. 2009, 46, 365–374. [Google Scholar] [CrossRef]
- Wu, S.; Wu, X.; Zhu, W.; Cai, W.J.; Schaper, J.; Schaper, W. Immunohistochemical study of the growth factors, aFGF, bFGF, PDGF-AB, VEGF-A and its receptor (Flk-1) during arteriogenesis. Mol. Cell Biochem. 2010, 343, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Grassot, J.; Gouy, M.; Perriere, G.; Mouchiroud, G. Origin and molecular evolution of receptor tyrosine kinases with immunoglobulin-like domains. Mol. Biol. Evol. 2006, 23, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Ferrara, N.; Damico, L.; Shams, N.; Lowman, H.; Kim, R. Development of ranibizumab, an anti–vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006, 26, 859–870. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug. Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Benjamin, L.E.; Hemo, I.; Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998, 125, 1591–1598. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008, 41, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Aznar, A.; Muhl, L.; Gaengel, K. VEGF Receptor Tyrosine Kinases: Key Regulators of Vascular Function. Curr. Top. Dev. Biol. 2017, 123, 433–482. [Google Scholar] [CrossRef] [PubMed]
- Autiero, M.; Luttun, A.; Tjwa, M.; Carmeliet, P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: Novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J. Thromb. Haemost. 2003, 1, 1356–1370. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, T.; Etienne, I.; Cunningham, F.; Moons, L.; Schlingemann, R.O.; Feyen, J.H.M.; Stitt, A.W. The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases. Prog. Retin. Eye Res. 2019, 69, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Kanda, A.; Noda, K.; Saito, W.; Ishida, S. Aflibercept Traps Galectin-1, an Angiogenic Factor Associated with Diabetic Retinopathy. Sci. Rep. 2015, 5, 17946. [Google Scholar] [CrossRef]
- Wu, D.; Kanda, A.; Liu, Y.; Kase, S.; Noda, K.; Ishida, S. Galectin-1 promotes choroidal neovascularization and subretinal fibrosis mediated via epithelial-mesenchymal transition. FASEB J. 2019, 33, 2498–2513. [Google Scholar] [CrossRef] [PubMed]
- Tjwa, M.; Luttun, A.; Autiero, M.; Carmeliet, P. VEGF and PlGF: Two pleiotropic growth factors with distinct roles in development and homeostasis. Cell Tissue Res. 2003, 314, 5–14. [Google Scholar] [CrossRef]
- Davis, S.; Yancopoulos, G.D. The angiopoietins: Yin and Yang in angiogenesis. Curr. Top. Microbiol. Immunol. 1999, 237, 173–185. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Abreu, F.; Adamis, A.P.; Basu, K.; Eichenbaum, D.A.; Haskova, Z.; Lin, H.; Loewenstein, A.; Mohan, S.; Pearce, I.A.; et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): Two randomised, double-masked, phase 3 trials. Lancet 2022, 399, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Wiegand, S.J.; Yancopoulos, G.D. New model of tumor angiogenesis: Dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene 1999, 18, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Tetzner, R.; Strunz, T.; Rittenhouse, K.D. Aflibercept Suppression of Angiopoietin-2 in a Rabbit Retinal Vascular Hyperpermeability Model. Transl. Vis. Sci. Technol. 2023, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Rojo Arias, J.E.; Jászai, J. Gene expression profile of the murine ischemic retina and its response to Aflibercept (VEGF-Trap). Sci. Rep. 2021, 11, 15313. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Boyer, D.S.; Csaky, K.; Vitti, R.; Perlee, L.; Chu, K.W.; Asmus, F.; Leal, S.; Zeitz, O.; Cheng, Y.; et al. INTRAVITREAL NESVACUMAB (ANTIANGIOPOIETIN 2) PLUS AFLIBERCEPT IN DIABETIC MACULAR EDEMA: Phase 2 RUBY Randomized Trial. Retina 2022, 42, 1111–1120. [Google Scholar] [CrossRef]
- Heier, J.S.; Ho, A.C.; Boyer, D.S.; Csaky, K.; Vitti, R.; Perlee, L.; Chu, K.W.; Asmus, F.; Leal, S.; Zeitz, O.; et al. Intravitreal Nesvacumab (Anti-Angiopoietin-2) Plus Aflibercept in Neovascular AMD: Phase 2 ONYX Randomized Trial. J. VitreoRetin. Dis. 2023, 7, 8–15. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Kelley, R.K.; Tolcher, A.W.; Razak, A.R.; Van Loon, K.; Patnaik, A.; Bedard, P.L.; Alfaro, A.A.; Beeram, M.; Adriaens, L.; et al. A Phase I First-in-Human Study of Nesvacumab (REGN910), a Fully Human Anti-Angiopoietin-2 (Ang2) Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Panos, G.D.; Lakshmanan, A.; Dadoukis, P.; Ripa, M.; Motta, L.; Amoaku, W.M. Faricimab: Transforming the Future of Macular Diseases Treatment-A Comprehensive Review of Clinical Studies. Drug. Des. Devel. Ther. 2023, 17, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Ciulla, T.A.; Ciardella, A.P.; Devin, F.; Dugel, P.U.; Eandi, C.M.; Masonson, H.; Mones, J.; Pearlman, J.A.; Quaranta-El Maftouhi, M.; et al. Dual Antagonism of PDGF and VEGF in Neovascular Age-Related Macular Degeneration: A Phase IIb, Multicenter, Randomized Controlled Trial. Ophthalmology 2017, 124, 224–234. [Google Scholar] [CrossRef]
- Kvanta, A.; Algvere, P.V.; Berglin, L.; Seregard, S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest. Ophthalmol. Vis. Sci. 1996, 37, 1929–1934. [Google Scholar] [CrossRef]
- Yi, X.; Ogata, N.; Komada, M.; Yamamoto, C.; Takahashi, K.; Omori, K.; Uyama, M. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Ogata, N.; Otsuji, T.; Uyama, M. Expression of vascular endothelial growth factor and its receptor (KDR/flk-1) mRNA in experimental choroidal neovascularization. Curr. Eye Res. 1999, 18, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Rakic, J.M.; Lambert, V.; Devy, L.; Luttun, A.; Carmeliet, P.; Claes, C.; Nguyen, L.; Foidart, J.M.; Noël, A.; Munaut, C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Hera, R.; Keramidas, M.; Peoc’h, M.; Mouillon, M.; Romanet, J.P.; Feige, J.J. Expression of VEGF and angiopoietins in subfoveal membranes from patients with age-related macular degeneration. Am. J. Ophthalmol. 2005, 139, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Schwesinger, C.; Yee, C.; Rohan, R.M.; Joussen, A.M.; Fernandez, A.; Meyer, T.N.; Poulaki, V.; Ma, J.J.; Redmond, T.M.; Liu, S.; et al. Intrachoroidal neovascularization in transgenic mice overexpressing vascular endothelial growth factor in the retinal pigment epithelium. Am. J. Pathol. 2001, 158, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Tobe, T.; Hackett, S.F.; Ozaki, H.; Vinores, M.A.; LaRochelle, W.; Zack, D.J.; Campochiaro, P.A. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: A new model of intraretinal and subretinal neovascularization. Am. J. Pathol. 1997, 151, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Marra, K.V.; Yu, G.; Wagley, S.; Ma, J.; Teague, G.C.; Nandakumar, N.; Lashkari, K.; Arroyo, J.G. Angiogenic and Inflammatory Vitreous Biomarkers Associated With Increasing Levels of Retinal Ischemia. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6523–6530. [Google Scholar] [CrossRef] [PubMed]
- Otani, A.; Takagi, H.; Oh, H.; Koyama, S.; Matsumura, M.; Honda, Y. Expressions of angiopoietins and Tie2 in human choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1912–1920. [Google Scholar]
- Lopez, P.F.; Sippy, B.D.; Lambert, H.M.; Thach, A.B.; Hinton, D.R. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1996, 37, 855–868. [Google Scholar]
- Grossniklaus, H.E.; Ling, J.X.; Wallace, T.M.; Dithmar, S.; Lawson, D.H.; Cohen, C.; Elner, V.M.; Elner, S.G.; Sternberg, P., Jr. Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol. Vis. 2002, 8, 119–126. [Google Scholar]
- Elner, S.G.; Strieter, R.M.; Elner, V.M.; Rollins, B.J.; Del Monte, M.A.; Kunkel, S.L. Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells. Lab. Investig. 1991, 64, 819–825. [Google Scholar] [PubMed]
- Elner, V.M.; Strieter, R.M.; Elner, S.G.; Baggiolini, M.; Lindley, I.; Kunkel, S.L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am. J. Pathol. 1990, 136, 745–750. [Google Scholar] [PubMed]
- Grossniklaus, H.E.; Cingle, K.A.; Yoon, Y.D.; Ketkar, N.; L’Hernault, N.; Brown, S. Correlation of histologic 2-dimensional reconstruction and confocal scanning laser microscopic imaging of choroidal neovascularization in eyes with age-related maculopathy. Arch. Ophthalmol. 2000, 118, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Takagi, H.; Takagi, C.; Suzuma, K.; Otani, A.; Ishida, K.; Matsumura, M.; Ogura, Y.; Honda, Y. The potential angiogenic role of macrophages in the formation of choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1891–1898. [Google Scholar]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Steen, B.; Sejersen, S.; Berglin, L.; Seregard, S.; Kvanta, A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2194–2200. [Google Scholar]
- Oshima, Y.; Oshima, S.; Nambu, H.; Kachi, S.; Hackett, S.F.; Melia, M.; Kaleko, M.; Connelly, S.; Esumi, N.; Zack, D.J.; et al. Increased expression of VEGF in retinal pigmented epithelial cells is not sufficient to cause choroidal neovascularization. J. Cell Physiol. 2004, 201, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Puklin, J.E.; Frank, R.N. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3178–3188. [Google Scholar]
- Reddy, V.M.; Zamora, R.L.; Kaplan, H.J. Distribution of growth factors in subfoveal neovascular membranes in age-related macular degeneration and presumed ocular histoplasmosis syndrome. Am. J. Ophthalmol. 1995, 120, 291–301. [Google Scholar] [CrossRef]
- Hangai, M.; Murata, T.; Miyawaki, N.; Spee, C.; Lim, J.I.; He, S.; Hinton, D.R.; Ryan, S.J. Angiopoietin-1 upregulation by vascular endothelial growth factor in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1617–1625. [Google Scholar]
- Yamashita, J.; Itoh, H.; Hirashima, M.; Ogawa, M.; Nishikawa, S.; Yurugi, T.; Naito, M.; Nakao, K.; Nishikawa, S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Luttun, A.; Dewerchin, M.; Collen, D.; Carmeliet, P. The role of proteinases in angiogenesis, heart development, restenosis, atherosclerosis, myocardial ischemia, and stroke: Insights from genetic studies. Curr. Atheroscler. Rep. 2000, 2, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Stanga, P.E.; Valentin-Bravo, F.J.; Stanga, S.E.F.; Reinstein, U.I.; Pastor-Idoate, S.; Downes, S.M. Faricimab in neovascular AMD: First report of real-world outcomes in an independent retina clinic. Eye 2023, 37, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Iglicki, M.; González, D.P.; Loewenstein, A.; Zur, D. Longer-acting treatments for neovascular age-related macular degeneration-present and future. Eye 2021, 35, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Bro, T.; Derebecka, M.; Jorstad, O.K.; Grzybowski, A. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 503–511. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Kaiser, P.K.; Korobelnik, J.F.; Brown, D.M.; Chong, V.; Nguyen, Q.D.; Ho, A.C.; Ogura, Y.; Simader, C.; Jaffe, G.J.; et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: Ninety-six-week results of the VIEW studies. Ophthalmology 2014, 121, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Guymer, R.H.; Basu, K.; Boston, H.; Heier, J.S.; Korobelnik, J.F.; Kotecha, A.; Lin, H.; Silverman, D.; Swaminathan, B.; et al. TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100076. [Google Scholar] [CrossRef]
- Khanani, A.M.; Kotecha, A.; Chang, A.; Chen, S.J.; Chen, Y.; Guymer, R.; Heier, J.S.; Holz, F.G.; Iida, T.; Ives, J.A.; et al. TENAYA and LUCERNE: 2-Year Results from the Phase 3 nAMD Trials of Faricimab with Treat-and-Extend Dosing in Year 2. Ophthalmology 2024. online ahead of print. [Google Scholar] [CrossRef]
- Moisseiev, E.; Waisbourd, M.; Ben-Artsi, E.; Levinger, E.; Barak, A.; Daniels, T.; Csaky, K.; Loewenstein, A.; Barequet, I.S. Pharmacokinetics of bevacizumab after topical and intravitreal administration in human eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ziemssen, F.; Henke-Fahle, S.; Tatar, O.; Szurman, P.; Aisenbrey, S.; Schneiderhan-Marra, N.; Xu, X.; Grisanti, S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology 2008, 115, 1750–1755.e1. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liang, X.H.; Ferrara, N. Comparing protein VEGF inhibitors: In vitro biological studies. Biochem. Biophys. Res. Commun. 2011, 408, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Blodi, B.A.; Shapiro, H.; Acharya, N.R.; Group, M.S. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007, 114, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Krohne, T.U.; Liu, Z.; Holz, F.G.; Meyer, C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012, 154, 682–686.e2. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Marcus, D.M.; Awh, C.C.; Regillo, C.; Adamis, A.P.; Bantseev, V.; Chiang, Y.; Ehrlich, J.S.; Erickson, S.; Hanley, W.D.; et al. The Port Delivery System with Ranibizumab for Neovascular Age-Related Macular Degeneration: Results from the Randomized Phase 2 Ladder Clinical Trial. Ophthalmology 2019, 126, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.V.; Wieland, M.R.; Tam, T.; Rea, J.C.; Horvath, J.; Hieb, A.R.; Jia, W.; Grace, L.; Barteselli, G.; Stewart, J.M. The Port Delivery System with ranibizumab: A new paradigm for long-acting retinal drug delivery. Drug Deliv. 2022, 29, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Kågedal, M.; Alskär, O.; Petersson, K.; Hanze, E.; Maia, M.; Lu, T.; Vakhavkar, S.; Quartino, A.; Willis, J.R.; Jin, J.Y.; et al. Population Pharmacokinetics of Ranibizumab Delivered via the Port Delivery System Implanted in the Eye in Patients with Neovascular Age-Related Macular Degeneration. J. Clin. Pharmacol. 2023, 63, 1210–1220. [Google Scholar] [CrossRef]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of Intravitreal Anti-VEGF Drugs in Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Do, D.V.; Rhoades, W.; Nguyen, Q.D. Pharmacokinetic study of intravitreal aflibercept in humans with neovascular age-related macular degeneration. Retina 2020, 40, 643–647. [Google Scholar] [CrossRef]
- Stewart, M.W.; Rosenfeld, P.J. Predicted biological activity of intravitreal VEGF Trap. Br. J. Ophthalmol. 2008, 92, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Le, K.; et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 2014, 98, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.L.; Castellarin, A.A.; Steinle, N.C.; Dhoot, D.S.; Pieramici, D.J.; See, R.; Couvillion, S.; Nasir, M.A.; Rabena, M.D.; Maia, M.; et al. Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 2017, 37, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, N.; Kuppermann, B.D.; Bandello, F. Brolucizimab-leading an era of structural revolution for long-term VEGF suppression. Eye 2020, 34, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, N.; Parachuri, N.; Sharma, R.; Bandello, F.; Kuppermann, B.D.; Loewenstein, A. Brolucizumab and immunogenicity. Eye 2020, 34, 1726–1728. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, N.; Parachuri, N.; Sahyoun, J.Y.; Kuppermann, B.D.; Bandello, F. Brolucizumab ─ termination of 4 weekly trials—rebalancing the immunogenicity risk. Expert Opin. Biol. Ther. 2022, 22, 441–443. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through Preclinical and Clinical Studies and the Implications for the Management of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef]
- Schubert, W.; Terjung, C.; Rafique, A.; Romano, C.; Ellinger, P.; Rittenhouse, K.D. Evaluation of Molecular Properties versus In Vivo Performance of Aflibercept, Brolucizumab, and Ranibizumab in a Retinal Vascular Hyperpermeability Model. Transl. Vis. Sci. Technol. 2022, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.T.; Lundh von Leithner, P.; Foxton, R.; Barathi, V.A.; Chui Ming, G.C.; Tun, S.B.B.; Wey, Y.S.; Iwata, D.; Dostalek, M.; Moelleken, J.; et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2019, 11, 1265–1288. [Google Scholar] [CrossRef]
- Khanani, A.M.; Russell, M.W.; Aziz, A.A.; Danzig, C.J.; Weng, C.Y.; Eichenbaum, D.A.; Singh, R.P. Angiopoietins as Potential Targets in Management of Retinal Disease. Clin. Ophthalmol. 2021, 15, 3747–3755. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lai, T.T.; Yang, C.H.; Hsieh, Y.T. Two-Year Real-World Results for Aflibercept Using the Treat-and-Extend Regimen in Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Ophthalmol. Ther. 2024, 13, 385–396. [Google Scholar] [CrossRef]
- Bailey, C.; Cackett, P.; Kotagiri, A.; Mahmood, S.; Minos, E.; Narendran, N.; Patwardhan, A.; Sim, D.A.; Morgan-Warren, P.; O’Neil, C.; et al. Practical implementation of a q4-q16 aflibercept treat-and-extend pathway for the treatment of neovascular age-related macular degeneration: Updated guidance from a UK expert panel. Eye 2023, 37, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hoshino, J.; Nakamura, K.; Akiyama, H. Two-year outcomes of treat-and-extend regimen with intravitreal brolucizumab for treatment-naïve neovascular age-related macular degeneration with type 1 macular neovascularization. Sci. Rep. 2023, 13, 3249. [Google Scholar] [CrossRef]
- Haensli, C.; Pfister, I.B.; Garweg, J.G. Switching to Brolucizumab in Neovascular Age-Related Macular Degeneration Incompletely Responsive to Ranibizumab or Aflibercept: Real-Life 6 Month Outcomes. J. Clin. Med. 2021, 10, 2666. [Google Scholar] [CrossRef]
- Kaiser, P.K.; Giani, A.; Fuchs, H.; Chong, V.; Heier, J.S. Factors That Can Prolong Ocular Treatment Duration in Age-Related Macular Degeneration. Ophthalmic. Res. 2023, 66, 653–663. [Google Scholar] [CrossRef]
- Veritti, D.; Sarao, V.; Di Bin, F.; Lanzetta, P. Pharmacokinetic and Pharmacodynamic Rationale for Extending VEGF Inhibition Increasing Intravitreal Aflibercept Dose. Pharmaceutics 2023, 15, 1416. [Google Scholar] [CrossRef]

| Ligands | Receptor | Biological Function | Role in AMD |
|---|---|---|---|
| VEGFA | VEGFR1, VEGFR2 |
| New vessel formation in NV Fluid extravasation Support of neovascular membrane Inflammatory response |
| PlGF | VEGFR1 |
| Stimulate vessel growth and remodeling of NV Vessel maturation |
| VEGFB | VEGFR1 |
| Stimulate vessel growth and remodeling of NV |
| VEGFC | VEGFR2, VEGFR3 | Regulates lymphatic vessels | None |
| VEGFD | VEGFR2, VEGFR3 | Regulates lymphatic vessels | None |
| Characteristics | Bevacizumab | Ranizibumab | Aflibercept | Brolucizumab | Faricimab |
|---|---|---|---|---|---|
| Class | Monoclonal Ab | Fab fragment | Fusion protein | scFv | Monoclonal Ab bispecific |
| Target | VEGFA | VEGFA | VEGFA, PlGF, VEGFB, galectin-1 | VEGFA | VEGFA, Ang-2 |
| Dose | 1.25 mg | 0.5 mg | 2 mg | 6 mg | 6 mg |
| Molecular weight | 149 kDa | 48 kDa | 115 kDa | 26 kDa | 150 kDa |
| Dissociation constant | 58 pM | 46 pM | 0.49 pM | 28.4 pM | 3 nM |
| Vitreous half-life (days) | 4.9 | 9 | 9.1 | 3.1 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragiotta, S.; Bassis, L.; Abdolrahimzadeh, B.; Marino, A.; Sepe, M.; Abdolrahimzadeh, S. Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents. Int. J. Mol. Sci. 2024, 25, 4433. https://doi.org/10.3390/ijms25084433
Fragiotta S, Bassis L, Abdolrahimzadeh B, Marino A, Sepe M, Abdolrahimzadeh S. Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents. International Journal of Molecular Sciences. 2024; 25(8):4433. https://doi.org/10.3390/ijms25084433
Chicago/Turabian StyleFragiotta, Serena, Lorena Bassis, Barmak Abdolrahimzadeh, Alessandra Marino, Massimiliano Sepe, and Solmaz Abdolrahimzadeh. 2024. "Exploring Current Molecular Targets in the Treatment of Neovascular Age-Related Macular Degeneration toward the Perspective of Long-Term Agents" International Journal of Molecular Sciences 25, no. 8: 4433. https://doi.org/10.3390/ijms25084433





