The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health
Abstract
1. Introduction
2. Prebiotics and Types of Prebiotics
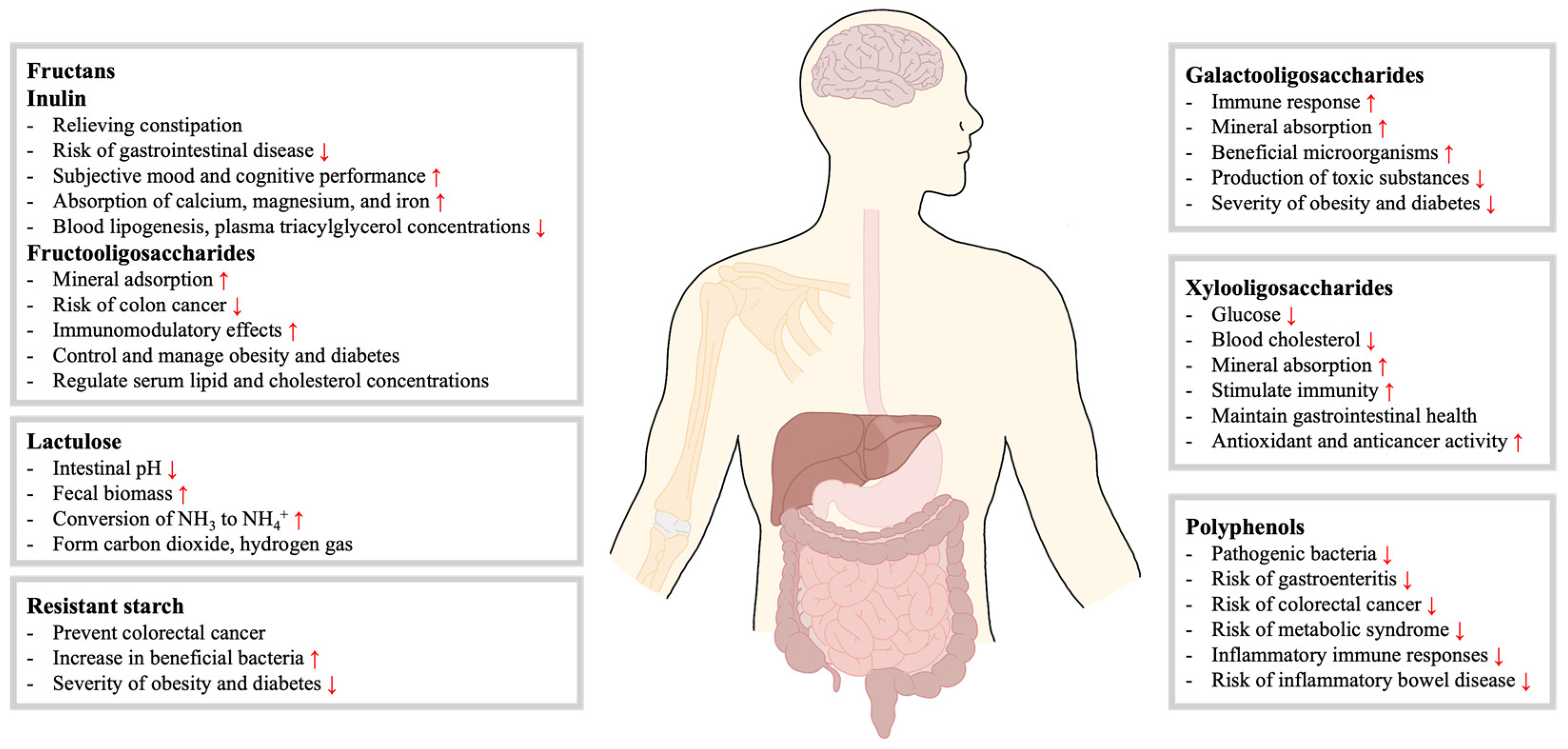
2.1. Fructans
2.1.1. Inulin
2.1.2. Fructooligosaccharides (FOS)
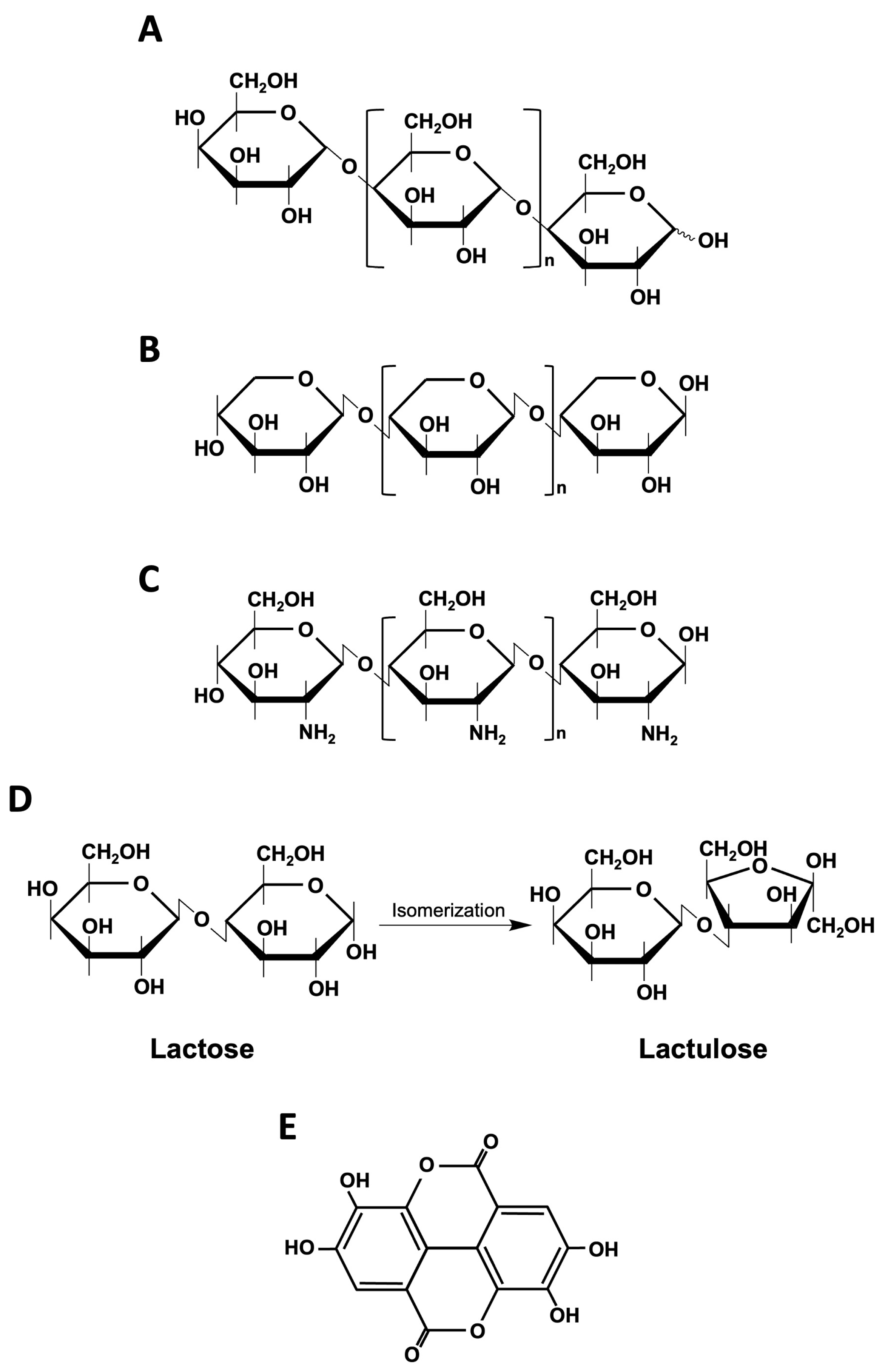
2.2. Galactooligosaccharides (GOS)
2.3. Xylooligosaccharides (XOS)
2.4. Chitooligosaccharides (COS)
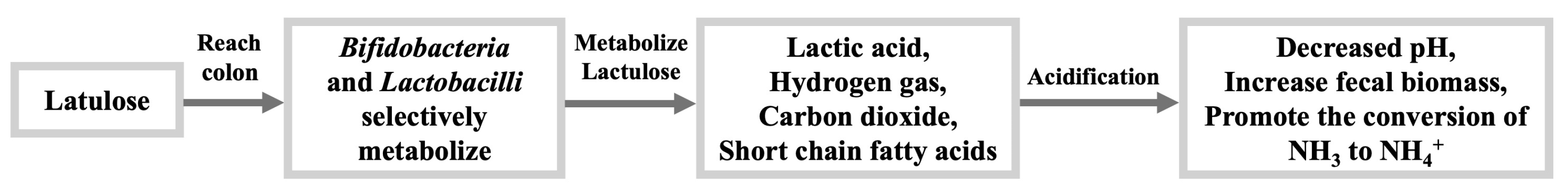
2.5. Lactulose
2.6. Resistant Starch (RS)
2.7. Polyphenols
3. Prebiotics Modulate Gut Microbiota
4. Influence of Prebiotics on Specific Health Conditions
4.1. Obesity
4.2. Inflammatory Bowel Disease (IBD)
4.3. Immune System
4.4. Mental Health
4.5. Other Diseases
5. Synbiotics
6. Market Potential of Prebiotics
7. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Lagier, J.C.; Khelaifia, S.; Alou, M.T.; Ndongo, S.; Dione, N.; Hugon, P.; Caputo, A.; Cadoret, F.; Traore, S.I.; Seck, E.H.; et al. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat. Microbiol. 2016, 1, 16203. [Google Scholar] [CrossRef] [PubMed]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr. J. Hematol. I 2016, 8, 2016025. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chi, L.; Zhu, Y.X.; Shi, X.C.; Tu, P.C.; Li, B.; Yin, J.; Gao, N.; Shen, W.S.; Schnabl, B. An Introduction to Next Generation Sequencing Bioinformatic Analysis in Gut Microbiome Studies. Biomolecules 2021, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- He, M.Q.; Shi, B.Y. Gut microbiota as a potential target of metabolic syndrome: The role of probiotics and prebiotics. Cell Biosci. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, F.; Startek, J.B.; Van den Ende, W. Prebiotics to fight diseases: Reality or fiction? Phytother. Res. 2013, 27, 1457–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cheong, K.L. Preparation, Structural Characterisation, and Bioactivities of Fructans: A Review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32 (Suppl. S1), 64–68. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Videla, S.; Vilaseca, J.; Antolin, M.; Garcia-Lafuente, A.; Guarner, F.; Crespo, E.; Casalots, J.; Salas, A.; Malagelada, J.R. Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am. J. Gastroenterol. 2001, 96, 1486–1493. [Google Scholar] [CrossRef]
- Hughes, R.; Rowland, I.R. Stimulation of apoptosis by two prebiotic chicory fructans in the rat colon. Carcinogenesis 2001, 22, 43–47. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Daubioul, C.; Neyrinck, A.; Lasa, M.; Taper, H.S. Inulin and oligofructose modulate lipid metabolism in animals: Review of biochemical events and future prospects. Br. J. Nutr. 2002, 87 (Suppl. S2), S255–S259. [Google Scholar] [CrossRef]
- Delgado, G.T.C.; Tamashiro, W.M.S.C.; Pastore, G.M. Immunomodulatory effects of fructans. Food Res. Int. 2010, 43, 1231–1236. [Google Scholar] [CrossRef]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Apolinario, A.C.; de Lima Damasceno, B.P.; de Macedo Beltrao, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Cherbut, C. Inulin and oligofructose in the dietary fibre concept. Br. J. Nutr. 2002, 87 (Suppl. S2), S159–S162. [Google Scholar] [CrossRef] [PubMed]
- Letexier, D.; Diraison, F.; Beylot, M. Addition of inulin to a moderately high-carbohydrate diet reduces hepatic lipogenesis and plasma triacylglycerol concentrations in humans. Am. J. Clin. Nutr. 2003, 77, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, A.L.; Rodrigues, L.R.; Lima, N.M.; Teixeira, J.A. An Overview of the Recent Developments on Fructooligosaccharide Production and Applications. Food Bioprocess. Tech. 2014, 7, 324–337. [Google Scholar] [CrossRef]
- Bali, V.; Panesar, P.S.; Bera, M.B.; Panesar, R. Fructo-oligosaccharides: Production, Purification and Potential Applications. Crit. Rev. Food Sci. 2015, 55, 1475–1490. [Google Scholar] [CrossRef]
- Gudiel-Urbano, M.; Goñi, I. Effect of fructooligosaccharide on nutritional parameters and mineral bioavailability in rats. J. Sci. Food Agr. 2002, 82, 913–917. [Google Scholar] [CrossRef]
- Bennett, N.; Greco, D.S.; Peterson, M.E.; Kirk, C.; Mathes, M.; Fettman, M.J. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus. J. Feline Med. Surg. 2006, 8, 73–84. [Google Scholar] [CrossRef]
- Lim, C.C.; Ferguson, L.R.; Tannock, G.W. Dietary fibres as “prebiotics”: Implications for colorectal cancer. Mol. Nutr. Food Res. 2005, 49, 609–619. [Google Scholar] [CrossRef]
- Schley, P.D.; Field, C.J. The immune-enhancing effects of dietary fibres and prebiotics. Brit J. Nutr. 2002, 87, S221–S230. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kaur, R.; Singh, R.S.; Kennedy, J.F. Biocatalytic strategies in the production of galacto-oligosaccharides and its global status. Int. J. Biol. Macromol. 2018, 111, 667–679. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Mahmood, S.; Liaqat, H.; Mushtaq, A.; Khan, S.; Amin, S.; Qi, X.H. Recent Advances in the Production, Analysis, and Application of Galacto-Oligosaccharides. Food Rev. Int. 2023, 39, 5814–5843. [Google Scholar] [CrossRef]
- Souza, A.F.C.E.; Gabardo, S.; Coelho, R.D.S. Galactooligosaccharides: Physiological benefits, production strategies, and industrial application. J. Biotechnol. 2022, 359, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Trends Food Sci. Tech. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Brienzo, M.; Carvalho, A.F.A.; Figueiredo, F.; Neto, P.O. Sugarcane bagasse hemicellulose properties, extraction technologies and xylooligosaccharides production. In Food Waste: Practices, Management and Challenges; Nova Science Publishers: New York, NY, USA, 2016; pp. 155–188. [Google Scholar]
- Moure, A.; Gullón, P.; Domínguez, H.; Parajó, J.C. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006, 41, 1913–1923. [Google Scholar] [CrossRef]
- Madhukumar, M.S.; Muralikrishna, G. Structural characterisation and determination of prebiotic activity of purified xylo-oligosaccharides obtained from Bengal gram husk (Cicer arietinum L.) and wheat bran (Triticum aestivum). Food Chem. 2010, 118, 215–223. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohyd. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.Q.; Zhao, Z.L.; Pi, X.G.; Meng, Y.Y.; Fei, D.B.; Liu, D.Q.; Wang, X. Effect of chitooligosaccharides on human gut microbiota and antiglycation. Carbohyd. Polym. 2020, 242, 116413. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef]
- Aït-Aissa, A.; Aïder, M. Lactulose: Production and use in functional food, medical and pharmaceutical applications. Practical and critical review. Int. J. Food Sci. Tech. 2014, 49, 1245–1253. [Google Scholar] [CrossRef]
- Olano, A.; Corzo, N. Lactulose as a food ingredient. J. Sci. Food Agr. 2009, 89, 1987–1990. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kumari, S. Lactulose: Production, purification and potential applications. Biotechnol. Adv. 2011, 29, 940–948. [Google Scholar] [CrossRef]
- Asp, N.-G.; Björck, I. Resistant starch. Trends Food Sci. Tech. 1992, 3, 111–114. [Google Scholar] [CrossRef]
- Klostermann, C.E.; Endika, M.F.; Kouzounis, D.; Buwalda, P.L.; de Vos, P.; Zoetendal, E.G.; Bitter, J.H.; Schols, H.A. Presence of digestible starch impacts fermentation of resistant starch. Food Funct. 2024, 15, 223–235. [Google Scholar] [CrossRef]
- Kang, M.S.; Jang, K.A.; Kim, H.R.; Song, S. Association of Dietary Resistant Starch Intake with Obesity and Metabolic Syndrome in Korean Adults. Nutrients 2024, 16, 158. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef]
- Baek, G.H.; Kim, Y.J.; Lee, Y.; Jung, S.C.; Seo, H.W.; Kim, J.S. Prebiotic potential of green banana flour: Impact on gut microbiota modulation and microbial metabolic activity in a murine model. Front. Nutr. 2023, 10, 1249358. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Danneskiold-Samsoe, N.B.; Barros, H.D.D.Q.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Marostica, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T. Gastrointestinal effects of prebiotics. Brit J. Nutr. 2002, 87, S145–S151. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- van Hoek, M.J.A.; Merks, R.M.H. Redox balance is key to explaining full. partial switching to low-yield metabolism. Bmc Syst. Biol. 2012, 6, 22. [Google Scholar] [CrossRef]
- Cook, S.I.; Sellin, J.H. Review article: Short chain fatty acids in health and disease. Aliment. Pharmacol. Ther. 1998, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.L.; Lin, T.L.; Chang, C.J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X. Regulation of bacterial pathogenesis by intestinal short-chain Fatty acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.B.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, J.; Wang, Y.Y.F.; Peng, B.; Liu, J.M.; Zhang, B.W.; Lv, H.; Wang, S. Protection of Galacto-Oligosaccharide against O157 Colonization through Enhancing Gut Barrier Function and Modulating Gut Microbiota. Foods 2020, 9, 1710. [Google Scholar] [CrossRef]
- Du, H.P.; Zhao, A.Q.; Wang, Q.; Yang, X.B.; Ren, D.Y. Supplementation of Inulin with Various Degree of Polymerization Ameliorates Liver Injury and Gut Microbiota Dysbiosis in High Fat-Fed Obese Mice. J. Agr. Food Chem. 2020, 68, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Hernot, D.C.; Boileau, T.W.; Bauer, L.L.; Middelbos, I.S.; Murphy, M.R.; Swanson, K.S.; Fahey, G.C. In Vitro Fermentation Profiles, Gas Production Rates, and Microbiota Modulation as Affected by Certain Fructans, Galactooligosaccharides, and Polydextrose. J. Agr. Food Chem. 2009, 57, 1354–1361. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; Leitch, E.C.M.; Child, M.W.; Flint, H.J. pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl. Env. Microb. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.S.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.J.; van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. Fems Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef]
- Schutz, F.; Figueiredo-Braga, M.; Barata, P.; Cruz-Martins, N. Obesity and gut microbiome: Review of potential role of probiotics. Porto Biomed. J. 2021, 6, e111. [Google Scholar] [CrossRef]
- Bothe, M.K.; Maathuis, A.J.H.; Bellmann, S.; van der Vossen, J.M.B.M.; Berressem, D.; Koehler, A.; Schwejda-Guettes, S.; Gaigg, B.; Kuchinka-Koch, A.; Stover, J.F. Dose-Dependent Prebiotic Effect of Lactulose in a Computer-Controlled In Vitro Model of the Human Large Intestine. Nutrients 2017, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Nicolucci, A.C.; Hume, M.P.; Martinez, I.; Mayengbam, S.; Walter, J.; Reimer, R.A. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology 2017, 153, 711–722. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Harpaz, N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology 2004, 126, 1634–1648. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, A.; Markandey, M.; Kedia, S.; Ahuja, V. Gut bacteriome in inflammatory bowel disease: An update on recent advances. Indian. J. Gastroenterol. 2024, 43, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Gerardi, V.; Lopetuso, L.R.; Del Zompo, F.; Mangiola, F.; Boskoski, I.; Bruno, G.; Petito, V.; Laterza, L.; Cammarota, G.; et al. Gut Microbial Flora, Prebiotics, and Probiotics in IBD: Their Current Usage and Utility. Biomed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Chan, B.D.; Leung, T.W.; Chen, M.X.; Tai, W.C.S. Beneficial and anti-inflammatory effects of formulated prebiotics, probiotics, and synbiotics in normal and acute colitis mice. J. Funct. Foods 2022, 88, 104871. [Google Scholar] [CrossRef]
- Du, Y.; Kusama, K.; Hama, K.; Chen, X.; Tahara, Y.; Kajiwara, S.; Shibata, S.; Orihara, K. Protective Effects of Inulin on Stress-Recurrent Inflammatory Bowel Disease. Int. J. Mol. Sci. 2024, 25, 2494. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Myhill, L.J.; Stolzenbach, S.; Hansen, T.V.A.; Skovgaard, K.; Stensvold, C.R.; Andersen, L.O.; Nejsum, P.; Mejer, H.; Thamsborg, S.M.; Williams, A.R. Mucosal Barrier and Th2 Immune Responses Are Enhanced by Dietary Inulin in Pigs Infected with Trichuris suis. Front. Immunol. 2018, 9, 2557. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.F.; Frokiær, H.; Christensen, A.G.; Bergström, A.; Licht, T.R.; Hansen, A.K.; Metzdorff, S.B. Dietary Xylooligosaccharide Downregulates IFN-γ and the Low-Grade Inflammatory Cytokine IL-1β Systemically in Mice. J. Nutr. 2013, 143, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Määttänen, P.; Napper, S.; Scruten, E.; Li, B.; Koike, Y.; Johnson-Henry, K.C.; Pierro, A.; Rossi, L.; Botts, S.R.; et al. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome 2017, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B.M. Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: Gut dysbiosis and altered brain function. Postgrad. Med. J. 2018, 94, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466. [Google Scholar] [CrossRef]
- Smith, A.P.; Sutherland, D.; Hewlett, P. An Investigation of the Acute Effects of Oligofructose-Enriched Inulin on Subjective Wellbeing, Mood and Cognitive Performance. Nutrients 2015, 7, 8887–8896. [Google Scholar] [CrossRef] [PubMed]
- Freijy, T.M.; Cribb, L.; Oliver, G.; Metri, N.J.; Opie, R.S.; Jacka, F.N.; Hawrelak, J.A.; Rucklidge, J.J.; Ng, C.H.; Sarris, J. Effects of a high-prebiotic diet versus probiotic supplements versus synbiotics on adult mental health: The “Gut Feelings” randomised controlled trial. Front. Neurosci-Switz. 2023, 16, 1097278. [Google Scholar] [CrossRef]
- Bauer, M.E.; Teixeira, A.L. Inflammation in psychiatric disorders: What comes first? Ann. N. Y. Acad. Sci. 2019, 1437, 57–67. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- Seijo, M.; Bryk, G.; Zeni Coronel, M.; Bonanno, M.; Rio, M.E.; Pita Martin de Portela, M.L.; Zeni, S.N. Effect of Adding a Galacto-Oligosaccharides/Fructo-Oligosaccharides (GOS/FOS(R)) Mixture to a Normal and Low Calcium Diet, on Calcium Absorption and Bone Health in Ovariectomy-Induced Osteopenic Rats. Calcif. Tissue Int. 2019, 104, 301–312. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Kulkarni, C.; Singh, P.; Sharma, S.; Singh, P.; Prajapati, G.; Gayen, J.R.; Ampapathi, R.S.; Mullick, A.; et al. A prebiotic, short-chain fructo-oligosaccharides promotes peak bone mass and maintains bone mass in ovariectomized rats by an osteogenic mechanism. Biomed. Pharmacother. 2020, 129, 110448. [Google Scholar] [CrossRef] [PubMed]
- Wang, X. The effect of chitooligosaccharides on gut microbiota in diabetic mice. Open Access Libr. J. 2019, 6, 1. [Google Scholar] [CrossRef]
- Ganguly, N.K.; Bhattacharya, S.K.; Sesikeran, B.; Nair, G.B.; Ramakrishna, B.S.; Sachdev, H.P.S.; Batish, V.K.; Kanagasabapathy, A.S.; Muthuswamy, V.; Kathuria, S.C.; et al. ICMR-DBT Guidelines for Evaluation of Probiotics in Food. Indian. J. Med. Res. 2011, 134, 22–25. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro Hepat. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with Claimed Probiotic Properties: An Overview of Recent Literature. Int. J. Env. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Hamasalim, H.J. Synbiotic as feed additives relating to animal health and performance. Adv. Microbiol. 2016, 6, 288–302. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef]
- Saini, R.; Patel, A.K.; Saini, J.K.; Chen, C.W.; Varjani, S.; Singhania, R.R.; Di Dong, C. Recent advancements in prebiotic oligomers synthesis via enzymatic hydrolysis of lignocellulosic biomass. Bioengineered 2022, 13, 2139–2172. [Google Scholar] [CrossRef]
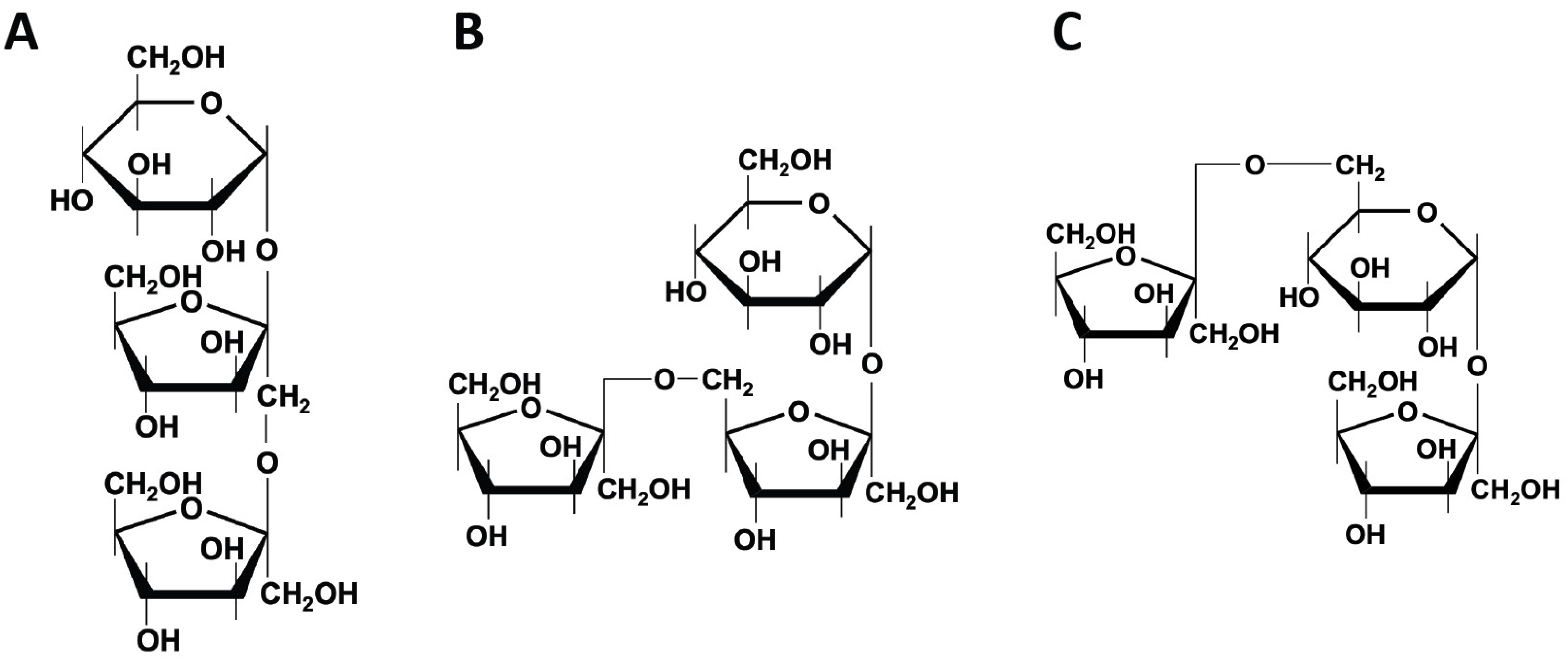

| Fructans | Linkage | Kestose Type |
|---|---|---|
| Inulin Fructooligosaccharides | β(2→1) | 1-kestose |
| Neo-inulin | β(2→1) | 6G-kestose |
| Levan | β(2→6) | 6-kestose |
| Mixed levan (graminan) | β(2→1) β(2→6) | 1-kestose 6-kestose |
| Neo-levan | β(2→1) β(2→6) | 6G-kestose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. https://doi.org/10.3390/ijms25094834
Yoo S, Jung S-C, Kwak K, Kim J-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. International Journal of Molecular Sciences. 2024; 25(9):4834. https://doi.org/10.3390/ijms25094834
Chicago/Turabian StyleYoo, Suyeon, Suk-Chae Jung, Kihyuck Kwak, and Jun-Seob Kim. 2024. "The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health" International Journal of Molecular Sciences 25, no. 9: 4834. https://doi.org/10.3390/ijms25094834
APA StyleYoo, S., Jung, S.-C., Kwak, K., & Kim, J.-S. (2024). The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. International Journal of Molecular Sciences, 25(9), 4834. https://doi.org/10.3390/ijms25094834







