HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology
Abstract
1. Introduction
2. Early Infiltration of HIV to the Central Nervous System
3. Loss of Blood–Brain Barrier Integrity
4. Activation of Immune Cells Exacerbates Neuroinflammation
4.1. Microglial Targeting of Synapses
4.2. Recruitment of Circulating Monocytes
4.3. Other Long-Lasting Cytokine Pathways
4.4. Biomarkers of HAND and Neurocognitive Impairment
5. Propagation of HIV-Associated Neuroinflammation
5.1. FKN Signaling
5.2. TNF-Alpha Signaling
5.3. Cofactors of HAND Progression
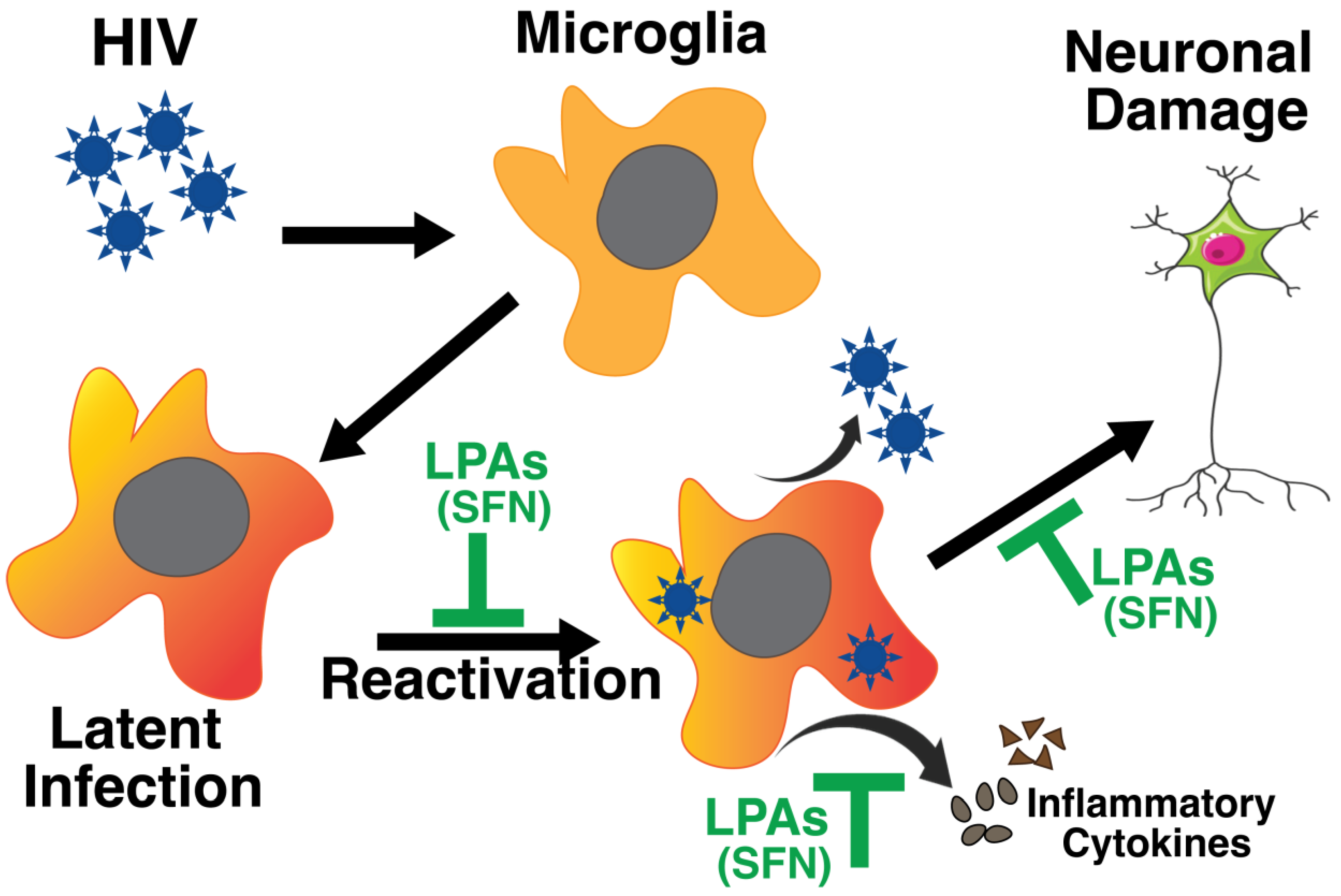
6. Impact of Latency on Neuroinflammation
6.1. Latency in the Central Nervous System
6.2. The Circulating Latent Pool
7. How to Address Neuropathogenesis and CNS Latent Reservoir
7.1. Shock and Kill Approach
7.2. Block and Lock Approach
7.3. Alternate Treatment and Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The End of AIDS: HIV Infection as a Chronic Disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef]
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 814362. [Google Scholar] [CrossRef]
- Rojas-Celis, V.; Valiente-Echeverría, F.; Toro-Ascuy, D.; Soto-Rifo, R. New Challenges of HIV-1 Infection: How HIV-1 Attacks and Resides in the Central Nervous System. Cells 2019, 8, 1245. [Google Scholar] [CrossRef]
- Davis, L.E.; Hjelle, B.L.; Miller, V.E.; Palmer, D.L.; Llewellyn, A.L.; Merlin, T.L.; Young, S.A.; Mills, R.G.; Wachsman, W.; Wiley, C.A. Early Viral Brain Invasion in Iatrogenic Human Immunodeficiency Virus Infection. Neurology 1992, 42, 1736. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Osiecki, K.; Lopez, L.; Goldstein, H.; Calderon, T.M.; Berman, J.W. CCL2/Monocyte Chemoattractant Protein-1 Mediates Enhanced Transmigration of Human Immunodeficiency Virus (HIV)-Infected Leukocytes across the Blood-Brain Barrier: A Potential Mechanism of HIV-CNS Invasion and NeuroAIDS. J. Neurosci. 2006, 26, 1098–1106. [Google Scholar] [CrossRef]
- Hazleton, J.E.; Berman, J.W.; Eugenin, E.A. Novel mechanisms of central nervous system damage in HIV infection. HIV/AIDS-Res. Palliat. Care 2010, 2, 39–49. [Google Scholar]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.; Michael, N.; Spudich, S.; et al. Central Nervous System Viral Invasion and Inflammation during Acute HIV Infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-Brain Barrier Pericytes as a Target for HIV-1 Infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef]
- Singh, M.V.; Davidson, D.C.; Kiebala, M.; Maggirwar, S.B. Detection of Circulating Platelet-Monocyte Complexes in Persons Infected with Human Immunodeficiency Virus Type-1. J. Virol. Methods 2012, 181, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Davidson, D.C.; Jackson, J.W.; Singh, V.B.; Silva, J.; Ramirez, S.H.; Maggirwar, S.B. Characterization of Platelet–Monocyte Complexes in HIV-1–Infected Individuals: Possible Role in HIV-Associated Neuroinflammation. J. Immunol. 2014, 192, 4674–4684. [Google Scholar] [CrossRef]
- Strauss-Ayali, D.; Conrad, S.M.; Mosser, D.M. Monocyte Subpopulations and Their Differentiation Patterns during Infection. J. Leukoc. Biol. 2007, 82, 244–252. [Google Scholar] [CrossRef]
- Fischer-Smith, T.; Bell, C.; Croul, S.; Lewis, M.; Rappaport, J. Monocyte/Macrophage Trafficking in Acquired Immunodeficiency Syndrome Encephalitis: Lessons from Human and Nonhuman Primate Studies. J. Neurovirol. 2008, 14, 318–326. [Google Scholar] [CrossRef]
- Kaul, M.; Garden, G.A.; Lipton, S.A. Pathways to Neuronal Injury and Apoptosis in HIV-Associated Dementia. Nature 2001, 410, 988–994. [Google Scholar] [CrossRef]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.-P.; Fenart, L. Modelling of the Blood–Brain Barrier in Drug Discovery and Development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef]
- Avison, M.J.; Nath, A.; Greene-Avison, R.; Schmitt, F.A.; Bales, R.A.; Ethisham, A.; Greenberg, R.N.; Berger, J.R. Inflammatory Changes and Breakdown of Microvascular Integrity in Early Human Immunodeficiency Virus Dementia. J. Neurovirol. 2004, 10, 223–232. [Google Scholar] [CrossRef]
- Toborek, M.; Lee, Y.W.; Pu, H.; Malecki, A.; Flora, G.; Garrido, R.; Hennig, B.; Bauer, H.; Nath, A. HIV-Tat Protein Induces Oxidative and Inflammatory Pathways in Brain Endothelium. J. Neurochem. 2003, 84, 169–179. [Google Scholar] [CrossRef]
- Wada, T.; Orphanides, G.; Hasegawa, J.; Kim, D.-K.; Shima, D.; Yamaguchi, Y.; Fukuda, A.; Hisatake, K.; Oh, S.; Reinberg, D.; et al. FACT Relieves DSIF/NELF-Mediated Inhibition of Transcriptional Elongation and Reveals Functional Differences between P-TEFb and TFIIH. Mol. Cell 2000, 5, 1067–1072. [Google Scholar] [CrossRef]
- Isel, C.; Karn, J. Direct Evidence That HIV-1 Tat Stimulates RNA Polymerase II Carboxyl-Terminal Domain Hyperphosphorylation during Transcriptional Elongation. J. Mol. Biol. 1999, 290, 929–941. [Google Scholar] [CrossRef]
- Wada, T.; Takagi, T.; Yamaguchi, Y.; Ferdous, A.; Imai, T.; Hirose, S.; Sugimoto, S.; Yano, K.; Hartzog, G.A.; Winston, F.; et al. DSIF, a Novel Transcription Elongation Factor That Regulates RNA Polymerase II Processivity, Is Composed of Human Spt4 and Spt5 Homologs. Genes. Dev. 1998, 12, 343–356. [Google Scholar] [CrossRef]
- Sui, Z.; Sniderhan, L.F.; Schifitto, G.; Phipps, R.P.; Gelbard, H.A.; Dewhurst, S.; Maggirwar, S.B. Functional Synergy between CD40 Ligand and HIV-1 Tat Contributes to Inflammation: Implications in HIV Type 1 Dementia. J. Immunol. 2007, 178, 3226–3236. [Google Scholar] [CrossRef]
- Zhong, Y.; Smart, E.J.; Weksler, B.; Couraud, P.-O.; Hennig, B.; Toborek, M. Caveolin-1 Regulates Human Immunodeficiency Virus-1 Tat-Induced Alterations of Tight Junction Protein Expression via Modulation of the Ras Signaling. J. Neurosci. 2008, 28, 7788–7796. [Google Scholar] [CrossRef]
- Jiang, W.; Huang, W.; Chen, Y.; Zou, M.; Peng, D.; Chen, D. HIV-1 Transactivator Protein Induces ZO-1 and Neprilysin Dysfunction in Brain Endothelial Cells via the Ras Signaling Pathway. Oxid. Med. Cell Longev. 2017, 2017, 3160360. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; Jiang, W.; Wu, X.; Ye, B.; Zhou, X. HIV-1 Tat Regulates Occludin and A β Transfer Receptor Expression in Brain Endothelial Cells via Rho/ROCK Signaling Pathway. Oxid. Med. Cell Longev. 2016, 2016, 4196572. [Google Scholar] [CrossRef]
- Khan, I.A.; Worrad, A.H.; Singh, M.V.; Maggirwar, S.B.; Singh, V.B. Human Immunodeficiency Virus-1 Tat Exerts Its Neurotoxic Effects by Downregulating Sonic Hedgehog Signaling. J. Neurovirol. 2022, 28, 305–311. [Google Scholar] [CrossRef]
- Singh, V.B.; Singh, M.V.; Piekna-Przybylska, D.; Gorantla, S.; Poluektova, L.Y.; Maggirwar, S.B. Sonic Hedgehog Mimetic Prevents Leukocyte Infiltration into the CNS during Acute HIV Infection. Sci. Rep. 2017, 7, 9578. [Google Scholar] [CrossRef]
- Singh, V.B.; Singh, M.V.; Gorantla, S.; Poluektova, L.Y.; Maggirwar, S.B. Smoothened Agonist Reduces Human Immunodeficiency Virus Type-1-Induced Blood-Brain Barrier Breakdown in Humanized Mice. Sci. Rep. 2016, 6, 26876. [Google Scholar] [CrossRef]
- Altmeyer, R.; Mordelet, E.; Girard, M.; Vidal, C. Expression and Detection of Macrophage-Tropic HIV-1 Gp120 in the Brain Using Conformation-Dependent Antibodies. Virology 1999, 259, 314–323. [Google Scholar] [CrossRef]
- Hesselgesser, J.; Taub, D.; Baskar, P.; Greenberg, M.; Hoxie, J.; Kolson, D.L.; Horuk, R. Neuronal Apoptosis Induced by HIV-1 Gp120 and the Chemokine SDF-1α Is Mediated by the Chemokine Receptor CXCR4. Curr. Biol. 1998, 8, 595–598. [Google Scholar] [CrossRef]
- Kaul, M.; Lipton, S.A. Chemokines and Activated Macrophages in HIV Gp120-Induced Neuronal Apoptosis. Proc. Natl. Acad. Sci. USA 1999, 96, 8212–8216. [Google Scholar] [CrossRef]
- Ullrich, C.K.; Groopman, J.E.; Ganju, R.K. HIV-1 Gp120- and Gp160-Induced Apoptosis in Cultured Endothelial Cells Is Mediated by Caspases. Blood 2000, 96, 1438–1442. [Google Scholar] [CrossRef]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 Gp120 Compromises Blood-Brain Barrier Integrity and Enhance Monocyte Migration across Blood-Brain Barrier: Implication for Viral Neuropathogenesis. J. Cereb. Blood Flow Metab. 2007, 27, 123–134. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System during Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harb. Perspect. Biol. 2015, 7, a020537. [Google Scholar] [CrossRef]
- Green, K.N.; Crapser, J.D.; Hohsfield, L.A. To Kill a Microglia: A Case for CSF1R Inhibitors. Trends Immunol. 2020, 41, 771–784. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-Stimulating Factor 1 Receptor Signaling Is Necessary for Microglia Viability, Unmasking a Microglia Progenitor Cell in the Adult Brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Karanikas, E.; Manganaris, S.; Ntouros, E.; Floros, G.; Antoniadis, D.; Garyfallos, G. Cytokines, Cortisol and IGF-1 in First Episode Psychosis and Ultra High Risk Males. Evidence for TNF-α, IFN-γ, ΤNF-β, IL-4 Deviation. Asian J. Psychiatr. 2017, 26, 99–103. [Google Scholar] [CrossRef]
- Planès, R.; Serrero, M.; Leghmari, K.; BenMohamed, L.; Bahraoui, E. HIV-1 Envelope Glycoproteins Induce the Production of TNF-α and IL-10 in Human Monocytes by Activating Calcium Pathway. Sci. Rep. 2018, 8, 17215. [Google Scholar] [CrossRef]
- Nosi, D.; Lana, D.; Giovannini, M.G.; Delfino, G.; Zecchi-Orlandini, S. Neuroinflammation: Integrated Nervous Tissue Response through Intercellular Interactions at the “Whole System” Scale. Cells 2021, 10, 1195. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef]
- Silveira, D.B.; Américo, M.F.; Flores, N.P.; Terenzi, H.; Pinto, A.R. Pharmacological Inhibition of UPR Sensor PERK Attenuates HIV Tat-induced Inflammatory M1 Phenotype in Microglial Cells. Cell Biochem. Funct. 2022, 40, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Kajaste-Rudnitski, A.; Coradin, T.; Saba, E.; Della Chiara, G.; Barbagallo, M.; Graziano, F.; Alfano, M.; Cassol, E.; Vicenzi, E.; et al. M1 Polarization of Human Monocyte-Derived Macrophages Restricts Pre and Postintegration Steps of HIV-1 Replication. AIDS 2013, 27, 1847–1856. [Google Scholar] [CrossRef]
- Graziano, F.; Aimola, G.; Forlani, G.; Turrini, F.; Accolla, R.S.; Vicenzi, E.; Poli, G. Reversible Human Immunodeficiency Virus Type-1 Latency in Primary Human Monocyte-Derived Macrophages Induced by Sustained M1 Polarization. Sci. Rep. 2018, 8, 14249. [Google Scholar] [CrossRef]
- Watson, Z.; Tang, S.-J. Aberrant Synaptic Pruning in CNS Diseases: A Critical Player in HIV-Associated Neurological Dysfunction? Cells 2022, 11, 1943. [Google Scholar] [CrossRef]
- Ru, W.; Liu, X.; Bae, C.; Shi, Y.; Walikonis, R.; Mo Chung, J.; Tang, S.-J. Microglia Mediate HIV-1 Gp120-Induced Synaptic Degeneration in Spinal Pain Neural Circuits. J. Neurosci. 2019, 39, 8408–8421. [Google Scholar] [CrossRef]
- Martins, P.A.d.C.; van Gils, J.M.; Mol, A.; Hordijk, P.L.; Zwaginga, J.J. Platelet Binding to Monocytes Increases the Adhesive Properties of Monocytes by Up-Regulating the Expression and Functionality of Β1 and Β2 Integrins. J. Leukoc. Biol. 2006, 79, 499–507. [Google Scholar] [CrossRef]
- Regal-McDonald, K.; Patel, R.P. Selective Recruitment of Monocyte Subsets by Endothelial N-Glycans. Am. J. Pathol. 2020, 190, 947–957. [Google Scholar] [CrossRef]
- Dhawan, S.; Puri, R.K.; Kumar, A.; Duplan, H.; Masson, J.M.; Aggarwal, B.B. Human Immunodeficiency Virus-1-Tat Protein Induces the Cell Surface Expression of Endothelial Leukocyte Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1, and Intercellular Adhesion Molecule-1 in Human Endothelial Cells. Blood 1997, 90, 1535–1544. [Google Scholar]
- Cartier, L.; Hartley, O.; Dubois-Dauphin, M.; Krause, K.-H. Chemokine Receptors in the Central Nervous System: Role in Brain Inflammation and Neurodegenerative Diseases. Brain Res. Rev. 2005, 48, 16–42. [Google Scholar] [CrossRef]
- Periyasamy, P.; Thangaraj, A.; Bendi, V.S.; Buch, S. HIV-1 Tat-Mediated Microglial Inflammation Involves a Novel MiRNA-34a-NLRC5-NFκB Signaling Axis. Brain Behav. Immun. 2019, 80, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, C.; Sun, T.; Wang, Q.; Xie, W.; Wang, R.; Cui, J. Reversible Ubiquitination Shapes NLRC5 Function and Modulates NF-ΚB Activation Switch. J. Cell Biol. 2015, 211, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Gargan, S.; Ahmed, S.; Mahony, R.; Bannan, C.; Napoletano, S.; O’Farrelly, C.; Borrow, P.; Bergin, C.; Stevenson, N.J. HIV-1 Promotes the Degradation of Components of the Type 1 IFN JAK/STAT Pathway and Blocks Anti-Viral ISG Induction. EBioMedicine 2018, 30, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Gavegnano, C.; Brehm, J.H.; Dupuy, F.P.; Talla, A.; Ribeiro, S.P.; Kulpa, D.A.; Cameron, C.; Santos, S.; Hurwitz, S.J.; Marconi, V.C.; et al. Novel Mechanisms to Inhibit HIV Reservoir Seeding Using Jak Inhibitors. PLoS Pathog. 2017, 13, e1006740. [Google Scholar] [CrossRef] [PubMed]
- Ances, B.M.; Sisti, D.; Vaida, F.; Liang, C.L.; Leontiev, O.; Perthen, J.E.; Buxton, R.B.; Benson, D.; Smith, D.M.; Little, S.J.; et al. Resting Cerebral Blood Flow. Neurology 2009, 73, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Ernst, T.; Leonido-Yee, M.; Speck, O. Perfusion MRI Detects RCBF Abnormalities in Early Stages of HIV–Cognitive Motor Complex. Neurology 2000, 54, 389. [Google Scholar] [CrossRef] [PubMed]
- Ances, B.M.; Roc, A.C.; Wang, J.; Korczykowski, M.; Okawa, J.; Stern, J.; Kim, J.; Wolf, R.; Lawler, K.; Kolson, D.L.; et al. Caudate Blood Flow and Volume Are Reduced in HIV+ Neurocognitively Impaired Patients. Neurology 2006, 66, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Callen, A.L.; Dupont, S.M.; Pyne, J.; Talbott, J.; Tien, P.; Calabrese, E.; Saloner, D.; Chow, F.C.; Narvid, J. The Regional Pattern of Abnormal Cerebrovascular Reactivity in HIV-Infected, Virally Suppressed Women. J. Neurovirol. 2020, 26, 734–742. [Google Scholar] [CrossRef]
- Chow, F.C.; Wang, H.; Li, Y.; Mehta, N.; Hu, Y.; Han, Y.; Xie, J.; Lu, W.; Xu, W.; Li, T. Cerebral Vasoreactivity Evaluated by the Breath-Holding Challenge Correlates with Performance on a Cognitive Screening Test in Persons Living with Treated HIV Infection in China. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 79, e101–e104. [Google Scholar] [CrossRef]
- Singh, M.V.; Uddin, M.N.; Singh, V.B.; Peterson, A.N.; Murray, K.D.; Zhuang, Y.; Tyrell, A.; Wang, L.; Tivarus, M.E.; Zhong, J.; et al. Initiation of Combined Antiretroviral Therapy Confers Suboptimal Beneficial Effects on Neurovascular Function in People with HIV. Front. Neurol. 2023, 14, 1240300. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.D.; Singh, M.V.; Zhuang, Y.; Uddin, M.N.; Qiu, X.; Weber, M.T.; Tivarus, M.E.; Wang, H.Z.; Sahin, B.; Zhong, J.; et al. Pathomechanisms of HIV-Associated Cerebral Small Vessel Disease: A Comprehensive Clinical and Neuroimaging Protocol and Analysis Pipeline. Front. Neurol. 2020, 11, 595463. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.D.; Uddin, M.N.; Tivarus, M.E.; Sahin, B.; Wang, H.Z.; Singh, M.V.; Qiu, X.; Wang, L.; Spincemaille, P.; Wang, Y.; et al. Increased Risk for Cerebral Small Vessel Disease Is Associated with Quantitative Susceptibility Mapping in HIV Infected and Uninfected Individuals. Neuroimage Clin. 2021, 32, 102786. [Google Scholar] [CrossRef] [PubMed]
- McMurtray, A.; Nakamoto, B.; Shikuma, C.; Valcour, V. Cortical Atrophy and White Matter Hyperintensities in HIV: The Hawaii Aging with HIV Cohort Study. J. Stroke Cerebrovasc. Dis. 2008, 17, 212–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, T.; Wit, F.W.N.M.; Caan, M.W.A.; Schouten, J.; Prins, M.; Geurtsen, G.J.; Cole, J.H.; Sharp, D.J.; Richard, E.; Reneman, L.; et al. White Matter Hyperintensities in Relation to Cognition in HIV-Infected Men with Sustained Suppressed Viral Load on Combination Antiretroviral Therapy. AIDS 2016, 30, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Filippi, C.G.; Ulug, A.M.; Ryan, E.; Ferrando, S.J.; van Gorp, W. Diffusion Tensor Imaging of Patients with HIV and Normal-Appearing White Matter on MR Images of the Brain. AJNR Am. J. Neuroradiol. 2001, 22, 277–283. [Google Scholar]
- Wu, Y.; Storey, P.; Cohen, B.A.; Epstein, L.G.; Edelman, R.R.; Ragin, A.B. Diffusion Alterations in Corpus Callosum of Patients with HIV. AJNR Am. J. Neuroradiol. 2006, 27, 656–660. [Google Scholar] [PubMed]
- Chang, L.; Wong, V.; Nakama, H.; Watters, M.; Ramones, D.; Miller, E.N.; Cloak, C.; Ernst, T. Greater Than Age-Related Changes in Brain Diffusion of HIV Patients after 1 Year. J. Neuroimmune Pharmacol. 2008, 3, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Rosenbloom, M.J.; Rohlfing, T.; Kemper, C.A.; Deresinski, S.; Sullivan, E. V Frontostriatal Fiber Bundle Compromise in HIV Infection without Dementia. AIDS 2009, 23, 1977–1985. [Google Scholar] [CrossRef]
- Tate, D.F.; Conley, J.; Paul, R.H.; Coop, K.; Zhang, S.; Zhou, W.; Laidlaw, D.H.; Taylor, L.E.; Flanigan, T.; Navia, B.; et al. Quantitative Diffusion Tensor Imaging Tractography Metrics Are Associated with Cognitive Performance among HIV-Infected Patients. Brain Imaging Behav. 2010, 4, 68–79. [Google Scholar] [CrossRef][Green Version]
- Zhu, T.; Zhong, J.; Hu, R.; Tivarus, M.; Ekholm, S.; Harezlak, J.; Ombao, H.; Navia, B.; Cohen, R.; Schifitto, G. Patterns of White Matter Injury in HIV Infection after Partial Immune Reconstitution: A DTI Tract-Based Spatial Statistics Study. J. Neurovirol. 2013, 19, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Gisslen, M.; Zetterberg, H.; Fuchs, D.; Shacklett, B.L.; Hagberg, L.; Yiannoutsos, C.T.; Spudich, S.S.; Price, R.W. Cerebrospinal Fluid (CSF) Neuronal Biomarkers across the Spectrum of HIV Infection: Hierarchy of Injury and Detection. PLoS ONE 2014, 9, e116081. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) Is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140. [Google Scholar] [CrossRef]
- Alagaratnam, J.; De Francesco, D.; Zetterberg, H.; Heslegrave, A.; Toombs, J.; Kootstra, N.A.; Underwood, J.; Gisslen, M.; Reiss, P.; Fidler, S.; et al. Correlation between Cerebrospinal Fluid and Plasma Neurofilament Light Protein in Treated HIV Infection: Results from the COBRA Study. J. Neurovirol. 2022, 28, 54–63. [Google Scholar] [CrossRef]
- Guha, D.; Mukerji, S.S.; Chettimada, S.; Misra, V.; Lorenz, D.R.; Morgello, S.; Gabuzda, D. Cerebrospinal Fluid Extracellular Vesicles and Neurofilament Light Protein as Biomarkers of Central Nervous System Injury in HIV-Infected Patients on Antiretroviral Therapy. AIDS 2019, 33, 615–625. [Google Scholar] [CrossRef]
- Ripamonti, E.; Edén, A.; Nilsson, S.; Sönnerborg, A.; Zetterberg, H.; Gisslén, M. Longitudinal Decline of Plasma Neurofilament Light Levels after Antiretroviral Initiation in People Living with HIV. J. Intern. Med. 2023, 293, 445–456. [Google Scholar] [CrossRef]
- de Menezes, E.G.M.; Liu, J.S.; Bowler, S.A.; Giron, L.B.; D’Antoni, M.L.; Shikuma, C.M.; Abdel-Mohsen, M.; Ndhlovu, L.C.; Norris, P.J. Circulating Brain-Derived Extracellular Vesicles Expressing Neuroinflammatory Markers Are Associated with HIV-Related Neurocognitive Impairment. Front. Immunol. 2022, 13, 1033712. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Chenna, A.; Petropoulos, C.J.; Lie, Y.; Curanovic, D.; Crescini, M.; Winslow, J.; Sundermann, E.; Tang, B.; Letendre, S.L. Higher Cerebrospinal Fluid Biomarkers of Neuronal Injury in HIV-Associated Neurocognitive Impairment. J. Neurovirol. 2022, 28, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Kollhoff, A.; Anderson, A.M.; Christina Howell, J.; Loring, D.W.; Waldrop-Valverde, D.; Franklin, D.; Letendre, S.; Tyor, W.R.; Hu, W.T. Linked CSF Reduction of Phosphorylated Tau and IL-8 in HIV Associated Neurocognitive Disorder. Sci. Rep. 2019, 9, 8733. [Google Scholar] [CrossRef]
- Sathler, M.F.; Doolittle, M.J.; Cockrell, J.A.; Nadalin, I.R.; Hofmann, F.; VandeWoude, S.; Kim, S. HIV and FIV Glycoproteins Increase Cellular Tau Pathology via CGMP-Dependent Kinase II Activation. J. Cell Sci. 2022, 135, jcs259764. [Google Scholar] [CrossRef]
- Rönsholt, F.F.; Ullum, H.; Katzenstein, T.L.; Gerstoft, J.; Ostrowski, S.R. Persistent Inflammation and Endothelial Activation in HIV-1 Infected Patients after 12 Years of Antiretroviral Therapy. PLoS ONE 2013, 8, e65182. [Google Scholar] [CrossRef]
- Ross, A.C.; O’Riordan, M.A.; Storer, N.; Dogra, V.; McComsey, G.A. Heightened Inflammation Is Linked to Carotid Intima-Media Thickness and Endothelial Activation in HIV-Infected Children. Atherosclerosis 2010, 211, 492–498. [Google Scholar] [CrossRef]
- Guha, D.; Misra, V.; Yin, J.; Horiguchi, M.; Uno, H.; Gabuzda, D. Vascular Injury Markers Associated with Cognitive Impairment in People with HIV on Suppressive Antiretroviral Therapy. AIDS 2023, 37, 2137–2147. [Google Scholar] [CrossRef]
- Saloner, R.; Sun-Suslow, N.; Morgan, E.E.; Lobo, J.; Cherner, M.; Ellis, R.J.; Heaton, R.K.; Grant, I.; Letendre, S.L.; Iudicello, J.E. Plasma Biomarkers of Vascular Dysfunction Uniquely Relate to a Vascular-Risk Profile of Neurocognitive Deficits in Virally-Suppressed Adults with HIV. Brain Behav. Immun. Health 2022, 26, 100560. [Google Scholar] [CrossRef]
- Shikuma, C.M.; Nakamoto, B.; Shiramizu, B.; Liang, C.-Y.; DeGruttola, V.; Bennett, K.; Paul, R.; Kallianpur, K.; Chow, D.; Gavegnano, C.; et al. Antiretroviral Monocyte Efficacy Score Linked to Cognitive Impairment in HIV. Antivir. Ther. 2012, 17, 1233–1242. [Google Scholar] [CrossRef]
- Kusao, I.; Shiramizu, B.; Liang, C.-Y.; Grove, J.; Agsalda, M.; Troelstrup, D.; Velasco, V.-N.; Marshall, A.; Whitenack, N.; Shikuma, C.; et al. Cognitive Performance Related to HIV-1-Infected Monocytes. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Nevárez, L.A.; Imp, B.M.; Eller, M.A.; Kiweewa, F.; Maswai, J.; Polyak, C.; Olwenyi, O.A.; Allen, I.E.; Rono, E.; Milanini, B.; et al. Monocyte Activation, HIV, and Cognitive Performance in East Africa. J. Neurovirol. 2020, 26, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Veenhuis, R.T.; Williams, D.W.; Shirk, E.N.; Monteiro Abreu, C.; Ferreira, E.A.; Coughlin, J.M.; Brown, T.T.; Maki, P.M.; Anastos, K.; Berman, J.W.; et al. Higher Circulating Intermediate Monocytes Are Associated with Cognitive Function in Women with HIV. JCI Insight 2021, 6, 146215. [Google Scholar] [CrossRef]
- Singh, M.V.; Uddin, N.; Covacevich Vidalle, M.; Sutton, K.R.; Boodoo, Z.D.; Peterson, A.N.; Tyrell, A.; Brenner, R.; Madalina Tivarus, E.; Wang, H.Z.; et al. Role of Non-Classical Monocytes in HIV-Associated Vascular Cognitive Impairment. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Li, L.-H.; Bian, C.; Yang, L.; Lv, N.; Zhang, Y.-Q. Involvement of NF-ΚB and the CX3CR1 Signaling Network in Mechanical Allodynia Induced by Tetanic Sciatic Stimulation. Neurosci. Bull. 2018, 34, 64–73. [Google Scholar] [CrossRef]
- Komissarov, A.; Potashnikova, D.; Freeman, M.L.; Gontarenko, V.; Maytesyan, D.; Lederman, M.M.; Vasilieva, E.; Margolis, L. Driving T Cells to Human Atherosclerotic Plaques: CCL3/CCR5 and CX3CL1/CX3CR1 Migration Axes. Eur. J. Immunol. 2021, 51, 1857–1859. [Google Scholar] [CrossRef]
- Cormican, S.; Griffin, M.D. Fractalkine (CX3CL1) and Its Receptor CX3CR1: A Promising Therapeutic Target in Chronic Kidney Disease? Front. Immunol. 2021, 12, 664202. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling Pathways of the TNF Superfamily: A Double-Edged Sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum Immune Markers and Disease Progression in an Incident Parkinson’s Disease Cohort. Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef]
- Mifflin, L.; Hu, Z.; Dufort, C.; Hession, C.C.; Walker, A.J.; Niu, K.; Zhu, H.; Liu, N.; Liu, J.S.; Levin, J.Z.; et al. A RIPK1-Regulated Inflammatory Microglial State in Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2025102118. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Garcia-Alvarez, L.; Garcia-Portilla, M.P.; Gonzalez-Blanco, L.; Saiz Martinez, P.A.; de la Fuente-Tomas, L.; Menendez-Miranda, I.; Iglesias, C.; Bobes, J. Biomarcadores Sanguíneos Diferenciales de Las Dimensiones Psicopatológicas de La Esquizofrenia. Rev. Psiquiatr. Salud Ment. 2016, 9, 219–227. [Google Scholar] [CrossRef]
- Korthuis, P.T.; Josephs, J.S.; Fleishman, J.A.; Hellinger, J.; Himelhoch, S.; Chander, G.; Morse, E.B.; Gebo, K.A. Substance Abuse Treatment in Human Immunodeficiency Virus: The Role of Patient–Provider Discussions. J. Subst. Abus. Treat. 2008, 35, 294–303. [Google Scholar] [CrossRef][Green Version]
- Bokhari, S.M.; Hegde, R.; Callen, S.; Yao, H.; Adany, I.; Li, Q.; Li, Z.; Pinson, D.; Yeh, H.-W.; Cheney, P.D.; et al. Morphine Potentiates Neuropathogenesis of SIV Infection in Rhesus Macaques. J. Neuroimmune Pharmacol. 2011, 6, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.C.G.; Flynn, C.; Watry, D.D.; Zandonatti, M.; Fox, H.S. Methamphetamine Increases Brain Viral Load and Activates Natural Killer Cells in Simian Immunodeficiency Virus-Infected Monkeys. Am. J. Pathol. 2010, 177, 355–361. [Google Scholar] [CrossRef]
- Gaskill, P.J.; Calderon, T.M.; Luers, A.J.; Eugenin, E.A.; Javitch, J.A.; Berman, J.W. Human Immunodeficiency Virus (HIV) Infection of Human Macrophages Is Increased by Dopamine. Am. J. Pathol. 2009, 175, 1148–1159. [Google Scholar] [CrossRef]
- Gaskill, P.J.; Calderon, T.M.; Coley, J.S.; Berman, J.W. Drug Induced Increases in CNS Dopamine Alter Monocyte, Macrophage and T Cell Functions: Implications for HAND. J. Neuroimmune Pharmacol. 2013, 8, 621–642. [Google Scholar] [CrossRef]
- Malik, S.; Khalique, H.; Buch, S.; Seth, P. A Growth Factor Attenuates HIV-1 Tat and Morphine Induced Damage to Human Neurons: Implication in HIV/AIDS-Drug Abuse Cases. PLoS ONE 2011, 6, e18116. [Google Scholar] [CrossRef]
- Fitting, S.; Xu, R.; Bull, C.; Buch, S.K.; El-Hage, N.; Nath, A.; Knapp, P.E.; Hauser, K.F. Interactive Comorbidity between Opioid Drug Abuse and HIV-1 Tat. Am. J. Pathol. 2010, 177, 1397–1410. [Google Scholar] [CrossRef]
- Byrd, D.A.; Fellows, R.P.; Morgello, S.; Franklin, D.; Heaton, R.K.; Deutsch, R.; Atkinson, J.H.; Clifford, D.B.; Collier, A.C.; Marra, C.M.; et al. Neurocognitive Impact of Substance Use in HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 58, 154–162. [Google Scholar] [CrossRef]
- Gill, A.J.; Kolson, D.L. Chronic Inflammation and the Role for Cofactors (Hepatitis C, Drug Abuse, Antiretroviral Drug Toxicity, Aging) in HAND Persistence. Curr. HIV/AIDS Rep. 2014, 11, 325–335. [Google Scholar] [CrossRef]
- Chak, E.; Talal, A.H.; Sherman, K.E.; Schiff, E.R.; Saab, S. Hepatitis C Virus Infection in USA: An Estimate of True Prevalence. Liver Int. 2011, 31, 1090–1101. [Google Scholar] [CrossRef]
- Wilkinson, J.; Radkowski, M.; Eschbacher, J.M.; Laskus, T. Activation of Brain Macrophages/Microglia Cells in Hepatitis C Infection. Gut 2010, 59, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; He, T.; Huang, D.; Pardo, C.A.; Ransohoff, R.M. TNF-α Mediates SDF-1α–Induced NF-ΚB Activation and Cytotoxic Effects in Primary Astrocytes. J. Clin. Investig. 2001, 108, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hokello, J.; Sharma, A.L.; Dimri, M.; Tyagi, M. Insights into the HIV Latency and the Role of Cytokines. Pathogens 2019, 8, 137. [Google Scholar] [CrossRef]
- Maksoud, S.; El Hokayem, J. The Cytokine/Chemokine Response in Leishmania/HIV Infection and Co-Infection. Heliyon 2023, 9, e15055. [Google Scholar] [CrossRef]
- Whalen, C.; Horsburgh, C.R.; Hom, D.; Lahart, C.; Simberkoff, M.; Ellner, J. Accelerated Course of Human Immunodeficiency Virus Infection after Tuberculosis. Am. J. Respir. Crit. Care Med. 1995, 151, 129–135. [Google Scholar] [CrossRef]
- Bell, L.C.K.; Noursadeghi, M. Pathogenesis of HIV-1 and Mycobacterium Tuberculosis Co-Infection. Nat. Rev. Microbiol. 2018, 16, 80–90. [Google Scholar] [CrossRef]
- Marais, S.; Meintjes, G.; Lesosky, M.; Wilkinson, K.A.; Wilkinson, R.J. Interleukin-17 Mediated Differences in the Pathogenesis of HIV-1-Associated Tuberculous and Cryptococcal Meningitis. AIDS 2015, 30, 395–404. [Google Scholar] [CrossRef]
- Goletti, D.; Weissman, D.; Jackson, R.W.; Graham, N.M.; Vlahov, D.; Klein, R.S.; Munsiff, S.S.; Ortona, L.; Cauda, R.; Fauci, A.S. Effect of Mycobacterium Tuberculosis on HIV Replication. Role of Immune Activation. J. Immunol. 1996, 157, 1271–1278. [Google Scholar] [CrossRef]
- Kalsdorf, B.; Scriba, T.J.; Wood, K.; Day, C.L.; Dheda, K.; Dawson, R.; Hanekom, W.A.; Lange, C.; Wilkinson, R.J. HIV-1 Infection Impairs the Bronchoalveolar T-Cell Response to Mycobacteria. Am. J. Respir. Crit. Care Med. 2009, 180, 1262–1270. [Google Scholar] [CrossRef]
- Sullivan, Z.A.; Wong, E.B.; Ndung’u, T.; Kasprowicz, V.O.; Bishai, W.R. Latent and Active Tuberculosis Infection Increase Immune Activation in Individuals Co-Infected with HIV. EBioMedicine 2015, 2, 334–340. [Google Scholar] [CrossRef]
- Ratto-Kim, S.; Schuetz, A.; Sithinamsuwan, P.; Barber, J.; Hutchings, N.; Lerdlum, S.; Fletcher, J.L.K.; Phuang-Ngern, Y.; Chuenarom, W.; Tipsuk, S.; et al. Characterization of Cellular Immune Responses in Thai Individuals with and without HIV-Associated Neurocognitive Disorders. AIDS Res. Hum. Retroviruses 2018, 34, 685–689. [Google Scholar] [CrossRef]
- Swanta, N.; Aryal, S.; Nejtek, V.; Shenoy, S.; Ghorpade, A.; Borgmann, K. Blood-Based Inflammation Biomarkers of Neurocognitive Impairment in People Living with HIV. J. Neurovirol. 2020, 26, 358–370. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal Fluid Immune Markers and HIV-Associated Neurocognitive Impairments: A Systematic Review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Livelli, A.; Vaida, F.; Ellis, R.J.; Ma, Q.; Ferrara, M.; Clifford, D.B.; Collier, A.C.; Gelman, B.B.; Marra, C.M.; McArthur, J.C.; et al. Correlates of HIV RNA Concentrations in Cerebrospinal Fluid during Antiretroviral Therapy: A Longitudinal Cohort Study. Lancet HIV 2019, 6, e456–e462. [Google Scholar] [CrossRef]
- Li, H.; McLaurin, K.A.; Mactutus, C.F.; Booze, R.M. A Rat Model of EcoHIV Brain Infection. J. Vis. Exp. 2021, 167, e62137. [Google Scholar] [CrossRef]
- Gumbs, S.B.H.; Berdenis van Berlekom, A.; Kübler, R.; Schipper, P.J.; Gharu, L.; Boks, M.P.; Ormel, P.R.; Wensing, A.M.J.; de Witte, L.D.; Nijhuis, M. Characterization of HIV-1 Infection in Microglia-Containing Human Cerebral Organoids. Viruses 2022, 14, 829. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.; Spencer, S.J. Microglia: Key Players in Neurodevelopment and Neuronal Plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Baxter, P.S.; Dando, O.; Emelianova, K.; He, X.; McKay, S.; Hardingham, G.E.; Qiu, J. Microglial Identity and Inflammatory Responses Are Controlled by the Combined Effects of Neurons and Astrocytes. Cell Rep. 2021, 34, 108882. [Google Scholar] [CrossRef]
- Holtman, I.R.; Skola, D.; Glass, C.K. Transcriptional Control of Microglia Phenotypes in Health and Disease. J. Clin. Investig. 2017, 127, 3220–3229. [Google Scholar] [CrossRef]
- Ryan, S.K.; Gonzalez, M.V.; Garifallou, J.P.; Bennett, F.C.; Williams, K.S.; Sotuyo, N.P.; Mironets, E.; Cook, K.; Hakonarson, H.; Anderson, S.A.; et al. Neuroinflammation and EIF2 Signaling Persist despite Antiretroviral Treatment in an HiPSC Tri-Culture Model of HIV Infection. Stem Cell Rep. 2020, 14, 703–716. [Google Scholar] [CrossRef]
- Garcia-Mesa, Y.; Jay, T.R.; Checkley, M.A.; Luttge, B.; Dobrowolski, C.; Valadkhan, S.; Landreth, G.E.; Karn, J.; Alvarez-Carbonell, D. Immortalization of Primary Microglia: A New Platform to Study HIV Regulation in the Central Nervous System. J. Neurovirol. 2017, 23, 47–66. [Google Scholar] [CrossRef]
- Desplats, P.; Dumaop, W.; Smith, D.; Adame, A.; Everall, I.; Letendre, S.; Ellis, R.; Cherner, M.; Grant, I.; Masliah, E. Molecular and Pathologic Insights from Latent HIV-1 Infection in the Human Brain. Neurology 2013, 80, 1415–1423. [Google Scholar] [CrossRef]
- Alvarez-Carbonell, D.; Ye, F.; Ramanath, N.; Garcia-Mesa, Y.; Knapp, P.E.; Hauser, K.F.; Karn, J. Cross-Talk between Microglia and Neurons Regulates HIV Latency. PLoS Pathog. 2019, 15, e1008249. [Google Scholar] [CrossRef]
- Zhan, L.; Krabbe, G.; Du, F.; Jones, I.; Reichert, M.C.; Telpoukhovskaia, M.; Kodama, L.; Wang, C.; Cho, S.; Sayed, F.; et al. Proximal Recolonization by Self-Renewing Microglia Re-Establishes Microglial Homeostasis in the Adult Mouse Brain. PLoS Biol. 2019, 17, e3000134. [Google Scholar] [CrossRef]
- Chhatbar, C.; Prinz, M. The Roles of Microglia in Viral Encephalitis: From Sensome to Therapeutic Targeting. Cell Mol. Immunol. 2021, 18, 250–258. [Google Scholar] [CrossRef]
- Alvarez-Carbonell, D.; Garcia-Mesa, Y.; Milne, S.; Das, B.; Dobrowolski, C.; Rojas, R.; Karn, J. Toll-like Receptor 3 Activation Selectively Reverses HIV Latency in Microglial Cells. Retrovirology 2017, 14, 9. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef]
- Pereira, C.F.; Middel, J.; Jansen, G.; Verhoef, J.; Nottet, H.S.L.M. Enhanced Expression of Fractalkine in HIV-1 Associated Dementia. J. Neuroimmunol. 2001, 115, 168–175. [Google Scholar] [CrossRef]
- Tong, N.; Perry, S.W.; Zhang, Q.; James, H.J.; Guo, H.; Brooks, A.; Bal, H.; Kinnear, S.A.; Fine, S.; Epstein, L.G.; et al. Neuronal Fractalkine Expression in HIV-1 Encephalitis: Roles for Macrophage Recruitment and Neuroprotection in the Central Nervous System. J. Immunol. 2000, 164, 1333–1339. [Google Scholar] [CrossRef]
- Erichsen, D.; Lopez, A.L.; Peng, H.; Niemann, D.; Williams, C.; Bauer, M.; Morgello, S.; Cotter, R.L.; Ryan, L.A.; Ghorpade, A.; et al. Neuronal Injury Regulates Fractalkine: Relevance for HIV-1 Associated Dementia. J. Neuroimmunol. 2003, 138, 144–155. [Google Scholar] [CrossRef]
- Nicolini, A.; Ajmone-Cat, M.A.; Bernardo, A.; Levi, G.; Minghetti, L. Human Immunodeficiency Virus Type-1 Tat Protein Induces Nuclear Factor (NF)-κB Activation and Oxidative Stress in Microglial Cultures by Independent Mechanisms. J. Neurochem. 2001, 79, 713–716. [Google Scholar] [CrossRef]
- Yeung, M.C.; Pulliam, L.; Lau, A.S. The HIV Envelope Protein Gp120 Is Toxic to Human Brain-Cell Cultures through the Induction of Interleukin-6 and Tumor Necrosis Factor-Alpha. AIDS 1995, 9, 137–143. [Google Scholar] [CrossRef]
- Zhao, M.-L.; Kim, M.-O.; Morgello, S.; Lee, S.C. Expression of Inducible Nitric Oxide Synthase, Interleukin-1 and Caspase-1 in HIV-1 Encephalitis. J. Neuroimmunol. 2001, 115, 182–191. [Google Scholar] [CrossRef]
- He, X.; Yang, W.; Zeng, Z.; Wei, Y.; Gao, J.; Zhang, B.; Li, L.; Liu, L.; Wan, Y.; Zeng, Q.; et al. NLRP3-Dependent Pyroptosis Is Required for HIV-1 Gp120-Induced Neuropathology. Cell Mol. Immunol. 2020, 17, 283–299. [Google Scholar] [CrossRef]
- Chivero, E.T.; Guo, M.-L.; Periyasamy, P.; Liao, K.; Callen, S.E.; Buch, S. HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J. Neurosci. 2017, 37, 3599–3609. [Google Scholar] [CrossRef]
- Trillo-Pazos, G.; Diamanturos, A.; Rislove, L.; Menza, T.; Chao, W.; Belem, P.; Sadiq, S.; Morgello, S.; Sharer, L.; Volsky, D.J. Detection of HIV-1 DNA in Microglia/ Macrophages, Astrocytes and Neurons Isolated from Brain Tissue with HIV-1 Encephalitis by Laser Capture Microdissection. Brain Pathol. 2003, 13, 144–154. [Google Scholar] [CrossRef]
- Valdebenito, S.; Castellano, P.; Ajasin, D.; Eugenin, E.A. Astrocytes Are HIV Reservoirs in the Brain: A Cell Type with Poor HIV Infectivity and Replication but Efficient Cell-to-cell Viral Transfer. J. Neurochem. 2021, 158, 429–443. [Google Scholar] [CrossRef]
- Carroll-Anzinger, D.; Al-Harthi, L. Gamma Interferon Primes Productive Human Immunodeficiency Virus Infection in Astrocytes. J. Virol. 2006, 80, 541–544. [Google Scholar] [CrossRef]
- Narasipura, S.D.; Kim, S.; Al-Harthi, L. Epigenetic Regulation of HIV-1 Latency in Astrocytes. J. Virol. 2014, 88, 3031–3038. [Google Scholar] [CrossRef]
- Mbonye, U.; Karn, J. The Molecular Basis for Human Immunodeficiency Virus Latency. Annu. Rev. Virol. 2017, 4, 261–285. [Google Scholar] [CrossRef]
- Agosto, L.; Gagne, M.; Henderson, A. Impact of Chromatin on HIV Replication. Genes 2015, 6, 957–976. [Google Scholar] [CrossRef]
- Ruelas, D.S.; Greene, W.C. An Integrated Overview of HIV-1 Latency. Cell 2013, 155, 519–529. [Google Scholar] [CrossRef]
- Jadlowsky, J.K.; Wong, J.Y.; Graham, A.C.; Dobrowolski, C.; Devor, R.L.; Adams, M.D.; Fujinaga, K.; Karn, J. Negative Elongation Factor Is Required for the Maintenance of Proviral Latency but Does Not Induce Promoter-Proximal Pausing of RNA Polymerase II on the HIV Long Terminal Repeat. Mol. Cell Biol. 2014, 34, 1911–1928. [Google Scholar] [CrossRef]
- Natarajan, M.; Lester, G.M.; Lee, C.; Missra, A.; Wasserman, G.A.; Steffen, M.; Gilmour, D.S.; Henderson, A.J. Negative Elongation Factor (NELF) Coordinates RNA Polymerase II Pausing, Premature Termination, and Chromatin Remodeling to Regulate HIV Transcription. J. Biol. Chem. 2013, 288, 25995–26003. [Google Scholar] [CrossRef]
- Soriano-Sarabia, N.; Bateson, R.E.; Dahl, N.P.; Crooks, A.M.; Kuruc, J.D.; Margolis, D.M.; Archin, N.M. Quantitation of Replication-Competent HIV-1 in Populations of Resting CD4+ T Cells. J. Virol. 2014, 88, 14070–14077. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M.; et al. HIV Reservoir Size and Persistence Are Driven by T Cell Survival and Homeostatic Proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Siliciano, R.F.; Greene, W.C. HIV Latency. Cold Spring Harb. Perspect. Med. 2011, 1, a007096. [Google Scholar] [CrossRef]
- Shan, L.; Deng, K.; Gao, H.; Xing, S.; Capoferri, A.A.; Durand, C.M.; Rabi, S.A.; Laird, G.M.; Kim, M.; Hosmane, N.N.; et al. Transcriptional Reprogramming during Effector-to-Memory Transition Renders CD4+ T Cells Permissive for Latent HIV-1 Infection. Immunity 2017, 47, 766–775. [Google Scholar] [CrossRef]
- Kumar, N.A.; Cheong, K.; Powell, D.R.; da Fonseca Pereira, C.; Anderson, J.; Evans, V.A.; Lewin, S.R.; Cameron, P.U. The Role of Antigen Presenting Cells in the Induction of HIV-1 Latency in Resting CD4+ T-Cells. Retrovirology 2015, 12, 76. [Google Scholar] [CrossRef]
- Evans, V.A.; Kumar, N.; Filali, A.; Procopio, F.A.; Yegorov, O.; Goulet, J.-P.; Saleh, S.; Haddad, E.K.; da Fonseca Pereira, C.; Ellenberg, P.C.; et al. Myeloid Dendritic Cells Induce HIV-1 Latency in Non-Proliferating CD4+ T Cells. PLoS Pathog. 2013, 9, e1003799. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Lorizate, M.; Puertas, M.C.; Rodriguez-Plata, M.T.; Zangger, N.; Erikson, E.; Pino, M.; Erkizia, I.; Glass, B.; Clotet, B.; et al. Siglec-1 Is a Novel Dendritic Cell Receptor That Mediates HIV-1 Trans-Infection through Recognition of Viral Membrane Gangliosides. PLoS Biol. 2012, 10, e1001448. [Google Scholar] [CrossRef]
- Fortin, J.-F.; Barat, C.; Beauséjour, Y.; Barbeau, B.; Tremblay, M.J. Hyper-Responsiveness to Stimulation of Human Immunodeficiency Virus-Infected CD4+ T Cells Requires Nef and Tat Virus Gene Products and Results from Higher NFAT, NF-ΚB, and AP-1 Induction. J. Biol. Chem. 2004, 279, 39520–39531. [Google Scholar] [CrossRef]
- Neri, F.; Giolo, G.; Potestà, M.; Petrini, S.; Doria, M. The HIV-1 Nef Protein Has a Dual Role in T Cell Receptor Signaling in Infected CD4+ T Lymphocytes. Virology 2011, 410, 316–326. [Google Scholar] [CrossRef]
- Fenard, D.; Yonemoto, W.; de Noronha, C.; Cavrois, M.; Williams, S.A.; Greene, W.C. Nef Is Physically Recruited into the Immunological Synapse and Potentiates T Cell Activation Early after TCR Engagement. J. Immunol. 2005, 175, 6050–6057. [Google Scholar] [CrossRef]
- Thompson, K.A.; Cherry, C.L.; Bell, J.E.; McLean, C.A. Brain Cell Reservoirs of Latent Virus in Presymptomatic HIV-Infected Individuals. Am. J. Pathol. 2011, 179, 1623–1629. [Google Scholar] [CrossRef]
- Sadowski, I.; Hashemi, F.B. Strategies to Eradicate HIV from Infected Patients: Elimination of Latent Provirus Reservoirs. Cell. Mol. Life Sci. 2019, 76, 3583–3600. [Google Scholar] [CrossRef]
- Nühn, M.M.; Gumbs, S.B.H.; Buchholtz, N.V.E.J.; Jannink, L.M.; Gharu, L.; de Witte, L.D.; Wensing, A.M.J.; Lewin, S.R.; Nijhuis, M.; Symons, J. Shock and Kill within the CNS: A Promising HIV Eradication Approach? J. Leukoc. Biol. 2022, 112, 1297–1315. [Google Scholar] [CrossRef]
- Sarabia, I.; Bosque, A. HIV-1 Latency and Latency Reversal: Does Subtype Matter? Viruses 2019, 11, 1104. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef]
- Abner, E.; Jordan, A. HIV “Shock and Kill” Therapy: In Need of Revision. Antivir. Res. 2019, 166, 19–34. [Google Scholar] [CrossRef]
- Vansant, G.; Bruggemans, A.; Janssens, J.; Debyser, Z. Block-And-Lock Strategies to Cure HIV Infection. Viruses 2020, 12, 84. [Google Scholar] [CrossRef]
- Kessing, C.F.; Nixon, C.C.; Li, C.; Tsai, P.; Takata, H.; Mousseau, G.; Ho, P.T.; Honeycutt, J.B.; Fallahi, M.; Trautmann, L.; et al. In Vivo Suppression of HIV Rebound by Didehydro-Cortistatin A, a “Block-and-Lock” Strategy for HIV-1 Treatment. Cell Rep. 2017, 21, 600–611. [Google Scholar] [CrossRef]
- Mousseau, G.; Kessing, C.F.; Fromentin, R.; Trautmann, L.; Chomont, N.; Valente, S.T. The Tat Inhibitor Didehydro-Cortistatin A Prevents HIV-1 Reactivation from Latency. mBio 2015, 6, e00465-15. [Google Scholar] [CrossRef]
- Mousseau, G.; Clementz, M.A.; Bakeman, W.N.; Nagarsheth, N.; Cameron, M.; Shi, J.; Baran, P.; Fromentin, R.; Chomont, N.; Valente, S.T. An Analog of the Natural Steroidal Alkaloid Cortistatin A Potently Suppresses Tat-Dependent HIV Transcription. Cell Host Microbe 2012, 12, 97–108. [Google Scholar] [CrossRef]
- Li, C.; Mousseau, G.; Valente, S.T. Tat Inhibition by Didehydro-Cortistatin A Promotes Heterochromatin Formation at the HIV-1 Long Terminal Repeat. Epigenetics Chromatin 2019, 12, 23. [Google Scholar] [CrossRef]
- Christ, F.; Shaw, S.; Demeulemeester, J.; Desimmie, B.A.; Marchand, A.; Butler, S.; Smets, W.; Chaltin, P.; Westby, M.; Debyser, Z.; et al. Small-Molecule Inhibitors of the LEDGF/P75 Binding Site of Integrase Block HIV Replication and Modulate Integrase Multimerization. Antimicrob. Agents Chemother. 2012, 56, 4365–4374. [Google Scholar] [CrossRef]
- Christ, F.; Voet, A.; Marchand, A.; Nicolet, S.; Desimmie, B.A.; Marchand, D.; Bardiot, D.; Van der Veken, N.J.; Van Remoortel, B.; Strelkov, S.V.; et al. Rational Design of Small-Molecule Inhibitors of the LEDGF/P75-Integrase Interaction and HIV Replication. Nat. Chem. Biol. 2010, 6, 442–448. [Google Scholar] [CrossRef]
- Jurado, K.A.; Wang, H.; Slaughter, A.; Feng, L.; Kessl, J.J.; Koh, Y.; Wang, W.; Ballandras-Colas, A.; Patel, P.A.; Fuchs, J.R.; et al. Allosteric Integrase Inhibitor Potency Is Determined through the Inhibition of HIV-1 Particle Maturation. Proc. Natl. Acad. Sci. USA 2013, 110, 8690–8695. [Google Scholar] [CrossRef]
- Nabel, G.; Baltimore, D. An Inducible Transcription Factor Activates Expression of Human Immunodeficiency Virus in T Cells. Nature 1987, 326, 711–713. [Google Scholar] [CrossRef]
- Anderson, I.; Low, J.S.; Weston, S.; Weinberger, M.; Zhyvoloup, A.; Labokha, A.A.; Corazza, G.; Kitson, R.A.; Moody, C.J.; Marcello, A.; et al. Heat Shock Protein 90 Controls HIV-1 Reactivation from Latency. Proc. Natl. Acad. Sci. USA 2014, 111, E1528–E1537. [Google Scholar] [CrossRef]
- Kim, H.; Choi, M.-S.; Inn, K.-S.; Kim, B.-J. Inhibition of HIV-1 Reactivation by a Telomerase-Derived Peptide in a HSP90-Dependent Manner. Sci. Rep. 2016, 6, 28896. [Google Scholar] [CrossRef]
- Joshi, P.; Maidji, E.; Stoddart, C.A. Inhibition of Heat Shock Protein 90 Prevents HIV Rebound. J. Biol. Chem. 2016, 291, 10332–10346. [Google Scholar] [CrossRef]
- Nandini, D.; Rao, R.; Deepak, B.; Reddy, P. Sulforaphane in Broccoli: The Green Chemoprevention!! Role in Cancer Prevention and Therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef]
- Li, Y.; Buckhaults, P.; Li, S.; Tollefsbol, T. Temporal Efficacy of a Sulforaphane-Based Broccoli Sprout Diet in Prevention of Breast Cancer through Modulation of Epigenetic Mechanisms. Cancer Prev. Res. 2018, 11, 451–464. [Google Scholar] [CrossRef]
- Jamal, I.; Paudel, A.; Thompson, L.; Abdelmalek, M.; Khan, I.A.; Singh, V.B. Sulforaphane Prevents the Reactivation of HIV-1 by Suppressing NFκB Signaling. J. Virus Erad. 2023, 9, 100341. [Google Scholar] [CrossRef]
- Mollace, V.; Nottet, H.S.L.M.; Clayette, P.; Turco, M.C.; Muscoli, C.; Salvemini, D.; Perno, C.F. Oxidative Stress and NeuroAIDS: Triggers, Modulators and Novel Antioxidants. Trends Neurosci. 2001, 24, 411–416. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane Inhibits NLRP3 Inflammasome Activation in Microglia through Nrf2-Mediated MiRNA Alteration. Immunol. Lett. 2021, 233, 20–30. [Google Scholar] [CrossRef]
- Tozzi, V.; Balestra, P.; Bellagamba, R.; Corpolongo, A.; Salvatori, M.F.; Visco-Comandini, U.; Vlassi, C.; Giulianelli, M.; Galgani, S.; Antinori, A.; et al. Persistence of Neuropsychologic Deficits Despite Long-Term Highly Active Antiretroviral Therapy in Patients with HIV-Related Neurocognitive Impairment. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 45, 174–182. [Google Scholar] [CrossRef]
- Robertson, K.R.; Su, Z.; Margolis, D.M.; Krambrink, A.; Havlir, D.V.; Evans, S.; Skiest, D.J. Neurocognitive Effects of Treatment Interruption in Stable HIV-Positive Patients in an Observational Cohort. Neurology 2010, 74, 1260–1266. [Google Scholar] [CrossRef]
- Caniglia, E.C.; Cain, L.E.; Justice, A.; Tate, J.; Logan, R.; Sabin, C.; Winston, A.; van Sighem, A.; Miro, J.M.; Podzamczer, D.; et al. Antiretroviral Penetration into the CNS and Incidence of AIDS-Defining Neurologic Conditions. Neurology 2014, 83, 134–141. [Google Scholar] [CrossRef]
- Perkins, D.O.; Stern, R.A.; Golden, R.N.; Murphy, C.; Naftolowitz, D.; Evans, D.L. Mood Disorders in HIV Infection: Prevalence and Risk Factors in a Nonepicenter of the AIDS Epidemic. Am. J. Psychiatry 1994, 151, 233–236. [Google Scholar] [CrossRef]
- Rabkin, J.G.; Rabkin, R.; Wagner, G. Effects of Fluoxetine on Mood and Immune Status in Depressed Patients with HIV Illness. J. Clin. Psychiatry 1994, 55, 92–97. [Google Scholar]
- D’Antoni, M.L.; Paul, R.H.; Mitchell, B.I.; Kohorn, L.; Fischer, L.; Lefebvre, E.; Seyedkazemi, S.; Nakamoto, B.K.; Walker, M.; Kallianpur, K.J.; et al. Improved Cognitive Performance and Reduced Monocyte Activation in Virally Suppressed Chronic HIV After Dual CCR2 and CCR5 Antagonism. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 79, 108–116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, L.J.-P.; Genovese, J.; Hong, Z.; Singh, M.V.; Singh, V.B. HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology. Int. J. Mol. Sci. 2024, 25, 4697. https://doi.org/10.3390/ijms25094697
Thompson LJ-P, Genovese J, Hong Z, Singh MV, Singh VB. HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology. International Journal of Molecular Sciences. 2024; 25(9):4697. https://doi.org/10.3390/ijms25094697
Chicago/Turabian StyleThompson, Landon John-Patrick, Jessica Genovese, Zhenzi Hong, Meera Vir Singh, and Vir Bahadur Singh. 2024. "HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology" International Journal of Molecular Sciences 25, no. 9: 4697. https://doi.org/10.3390/ijms25094697
APA StyleThompson, L. J.-P., Genovese, J., Hong, Z., Singh, M. V., & Singh, V. B. (2024). HIV-Associated Neurocognitive Disorder: A Look into Cellular and Molecular Pathology. International Journal of Molecular Sciences, 25(9), 4697. https://doi.org/10.3390/ijms25094697





