Kaempferol as an Alternative Cryosupplement for Bovine Spermatozoa: Cytoprotective and Membrane-Stabilizing Effects
Abstract
:1. Introduction
2. Results
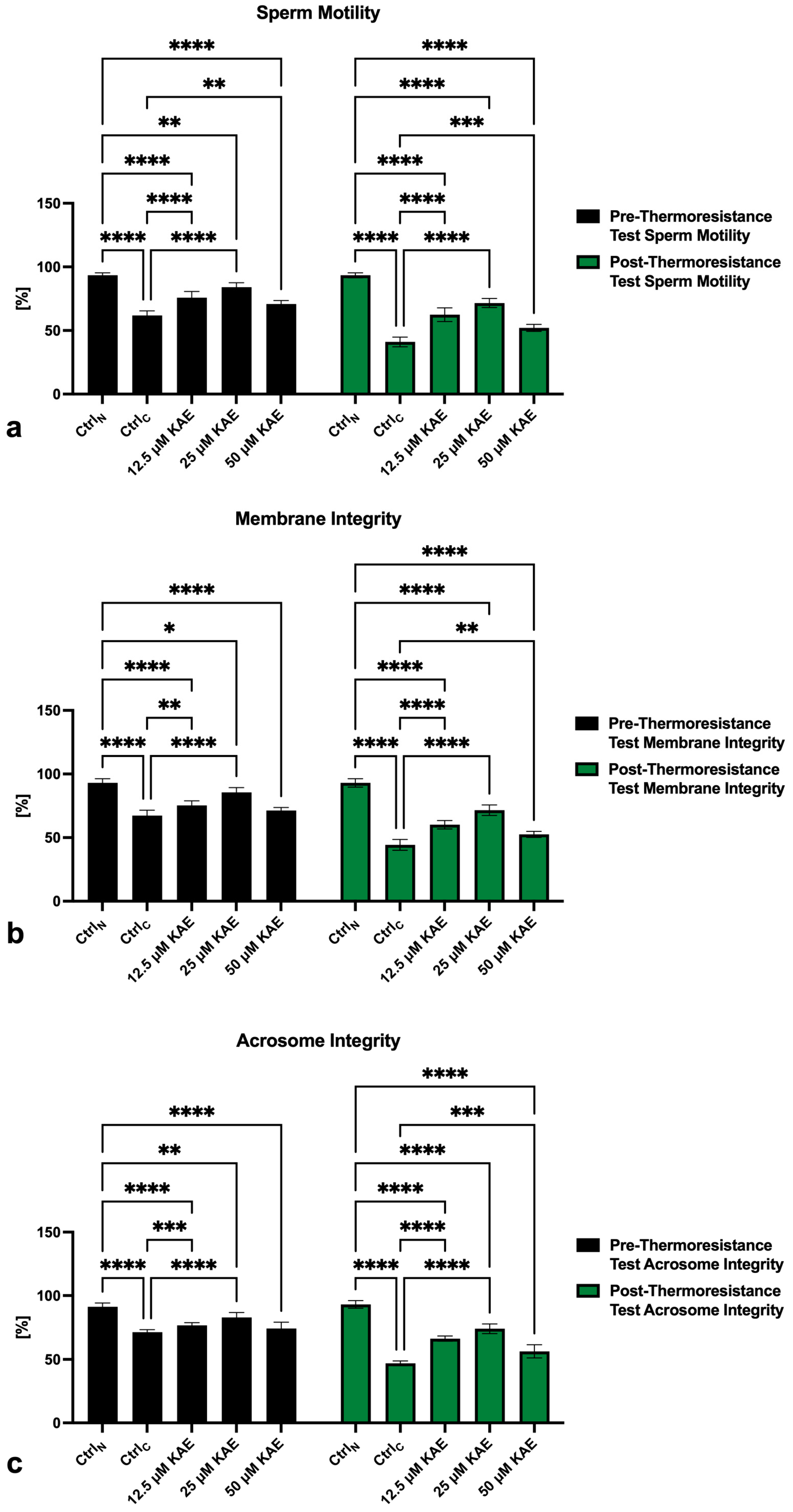
2.1. Conventional Sperm Quality
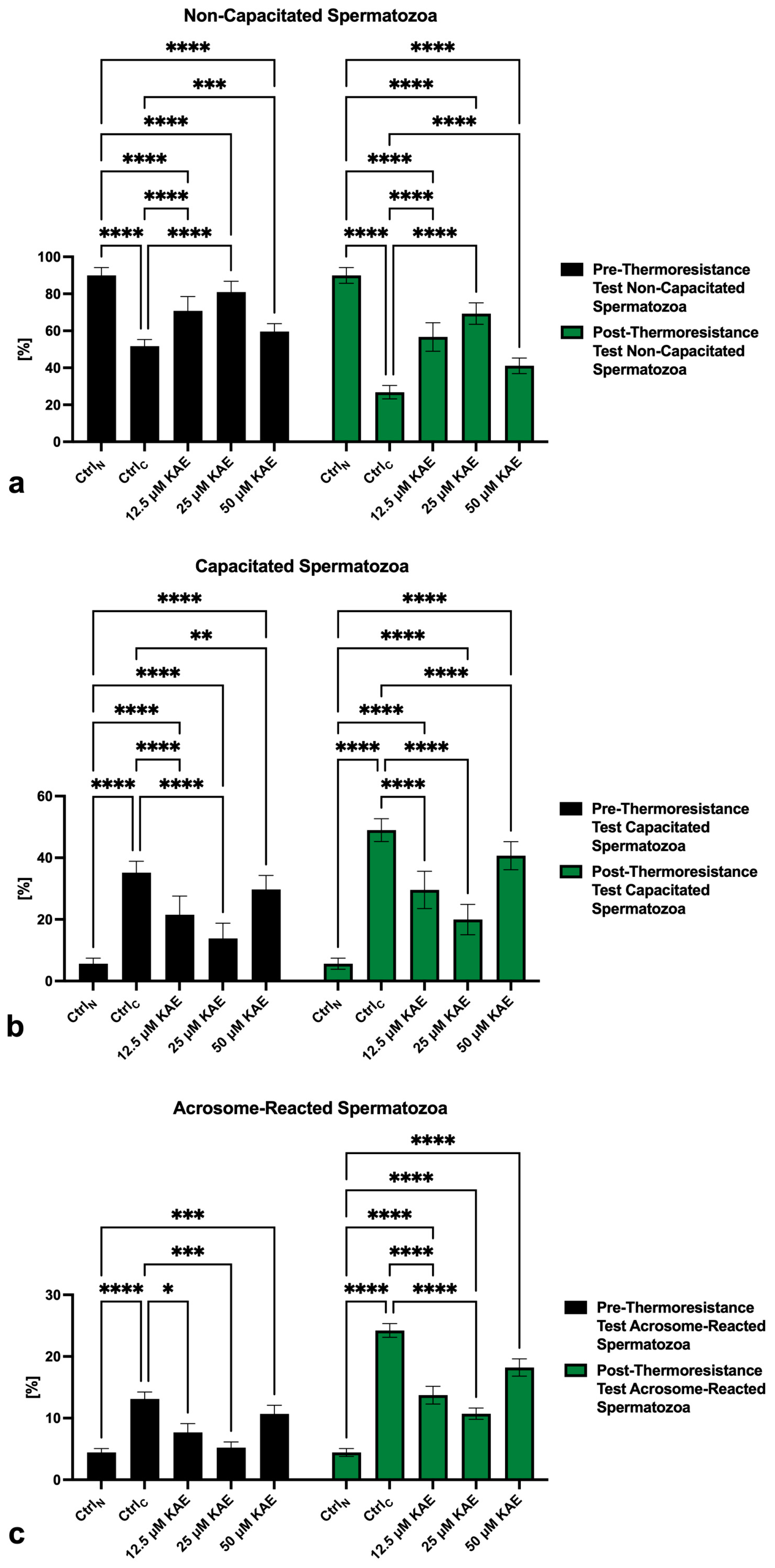
2.2. Capacitation Patterns
2.3. Activity of ATP-Ases
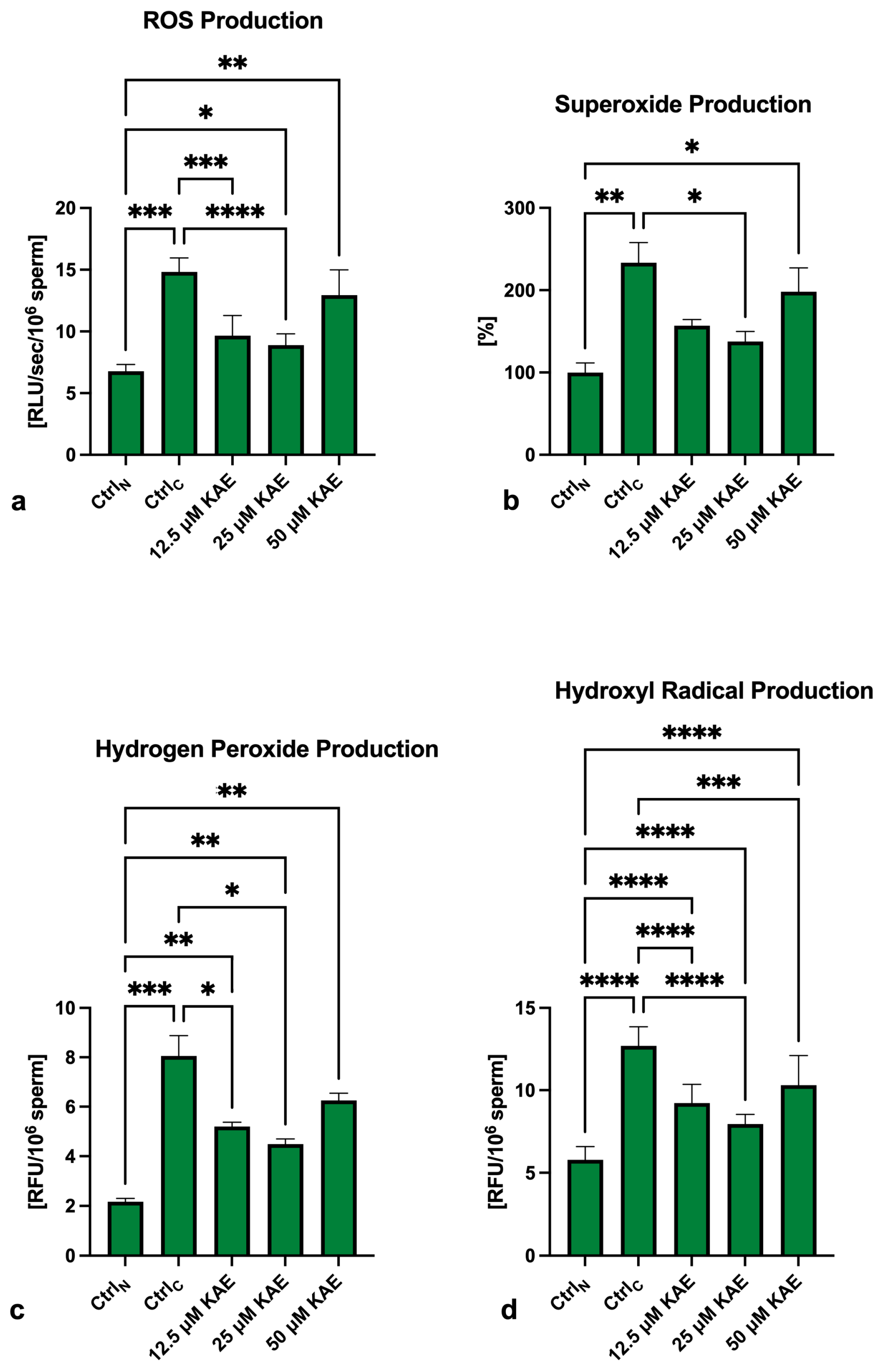
2.4. Oxidative Profile
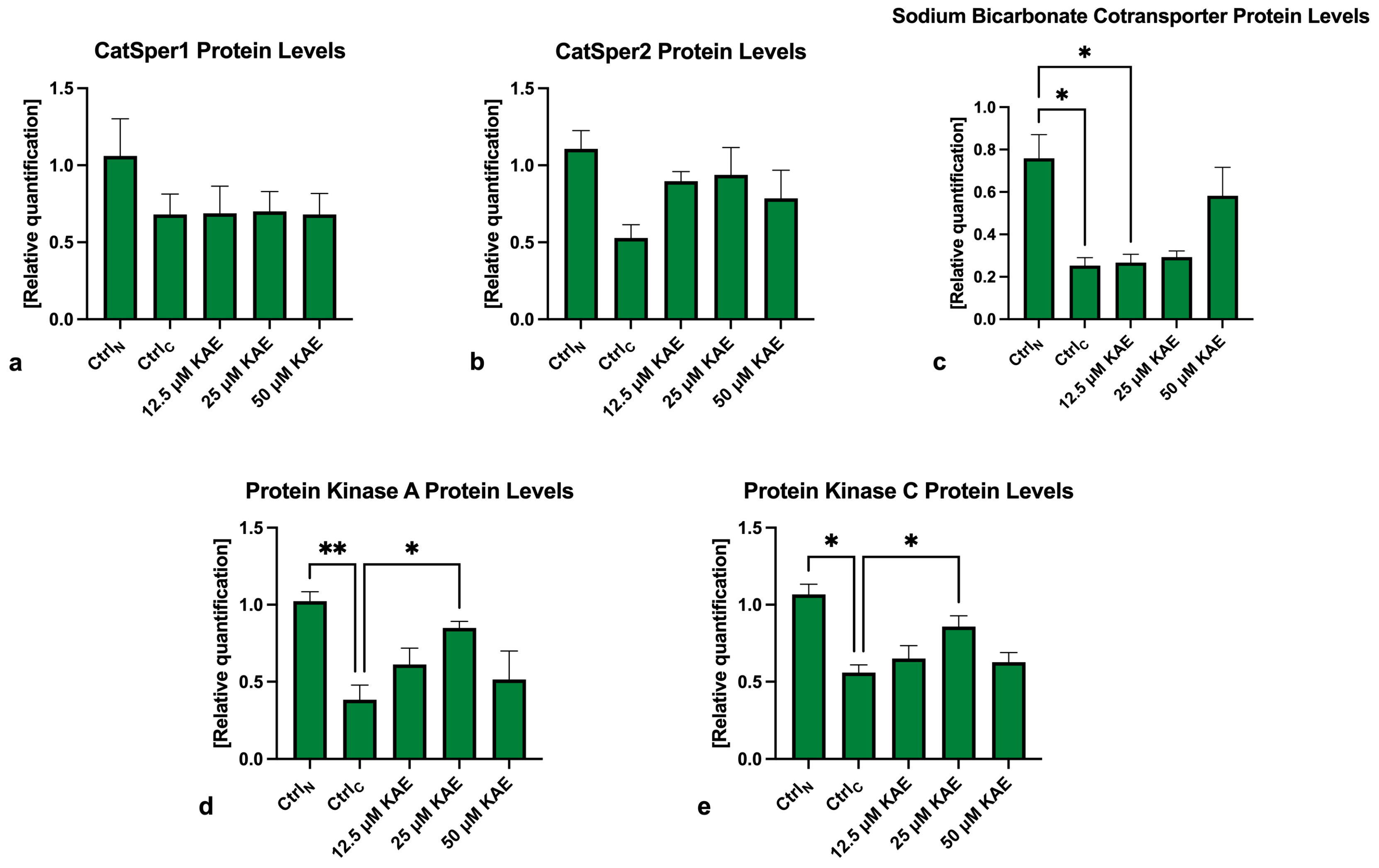
2.5. Western Blots
3. Discussion
4. Materials and Methods
4.1. Collection and Cryopreservation of Ejaculates
- Immediately after thawing (pre-thermoresistance test).
- Following a thermoresistance test (post-thermoresistance test): The samples were distributed into test tubes with support grids and placed in a water bath (37 °C) for 2 h. After that, an aliquot of each sample was withdrawn for evaluation [57].
4.2. Conventional Sperm Quality Assessment
4.3. Capacitation Status
4.4. Activity of ATP-Ases
4.5. Oxidative Profile
4.6. Western Blots
4.7. Statistics
- −
- The native control (fresh semen; CtrlN) was compared to the cryopreserved control (CtrlC);
- −
- The experimental groups were compared to both controls.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef] [PubMed]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Van Robays, J. Artificial insemination history: Hurdles and milestones. Facts Views Vis. Obgyn. 2015, 7, 137–143. [Google Scholar] [PubMed]
- Peris-Frau, P.; Soler, A.J.; Iniesta-Cuerda, M.; Martín-Maestro, A.; Sánchez-Ajofrín, I.; Medina-Chávez, D.A.; Fernández-Santos, M.R.; García-Álvarez, O.; Maroto-Morales, A.; Montoro, V.; et al. Sperm Cryodamage in Ruminants: Understanding the Molecular Changes Induced by the Cryopreservation Process to Optimize Sperm Quality. Int. J. Mol. Sci. 2020, 21, 2781. [Google Scholar] [CrossRef] [PubMed]
- John Morris, G.; Acton, E.; Murray, B.J.; Fonseca, F. Freezing injury: The special case of the sperm cell. Cryobiology 2012, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Mohammadi-Sangcheshmeh, A.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In vitro versus cryo-induced capacitation of bovine spermatozoa, part 1: Structural, functional, and oxidative similarities and differences. PLoS ONE 2022, 17, e0276683. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Fialková, V.; Žiarovská, J.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. In Vitro versus Cryo-Induced Capacitation of Bovine Spermatozoa, Part 2: Changes in the Expression Patterns of Selected Transmembrane Channels and Protein Kinase A. Int. J. Mol. Sci. 2022, 23, 14646. [Google Scholar] [CrossRef] [PubMed]
- Vigolo, V.; Giaretta, E.; Da Dalt, L.; Damiani, J.; Gabai, G.; Bertuzzo, F.; Falomo, M.E. Relationships between Biomarkers of Oxidative Stress in Seminal Plasma and Sperm Motility in Bulls before and after Cryopreservation. Animals 2022, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Benko, F.; Duracka, M.; Lukac, N.; Tvrda, E. Cryocapacitation and its association with oxidative features in cryopreserved bovine spermatozoa. J. Microbiol. Biotech. Food Sci. 2022, 12, e9268. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Beorlegui, N.; Beconi, M.T. Heparin- and superoxide anion-dependent capacitation of cryopreserved bovine spermatozoa: Requirement of dehydrogenases and protein kinases. Free Radic. Res. 2006, 40, 427–432. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Epicatechin Prevents Cryocapacitation of Bovine Spermatozoa through Antioxidant Activity and Stabilization of Transmembrane Ion Channels. Int. J. Mol. Sci. 2023, 24, 2510. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Beconi, M.T.; Francia, C.R.; Mora, N.G.; Affranchino, M.A. Effect of natural antioxidants on frozen bovine semen preservation. Theriogenology 1993, 40, 841–851. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Beconi, M.; Beorlegui, N. Effect of natural antioxidants, superoxide dismutase and hydrogen peroxide on capacitation of frozen-thawed bull spermatozoa. Andrologia 1997, 29, 269–275. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Antifreeze Proteins from Diverse Organisms and their Applications: An Overview. Curr. Protein Pept. Sci. 2017, 18, 262–283. [Google Scholar] [CrossRef] [PubMed]
- Sieme, H.; Oldenhof, H.; Wolkers, W.F. Mode of action of cryoprotectants for sperm preservation. Anim. Reprod. Sci. 2016, 169, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, J.; Lu, W. Regulation of semen quality by fatty acids in diets, extender, and semen. Front. Vet. Sci. 2023, 10, 1119153. [Google Scholar] [CrossRef]
- Sapanidou, V.; Tsantarliotou, M.P.; Lavrentiadou, S.N. A review of the use of antioxidants in bovine sperm preparation protocols. Anim. Reprod. Sci. 2023, 251, 107215. [Google Scholar] [CrossRef]
- Barakat, I.A.; Danfour, M.A.; Galewan, F.A.; Dkhil, M.A. Effect of various concentrations of caffeine, pentoxifylline, and kallikrein on hyperactivation of frozen bovine semen. BioMed Res. Int. 2015, 2015, 948575. [Google Scholar] [CrossRef]
- Ros-Santaella, J.L.; Pintus, E. Plant Extracts as Alternative Additives for Sperm Preservation. Antioxidants 2021, 10, 772. [Google Scholar] [CrossRef]
- Adewoyin, M.; Ibrahim, M.; Roszaman, R.; Isa, M.L.M.; Alewi, N.A.M.; Rafa, A.A.A.; Anuar, M.N.N. Male Infertility: The Effect of Natural Antioxidants and Phytocompounds on Seminal Oxidative Stress. Diseases 2017, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and Mechanisms of Kaempferol in the Management of Cancers through Modulation of Inflammation and Signal Transduction Pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–523. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Debacker, M.; Bučko, O.; Lukáč, N.; Tvrdá, E. The effect of kaempferol and naringenin may improve the in vitro quality of stored boar semen. J. Cent. Eur. Agric. 2019, 20, 1069–1075. [Google Scholar] [CrossRef]
- Baňas, Š.; Benko, F.; Ďuračka, M.; Lukáč, N.; Tvrdá, E. Kaempferol Enhances Sperm Post-Thaw Survival by Its Cryoprotective and Antioxidant Behavior. Stresses 2023, 3, 687–700. [Google Scholar] [CrossRef]
- Al-Mutary, M.G. Use of antioxidants to augment semen efficiency during liquid storage and cryopreservation in livestock animals: A review. J. King Saud Univ. Sci. 2021, 33, 101226. [Google Scholar] [CrossRef]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant Role of Kaempferol in Prevention of Hepatocellular Carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Azab, R.E. The Effect of Kaempferol on Buffalo Semen Freezability and Redox State. Benha Vet. Med. J. 2022, 42, 164–169. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, U.H.; Lee, M.S.; Lee, S.D.; Cho, S.R. Spermatozoa motility, viability, acrosome integrity, mitochondrial membrane potential and plasma membrane integrity in 0.25 mL and 0.5 mL straw after frozen-thawing in Hanwoo bull. J. Anim. Reprod. Biotechnol. 2020, 35, 307–314. [Google Scholar] [CrossRef]
- Bernecic, N.C.; Donnellan, E.; O’Callaghan, E.; Kupisiewicz, K.; O’Meara, C.; Weldon, K.; Lonergan, P.; Kenny, D.A.; Fair, S. Comprehensive functional analysis reveals that acrosome integrity and viability are key variables distinguishing artificial insemination bulls of varying fertility. J. Dairy Sci. 2021, 104, 11226–11241. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Lobatón, C.D.; Hernández-Sanmiguel, E.; Santodomingo, J.; Vay, L.; Moreno, A.; Alvarez, J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004, 384, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Podralska, M.; Nadel, A.; Pszczola, M.; Pawlak, P.; Rozwadowska, N. Mitochondria Content and Activity Are Crucial Parameters for Bull Sperm Quality Evaluation. Antioxidants 2021, 10, 1204. [Google Scholar] [CrossRef] [PubMed]
- Tejero, I.; Gonzalez-Lafont, A.; Lluch, J.M.; Eriksson, L.A. Theoretical modeling of hydroxyl-radical-induced lipid peroxidation reactions. J. Phys. Chem. B 2007, 111, 5684–5693. [Google Scholar] [CrossRef]
- Heo, S.; Kim, S.; Kang, D. The Role of Hydrogen Peroxide and Peroxiredoxins throughout the Cell Cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- Gosalvez, J.; Tvrda, E.; Agarwal, A. Free radical and superoxide reactivity detection in semen quality assessment: Past, present, and future. J. Assist. Reprod. Genet. 2017, 34, 697–707. [Google Scholar] [CrossRef]
- O’Flaherty, C.M.; Beorlegui, N.B.; Beconi, M.T. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology 1999, 52, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Baro Graf, C.; Ritagliati, C.; Stival, C.; Luque, G.M.; Gentile, I.; Buffone, M.G.; Krapf, D. Everything you ever wanted to know about PKA regulation and its involvement in mammalian sperm capacitation. Mol. Cell. Endocrinol. 2020, 518, 110992. [Google Scholar] [CrossRef] [PubMed]
- Kalina, M.; Socher, R.; Rotem, R.; Naor, Z. Ultrastructural localization of protein kinase C in human sperm. J. Histochem. Cytochem. 1995, 43, 439–445. [Google Scholar] [CrossRef]
- Naor, Z.; Breitbart, H. Protein kinase C and mammalian spermatozoa acrosome reaction. Trends Endocrinol. Metabol. 1997, 8, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Hassan, N.A.; El-Halawany, A.M.; Mohamed, G.A.; Safo, M.K.; El-Bassossy, H.M. Major flavonoids from Psiadia punctulata produce vasodilation via activation of endothelial dependent NO signaling. J. Adv. Res. 2020, 24, 273–279. [Google Scholar] [CrossRef]
- Richter-Laskowska, M.; Trybek, P.; Delfino, D.V.; Wawrzkiewicz-Jałowiecka, A. Flavonoids as Modulators of Potassium Channels. Int. J. Mol. Sci. 2023, 24, 1311. [Google Scholar] [CrossRef]
- Illek, B.; Fischer, H. Flavonoids stimulate Cl conductance of human airway epithelium in vitro and in vivo. Am. J. Physiol. 1998, 275, L902–L910. [Google Scholar] [CrossRef]
- Morrell, J.M.; Valeanu, A.S.; Lundeheim, N.; Johannisson, A. Sperm quality in frozen beef and dairy bull semen. Acta Vet. Scand. 2018, 60, 41. [Google Scholar] [CrossRef]
- Hirwa, C.D.; Kugonza, D.R.; Amahoro, E.; Ingabire, C.; Niyiragira, V.; Myambi, C.; Manzi, M.; Murekezi, T.; Nyabinwa, P.; Nshimiyimana, A.M.; et al. Influence of breed, season and age on quality bovine semen used for artificial insemination. Int. J. Livest. Prod. 2017, 8, 72–78. [Google Scholar] [CrossRef]
- Dias, E.A.R.; Campanholi, S.P.; Rossi, G.F.; Freitas Dell’Aqua, C.P.; Dell’Aqua Junior, J.A.; Papa, F.O.; Zorzetto, M.F.; de Paz, C.C.P.; Oliveira, L.Z.; Mercadante, M.E.Z.; et al. Evaluation of cooling and freezing systems of bovine semen. Anim. Reprod. Sci. 2018, 195, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Forero-Gonzalez, R.A.; Celeghini, E.C.; Raphael, C.F.; Andrade, A.F.; Bressan, F.F.; Arruda, R.P. Effects of bovine sperm cryopreservation using different freezing techniques and cryoprotective agents on plasma, acrosomal and mitochondrial membranes. Andrologia 2012, 44, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Arriaga, R.; Pérez-González, A.; Reina, M.; Alvarez-Idaboy, J.R.; Galano, A. Comprehensive Investigation of the Antioxidant and Pro-oxidant Effects of Phenolic Compounds: A Double-Edged Sword in the Context of Oxidative Stress? J. Phys. Chem. B 2018, 122, 6198–6214. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Lu, H.F.; Chou, Y.C.; Shih, Y.L.; Bau, D.T.; Chen, J.C.; Hsu, S.C.; Chung, J.G. Kaempferol induces DNA damage and inhibits DNA repair associated protein expressions in human promyelocytic leukemia HL-60 cells. Am. J. Chin. Med. 2015, 43, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Wallgren, M. Alternatives to antibiotics in semen extenders: A review. Pathogens 2014, 3, 934–946. [Google Scholar] [CrossRef] [PubMed]
- Talini, R.; Kozicki, L.E.; FGaievski, F.R.; Polo, G.; Lima, L.G.F.; Santiago, J.; Segui, M.S.; Weiss, R.R.; Galan, T.G.B. Bovine semen thermoresistance tests and their correlation with pregnancy rates after fixed-time artificial insemination. Arq. Bras. Med. Vet. Zootec. 2019, 71, 2085–2092. [Google Scholar] [CrossRef]
- Zhao, Y.; Buhr, M.M. Localization of various ATPases in fresh and cryopreserved bovine spermatozoa. Anim. Reprod. Sci. 1996, 44, 139–148. [Google Scholar] [CrossRef]
- Breitbart, H.; Stem, B.; Rubinstein, S. Calcium transport and Ca2+-ATPase activity in ram spermatozoa plasma membrane vesicles. Biochim. Biophys. Acta 1983, 728, 349–355. [Google Scholar] [CrossRef]
- Beltowski, J.; Wójcicka, G. Spectrophotometric method for the determination of renal ouabain-sensitive H+, K+-ATPase activity. Acta Biochim. Pol. 2002, 49, 515–527. [Google Scholar] [CrossRef]
- Taylor, S.C.; Berkelman, T.; Yadav, G.; Hammond, M. A defined methodology for reliable quantification of Western blot data. Mol. Biotechnol. 2013, 55, 217–226. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baňas, Š.; Tvrdá, E.; Benko, F.; Ďuračka, M.; Čmiková, N.; Lukáč, N.; Kačániová, M. Kaempferol as an Alternative Cryosupplement for Bovine Spermatozoa: Cytoprotective and Membrane-Stabilizing Effects. Int. J. Mol. Sci. 2024, 25, 4129. https://doi.org/10.3390/ijms25074129
Baňas Š, Tvrdá E, Benko F, Ďuračka M, Čmiková N, Lukáč N, Kačániová M. Kaempferol as an Alternative Cryosupplement for Bovine Spermatozoa: Cytoprotective and Membrane-Stabilizing Effects. International Journal of Molecular Sciences. 2024; 25(7):4129. https://doi.org/10.3390/ijms25074129
Chicago/Turabian StyleBaňas, Štefan, Eva Tvrdá, Filip Benko, Michal Ďuračka, Natália Čmiková, Norbert Lukáč, and Miroslava Kačániová. 2024. "Kaempferol as an Alternative Cryosupplement for Bovine Spermatozoa: Cytoprotective and Membrane-Stabilizing Effects" International Journal of Molecular Sciences 25, no. 7: 4129. https://doi.org/10.3390/ijms25074129
APA StyleBaňas, Š., Tvrdá, E., Benko, F., Ďuračka, M., Čmiková, N., Lukáč, N., & Kačániová, M. (2024). Kaempferol as an Alternative Cryosupplement for Bovine Spermatozoa: Cytoprotective and Membrane-Stabilizing Effects. International Journal of Molecular Sciences, 25(7), 4129. https://doi.org/10.3390/ijms25074129







