Anticancer Effects of Mitoquinone via Cell Cycle Arrest and Apoptosis in Canine Mammary Gland Tumor Cells
Abstract
1. Introduction
2. Results
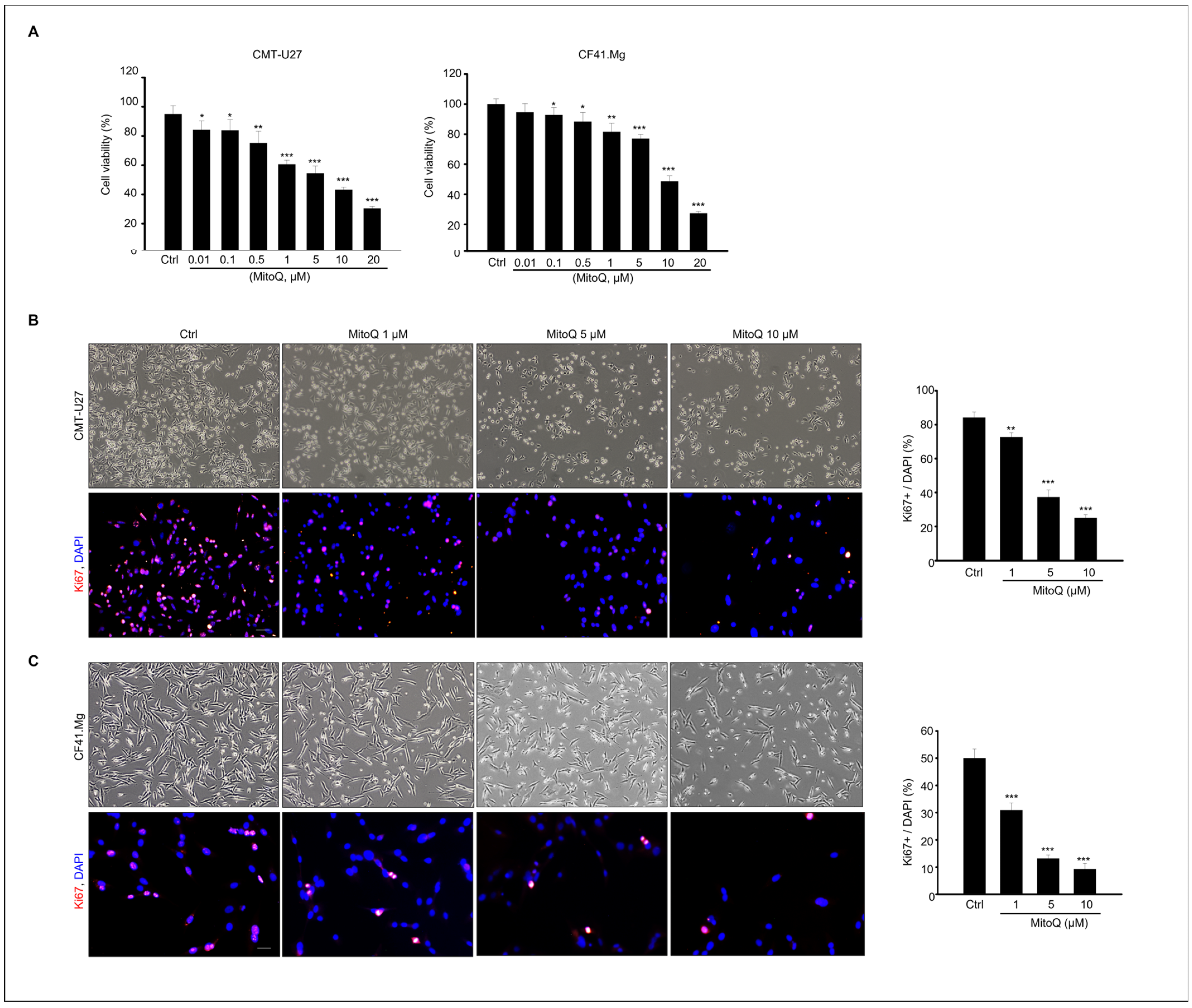
2.1. Effects of MitoQ on Cell Viability and Proliferation in Canine Mammary Tumor Cells
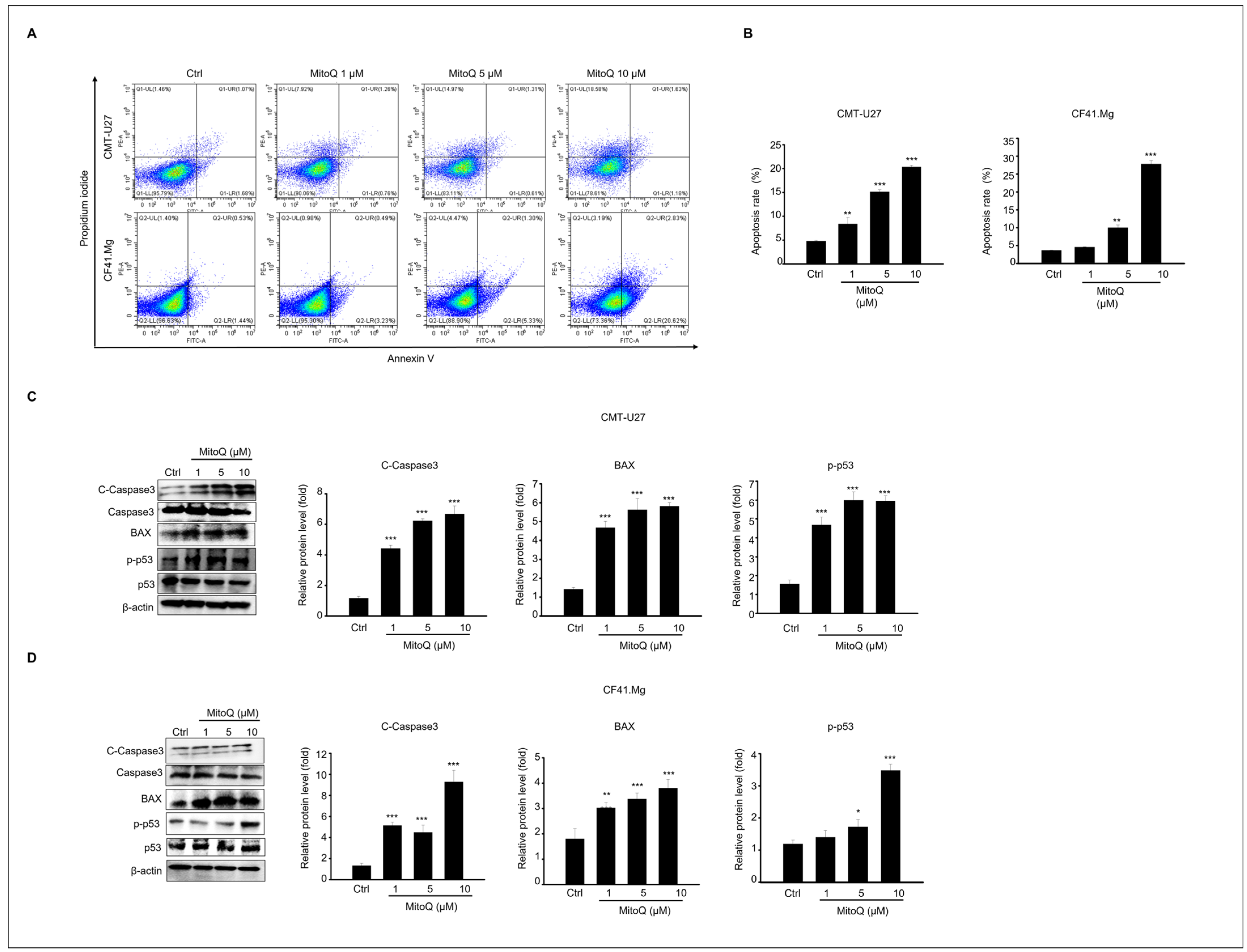
2.2. Apoptotic Effects of MitoQ on Canine Mammary Gland Tumor Cell Culture
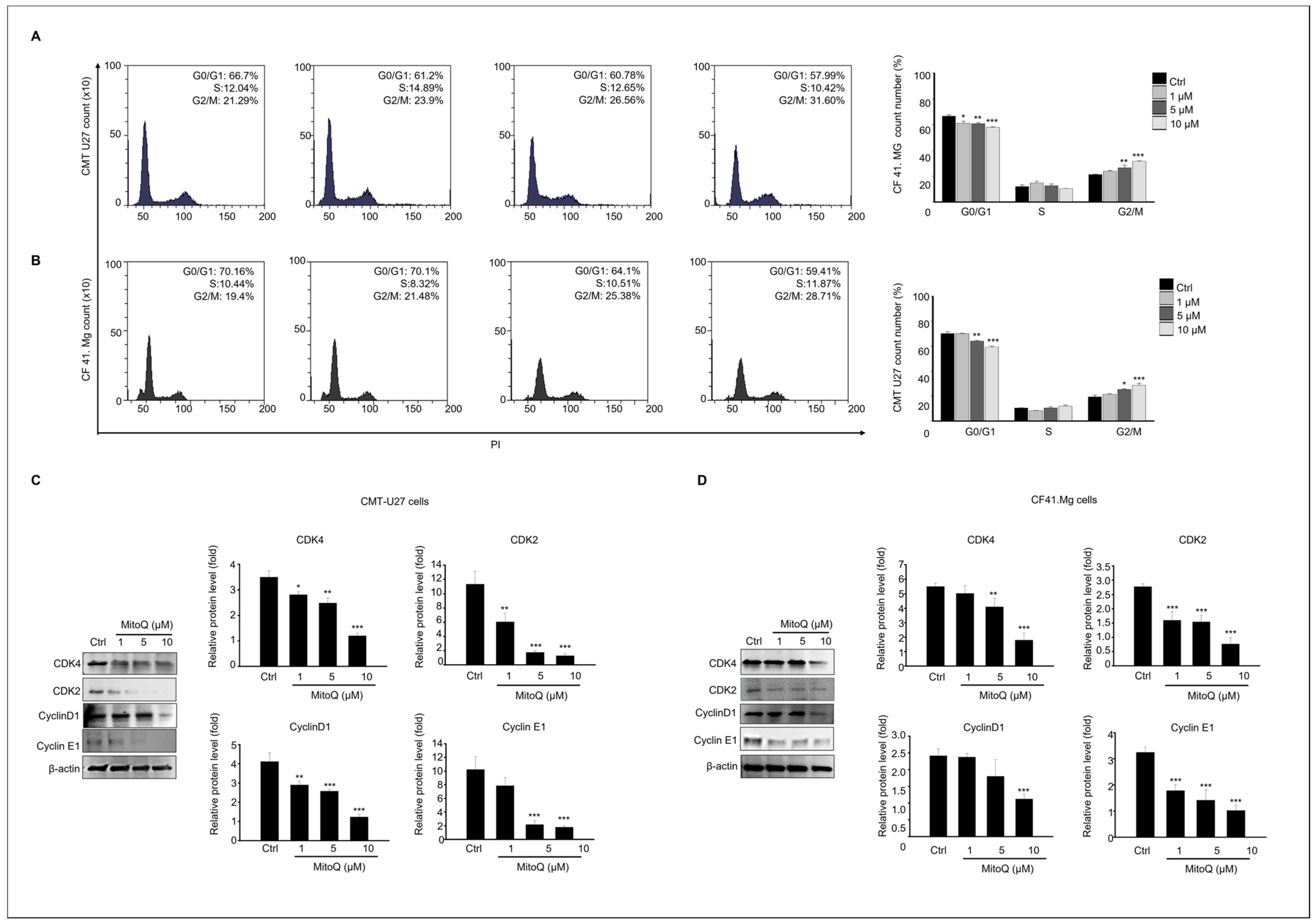
2.3. Effects of MitoQ on Cell Cycle Arrest and Expression of Cell Cycle Regulatory Proteins in Canine Mammary Gland Tumor Cells
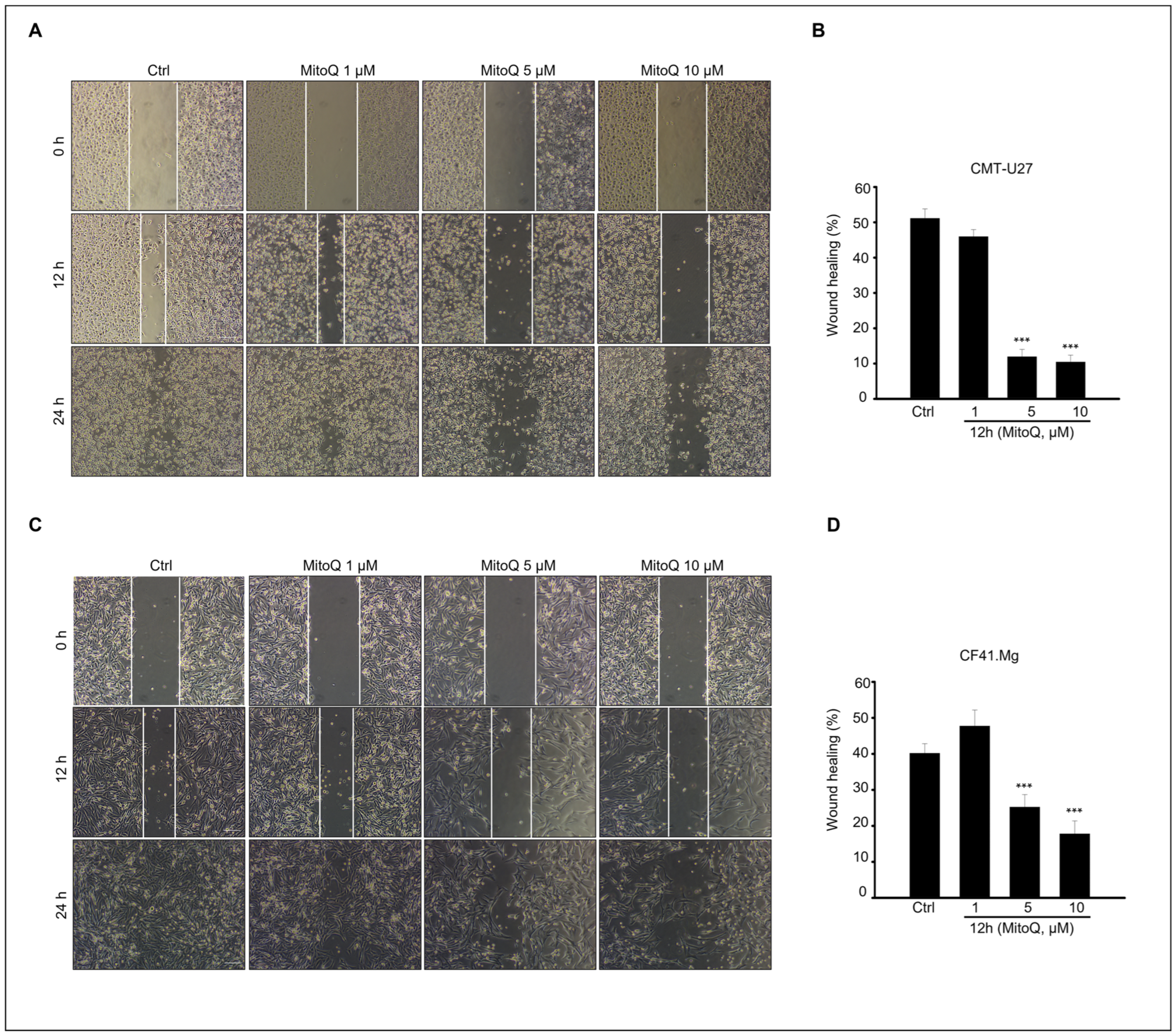
2.4. Effects of MitoQ on Canine Mammary Cancer Cell Migration
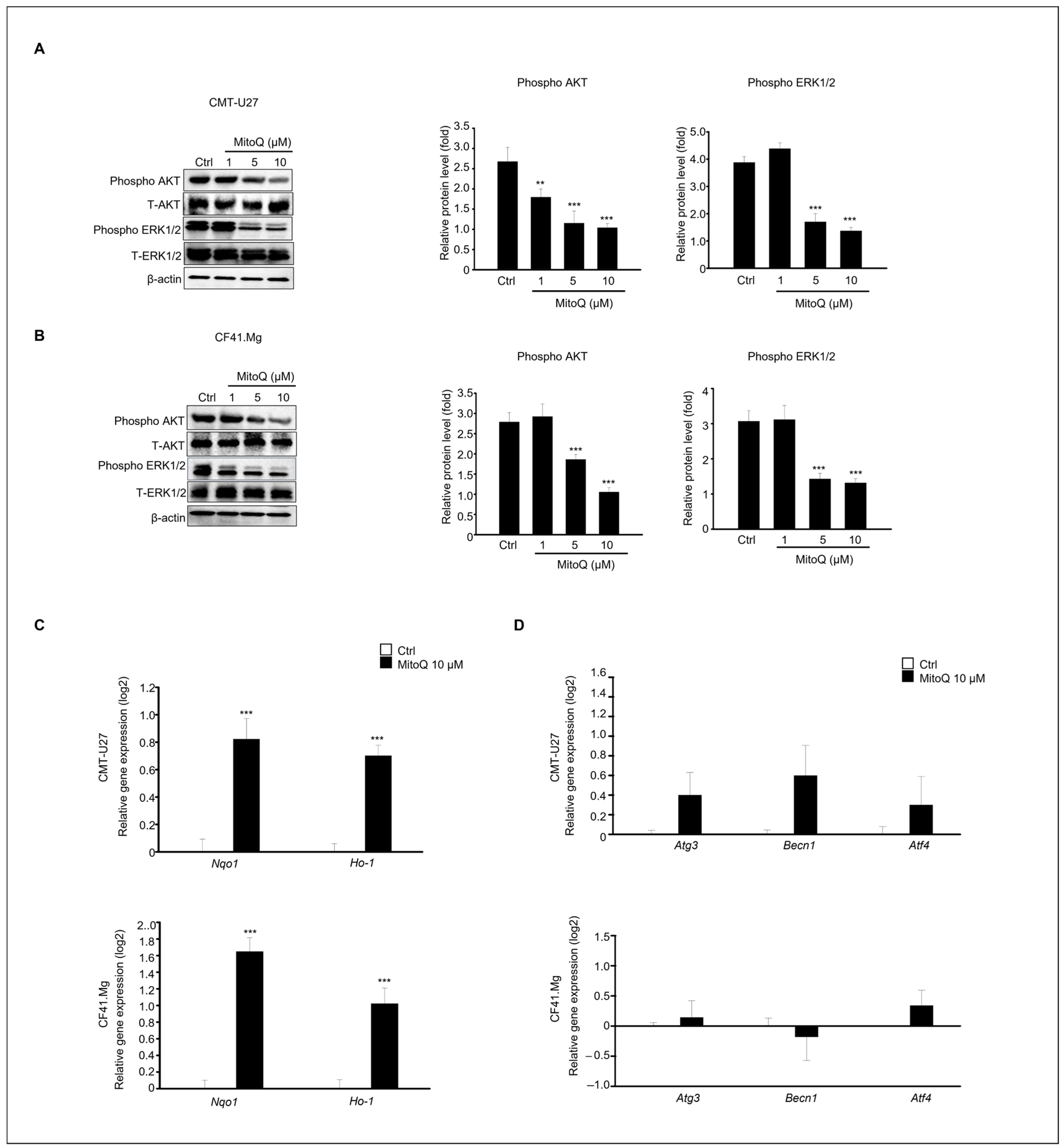
2.5. Effects of MitoQ on Canine Mammary Cancer Cell Death Involving Extracellular Signal-Regulated Kinase and Protein Kinase B Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Viability Assay
4.3. Immunostaining
4.4. Flow Cytometry
4.5. Wound-Healing Assay
4.6. Quantitative Polymerase Chain Reaction (qPCR)
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L.N. Canine neoplasia in the UK: Estimates of incidence rates from a population of insured dogs. J. Small Animal Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Egenvall, A.; Bonnett, B.N.; Ohagen, P.; Olson, P.; Hedhammar, A.; Von Euler, H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005, 69, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Canadas, A.; França, M.; Pereira, C.; Vilaça, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Vet. Pathol. 2019, 56, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Peña, L.; Perez-Alenza, M.D.; Rodriguez-Bertos, A.; Nieto, A. Canine inflammatory mammary carcinoma: Histopathology, immunohistochemistry and clinical implications of 21 cases. Breast Cancer Res. Treat. 2003, 78, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, G.K.; Reynolds, H.A.; Richardson, R.C.; Withrow, S.J.; Norris, A.M.; Henderson, R.A.; Klausner, J.S.; Fowler, J.D.; McCaw, D. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J. Am. Vet. Med. Assoc. 1989, 195, 1580–1583. [Google Scholar] [PubMed]
- Zambrano-Estrada, X.; Landaverde-Quiroz, B.; Dueñas-Bocanegra, A.A.; De Paz-Campos, M.A.; Hernández-Alberto, G.; Solorio-Perusquia, B.; Trejo-Mandujano, M.; Pérez-Guerrero, L.; Delgado-González, E.; Anguiano, B.; et al. Molecular iodine/doxorubicin neoadjuvant treatment impair invasive capacity and attenuate side effect in canine mammary cancer. BMC Vet. Res. 2018, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small. Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Lavalle, G.E.; De Campos, C.B.; Bertagnolli, A.C.; Cassali, G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. Randomized Control. Trial Vivo 2012, 26, 375–379. [Google Scholar]
- Poirier, V.J.; Hershey, A.E.; Burgess, K.E.; Phillips, B.; Turek, M.M.; Forrest, L.J.; Beaver, L.; Vail, D.M. Efficacy and toxicity of paclitaxel (Taxol) for the treatment of canine malignant tumors. J. Vet. Intern. Med. 2004, 18, 219–222. [Google Scholar] [CrossRef] [PubMed]
- von Euler, H.; Rivera, P.; Nyman, H.; Häggström, J.; Borgå, O. A dose-finding study with a novel water-soluble formulation of paclitaxel for the treatment of malignant high-grade solid tumours in dogs. Vet. Comp. Oncol. 2013, 11, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Lorenzo, R.M.; Abramo, F.; Ratto, A.; Zini, E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet. Comp. Oncol. 2008, 6, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Schoenrock, D.; Baumgärtner, W.; Nolte, I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J. Vet. Intern. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2016, 14, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, J.M.; Prerana, P.; Dharmarajan, A.; Warrier, S.; Gandhirajan, R.K. Modulation of reactive oxygen species in cancers: Recent advances. Free Radic. Res. 2022, 56, 447–470. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Goldstein, H.P.; Reinecke, R.D. Absence of visual sampling in infantile nystagmus. Korean J. Ophthalmol. 1989, 3, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, T.G.; Park, S.; Yun, H.R.; Nguyen, N.N.Y.; Jo, Y.H.; Jang, M.; Kim, J.; Kim, J.; Kang, I.; et al. Mitochondrial ROS-derived PTEN oxidation activates PI3K pathway for mTOR-induced myogenic autophagy. Cell Death Differ. 2018, 25, 1921–1937. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Ambrosone, C.B.; Zirpoli, G.R.; Hutson, A.D.; McCann, W.E.; McCann, S.E.; Barlow, W.E.; Kelly, K.M.; Cannioto, R.; Sucheston-Campbell, L.E.; Hershman, D.L.; et al. Dietary Supplement Use During Chemotherapy and Survival Outcomes of Patients With Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 2020, 38, 804–814. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer. 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 7260, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Yang, K.S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Meng, T.C.; Hinks, J.A.; Tonks, N.K.; Barford, D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 2003, 423, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Gírio, A.; Cebola, I.; Santos, C.I.; Antunes, F.; Barata, J.T. Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells. Leukemia 2011, 25, 960–967. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Sharpley, M.S.; Manas, A.R.; Frerman, F.E.; Hirst, J.; Smith, R.A.; Murphy, M.P. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J. Biol. Chem. 1999, 18, 14708–14718. [Google Scholar] [CrossRef]
- Faubert, B.; Boily, G.; Izreig, S.; Griss, T.; Samborska, B.; Dong, Z.; Dupuy, F.; Chambers, C.; Fuerth, B.J.; Viollet, B.; et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013, 17, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Availability, not respiratory capacity governs oxygen consumption of solid tumors. Int. J. Biochem. Cell Biol. 2012, 44, 1477–1481. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Chang, Y.S.; di Tomaso, E.; McDonald, D.M.; Jones, R.; Jain, R.K.; Munn, L.L. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 2000, 97, 14608–14613. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, T.V.; Gorbunova, A.S.; Zhivotovsky, B. Mitochondrial Involvement in Migration, Invasion and Metastasis. Front. Cell Dev. Biol. 2019, 20, 355. [Google Scholar] [CrossRef] [PubMed]
- Capeloa, T.; Krzystyniak, J.; Rodriguez, A.C.; Payen, V.L.; Zampieri, L.X.; Pranzini, E.; Derouane, F.; Vazeille, T.; Bouzin, C.; Duhoux, F.P.; et al. MitoQ Prevents Human Breast Cancer Recurrence and Lung Metastasis in Mice. Cancers 2022, 14, 1488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Karoui, H.; Hardy, M.; Kalyanaraman, B. Redox-crippled MitoQ potently inhibits breast cancer and glioma cell proliferation: A negative control for verifying the antioxidant mechanism of MitoQ in cancer and other oxidative pathologies. Free Radic. Biol. Med. 2023, 205, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.Y.; Gudup, S.; Sunkaria, A.; Bal, A.; Singh, P.P.; Kandimalla, R.J.; Sharma, D.R.; Gill, K.D. Protective efficacy of mitochondrial targeted antioxidant MitoQ against dichlorvos induced oxidative stress and cell death in rat brain. Neuropharmacology 2011, 61, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Murphy, M.P. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 2010, 1201, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 5, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Capeloa, T.; Krzystyniak, J.; d’Hose, D.; Canas Rodriguez, A.; Payen, V.L.; Zampieri, L.X.; Van de Velde, J.A.; Benyahia, Z.; Pranzini, E.; Vazeille, T.; et al. MitoQ Inhibits Human Breast Cancer Cell Migration, Invasion and Clonogenicity. Cancers 2022, 14, 1516. [Google Scholar] [CrossRef] [PubMed]
- Magwere, T.; West, M.; Riyahi, K.; Murphy, M.P.; Smith, R.A.; Partridge, L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech. Ageing Dev. 2006, 127, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Saretzki, G.; Murphy, M.P.; von Zglinicki, T. MitoQ counteracts telomere shortening and elongates lifespan of fibroblasts under mild oxidative stress. Aging Cell 2003, 2, 141–143. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Huot, J.R.; Bonetto, A. The Mitochondria-Targeting Agent MitoQ Improves Muscle Atrophy, Weakness and Oxidative Metabolism in C26 Tumor-Bearing Mice. Front. Cell Dev. Biol. 2022, 10, 861622. [Google Scholar] [CrossRef] [PubMed]
- Vergeade, A.; Mulder, P.; Vendeville-Dehaudt, C.; Estour, F.; Fortin, D.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: Prevention by the targeted antioxidant MitoQ. Free Radic. Biol. Med. 2010, 49, 748–756. [Google Scholar] [CrossRef] [PubMed]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The mitochondria-targeted antioxidant MitoQ prevents loss of spatial memory retention and early neuropathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Dall, G.V.; Hawthorne, S.; Seyed-Razavi, Y.; Vieusseux, J.; Wu, W.; Gustafsson, J.A.; Byrne, D.; Murphy, L.; Risbridger, G.P.; Britt, K.L. Estrogen receptor subtypes dictate the proliferative nature of the mammary gland. J. Endocrinol. 2018, 237, 323–336. [Google Scholar] [CrossRef]
- Mei, C.; Xin, L.; Liu, Y.; Lin, J.; Xian, H.; Zhang, X.; Hu, W.; Xia, Z.; Wang, H.; Lyu, Y. Establishment of a New Cell Line of Canine Mammary Tumor CMT-1026. Front. Vet. Sci. 2021, 12, 744032. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.G.; Olivares, A.; Stoore, C. Simvastatin exhibits antiproliferative effects on spheres derived from canine mammary carcinoma cells. Oncol. Rep. 2015, 33, 2235–2244. [Google Scholar] [CrossRef][Green Version]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Okon, I.S.; Zou, M.H. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol. Res. 2015, 100, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Capeloa, T.; Van de Velde, J.A.; d’Hose, D.; Lipari, S.G.; Derouane, F.; Hamelin, L.; Bedin, M.; Vazeille, T.; Duhoux, F.P.; Murphy, M.P.; et al. Inhibition of Mitochondrial Redox Signaling with MitoQ Prevents Metastasis of Human Pancreatic Cancer in Mice. Cancers 2022, 14, 4918. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Klein, S.R.; Bonar, S.J.; Zielonka, J.; Mizuno, N.; Dickey, J.S.; Keller, P.W.; Joseph, J.; Kalyanaraman, B.; Shacter, E. The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. J. Biol. Chem. 2010, 5, 34447–34459. [Google Scholar] [CrossRef]
- Liu, L.; Cui, H.; Xu, Y. Quantitative Estimation of Oxidative Stress in Cancer Tissue Cells Through Gene Expression Data Analyses. Front. Genet. 2020, 19, 494. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 10, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Chemoprevention by isothiocyanates. J. Cell Biochem. Suppl. 1995, 22, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.C.; Jiao, D.; Chung, F.L. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: A structure-activity relationship study. Carcinogenesis 1996, 17, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front. Oncol. 2022, 12, 862743. [Google Scholar] [CrossRef]
- Nazarewicz, R.R.; Dikalova, A.; Bikineyeva, A.; Ivanov, S.; Kirilyuk, I.A.; Grigor’ev, I.A.; Dikalov, S.I. Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid. Redox Signal. 2013, 19, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Balmanno, K.; Cook, S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009, 16, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Arboleda, M.J.; Lyons, J.F.; Kabbinavar, F.F.; Bray, M.R.; Snow, B.E.; Ayala, R.; Danino, M.; Karlan, B.Y.; Slamon, D.J. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003, 63, 196–206. [Google Scholar] [PubMed]
- Lee, M.W.; Kim, D.S.; Lee, J.H.; Lee, B.S.; Lee, S.H.; Jung, H.L.; Sung, K.W.; Kim, H.T.; Yoo, K.H.; Koo, H.H. Roles of AKT1 and AKT2 in non-small cell lung cancer cell survival, growth, and migration. Cancer Sci. 2011, 102, 1822–1828. [Google Scholar] [CrossRef]
- Rashmi, R.; DeSelm, C.; Helms, C.; Bowcock, A.; Rogers, B.E.; Rader, J.L.; Rader, J.; Grigsby, P.W.; Schwarz, J.K. AKT inhibitors promote cell death in cervical cancer through disruption of mTOR signaling and glucose uptake. PLoS ONE 2014, 9, e92948. [Google Scholar] [CrossRef] [PubMed]
- Rudner, J.; Ruiner, C.E.; Handrick, R.; Eibl, H.J.; Belka, C.; Jendrossek, V. The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation. Radiat. Oncol. 2010, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, G.; Qiu, Z. Akt inhibitor MK-2206 reduces pancreatic cancer cell viability and increases the efficacy of gemcitabine. Oncol. Lett. 2020, 19, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Degtyarev, M.; De Mazière, A.; Orr, C.; Lin, J.; Lee, B.B.; Tien, J.Y.; Prior, W.W.; van Dijk, S.; Wu, H.; Gray, D.C.; et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol. 2008, 183, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.W.; Yuca, E.; Scott, S.S.; Zhao, M.; Paez-Arango, N.; Cruz-Pico, C.X.; Saridogan, T.; Shariati, M.; Class, C.A.; Bristow, C.A.; et al. Oxidative Phosphorylation Is a Metabolic Vulnerability in Chemotherapy-Resistant Triple-Negative Breast Cancer. Cancer Res. 2021, 1, 5572–5581. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 1, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; O’Connell, J.T.; Gonzalez-Herrera, K.N.; Wikman, H.; Pantel, K.; Haigis, M.C.; de Carvalho, F.M.; Damascena, A.; Domingos-Chinen, L.T.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Lim, C.Y.; Kim, H.J.; Lee, M.; An, H.J. Antioxidant mitoquinone suppresses benign prostatic hyperplasia by regulating the AR-NLRP3 pathway. Redox Bio. 2023, 65, 102816. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Lee, R.; Park, H.J. Tebuconazole Induces ER-Stress-Mediated Cell Death in Bovine Mammary Epithelial Cell Lines. Toxics 2023, 21, 397. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Park, H.J. Perfluorooctanoic acid induces cell death in TM3 cells via the ER stress-mitochondrial apoptosis pathway. Reprod. Toxicol. 2023, 118, 108383. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J. Anti-inflammatory properties of broccoli sprout extract in a lipopolysaccharide-induced testicular dysfunction. J. Anim. Reprod. Biotechnol. 2023, 38, 17–25. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, H.J. T-2 mycotoxin Induces male germ cell apoptosis by ROS-mediated JNK/p38 MAPK pathway. Ecotoxicol. Environ. Saf. 2023, 262, 115323. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kim, D.W.; Lee, W.Y.; Park, H.J. Zearalenone Induces Apoptosis and Autophagy in a Spermatogonia Cell Line. Toxins 2022, 17, 148. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| NQO1 | 5′-GAAGCCGCAGACCTGGTGAT-3′ | 5′-GCACTCGCTCGAACCAGCCT-3′ |
| HMOX1 | 5′-CTTTCAGAAGGGCCAGGTGAC-3′ | 5′-TGCTCGATCTCCTCCTCCAG-3′ |
| ATG3 | 5′-TACCAGACACCACGGCTATG-3′ | 5′-CCTGCATGGGTGAACTGAAC-3′ |
| BECN1 | 5′-GGCTGAGAGACTGGATCAGG-3′ | 5′-TGTGCCAGATGTGAAAGGTC-3′ |
| ATF4 | 5′-ACCTTTCTGCAACCACTTCC-3′ | 5′-TTATGCACTGAGGGATCACG-3′ |
| GAPDH | 5′-AATTCCACGGCACAGTCAAG-3′ | 5′-TACTCAGCACCAGCATCACC-3′ |
| Antibody | Manufacturer | Catalog Number | Dilution (Usage) |
|---|---|---|---|
| Cleaved-caspase3 | Cell Signaling (Danvers, MA, USA) | #9661 | 1:2000 |
| Caspase3 | Cell Signaling | #9662 | 1:2000 |
| BAX | Cell Signaling | #5023 | 1:2000 |
| p-p53 | Cell Signaling | #9284 | 1:2000 |
| p53 | Cell Signaling | #2524 | 1:2000 |
| CDK4 | Cell Signaling | #12790 | 1:2000 |
| CDK2 | Cell Signaling | #2546 | 1:2000 |
| Cyclin D1 | Cell Signaling | #2978 | 1:2000 |
| Cyclin E1 | Cell Signaling | #55506 | 1:2000 |
| P-AKT | Cell Signaling | #4060 | 1:2000 |
| T-AKT | Cell Signaling | #9272 | 1:2000 |
| P-erk | Cell Signaling | #9101 | 1:2000 |
| T-erk | Cell Signaling | #4695 | 1:2000 |
| Ki-67 | Abcam | ab15580 | 1:200 |
| β-actin | Santa Cruz Biotech (Dallas, TX, USA) | sc47778 | 1:2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, R.; Lee, W.-Y.; Park, H.-J. Anticancer Effects of Mitoquinone via Cell Cycle Arrest and Apoptosis in Canine Mammary Gland Tumor Cells. Int. J. Mol. Sci. 2024, 25, 4923. https://doi.org/10.3390/ijms25094923
Lee R, Lee W-Y, Park H-J. Anticancer Effects of Mitoquinone via Cell Cycle Arrest and Apoptosis in Canine Mammary Gland Tumor Cells. International Journal of Molecular Sciences. 2024; 25(9):4923. https://doi.org/10.3390/ijms25094923
Chicago/Turabian StyleLee, Ran, Won-Young Lee, and Hyun-Jung Park. 2024. "Anticancer Effects of Mitoquinone via Cell Cycle Arrest and Apoptosis in Canine Mammary Gland Tumor Cells" International Journal of Molecular Sciences 25, no. 9: 4923. https://doi.org/10.3390/ijms25094923
APA StyleLee, R., Lee, W.-Y., & Park, H.-J. (2024). Anticancer Effects of Mitoquinone via Cell Cycle Arrest and Apoptosis in Canine Mammary Gland Tumor Cells. International Journal of Molecular Sciences, 25(9), 4923. https://doi.org/10.3390/ijms25094923





