Abstract
Glycosphingolipids (GSLs), a subtype of glycolipids containing sphingosine, are critical components of vertebrate plasma membranes, playing a pivotal role in cellular signaling and interactions. In human articular cartilage in osteoarthritis (OA), GSL expression is known notably to decrease. This review focuses on the roles of gangliosides, a specific type of GSL, in cartilage degeneration and regeneration, emphasizing their regulatory function in signal transduction. The expression of gangliosides, whether endogenous or augmented exogenously, is regulated at the enzymatic level, targeting specific glycosyltransferases. This regulation has significant implications for the composition of cell-surface gangliosides and their impact on signal transduction in chondrocytes and progenitor cells. Different levels of ganglioside expression can influence signaling pathways in various ways, potentially affecting cell properties, including malignancy. Moreover, gene manipulations against gangliosides have been shown to regulate cartilage metabolisms and chondrocyte differentiation in vivo and in vitro. This review highlights the potential of targeting gangliosides in the development of therapeutic strategies for osteoarthritis and cartilage injury and addresses promising directions for future research and treatment.
1. Introduction
Osteoarthritis (OA), a prevalent joint disorder, imposes a significant economic strain, costing the medical economy over USD 80 billion annually [1,2,3]. Despite extensive research, the pathogenesis of OA, marked by the progressive deterioration of articular cartilage and extracellular matrix (ECM), remains largely elusive [4]. The articular cartilage, also known as hyaline cartilage, is required by its elasticity, crucial for load absorption and distribution, and its smooth, lubricated surface, which facilitates motion and minimizes friction [5]. The avascular, aneural, lymphatic, and hypocellular nature of articular cartilage, a specialized connective tissue, restricts its access to nutrients and circulating chondrogenic progenitor cells, thereby limiting its intrinsic healing capacity [6,7]. This limitation can promote cartilage degeneration, culminating in OA. Recent attention has been drawn to the potential role of glycolipids in OA pathogenesis, following the discovery of significant alterations in the composition of glycosphingolipids (GSLs) in the articular cartilage of OA patients [8,9,10]. This has led to the consideration of biomembrane glycolipids as potential contributors to OA pathogenesis in post-genomic studies.
GSLs are key components of cell membranes, comprising a hydrophobic ceramide and a hydrophilic oligosaccharide residue (Figure 1). Ceramides are embedded in the outer leaflet of the plasma membrane, while oligosaccharides project into the extracellular space [11,12]. GSLs cluster on the cell-membrane surface, modulating transmembrane signaling and mediating intercellular and cell–matrix interactions [11,12,13,14]. An enzyme called glucosylceramide synthase encoded by the Uridine diphosphate (UDP)-glucose ceramide glucosyltransferase (Ugcg) gene is responsible for directing the first committed step in GSL synthesis [11,15,16,17]. Glucosylceramide is formed when a glucose moiety is transferred from UDP-glucose to ceramide, which is the precursor of most cellular GSLs. Mice with a global disruption in UGCG are embryonically lethal (E7.5), suggesting that GSLs are essential for embryonic development and differentiation [15,16,17,18]. It is now well established that some sphingolipids can regulate key biological functions, and these include cell growth and survival, cell differentiation, angiogenesis, autophagy, cell migration, and organogenesis [19]. GSLs are expressed not only in cartilage, but also in the nucleus pulposus tissue of the intervertebral disc [20], and are also abundant in nerve tissue [15,21,22], suggesting an association with pain [23,24]. Furthermore, specific bioactive sphingolipids have been linked to various pathologies, including inflammation-related diseases like atherosclerosis, rheumatoid arthritis, type II diabetes, obesity, cancer, and OA.
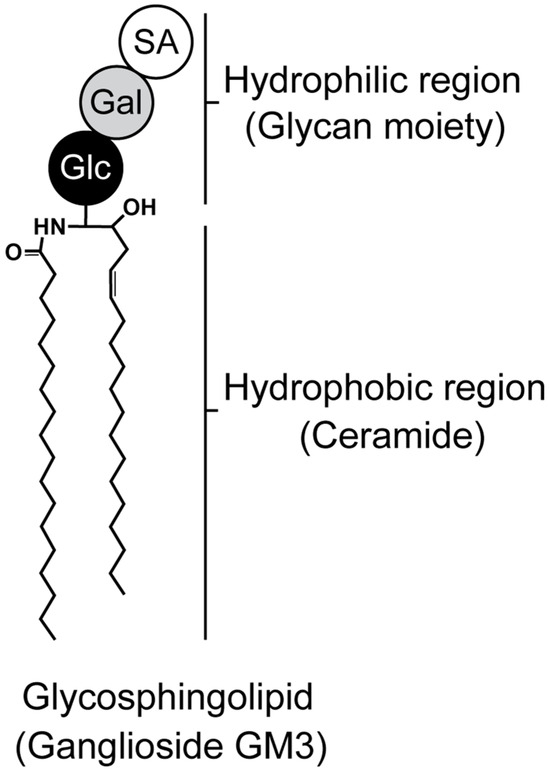
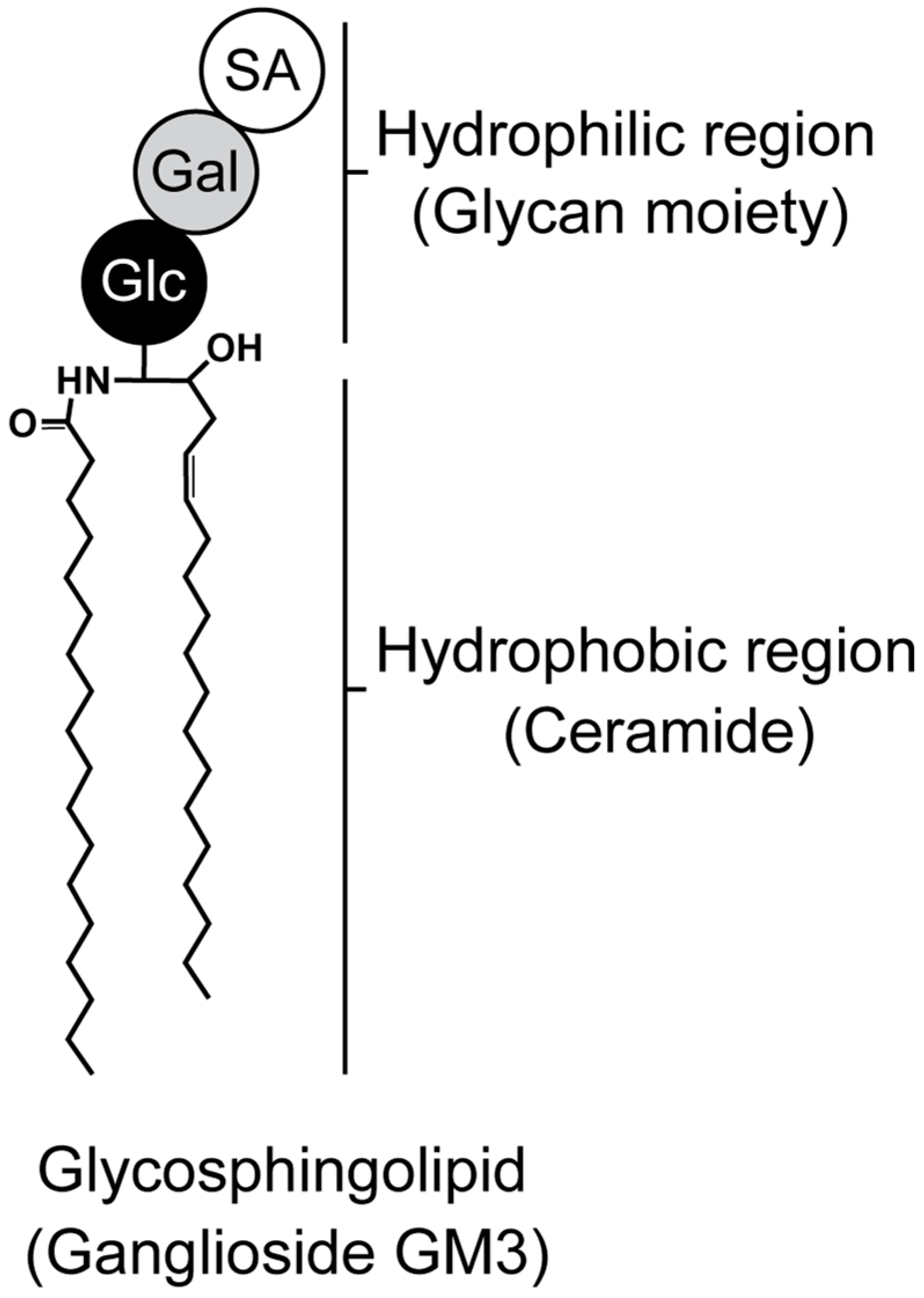
Figure 1.
Structure of glycosphingolipid. Ganglioside monosialodihexosylganglioside (GM3) is composed of both hydrophilic (glycan) and hydrophobic (ceramide) regions. Glc, Gal, and SA indicate glucose, galactose, and sialic acid, respectively.
The role of GSLs in articular cartilage, OA pathogenesis, and therapeutic prospects has not provided a comprehensive overview of the role of GSLs in articular cartilage. A brief description of the role of GSL on cartilage, focusing on the processes of homeostasis and the differentiation of chondrocytes, will be followed by an explanation of the endogenous ability of articular cartilage to heal. Finally, the usefulness and prospects of GSLs expressed on cell membranes as biomarkers for quality control in cartilage-regenerative medicine and as therapeutic target molecules for OA will be discussed.
2. Impact of GSLs on Cartilage Homeostasis
Ceramide, a principal constituent of glycolipids, instigates the mRNA expression of collagenase-1/matrix metalloproteinase (MMP)-1 and stromelysin-1/MMP-3 in human fibroblasts via the activation of three distinct mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK)1/2, stress-activated protein kinase/Jun N-terminal-kinase (SAPK/JNK), and p38 in cartilage [25]. Moreover, ceramide has also been discovered to be implicated in cartilage degeneration and apoptosis [26]. The ceramide pathway activator curtailed the production of inflammatory cytokines (interleukin [IL]-1β, IL-6, and IL-18) and the activation of the MAPK pathways (p-ERK, p-JUK, and p-p38) [27]. As mentioned above, systemic knockout mice of the UDP-glucose ceramide glucosyltransferase gene prove to be embryonically lethal because UGCG is the initial committed step in the synthesis of the majority of GSLs [11,15,16,17]. GSLs form clusters on the plasma membrane and undertake diverse roles in regulating membrane-mediated signal transduction and in mediating cell–cell and cell–extracellular matrix interactions [28,29,30,31]. Consequently, an endeavor is undertaken to identify even the most characteristic downstream glycolipid molecules by the sequential knockout of upstream glycosyltransferase genes implicated in the impairment of cartilage homeostasis in chondrocytes. The contents of such research studies are encapsulated in Figure 2 and Table 1.
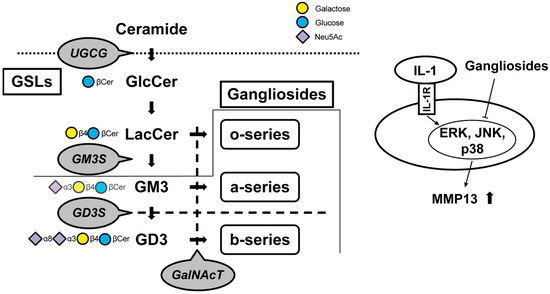
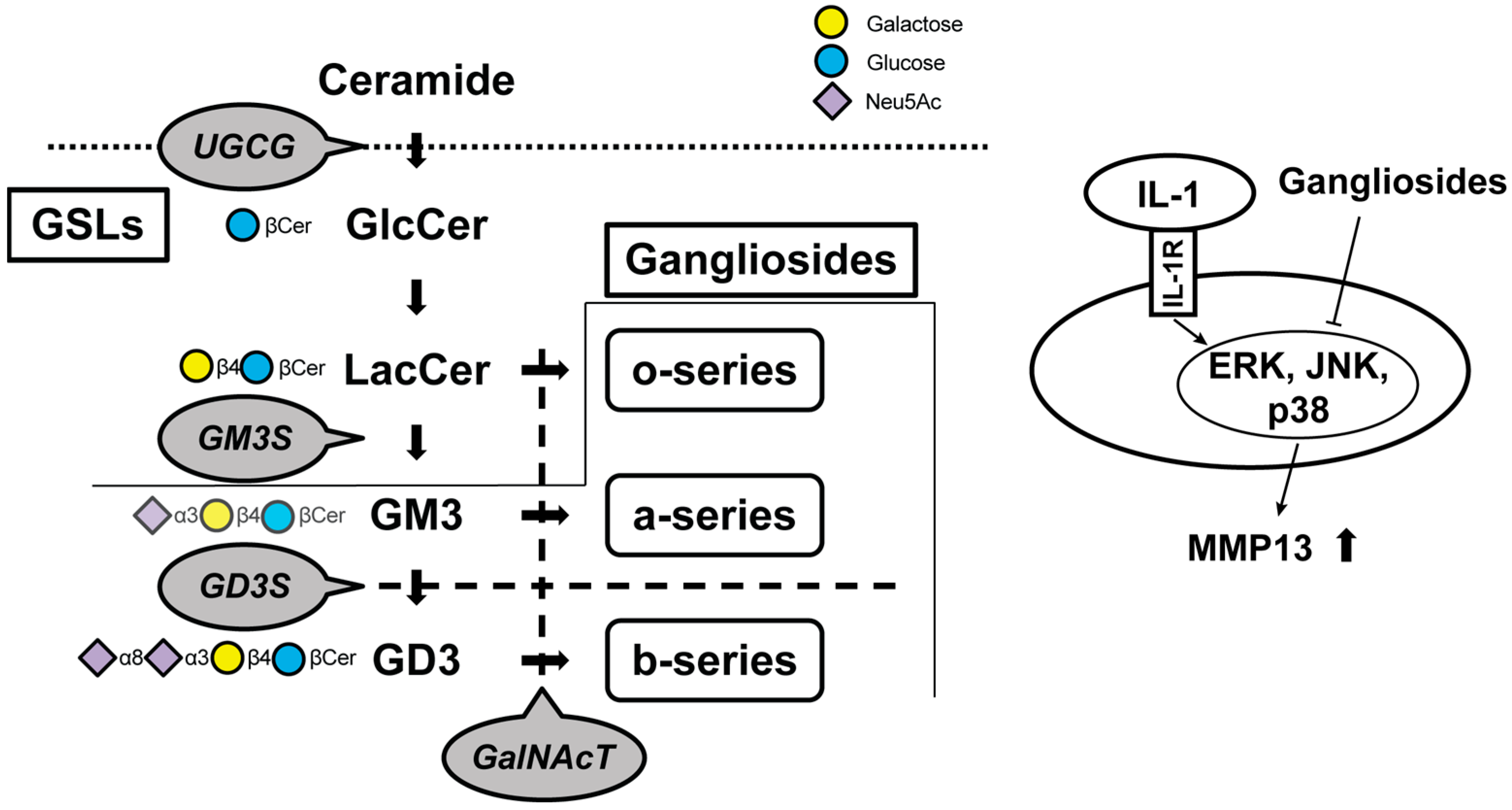
Figure 2.
Schematic of the biosynthetic pathway for gangliosides. Glucosylceramide (GlcCer) synthase, encoded by the UDP-glucose ceramide glucosyltransferase (Ugcg) gene, synthesizes GlcCer from ceramide. Gangliosides are classified as o-, a-, or b-series according to the number of sialic acids attached to galactose. Monosialodihexosylganglioside (GM3) synthase (GM3S) is required for the GSL synthesis downstream of lactosylceramide (LacCer), including the a-series and b-series. The b-series gangliosides are synthesized from the common precursor molecule Disialosyllactosylceramide (GD3), which is the product of GD3 synthase (GD3S, encoded by the Gd3s gene). β1, 4-N-acetylgalactosaminyltransferase (GalNAcT) activity is required for the elaboration of the o-, a-, and b-series precursors LacCer, GM3, and GD3, respectively. Gangliosides suppress protease upregulation through the mitogen-activated protein kinase signaling pathways. Cer, ceramide; GSLs, glycosphingolipids; IL, interleukin; MMP, matrix metalloproteinase; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; p38, Thr180/Tyr182.
A decrease in all major gangliosides, contrasting with a marked increase in the monosialodihexosylganglioside (GM3), has been demonstrated in osteoarthritic fibrillated cartilage. Some previous studies have shown that gangliosides have tissue-protective effects against oxidative stress or apoptosis in neuronal, cardiac, and hepatic cells [32,33,34,35,36]. The results of the series of studies indicate that the loss of gangliosides results in greater cartilage vulnerability to IL-1α/β stimulation in the cartilage-degradation process by increasing MMP-13 secretion and chondrocyte apoptosis. The mechanical properties of cartilage have been mainly focused on hyaluronan and chondroitin sulfate [37,38]. Since these increases and decreases affect the physical properties of cartilage, they have been relatively well studied and are attracting attention as a therapeutic approach [39]. Sphingomyelin is involved in the boundary lubrication of articular cartilage [40], and the presence of GSLs on chondrocytes appears to raise the threshold of sensitivity to mechanical stress and resist catabolic reactions [41]. This is not unrelated to the fact that GSLs are expressed on the plasma membrane. On the other hand, there are indications that replenishing the cells with the missing gangliosides can restore normal activation [42,43]. The fact that β1, 4-N-acetylgalactosaminyltransferase (GalNAcT) knockout mice must be supplemented with all three series to be rescued indicates that each series of gangliosides is essential for cartilage maintenance (Figure 2) [43]. Considering previous studies [18,42,43,44], a treatment supplementing the o-, a-, and b-series gangliosides below GalNAcT appears promising, with future therapeutic trials anticipated.
Glycosidase inhibitors are also considered to be an important target for cartilage regeneration. They are directly linked to osteoarthritis because N-acetyl-beta-hexosaminidase is the predominant glycosidase released by chondrocytes to degrade glycosaminoglycan [45]. The stimulation of chondrocytes with IL-1β selectively increases extracellular hexosaminidase activity among many enzymes, suggesting that hexosaminidase is the cartilage matrix-degrading enzyme activated by inflammatory stimuli [45]. The inhibitor of this hexosaminidase is shown to modulate intracellular levels of glycolipids, including Galβ1,4(Neu5Acα2,3)Galβ1,4Glc (GM2) and asialo ganglioside GM2 (GA2) (o- and a-series gangliosides) [46]. Since these kinetics have been studied in adults and in the elderly who have formed permanent articular cartilage, caution should be exercised when applying them to children or infants.

Table 1.
Genetic defects in mouse glycan formation and physiologic consequences.
Table 1.
Genetic defects in mouse glycan formation and physiologic consequences.
| Glycosyltransferase | Lost Glycolipids | Consequences of Depletion of Its Glycolipid | References |
|---|---|---|---|
| UGCG (Glucosylceramide synthase) | GSLs | Embryonic death. Reduced insulative capacity of the myelin sheath. Col2-Ugcg−/− mice enhance the development of OA. | [16,17,18,41,47,48] |
| ST3GalIV (GM3S) | Gangliosides other than the o-series | GM3 plays an immunologic role. Heightened sensitivity to insulin. Severely reduced CD4+ T cell proliferative response and cytokine production. Promotes OA and RA but cartilage regeneration. | [42,44,49,50,51] |
| ST8SiaI (GD3S) | b-series ganglioside | Tumor-associated carbohydrate antigens (TACA) in neuro-ectoderm-derived cancers. Suppression of age-related bone loss. Deteriorates OA with aging. | [43,52,53,54,55] |
| GalNAcT (GM2/GD2S) | Almost all gangliosides except GM3, GD3, and GT3 | Age-dependent neurodegeneration and movement disorders associated with it. Defects in spermatogenesis and learning. Exacerbating OA progression. | [43,56,57,58] |
3. Role of GSLs in Cartilage Repair and Differentiation Processes
GSLs play a crucial role in the repair and differentiation processes of articular cartilage [50,59]. These processes are essential for addressing cartilage defects, commonly observed in osteoarthritis. The unique properties of GSLs facilitate the regeneration of cartilage tissue, which is key to restoring joint function [43,60]. Understanding the mechanisms behind cartilage regeneration, particularly the role of GSLs, is critical.
Here, various aspects of cartilage repair will be explored, with a focus on the role of glycolipids in promoting the regeneration and repair process through chondrogenic differentiation.
3.1. Endogenous Potential to Heal in Articular Cartilage
To enhance endogenous cell recruitment to the injury site, the biological process within a living organism after articular cartilage injury needs to be clarified. Models have been constructed in mice [61,62,63], rats [64], rabbits [65,66,67], horses [68], and canines [69] to study the repair process of articular cartilage. This has successively revealed various molecular reactions after articular cartilage injury [70,71,72,73,74]. Even though the species are different, the major ganglioside in articular cartilage appears to be GM3 [75,76]. GSLs consist of several types of glycolipids and are classified into several groups depending on their structural features, which include neo-lacto-series, globo-series, isoglobo-series, and ganglio-series (gangliosides) [28,44]. GM3 functions as a precursor molecule for the majority of the more intricate ganglioside species [49], and ganglioside expression patterns in cells during differentiation undergo alterations in response to cytokine and growth factor stimulation [77,78,79]. Many receptor tyrosine kinases, including the epidermal growth factor receptor (EGFR), an N-glycosylated transmembrane protein with an intracellular kinase domain, are localized in lipid rafts and inhibit the tyrosine kinase activity of EGFR through the inter-glycan interaction between N-glycans on EGFR and oligosaccharides on GM3 [78,79]. Equally, GM3 inhibits vascular endothelial growth factor (VEGF)-induced activation of VEGF receptor-2 (VEGFR-2) by blocking VEGF dimerization and inhibits VEGF binding to VEGFR-2 through GM3-specific interactions with the extracellular domain of VEGFR-2 [77]. Predicated on the outcomes of these in vitro studies, it is anticipated that GM3 would assume a significant role in the process of articular cartilage repair, as it is implicated in the regulation of tyrosine phosphorylation of growth factor receptors and angiogenesis. During the articular cartilage healing process, GM3 has dual roles in recruiting chondrogenic precursor cells to the injury site and in the induction of hypertrophic differentiation in chondrocytes [50]. The manipulation of ganglioside expression may be the future direction in articular cartilage regeneration. Accordingly, a site- and time-specific intervention is needed to manipulate glycosphingolipids in articular cartilage. Genome editing can overcome these difficulties, with CRISPR (clustered regularly interspaced short palindromic repeats)-Cas9 being the simplest and most rapid technology [80]. Previously, CRISPR-Cas9 has been used to modify GSL glycans on leukocyte cells [81], and it might be useful for regenerating cartilage [82]. The multi-omics of cartilage from a spatio-temporal perspective is currently of vital interest [83,84]. On the other hand, spatial and temporal analysis of glycome expression patterns on tissues is limited to N-glycans due to technical difficulties [85,86,87,88]. Advances in innovative spatial biology techniques are expected to allow intact tissue sections to be examined using the glycome of GSLs with spatial data.
3.2. Changes in the Glycan Structure during Chondrogenic Differentiation
Chondrogenic differentiation is a well-organized process; cartilage is formed from condensed mesenchymal tissue that differentiates into chondrocytes and begins to secrete the molecules that make up the extracellular matrix [89]. Extracellular enzymes, which include the matrix metallopeptidases, lead to the activation of cell signaling pathways and gene expression in a temporal-spatial-specific manner during the development process. The recruited mesenchymal stem cells may attempt to differentiate into chondrocytes after articular cartilage injury [90,91]. However, the response after injury is not a fully recapitulated process of development, resulting in regenerated cartilage-like tissue that does not possess typical biomolecules of hyaline cartilage such as type II collagen and aggrecan, and a proportion of their chemical constitutes differ from those in the original cartilage [92,93]. Molecules promoting the selective differentiation of multipotent mesenchymal stem cells into chondrocytes have been reported to stimulate the repair of injured articular cartilage (MMP14, microphthalmia-associated transcription factor [MTIF], Glycoprotein Nmb [GPNM], Secreted Phosphoprotein 1 [SPP1], Prostaglandin-Endoperoxide Synthase 2 [PTGS2], Vascular Endothelial Growth Factor A [VEGFa], and JunD Proto-Oncogene [JUND] [94]. Therefore, the regulation system for chondrogenic differentiation is attracting attention.
Glycosylation is one of the post-translational modifications in cell-surface proteins and extracellular matrix proteins which regulate a variety of biological functions, including the enhancement of protein stability, controlling cell-to-cell communication, and adhesion [95]. In addition, this process is known to contribute to the pathogenesis of various kinds of diseases [96,97]. Quantitative and qualitative analyses of N-glycans during chondrogenic differentiation were previously performed using the glycoblotting method [98], followed by glycoform-focused reverse proteomics and genomics using a mouse pre-chondrogenic cell line, an embryonal carcinoma-derived chondrogenic cell line ATDC5 [99]. The levels of high-mannose-type N-glycans increase during chondrogenic differentiation, suggesting that N-glycans may have key roles in the differentiation and/or homeostatic maintenance of chondrocytes [100]. As for hypertrophic differentiation in chondrocytes, Yan et al. reported that resting chondrocytes exposed to concanavalin A, which binds specifically to high-mannose-type structures, selectively differentiated to the hypertrophic stage [101,102]. Concanavalin A is known to be a potent mitogen and is found to promote differentiation by inducing the cross-linking of high-mannose-type N-glycans. A comprehensive analysis of all classes of glycoconjugates on articular cartilage, including N-glycans, O-glycans, free oligosaccharides (fOSs), glycosaminoglycans (GAGs), and GSLs, revealed dynamic alterations [76,103]; the quantitative glycan profile showed that several N-glycans increased significantly with hypertrophy, whereas GSL and fOS decreased significantly. These findings suggest that glycan markers can be used as differentiation biomarkers for chondrogenic differentiation and may help to evaluate the regenerative product after articular cartilage injury.
4. Cell Sources
As stated in the Introduction Section, articular cartilage is a type of hyaline cartilage that enables smooth movements between bones in articulating joints, which requires both weight-bearing and low-friction capability [104]. Therefore, cartilage regeneration with these high qualities is important. The ultimate goal for ideal cartilage regeneration is to restore these key properties of the original hyaline cartilage in terms of histological structure and biomechanical functions, which seems to be only achieved by replacing it with healthy cartilage tissue [105]. Several types of cell-based approaches have been introduced at the present moment [106]. Representative strategies include autologous cartilage implantation, mesenchymal stem cells, and induced pluripotent stem cells. Table 2 summarizes three representative cell types and reports on cartilage regenerative medicine. This article covers cell-based regenerative medicine in articular cartilage, primarily based on glycobiology.

Table 2.
Cell sources and cartilage-regenerative medicine.
4.1. Autologous Chondrocyte Implantation
Autologous chondrocyte implantation is the beginning of a major trend in regenerative medicine for cartilage injuries, published by Lars Peterson and his group in Sweden in 1994 [144]. Articular cartilage does not possess access to the nutrients or circulating chondrogenic progenitor cells, and cartilage lacks the natural potential to overcome a sufficient healing response by possessing a nearly acellular nature [145]. Consequently, articular cartilage has limited healing potential; therefore, it can lead to cartilage degeneration and ultimately result in OA. Autologous chondrocyte implantation is conceived to compensate for the sparse and nonvascular nature of cartilage by providing cultured chondrocytes: a cell-based therapy consisting of two-staged procedures for full-thickness defects of articular cartilage in the knee [144]. This procedure requires the first harvest of chondrocytes from a non-weight-bearing area of articular cartilage. After a culture of 4 to 6 weeks, a second-stage procedure is undertaken to implant amplified chondrocytes into the defect. Considering the limited healing potential for articular cartilage, these procedures seem to be ideal.
Since the first clinical report was published, several authors have demonstrated successful clinical outcomes of this procedure for cartilaginous lesions [146,147,148]. However, there remain concerns about the dedifferentiation of chondrocytes during the culture period due to the limited proliferative capacity [149,150,151]. The cartilage extracellular matrix possesses various glycosylated proteins, which contribute to the maintenance of its specific functions [152]. Sialic acids are negatively charged sugars expressed at the terminal positions of N- and O-linked oligosaccharides, which are attached to cell surfaces or secreted glycoproteins. As a result of their non-reducing terminal position, sialic acids are involved in highly specific recognition phenomena [153,154]. Primary human chondrocytes predominantly express α2,6-specific sialyltransferases and α2,6-linked sialic acid residues in glycoprotein N-glycans [155]. Interestingly, inflammation stimuli induced a shift from α2,6-linked to α2,3-linked sialic acid, suggesting that α2,6-linked sialic acid can be used as a biomarker for quality control in amplified human cartilage.
4.2. Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells are multipotent stem cells and can be obtained from various organs including bone marrow, synovium, periosteum, adipose tissue, and skeletal muscle [156]. MSCs are attractive cell sources in regenerative medicine based on their abilities to self-renew and differentiate into mesenchymal tissue lineage [157]. During the last few years, the use of MSCs and their cell-free derivatives has seen an increasing number of applications in disparate medical fields, including chronic musculoskeletal conditions [60,158,159,160,161]. The advantage of autologous MSC transplantation is that it avoids the problem of defects caused by cartilage harvesting to secure cells in autologous cartilage transplantation. MSCs are not considered to be tumorigenic like ES cells, and only minor side effects such as fever, chills, and liver damage have been reported as side effects of MSC therapy. Furthermore, the potency of MSCs can be enhanced using the bone marrow aspirate concentrate (BMAC) method [142,162]. BMAC therapy has a slow onset of benefit (2 to 4 years) [143], so cell quality is crucial. One of the concerns is that MSCs are heterogeneous populations, whose capability to differentiate varies depending on the tissues harvested and the donor age. MSCs are grouped by the common characteristics of CD44, 73, 90, and 105 positivity and CD31 and 45 negativity, but these do not necessarily define a “stem cell”. In addition, since the properties of these cells change depending on the isolation method, culture conditions, and passages when they are grown in vitro until the required cell number is reached, there has been a need for an appropriate biomarker that could define “stem cells”. Considering how OA cartilage is characterized by a reduction of most gangliosides, these gangliosides could be combined with expression information such as high-mannose-type N-glycans, linear poly-N-acetyllactosamine chains, and α2,3-sialylation to more accurately define MSC undifferentiated characteristics.
Glycan expression changes rapidly upon differentiation; thus, many of the typical cell markers are glycans [163,164]. This is in contrast to the little change in the protein expression profiles between differentiated and undifferentiated cells [165]. Glycosylation features associated with bone marrow-derived MSCs included high-mannose-type N-glycans, linear poly-N-acetyllactosamine chains, and α2,3-sialylation [166]. Their cellular differentiation stage can be determined using these glycomics. Tateno et al. carried out glycome analysis on different passages of adipose-derived human MSCs (hMSCs) using high-density lectin microarrays to identify glycan markers that distinguish MSCs to have enough capability to differentiate [167]. This report indicated that α2,6-linked sialic acid-specific lectins showed stronger binding to the early passage of adipose-derived hMSCs with the ability to differentiate to adipocytes and osteoblasts than late passage cells without the ability did. They also reported quantitative glycome analysis targeting both N- and O-glycans from early and late passages of adipose tissue-derived hMSCs and showed that the expression of α2,6-sialylated N-glycans varies depending on the differentiation potential of stem cells but not O-glycans, suggesting that α2,6-sialylated N-glycans can be used as biomarkers for the quality control of hMSCs [168]. The presence of α2,6-linked sialic acid structure is a characteristic of pluripotent stem cells that possess higher differentiation potential. This may serve as an indicator of their differentiation potential. Ryu et al. showed that the gangliosides GM3 and GD3, which contain α2,3- and α2,8-linked sialic acids, were expressed after the chondrogenic differentiation of synovium-derived hMSC aggregates [59]. In the same way, GM3 expression increased temporarily following the chondrogenic differentiation of hMSCs derived from bone marrow [169]. Considering OA cartilage is characterized by a decrease in most gangliosides, these gangliosides may be useful in developing therapeutic agents for MSC-based articular cartilage regeneration in articular cartilage disease.
4.3. Induced Pluripotent Stem Cells (iPSCs)
In 2006, Takahashi and Yamanaka reported pluripotential stem cells from mouse embryonic or adult fibroblasts by introducing four factors, Oct3/4, Sox2, c-Myc, and Klf4 [170]. The method to establish human iPSCs dramatically evolved and simplified [171]. This progress provides us with opportunities to understand the disease mechanisms and promote regenerative medicine [172,173]. To avoid the rejection of differentiated cells originating from iPSC transplantation, autogenous transplantation with iPSCs originating from the individual’s own cells is ideal. However, these procedures require time to establish iPSCs of high enough quality for transplantation as well as the cost. In contrast, iPSCs induced from healthy donors with a homozygous human leukocyte antigen haplotype (HLA-homo) is a significant candidate for allogenic transplantation on the basis that HLA-homo iPSCs might not be rejected by HLA haplotype-matched patients [174,175,176]. iPSC banking uses cells recruited from healthy, consenting HLA-type homozygous donors and is made with peripheral blood-derived mononuclear cells or umbilical cord blood [177]. Many research groups have been trying to apply iPSCs-based therapy to patients, and some of them are already being administered in clinical trials [178]. The allogeneic transplantation of iPS cell-derived cartilage has shown comparable results to the allogeneic transplantation of polydactyly-derived chondrocyte sheets in preclinical studies [129], indicating its potential to provide a vast cellular resource for creating artificial cartilage tissue [128,130]. On the other hand, the argument remains that there are differences between iPSCs and their derivatives from healthy and diseased donors [131], and proving the quality of iPS cell-derived cartilage is key to optimization.
As for cartilage metabolisms, the main techniques to confirm chondrogenic differentiation from iPSCs are based on the detection of upregulated chondrogenic genes or the histological analysis of the extracellular matrix. When human iPSCs, iPSC-derived MSC-like cells, iPS-MSC-derived chondrocytes (iPS-MSC-CDs), and bone marrow-derived MSCs are induced to differentiate into chondrocytes, respectively, the cartilage ultimately produced is comparable in general evaluation (type 2 collagen, aggrecan) but different in GSL-glycan profile [169,179]. It remains uncertain how this difference in glycan structure will behave in vivo. These data should, however, assist in assessing the quality of cartilage obtained through modern regenerative medicine.
In terms of tumorigenicity for iPSCs themselves, while clinical applications are going forward, the concerns that the transplantation of differentiated iPSC might lead to teratoma formation in the recipient should be clarified [180,181]. Matsumoto et al. reported that the R-17F antibody detects undifferentiated iPSCs harboring the Lacto-N-fucopentaose I (LNFP I) of GSLs, and as a result, exerts cytotoxic activity [182]. Recently, several methods have been developed to identify sialic acid-linked isomers on glycans [183,184,185], and α2,3-linked sialic acids on glycolipid glycans can also be detected [186]. If teratoma formation can be prevented by modifying the glycan structures involved in maintaining the undifferentiated nature of iPS cells, iPS cells can be used more safely [187,188]. The GSLs–glycome analysis is useful to determine the optimal conditions for removing undifferentiated iPSCs to a level safe for transplantation.
5. Conclusions
The field of GSLs and their biological functions is still in its infancy and lacks a rigorous review process such as defined inclusion and exclusion criteria. This limitation may affect the comprehensiveness and accuracy of the review. Despite these challenges, the impact of glycolipids on cartilage is not negligible, and a glycolipid-based cartilage-regeneration strategy is attractive. Although there have been no clinical trials using GSLs in cartilage-treatment strategies, and all of the related literature is in the experimental phase, the glycomics of mesenchymal stem/progenitor cells can be utilized to evaluate their stage of cellular differentiation. Furthermore, this review highlighted the possibility that supplementing missing GSLs could play significant roles in tissue regeneration and disease modification. To guide the regeneration of degenerated or injured cartilage into articular cartilage, a multifactorial methodology that incorporates GSLs, which are closely related to cartilage homeostasis, should be developed in the future.
Author Contributions
Conceptualization, K.H. and T.O.; investigation, K.H., T.O. and M.M.; writing—original draft preparation, K.H. and T.O.; writing—review and editing, K.H., T.O. and M.M.; supervision, N.I.; funding acquisition, K.H., T.O. and N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds for the Japan Society for the Promotion of Science KAKENHI (Grant-in-Aid for Challenging Exploratory Research: funding numbers: 19K22672 [to N.I.]; Grant-in-Aid for Research (B): funding numbers: 22H03917 [to T.O.]; and Grant-in-Aid for Early-Career Scientists: funding number: 23K16633 [to K.H.]) and AMED (Grant Number JP22zf01270004h0002 [to N.I., T.O., K.H.]).
Acknowledgments
The sponsor of the study had no role in the report preparation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chua, J.R.; Jamal, S.; Riad, M.; Castrejon, I.; Malfait, A.-M.; Block, J.A.; Pincus, T. Disease Burden in Osteoarthritis Is Similar to That of Rheumatoid Arthritis at Initial Rheumatology Visit and Significantly Greater Six Months Later. Arthritis Rheumatol. 2019, 71, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, J.L.; Cao, J.; Chapin, A.; Chen, C.; Li, Z.; Liu, A.; Horst, C.; Kaldjian, A.; Matyasz, T.; Scott, K.W.; et al. US Health Care Spending by Payer and Health Condition, 1996–2016. JAMA 2020, 323, 863–884. [Google Scholar] [CrossRef] [PubMed]
- Peat, G.; Thomas, M.J. Osteoarthritis Year in Review 2020: Epidemiology & Therapy. Osteoarthr. Cartil. 2021, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.J.; Beier, F.; Young, D.A.; Loughlin, J. Interplay between Genetics and Epigenetics in Osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. Articular Cartilage Repair Biomaterials: Strategies and Applications. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, A.; Lane, J.G.; Longo, U.G.; Dallo, I. Joint Function Preservation: A Focus on the Osteochondral Unit; Springer Nature: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-82958-2. [Google Scholar]
- Muthu, S.; Korpershoek, J.V.; Novais, E.J.; Tawy, G.F.; Hollander, A.P.; Martin, I. Failure of Cartilage Regeneration: Emerging Hypotheses and Related Therapeutic Strategies. Nat. Rev. Rheumatol. 2023, 19, 403–416. [Google Scholar] [CrossRef]
- David, M.J.; Portoukalian, J.; Rebbaa, A.; Vignon, E.; Carret, J.P.; Richard, M. Characterization of Gangliosides from Normal and Osteoarthritic Human Articular Cartilage. Arthritis Rheum. 1993, 36, 938–942. [Google Scholar] [CrossRef] [PubMed]
- David, M.J.; Hellio, M.P.; Portoukalian, J.; Richard, M.; Caton, J.; Vignon, E. Gangliosides from Normal and Osteoarthritic Joints. J. Rheumatol. Suppl. 1995, 43, 133–135. [Google Scholar] [PubMed]
- Bonner, W.M.; Jonsson, H.; Malanos, C.; Bryant, M. Changes in the Lipids of Human Articular Cartilage with Age. Arthritis Rheum. 1975, 18, 461–473. [Google Scholar] [CrossRef]
- Ichikawa, S.; Hirabayashi, Y. Glucosylceramide Synthase and Glycosphingolipid Synthesis. Trends Cell Biol. 1998, 8, 198–202. [Google Scholar] [CrossRef]
- Varki, A. Biological Roles of Glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef]
- Degroote, S.; Wolthoorn, J.; van Meer, G. The Cell Biology of Glycosphingolipids. Semin. Cell Dev. Biol. 2004, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Allende, M.L.; Kalkofen, D.N.; Werth, N.; Sandhoff, K.; Proia, R.L. Conditional LoxP-Flanked Glucosylceramide Synthase Allele Controlling Glycosphingolipid Synthesis. Genesis 2005, 43, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wada, R.; Proia, R.L. Early Developmental Expression of the Gene Encoding Glucosylceramide Synthase, the Enzyme Controlling the First Committed Step of Glycosphingolipid Synthesis. Biochim. Biophys. Acta 2002, 1573, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Wada, R.; Sasaki, T.; Deng, C.; Bierfreund, U.; Sandhoff, K.; Proia, R.L. A Vital Role for Glycosphingolipid Synthesis during Development and Differentiation. Proc. Natl. Acad. Sci. USA 1999, 96, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Seito, N.; Yamashita, T.; Tsukuda, Y.; Matsui, Y.; Urita, A.; Onodera, T.; Mizutani, T.; Haga, H.; Fujitani, N.; Shinohara, Y.; et al. Interruption of Glycosphingolipid Synthesis Enhances Osteoarthritis Development in Mice. Arthritis Rheum. 2012, 64, 2579–2588. [Google Scholar] [CrossRef]
- Khavandgar, Z.; Murshed, M. Sphingolipid Metabolism and Its Role in the Skeletal Tissues. Cell. Mol. Life Sci. CMLS 2015, 72, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.E.; et al. Exhaustion of Nucleus Pulposus Progenitor Cells with Ageing and Degeneration of the Intervertebral Disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Sixma, T.K.; Pronk, S.E.; Kalk, K.H.; Wartna, E.S.; van Zanten, B.A.; Witholt, B.; Hol, W.G. Crystal Structure of a Cholera Toxin-Related Heat-Labile Enterotoxin from E. coli. Nature 1991, 351, 371–377. [Google Scholar] [CrossRef]
- Takamiya, K.; Yamamoto, A.; Furukawa, K.; Yamashiro, S.; Shin, M.; Okada, M.; Fukumoto, S.; Haraguchi, M.; Takeda, N.; Fujimura, K.; et al. Mice with Disrupted GM2/GD2 Synthase Gene Lack Complex Gangliosides but Exhibit Only Subtle Defects in Their Nervous System. Proc. Natl. Acad. Sci. USA 1996, 93, 10662–10667. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.; Kuraoka, S.; Watanabe, S.; Iwai, T.; Tanabe, M. Electrophysiological Evidence of Increased Glycine Receptor-Mediated Phasic and Tonic Inhibition by Blockade of Glycine Transporters in Spinal Superficial Dorsal Horn Neurons of Adult Mice. J. Pharmacol. Sci. 2017, 133, 162–167. [Google Scholar] [CrossRef]
- Watanabe, S.; Iwai, T.; Tanabe, M. Intraplantar Injection of Sialidase Reduces Mechanical Allodynia during Inflammatory Pain. J. Pharmacol. Sci. 2017, 133, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, N.; Westermarck, J.; Häkkinen, L.; Holmström, T.H.; Elo, I.; Eriksson, J.E.; Kähäri, V.M. Enhancement of Fibroblast Collagenase (Matrix Metalloproteinase-1) Gene Expression by Ceramide Is Mediated by Extracellular Signal-Regulated and Stress-Activated Protein Kinase Pathways. J. Biol. Chem. 1998, 273, 5137–5145. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.; Rolland, G.; Léonce, S.; Thomas, M.; Lesur, C.; Pérez, V.; de Nanteuil, G.; Bonnet, J. Effects of Ceramide on Apoptosis, Proteoglycan Degradation, and Matrix Metalloproteinase Expression in Rabbit Articular Cartilage. Biochem. Biophys. Res. Commun. 2000, 267, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Gu, J.; Xu, F.; Wang, Y.; Wang, H.; Zhang, B. The Protective Role of Glucocerebrosidase/Ceramide in Rheumatoid Arthritis. Connect. Tissue Res. 2022, 63, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Structure and Function of Glycosphingolipids and Sphingolipids: Recollections and Future Trends. Biochim. Biophys. Acta 2008, 1780, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Yamamura, S.; Prinetti, A.; Handa, K.; Hakomori, S. GM3-Enriched Microdomain Involved in Cell Adhesion and Signal Transduction through Carbohydrate-Carbohydrate Interaction in Mouse Melanoma B16 Cells. J. Biol. Chem. 1998, 273, 9130–9138. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Shiota, M.; Sadahira, Y.; Handa, K.; Hakomori, S. Cell Adhesion in a Dynamic Flow System as Compared to Static System. Glycosphingolipid-Glycosphingolipid Interaction in the Dynamic System Predominates over Lectin- or Integrin-Based Mechanisms in Adhesion of B16 Melanoma Cells to Non-Activated Endothelial Cells. J. Biol. Chem. 1992, 267, 17264–17270. [Google Scholar]
- Kojima, N.; Hakomori, S. Specific Interaction between Gangliotriaosylceramide (Gg3) and Sialosyllactosylceramide (GM3) as a Basis for Specific Cellular Recognition between Lymphoma and Melanoma Cells. J. Biol. Chem. 1989, 264, 20159–20162. [Google Scholar] [CrossRef]
- Ferrari, G.; Anderson, B.L.; Stephens, R.M.; Kaplan, D.R.; Greene, L.A. Prevention of Apoptotic Neuronal Death by GM1 Ganglioside. Involvement of Trk Neurotrophin Receptors. J. Biol. Chem. 1995, 270, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Fighera, M.R.; Royes, L.F.F.; Furian, A.F.; Oliveira, M.S.; Fiorenza, N.G.; Frussa-Filho, R.; Petry, J.C.; Coelho, R.C.; Mello, C.F. GM1 Ganglioside Prevents Seizures, Na+,K+-ATPase Activity Inhibition and Oxidative Stress Induced by Glutaric Acid and Pentylenetetrazole. Neurobiol. Dis. 2006, 22, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T.V.; Zakharova, I.O.; Furaev, V.V.; Rychkova, M.P.; Avrova, N.F. Neuroprotective Effect of Ganglioside GM1 on the Cytotoxic Action of Hydrogen Peroxide and Amyloid Beta-Peptide in PC12 Cells. Neurochem. Res. 2007, 32, 1302–1313. [Google Scholar] [CrossRef]
- Cavallini, L.; Venerando, R.; Miotto, G.; Alexandre, A. Ganglioside GM1 Protection from Apoptosis of Rat Heart Fibroblasts. Arch. Biochem. Biophys. 1999, 370, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Sergent, O.; Pereira, M.; Belhomme, C.; Chevanne, M.; Huc, L.; Lagadic-Gossmann, D. Role for Membrane Fluidity in Ethanol-Induced Oxidative Stress of Primary Rat Hepatocytes. J. Pharmacol. Exp. Ther. 2005, 313, 104–111. [Google Scholar] [CrossRef]
- Karimizade, A.; Hasanzadeh, E.; Abasi, M.; Enderami, S.E.; Mirzaei, E.; Annabi, N.; Mellati, A. Collagen Short Nanofiber-Embedded Chondroitin Sulfate-Hyaluronic Acid Nanocomposite: A Cartilage-Mimicking in Situ-Forming Hydrogel with Fine-Tuned Properties. Int. J. Biol. Macromol. 2024, 266, 131051. [Google Scholar] [CrossRef]
- Carton, F.; Malatesta, M. Nanotechnological Research for Regenerative Medicine: The Role of Hyaluronic Acid. Int. J. Mol. Sci. 2024, 25, 3975. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, M.; Qian, Y.; Yuan, K.; Han, X.; Wang, W.; Guo, J.J.; Chen, Q.; Li, B. Enhancing Endogenous Hyaluronic Acid in Osteoarthritic Joints with an Anti-Inflammatory Supramolecular Nanofiber Hydrogel Delivering HAS2 Lentivirus. Small 2024, e2400542. [Google Scholar] [CrossRef]
- Cao, Y.; Kampf, N.; Lin, W.; Klein, J. Normal and Shear Forces between Boundary Sphingomyelin Layers under Aqueous Conditions. Soft Matter 2020, 16, 3973–3980. [Google Scholar] [CrossRef]
- Matsubara, S.; Onodera, T.; Maeda, E.; Momma, D.; Matsuoka, M.; Homan, K.; Ohashi, T.; Iwasaki, N. Depletion of Glycosphingolipids Induces Excessive Response of Chondrocytes under Mechanical Stress. J. Biomech. 2019, 94, 22–30. [Google Scholar] [CrossRef]
- Nagafuku, M.; Okuyama, K.; Onimaru, Y.; Suzuki, A.; Odagiri, Y.; Yamashita, T.; Iwasaki, K.; Fujiwara, M.; Takayanagi, M.; Ohno, I.; et al. CD4 and CD8 T Cells Require Different Membrane Gangliosides for Activation. Proc. Natl. Acad. Sci. USA 2012, 109, E336–E342. [Google Scholar] [CrossRef] [PubMed]
- Momma, D.; Onodera, T.; Homan, K.; Matsubara, S.; Sasazawa, F.; Furukawa, J.; Matsuoka, M.; Yamashita, T.; Iwasaki, N. Coordinated Existence of Multiple Gangliosides Is Required for Cartilage Metabolism. Osteoarthr. Cartil. 2019, 27, 314–325. [Google Scholar] [CrossRef]
- Sasazawa, F.; Onodera, T.; Yamashita, T.; Seito, N.; Tsukuda, Y.; Fujitani, N.; Shinohara, Y.; Iwasaki, N. Depletion of Gangliosides Enhances Cartilage Degradation in Mice. Osteoarthr. Cartil. 2014, 22, 313–322. [Google Scholar] [CrossRef]
- Ho, C.-W.; Popat, S.D.; Liu, T.-W.; Tsai, K.-C.; Ho, M.-J.; Chen, W.-H.; Yang, A.-S.; Lin, C.-H. Development of GlcNAc-Inspired Iminocyclitiols as Potent and Selective N-Acetyl-Beta-Hexosaminidase Inhibitors. ACS Chem. Biol. 2010, 5, 489–497. [Google Scholar] [CrossRef]
- Shikhman, A.R.; Brinson, D.C.; Lotz, M. Profile of Glycosaminoglycan-Degrading Glycosidases and Glycoside Sulfatases Secreted by Human Articular Chondrocytes in Homeostasis and Inflammation. Arthritis Rheum. 2000, 43, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.; Golde, D.W. The Sphingomyelin Pathway in Tumor Necrosis Factor and Interleukin-1 Signaling. Cell 1994, 77, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. The Sphingomyelin Cycle and the Second Messenger Function of Ceramide. J. Biol. Chem. 1994, 269, 3125–3128. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hashiramoto, A.; Haluzik, M.; Mizukami, H.; Beck, S.; Norton, A.; Kono, M.; Tsuji, S.; Daniotti, J.L.; Werth, N.; et al. Enhanced Insulin Sensitivity in Mice Lacking Ganglioside GM3. Proc. Natl. Acad. Sci. USA 2003, 100, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Onodera, T.; Homan, K.; Sasazawa, F.; Furukawa, J.-I.; Momma, D.; Baba, R.; Hontani, K.; Joutoku, Z.; Matsubara, S.; et al. Depletion of Gangliosides Enhances Articular Cartilage Repair in Mice. Sci. Rep. 2017, 7, 43729. [Google Scholar] [CrossRef]
- Tsukuda, Y.; Iwasaki, N.; Seito, N.; Kanayama, M.; Fujitani, N.; Shinohara, Y.; Kasahara, Y.; Onodera, T.; Suzuki, K.; Asano, T.; et al. Ganglioside GM3 Has an Essential Role in the Pathogenesis and Progression of Rheumatoid Arthritis. PLoS ONE 2012, 7, e40136. [Google Scholar] [CrossRef]
- Kasprowicz, A.; Sophie, G.-D.; Lagadec, C.; Delannoy, P. Role of GD3 Synthase ST8Sia I in Cancers. Cancers 2022, 14, 1299. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, X.; Pang, X.; Li, L.; Wang, J.; Yang, C.; Du, G. Ganglioside GD3 Synthase (GD3S), a Novel Cancer Drug Target. Acta Pharm. Sin. B 2018, 8, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hu, X.; Ren, S.; Wang, Y.; Shao, Y.; Wu, K.; Yang, Z.; Yang, W.; He, G.; Li, X. The Biological Role and Immunotherapy of Gangliosides and GD3 Synthase in Cancers. Front. Cell Dev. Biol. 2023, 11, 1076862. [Google Scholar] [CrossRef] [PubMed]
- Yo, S.; Hamamura, K.; Mishima, Y.; Hamajima, K.; Mori, H.; Furukawa, K.; Kondo, H.; Tanaka, K.; Sato, T.; Miyazawa, K.; et al. Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging. Int. J. Mol. Sci. 2019, 20, 2825. [Google Scholar] [CrossRef] [PubMed]
- McGonigal, R.; Barrie, J.A.; Yao, D.; Black, L.E.; McLaughlin, M.; Willison, H.J. Neuronally Expressed A-Series Gangliosides Are Sufficient to Prevent the Lethal Age-Dependent Phenotype in GM3-Only Expressing Mice. J. Neurochem. 2021, 158, 217–232. [Google Scholar] [CrossRef]
- Sheikh, K.A.; Sun, J.; Liu, Y.; Kawai, H.; Crawford, T.O.; Proia, R.L.; Griffin, J.W.; Schnaar, R.L. Mice Lacking Complex Gangliosides Develop Wallerian Degeneration and Myelination Defects. Proc. Natl. Acad. Sci. USA 1999, 96, 7532–7537. [Google Scholar] [CrossRef]
- Chiavegatto, S.; Sun, J.; Nelson, R.J.; Schnaar, R.L. A Functional Role for Complex Gangliosides: Motor Deficits in GM2/GD2 Synthase Knockout Mice. Exp. Neurol. 2000, 166, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-S.; Seo, S.Y.; Jeong, E.-J.; Kim, J.-Y.; Koh, Y.-G.; Kim, Y.I.; Choo, Y.-K. Ganglioside GM3 Up-Regulate Chondrogenic Differentiation by Transform Growth Factor Receptors. Int. J. Mol. Sci. 2020, 21, 1967. [Google Scholar] [CrossRef]
- Giannasi, C.; Della Morte, E.; Cadelano, F.; Valenza, A.; Casati, S.; Dei Cas, M.; Niada, S.; Brini, A.T. Boosting the Therapeutic Potential of Cell Secretome against Osteoarthritis: Comparison of Cytokine-Based Priming Strategies. Biomed. Pharmacother. 2023, 170, 115970. [Google Scholar] [CrossRef]
- Matsuoka, M.; Onodera, T.; Sasazawa, F.; Momma, D.; Baba, R.; Hontani, K.; Iwasaki, N. An Articular Cartilage Repair Model in Common C57Bl/6 Mice. Tissue Eng. Part C Methods 2015, 21, 767–772. [Google Scholar] [CrossRef]
- Fan, A.; Wu, G.; Wang, J.; Lu, L.; Wang, J.; Wei, H.; Sun, Y.; Xu, Y.; Mo, C.; Zhang, X.; et al. Inhibition of Fibroblast Activation Protein Ameliorates Cartilage Matrix Degradation and Osteoarthritis Progression. Bone Res. 2023, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Molin, A.N.; Contentin, R.; Angelozzi, M.; Karvande, A.; Kc, R.; Haseeb, A.; Voskamp, C.; de Charleroy, C.; Lefebvre, V. Skeletal Growth Is Enhanced by a Shared Role for SOX8 and SOX9 in Promoting Reserve Chondrocyte Commitment to Columnar Proliferation. Proc. Natl. Acad. Sci. USA 2024, 121, e2316969121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-Inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sports Med. 2020, 48, 2808–2818. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Urita, A.; Onodera, T.; Hishimura, R.; Nonoyama, T.; Hamasaki, M.; Liang, D.; Homan, K.; Gong, J.P.; Iwasaki, N. Ultrapurified Alginate Gel Containing Bone Marrow Aspirate Concentrate Enhances Cartilage and Bone Regeneration on Osteochondral Defects in a Rabbit Model. Am. J. Sports Med. 2021, 49, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dai, W.; Gao, C.; Wei, W.; Huang, R.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q. Multileveled Hierarchical Hydrogel with Continuous Biophysical and Biochemical Gradients for Enhanced Repair of Full-Thickness Osteochondral Defect. Adv. Mater. 2023, 35, e2209565. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Han, X.; Li, Z.; Bian, S.; Zeng, W.; Ding, M.; Liang, J.; Jiang, Q.; Zhou, Z.; et al. Molecular Co-Assembled Strategy Tuning Protein Conformation for Cartilage Regeneration. Nat. Commun. 2024, 15, 1488. [Google Scholar] [CrossRef] [PubMed]
- González Vázquez, A.G.; Blokpoel Ferreras, L.A.; Bennett, K.E.; Casey, S.M.; Brama, P.A.; O’Brien, F.J. Systematic Comparison of Biomaterials-Based Strategies for Osteochondral and Chondral Repair in Large Animal Models. Adv. Healthc. Mater. 2021, 10, e2100878. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, B.; Liu, W.; Li, X.; Liang, K.; Fan, Z.; Li, J.J.; Niu, Y.; He, Z.; Li, H.; et al. In Situ Self-Assembled Organoid for Osteochondral Tissue Regeneration with Dual Functional Units. Bioact. Mater. 2023, 27, 200–215. [Google Scholar] [CrossRef]
- Ibaraki, K.; Hayashi, S.; Kanzaki, N.; Hashimoto, S.; Kihara, S.; Haneda, M.; Takeuchi, K.; Niikura, T.; Kuroda, R. Deletion of P21 Expression Accelerates Cartilage Tissue Repair via Chondrocyte Proliferation. Mol. Med. Rep. 2020, 21, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, K.; Hettinghouse, A.; Liu, C. Atsttrin Promotes Cartilage Repair Primarily Through TNFR2-Akt Pathway. Front. Cell Dev. Biol. 2020, 8, 577572. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Schwab, A.; Tong, W.; Xu, J.; Zheng, L.; Li, Y.; Li, Z.; Xu, S.; Chen, Z.; et al. Engineered Biochemical Cues of Regenerative Biomaterials to Enhance Endogenous Stem/Progenitor Cells (ESPCs)-Mediated Articular Cartilage Repair. Bioact. Mater. 2023, 26, 490–512. [Google Scholar] [CrossRef]
- Massengale, M.; Massengale, J.L.; Benson, C.R.; Baryawno, N.; Oki, T.; Steinhauser, M.L.; Wang, A.; Balani, D.; Oh, L.S.; Randolph, M.A.; et al. Adult Prg4+ Progenitors Repair Long-Term Articular Cartilage Wounds in Vivo. JCI Insight 2023, 8, e167858. [Google Scholar] [CrossRef] [PubMed]
- Trengove, A.; Duchi, S.; Onofrillo, C.; Sooriyaaratchi, D.; Di Bella, C.; O’Connor, A.J. Bridging Bench to Body: Ex Vivo Models to Understand Articular Cartilage Repair. Curr. Opin. Biotechnol. 2024, 86, 103065. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, N.; Ito, M.; Iwata, H.; Yamagata, T. Characterization of the Glycosphingolipids of Pig Cortical Bone and Cartilage. Biochim. Biophys. Acta 1989, 1004, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.; Onodera, T.; Hanamatsu, H.; Furukawa, J.-I.; Momma, D.; Matsuoka, M.; Iwasaki, N. Articular Cartilage Corefucosylation Regulates Tissue Resilience in Osteoarthritis. eLife 2024, 12, RP92275. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Kim, S.-J.; Choi, H.-J.; Kim, K.-J.; Kim, M.-J.; Kim, S.-H.; Lee, H.-J.; Ko, J.-H.; Lee, Y.-C.; Suzuki, A.; et al. Ganglioside GM3 Inhibits VEGF/VEGFR-2-Mediated Angiogenesis: Direct Interaction of GM3 with VEGFR-2. Glycobiology 2009, 19, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kabayama, K.; Sato, T.; Saito, K.; Loberto, N.; Prinetti, A.; Sonnino, S.; Kinjo, M.; Igarashi, Y.; Inokuchi, J. Dissociation of the Insulin Receptor and Caveolin-1 Complex by Ganglioside GM3 in the State of Insulin Resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 13678–13683. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Yoon, S.-J.; Itoh, K.; Nakayama, K. Tyrosine Kinase Activity of Epidermal Growth Factor Receptor Is Regulated by GM3 Binding through Carbohydrate to Carbohydrate Interactions. J. Biol. Chem. 2009, 284, 6147–6155. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR Technologies in Research and Beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Stolfa, G.; Mondal, N.; Zhu, Y.; Yu, X.; Buffone, A.; Neelamegham, S. Using CRISPR-Cas9 to Quantify the Contributions of O-Glycans, N-Glycans and Glycosphingolipids to Human Leukocyte-Endothelium Adhesion. Sci. Rep. 2016, 6, 30392. [Google Scholar] [CrossRef]
- Li, C.; Du, Y.; Zhang, T.; Wang, H.; Hou, Z.; Zhang, Y.; Cui, W.; Chen, W. “Genetic Scissors” CRISPR/Cas9 Genome Editing Cutting-Edge Biocarrier Technology for Bone and Cartilage Repair. Bioact. Mater. 2023, 22, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sun, A.R.; Young, R.S.E.; Afara, I.O.; Hamilton, B.R.; Ong, L.J.Y.; Crawford, R.; Prasadam, I. Spatial Analysis of the Osteoarthritis Microenvironment: Techniques, Insights, and Applications. Bone Res. 2024, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Bian, X.; Meng, X.; Li, L.; Fu, L.; Zhang, Y.; Wang, L.; Zhang, Y.; Gao, D.; Guo, X.; et al. Unveiling Inflammatory and Prehypertrophic Cell Populations as Key Contributors to Knee Cartilage Degeneration in Osteoarthritis Using Multi-Omics Data Integration. Ann. Rheum. Dis. 2023, ard-2023-224420. [Google Scholar] [CrossRef]
- Briggs, M.T.; Kuliwaba, J.S.; Muratovic, D.; Everest-Dass, A.V.; Packer, N.H.; Findlay, D.M.; Hoffmann, P. MALDI Mass Spectrometry Imaging of N-Glycans on Tibial Cartilage and Subchondral Bone Proteins in Knee Osteoarthritis. Proteomics 2016, 16, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ha, S.; Kim, M.; Kim, S.-W.; Yun, J.; Ozcan, S.; Hwang, H.; Ji, I.J.; Yin, D.; Webster, M.J.; et al. Spatial and Temporal Diversity of Glycome Expression in Mammalian Brain. Proc. Natl. Acad. Sci. USA 2020, 117, 28743–28753. [Google Scholar] [CrossRef] [PubMed]
- Denti, V.; Capitoli, G.; Piga, I.; Clerici, F.; Pagani, L.; Criscuolo, L.; Bindi, G.; Principi, L.; Chinello, C.; Paglia, G.; et al. Spatial Multiomics of Lipids, N-Glycans, and Tryptic Peptides on a Single FFPE Tissue Section. J. Proteome Res. 2022, 21, 2798–2809. [Google Scholar] [CrossRef]
- Conroy, L.R.; Clarke, H.A.; Allison, D.B.; Valenca, S.S.; Sun, Q.; Hawkinson, T.R.; Young, L.E.A.; Ferreira, J.E.; Hammonds, A.V.; Dunne, J.B.; et al. Spatial Metabolomics Reveals Glycogen as an Actionable Target for Pulmonary Fibrosis. Nat. Commun. 2023, 14, 2759. [Google Scholar] [CrossRef]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular Interactions and Signaling in Cartilage Development. Osteoarthr. Cartil. 2000, 8, 309–334. [Google Scholar] [CrossRef]
- Vainieri, M.L.; Lolli, A.; Kops, N.; D’Atri, D.; Eglin, D.; Yayon, A.; Alini, M.; Grad, S.; Sivasubramaniyan, K.; van Osch, G.J.V.M. Evaluation of Biomimetic Hyaluronic-Based Hydrogels with Enhanced Endogenous Cell Recruitment and Cartilage Matrix Formation. Acta Biomater. 2020, 101, 293–303. [Google Scholar] [CrossRef]
- Martin, A.R.; Patel, J.M.; Locke, R.C.; Eby, M.R.; Saleh, K.S.; Davidson, M.D.; Sennett, M.L.; Zlotnick, H.M.; Chang, A.H.; Carey, J.L.; et al. Nanofibrous Hyaluronic Acid Scaffolds Delivering TGF-Β3 and SDF-1α for Articular Cartilage Repair in a Large Animal Model. Acta Biomater. 2021, 126, 170–182. [Google Scholar] [CrossRef]
- Xiang, S.; Lin, Z.; Makarcyzk, M.J.; Riewruja, K.; Zhang, Y.; Zhang, X.; Li, Z.; Clark, K.L.; Li, E.; Liu, S.; et al. Differences in the Intrinsic Chondrogenic Potential of Human Mesenchymal Stromal Cells and iPSC-Derived Multipotent Cells. Clin. Transl. Med. 2022, 12, e1112. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jin, M.; Sun, K.; Zhang, Z.; Wu, Z.; Shi, J.; Liu, P.; Yao, H.; Wang, D.-A. Type II Collagen Scaffolds Repair Critical-Sized Osteochondral Defects under Induced Conditions of Osteoarthritis in Rat Knee Joints via Inhibiting TGF-β-Smad1/5/8 Signaling Pathway. Bioact. Mater. 2024, 35, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wei, Y.; Zeng, C.; Yang, G.; Dong, C.; Wan, W.; Chen, S. Hierarchically Assembled Nanofiber Scaffold Guides Long Bone Regeneration by Promoting Osteogenic/Chondrogenic Differentiation of Endogenous Mesenchymal Stem Cells. Small 2024, e2309868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Jiang, X.; Xu, M.; Wang, M.; Wang, R.; Zheng, B.; Chen, M.; Ke, Q.; Long, J. Unleashing the Power of Immune Checkpoints: Post-Translational Modification of Novel Molecules and Clinical Applications. Cancer Lett. 2024, 588, 216758. [Google Scholar] [CrossRef] [PubMed]
- Alghazali, R.; Nugud, A.; El-Serafi, A. Glycan Modifications as Regulators of Stem Cell Fate. Biology 2024, 13, 76. [Google Scholar] [CrossRef]
- Liu, B.; Zou, X.; Zhang, Y.; Yang, Y.; Xu, H.; Tang, F.; Yu, H.; Xia, F.; Liu, Z.; Zhao, J.; et al. Site- and Stereoselective Glycomodification of Biomolecules through Carbohydrate-Promoted Pictet-Spengler Reaction. Angew. Chem. Int. Ed. 2024, 63, e202401394. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.; Shinohara, Y.; Kuramoto, H.; Miura, Y.; Shimaoka, H.; Kurogochi, M.; Nakano, M.; Nishimura, S.-I. Comprehensive Approach to Structural and Functional Glycomics Based on Chemoselective Glycoblotting and Sequential Tag Conversion. Anal. Chem. 2008, 80, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Miwa, Y.; Kimata, K.; Ikawa, Y. A Chondrogenic Cell Line Derived from a Differentiating Culture of AT805 Teratocarcinoma Cells. Cell Differ. Dev. 1990, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Kakiya, K.; Takahashi, K.; Miwa, H.; Rokushima, M.; Yoshinaga, T.; Tanaka, Y.; Ito, T.; Togame, H.; Takemoto, H.; et al. Discovery of Novel Differentiation Markers in the Early Stage of Chondrogenesis by Glycoform-Focused Reverse Proteomics and Genomics. Biochim. Biophys. Acta 2014, 1840, 645–655. [Google Scholar] [CrossRef]
- Yan, W.Q.; Nakashima, K.; Iwamoto, M.; Kato, Y. Stimulation by Concanavalin A of Cartilage-Matrix Proteoglycan Synthesis in Chondrocyte Cultures. J. Biol. Chem. 1990, 265, 10125–10131. [Google Scholar] [CrossRef]
- Yan, W.; Pan, H.; Ishida, H.; Nakashima, K.; Suzuki, F.; Nishimura, M.; Jikko, A.; Oda, R.; Kato, Y. Effects of Concanavalin A on Chondrocyte Hypertrophy and Matrix Calcification. J. Biol. Chem. 1997, 272, 7833–7840. [Google Scholar] [CrossRef] [PubMed]
- Homan, K.; Hanamatsu, H.; Furukawa, J.-I.; Okada, K.; Yokota, I.; Onodera, T.; Iwasaki, N. Alteration of the Total Cellular Glycome during Late Differentiation of Chondrocytes. Int. J. Mol. Sci. 2019, 20, 3546. [Google Scholar] [CrossRef]
- Lin, P.; Fu, D.; Zhang, T.; Ma, S.; Zhou, F. Microgel-Modified Bilayered Hydrogels Dramatically Boosting Load-Bearing and Lubrication. ACS Macro Lett. 2023, 12, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Alkaya, D.; Gurcan, C.; Kilic, P.; Yilmazer, A.; Gurman, G. Where Is Human-Based Cellular Pharmaceutical R&D Taking Us in Cartilage Regeneration? 3 Biotech 2020, 10, 161. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Sun, C.; Wang, Q.; Yang, W.; Zhang, Z.; Xia, Z.; Shao, Z.; Wang, B. Endogenous Repair and Regeneration of Injured Articular Cartilage: A Challenging but Promising Therapeutic Strategy. Aging Dis. 2021, 12, 886–901. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Baroncini, A.; Bell, A.; Hildebrand, F.; Schenker, H. Autologous Matrix-Induced Chondrogenesis Is Effective for Focal Chondral Defects of the Knee. Sci. Rep. 2022, 12, 9328. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Götze, C.; Hildebrand, F.; Betsch, M. Prognostic Factors for the Management of Chondral Defects of the Knee and Ankle Joint: A Systematic Review. Eur. J. Trauma Emerg. Surg. 2023, 49, 723–745. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, J.; Kraeutler, M.J.; Fasulo, S.M.; Belk, J.W.; Mulcahey, M.K.; Scillia, A.J.; McCulloch, P.C. Cartilage Repair of the Tibiofemoral Joint With Versus Without Concomitant Osteotomy: A Systematic Review of Clinical Outcomes. Orthop. J. Sports Med. 2023, 11, 23259671231151707. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Y.; Ma, Y.; Huang, L.; Wang, X.; Lin, Q.; Xing, Y.; Yang, W.; Duan, W.; Wei, X. Treatment of Articular Cartilage Defects: A Descriptive Analysis of Clinical Characteristics and Global Trends Reported from 2001 to 2020. Cartilage 2023, 19476035231205695. [Google Scholar] [CrossRef]
- Dhillon, J.; Orozco, E.; Keeter, C.; Scillia, A.J.; Harris, J.D.; Kraeutler, M.J. Microfracture of Acetabular Chondral Lesions Is Not Superior to Other Cartilage Repair Techniques in Patients with Femoroacetabular Impingement Syndrome: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 602–611. [Google Scholar] [CrossRef]
- Götze, C.; Nieder, C.; Felder, H.; Migliorini, F. AMIC for Focal Osteochondral Defect of the Talar Shoulder. Life 2020, 10, 328. [Google Scholar] [CrossRef]
- Waltenspül, M.; Suter, C.; Ackermann, J.; Kühne, N.; Fucentese, S.F. Autologous Matrix-Induced Chondrogenesis (AMIC) for Isolated Retropatellar Cartilage Lesions: Outcome after a Follow-Up of Minimum 2 Years. Cartilage 2021, 13, 1280S–1290S. [Google Scholar] [CrossRef]
- Tradati, D.; De Luca, P.; Maione, A.; Uboldi, F.M.; Volpi, P.; de Girolamo, L.; Berruto, M. AMIC-Autologous Matrix-Induced Chondrogenesis Technique in Patellar Cartilage Defects Treatment: A Retrospective Study with a Mid-Term Follow-Up. J. Clin. Med. 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Thorey, F.; Malahias, M.-A.; Giotis, D. Sustained Benefit of Autologous Matrix-Induced Chondrogenesis for Hip Cartilage Repair in a Recreational Athletic Population. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Salonius, E.; Meller, A.; Paatela, T.; Vasara, A.; Puhakka, J.; Hannula, M.; Haaparanta, A.-M.; Kiviranta, I.; Muhonen, V. Cartilage Repair Capacity within a Single Full-Thickness Chondral Defect in a Porcine Autologous Matrix-Induced Chondrogenesis Model Is Affected by the Location within the Defect. Cartilage 2021, 13, 744S–754S. [Google Scholar] [CrossRef] [PubMed]
- Bąkowski, P.; Grzywacz, K.; Prusińska, A.; Ciemniewska-Gorzela, K.; Gille, J.; Piontek, T. Autologous Matrix-Induced Chondrogenesis (AMIC) for Focal Chondral Lesions of the Knee: A 2-Year Follow-Up of Clinical, Proprioceptive, and Isokinetic Evaluation. J. Funct. Biomater. 2022, 13, 277. [Google Scholar] [CrossRef] [PubMed]
- De Lucas Villarrubia, J.C.; Méndez Alonso, M.Á.; Sanz Pérez, M.I.; Trell Lesmes, F.; Panadero Tapia, A. Acellular Matrix-Induced Chondrogenesis Technique Improves the Results of Chondral Lesions Associated with Femoroacetabular Impingement. Arthrosc. J. Arthrosc. Relat. Surg. 2022, 38, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, P.; Laute, V.; Zinser, W.; John, T.; Becher, C.; Diehl, P.; Kolombe, T.; Fay, J.; Siebold, R.; Fickert, S. Safety and Efficacy of Matrix-Associated Autologous Chondrocyte Implantation with Spheroid Technology Is Independent of Spheroid Dose after 4 Years. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1130–1143. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, M.; Henninger, B.; Petry, B.; Schuster, P.; Herbst, E.; Wagner, M.; Rosenberger, R.; Mayr, R. Treatment of Cartilage Defects in the Patellofemoral Joint with Matrix-Associated Autologous Chondrocyte Implantation Effectively Improves Pain, Function, and Radiological Outcomes after 5–7 Years. Arch. Orthop. Trauma Surg. 2024, 144, 1655–1665. [Google Scholar] [CrossRef]
- Yoon, K.-H.; Lee, J.; Park, J.-Y. Costal Chondrocyte-Derived Pellet-Type Autologous Chondrocyte Implantation Versus Microfracture for the Treatment of Articular Cartilage Defects: A 5-Year Follow-up of a Prospective Randomized Trial. Am. J. Sports Med. 2024, 52, 362–367. [Google Scholar] [CrossRef]
- Snow, M.; Middleton, L.; Mehta, S.; Roberts, A.; Gray, R.; Richardson, J.; Kuiper, J.H.; ACTIVE Consortium; Smith, A.; White, S.; et al. A Randomized Trial of Autologous Chondrocyte Implantation Versus Alternative Forms of Surgical Cartilage Management in Patients with a Failed Primary Treatment for Chondral or Osteochondral Defects in the Knee. Am. J. Sports Med. 2023, 51, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Yamada, S.; Nejima, S.; Sotozawa, M.; Inaba, Y. Minimum 5-Year Outcomes of Osteochondral Autograft Transplantation with a Concomitant High Tibial Osteotomy for Spontaneous Osteonecrosis of the Knee with a Large Lesion. Cartilage 2022, 13, 19476035221126341. [Google Scholar] [CrossRef]
- Tsoukas, D.; Muntean, I.; Simos, C.; Sabido-Vera, R. Prospective Observational Study of a Non-Arthroscopic Autologous Cartilage Micrografting Technology for Knee Osteoarthritis. Bioengineering 2023, 10, 1294. [Google Scholar] [CrossRef] [PubMed]
- Trofa, D.P.; Hong, I.S.; Lopez, C.D.; Rao, A.J.; Yu, Z.; Odum, S.M.; Moorman, C.T.; Piasecki, D.P.; Fleischli, J.E.; Saltzman, B.M. Isolated Osteochondral Autograft Versus Allograft Transplantation for the Treatment of Symptomatic Cartilage Lesions of the Knee: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2023, 51, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.; Lee, M.S.; Pettinelli, N.; Norman, M.; Park, N.; Gillinov, S.M.; Zhu, J.; Gagné, J.; Lee, A.Y.; Mahatme, R.J.; et al. Osteochondral Allograft or Autograft Transplantation of the Femoral Head Leads to Improvement in Outcomes But Variable Survivorship: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2024; in press. [Google Scholar] [CrossRef]
- Takei, Y.; Morioka, M.; Yamashita, A.; Kobayashi, T.; Shima, N.; Tsumaki, N. Quality Assessment Tests for Tumorigenicity of Human iPS Cell-Derived Cartilage. Sci. Rep. 2020, 10, 12794. [Google Scholar] [CrossRef]
- Tam, W.L.; Freitas Mendes, L.; Chen, X.; Lesage, R.; Van Hoven, I.; Leysen, E.; Kerckhofs, G.; Bosmans, K.; Chai, Y.C.; Yamashita, A.; et al. Human Pluripotent Stem Cell-Derived Cartilaginous Organoids Promote Scaffold-Free Healing of Critical Size Long Bone Defects. Stem Cell Res. Ther. 2021, 12, 513. [Google Scholar] [CrossRef]
- Hamahashi, K.; Toyoda, E.; Ishihara, M.; Mitani, G.; Takagaki, T.; Kaneshiro, N.; Maehara, M.; Takahashi, T.; Okada, E.; Watanabe, A.; et al. Polydactyly-Derived Allogeneic Chondrocyte Cell-Sheet Transplantation with High Tibial Osteotomy as Regenerative Therapy for Knee Osteoarthritis. NPJ Regen. Med. 2022, 7, 71. [Google Scholar] [CrossRef]
- Abe, K.; Yamashita, A.; Morioka, M.; Horike, N.; Takei, Y.; Koyamatsu, S.; Okita, K.; Matsuda, S.; Tsumaki, N. Engraftment of Allogeneic iPS Cell-Derived Cartilage Organoid in a Primate Model of Articular Cartilage Defect. Nat. Commun. 2023, 14, 804. [Google Scholar] [CrossRef]
- Eremeev, A.; Pikina, A.; Ruchko, Y.; Bogomazova, A. Clinical Potential of Cellular Material Sources in the Generation of iPSC-Based Products for the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 14408. [Google Scholar] [CrossRef]
- Mologne, T.S.; Bugbee, W.D.; Kaushal, S.; Locke, C.S.; Goulet, R.W.; Casden, M.; Grant, J.A. Osteochondral Allografts for Large Oval Defects of the Medial Femoral Condyle: A Comparison of Single Lateral Versus Medial Femoral Condyle Oval Grafts Versus 2 Overlapping Circular Grafts. Am. J. Sports Med. 2023, 51, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, Z.; Liu, Y.; Ma, Y.; Huang, L.; Song, W.; Lin, Q.; Zhang, Z.; Li, P.; Wei, X.; et al. Characteristics and Clinical Outcomes after Osteochondral Allograft Transplantation for Treating Articular Cartilage Defects: Systematic Review and Single-Arm Meta-Analysis of Studies from 2001 to 2020. Orthop. J. Sports Med. 2023, 11, 23259671231199418. [Google Scholar] [CrossRef] [PubMed]
- Nuelle, C.W.; Gelber, P.E.; Waterman, B.R. Osteochondral Allograft Transplantation in the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 663–665. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-Articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, E.D.; Cui, X.; Murphy, C.A.; Lim, K.S.; Hooper, G.J.; McIlwraith, C.W.; Woodfield, T.B.F. Allogeneic Mesenchymal Stromal Cells for Cartilage Regeneration: A Review of in Vitro Evaluation, Clinical Experience, and Translational Opportunities. Stem Cells Transl. Med. 2021, 10, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-F.; Hu, C.-C.; Wu, C.-T.; Wu, H.-T.H.; Chang, C.-S.; Hung, Y.-P.; Tsai, C.-C.; Chang, Y. Treatment of Knee Osteoarthritis with Intra-Articular Injection of Allogeneic Adipose-Derived Stem Cells (ADSCs) ELIXCYTE®: A Phase I/II, Randomized, Active-Control, Single-Blind, Multiple-Center Clinical Trial. Stem Cell Res. Ther. 2021, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Saris, T.F.F.; de Windt, T.S.; Kester, E.C.; Vonk, L.A.; Custers, R.J.H.; Saris, D.B.F. Five-Year Outcome of 1-Stage Cell-Based Cartilage Repair Using Recycled Autologous Chondrons and Allogenic Mesenchymal Stromal Cells: A First-in-Human Clinical Trial. Am. J. Sports Med. 2021, 49, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, I.; Katano, H.; Mizuno, M.; Koga, H.; Masumoto, J.; Tomita, M.; Ozeki, N. Alterations in Cartilage Quantification before and after Injections of Mesenchymal Stem Cells into Osteoarthritic Knees. Sci. Rep. 2021, 11, 13832. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.d.C.; Araújo, L.T.d.; Santos, G.C.; Damasceno, P.K.F.; Vieira, J.L.; Santos, R.R.D.; Barbosa, J.D.V.; Soares, M.B.P. Clinical Trials with Mesenchymal Stem Cell Therapies for Osteoarthritis: Challenges in the Regeneration of Articular Cartilage. Int. J. Mol. Sci. 2023, 24, 9939. [Google Scholar] [CrossRef]
- Razak, H.R.B.A.; Corona, K.; Totlis, T.; Chan, L.Y.T.; Salreta, J.F.; Sleiman, O.; Vasso, M.; Baums, M.H. Mesenchymal Stem Cell Implantation Provides Short-Term Clinical Improvement and Satisfactory Cartilage Restoration in Patients with Knee Osteoarthritis but the Evidence Is Limited: A Systematic Review Performed by the Early-Osteoarthritis Group of ESSKA-European Knee Associates Section. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 5306–5318. [Google Scholar] [CrossRef]
- Pintore, A.; Notarfrancesco, D.; Zara, A.; Oliviero, A.; Migliorini, F.; Oliva, F.; Maffulli, N. Intra-Articular Injection of Bone Marrow Aspirate Concentrate (BMAC) or Adipose-Derived Stem Cells (ADSCs) for Knee Osteoarthritis: A Prospective Comparative Clinical Trial. J. Orthop. Surg. 2023, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, C.; Lothaller, H.; Kobinia, G.S. Intra-Articular Injection of Bone Marrow Aspirate Concentrate (Mesenchymal Stem Cells) in KL Grade III and IV Knee Osteoarthritis: 4 Year Results of 37 Knees. Sci. Rep. 2024, 14, 2665. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Huey, D.J.; Hu, J.C.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Brittberg, M.; Peterson, L.; Sjögren-Jansson, E.; Tallheden, T.; Lindahl, A. Articular Cartilage Engineering with Autologous Chondrocyte Transplantation. A Review of Recent Developments. J. Bone Jt. Surg. Am. 2003, 85 (Suppl. 3), 109–115. [Google Scholar] [CrossRef]
- Steinwachs, M.; Kreuz, P.C. Autologous Chondrocyte Implantation in Chondral Defects of the Knee with a Type I/III Collagen Membrane: A Prospective Study with a 3-Year Follow-Up. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, H.; Yasuda, K.; Minami, A.; Majima, T.; Iwasaki, N.; Muneta, T.; Sekiya, I.; Yagishita, K.; Takahashi, S.; Kurokouchi, K.; et al. Atelocollagen-Associated Autologous Chondrocyte Implantation for the Repair of Chondral Defects of the Knee: A Prospective Multicenter Clinical Trial in Japan. J. Orthop. Sci. 2009, 14, 579–588. [Google Scholar] [CrossRef]
- Roberts, S.; Menage, J.; Sandell, L.J.; Evans, E.H.; Richardson, J.B. Immunohistochemical Study of Collagen Types I and II and Procollagen IIA in Human Cartilage Repair Tissue Following Autologous Chondrocyte Implantation. Knee 2009, 16, 398–404. [Google Scholar] [CrossRef]
- Benya, P.D.; Padilla, S.R.; Nimni, M.E. Independent Regulation of Collagen Types by Chondrocytes during the Loss of Differentiated Function in Culture. Cell 1978, 15, 1313–1321. [Google Scholar] [CrossRef]
- Tan Timur, U.; Caron, M.; van den Akker, G.; van der Windt, A.; Visser, J.; van Rhijn, L.; Weinans, H.; Welting, T.; Emans, P.; Jahr, H. Increased TGF-β and BMP Levels and Improved Chondrocyte-Specific Marker Expression In Vitro under Cartilage-Specific Physiological Osmolarity. Int. J. Mol. Sci. 2019, 20, 795. [Google Scholar] [CrossRef]
- Knudson, C.B.; Knudson, W. Cartilage Proteoglycans. Semin. Cell Dev. Biol. 2001, 12, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Glycan-Based Interactions Involving Vertebrate Sialic-Acid-Recognizing Proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Malagolini, N.; Chiricolo, M.; Marini, M.; Dall’Olio, F. Exposure of Alpha2,6-Sialylated Lactosaminic Chains Marks Apoptotic and Necrotic Death in Different Cell Types. Glycobiology 2009, 19, 172–181. [Google Scholar] [CrossRef]
- Toegel, S.; Pabst, M.; Wu, S.Q.; Grass, J.; Goldring, M.B.; Chiari, C.; Kolb, A.; Altmann, F.; Viernstein, H.; Unger, F.M. Phenotype-Related Differential Alpha-2,6- or Alpha-2,3-Sialylation of Glycoprotein N-Glycans in Human Chondrocytes. Osteoarthr. Cartil. OARS Osteoarthr. Res. Soc. 2010, 18, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kahrizi, M.S.; Mousavi, E.; Khosravi, A.; Rahnama, S.; Salehi, A.; Nasrabadi, N.; Ebrahimzadeh, F.; Jamali, S. Recent Advances in Pre-Conditioned Mesenchymal Stem/Stromal Cell (MSCs) Therapy in Organ Failure; a Comprehensive Review of Preclinical Studies. Stem Cell Res. Ther. 2023, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.U.; Mehta, A.; Vũ, T.T.; Yeo, G.C. Cellular Modifications and Biomaterial Design to Improve Mesenchymal Stem Cell Transplantation. Biomater. Sci. 2023, 11, 4752–4773. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Homan, K.; Fukushima, A.; Ukeba, D.; Iwasaki, N.; Sudo, H. A Review: Methodologies to Promote the Differentiation of Mesenchymal Stem Cells for the Regeneration of Intervertebral Disc Cells Following Intervertebral Disc Degeneration. Cells 2023, 12, 2161. [Google Scholar] [CrossRef]
- Ma, C.-Y.; Zhai, Y.; Li, C.T.; Liu, J.; Xu, X.; Chen, H.; Tse, H.-F.; Lian, Q. Translating Mesenchymal Stem Cell and Their Exosome Research into GMP Compliant Advanced Therapy Products: Promises, Problems and Prospects. Med. Res. Rev. 2023, 44, 919–938. [Google Scholar] [CrossRef]
- Fernández-Garza, L.E.; Barrera-Barrera, S.A.; Barrera-Saldaña, H.A. Mesenchymal Stem Cell Therapies Approved by Regulatory Agencies around the World. Pharmaceuticals 2023, 16, 1334. [Google Scholar] [CrossRef]
- Luo, D.; Zhu, H.; Li, S.; Wang, Z.; Xiao, J. Mesenchymal Stem Cell-Derived Exosomes as a Promising Cell-Free Therapy for Knee Osteoarthritis. Front. Bioeng. Biotechnol. 2024, 12, 1309946. [Google Scholar] [CrossRef]
- Belk, J.W.; Lim, J.J.; Keeter, C.; McCulloch, P.C.; Houck, D.A.; McCarty, E.C.; Frank, R.M.; Kraeutler, M.J. Patients with Knee Osteoarthritis Who Receive Platelet-Rich Plasma or Bone Marrow Aspirate Concentrate Injections Have Better Outcomes Than Patients Who Receive Hyaluronic Acid: Systematic Review and Meta-Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 1714–1734. [Google Scholar] [CrossRef]
- Nagano, K.; Yoshida, Y.; Isobe, T. Cell Surface Biomarkers of Embryonic Stem Cells. Proteomics 2008, 8, 4025–4035. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Go, S.; Veillon, L.; Ciampa, M.G.; Mauri, L.; Sato, C.; Kitajima, K.; Prinetti, A.; Sonnino, S.; Inokuchi, J.-I. Altered Expression of Ganglioside GM3 Molecular Species and a Potential Regulatory Role during Myoblast Differentiation. J. Biol. Chem. 2017, 292, 7040–7051. [Google Scholar] [CrossRef]
- Amano, M.; Yamaguchi, M.; Takegawa, Y.; Yamashita, T.; Terashima, M.; Furukawa, J.-I.; Miura, Y.; Shinohara, Y.; Iwasaki, N.; Minami, A.; et al. Threshold in Stage-Specific Embryonic Glycotypes Uncovered by a Full Portrait of Dynamic N-Glycan Expression during Cell Differentiation. Mol. Cell. Proteomics MCP 2010, 9, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, A.; Hirvonen, T.; Salo, H.; Impola, U.; Olonen, A.; Laitinen, A.; Tiitinen, S.; Natunen, S.; Aitio, O.; Miller-Podraza, H.; et al. Glycomics of Bone Marrow-Derived Mesenchymal Stem Cells Can Be Used to Evaluate Their Cellular Differentiation Stage. Glycoconj. J. 2009, 26, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Saito, S.; Hiemori, K.; Kiyoi, K.; Hasehira, K.; Toyoda, M.; Onuma, Y.; Ito, Y.; Akutsu, H.; Hirabayashi, J. A2-6 Sialylation Is a Marker of the Differentiation Potential of Human Mesenchymal Stem Cells. Glycobiology 2016, 26, 1328–1337. [Google Scholar] [CrossRef]
- Hasehira, K.; Hirabayashi, J.; Tateno, H. Structural and Quantitative Evidence of A2-6-Sialylated N-Glycans as Markers of the Differentiation Potential of Human Mesenchymal Stem Cells. Glycoconj. J. 2017, 34, 797–806. [Google Scholar] [CrossRef][Green Version]
- Xu, L.; Hanamatsu, H.; Homan, K.; Onodera, T.; Miyazaki, T.; Furukawa, J.-I.; Hontani, K.; Tian, Y.; Baba, R.; Iwasaki, N. Alterations of Glycosphingolipid Glycans and Chondrogenic Markers during Differentiation of Human Induced Pluripotent Stem Cells into Chondrocytes. Biomolecules 2020, 10, 1622. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A More Efficient Method to Generate Integration-Free Human iPS Cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef]
- Nikolouli, E.; Reichstein, J.; Hansen, G.; Lachmann, N. In Vitro Systems to Study Inborn Errors of Immunity Using Human Induced Pluripotent Stem Cells. Front. Immunol. 2022, 13, 1024935. [Google Scholar] [CrossRef] [PubMed]
- Şen, B.; Balcı-Peynircioğlu, B. Cellular Models in Autoinflammatory Disease Research. Clin. Transl. Immunol. 2024, 13, e1481. [Google Scholar] [CrossRef] [PubMed]
- de Rham, C.; Villard, J. Potential and Limitation of HLA-Based Banking of Human Pluripotent Stem Cells for Cell Therapy. J. Immunol. Res. 2014, 2014, 518135. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Preynat-Seauve, O.; Tiercy, J.-M.; Krause, K.-H.; Villard, J. Haplotype-Based Banking of Human Pluripotent Stem Cells for Transplantation: Potential and Limitations. Stem Cells Dev. 2012, 21, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Morishima, Y.; Azuma, F.; Kashiwase, K.; Matsumoto, K.; Orihara, T.; Yabe, H.; Kato, S.; Kato, K.; Kai, S.; Mori, T.; et al. Risk of HLA Homozygous Cord Blood Transplantation: Implications for Induced Pluripotent Stem Cell Banking and Transplantation. Stem Cells Transl. Med. 2018, 7, 173–179. [Google Scholar] [CrossRef]
- Umekage, M.; Sato, Y.; Takasu, N. Overview: An iPS Cell Stock at CiRA. Inflamm. Regen. 2019, 39, 17. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Hontani, K.; Onodera, T.; Terashima, M.; Momma, D.; Matsuoka, M.; Baba, R.; Joutoku, Z.; Matsubara, S.; Homan, K.; Hishimura, R.; et al. Chondrogenic Differentiation of Mouse Induced Pluripotent Stem Cells Using the Three-Dimensional Culture with Ultra-Purified Alginate Gel. J. Biomed. Mater. Res. A 2019, 107, 1086–1093. [Google Scholar] [CrossRef]
- Gropp, M.; Shilo, V.; Vainer, G.; Gov, M.; Gil, Y.; Khaner, H.; Matzrafi, L.; Idelson, M.; Kopolovic, J.; Zak, N.B.; et al. Standardization of the Teratoma Assay for Analysis of Pluripotency of Human ES Cells and Biosafety of Their Differentiated Progeny. PLoS ONE 2012, 7, e45532. [Google Scholar] [CrossRef]
- Kawamata, S.; Kanemura, H.; Sakai, N.; Takahashi, M.; Go, M.J. Design of a Tumorigenicity Test for Induced Pluripotent Stem Cell (iPSC)-Derived Cell Products. J. Clin. Med. 2015, 4, 159–171. [Google Scholar] [CrossRef]
- Matsumoto, S.; Nakao, H.; Kawabe, K.; Nonaka, M.; Toyoda, H.; Takishima, Y.; Kawabata, K.; Yamaguchi, T.; Furue, M.K.; Taki, T.; et al. A Cytotoxic Antibody Recognizing Lacto-N-Fucopentaose I (LNFP I) on Human Induced Pluripotent Stem (hiPS) Cells. J. Biol. Chem. 2015, 290, 20071–20085. [Google Scholar] [CrossRef]
- Li, H.; Gao, W.; Feng, X.; Liu, B.-F.; Liu, X. MALDI-MS Analysis of Sialylated N-Glycan Linkage Isomers Using Solid-Phase Two Step Derivatization Method. Anal. Chim. Acta 2016, 924, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lageveen-Kammeijer, G.S.M.; de Haan, N.; Mohaupt, P.; Wagt, S.; Filius, M.; Nouta, J.; Falck, D.; Wuhrer, M. Highly Sensitive CE-ESI-MS Analysis of N-Glycans from Complex Biological Samples. Nat. Commun. 2019, 10, 2137. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Abe, T.; Natsuka, S. Quantitative LC-MS and MS/MS Analysis of Sialylated Glycans Modified by Linkage-Specific Alkylamidation. Anal. Biochem. 2019, 567, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hanamatsu, H.; Nishikaze, T.; Miura, N.; Piao, J.; Okada, K.; Sekiya, S.; Iwamoto, S.; Sakamoto, N.; Tanaka, K.; Furukawa, J.-I. Sialic Acid Linkage Specific Derivatization of Glycosphingolipid Glycans by Ring-Opening Aminolysis of Lactones. Anal. Chem. 2018, 90, 13193–13199. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hanamatsu, H.; Xu, L.; Onodera, T.; Furukawa, J.-I.; Homan, K.; Baba, R.; Kawasaki, T.; Iwasaki, N. Evaluation of Residual Human-Induced Pluripotent Stem Cells in Human Chondrocytes by Cell Type-Specific Glycosphingolipid Glycome Analysis Based on the Aminolysis-SALSA Technique. Int. J. Mol. Sci. 2019, 21, 231. [Google Scholar] [CrossRef]
- Miyazaki, T.; Hanamatsu, H.; Onodera, T.; Furukawa, J.-I.; Xu, L.; Homan, K.; Baba, R.; Kawasaki, T.; Iwasaki, N. Establishment of the Removal Method of Undifferentiated Induced Pluripotent Stem Cells Coexisting with Chondrocytes Using R-17F Antibody. Regen. Med. 2022, 17, 793–803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).