Stress-Induced Proteasome Sub-Cellular Translocation in Cardiomyocytes Causes Altered Intracellular Calcium Handling and Arrhythmias
Abstract
:1. Introduction
2. Results
2.1. Starving CMs to Amino Acids Results in Nucleo-Cytoplasmic Proteasome Shuttling, Which Is Inhibited by the Triad of Aromatic Amino Acids
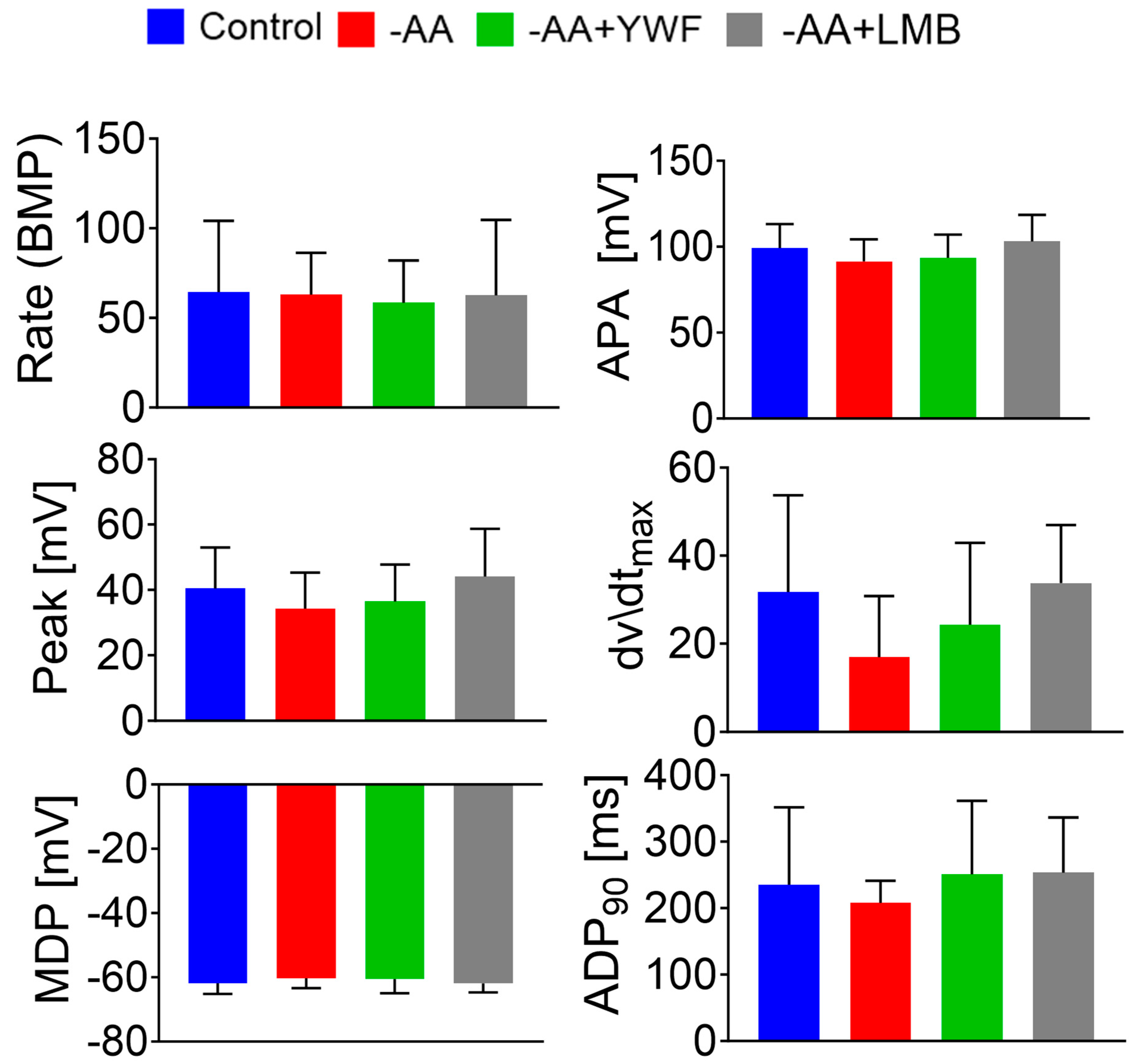
2.2. Amino Acid Starvation and YWF Supplementation Do Not Affect Action Potential Characteristics, and Are Not Toxic to Cardiomyocytes
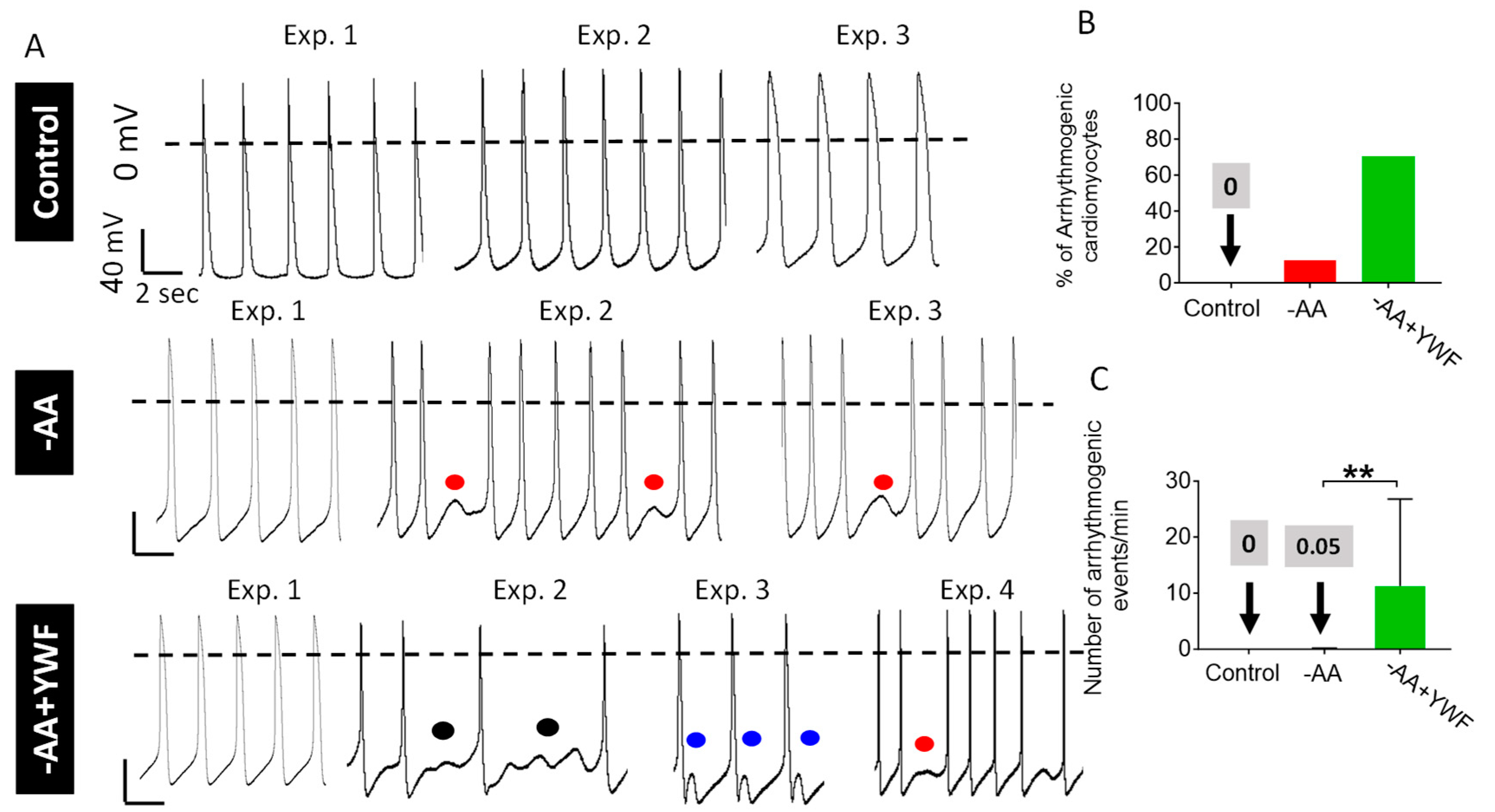
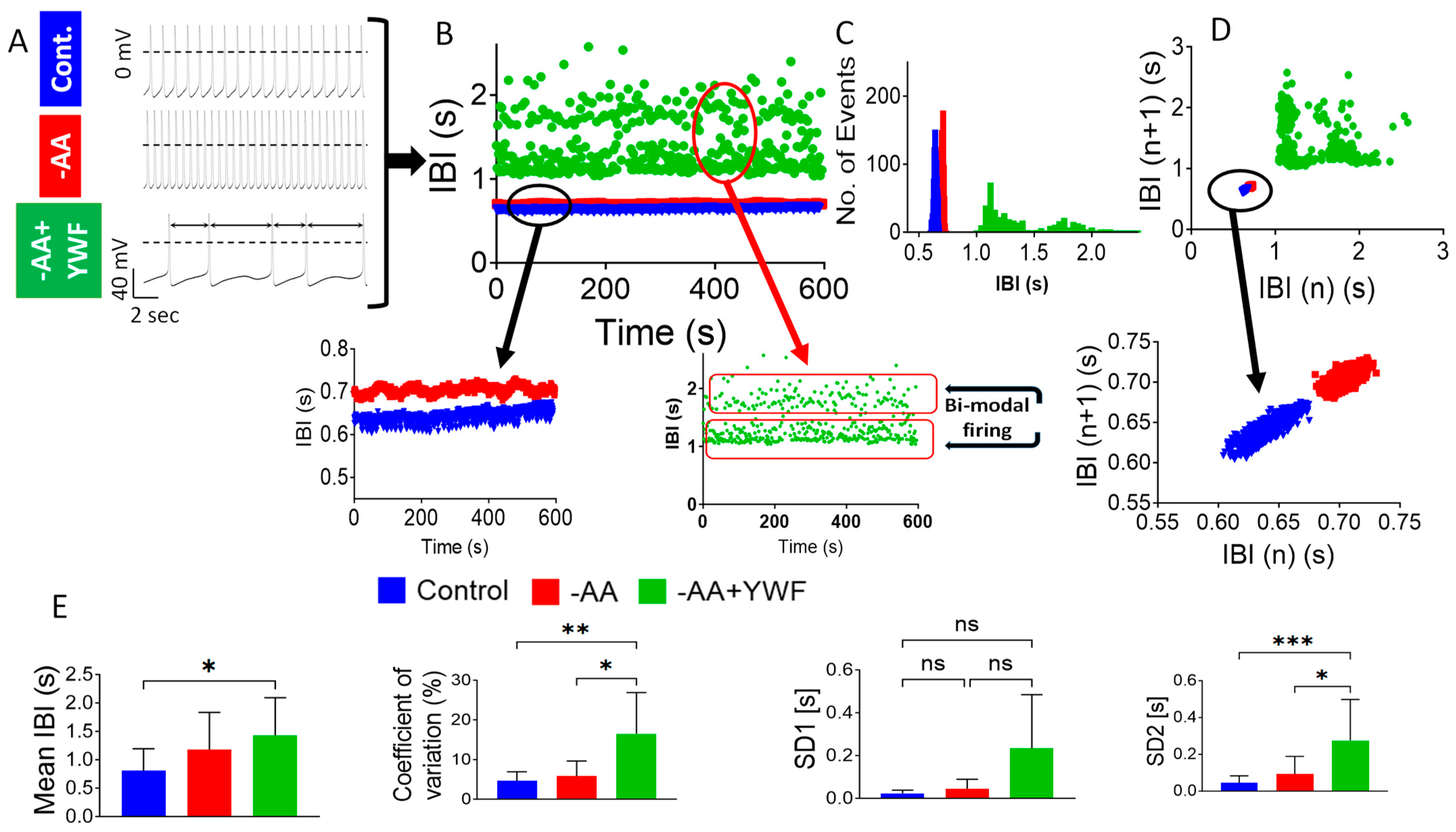
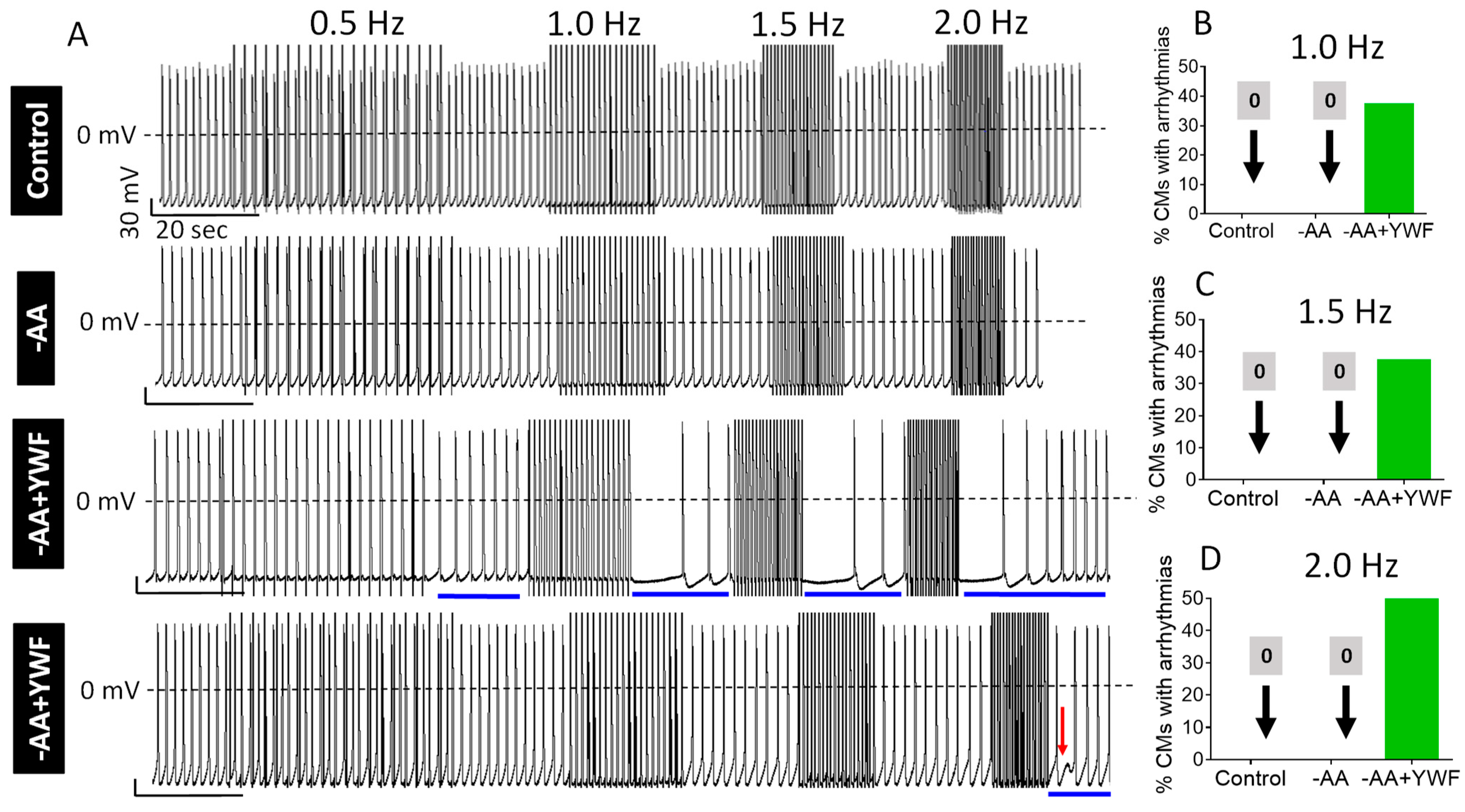
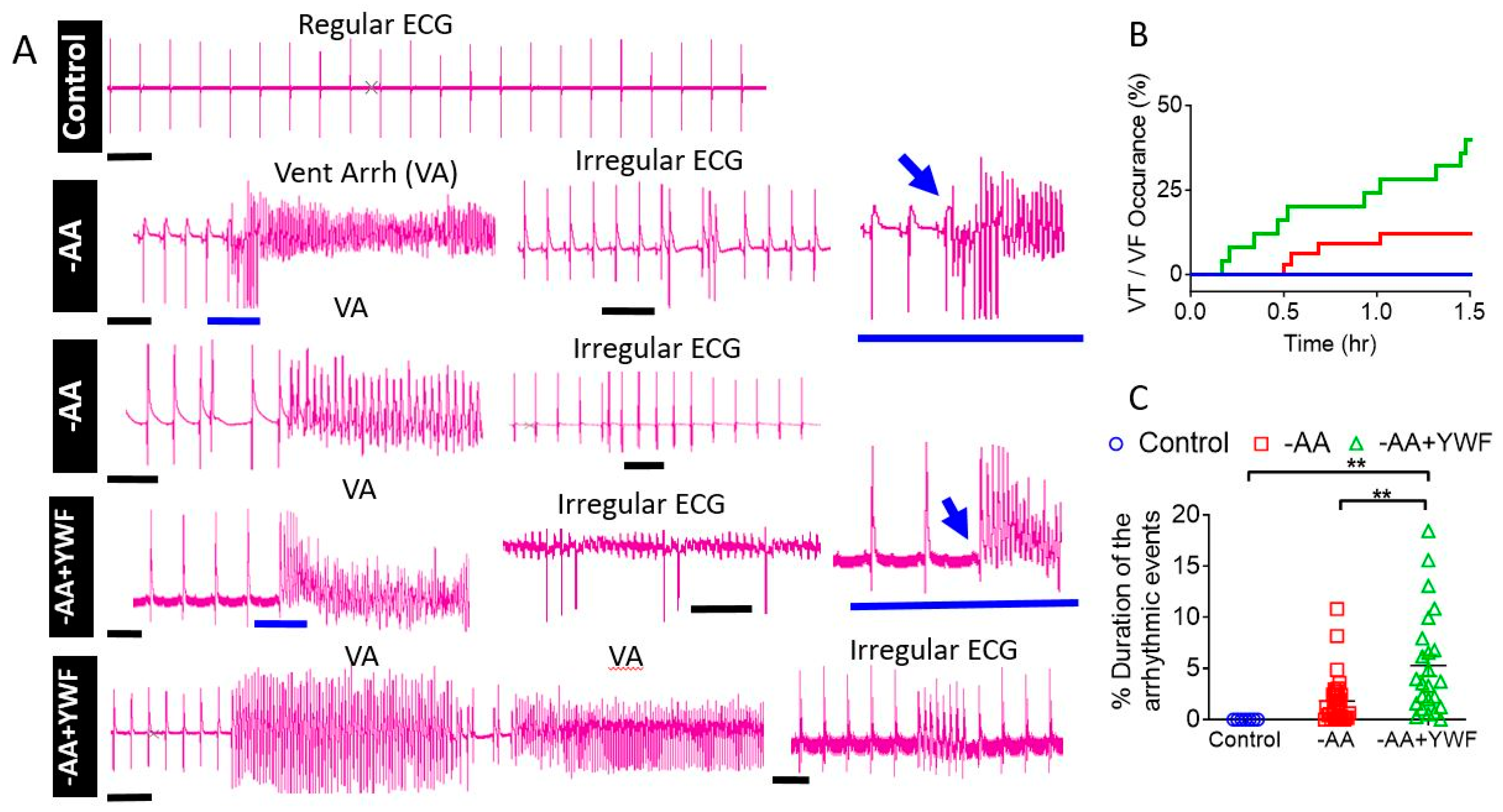
2.3. AA-Deficient Treatments Cause Arrhythmias
2.4. Supplementing -AA with Leptomycin B (LMB) Causes Proteasome Nuclear Sequestration and Arrhythmias
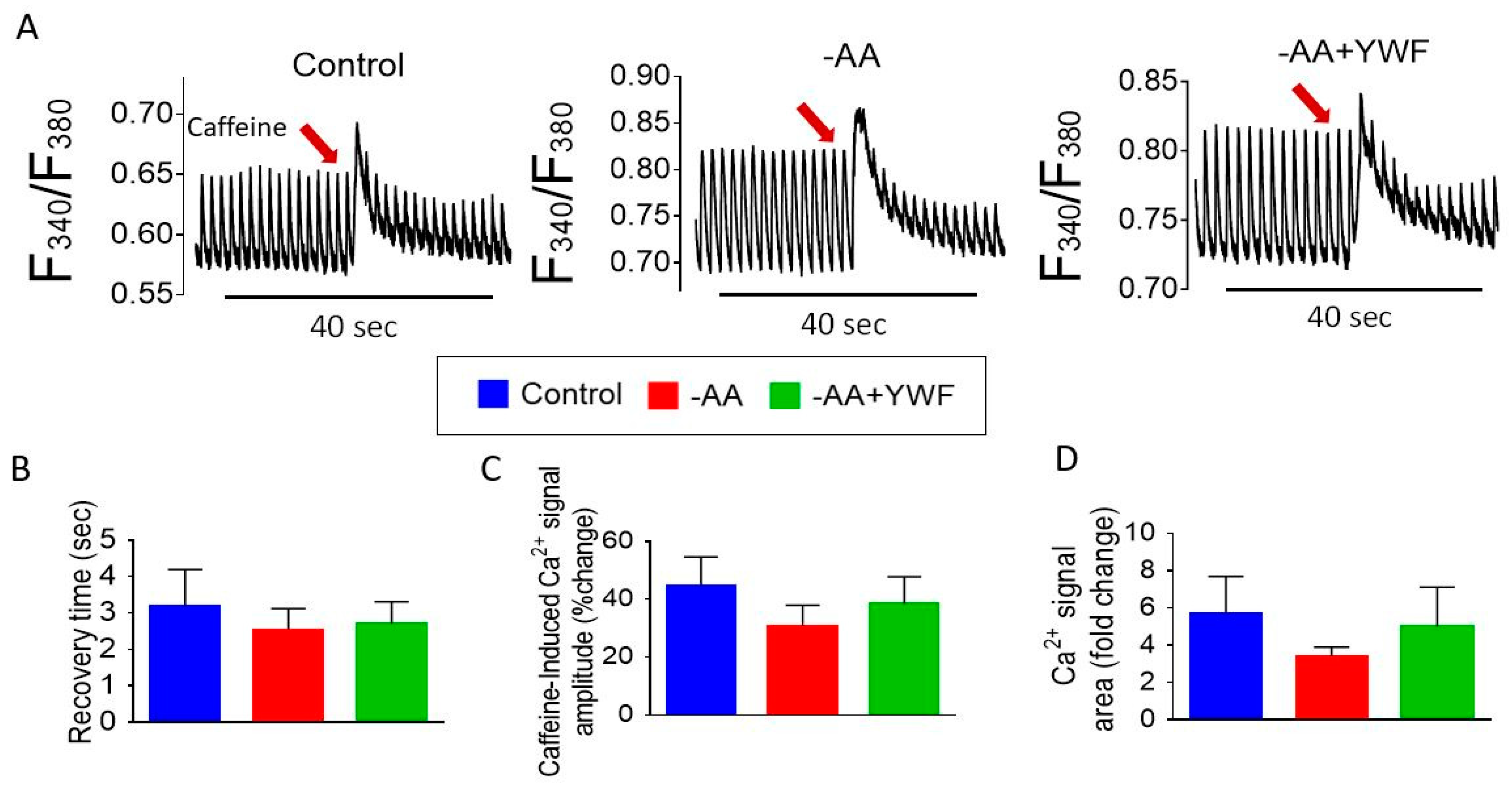
2.5. Do AA-Deficient Treatments Affect Intracellular Ca2+ Handling?
- (i)
- Measuring Ca2+ transients and caffeine-induced RyR-mediated SR Ca2+ release
- (ii)
- Testing the ability of rapid pacing to cause arrhythmias
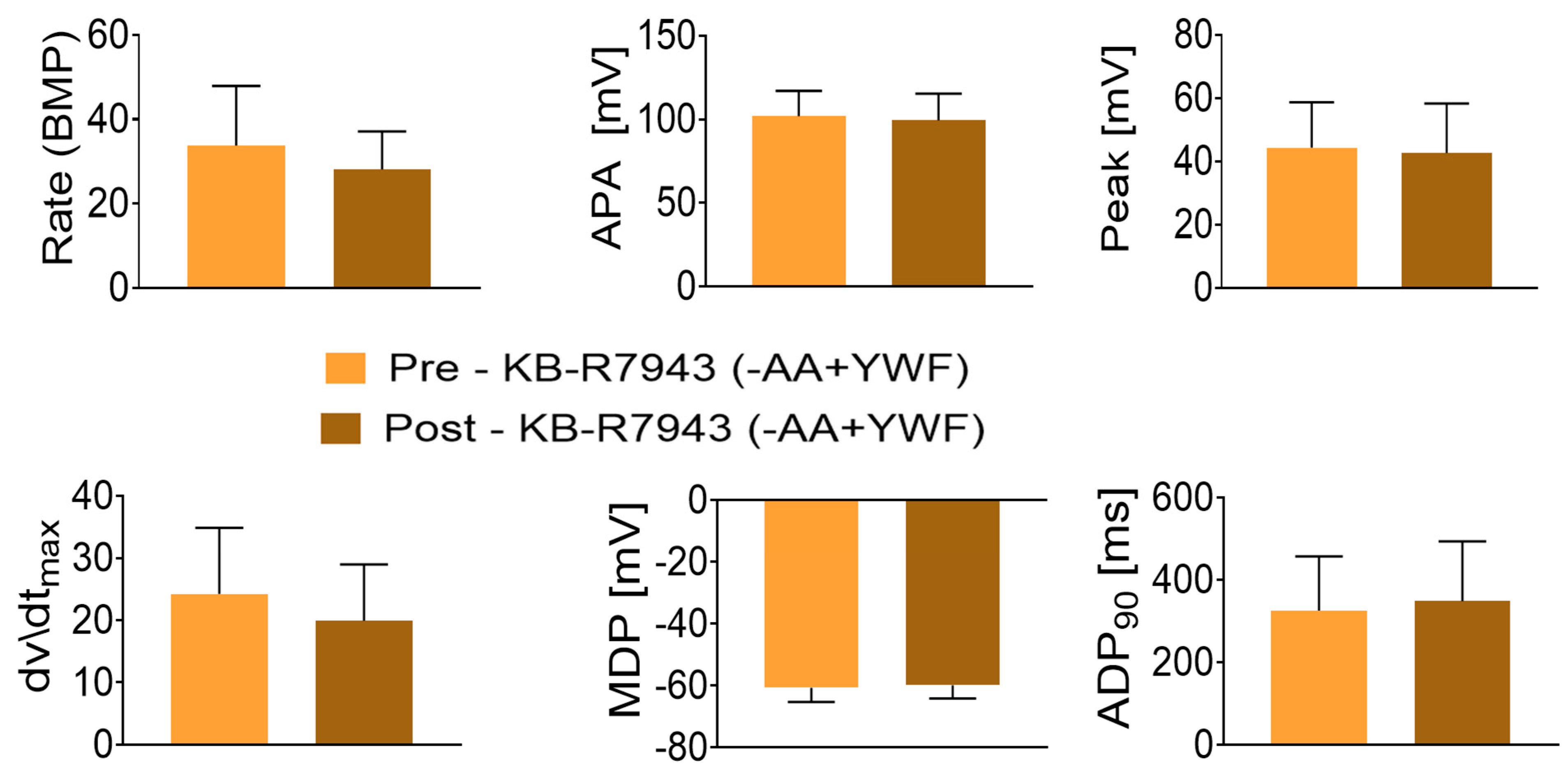
- (iii)
- Testing the involvement of NCX in the arrhythmias caused by -AA+YWF
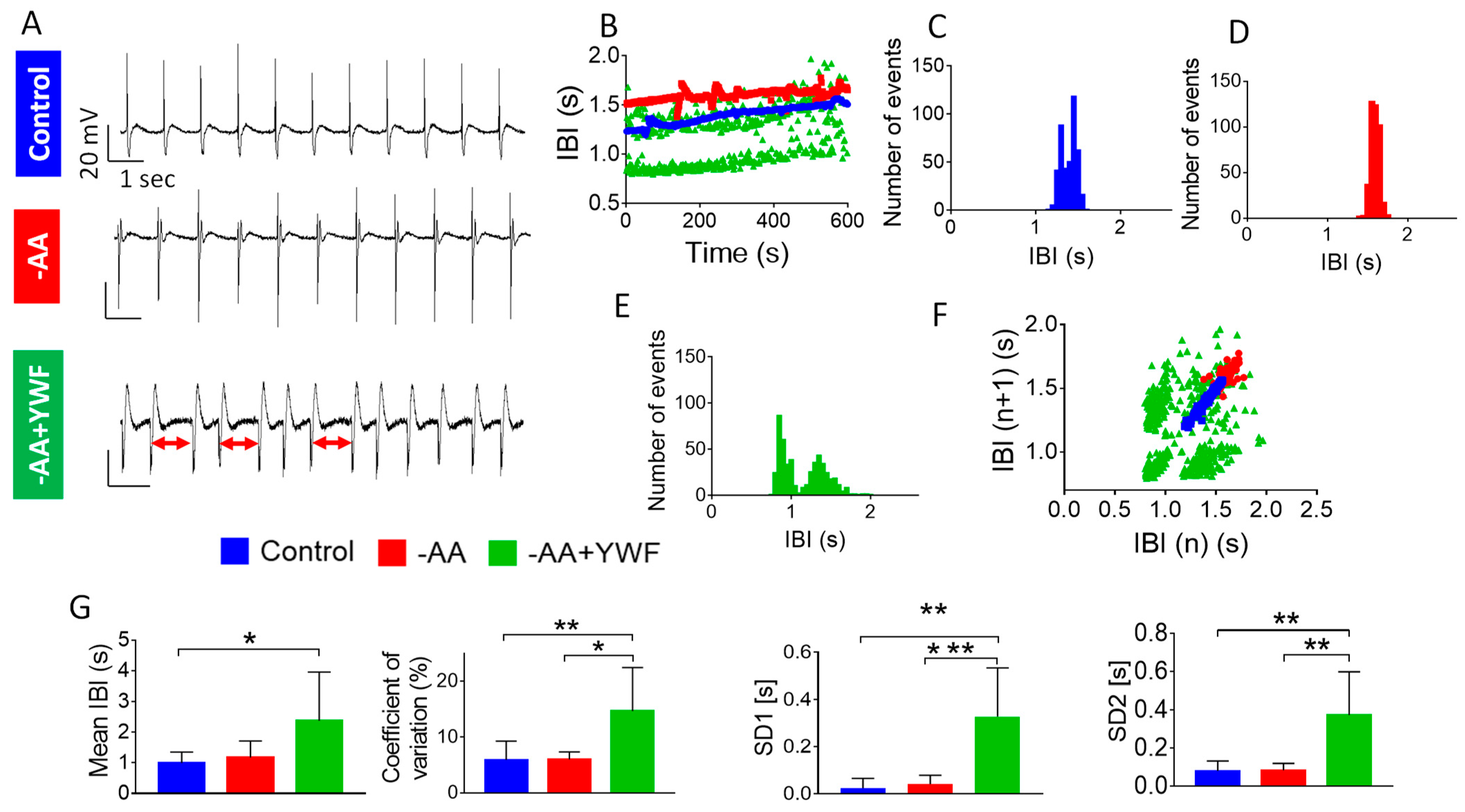
2.6. Retrograde Perfusion of Isolated Rat Hearts with AA-Deficient Media
3. Discussion
3.1. AA-Deficient Media Cause Nucleo-Cytoplasmicc Proteasome Translocation
3.2. The Effects of AA-Deficient Media on the Electrophysiological Characteristics of CMs
3.2.1. AA-Deficient Media Do Not Affect Action Potential Characteristics
3.2.2. AA-Deficient Media Cause Arrhythmias in CMs and in Isolated Rat Hearts
3.3. The Mechanism Underlying the Arrhythmias in -AA+YWF-Treated CMs
- (i) Measuring Ca2+ transients
- (ii) Testing the ability of rapid pacing to cause arrhythmias
- (iii) Testing the involvement of NCX in the arrhythmias caused by -AA+YWF
3.4. The Association between Proteasome Translocation and the Arrhythmias
3.5. Summary
4. Materials and Methods
4.1. iPSCs Culture and Differentiation
4.2. Enrichment of Cardiomyocytes
4.3. Langendorff Retrogradely Perfused Isolated Rat Hearts
4.4. Immunofluorescence Staining
4.5. Live Imaging of Proteasome Activity
4.6. Image Analysis
4.7. Drugs and Chemicals
4.8. Action Potential Recordings from Small iPSC-CMs Clusters
4.9. Recordings of Extracellular Electrograms from Small iPSC-CMs Clusters
4.10. Measurements of Intracellular Ca2+ Transients
4.11. Analysis of Beat Rate Variability (BRV)
4.12. The Composition of the Control, -AA and -AA+YWF Solutions
- (i).
- Tyrode’s solution to which physiological amounts of all amino acids (AAs) were added, Control: L-Alanine 4.45 mg/L, L-Arginine 69.46 mg/L, L-Asparagine 6.6 mg/L, L-Aspartic acid 6.65 mg/L, L-Cysteine 48 mg/L, L-Glutamic acid 7.35 mg/L, L-Glutamine 292 mg/L, L-Glycine 30 mg/L, L-Histidine 31.09 mg/L, L-Isoleucine 105 mg/L, L-Leucine 105 mg/L, L-Lysine 116.86 mg/L, L-Methionine 30 mg/L, L-Phenylalanine 66 mg/L, L-Proline 17.25 mg/L, L-Serine 42 mg/L, L-Threonine 95 mg/L, L-Tryptophan 16 mg/L, L-Tyrosine 72 mg/L, and L-Valine 94 mg/L). pH levels were adjusted to 7.4 with NaOH.
- (ii).
- -AA, amino acid-free Tyrode’s solution.
- (iii).
- -AA+YWF: Tyrode’s solution containing L-Tyrosine 362.38 mg/L, L-Tryptophan 408.46 mg/L and L-Phenylalanine 330.4 mg/L.
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livneh, I.; Cohen-Kaplan, V.; Cohen-Rosenzweig, C.; Avni, N.; Ciechanover, A. The life cycle of the 26S proteasome: From birth, through regulation and function, and onto its death. Cell Res. 2016, 26, 869–885. [Google Scholar] [CrossRef]
- Sweeney, P.; Park, H.; Baumann, M.; Dunlop, J.; Frydman, J.; Kopito, R.; McCampbell, A.; Leblanc, G.; Venkateswaran, A.; Nurmi, A.; et al. Protein misfolding in neurodegenerative diseases: Implications and strategies. Transl. Neurodegener. 2017, 6, 6. [Google Scholar] [CrossRef]
- Fabre, B.; Livneh, I.; Ziv, T.; Ciechanover, A. Identification of proteins regulated by the proteasome following induction of endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2019, 517, 188–192. [Google Scholar] [CrossRef]
- Fabre, B.; Livneh, I.; Ziv, T.; Ciechanover, A. Modulation of the cell cycle regulating transcription factor E2F1 pathway by the proteasome following amino acid starvation. Biochem. Biophys. Res. Commun. 2019, 513, 721–725. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef] [PubMed]
- Schlossarek, S.; Frey, N.; Carrier, L. Ubiquitin-proteasome system and hereditary cardiomyopathies. J. Mol. Cell. Cardiol. 2014, 71, 25–31. [Google Scholar] [CrossRef]
- Scofield, S.L.C.; Amin, P.; Singh, M.; Singh, K. Extracellular ubiquitin: Role in myocyte apoptosis and myocardial remodeling. Compr. Physiol. 2015, 6, 527–560. [Google Scholar] [CrossRef]
- Barac, Y.D.; Emrich, F.; Krutzwakd-Josefson, E.; Schrepfer, S.; Sampaio, L.C.; Willerson, J.T.; Robbins, R.C.; Ciechanover, A.; Mohr, F.-W.; Aravot, D.; et al. The ubiquitin-proteasome system: A potential therapeutic target for heart failure. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2017, 36, 708–714. [Google Scholar] [CrossRef]
- Patterson, C.; Ike, C.; Willis, P.W.; Stouffer, G.A.; Willis, M.S. The bitter end: The ubiquitin-proteasome system and cardiac dysfunction. Circulation 2007, 115, 1456–1463. [Google Scholar] [CrossRef]
- Powell, S.R.; Wang, P.; Katzeff, H.; Shringarpure, R.; Teoh, C.; Khaliulin, I.; Das, D.K.; Davies, K.J.A.; Schwalb, H. Oxidized and ubiquitinated proteins may predict recovery of postischemic cardiac function: Essential role of the proteasome. Antioxid. Redox Signal. 2005, 7, 538–546. [Google Scholar] [CrossRef]
- Bulteau, A.L.; Lundberg, K.C.; Humphries, K.M.; Sadek, H.A.; Szweda, P.A.; Friguet, B.; Szweda, L.I. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J. Biol. Chem. 2001, 276, 30057–30063. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, O.; Minamino, T.; Okada, K.; Shintani, Y.; Takashima, S.; Kato, H.; Liao, Y.; Okazaki, H.; Asai, M.; Hirata, A.; et al. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem. Biophys. Res. Commun. 2006, 340, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.C.B.; Boer, B.N.; Grinberg, M.; Brum, P.C.; Mochly-Rosen, D. Protein quality control disruption by PKCβII in heart failure; rescue by the selective PKCβII inhibitor, βIIV5-3. PLoS ONE 2012, 7, e33175. [Google Scholar] [CrossRef] [PubMed]
- Day, S.M.; Divald, A.; Wang, P.; Davis, F.; Bartolone, S.; Jones, R.; Powell, S.R. Impaired assembly and post-translational regulation of 26S proteasome in human end-stage heart failure. Circ. Heart Fail. 2013, 6, 544–549. [Google Scholar] [CrossRef]
- Predmore, J.M.; Wang, P.; Davis, F.; Bartolone, S.; Westfall, M.V.; Dyke, D.B.; Pagani, F.; Powell, S.R.; Day, S.M. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 2010, 121, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, M.; Mestril, R.; Wang, X. Aberrant protein aggregation is essential for a mutant desmin to impair the proteolytic function of the ubiquitin-proteasome system in cardiomyocytes. J. Mol. Cell. Cardiol. 2006, 40, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Bahrudin, U.; Morikawa, K.; Takeuchi, A.; Kurata, Y.; Miake, J.; Mizuta, E.; Adachi, K.; Higaki, K.; Yamamoto, Y.; Shirayoshi, Y.; et al. Impairment of ubiquitin-proteasome system by E334K cMyBPC modifies channel proteins, leading to electrophysiological dysfunction. J. Mol. Biol. 2011, 413, 857–878. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zheng, H.; Li, J.; Li, Y.; Su, H.; Wang, X. Genetically induced moderate inhibition of the proteasome in cardiomyocytes exacerbates myocardial ischemia-reperfusion injury in mice. Circ. Res. 2012, 111, 532–542. [Google Scholar] [CrossRef]
- Shukla, S.K.; Rafiq, K. Proteasome biology and therapeutics in cardiac diseases. Transl. Res. J. Lab. Clin. Med. 2019, 205, 64–76. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Sabatini, D.M. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 2017, 26, 301–309. [Google Scholar] [CrossRef]
- Livneh, I.; Cohen-Kaplan, V.; Fabre, B.; Abramovitch, I.; Lulu, C.; Nataraj, N.B.; Lazar, I.; Ziv, T.; Yarden, Y.; Zohar, Y.; et al. Regulation of nucleo-cytosolic 26S proteasome translocation by aromatic amino acids via mTOR is essential for cell survival under stress. Mol. Cell 2023, 83, 3333–3346.e5. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Leestemaker, Y.; Sapmaz, A.; Ovaa, H. Highlighting the proteasome: Using fluorescence to visualize proteasome activity and distribution. Front. Mol. Biosci. 2019, 6, 14. [Google Scholar] [CrossRef]
- Mandel, Y.; Weissman, A.; Schick, R.; Barad, L.; Novak, A.; Meiry, G.; Goldberg, S.; Lorber, A.; Rosen, M.R.; Itskovitz-Eldor, J.; et al. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation 2012, 125, 883–893. [Google Scholar] [CrossRef]
- Ben-Ari, M.; Schick, R.; Barad, L.; Novak, A.; Ben-Ari, E.; Lorber, A.; Itskovitz-Eldor, J.; Rosen, M.R.; Weissman, A.; Binah, O. From beat rate variability in induced pluripotent stem cell-derived pacemaker cells to heart rate variability in human subjects. Heart Rhythm. Off. J. Heart Rhythm. Soc. 2014, 11, 1808–1818. [Google Scholar] [CrossRef]
- Ben-Ari, M.; Naor, S.; Zeevi-Levin, N.; Schick, R.; Jehuda, R.B.; Reiter, I.; Raveh, A.; Grijnevitch, I.; Barak, O.; Rosen, M.R.; et al. Developmental changes in electrophysiological characteristics of human induced Pluripotent Stem Cell-derived cardiomyocytes. Heart Rhythm. Soc. 2016, 13, 2379–2387. [Google Scholar] [CrossRef]
- Jans, D.A.; Martin, A.J.; Wagstaff, K.M. Inhibitors of nuclear transport. Curr. Opin. Cell Biol. 2019, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Veldkamp, M.W.; Baartscheer, A.; Schumacher, C.A.; Klöpping, C.; van Ginneken, A.C.; Ravesloot, J.H. Ionic mechanism of delayed afterdepolarizations in ventricular cells isolated from human end-stage failing hearts. Circulation 2001, 104, 2728–2733. [Google Scholar] [CrossRef]
- Sung, R.J.; Wu, S.-N.; Wu, J.-S.; Chang, H.-D.; Luo, C.-H. Electrophysiological mechanisms of ventricular arrhythmias in relation to Andersen-Tawil syndrome under conditions of reduced IK1: A simulation study. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2597–H2605. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Noble, P.J.; Noble, D. Ca2+-induced delayed afterdepolarizations are triggered by dyadic subspace Ca2+ affirming that increasing SERCA reduces aftercontractions. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H921–H935. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium signaling and cardiac arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Vassalle, M.; Lin, C.-I. Calcium overload and cardiac function. J. Biomed. Sci. 2004, 11, 542–565. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, J.N.; Nett, M.P.; Rota, M.; Vassalle, M. On the mechanisms underlying diastolic voltage oscillations in the sinoatrial node. J. Electrocardiol. 2006, 39, 342. [Google Scholar] [CrossRef] [PubMed]
- Sedan, O.; Dolnikov, K.; Zeevi-Levin, N.; Leibovich, N.; Amit, M.; Itskovitz-Eldor, J.; Binah, O. 1,4,5-Inositol trisphosphate-operated intracellular Ca(2+) stores and angiotensin-II/endothelin-1 signaling pathway are functional in human embryonic stem cell-derived cardiomyocytes. Stem Cells Dayt. Ohio 2008, 26, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Schick, R.; Mekies, L.N.; Shemer, Y.; Eisen, B.; Hallas, T.; Ben Jehuda, R.; Ben-Ari, M.; Szantai, A.; Willi, L.; Shulman, R.; et al. Functional abnormalities in induced Pluripotent Stem Cell-derived cardiomyocytes generated from titin-mutated patients with dilated cardiomyopathy. PLoS ONE 2018, 13, e0205719. [Google Scholar] [CrossRef] [PubMed]
- Mekies, L.N.; Regev, D.; Eisen, B.; Fernandez-Gracia, J.; Baskin, P.; Ben Jehuda, R.; Shulman, R.; Reiter, I.; Palty, R.; Arad, M.; et al. Depressed β-adrenergic inotropic responsiveness and intracellular calcium handling abnormalities in Duchenne Muscular Dystrophy patients’ induced pluripotent stem cell–derived cardiomyocytes. J. Cell. Mol. Med. 2021, 25, 3922–3934. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Barad, L.; Lorber, A.; Gherghiceanu, M.; Reiter, I.; Eisen, B.; Eldor, L.; Itskovitz-Eldor, J.; Eldar, M.; Arad, M.; et al. Functional abnormalities in iPSC-derived cardiomyocytes generated from CPVT1 and CPVT2 patients carrying ryanodine or calsequestrin mutations. J. Cell. Mol. Med. 2015, 19, 2006–2018. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D.C. The control of diastolic calcium in the heart: Basic mechanisms and functional implications. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef]
- Ottolia, M.; Torres, N.; Bridge, J.H.B.; Philipson, K.D.; Goldhaber, J.I. Na/Ca exchange and contraction of the heart. J. Mol. Cell. Cardiol. 2013, 61, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Eckardt, L.; Goldhaber, J.I. Triple threat: The Na+/Ca2+ exchanger in the pathophysiology of cardiac arrhythmia, ischemia and heart failure. Curr. Drug Targets 2011, 12, 737–747. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Hartl, F.U. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 2005, 310, 1960–1963. [Google Scholar] [CrossRef]
- Enenkel, C.; Kang, R.W.; Wilfling, F.; Ernst, O.P. Intracellular localization of the proteasome in response to stress conditions. J. Biol. Chem. 2022, 298, 102083. [Google Scholar] [CrossRef]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [PubMed]
- He, X.-D.; Gong, W.; Zhang, J.-N.; Nie, J.; Yao, C.-F.; Guo, F.-S.; Lin, Y.; Wu, X.-H.; Li, F.; Li, J.; et al. Sensing and transmitting intracellular amino acid signals through reversible lysine aminoacylations. Cell Metab. 2018, 27, 151–166.e6. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell 2018, 34, 724–740.e4. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Wei, S.-K.; Haigney, M.C.P.; Ocampo, C.J.; Stern, M.D.; Silverman, H.S. Inhibition of mitochondrial calcium efflux by clonazepam in intact single rat cardiomyocytes and effects on NADH production. Cell Calcium 1997, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, J.; Nathan, R.D. Ionic basis of ryanodine’s negative chronotropic effect on pacemaker cells isolated from the sinoatrial node. Am. J. Physiol. 1997, 273, H2481–H2489. [Google Scholar] [CrossRef]
- Schillinger, W.; Fiolet, J.W.; Schlotthauer, K.; Hasenfuss, G. Relevance of Na+–Ca2+ exchange in heart failure. Cardiovasc. Res. 2003, 57, 921–933. [Google Scholar] [CrossRef]
- Wagner, S.; Maier, L.S.; Bers, D.M. Role of Sodium and Calcium Dysregulation in Tachyarrhythmias in Sudden Cardiac Death. Circ. Res. 2015, 116, 1956–1970. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.; Barad, L.; Zeevi-Levin, N.; Shick, R.; Shtrichman, R.; Lorber, A.; Itskovitz-Eldor, J.; Binah, O. Cardiomyocytes generated from CPVTD307H patients are arrhythmogenic in response to β-adrenergic stimulation. J. Cell. Mol. Med. 2012, 16, 468–482. [Google Scholar] [CrossRef]
- Itzhaki, I.; Maizels, L.; Huber, I.; Gepstein, A.; Arbel, G.; Caspi, O.; Miller, L.; Belhassen, B.; Nof, E.; Glikson, M.; et al. Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. J. Am. Coll. Cardiol. 2012, 60, 990–1000. [Google Scholar] [CrossRef]
- Shinnawi, R.; Gepstein, L. iPCS cell modeling of inherited cardiac arrhythmias. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 331. [Google Scholar] [CrossRef] [PubMed]
- Sander, P.; Feng, M.; Schweitzer, M.K.; Wilting, F.; Gutenthaler, S.M.; Arduino, D.M.; Fischbach, S.; Dreizehnter, L.; Moretti, A.; Gudermann, T.; et al. Approved drugs ezetimibe and disulfiram enhance mitochondrial Ca2+ uptake and suppress cardiac arrhythmogenesis. Br. J. Pharmacol. 2021, 178, 4518–4532. [Google Scholar] [CrossRef] [PubMed]
- Brustovetsky, T.; Brittain, M.K.; Sheets, P.L.; Cummins, T.R.; Pinelis, V.; Brustovetsky, N. KB-R7943, an inhibitor of the reverse Na+ /Ca2+ exchanger, blocks N-methyl-D-aspartate receptor and inhibits mitochondrial complex I. Br. J. Pharmacol. 2011, 162, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Antoons, G.; Willems, R.; Sipido, K.R. Alternative strategies in arrhythmia therapy: Evaluation of Na/Ca exchange as an anti-arrhythmic target. Pharmacol. Ther. 2012, 134, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Knappe, V.; Pauls, P.; Schulte, J.S.; Goldhaber, J.I.; Müller, F.U.; Nickenig, G.; Eckardt, L.; Schrickel, J.W.; Beiert, T. Increased in vivo perpetuation of whole-heart ventricular arrhythmia in heterozygous Na+/Ca2+ exchanger knockout mice. Int. J. Cardiol. Heart Vasc. 2023, 44, 101168. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Muszynski, A.; Ruhe, M.; Bögeholz, N.; Schulte, J.S.; Milberg, P.; Mönnig, G.; Fabritz, L.; Goldhaber, J.I.; Breithardt, G.; et al. Proarrhythmia in a non-failing murine model of cardiac-specific Na+/Ca2+ exchanger overexpression: Whole heart and cellular mechanisms. Basic. Res. Cardiol. 2012, 107, 247. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Pauls, P.; Bauer, B.K.; Schulte, J.S.; Dechering, D.G.; Frommeyer, G.; Kirchhefer, U.; Goldhaber, J.I.; Müller, F.U.; Eckardt, L.; et al. Suppression of early and late afterdepolarizations by heterozygous knockout of the Na+/Ca2+ exchanger in a murine model. Circ. Arrhythm. Electrophysiol. 2015, 8, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Bögeholz, N.; Schulte, J.S.; Kaese, S.; Bauer, B.K.; Pauls, P.; Dechering, D.G.; Frommeyer, G.; Goldhaber, J.I.; Kirchhefer, U.; Eckardt, L.; et al. The effects of SEA0400 on Ca2+ transient amplitude and proarrhythmia depend on the Na+/Ca2+ exchanger expression level in murine models. Front. Pharmacol. 2017, 8, 649. [Google Scholar] [CrossRef]
- Yehezkel, S.; Rebibo-Sabbah, A.; Segev, Y.; Tzukerman, M.; Shaked, R.; Huber, I.; Gepstein, L.; Skorecki, K.; Selig, S. Reprogramming of telomeric regions during the generation of human induced pluripotent stem cells and subsequent differentiation into fibroblast-like derivatives. Epigenetics 2011, 6, 63–75. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kaplan, V.; Livneh, I.; Kwon, Y.T.; Ciechanover, A. Chapter Fifteen—Monitoring stress-induced autophagic engulfment and degradation of the 26S proteasome in mammalian cells. In Methods in Enzymology; Hochstrasser, M., Ed.; Ubiquitin-dependent Protein Degradation; Academic Press: Cambridge, MA, USA, 2019; Volume 619, pp. 337–366. [Google Scholar]
- Berkers, C.R.; van Leeuwen, F.W.B.; Groothuis, T.A.; Peperzak, V.; van Tilburg, E.W.; Borst, J.; Neefjes, J.J.; Ovaa, H. Profiling Proteasome Activity in Tissue with Fluorescent Probes. Mol. Pharm. 2007, 4, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Dolnikov, K.; Shilkrut, M.; Zeevi-Levin, N.; Gerecht-Nir, S.; Amit, M.; Danon, A.; Itskovitz-Eldor, J.; Binah, O. Functional properties of human embryonic stem cell-derived cardiomyocytes: Intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells Dayt. Ohio 2006, 24, 236–245. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neeman-Egozi, S.; Livneh, I.; Dolgopyat, I.; Nussinovitch, U.; Milman, H.; Cohen, N.; Eisen, B.; Ciechanover, A.; Binah, O. Stress-Induced Proteasome Sub-Cellular Translocation in Cardiomyocytes Causes Altered Intracellular Calcium Handling and Arrhythmias. Int. J. Mol. Sci. 2024, 25, 4932. https://doi.org/10.3390/ijms25094932
Neeman-Egozi S, Livneh I, Dolgopyat I, Nussinovitch U, Milman H, Cohen N, Eisen B, Ciechanover A, Binah O. Stress-Induced Proteasome Sub-Cellular Translocation in Cardiomyocytes Causes Altered Intracellular Calcium Handling and Arrhythmias. International Journal of Molecular Sciences. 2024; 25(9):4932. https://doi.org/10.3390/ijms25094932
Chicago/Turabian StyleNeeman-Egozi, Shunit, Ido Livneh, Irit Dolgopyat, Udi Nussinovitch, Helena Milman, Nadav Cohen, Binyamin Eisen, Aaron Ciechanover, and Ofer Binah. 2024. "Stress-Induced Proteasome Sub-Cellular Translocation in Cardiomyocytes Causes Altered Intracellular Calcium Handling and Arrhythmias" International Journal of Molecular Sciences 25, no. 9: 4932. https://doi.org/10.3390/ijms25094932
APA StyleNeeman-Egozi, S., Livneh, I., Dolgopyat, I., Nussinovitch, U., Milman, H., Cohen, N., Eisen, B., Ciechanover, A., & Binah, O. (2024). Stress-Induced Proteasome Sub-Cellular Translocation in Cardiomyocytes Causes Altered Intracellular Calcium Handling and Arrhythmias. International Journal of Molecular Sciences, 25(9), 4932. https://doi.org/10.3390/ijms25094932






