The Rise in Tubular pH during Hypercalciuria Exacerbates Calcium Stone Formation
Abstract
1. Introduction
2. Results
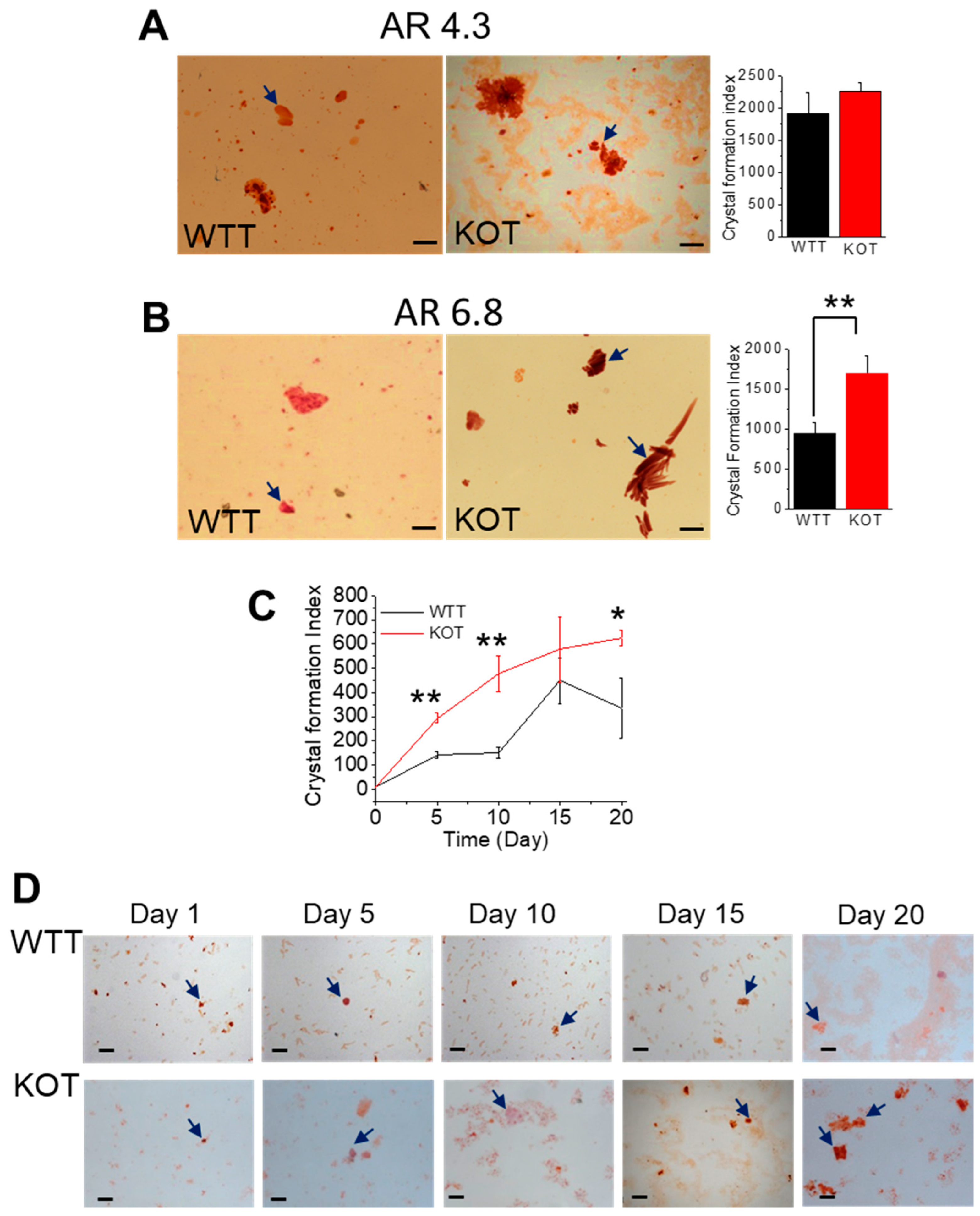
2.1. Hypercalciuria in Combination with Alkaline Urine Results in Larger CaP + CaOx Crystal Formation in the Absence of TRPC3
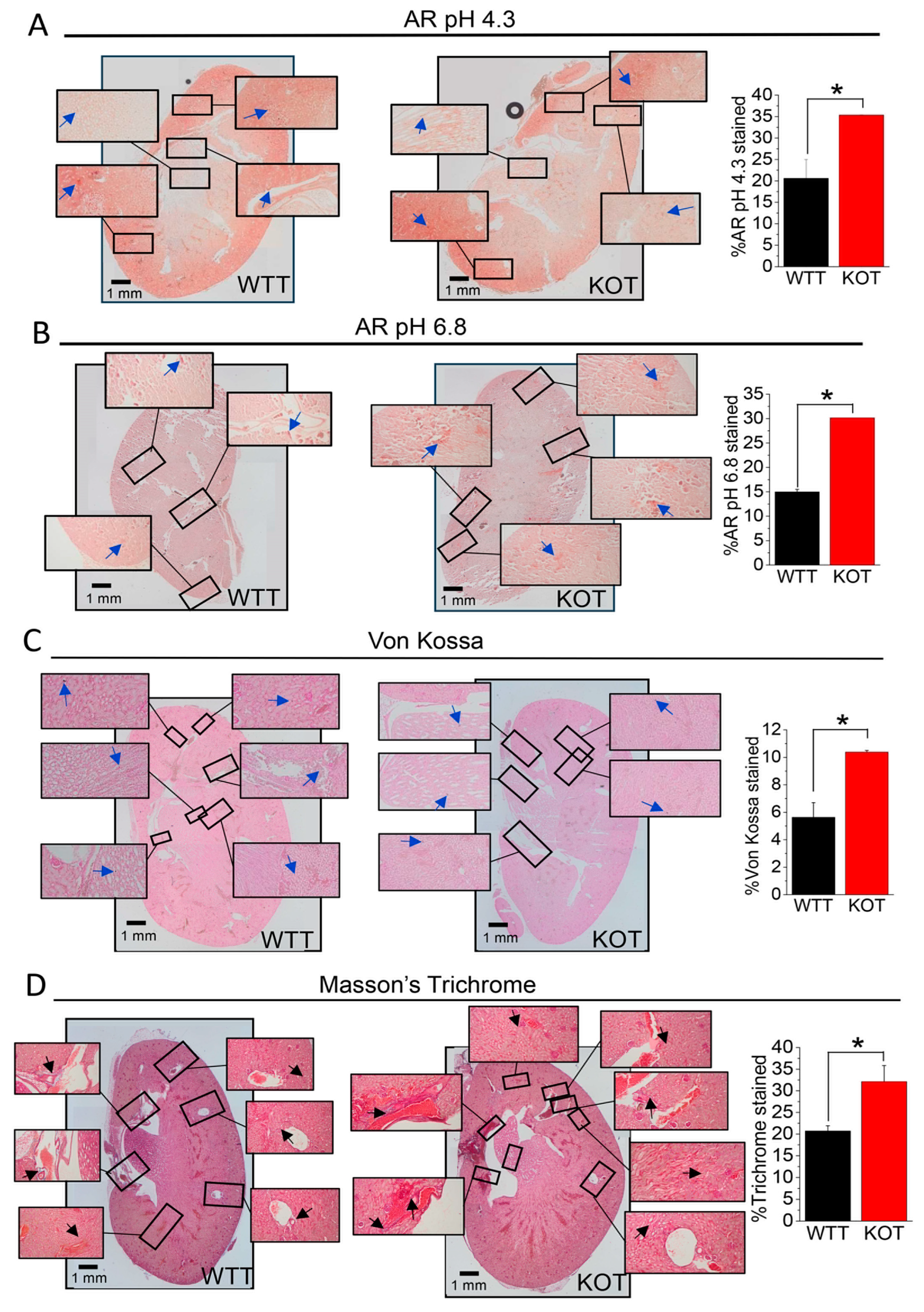
2.2. Supplementation of CaG + ACZ Imparts Diffuse Fibrosis and Calcification in TRPC3 KO Kidney Sections
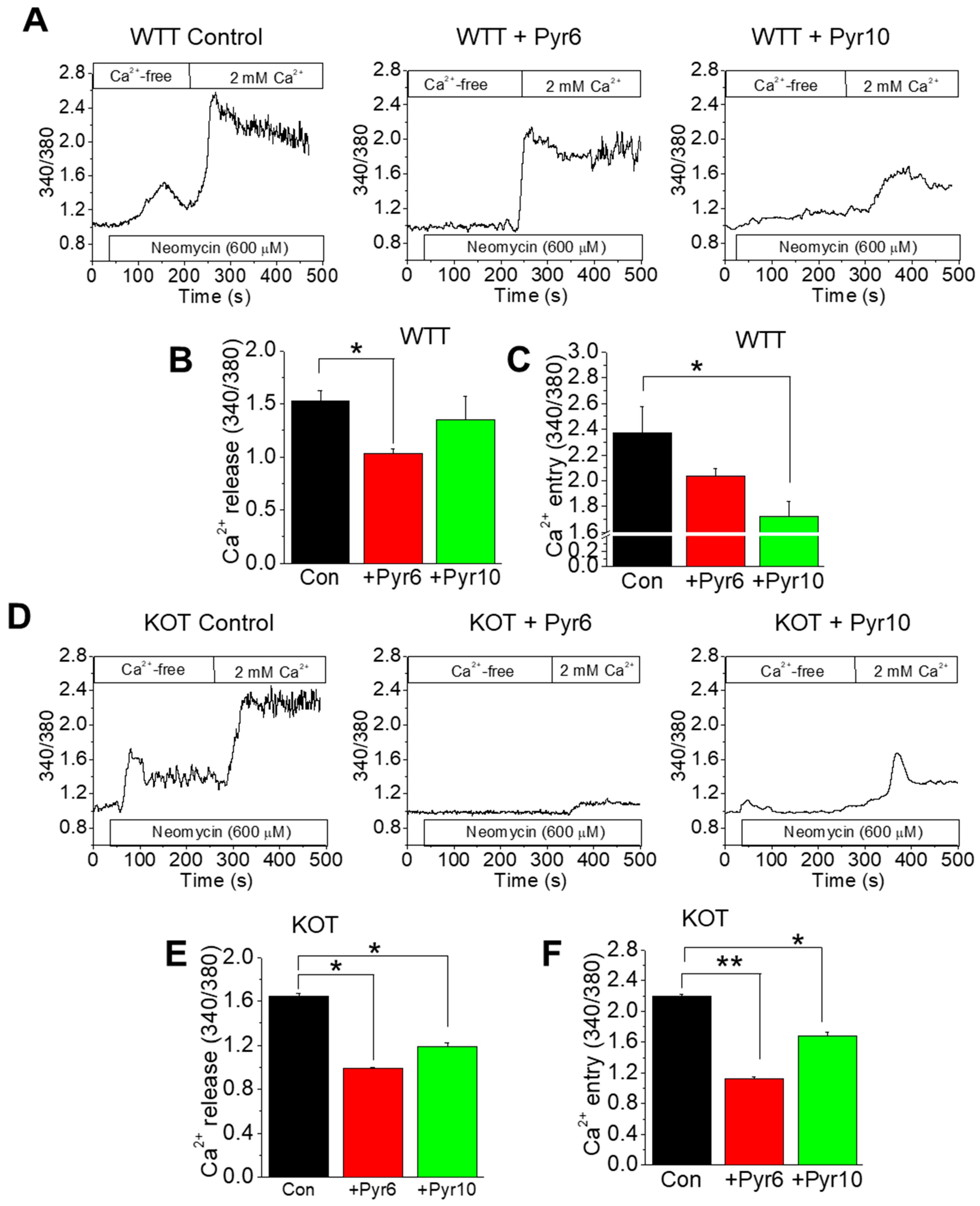
2.3. CaG + ACZ Treatment Increases SOCE Sensitivity in WTT and KOT Mice Proximal Tubular Cells
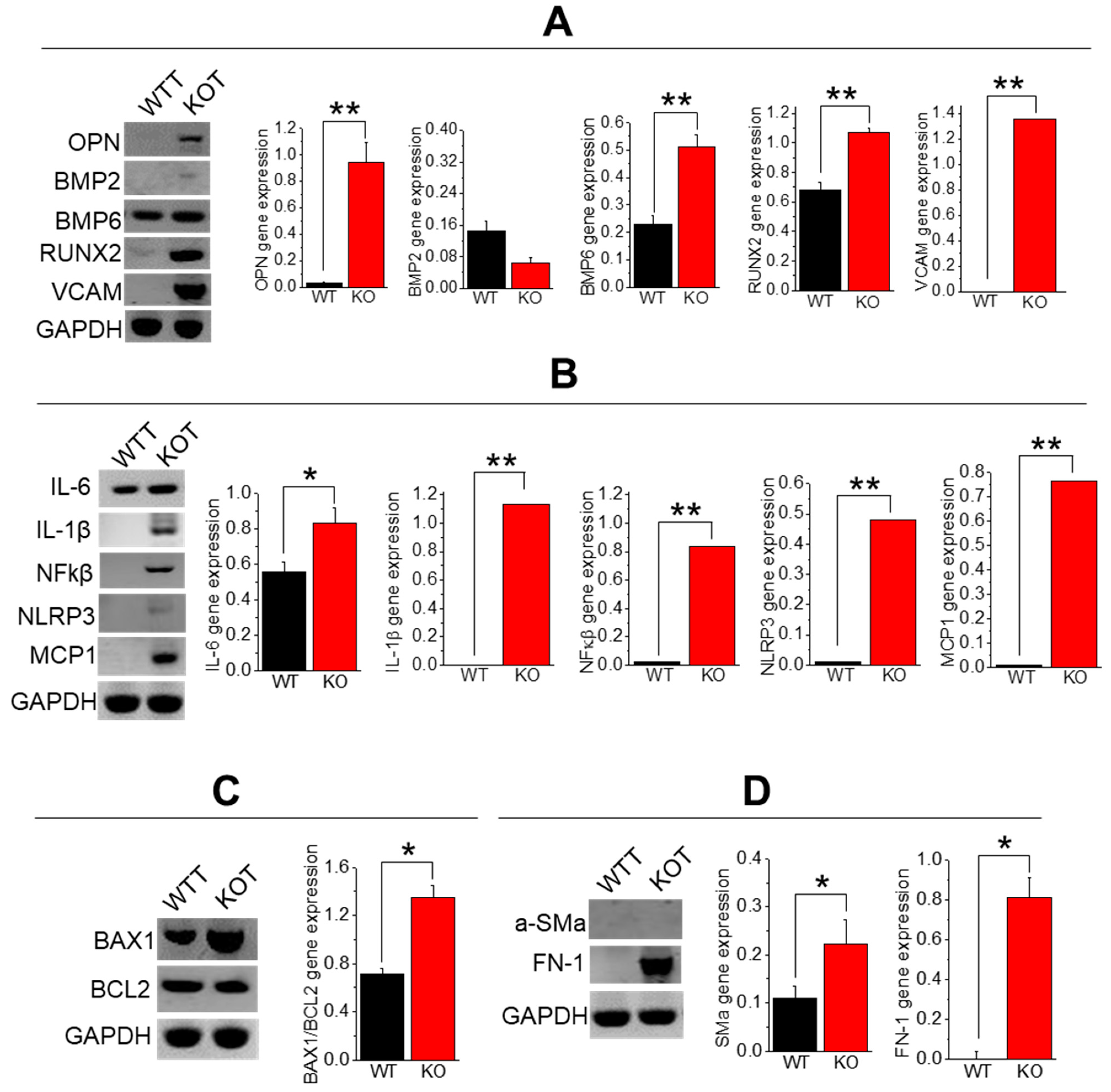
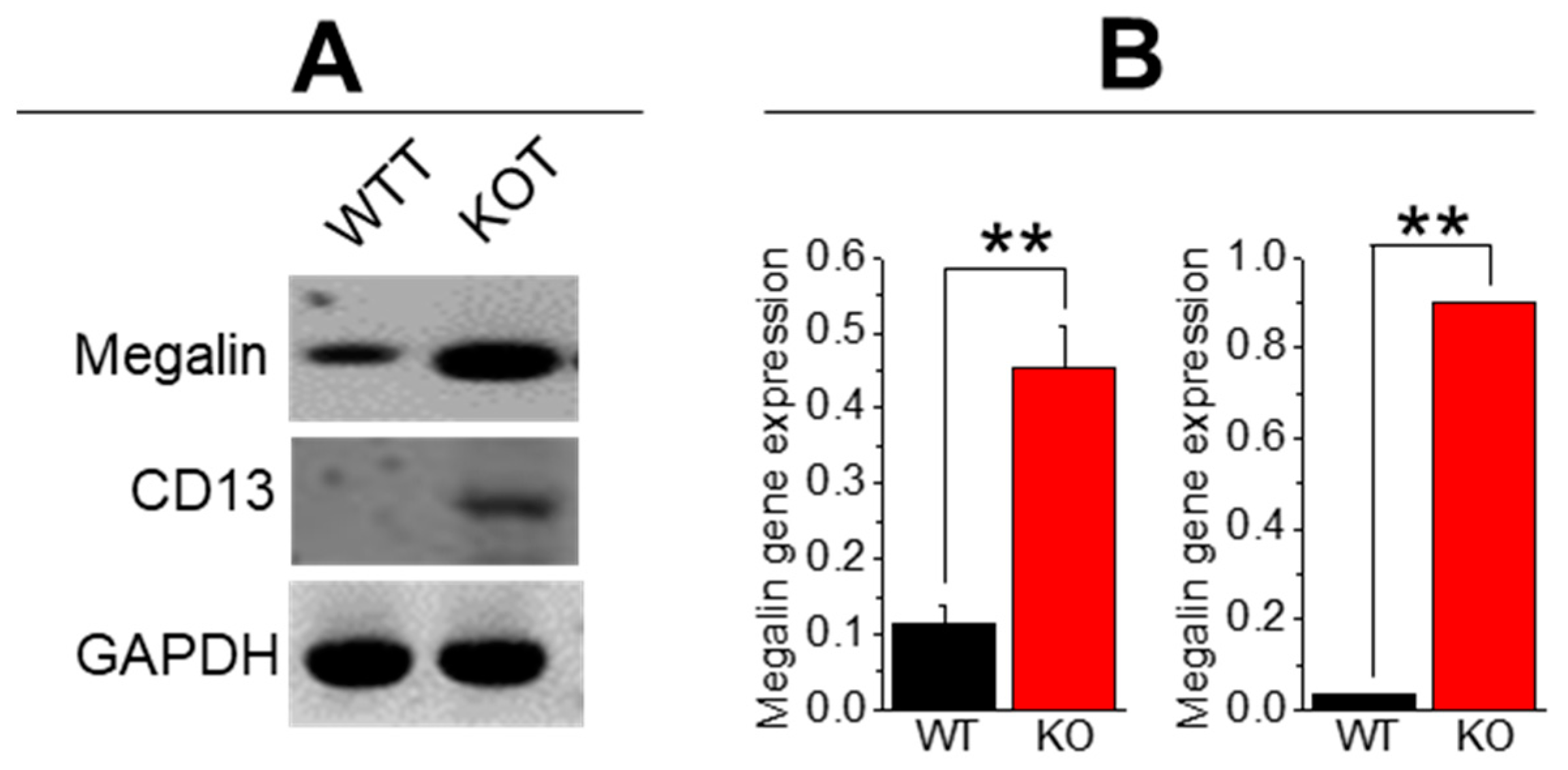
2.4. Enhanced Calcification, Inflammation, Fibrosis, and Apoptosis in TRPC3 KO PT Cells upon CaG+ACZ Treatment
3. Discussion
4. Methods and Materials
4.1. Animals
4.2. Chemicals
4.3. Isolation and Primary Culture of PT Cells
4.4. Fura-2 [Ca2+]o Measurements
4.5. Alizarin Red Staining of Urine Crystals
4.6. RT-PCR from Isolated PT Cells
4.7. Histochemistry of Kidney Sections
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Parks, J.H.; Coe, F.L.; Evan, A.P.; Worcester, E.M. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol. Dial. Transplant. 2009, 24, 130–136. [Google Scholar] [CrossRef]
- Wagner, C.A.; Mohebbi, N. Urinary pH and stone formation. J. Nephrol. 2010, 23 (Suppl. 16), S165–S169. [Google Scholar] [PubMed]
- Goldfarb, D.S. A Woman with Recurrent Calcium Phosphate Kidney Stones. Clin. J. Am. Soc. Nephrol. 2012, 7, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.A.; Gesek, F.A.; Stafford, N.; Wilson, C.; Oceandy, D.; Neyses, L.; Cartwright, E.J.; Alexander, R.T.; Dimke, H.; Edwards, A.; et al. Cellular calcium transport in renal epithelia: Measurement, mechanisms, and regulation. Physiol. Rev. 1995, 75, 429–471. [Google Scholar] [CrossRef] [PubMed]
- Worcester, E.M.; Coe, F.L. Does idiopathic hypercalciuria trigger calcium-sensing receptor-mediated protection from urinary supersaturation? J. Am. Soc. Nephrol. 2009, 20, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Pirson, Y. Genetics of hypercalciuric stone forming diseases. Kidney Int. 2007, 72, 1065–1072. [Google Scholar] [CrossRef]
- Hamm, L.; Nakhoul, N. Renal Acidification. In Brenner and Rector’s the Kidney, 8th ed.; Brenner, B.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 248–267. [Google Scholar]
- Worcester, E.M.; Coe, F.L. Clinical Practice Calcium Kidney Stones. N. Engl. J. Med. 2010, 363, 954–963. [Google Scholar] [CrossRef]
- Tiselius, H.-G. The role of calcium phosphate in the development of Randall’s plaques. Urolithiasis 2013, 41, 369–377. [Google Scholar] [CrossRef]
- Awuah Boadi, E.; Shin, S.; Yeroushalmi, S.; Choi, B.E.; Li, P.; Bandyopadhyay, B.C. Modulation of Tubular pH by Acetazolamide in a Ca2+ Transport Deficient Mice Facilitates Calcium Nephrolithiasis. Int. J. Mol. Sci. 2021, 22, 3050. [Google Scholar] [CrossRef]
- Ibeh, C.-L.; Yiu, A.J.; Kanaras, Y.L.; Paal, E.; Birnbaumer, L.; Jose, P.A.; Bandyopadhyay, B.C. Evidence for a regulated Ca2+ entry in proximal tubular cells and its implication in calcium stone formation. J. Cell Sci. 2019, 132, jcs225268. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ibeh, C.-L.; Boadi, E.A.; Choi, B.-E.; Roy, S.K.; Bandyopadhyay, B.C. Hypercalciuria switches Ca2+ signaling in proximal tubular cells, induces oxidative damage to promote calcium nephrolithiasis. Genes Dis. 2021, 9, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Potluri, A.; Ibeh, C.L.; Redman, R.S.; Paal, E.; Bandyopadhyay, B.C. Microcalcifications in stone-obstructed human submandibular gland are associated with apoptosis and cell proliferation. Arch. Oral. Biol. 2017, 82, 99–108. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, S.; Holden, D.; Ward, D.; Jones, M.; Elliott, A.; Riccardi, D. Aminoglycoside antibiotics induce pH-sensitive activation of the calcium-sensing receptor. Biochem. Biophys. Res. Commun. 2002, 297, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Caballero, D.; Ponsetto, J.; Chen, A.; Zhu, C.; Guo, J.; Bergwitz, C. Response of Npt2a knockout mice to dietary calcium and phosphorus. PLoS ONE, 2017; 12, e0176232. [Google Scholar]
- Christensen, E.I.; Willnow, T.E. Essential Role of Megalin in Renal Proximal Tubule for Vitamin Homeostasis. J. Am. Soc. Nephrol. 1999, 10, 2224–2236. [Google Scholar] [CrossRef] [PubMed]
- Gildea, J.J.; Shah, I.; Weiss, R.; Casscells, N.D.; McGrath, H.E.; Zhang, J.; Jones, J.E.; Felder, R.A. HK-2 Human Renal Proximal Tubule Cells as a Model for G Protein–Coupled Receptor Kinase Type 4–Mediated Dopamine 1 Receptor Uncoupling. Hypertension 2010, 56, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Awuah Boadi, E.; Shin, S.; Gombedza, F.; Bandyopadhyay, B.C. Differential biomolecular recognition by synthetic vs. biologically-derived components in the stone-forming process using 3D microfluidics. J. Mater. Chem. B 2021, 10, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Fluck, E.C.; Yazici, A.T.; Rohacs, T.; Moiseenkova-Bell, V.Y. Structural basis of TRPV5 regulation by physiological and pathophysiological modulators. Cell Rep. 2022, 39, 110737. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, N.P.; Moe, O.W. Proximal Tubule Function and Response to Acidosis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1627–1638. [Google Scholar] [CrossRef]
- Khan, S.R. Nephrocalcinosis in animal models with and without stones. Urol. Res. 2010, 38, 429–438. [Google Scholar] [CrossRef]
- Gombedza, F.C.; Shin, S.; Kanaras, Y.L.; Bandyopadhyay, B.C. Abrogation of store-operated Ca2+ entry protects against crystal-induced ER stress in human proximal tubular cells. Cell Death Discov. 2019, 5, 124. [Google Scholar] [CrossRef]
- Bihl, G.; Meyers, A. Recurrent renal stone disease—Advances in pathogenesis and clinical management. Lancet 2001, 358, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Ilzkovitz, M.; Kayembe, E.E.; Geers, C.; Pozdzik, A. Kidney Stones, Proteinuria and Renal Tubular Metabolic Acidosis: What Is the Link? Healthcare 2022, 10, 836. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Bin Liu, B.; Zhang, Y.; Pan, X.; Yu, X.-Y.; Shen, Z.; Song, Y.-H. Inflammasomes as therapeutic targets in human diseases. Signal Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E.; Zhu, Y.; Yu, C.; Zhuang, S.; Taylor, E.B.; Sasser, J.M.; Maeda, K.J.; Ryan, M.J.; et al. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Kim, Y.G.; Kim, D.-J.; Park, S.H.; Jeong, K.-H.; Lee, Y.H.; Lim, S.J.; Lee, S.-H.; Moon, J.-Y. Inflammasome-Independent Role of NLRP3 Mediates Mitochondrial Regulation in Renal Injury. Front. Immunol. 2018, 9, 2563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z. Effect and Regulation of the NLRP3 Inflammasome During Renal Fibrosis. Front. Cell Dev. Biol. 2020, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Katsnelson, M.A.; Rucker, L.G.; Russo, H.M.; Dubyak, G.R. K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J. Immunol. 2015, 194, 3937–3952. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.-T. Urine pH is an indicator of dietary acid–base load, fruit and vegetables and meat intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk population study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef]
- Kanbara, A.; Miura, Y.; Hyogo, H.; Chayama, K.; Seyama, I. Effect of urine pH changed by dietary intervention on uric acid clearance mechanism of pH-dependent excretion of urinary uric acid. Nutr. J. 2012, 11, 39. [Google Scholar] [CrossRef]
- Matlaga, B.R.; Shah, O.D.; Assimos, D.G. Drug-induced urinary calculi. Rev. Urol. 2003, 5, 227–231. [Google Scholar] [PubMed]
- Khan, S.R.; Canales, B.K.; Dominguez-Gutierrez, P.R. Randall’s plaque and calcium oxalate stone formation: Role for immunity and inflammation. Nat. Rev. Nephrol. 2021, 17, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Kos, C.H.; Karaplis, A.C.; Peng, J.B.; Hediger, M.A.; Goltzman, D.; Mohammad, K.S.; Pollak, M.R. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J. Clin. Investig. 2003, 111, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Englisch, C.N.; Paulsen, F.; Tschernig, T. TRPC Channels in the Physiology and Pathophysiology of the Renal Tubular System: What Do We Know? Int. J. Mol. Sci. 2022, 24, 181. [Google Scholar] [CrossRef] [PubMed]
- Khayyat, N.H.; Tomilin, V.N.; Zaika, O.; Pochynyuk, O. Polymodal roles of TRPC3 channel in the kidney. Channels 2020, 14, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Diebolt, C.M.; Schaudien, D.; Junker, K.; Krasteva-Christ, G.; Tschernig, T.; Englisch, C.N. New insights in the renal distribution profile of TRPC3—Of mice and men. Ann. Anat. 2024, 252, 152192. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, M.; Garner, M.K.; Farragut, K.M.; Kobori, H. The Establishment of a Primary Culture System of Proximal Tubule Segments Using Specific Markers from Normal Mouse Kidneys. Int. J. Mol. Sci. 2012, 13, 5098–5111. [Google Scholar] [CrossRef]
- Thompson, M.E.; Lewin-Smith, M.R.; Kalasinsky, V.F.; Pizzolato, K.M.; Fleetwood, M.L.; McElhaney, M.R.; Johnson, T.O. Characterization of Melamine-containing and Calcium Oxalate Crystals in Three Dogs with Suspected Pet Food-induced Nephrotoxicosis. Veter- Pathol. 2008, 45, 417–426. [Google Scholar] [CrossRef]
| Primer | Sequence (Sense, Antisense) | Product Size (bp) |
|---|---|---|
| GAPDH | 5′-ACTCCACTCACGGCAAATTC-3′ 5′-TCTCCATGGTGGTGAAGACA-3′ | 171 |
| OPN | 5′-GAGGAAACCAGCCAAGGACTAA-3′ 5′-TCCAGACTTGGTTCATCCAGC-3′ | 695 |
| BMP2 | 5′-TGGAAGTGGCCCATTTAGAG-3′ 5′-TGACGCTTTTCTCGTTTGTG-3′ | 166 |
| BMP6 | 5′-CCCGCCCGGAGTAGTTTTAGC-3′ 5′-AGTGCCCTTCTCCCCTCCATT-3′ | 168 |
| RUNX2 | 5′-CGGCCCTCCCTGAACTCT-3′ 5′-TGCCTGCCTGGGATCTGTA-3′ | 330 |
| VCAM | 5′-CCCAAGGATCCAGAGATTCA-3′ 5′-TAAGGTGAGGGTGGCATTTC-3′ | 489 |
| IL-6 | 5′-TGGAGTCACAGAAGGAGTGGCTAA-3′ 5′-TCTGACCACAGTGAGGAATGTCCA-3′ | 155 |
| IL-1β | 5′-TCCATGAGCTTTGTACAAGGA-3′ 5′-AGCCCATACTTTAGGAAGACA-3′ | 343 |
| NFκβ | 5′-GTGGAGGCATGTTCGGTAGT-3′ 5′-AGCTGCAGAGCCTTCTCAAG-3′ | 367 |
| NLRP3 | 5′-TGGGTTCTGGTCAGACACGAG-3′ 5′-GTCATTCCACTCTGGCTGGT-3′ | 486 |
| MCP1 | 5′-AGGTGTCCCAAAGAAGCTGT-3′ 5′-AAGACCTTAGGGCAGATGCA-3′ | 163 |
| BAX1 | 5′-GGAGACACCTGAGCTGACCT-3′ 5′-CTCAGCCCATCTTCTTCCAG-3′ | 510 |
| BCL2 | 5′-TCGTCGCTACCGTCGTGACTTCG-′ 5′-AGAGTCCGGTTCAGGTACTCAGTC-3′ | 242 |
| Megalin | 5-GTTCGGGTTGATGTTCTGGA-3′ 5′-ACTTGGGTAAGCCAGGGGTT-3′ | 369 |
| aSMa | 5′-AGATTGTCCGTGACATCAAGG -3′ 5′-TTGTGTGCTAGAGGCAGAGC-3′ | 538 |
| FN-1 | 5′-AATCCGGGAGCTTTTCCCTG-3′ 5′-GAGCTTCCTGTCCTGTCTTCT-3′ | 971 |
| mCD13 | 5′-TCACAGTGATAACGGGAAAGCCCA-3′ 5′-ATAAGCTCCGTCTCAGCCAATGGT-3′ | 799 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gombedza, F.C.; Shin, S.; Sadiua, J.; Stackhouse, G.B.; Bandyopadhyay, B.C. The Rise in Tubular pH during Hypercalciuria Exacerbates Calcium Stone Formation. Int. J. Mol. Sci. 2024, 25, 4787. https://doi.org/10.3390/ijms25094787
Gombedza FC, Shin S, Sadiua J, Stackhouse GB, Bandyopadhyay BC. The Rise in Tubular pH during Hypercalciuria Exacerbates Calcium Stone Formation. International Journal of Molecular Sciences. 2024; 25(9):4787. https://doi.org/10.3390/ijms25094787
Chicago/Turabian StyleGombedza, Farai C., Samuel Shin, Jaclyn Sadiua, George B. Stackhouse, and Bidhan C. Bandyopadhyay. 2024. "The Rise in Tubular pH during Hypercalciuria Exacerbates Calcium Stone Formation" International Journal of Molecular Sciences 25, no. 9: 4787. https://doi.org/10.3390/ijms25094787
APA StyleGombedza, F. C., Shin, S., Sadiua, J., Stackhouse, G. B., & Bandyopadhyay, B. C. (2024). The Rise in Tubular pH during Hypercalciuria Exacerbates Calcium Stone Formation. International Journal of Molecular Sciences, 25(9), 4787. https://doi.org/10.3390/ijms25094787





