Lethal Arrhythmogenic Role of Left Ventricular Myocardial Interstitial Fibrosis in Apolipoprotein E/Low-Density Lipoprotein Receptor Double-Knockout Mice with Metabolic Dysfunction-Associated Steatohepatitis
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Mice
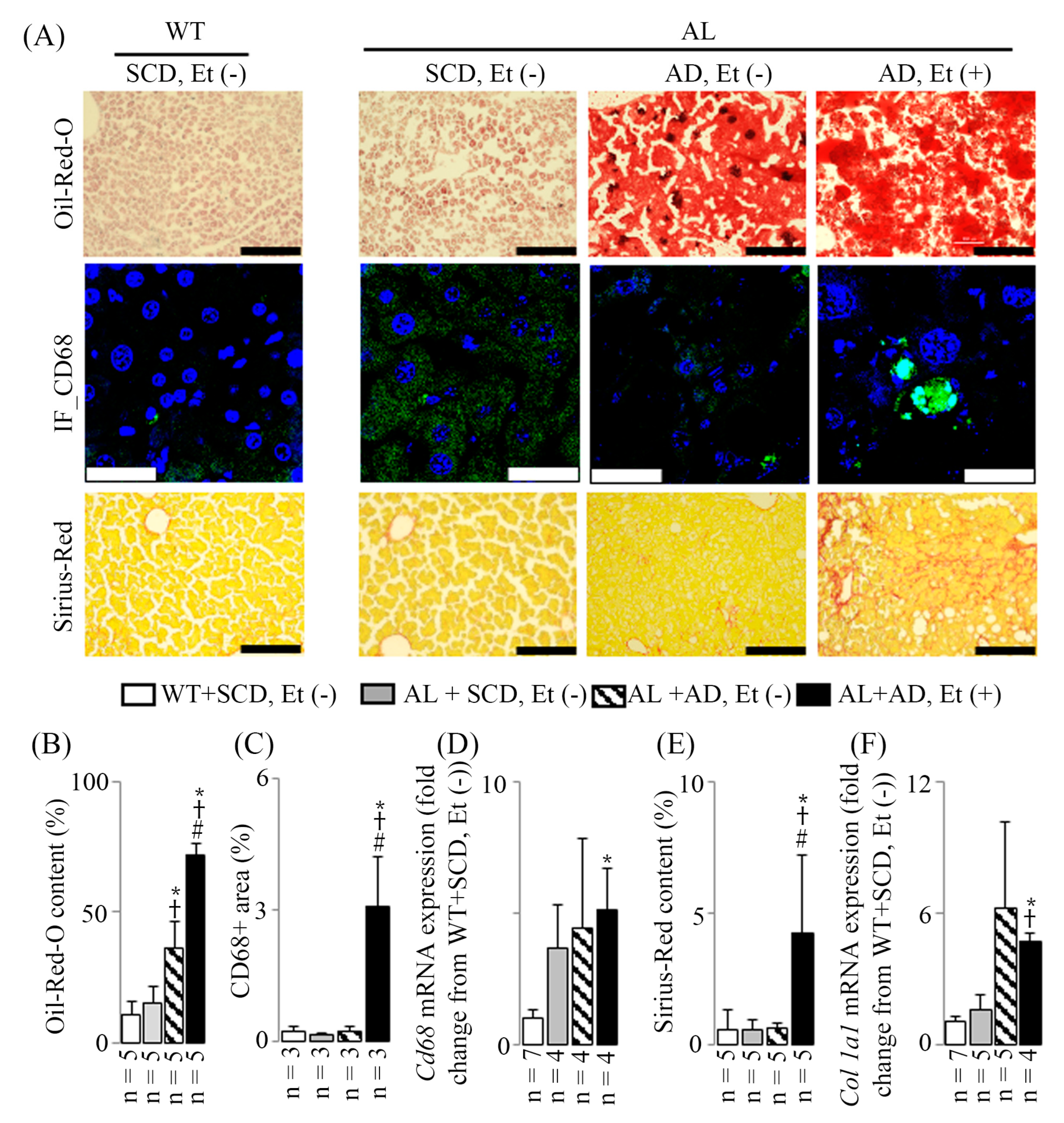
2.2. MASH Induced by Addition of Ethanol to AD
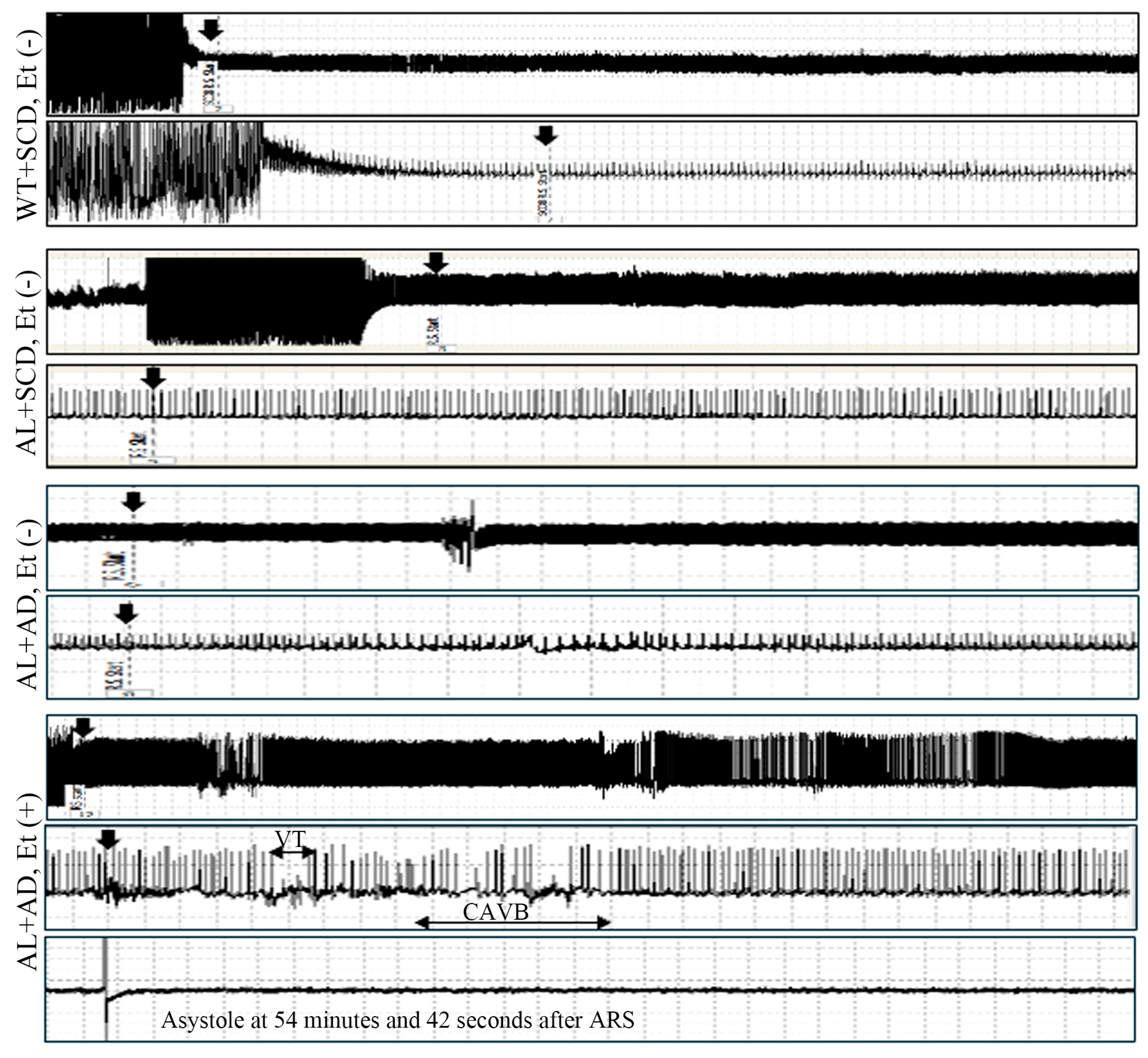
2.3. Increased Susceptibility to Lethal Arrhythmia in AL Mice with MASH
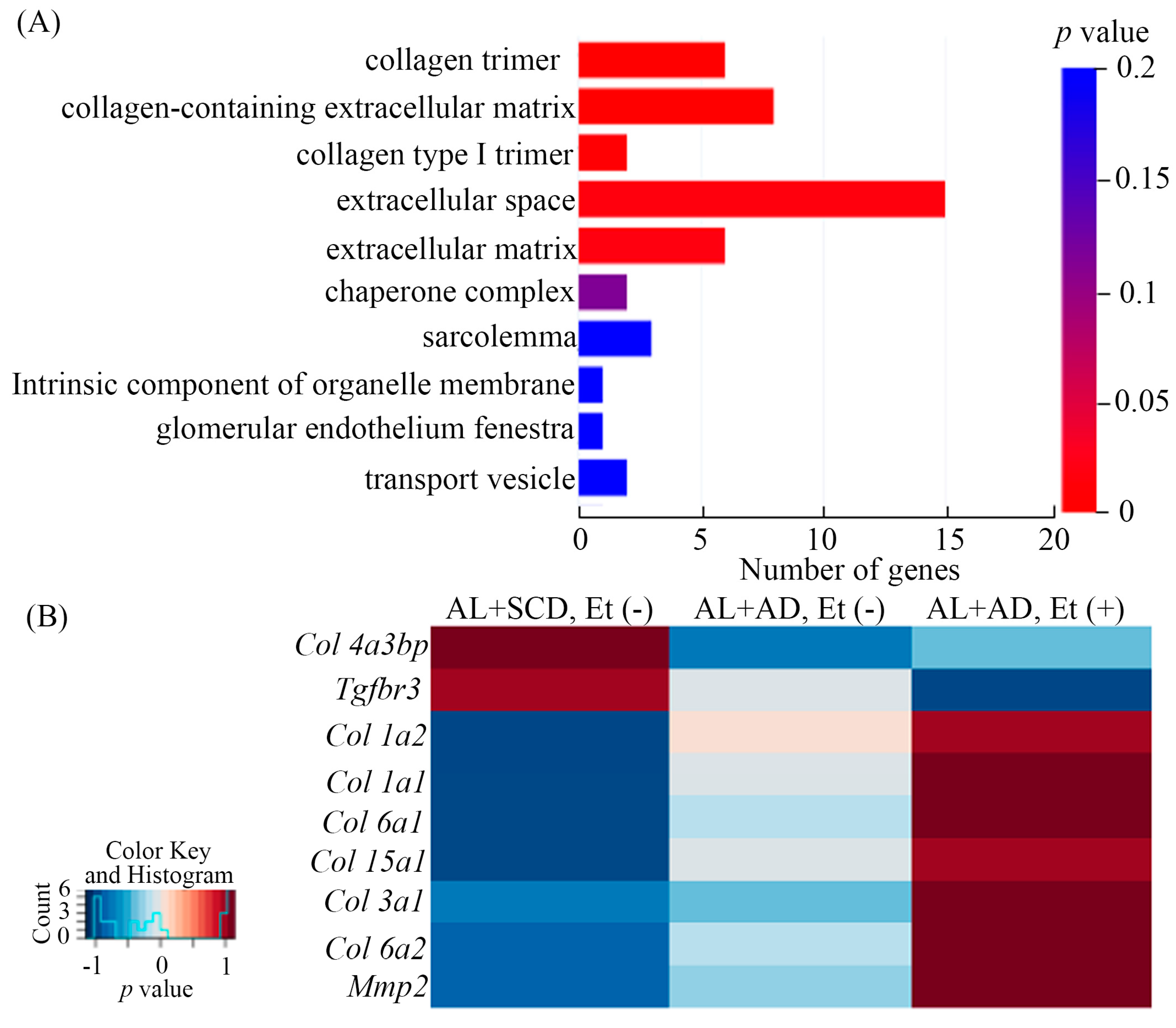
2.4. Ethanol and AD Additively Upregulated LV Gene Expression Related to Fibrosis in Mice with a Co-Diet of Ethanol and AD-Induced MASH
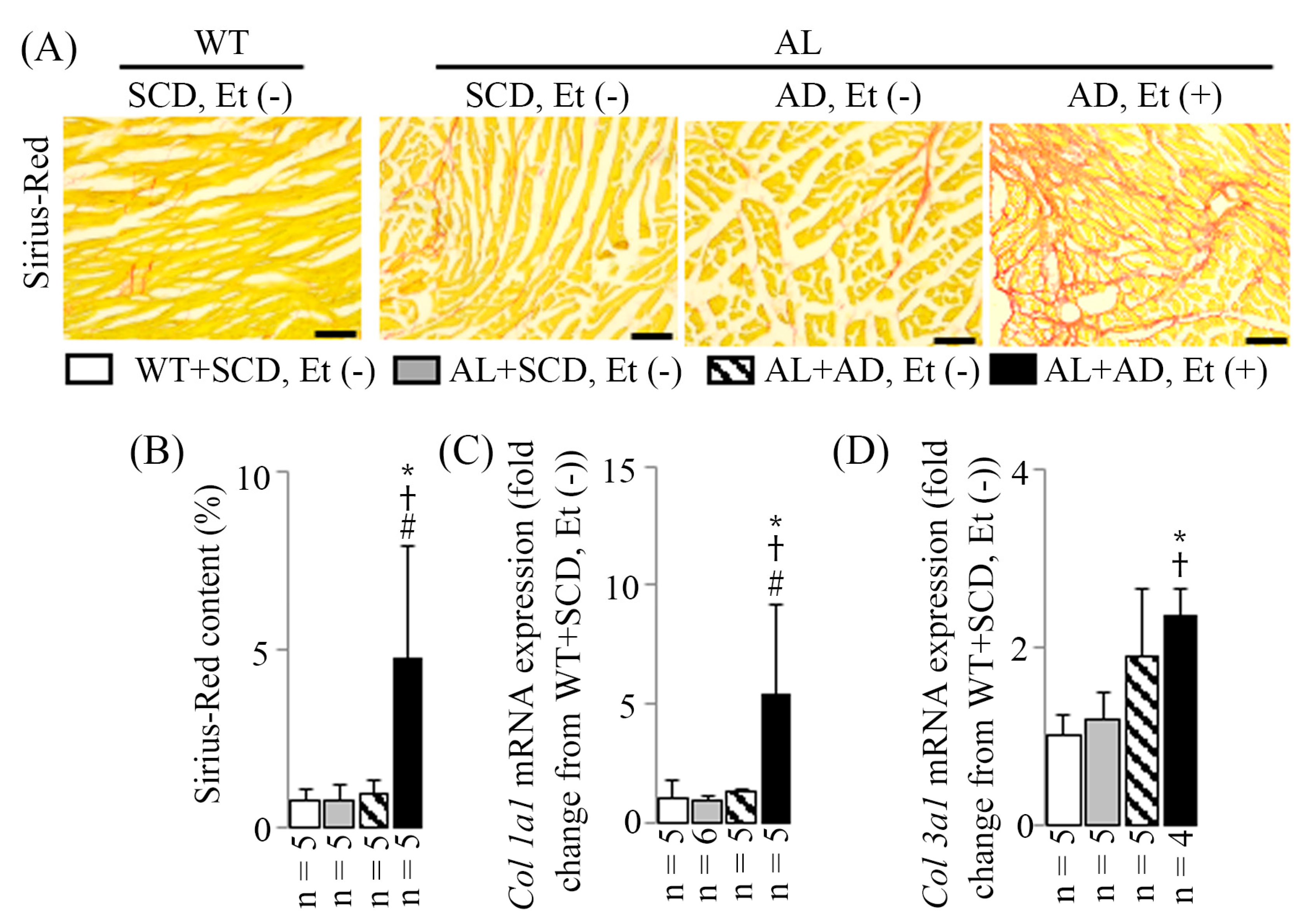
2.5. LV MIF in Mice with a Co-Diet of Ethanol and AD-Induced MASH
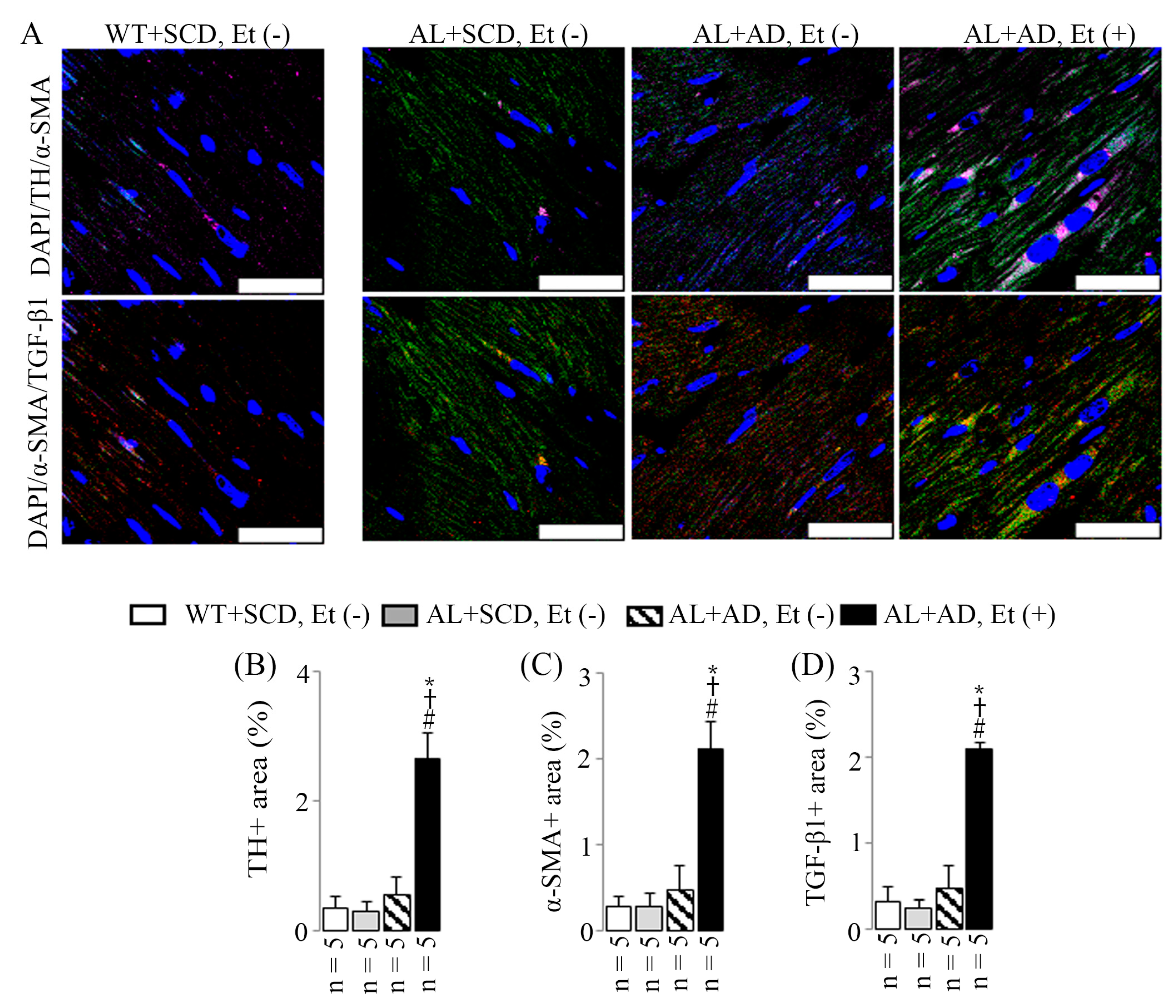
2.6. Co-Diet of Ethanol and AD Induced Features of LV Sympathetic Activation in Cardiac Myofibroblasts, Which in Turn Led to Upregulated Productions of Cardiac Myofibroblast-Derived TGF-β1
3. Discussion
4. Materials and Methods
4.1. Mouse Model and Diets
4.2. Lethal Arrhythmia-Evoked Test
4.3. Blood, Liver, and LV Tissues Collection
4.4. Histopathological and Fluorescence Immunohistochemical Examination
4.5. Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.6. RNA-Seq and Pathway Analyses
4.7. Analysis of Blood Ethanol Level at the End of the Lethal Arrhythmia-Evoked Test
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiuchi, M.G.; Nolde, J.M.; Villacorta, H.; Carnagarin, R.; Chan, J.J.S.; Lugo-Gavidia, L.M.; Ho, J.K.; Matthews, V.B.; Dwivedi, G.; Schlaich, M.P. New Approaches in the Management of Sudden Cardiac Death in Patients with Heart Failure-Targeting the Sympathetic Nervous System. Int. J. Mol. Sci. 2019, 20, E2430. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Mahtab, M.A.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A Lancet Gastroenterology & hepatology Commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e272–e391. [Google Scholar]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Ciaula, A.D. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Natta, M.L.V.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.M.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
- Shiraishi, S.; Liu, J.; Saito, Y.; Oba, Y.; Nishihara, Y.; Yoshimura, S. A New Non-Obese Steatohepatitis Mouse Model with Cardiac Dysfunction Induced by Addition of Ethanol to a High-Fat/High-Cholesterol Diet. Biology 2024, 13, 91. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Hagström, H.; Sundström, J.; Ludvigsson, J.F. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut 2022, 71, 1867–1875. [Google Scholar] [CrossRef]
- Zhu, Y.; Llamosas-Falcón, L.; Kerr, W.; Puka, K.; Probst, C. Differential associations of alcohol use with ischemic heart disease mortality by socioeconomic status in the US, 1997–2018. JAMA 2024, 7, e2354270. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Gu, S.; Kim, H.; Seong, D.; Cho, S.J.; Yi, B.K.; Park, H.D.; et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: A retrospective cohort study. Gut 2017, 66, 323–329. [Google Scholar] [CrossRef]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- Liu, J.; Oba, Y.; Yamano, S. Chronic ethanol consumption plus an atherogenic diet cause metabolic steatohepatitis with advanced liver fibrosis in apolipoprotein E/low-density lipoprotein receptor double-knockout mice. Alcohol. Clin. Exp. Res. 2022, 46, 1192–1203. [Google Scholar] [CrossRef]
- Furuta, Y.; Liu, J.; Himemiya-Hakucho, A.; Yoshimura, K.; Fujimiya, T. Alcohol consumption in combination with an atherogenic diet increased indices of atherosclerosis in apolipoprotein E/low-density lipoprotein receptor double-knockout mice. Alcohol. Clin. Exp. Res. 2019, 43, 227–242. [Google Scholar] [CrossRef]
- Liu, J. Alcohol consumption combined with dietary low-carbohydrate/high-protein intake increased the left ventricular systolic dysfunction risk and lethal ventricular arrhythmia susceptibility in apolipoprotein E/low-density lipoprotein receptor double-knockout mice. Alcohol 2020, 89, 63–74. [Google Scholar]
- Packer, M. Sudden unexpected death in patients with congestive heart failure: A second frontier. Circulation 1985, 72, 681–685. [Google Scholar] [CrossRef]
- van Opstal, J.M.; Verduyn, S.C.; Leunissen, H.D.; de Groot, S.H.; Wellens, H.J.; Vos, M.A. Electrophysiological parameters indicative of sudden cardiac death in the dog with chronic complete AV-block. Cardiovasc. Res. 2001, 50, 354–361. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.J.; Ji, Y.X.; Zhang, P.; She, Z.G.; Li, H. Nonalcoholic fatty liver disease pandemic fuels the upsurge in cardiovascular diseases. Circ. Res. 2020, 126, 679–704. [Google Scholar] [CrossRef]
- Zhang, X.J.; She, Z.G.; Li, H. Time to step-up the fight against NAFLD. Hepatology 2018, 67, 2068–2071. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Mantovani, A.; Tilg, H.; Targher, G. Risk of cardiomyopathy and cardiac arrhythmias in patients with nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 425–439. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic fatty liver disease and the heart: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical practice guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; Spragg, D.D.; Tunin, R.S.; Kass, D.A.; Tomaselli, G.F. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ. Res. 2004, 95, 717–725. [Google Scholar] [CrossRef]
- Glukhov, A.V.; Fedorov, V.V.; Kalish, P.W.; Ravikumar, V.K.; Lou, Q.; Janks, D.; Schuessler, R.B.; Moazami, N.; Efimov, I.R. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation 2012, 125, 1835–1847. [Google Scholar] [CrossRef]
- Yan, J.; Killingsworth, C.; Walcott, G.; Zhu, Y.; Litovsky, S.; Huang, J.; Ai, X.; Pogwizd, S.M. Molecular remodeling of Cx43, but not structural remodeling, promotes arrhythmias in an arrhythmogenic canine model of nonischemic heart failure. J. Mol. Cell Cardiol. 2021, 158, 72–81. [Google Scholar] [CrossRef]
- Bernaba, B.N.; Chan, J.B.; Lai, C.K.; Fishbein, M.C. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc. Pathol. 2010, 19, 308–311. [Google Scholar] [CrossRef]
- Asbun, J.; Villarreal, F.J. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2006, 47, 693–700. [Google Scholar] [CrossRef]
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell Mol. Life Sci. 2014, 71, 549–574. [Google Scholar] [CrossRef]
- Weber, K.T.; Sun, Y.; Bhattacharya, S.K.; Ahokas, R.A.; Gerling, I.C. Myofibroblast-mediated mechanisms of pathological remodeling of the heart. Nat. Rev. Cardiol. 2013, 10, 15–26. [Google Scholar] [CrossRef]
- Lin, R.; Rahtu-Korpela, L.; Szabo, Z.; Kemppi, A.; Skarp, S.; Kiviniemi, A.M.; Lepojärvi, E.S.; Halmetoja, E.; Kilpiö, T.; Porvari, K.; et al. MiR-185-5p regulates the development of myocardial fibrosis. J. Mol. Cell Cardiol. 2022, 165, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bomb, R.; Heckle, M.R.; Sun, Y.; Mancarella, S.; Guntaka, R.V.; Gerling, I.C.; Weber, K.T. Myofibroblast secretome and its auto-paracrine signaling. Expert. Rev. Cardiovasc. Ther. 2016, 14, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Targeting metabolically activated fibroblasts in the failing heart. Nat. Cardiovasc. Res. 2024, 3, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qiao, Y.; Yan, D.; Meng, Q. Targeting Interactions between Fibroblasts and Macrophages to Treat Cardiac Fibrosis. Cells 2024, 13, 764. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Oba, Y.; Kondo, Y.; Nakaki, R.; Yamano, S. Lethal Arrhythmogenic Role of Left Ventricular Myocardial Interstitial Fibrosis in Apolipoprotein E/Low-Density Lipoprotein Receptor Double-Knockout Mice with Metabolic Dysfunction-Associated Steatohepatitis. Int. J. Mol. Sci. 2025, 26, 144. https://doi.org/10.3390/ijms26010144
Liu J, Oba Y, Kondo Y, Nakaki R, Yamano S. Lethal Arrhythmogenic Role of Left Ventricular Myocardial Interstitial Fibrosis in Apolipoprotein E/Low-Density Lipoprotein Receptor Double-Knockout Mice with Metabolic Dysfunction-Associated Steatohepatitis. International Journal of Molecular Sciences. 2025; 26(1):144. https://doi.org/10.3390/ijms26010144
Chicago/Turabian StyleLiu, Jinyao, Yumiko Oba, Yosuke Kondo, Ryo Nakaki, and Seiko Yamano. 2025. "Lethal Arrhythmogenic Role of Left Ventricular Myocardial Interstitial Fibrosis in Apolipoprotein E/Low-Density Lipoprotein Receptor Double-Knockout Mice with Metabolic Dysfunction-Associated Steatohepatitis" International Journal of Molecular Sciences 26, no. 1: 144. https://doi.org/10.3390/ijms26010144
APA StyleLiu, J., Oba, Y., Kondo, Y., Nakaki, R., & Yamano, S. (2025). Lethal Arrhythmogenic Role of Left Ventricular Myocardial Interstitial Fibrosis in Apolipoprotein E/Low-Density Lipoprotein Receptor Double-Knockout Mice with Metabolic Dysfunction-Associated Steatohepatitis. International Journal of Molecular Sciences, 26(1), 144. https://doi.org/10.3390/ijms26010144





