Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders
Abstract
1. Introduction
2. Endocannabinoid System (ECS)
2.1. Arachidonoyl Ethanolamine (AEA): Biosynthesis and Functions
2.2. 2-Arachidonoylglycerol (2-AG)
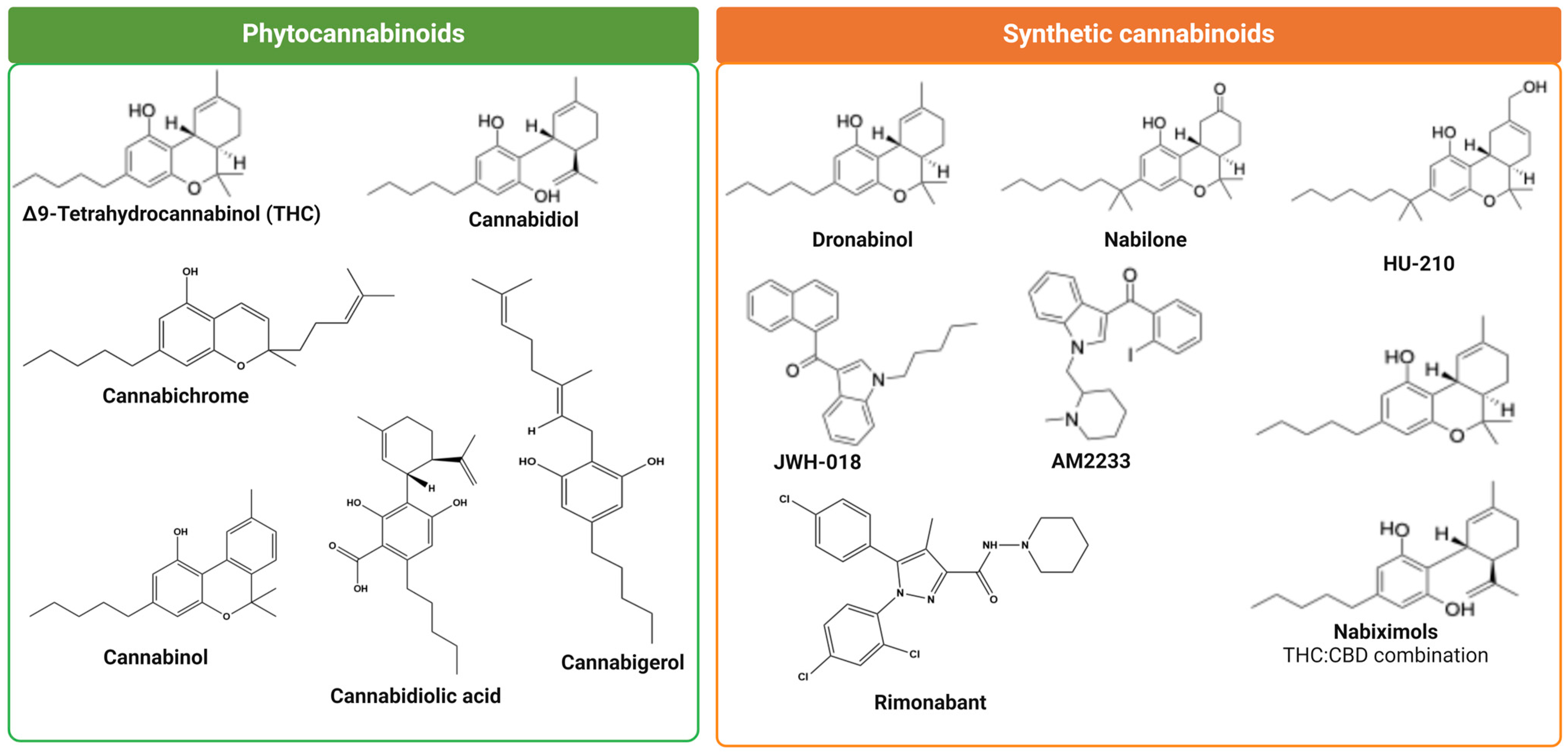
3. Phytocannabinoids
3.1. (−)-Trans-Δ9-Tetrahydrocannabinol
3.2. Cannabidiol (CBD)
3.3. Cannabidiolic Acid (CBDA)
3.4. Cannabigerol (CBG)
3.5. Cannabinol (CBN)
3.6. Cannabichromene (CBC)
4. Synthetic Cannabinoids
5. Cannabinoid Receptors: Structure, Function, and Distribution
5.1. Cannabinoid Receptor 1
5.2. Cannabinoid Receptor 1 Distribution and Functions
5.3. Cannabinoid Receptor 2: Distribution and Functions
6. Cannabinoid Receptors Internalization
6.1. Cannabinoid Receptors 1 Internalization
6.2. Cannabinoid Receptors 2 Internalization
7. Endocannabinoid Retrograde Signaling and Synaptic Plasticity
8. Cannabinoid Receptors and Signal Transduction Pathways
9. Central Nervous System and Cannabinoid Receptors Mediated Signaling Pathways
10. Cannabinoid Receptor-Mediated Regulation of Signaling in a G Protein-Independent Manner
11. Role of Cannabinoids in Regulation of Cell Survival Signaling Pathways
12. Morphological Changes in Central Nervous System with the Use of Cannabis
13. Addiction, Withdrawal, and Cannabis Use Disorder: A Challenge or Limitation for Medical Use
14. Cannabinoids Therapeutic Use and Diseases
15. Cannabinoid and Its Relevance to Clinical Implication
16. Early Use of Cannabinoids and Vulnerability to Neurological Diseases
17. Aging, Brain Development, and Cannabinoid Receptors
18. Cannabinoids and Neurodegenerative Diseases: What Is the Link?
18.1. Genetic Variation in Endocannabinoid System and Neurological and Psychiatric Disorders
18.2. Cannabinoids and Regulation of Inflammation
18.3. Cannabinoids as Suppressant of Oxidative Stress
18.4. Excitotoxicity and Cannabinoids: Neuroprotection
18.5. Neurogenesis and Cannabinoids: Role in Neurodegenerative Diseases
19. Cannabinoids and Perception of Pain
20. Therapeutic Implications of Cannabinoids in Neurological Diseases
21. Epilepsy and Cannabinoids
22. Alzheimer’s Disease and Cannabinoid Receptors
22.1. Neurotransmitters Modulation and AD: Role of Cannabinoids
22.2. Changes in Endogenous Cannabinoid and Cannabinoid Receptors in AD Brain
23. Huntington’s Disease and Cannabinoid Receptors
24. Parkinson’s Disease and Cannabinoid Receptors
25. Multiple Sclerosis and Cannabinoid Receptors
25.1. Role of CB1R in Pathogenesis of Multiple Sclerosis
25.2. CB2R and Implication in Multiple Sclerosis Pathogenesis
25.3. Immune Cells, Cytokines, Inflammation, and Cannabinoids: Supporting Role of CB2R in Multiple Sclerosis
25.4. CD4+ T Cells and CB2R and Immune Response and Multiple Sclerosis
25.5. CB2R, B Lymphocytes and Multiple Sclerosis
25.6. Microglia and Macrophages in Multiple Sclerosis and Role of Cannabinoid
25.7. CBR Subtypes and Astrocytes: Association with Pathogenesis of Multiple Sclerosis
26. Amyotrophic Lateral Sclerosis and Cannabinoid Receptors
27. Therapeutic Implications of Cannabis in the Treatment of Neuropsychiatric Disorders
28. Cannabinoid Hypothesis of Schizophrenia: Schizophrenia and Use of Cannabis
28.1. The Dopamine Hypothesis of Schizophrenia and the Role of Cannabinoids
28.2. NMDA Receptor Hypothesis of Schizophrenia with the Implication of Cannabinoids
28.3. Neurodevelopmental Hypothesis of Schizophrenia
28.4. Independent Risk of Schizophrenia
29. Role of Cannabinoids in Depression, Stress, and Anxiety
30. Cannabinoids and Acute Brain Damage: Stroke and Brain Trauma
Role of Cannabinoids in Stroke
31. Role of Cannabinoid in Brain Tumor Glioma
32. Beneficial Effects of Cannabis at the Cost of Risk
33. Cannabinoid Receptors Cross-Talk with Other Cell Surface Receptors: A New Way for Cannabinoids Improved Functionality and Diversity
33.1. Homo-And Heterodimerization of CB1R and CB2R
33.2. Heterodimerization of Cannabinoid Receptor with Other GPCRs
33.3. CBR and Opioid Receptors Heteromeric Interaction and Consequences
33.4. Heterodimerization Between CBR and Dopamine Receptors
33.5. Heterodimerization Between CBR and Somatostatin Receptors
34. Perspective, Future Direction, and Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Touw, M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Report, U.N.W.D. Drug Use and Health Consequences. 2020. Available online: https://wdr.unodc.org/wdr2020/en/drug-use-health.html (accessed on 20 March 2024).
- Dornbush, R.L.; Freedman, A.M. Chronic Cannabis Use—Introduction. Ann. N. Y. Acad. Sci. 1976, 282, R7–R8. [Google Scholar]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef]
- Xing, C.R.; Zhuang, Y.W.; Xu, T.H.; Feng, Z.W.; Zhou, X.E.; Chen, M.Z.; Wang, L.; Meng, X.; Xue, Y.; Wang, J.M.; et al. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-G(i) Signaling Complex. Cell 2020, 180, 645. [Google Scholar] [CrossRef]
- Bayer, R.E. Therapeutic Cannabis (Marijuana) as an Antiemetic and Appetite Stimulant in Persons with Acquired Immunodeficiency Syndrome (AIDS). J. Cannabis Ther. 2001, 1, 5–16. [Google Scholar] [CrossRef]
- Razmovski-Naumovski, V.; Luckett, T.; Amgarth-Duff, I.; Agar, M.R. Efficacy of medicinal cannabis for appetite-related symptoms in people with cancer: A systematic review. Palliat Med. 2022, 36, 912–927. [Google Scholar] [CrossRef]
- Riggs, P.K.; Vaida, F.; Rossi, S.S.; Sorkin, L.S.; Gouaux, B.; Grant, I.; Ellis, R.J. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012, 1431, 46–52. [Google Scholar] [CrossRef]
- Yang, P.; Myint, K.Z.; Tong, Q.; Peng, R.T.; Cao, H.P.; Almehizia, A.A.; Alqarni, M.H.; Wang, L.R.; Bartlow, P.; Gao, Y.D.; et al. Lead Discovery, Chemistry Optimization, and Biological Evaluation Studies of Novel Biamide Derivatives as CB2 Receptor Inverse Agonists and Osteoclast Inhibitors. J. Med. Chem. 2012, 55, 9973–9987. [Google Scholar] [CrossRef]
- Galiegue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carriere, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Lefur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned Cdna. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abushaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Elphick, M.R.; Egertova, M. The neurobiology and evolution of cannabinoid signalling. Philos. Trans. R. Soc. B-Biol. Sci. 2001, 356, 381–408. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Grotenhermen, F. Cannabinoids. Curr. Drug Targets. CNS Neurol. Disord. 2005, 4, 507–530. [Google Scholar] [CrossRef]
- Mechoulam, R.; Benshabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R.; et al. Identification of an Endogenous 2-Monoglyceride, Present in Canine Gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylgylcerol—A Possible Endogenous Cannabinoid Receptor-Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef]
- Battistella, G.; Fornari, E.; Annoni, J.M.; Chtioui, H.; Dao, K.; Fabritius, M.; Favrat, B.; Mall, J.F.; Maeder, P.; Giroud, C. Long-term effects of cannabis on brain structure. Neuropsychopharmacology 2014, 39, 2041–2048. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Onaivi, E.S.; Chaudhuri, G. Cloning and sequencing of a cDNA encoding the mouse brain-type cannabinoid receptor protein. DNA Seq. 1995, 5, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L. Cannabis and alcohol—A close friendship. Trends Pharmacol. Sci. 2003, 24, 266–268. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Leonard, C.M.; Ishiguro, H.; Zhang, P.W.; Lin, Z.; Akinshola, B.E.; Uhl, G.R. Endocannabinoids and cannabinoid receptor genetics. Prog. Neurobiol. 2002, 66, 307–344. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Takahashi, K.A.; Castillo, P.E. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 2006, 29, 37–76. [Google Scholar] [CrossRef]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef]
- Maccarrone, M.; Guzman, M.; Mackie, K.; Doherty, P.; Harkany, T. Programming of neural cells by (endo)cannabinoids: From physiological rules to emerging therapies. Nat. Rev. Neurosci. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Ruggiero, R.N.; Rossignoli, M.T.; De Ross, J.B.; Hallak, J.E.C.; Leite, J.P.; Bueno-Junior, L.S. Cannabinoids and Vanilloids in Schizophrenia: Neurophysiological Evidence and Directions for Basic Research. Front. Pharmacol. 2017, 8, 399. [Google Scholar] [CrossRef]
- Begg, M.; Pacher, P.; Batkai, S.; Osei-Hyiaman, D.; Offertaler, L.; Mo, F.M.; Liu, J.; Kunos, G. Evidence for novel cannabinoid receptors. Pharmacol. Ther. 2005, 106, 133–145. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chavez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef] [PubMed]
- .Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Kunos, G. Modulating the endocannabinoid system in human health and disease successes and failures. Febs J. 2013, 280, 1918–1943. [Google Scholar] [CrossRef]
- Greco, R.; Gasperi, V.; Maccarrone, M.; Tassorelli, C. The endocannabinoid system and migraine. Exp. Neurol. 2010, 224, 85–91. [Google Scholar] [CrossRef]
- El Manira, A.; Kyriakatos, A. The role of endocannabinoid signaling in motor control. Physiology 2010, 25, 230–238. [Google Scholar] [CrossRef]
- Dimarzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and Inactivation of Endogenous Cannabinoid Anandamide in Central Neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef]
- Freund, T.F.; Katona, I.; Piomelli, D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Tonegawa, T.; Nakane, S.; Yamashita, A.; Ishima, Y.; Waku, K. Transacylase-mediated and phosphodiesterase-mediated synthesis of N-arachidonoylethanolamine, an endogenous cannabinoid-receptor ligand, in rat brain microsomes—Comparison with synthesis from free arachidonic acid and ethanolamine. Eur. J. Biochem. 1996, 240, 53–62. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Tonegawa, T.; Nakane, S.; Yamashita, A.; Waku, K. Enzymatic synthesis of anandamide, an endogenous cannabinoid receptor ligand, through N-acylphosphatidylethanolamine pathway in testis: Involvement of Ca2+-dependent transacylase and phosphodiesterase activities. Biochem. Biophys. Res. Commun. 1996, 218, 113–117. [Google Scholar]
- Mallet, P.E.; Beninger, R.J. The endogenous cannabinoid receptor agonist anandamide impairs memory in rats. Behav. Pharmacol. 1996, 7, 276–284. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Gunduz-Cinar, O.; Hill, M.N.; McEwen, B.S.; Holmes, A. Amygdala FAAH and anandamide: Mediating protection and recovery from stress. Trends Pharmacol. Sci. 2013, 34, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Hillard, C.J.; Calero, M.; Rabano, A. Fatty acid amide hydrolase localization in the human central nervous system: An immunohistochemical study. Brain Res. Mol. Brain Res. 2002, 100, 85–93. [Google Scholar] [CrossRef]
- den Boon, F.S.; Chameau, P.; Schaafsma-Zhao, Q.; van Aken, W.; Bari, M.; Oddi, S.; Kruse, C.G.; Maccarrone, M.; Wadman, W.J.; Werkman, T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 3534–3539. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agro, A. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 2003, 10, 946–955. [Google Scholar] [CrossRef]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 2006, 49, 67–79. [Google Scholar] [CrossRef]
- Giuffrida, A.; Parsons, L.H.; Kerr, T.M.; de Fonseca, F.R.; Navarro, M.; Piomelli, D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999, 2, 358–363. [Google Scholar] [CrossRef]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.; Piomelli, D. A second endogenous cannabinoid that modulates long-term potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Nakane, S.; Oka, S.; Arai, S.; Waku, K.; Ishima, Y.; Tokumura, A.; Sugiura, T. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: Occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch. Biochem. Biophys. 2002, 402, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Sigel, E.; Baur, R.; Racz, I.; Marazzi, J.; Smart, T.G.; Zimmer, A.; Gertsch, J. The major central endocannabinoid directly acts at GABA(A) receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 18150–18155. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Lerner, T.N.; Kreitzer, A.C. RGS4 Is Required for Dopaminergic Control of Striatal LTD and Susceptibility to Parkinsonian Motor Deficits. Neuron 2012, 73, 347–359. [Google Scholar] [CrossRef]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Fezza, F.; Musella, A.; Gasperi, V.; Prosperetti, C.; Bernardi, G.; Finazzi-Agro, A.; et al. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat. Neurosci. 2008, 11, 152–159. [Google Scholar] [CrossRef]
- Puente, N.; Cui, Y.H.; Lassalle, O.; Lafourcade, M.; Georges, F.; Venance, L.; Grandes, P.; Manzoni, O.J. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 2011, 14, 1542–1547. [Google Scholar] [CrossRef]
- Zlebnik, N.E.; Cheer, J.F. Drug-Induced Alterations of Endocannabinoid-Mediated Plasticity in Brain Reward Regions. J. Neurosci. 2016, 36, 10230–10238. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Ozturk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Hanus, L.O.; Meyer, S.M.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef]
- Izzo, A.A.; Borrelli, F.; Capasso, R.; Di Marzo, V.; Mechoulam, R. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 2009, 30, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. Marihuana chemistry. Science 1970, 168, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Gaoni, Y. Recent advances in the chemistry of hashish. Fortschr. Chem Org. Naturst. 1967, 25, 175–213. [Google Scholar]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165771. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- Garrett, E.R.; Hunt, C.A. Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef]
- Mechoulam, R.; Gaoni, Y. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Lett. 1967, 12, 1109–1111. [Google Scholar] [CrossRef]
- Hillard, C.J.; Harris, R.A.; Bloom, A.S. Effects of the Cannabinoids on Physical-Properties of Brain Membranes and Phospholipid-Vesicles—Fluorescence Studies. J. Pharmacol. Exp. Ther. 1985, 232, 579–588. [Google Scholar]
- Lawrence, D.K.; Gill, E.W. Effects of Delta1-Tetrahydrocannabinol and Other Cannabinoids on Spin-Labeled Liposomes and Their Relationship to Mechanisms of General Anesthesia. Mol. Pharmacol. 1975, 11, 595–602. [Google Scholar]
- Pertwee, R.G. The central neuropharmacology of psychotropic cannabinoids. Pharmacol. Ther. 1988, 36, 189–261. [Google Scholar] [CrossRef]
- De Luca, M.A.; Solinas, M.; Bimpisidis, Z.; Goldberg, S.R.; Di Chiara, G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 2012, 63, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Hanus, L.O.; Pertwee, R.; Howlett, A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S163–S171. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Y.; Sun, L.; Wang, H. Role of integrating cannabinoids and the endocannabinoid system in neonatal hypoxic-ischaemic encephalopathy. Front. Mol. Neurosci. 2023, 16, 1152167. [Google Scholar] [CrossRef]
- Ibeas Bih, C.; Chen, T.; Nunn, A.V.; Bazelot, M.; Dallas, M.; Whalley, B.J. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics 2015, 12, 699–730. [Google Scholar] [CrossRef]
- Miller, S.; Daily, L.; Leishman, E.; Bradshaw, H.; Straiker, A. Delta9-Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5904–5911. [Google Scholar] [CrossRef]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allara, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARalpha/gamma agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta. Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Fernandes, M.; Warning, N.; Christ, W.; Hill, R. Interactions of several cannabinoids with the hepatic drug metabolizing system. Biochem. Pharmacol. 1973, 22, 2981–2987. [Google Scholar] [CrossRef]
- Anderson, P.F.; Jackson, D.M.; Chesher, G.B.; Malor, R. Tolerance to the effect of delta9-tetrahydrocannabinol in mice on intestinal motility, temperature and locomotor activity. Psychopharmacologia 1975, 43, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Orsingher, O.A.; Berardi, A.C. Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia 1973, 28, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Karler, R.; Cely, W.; Turkanis, S.A. Anticonvulsant properties of delta 9-tetrahydrocannabinol and other cannabinoids. Life Sci. 1974, 15, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Udoh, M.; Santiago, M.; Devenish, S.; McGregor, I.S.; Connor, M. Cannabichromene is a cannabinoid CB(2) receptor agonist. Br. J. Pharmacol. 2019, 176, 4537–4547. [Google Scholar] [CrossRef]
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta(9)-tetrahydrocannabinol, cannabidiol and Delta(9)-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef]
- Vasquez, C.; Lewis, D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999, 19, 9271–9280. [Google Scholar] [CrossRef]
- Molina-Holgado, E.; Vela, J.M.; Arevalo-Martin, A.; Almazan, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002, 22, 9742–9753. [Google Scholar] [CrossRef]
- Romero, J.; Garcia-Palomero, E.; Berrendero, F.; Garcia-Gil, L.; Hernandez, M.L.; Ramos, J.A.; Fernandez-Ruiz, J.J. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse 1997, 26, 317–323. [Google Scholar] [CrossRef]
- .Camilleri, M.; Kolar, G.J.; Vazquez-Roque, M.I.; Carlson, P.; Burton, D.D.; Zinsmeister, A.R. Cannabinoid receptor 1 gene and irritable bowel syndrome: Phenotype and quantitative traits. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G553–G560. [Google Scholar] [CrossRef] [PubMed]
- Gerard, C.; Mollereau, C.; Vassart, G.; Parmentier, M. Nucleotide-Sequence of a Human Cannabinoid Receptor Cdna. Nucleic Acids Res. 1990, 18, 7142. [Google Scholar] [CrossRef] [PubMed]
- Ryberg, E.; Vu, H.K.; Larsson, N.; Groblewski, T.; Hjorth, S.; Elebring, T.; Sjogren, S.; Greasley, P.J. Identification and characterisation of a novel splice variant of the human CB1 receptor. FEBS Lett. 2005, 579, 259–264. [Google Scholar] [CrossRef]
- Shire, D.; Calandra, B.; Rinaldi-Carmona, M.; Oustric, D.; Pessegue, B.; Bonnin-Cabanne, O.; Le Fur, G.; Caput, D.; Ferrara, P. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim. Biophys. Acta 1996, 1307, 132–136. [Google Scholar] [CrossRef]
- Chavarria-Siles, I.; Contreras-Rojas, J.; Hare, E.; Walss-Bass, C.; Quezada, P.; Dassori, A.; Contreras, S.; Medina, R.; Ramirez, M.; Salazar, R.; et al. Cannabinoid receptor 1 gene (CNR1) and susceptibility to a quantitative phenotype for hebephrenic schizophrenia. Am. J. Med. Genet B 2008, 147B, 279–284. [Google Scholar] [CrossRef]
- Ujike, H.; Morita, Y. New perspectives in the studies on endocannabinoid and cannabis: Cannabinoid receptors and schizophrenia. J. Pharmacol. Sci. 2004, 96, 376–381. [Google Scholar] [CrossRef]
- Ponce, G.; Hoenicka, J.; Rubio, G.; Ampuero, I.; Jimenez-Arriero, M.A.; Rodriguez-Jimenez, R.; Palomo, T.; Ramos, J.A. Association between cannabinoid receptor gene (CNR1) and childhood attention deficit/hyperactivity disorder in Spanish male alcoholic patients. Mol. Psychiatry 2003, 8, 466–467. [Google Scholar] [CrossRef]
- Barrero, F.J.; Ampuero, I.; Morales, B.; Vives, F.; del Castillo, J.D.L.; Hoenicka, J.; Yebenes, J.G. Depression in Parkinson’s disease is related to a genetic polymorphism of the cannabinoid receptor gene (CNR1). Pharmacogenomics J. 2005, 5, 135–141. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Multiple Functions of Endocannabinoid Signaling in the Brain. Annu. Rev. Neurosci. 2012, 35, 529–558. [Google Scholar] [CrossRef]
- Katona, I.; Sperlagh, B.; Sik, A.; Kafalvi, A.; Vizi, E.S.; Mackie, K.; Freund, T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999, 19, 4544–4558. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Howlett, A.C. Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci. 1995, 56, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.L.; Salles, J.; Meana, J.J.; Callado, L.F. Characterization of CB1 cannabinoid receptor immunoreactivity in postmortem human brain homogenates. Neuroscience 2006, 140, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Oddi, S.; Dainese, E.; Sandiford, S.; Fezza, F.; Lanuti, M.; Chiurchiu, V.; Totaro, A.; Catanzaro, G.; Barcaroli, D.; De Laurenzi, V.; et al. Effects of palmitoylation of Cys(415) in helix 8 of the CB(1) cannabinoid receptor on membrane localization and signalling. Br. J. Pharmacol. 2012, 165, 2635–2651. [Google Scholar] [CrossRef]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar]
- Howlett, A.C. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol. Pharmacol. 1985, 27, 429–436. [Google Scholar]
- Howlett, A.C.; Fleming, R.M. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol. Pharmacol. 1984, 26, 532–538. [Google Scholar]
- Howlett, A.C.; Qualy, J.M.; Khachatrian, L.L. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 1986, 29, 307–313. [Google Scholar]
- Devane, W.A.; Dysarz, F.A.; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and Characterization of a Cannabinoid Receptor in Rat-Brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; Decosta, B.R.; Rice, K.C. Characterization and Localization of Cannabinoid Receptors in Rat-Brain—A Quantitative Invitro Autoradiographic Study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- .Huestis, M.A.; Gorelick, D.A.; Heishman, S.J.; Preston, K.L.; Nelson, R.A.; Moolchan, E.T.; Frank, R.A. Blockade of effects of smoked marijuana by the CBl-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry 2001, 58, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Borgan, F.; Kokkinou, M.; Howes, O. The Cannabinoid CB1 Receptor in Schizophrenia. Biol. Psychiatry-Cogn. Neurosci. Neuroimaging 2021, 6, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Nyiri, G.; Cserep, C.; Szabadits, E.; Mackie, K.; Freund, T.F. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 2005, 136, 811–822. [Google Scholar] [CrossRef]
- McLemore, G.L.; Cooper, R.Z.; Richardson, K.A.; Mason, A.V.; Marshall, C.; Northington, F.J.; Gauda, E.B. Cannabinoid receptor expression in peripheral arterial chemoreceptors during postnatal development. J. Appl. Physiol. (1985) 2004, 97, 1486–1495. [Google Scholar] [CrossRef]
- Pertwee, R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Int. J. Obes. 2006, 30, S13–S18. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb. Exp. Pharmacol. 2005, 299–325. [Google Scholar]
- Petroff, O.A. GABA and glutamate in the human brain. Neuroscientist 2002, 8, 562–573. [Google Scholar] [CrossRef]
- Benard, G.; Massa, F.; Puente, N.; Lourenco, J.; Bellocchio, L.; Soria-Gomez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Morozov, Y.M.; Dominguez, M.H.; Varela, L.; Shanabrough, M.; Koch, M.; Horvath, T.L.; Rakic, P. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur. J. Neurosci. 2013, 38, 2341–2348. [Google Scholar] [CrossRef]
- Zou, S.L.; Kumar, U. Colocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hippocampus. Brain Res. 2015, 1622, 114–126. [Google Scholar] [CrossRef]
- Zou, S.L.; Somvanshi, R.K.; Paik, S.; Kumar, U. Colocalization of Cannabinoid Receptor 1 with Somatostatin and Neuronal Nitric Oxide Synthase in Rat Brain Hypothalamus. J. Mol. Neurosci. 2015, 55, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.K.; Devi, L.A. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol. Rev. 2011, 63, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.L.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 2005, 315, 828–838. [Google Scholar] [CrossRef]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef]
- Jhaveri, M.D.; Elmes, S.J.R.; Richardson, D.; Barrett, D.A.; Kendall, D.A.; Mason, R.; Chapman, V. Evidence for a novel functional role of cannabinoid CB2 receptors in the thalamus of neuropathic rats. Eur. J. Neurosci. 2008, 27, 1722–1730. [Google Scholar] [CrossRef]
- Elmes, S.J.R.; Jhaveri, M.D.; Smart, D.; Kendall, D.A.; Chapman, V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur. J. Neurosci. 2004, 20, 2311–2320. [Google Scholar] [CrossRef]
- Morgan, N.H.; Stanford, I.M.; Woodhall, G.L. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 2009, 57, 356–368. [Google Scholar] [CrossRef]
- Sagar, D.R.; Kelly, S.; Millns, P.J.; O’Shaughnessey, C.T.; Kendall, D.A.; Chapman, V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur. J. Neurosci. 2005, 22, 371–379. [Google Scholar] [CrossRef]
- Xi, Z.X.; Peng, X.Q.; Li, X.; Song, R.; Zhang, H.Y.; Liu, Q.R.; Yang, H.J.; Bi, G.H.; Li, J.; Gardner, E.L. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011, 14, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Pettit, D.A.D.; Harrison, M.P.; Olson, J.M.; Spencer, R.F.; Cabral, G.A. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J. Neurosci. Res. 1998, 51, 391–402. [Google Scholar] [CrossRef]
- Tsou, K.; Brown, S.; Sanudo-Pena, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; Gonzalez, S.; Tolon, R.M.; Romero, J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.X.; Min, X.N.; Cabral, G.A.; Lokensgard, J.R.; Peterson, P.K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1 beta-stimulated human astrocytes. Glia 2005, 49, 211–219. [Google Scholar] [CrossRef]
- Ashton, J.C.; Friberg, D.; Darlington, C.L.; Smith, P.F. Expression of the cannabinoid CB2 receptor in the rat cerebellum: An immunohistochemical study. Neurosci. Lett. 2006, 396, 113–116. [Google Scholar] [CrossRef]
- Stella, N. Cannabinoid and Cannabinoid-Like Receptors in Microglia, Astrocytes, and Astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef]
- .Morales, M.; Pickel, V.M. Insights to drug addiction derived from ultrastructural views of the mesocorticolimbic system. Addict. Rev. 2012, 1248, 71–88. [Google Scholar] [CrossRef]
- Benito, C.; Nunez, E.; Tolon, R.M.; Carrier, E.J.; Rabano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef]
- Pazos, M.R.; Nunez, E.; Benito, C.; Tolon, R.M.; Romero, J. Functional neuroanatomy of the endocannabinoid system. Pharmacol. Biochem. Behav. 2005, 81, 239–247. [Google Scholar] [CrossRef]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Kim, W.K.; Chavarria, I.; Hillard, C.J.; Mackie, K.; Tolon, R.M.; Williams, K.; Romero, J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J. Neurosci. 2005, 25, 2530–2536. [Google Scholar] [CrossRef] [PubMed]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Ozdogan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Racz, I.; Ponomarenko, A.; et al. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.; von Zastrow, M. Downregulation of G protein-coupled receptors. Curr. Opin. Neurobiol. 2000, 10, 365–369. [Google Scholar] [CrossRef]
- von Zastrow, M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003, 74, 217–224. [Google Scholar] [CrossRef]
- Canals, M.; Milligan, G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed mu opioid receptors. J. Biol. Chem. 2008, 283, 11424–11434. [Google Scholar] [CrossRef]
- Ellis, J.; Pediani, J.D.; Canals, M.; Milasta, S.; Milligan, G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J. Biol. Chem. 2006, 281, 38812–38824. [Google Scholar] [CrossRef]
- Leterrier, C.; Laine, J.; Darmon, M.; Boudin, H.; Rossier, J.; Lenkei, Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J. Neurosci. 2006, 26, 3141–3153. [Google Scholar] [CrossRef]
- McDonald, N.A.; Henstridge, C.M.; Connolly, C.N.; Irving, A.J. An essential role for constitutive endocytosis, but not activity, in the axonal targeting of the CB1 cannabinoid receptor. Mol. Pharmacol. 2007, 71, 976–984. [Google Scholar] [CrossRef]
- Nie, J.; Lewis, D.L. Structural domains of the CB1 cannabinoid receptor that contribute to constitutive activity and G-protein sequestration. J. Neurosci. 2001, 21, 8758–8764. [Google Scholar] [CrossRef]
- Roche, J.P.; Bounds, S.; Brown, S.; Mackie, K. A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G protein signaling and prevents receptor internalization. Mol. Pharmacol. 1999, 56, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Abood, M.E. Mutation of a highly conserved aspartate residue in the second transmembrane domain of the cannabinoid receptors, CB1 and CB2, disrupts G-protein coupling. J. Pharmacol. Exp. Ther. 1998, 285, 651–658. [Google Scholar] [PubMed]
- Daigle, T.L.; Kearn, C.S.; Mackie, K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology 2008, 54, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.A.; Anavi-Goffer, S.; Ross, R.A.; MacEwan, D.J.; Mackie, K.; Pertwee, R.G.; Irving, A.J. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J. Neurosci. 2001, 21, 2425–2433. [Google Scholar] [CrossRef]
- Hsieh, C.; Brown, S.; Derleth, C.; Mackie, K. Internalization and recycling of the CB1 cannabinoid receptor. J. Neurochem. 1999, 73, 493–501. [Google Scholar] [CrossRef]
- Leterrier, C.; Bonnard, D.; Carrel, D.; Rossier, J.; Lenkei, Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 2004, 279, 36013–36021. [Google Scholar] [CrossRef]
- Rinaldi-Carmona, M.; Le Duigou, A.; Oustric, D.; Barth, F.; Bouaboula, M.; Carayon, P.; Casellas, P.; Le Fur, G. Modulation of CB1 cannabinoid receptor functions after a long-term exposure to agonist or inverse agonist in the Chinese hamster ovary cell expression system. J. Pharmacol. Exp. Ther. 1998, 287, 1038–1047. [Google Scholar]
- Atwood, B.K.; Wager-Miller, J.; Haskins, C.; Straiker, A.; Mackie, K. Functional selectivity in CB(2) cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB(2) ligands. Mol. Pharmacol. 2012, 81, 250–263. [Google Scholar] [CrossRef]
- .Bouaboula, M.; Dussossoy, D.; Casellas, P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528—Implications for receptor biological responses. J. Biol. Chem. 1999, 274, 20397–20405. [Google Scholar] [CrossRef]
- Atwood, B.K.; Huffman, J.; Straiker, A.; Mackie, K. JWH018, a common constituent of ’Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br. J. Pharmacol. 2010, 160, 585–593. [Google Scholar] [CrossRef]
- Atwood, B.K.; Lee, D.; Straiker, A.; Widlanski, T.S.; Mackie, K. CP47,497-C8 and JWH073, commonly found in ’Spice’ herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur. J. Pharmacol. 2011, 659, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. THC and CBD: Similarities and differences between siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.A.; Castillo, P.E. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience 2006, 139, 795–802. [Google Scholar] [CrossRef]
- Lau, T.; Schloss, P. The cannabinoid CB1 receptor is expressed on serotonergic and dopaminergic neurons. Eur. J. Pharmacol. 2008, 578, 137–141. [Google Scholar] [CrossRef]
- Ohno-Shosaku, T.; Maejima, T.; Kano, M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 2001, 29, 729–738. [Google Scholar] [CrossRef]
- Wilson, R.I.; Nicoll, R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 2001, 410, 588–592. [Google Scholar] [CrossRef]
- Kreitzer, A.C.; Regehr, W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 2001, 29, 717–727. [Google Scholar] [CrossRef]
- Katona, I.; Urban, G.M.; Wallace, M.; Ledent, C.; Jung, K.M.; Piomelli, D.; Mackie, K.; Freund, T.F. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 2006, 26, 5628–5637. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Maejima, T. Retrograde signaling at central synapses via endogenous cannabinoids. Mol. Psychiatry 2002, 7, 234–235. [Google Scholar] [CrossRef]
- Hashimotodani, Y.; Ohno-Shosaku, T.; Kano, M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J. Neurosci. 2007, 27, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Uchigashima, M.; Narushima, M.; Fukaya, M.; Katona, I.; Kano, M.; Watanabe, M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J. Neurosci. 2007, 27, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Di Marzo, V. Non-CB1, Non-CB2 Receptors for Endocannabinoids, Plant Cannabinoids, and Synthetic Cannabimimetics: Focus on G-protein-coupled Receptors and Transient Receptor Potential Channels. J. Neuroimmune Pharm. 2010, 5, 103–121. [Google Scholar] [CrossRef]
- .Liu, J.; Wang, L.; Harvey-White, J.; Huang, B.X.; Kim, H.Y.; Luquet, S.; Palmiter, R.D.; Krystal, G.; Rai, R.; Mahadevan, A.; et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 2008, 54, 1–7. [Google Scholar] [CrossRef]
- Chavez, A.E.; Chiu, C.Q.; Castillo, P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010, 13, 1511–1518. [Google Scholar] [CrossRef]
- .Grueter, B.A.; Brasnjo, G.; Malenka, R.C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 2010, 13, 1519–1525. [Google Scholar] [CrossRef]
- Bacci, A.; Huguenard, J.R.; Prince, D.A. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature 2004, 431, 312–316. [Google Scholar] [CrossRef]
- Gomez, O.; Arevalo-Martin, A.; Garcia-Ovejero, D.; Ortega-Gutierrez, S.; Cisneros, J.A.; Almazan, G.; Sanchez-Rodriguez, M.A.; Molina-Holgado, F.; Molina-Holgado, E. The Constitutive Production of the Endocannabinoid 2-Arachidonoylglycerol Participates in Oligodendrocyte Differentiation. Glia 2010, 58, 1913–1927. [Google Scholar] [CrossRef]
- Hegyi, Z.; Hollo, K.; Kis, G.; Mackie, K.; Antal, M. Differential distribution of diacylglycerol lipase-alpha and N-acylphosphatidylethanolamine-specific phospholipase D immunoreactivity in the superficial spinal dorsal horn of rats. Glia 2012, 60, 1316–1329. [Google Scholar] [CrossRef]
- .Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef]
- Bosier, B.; Muccioli, G.G.; Hermans, E.; Lambert, D.M. Functionally selective cannabinoid receptor signalling: Therapeutic implications and opportunities. Biochem. Pharmacol. 2010, 80, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Felder, C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997, 17, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Northup, J.K. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol. Pharmacol. 1999, 56, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.H.; Bayewitch, M.; Avidor-Reiss, T.; Levy, R.; Vogel, Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J. Neurochem. 1998, 71, 1525–1534. [Google Scholar] [CrossRef]
- Maneuf, Y.P.; Brotchie, J.M. Paradoxical action of the cannabinoid WIN 55,212-2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br. J. Pharmacol. 1997, 120, 1397–1398. [Google Scholar] [CrossRef]
- Bonhaus, D.W.; Chang, L.K.; Kwan, J.; Martin, G.R. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: Evidence for agonist-specific trafficking of intracellular responses. J. Pharmacol. Exp. Ther. 1998, 287, 884–888. [Google Scholar]
- Lauckner, J.E.; Hille, B.; Mackie, K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 19144–19149. [Google Scholar] [CrossRef]
- Navarrete, M.; Araque, A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef]
- Thibault, K.; Carrel, D.; Bonnard, D.; Gallatz, K.; Simon, A.; Biard, M.; Pezet, S.; Palkovits, M.; Lenkei, Z. Activation-Dependent Subcellular Distribution Patterns of CB1 Cannabinoid Receptors in the Rat Forebrain. Cereb. Cortex 2013, 23, 2581–2591. [Google Scholar] [CrossRef]
- Gergely, G.S.; Nora, L.; Noemi, H.; Tibor, A.; Zoltan, N.; Norbert, H. Presynaptic Calcium Channel Inhibition Underlies CB1 Cannabinoid Receptor-Mediated Suppression of GABA Release. J. Neurosci. 2014, 34, 7958–7963. [Google Scholar]
- Fisyunov, A.; Tsintsadze, V.; Min, R.; Burnashev, N.; Lozovaya, N. Cannabinoids modulate the P-type high-voltage-activated calcium currents in Purkinje neurons. J. Neurophysiol. 2006, 96, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Safo, P.K.; Regehr, W.G. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J. Neurosci. 2004, 24, 5623–5631. [Google Scholar] [CrossRef] [PubMed]
- Twitchell, W.; Brown, S.; Mackie, K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997, 78, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K.; Lai, Y.; Westenbroek, R.; Mitchell, R. Cannabinoids Activate an Inwardly Rectifying Potassium Conductance and Inhibit Q-Type Calcium Currents in Att20 Cells Transfected with Rat-Brain Cannabinoid Receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef]
- Guo, J.; Ikeda, S.R. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 2004, 65, 665–674. [Google Scholar] [CrossRef]
- Robbe, D.; Alonso, G.; Duchamp, F.; Bockaert, J.; Manzoni, O.J. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J. Neurosci. 2001, 21, 109–116. [Google Scholar] [CrossRef]
- Turu, G.; Hunyady, L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [Google Scholar] [CrossRef]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB1 Cannabinoid Receptors and their Associated Proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Rueda, D.; Del Pulgar, T.G.; Velasco, G.; Guzman, M. Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor. Mol. Pharmacol. 2002, 62, 1385–1392. [Google Scholar] [CrossRef]
- Flores-Otero, J.; Ahn, K.H.; Delgado-Peraza, F.; Mackie, K.; Kendall, D.A.; Yudowski, G.A. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat. Commun. 2014, 5, 4589. [Google Scholar] [CrossRef]
- Bouaboula, M.; Poinotchazel, C.; Bourrie, B.; Canat, X.; Calandra, B.; Rinaldicarmona, M.; Lefur, G.; Casellas, P. Activation of Mitogen-Activated Protein-Kinases by Stimulation of the Central Cannabinoid Receptor Cb1. Biochem. J. 1995, 312, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Derkinderen, P.; Ledent, C.; Parmentier, M.; Girault, J.A. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. J. Neurochem. 2001, 77, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Rueda, D.; Galve-Roperh, I.; Haro, A.; Guzman, M. The CB1 cannabinoid receptor is coupled to the activation of c-Jun N-terminal kinase. Mol. Pharmacol. 2000, 58, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, B.; Mirshahi, F.; Sanyal, A.J.; Khanolkar, A.D.; Makriyannis, A.; Kunos, G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000, 346, 835–840. [Google Scholar] [CrossRef]
- He, J.C.J.; Gomes, I.; Nguyen, T.; Jayaram, G.; Ram, P.T.; Devi, L.A.; Iyengar, R. The G alpha(o/)i-coupled cannabinoid receptor-mediated neurite outgrowth involves rap regulation of Src and Stat3. J. Biol. Chem. 2005, 280, 33426–33434. [Google Scholar] [CrossRef]
- Valjent, E.; Pages, C.; Rogard, M.; Besson, M.J.; Maldonado, R.; Caboche, J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 2001, 14, 342–352. [Google Scholar] [CrossRef]
- Derkinderen, P.; Valjent, E.; Toutant, M.; Corvol, J.C.; Enslen, H.; Ledent, C.; Trzaskos, J.; Caboche, J.; Girault, J.A. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J. Neurosci. 2003, 23, 2371–2382. [Google Scholar] [CrossRef]
- Rubino, T.; Forlani, G.; Vigano, D.; Zippel, R.; Parolaro, D. Modulation of extracellular signal-regulated kinases cascade by chronic Delta(9)-tetrahydrocannabinol treatment. Mol. Cell Neurosci. 2004, 25, 355–362. [Google Scholar] [CrossRef]
- Daigle, T.L.; Madisen, L.; Hage, T.A.; Valley, M.T.; Knoblich, U.; Larsen, R.S.; Takeno, M.M.; Huang, L.; Gu, H.; Larsen, R.; et al. A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 2018, 174, 465. [Google Scholar] [CrossRef]
- McCudden, C.R.; Hains, M.D.; Kimple, R.J.; Siderovski, D.P.; Willard, F.S. G-protein signaling: Back to the future. Cell. Mol. Life Sci. 2005, 62, 551–577. [Google Scholar] [CrossRef]
- Kouznetsova, M.; Kelley, B.; Shen, M.X.; Thayer, S.A. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol. Pharmacol. 2002, 61, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.Z.; Brown, S.; Roche, J.P.; Hsieh, C.; Celver, J.P.; Kovoor, A.; Chavkin, C.; Mackie, K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999, 19, 3773–3780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Schmid, C.L.; Raehal, K.M.; Selley, D.E.; Bohn, L.M.; Sim-Selley, L.J. beta-Arrestin2 Regulates Cannabinoid CB1 Receptor Signaling and Adaptation in a Central Nervous System Region-Dependent Manner. Biol. Psychiatry 2012, 71, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, C.S.; Lambert, J.M.; Gerfin, S.; Huffman, J.W.; Razdan, R.K. Sensitivity to Delta 9-tetrahydrocannabinol is selectively enhanced in beta-arrestin2-/- mice. Behav. Pharmacol. 2008, 19, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Mahmoud, M.M.; Shim, J.Y.; Kendall, D.A. Distinct Roles of beta-Arrestin 1 and beta-Arrestin 2 in ORG27569-induced Biased Signaling and Internalization of the Cannabinoid Receptor 1 (CB1). J. Biol. Chem. 2013, 288, 9790–9800. [Google Scholar] [CrossRef]
- Gomez del Pulgar, T.; Velasco, G.; Guzman, M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem. J. 2000, 347 Pt 2, 369–373. [Google Scholar] [CrossRef]
- Gomez, O.; Sanchez-Rodriguez, A.; Le, M.Q.U.; Sanchez-Caro, C.; Molina-Holgado, F.; Molina-Holgado, E. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 2011, 163, 1520–1532. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Pinteaux, E.; Heenan, L.; Moore, J.D.; Rothwell, N.J.; Gibson, R.M. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol. Cell. Neurosci. 2005, 28, 189–194. [Google Scholar] [CrossRef]
- Ozaita, A.; Puighermanal, E.; Maldonado, R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J. Neurochem. 2007, 102, 1105–1114. [Google Scholar] [CrossRef]
- Blazquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; Garcia-Rincon, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef]
- Lopez-Cardona, A.P.; Perez-Cerezales, S.; Fernandez-Gonzalez, R.; Laguna-Barraza, R.; Pericuesta, E.; Agirregoitia, N.; Gutierrez-Adan, A.; Agirregoitia, E. CB1 cannabinoid receptor drives oocyte maturation and embryo development via PI3K/Akt and MAPK pathways. Faseb J. 2017, 31, 3372–3382. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Yucel, M.; Solowij, N.; Respondek, C.; Whittle, S.; Fornito, A.; Pantelis, C.; Lubman, D.I. Regional brain abnormalities associated with heavy long-term cannabis use. Eur. Neuropsychopharmacol. 2008, 18, S545–S546. [Google Scholar] [CrossRef]
- Solowij, N.; Yucel, M.; Respondek, C.; Whittle, S.; Lindsay, E.; Pantelis, C.; Lubman, D.I. Cerebellar white-matter changes in cannabis users with and without schizophrenia. Psychol. Med. 2011, 41, 2349–2359. [Google Scholar] [CrossRef]
- Cohen, M.; Rasser, P.E.; Peck, G.; Carr, V.J.; Ward, P.B.; Thompson, P.M.; Johnston, P.; Baker, A.; Schall, U. Cerebellar grey-matter deficits, cannabis use and first-episode schizophrenia in adolescents and young adults. Int. J. Neuropsychopharmacol. 2012, 15, 297–307. [Google Scholar] [CrossRef]
- Tzilos, G.K.; Cintron, C.B.; Wood, J.B.R.; Simpson, N.S.; Young, A.D.; Pope, H.G.; Yurgelun-Todd, D.A. Lack of hippocampal volume change in long-term heavy cannabis users. Am J. Addict. 2005, 14, 64–72. [Google Scholar] [CrossRef]
- Jager, G.; Van Hell, H.H.; De Win, M.M.L.; Kahn, R.S.; Van den Brink, W.; Van Ree, J.M.; Ramsey, N.F. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur. Neuropsychopharmacol. 2007, 17, 289–297. [Google Scholar] [CrossRef]
- Zalesky, A.; Solowij, N.; Yucel, M.; Lubman, D.I.; Takagi, M.; Harding, I.H.; Lorenzetti, V.; Wang, R.P.; Searle, K.; Pantelis, C.; et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain 2012, 135, 2245–2255. [Google Scholar] [CrossRef]
- Dalton, V.S.; Zavitsanou, K. Cannabinoid Effects on CB1 Receptor Density in the Adolescent Brain: An Autoradiographic Study Using the Synthetic Cannabinoid HU210. Synapse 2010, 64, 845–854. [Google Scholar] [CrossRef]
- Barres, B.A.; Hart, I.K.; Coles, H.S.R.; Burne, J.F.; Voyyodic, J.T.; Richardson, W.D.; Raff, M.C. Cell-Death and Control of Cell-Survival in the Oligodendrocyte Lineage. Cell 1992, 70, 31–46. [Google Scholar] [CrossRef]
- Gonzalez, S.; Cebeira, M.; Fernandez-Ruiz, J. Cannabinoid tolerance and dependence: A review of studies in laboratory animals. Pharmacol. Biochem. Behav. 2005, 81, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Hasin, D.S.; Saha, T.D.; Kerridge, B.T.; Goldstein, R.B.; Chou, S.P.; Zhang, H.; Jung, J.; Pickering, R.P.; Ruan, W.J.; Smith, S.M.; et al. Prevalence of Marijuana Use Disorders in the United States Between 2001–2002 and 2012–2013. JAMA Psychiatry 2015, 72, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Martin, B.R. Cannabinoid tolerance and dependence. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 691–717. [Google Scholar]
- Murray, R.M.; Morrison, P.D.; Henquet, C.; Di Forti, M. Science and society—Cannabis, the mind and society: The hash realities. Nat. Rev. Neurosci. 2007, 8, 885–895. [Google Scholar] [CrossRef]
- WB, O.S. On the preparations of the Indian hemp, or gunjah (Cannabis indica). Their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov. Med. J. Retrosp. Med. Sci 1843, 5, 343–347, 363–369, 397–398. [Google Scholar]
- Nurmikko, T.J.; Serpell, M.G.; Hoggart, B.; Toomey, P.J.; Morlion, B.J.; Haines, D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain 2007, 133, 210–220. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Young, C.A. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: An uncontrolled, open-label, 2-year extension trial. Clin. Ther. 2007, 29, 2068–2079. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Kunos, G.; Tam, J. The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br. J. Pharmacol. 2011, 163, 1423–1431. [Google Scholar] [CrossRef]
- Horvath, B.; Mukhopadhyay, P.; Hasko, G.; Pacher, P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am. J. Pathol. 2012, 180, 432–442. [Google Scholar] [CrossRef]
- Centonze, D.; Finazzi-Agro, A.; Bernardi, G.; Maccarrone, M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol. Sci. 2007, 28, 180–187. [Google Scholar] [CrossRef]
- Skaper, S.D.; Di Marzo, V. Endocannabinoids in nervous system health and disease: The big picture in a nutshell. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2012, 367, 3193–3200. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005, 5, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Mukhopadhyay, P.; Mohanraj, R.; Godlewski, G.; Batkai, S.; Kunos, G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension 2008, 52, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Pacher, P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: Promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323. [Google Scholar] [CrossRef]
- Montecucco, F.; Di Marzo, V. At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction. Trends Pharmacol. Sci. 2012, 33, 331–340. [Google Scholar] [CrossRef]
- Lotersztajn, S.; Teixeira-Clerc, F.; Julien, B.; Deveaux, V.; Ichigotani, Y.; Manin, S.; Tran-Van-Nhieu, J.; Karsak, M.; Zimmer, A.; Mallat, A. CB2 receptors as new therapeutic targets for liver diseases. Br. J. Pharmacol. 2008, 153, 286–289. [Google Scholar] [CrossRef]
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in Liver Disease. Hepatology 2011, 53, 346–355. [Google Scholar] [CrossRef]
- Izzo, A.A.; Camilleri, M. Emerging role of cannabinoids in gastrointestinal and liver diseases: Basic and clinical aspects. Gut 2008, 57, 1140–1155. [Google Scholar] [CrossRef]
- Biro, T.; Toth, B.I.; Hasko, G.; Paus, R.; Pacher, P. The endocannabinoid system of the skin in health and disease: Novel perspectives and therapeutic opportunities. Trends Pharmacol. Sci. 2009, 30, 411–420. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and Pain. Cns Neurol. Disord.-Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Hillard, C.J.; Weinlander, K.M.; Stuhr, K.L. Contributions of Endocannabinoid Signaling to Psychiatric Disorders in Humans: Genetic and Biochemical Evidence. Neuroscience 2012, 204, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Batkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and cancer: Therapeutic implication. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Sanchez, C.; Guzman, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef]
- Talarico, G.; Trebbastoni, A.; Bruno, G.; de Lena, C. Modulation of the Cannabinoid System: A New Perspective for the Treatment of the Alzheimer’s Disease. Curr. Neuropharmacol. 2019, 17, 176–183. [Google Scholar] [CrossRef]
- Walther, S.; Mahlberg, R.; Eichmann, U.; Kunz, D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 2006, 185, 524–528. [Google Scholar] [CrossRef]
- Volicer, L.; Stelly, M.; Morris, J.; McLaughlin, J.; Volicer, B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Fagan, S.G.; Campbell, V.A. The influence of cannabinoids on generic traits of neurodegeneration. Br. J. Pharmacol. 2014, 171, 1347–1360. [Google Scholar] [CrossRef]
- Notcutt, W.; Langford, R.; Davies, P.; Ratcliffe, S.; Potts, R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex (R) (nabiximols). Mult. Scler. J. 2012, 18, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.D.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB(1) on neurons and CB(2) on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Valdeolivas, S.; Satta, V.; Pertwee, R.G.; Fernandez-Ruiz, J.; Sagredo, O. Sativex-like Combination of Phytocannabinoids is Neuroprotective in Malonate-Lesioned Rats, an Inflammatory Model of Huntington’s Disease: Role of CB1 and CB2 Receptors. Acs Chem. Neurosci. 2012, 3, 400–406. [Google Scholar] [CrossRef]
- Muller-Vahl, K.R. Treatment of Tourette syndrome with cannabinoids. Behav. Neurol. 2013, 27, 119–124. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Sagredo, O.; Pazos, M.R.; Garcia, C.; Pertwee, R.; Mechoulam, R.; Martinez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Garcia, C.; Palomo-Garo, C.; Garcia-Arencibia, M.; Ramos, J.A.; Pertwee, R.G.; Fernandez-Ruiz, J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Delta(9)-THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef]
- Harkany, T.; Guzman, M.; Galve-Roperh, I.; Berghuis, P.; Devi, L.A.; Mackie, K. The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 2007, 28, 83–92. [Google Scholar] [CrossRef]
- Bockmann, E.C.; Brito, R.; Madeira, L.F.; da Silva Sampaio, L.; de Melo Reis, R.A.; Franca, G.R.; Calaza, K.D.C. The Role of Cannabinoids in CNS Development: Focus on Proliferation and Cell Death. Cell. Mol. Neurobiol. 2023, 43, 1469–1485. [Google Scholar] [CrossRef]
- Navarrete, F.; Garcia-Gutierrez, M.S.; Jurado-Barba, R.; Rubio, G.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Endocannabinoid System Components as Potential Biomarkers in Psychiatry. Front. Psychiatry 2020, 11, 315. [Google Scholar] [CrossRef]
- Landfield, P.W.; Pitler, T.A. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science 1984, 226, 1089–1092. [Google Scholar] [CrossRef]
- Thibault, O.; Landfield, P.W. Increase in single L-type calcium channels in hippocampal neurons during aging. Science 1996, 272, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin. Neurochem. Int. 2011, 58, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Wu, Z. Microglia-aging: Roles of microglial lysosome- and mitochondria-derived reactive oxygen species in brain aging. Behav. Brain Res. 2009, 201, 1–7. [Google Scholar] [CrossRef]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc. Natl. Acad. Sci. USA 2011, 108, 11256–11261. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Racz, I.; Valverde, O.; Otto, M.; Michel, K.; Sastre, M.; Zimmer, A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 15670–15675. [Google Scholar] [CrossRef]
- Bilkei-Gorzo, A.; Drews, E.; Albayram, O.; Piyanova, A.; Gaffal, E.; Tueting, T.; Michel, K.; Mauer, D.; Maier, W.; Zimmer, A. Early onset of aging-like changes is restricted to cognitive abilities and skin structure in Cnr1(-)/(-) mice. Neurobiol. Aging 2012, 33, 200.e11–200.e22. [Google Scholar] [CrossRef]
- Domenici, M.R.; Azad, S.C.; Marsicano, G.; Schierloh, A.; Wotjak, C.T.; Dodt, H.U.; Zieglgansberger, W.; Lutz, B.; Rammes, G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J. Neurosci. 2006, 26, 5794–5799. [Google Scholar] [CrossRef]
- Kawamura, Y.; Fukaya, M.; Maejima, T.; Yoshida, T.; Miura, E.; Watanabe, M.; Ohno-Shosaku, T.; Kano, M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006, 26, 2991–3001. [Google Scholar]
- Mulder, J.; Aguado, T.; Keimpema, E.; Barabas, K.; Rosado, C.J.B.; Nguyen, L.; Monory, K.; Marsicano, G.; Di Marzo, V.; Hurd, Y.L.; et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. USA 2008, 105, 8760–8765. [Google Scholar]
- Berrendero, F.; Romero, J.; Garcia-Gil, L.; Suarez, I.; De la Cruz, P.; Ramos, J.A.; Fernandez-Ruiz, J.J. Changes in cannabinoid receptor binding and mRNA levels in several brain regions of aged rats. Biochim. Biophys. Acta-Mol. Basis Dis. 1998, 1407, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, H.; Harvey-White, J.; Zimmer, A.; Kunos, G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Vuic, B.; Milos, T.; Tudor, L.; Konjevod, M.; Nikolac Perkovic, M.; Jazvinscak Jembrek, M.; Nedic Erjavec, G.; Svob Strac, D. Cannabinoid CB2 Receptors in Neurodegenerative Proteinopathies: New Insights and Therapeutic Potential. Biomedicines 2022, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Gorka, A.; Hyde, L.W.; Kimak, M.; Halder, I.; Ducci, F.; Ferrell, R.E.; Goldman, D.; Manuck, S.B. Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry 2009, 66, 9–16. [Google Scholar] [CrossRef]
- Martin, M.; Ledent, C.; Parmentier, M.; Maldonado, R.; Valverde, O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology 2002, 159, 379–387. [Google Scholar] [CrossRef]
- Moreira, F.A.; Kaiser, N.; Monory, K.; Lutz, B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 2008, 54, 141–150. [Google Scholar] [CrossRef]
- Ibarra-Lecue, I.; Pilar-Cuellar, F.; Muguruza, C.; Florensa-Zanuy, E.; Diaz, A.; Uriguen, L.; Castro, E.; Pazos, A.; Callado, L.F. The endocannabinoid system in mental disorders: Evidence from human brain studies. Biochem. Pharmacol. 2018, 157, 97–107. [Google Scholar] [CrossRef]
- Ishiguro, H.; Carpio, O.; Horiuchi, Y.; Shu, A.; Higuchi, S.; Schanz, N.; Benno, R.; Arinami, T.; Onaivi, E.S. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 2010, 64, 92–96. [Google Scholar] [CrossRef]
- Smith, D.R.; Stanley, C.M.; Foss, T.; Boles, R.G.; McKernan, K. Rare genetic variants in the endocannabinoid system genes CNR1 and DAGLA are associated with neurological phenotypes in humans. PLoS ONE 2017, 12, e0187926. [Google Scholar] [CrossRef]
- Chakrabarti, B.; Kent, L.; Suckling, J.; Bullmore, E.; Baron-Cohen, S. Variations in the human cannabinoid receptor (CNR1) gene modulate striatal responses to happy faces. Eur. J. Neurosci. 2006, 23, 1944–1948. [Google Scholar] [CrossRef]
- Kong, X.; Miao, Q.; Lu, X.; Zhang, Z.; Chen, M.; Zhang, J.; Zhai, J. The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: A meta-analysis. Medicine 2019, 98, e17403. [Google Scholar] [CrossRef] [PubMed]
- .Martinez-Gras, I.; Hoenicka, J.; Ponce, G.; Rodriguez-Jimenez, R.; Jimenez-Arriero, M.A.; Perez-Hernandez, E.; Ampuero, I.; Ramos-Atance, J.A.; Palomo, T.; Rubio, G. (AAT)n repeat in the cannabinoid receptor gene, CNR1: Association with schizophrenia in a Spanish population. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: From mice to human subjects. PLoS ONE 2008, 3, e1640. [Google Scholar] [CrossRef] [PubMed]
- Peiro, A.M.; Garcia-Gutierrez, M.S.; Planelles, B.; Femenia, T.; Mingote, C.; Jimenez-Trevino, L.; Martinez-Barrondo, S.; Garcia-Portilla, M.P.; Saiz, P.A.; Bobes, J.; et al. Association of cannabinoid receptor genes (CNR1 and CNR2) polymorphisms and panic disorder. Anxiety Stress Coping 2020, 33, 256–265. [Google Scholar] [CrossRef]
- Garcia-Rincon, D.; Diaz-Alonso, J.; Paraiso-Luna, J.; Ortega, Z.; Aguareles, J.; de Salas-Quiroga, A.; Jou, C.; de Prada, I.; Martinez-Cerdeno, V.; Aronica, E.; et al. Contribution of Altered Endocannabinoid System to Overactive mTORC1 Signaling in Focal Cortical Dysplasia. Front. Pharmacol. 2018, 9, 1508. [Google Scholar] [CrossRef]
- Wei, B.Q.; Mikkelsen, T.S.; McKinney, M.K.; Lander, E.S.; Cravatt, B.F. A second fatty acid amide hydrolase with variable distribution among placental mammals. J. Biol. Chem. 2006, 281, 36569–36578. [Google Scholar] [CrossRef]
- Sirrs, S.; van Karnebeek, C.D.; Peng, X.; Shyr, C.; Tarailo-Graovac, M.; Mandal, R.; Testa, D.; Dubin, D.; Carbonetti, G.; Glynn, S.E.; et al. Defects in fatty acid amide hydrolase 2 in a male with neurologic and psychiatric symptoms. Orphanet J. Rare Dis. 2015, 10, 38. [Google Scholar] [CrossRef]
- Colizzi, M.; Fazio, L.; Ferranti, L.; Porcelli, A.; Masellis, R.; Marvulli, D.; Bonvino, A.; Ursini, G.; Blasi, G.; Bertolino, A. Functional genetic variation of the cannabinoid receptor 1 and cannabis use interact on prefrontal connectivity and related working memory behavior. Neuropsychopharmacology 2015, 40, 640–649. [Google Scholar] [CrossRef]
- Ruiz-Contreras, A.E.; Carrillo-Sanchez, K.; Gomez-Lopez, N.; Vadillo-Ortega, F.; Hernandez-Morales, S.; Carnevale-Cantoni, A.; Espejel-Nunez, A.; Mendez-Diaz, M.; Prospero-Garcia, O. Working memory performance in young adults is associated to the AATn polymorphism of the CNR1 gene. Behav. Brain Res. 2013, 236, 62–66. [Google Scholar] [CrossRef]
- Gibson, C.L.; Nia, A.B.; Spriggs, S.A.; DeFrancisco, D.; Swift, A.; Perkel, C.; Zhong, X.B.; Mazumdar, M.; Fernandez, N.; Patel, M.; et al. Cannabinoid use in psychotic patients impacts inflammatory levels and their association with psychosis severity. Psychiatry Res. 2020, 293. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Dantzer, R. Is there a role for immune-to-brain communication in schizophrenia? Psychopharmacology 2016, 233, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Muller, N.; Schwarz, M.J. Immune System and Schizophrenia. Curr. Immunol. Rev. 2010, 6, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, A.; Martorell, L.; Montalvo, I.; Ortega, L.; Monseny, R.; Vilella, E.; Labad, J. Increased serum interleukin-6 levels in early stages of psychosis: Associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 2014, 41, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lunn, C.A.; Reich, E.P.; Fine, J.S.; Lavey, B.; Kozlowski, J.A.; Hipkin, R.W.; Lundell, D.J.; Bober, L. Biology and therapeutic potential of cannabinoid CB2 receptor inverse agonists. Br. J. Pharmacol. 2008, 153, 226–239. [Google Scholar] [CrossRef]
- Kaplan, B.L.; Rockwell, C.E.; Kaminski, N.E. Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J. Pharmacol. Exp. Ther. 2003, 306, 1077–1085. [Google Scholar] [CrossRef]
- Benamar, K.; Yondorf, M.; Meissler, J.J.; Geller, E.B.; Tallarida, R.J.; Eisenstein, T.K.; Adler, M.W. A novel role of cannabinoids: Implication in the fever induced by bacterial lipopolysaccharide. J. Pharmacol. Exp. Ther. 2007, 320, 1127–1133. [Google Scholar] [CrossRef]
- Croci, T.; Landi, M.; Galzin, A.M.; Marini, P. Role of cannabinoid CB1 receptors and tumor necrosis factor-alpha in the gut and systemic anti-inflammatory activity of SR 141716 (rimonabant) in rodents. Br. J. Pharmacol. 2003, 140, 115–122. [Google Scholar] [CrossRef]
- Smith, S.R.; Terminelli, C.; Denhardt, G. Effects of cannabinoid receptor agonist and antagonist ligands on production of inflammatory cytokines and anti-inflammatory interleukin-10 in endotoxemic mice. J. Pharmacol. Exp. Ther. 2000, 293, 136–150. [Google Scholar]
- Smith, S.R.; Terminelli, C.; Denhardt, G. Modulation of cytokine responses in Corynebacterium parvum-primed endotoxemic mice by centrally administered cannabinoid ligands. Eur. J. Pharmacol. 2001, 425, 73–83. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Chen, C. Endocannabinoid 2-arachidonoylglycerol protects neurons against beta-amyloid insults. Neuroscience 2011, 178, 159–168. [Google Scholar] [CrossRef]
- Du, H.; Chen, X.; Zhang, J.; Chen, C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-gamma. Br. J. Pharmacol. 2011, 163, 1533–1549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C. Endocannabinoid 2-arachidonoylglycerol protects neurons by limiting COX-2 elevation. J. Biol. Chem. 2008, 283, 22601–22611. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Governa, P.; Montopoli, M.; Biagi, M. Cannabis sativa L. Constituents and Their Role in Neuroinflammation. Curr. Bioact. Compd. 2019, 15, 147–158. [Google Scholar] [CrossRef]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef]
- Marsicano, G.; Moosmann, B.; Hermann, H.; Lutz, B.; Behl, C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. J. Neurochem. 2002, 80, 448–456. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc. Natl. Acad. Sci. USA 1999, 96, 8867–8872. [Google Scholar] [CrossRef]
- Jan, T.R.; Kaminski, N.E. Role of mitogen-activated protein kinases in the differential regulation of interleukin-2 by cannabinol. J. Leukoc. Biol. 2001, 69, 841–849. [Google Scholar] [CrossRef]
- Shen, M.X.; Thayer, S.A. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998, 783, 77–84. [Google Scholar] [CrossRef]
- .Chiarlone, A.; Bellocchio, L.; Blazquez, C.; Resel, E.; Soria-Gomez, E.; Cannich, A.; Ferrero, J.J.; Sagredo, O.; Benito, C.; Romero, J.; et al. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc. Natl. Acad. Sci. USA 2014, 111, 8257–8262. [Google Scholar] [CrossRef]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.M.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef]
- More, S.V.; Choi, D.K. Promising cannabinoid-based therapies for Parkinson’s disease: Motor symptoms to neuroprotection. Mol. Neurodegener. 2015, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Xie, L.; Kim, S.H.; Parmentier-Batteur, S.; Sun, Y.; Mao, X.O.; Childs, J.; Greenberg, D.A. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004, 66, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, C.; Di Siena, S.; Bustamante, M.B.; Di Giacomo, D.; Di Tommaso, M.; Maccarrone, M.; Grimaldi, P.; Sette, C. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS ONE 2013, 8, e54271. [Google Scholar] [CrossRef] [PubMed]
- Khaspekov, L.G.; Brenz Verca, M.S.; Frumkina, L.E.; Hermann, H.; Marsicano, G.; Lutz, B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur. J. Neurosci. 2004, 19, 1691–1698. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. Cannabinoids for treatment of Alzheimer’s disease: Moving toward the clinic. Front. Pharmacol. 2014, 5, 37. [Google Scholar] [CrossRef]
- Crews, L.; Adame, A.; Patrick, C.; Delaney, A.; Pham, E.; Rockenstein, E.; Hansen, L.; Masliah, E. Increased BMP6 levels in the brains of Alzheimer’s disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J. Neurosci. 2010, 30, 12252–12262. [Google Scholar] [CrossRef]
- Molero, A.E.; Gokhan, S.; Gonzalez, S.; Feig, J.L.; Alexandre, L.C.; Mehler, M.F. Impairment of developmental stem cell-mediated striatal neurogenesis and pluripotency genes in a knock-in model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2009, 106, 21900–21905. [Google Scholar] [CrossRef]
- Prenderville, J.A.; Kelly, A.M.; Downer, E.J. The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef]
- de Oliveira, R.W.; Oliveira, C.L.; Guimaraes, F.S.; Campos, A.C. Cannabinoid signalling in embryonic and adult neurogenesis: Possible implications for psychiatric and neurological disorders. Acta Neuropsychiatr. 2019, 31, 1–16. [Google Scholar] [CrossRef]
- Farquhar-Smith, W.P.; Egertova, M.; Bradbury, E.J.; McMahon, S.B.; Rice, A.S.C.; Elphick, M.R. Cannabinoid CB1 receptor expression in rat spinal cord. Mol. Cell. Neurosci. 2000, 15, 510–521. [Google Scholar] [CrossRef]
- Iversen, L.; Chapman, V. Cannabinoids: A real prospect for pain relief? Curr. Opin. Pharmacol. 2002, 2, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Urban, L.; Capogna, M.; Bevan, S.; Nagy, I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 2000, 100, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Clapper, J.R.; Moreno-Sanz, G.; Russo, R.; Guijarro, A.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Sciolino, N.R.; Spradley, J.M.; et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat. Neurosci. 2010, 13, 1265–1270. [Google Scholar] [CrossRef]
- Stein, C.; Zollner, C. Opioids and sensory nerves. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 495–518. [Google Scholar]
- Jaggar, S.I.; Sellaturay, S.; Rice, A.S.C. The endogenous cannabinoid anandamide, but not the CB2 ligand palmitoylethanolamide, prevents the viscero-visceral hyperreflexia associated with inflammation of the rat urinary bladder. Neurosci. Lett. 1998, 253, 123–126. [Google Scholar] [CrossRef]
- Nackley, A.G.; Suplita, R.L., 2nd; Hohmann, A.G. A peripheral cannabinoid mechanism suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 2003, 117, 659–670. [Google Scholar] [CrossRef]
- Dziadulewicz, E.K.; Bevan, S.J.; Brain, C.T.; Coote, P.R.; Culshaw, A.J.; Davis, A.J.; Edwards, L.J.; Fisher, A.J.; Fox, A.J.; Gentry, C.; et al. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: A potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J. Med. Chem. 2007, 50, 3851–3856. [Google Scholar] [CrossRef]
- Anand, P.; Whiteside, G.; Fowler, C.J.; Hohmann, A.G. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res. Rev. 2009, 60, 255–266. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef]
- Kaufmann, I.; Hauer, D.; Huge, V.; Vogeser, M.; Campolongo, P.; Chouker, A.; Thiel, M.; Schelling, G. Enhanced Anandamide Plasma Levels in Patients with Complex Regional Pain Syndrome following Traumatic Injury: A Preliminary Report. Eur. Surg. Res. 2009, 43, 325–329. [Google Scholar] [CrossRef]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Tabrizi, M.A.; Merighi, S.; Gessi, S.; Saponaro, G.; Baraldi, P.G.; Borea, P.A.; Varani, K. Antinociceptive effects of the selective CB2 agonist MT178 in inflammatory and chronic rodent pain models. Pain 2013, 154, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. Endocannabinoid signaling in the brain: Biosynthetic mechanisms in the limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.R.; Willenbring, D.; Guan, Y.; Pan, H.L.; Ren, K.; Xu, Y.; et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef]
- Benito, C.; Nunez, E.; Pazos, M.R.; Tolon, R.M.; Romero, J. The endocannabinoid system and Alzheimer’s disease. Mol. Neurobiol. 2007, 36, 75–81. [Google Scholar] [CrossRef]
- .De Marchi, N.; De Petrocellis, L.; Orlando, P.; Daniele, F.; Fezza, F.; Di Marzo, V. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003, 2, 5. [Google Scholar] [CrossRef]
- Di Filippo, M.; Pini, L.A.; Pelliccioli, G.P.; Calabresi, P.; Sarchielli, P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1224–1229. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef]
- Giuffrida, A.; Leweke, F.M.; Gerth, C.W.; Schreiber, D.; Koethe, D.; Faulhaber, J.; Klosterkotter, J.; Piomelli, D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology 2004, 29, 2108–2114. [Google Scholar] [CrossRef]
- Hill, M.N.; Miller, G.E.; Ho, W.S.; Gorzalka, B.B.; Hillard, C.J. Serum endocannabinoid content is altered in females with depressive disorders: A preliminary report. Pharmacopsychiatry 2008, 41, 48–53. [Google Scholar] [CrossRef]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Koppel, J.; Bradshaw, H.; Goldberg, T.E.; Khalili, H.; Marambaud, P.; Walker, M.J.; Pazos, M.; Gordon, M.L.; Christen, E.; Davies, P. Endocannabinoids in Alzheimer’s disease and their impact on normative cognitive performance: A case-control and cohort study. Lipids Health Dis. 2009, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Koppel, J.; Davies, P. Targeting the endocannabinoid system in Alzheimer’s disease. J. Alzheimer’s Dis. 2008, 15, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Matias, I.; Martiadis, V.; De Petrocellis, L.; Maj, M.; Di Marzo, V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 2005, 30, 1216–1221. [Google Scholar] [CrossRef]
- Rossi, C.; Pini, L.A.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: Relation with serotonin levels. Eur. J. Clin. Pharmacol. 2008, 64, 1–8. [Google Scholar] [CrossRef]
- Sarchielli, P.; Pini, L.A.; Coppola, F.; Rossi, C.; Baldi, A.; Mancini, M.L.; Calabresi, P. Endocannabinoids in chronic migraine: CSF findings suggest a system failure. Neuropsychopharmacology 2007, 32, 1384–1390. [Google Scholar] [CrossRef]
- van der Stelt, M.; Mazzola, C.; Esposito, G.; Matias, I.; Petrosino, S.; De Filippis, D.; Micale, V.; Steardo, L.; Drago, F.; Iuvone, T.; et al. Endocannabinoids and beta-amyloid-induced neurotoxicity in vivo: Effect of pharmacological elevation of endocannabinoid levels. Cell. Mol. Life Sci. 2006, 63, 1410–1424. [Google Scholar] [CrossRef]
- Mechoulam, R. The Pharmacohistory of Cannabis Sativa, in Cannabis as Therapeutic Agent; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Ellison, J.M.; Gelwan, E.; Ogletree, J. Complex Partial Seizure Symptoms Affected by Marijuana Abuse. J. Clin. Psychiatry 1990, 51, 439–440. [Google Scholar]
- Consroe, P.F.; Wood, G.C.; Buchsbaum, H. Anticonvulsant Nature of Marihuana Smoking. Jama-J. Am. Med. Assoc. 1975, 234, 306–307. [Google Scholar] [CrossRef]
- Keeler, M.H.; Reifler, C.B. Grand Mal Convulsions Subsequent to Marijuana Use—Case Report. Dis. Nerv. Syst. 1967, 28, 474. [Google Scholar]
- Clement, A.B.; Hawkins, E.G.; Lichtman, A.H.; Cravatt, B.F. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J. Neurosci. 2003, 23, 3916–3923. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.J.; Martin, B.R.; DeLorenzo, R.J. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur. J. Pharmacol. 2002, 452, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Neu, A.; Howard, A.L.; Foldy, C.; Echegoyen, J.; Hilgenberg, L.; Smith, M.; Mackie, K.; Soltesz, I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J. Neurosci. 2007, 27, 46–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, K.; Ratzliff, A.; Hilgenberg, L.; Gulyas, A.; Freund, T.F.; Smith, M.; Dinh, T.P.; Piomelli, D.; Mackie, K.; Soltesz, I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron 2003, 39, 599–611. [Google Scholar] [CrossRef]
- Bhaskaran, M.D.; Smith, B.N. Cannabinoid-Mediated Inhibition of Recurrent Excitatory Circuitry in the Dentate Gyrus in a Mouse Model of Temporal Lobe Epilepsy. PLoS ONE 2010, 5, e10683. [Google Scholar] [CrossRef]
- Falenski, K.W.; Blair, R.E.; Sim-Selley, L.J.; Martin, B.R.; DeLorenzo, R.J. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience 2007, 146, 1232–1244. [Google Scholar] [CrossRef][Green Version]
- Wallace, M.J.; Blair, R.E.; Falenski, K.W.; Martin, B.R.; DeLorenzo, R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003, 307, 129–137. [Google Scholar] [CrossRef]
- Falenski, K.W.; Carter, D.S.; Harrison, A.J.; Martin, B.R.; Blair, R.E.; DeLorenzo, R.J. Temporal characterization of changes in hippocampal cannabinoid CB1 receptor expression following pilocarpine-induced status epilepticus. Brain Res. 2009, 1262, 64–72. [Google Scholar] [CrossRef]
- Hao, E.K.; Mukhopadhyay, P.; Cao, Z.X.; Erdelyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Hasko, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Reggio, P.H. Emerging Roles of Cannabinoids and Synthetic Cannabinoids in Clinical Experimental Models. Cannabinoids Neuropsychiatr. Disord. 2021, 1264, 47–65. [Google Scholar]
- Rosenberg, E.C.; Tsien, R.W.; Whalley, B.J.; Devinsky, O. Cannabinoids and Epilepsy. Neurotherapeutics 2015, 12, 747–768. [Google Scholar] [CrossRef] [PubMed]
- Umathe, S.N.; Manna, S.S.S.; Jain, N.S. Endocannabinoid analogues exacerbate marble-burying behavior in mice via TRPV1 receptor. Neuropharmacology 2012, 62, 2024–2033. [Google Scholar] [CrossRef]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77sr1. [Google Scholar] [CrossRef]
- Dong, S.; Duan, Y.; Hu, Y.; Zhao, Z. Advances in the pathogenesis of Alzheimer’s disease: A re-evaluation of amyloid cascade hypothesis. Transl. Neurodegener. 2012, 1, 18. [Google Scholar] [CrossRef]
- .Weggen, S.; Beher, D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimers Res. Ther. 2012, 4, 9. [Google Scholar] [CrossRef]
- Monteiro, K.L.C.; Dos Santos Alcantara, M.G.; de Aquino, T.M.; da Silva-Junior, E.F. Cannabinoid pharmacology and its therapeutic uses in Alzheimer’s disease. Neural Regen. Res. 2021, 16, 990–991. [Google Scholar]
- Aso, E.; Juves, S.; Maldonado, R.; Ferrer, I. CB2 Cannabinoid Receptor Agonist Ameliorates Alzheimer-Like Phenotype in A beta PP/PS1 Mice. J. Alzheimers Dis. 2013, 35, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; Garcia-Garcia, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Fakhfouri, G.; Ahmadiani, A.; Rahimian, R.; Grolla, A.A.; Moradi, F.; Haeri, A. WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-gamma pathway. Neuropharmacology 2012, 63, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bie, B.H.; Yang, H.; Xu, J.J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Grunblatt, E.; Bartl, J.; Zehetmayer, S.; Ringel, T.M.; Bauer, P.; Riederer, P.; Jacob, C.P. Gene Expression as Peripheral Biomarkers for Sporadic Alzheimer’s Disease. J. Alzheimers Dis. 2009, 16, 627–634. [Google Scholar] [CrossRef]
- D’Addario, C.; Di Francesco, A.; Arosio, B.; Gussago, C.; Dell’Osso, B.; Bari, M.; Galimberti, D.; Scarpini, E.; Altamura, A.C.; Mari, D.; et al. Epigenetic Regulation of Fatty Acid Amide Hydrolase in Alzheimer Disease. PLoS ONE 2012, 7, e39186. [Google Scholar]
- Casarejos, M.J.; Perucho, J.; Gomez, A.; Munoz, M.P.; Fernandez-Estevez, M.; Sagredo, O.; Fernandez Ruiz, J.; Guzman, M.; de Yebenes, J.G.; Mena, M.A. Natural cannabinoids improve dopamine neurotransmission and tau and amyloid pathology in a mouse model of tauopathy. J. Alzheimer’s Dis. 2013, 35, 525–539. [Google Scholar] [CrossRef]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratu, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Abeta-induced neuroinflammation and promotes hippocampal neurogenesis through PPARgamma involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef]
- Mulder, J.; Zilberter, M.; Pasquare, S.J.; Alpar, A.; Schulte, G.; Ferreira, S.G.; Kofalvi, A.; Martin-Moreno, A.M.; Keimpema, E.; Tanila, H.; et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain 2011, 134, 1041–1060. [Google Scholar] [CrossRef]
- Eubanks, L.M.; Rogers, C.J.; Beuscher, A.E.t.; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 2006, 3, 773–777. [Google Scholar] [CrossRef]
- Lee, J.H.; Agacinski, G.; Williams, J.H.; Wilcock, G.K.; Esiri, M.M.; Francis, P.T.; Wong, P.T.H.; Chen, C.P.; Lai, M.K. Intact cannabinoid CB1 receptors in the Alzheimer’s disease cortex. Neurochem. Int. 2010, 57, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, B.G.; Blazquez, C.; del Pulgar, T.G.; Guzman, N.; de Ceballos, M.A.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005, 25, 1904–1913. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Francis, P.T.; Franco, R.; Ramirez, M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 805–808. [Google Scholar] [CrossRef]
- Ahmad, R.; Goffin, K.; Van den Stock, J.; De Winter, F.L.; Cleeren, E.; Bormans, G.; Tournoy, J.; Persoons, P.; Van Laere, K.; Vandenbulcke, M. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur. Neuropsychopharmacol. 2014, 24, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.E.; Thorpe, A.J.; Lichtman, A.H. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology 2009, 34, 2072–2080. [Google Scholar] [CrossRef]
- Halleskog, C.; Mulder, J.; Dahlstrom, J.; Mackie, K.; Hortobagyi, T.; Tanila, H.; Puli, L.K.; Farber, K.; Harkany, T.; Schulte, G. WNT Signaling in Activated Microglia Is Proinflammatory. Glia 2011, 59, 119–131. [Google Scholar] [CrossRef]
- Mazzola, C.; Micale, V.; Drago, F. Amnesia induced by beta-amyloid fragments is counteracted by cannabinoid CB1 receptor blockade. Eur. J. Pharmacol. 2003, 477, 219–225. [Google Scholar] [CrossRef]
- Micale, V.; Cristino, L.; Tamburella, A.; Petrosino, S.; Leggio, G.M.; Di Marzo, V.; Drago, F. Enhanced cognitive performance of dopamine D3 receptor “knock-out” mice in the step-through passive-avoidance test: Assessing the role of the endocannabinoid/endovanilloid systems. Pharmacol. Res. 2010, 61, 531–536. [Google Scholar] [CrossRef]
- Marsicano, G.; Goodenough, S.; Monory, K.; Hermann, H.; Eder, M.; Cannich, A.; Azad, S.C.; Cascio, M.G.; Gutierrez, S.O.; van der Stelt, M.; et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003, 302, 84–88. [Google Scholar] [CrossRef]
- Hassel, B.; Tessler, S.; Faull, R.L.M.; Emson, P.C. Glutamate uptake is reduced in prefrontal cortex in Huntington’s disease. Neurochem. Res. 2008, 33, 232–237. [Google Scholar] [CrossRef]
- Ross, C.A.; Tabrizi, S.J. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011, 10, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.I.; Xu, H.; Igarashi, S.; Fujimuro, M.; Agrawal, N.; Taya, Y.; Hayward, S.D.; Moran, T.H.; Montell, C.; Ross, C.A.; et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron 2005, 47, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Horne, E.A.; Coy, J.; Swinney, K.; Fung, S.; Cherry, A.E.T.; Marrs, W.R.; Naydenov, A.V.; Lin, Y.H.; Sun, X.C.; Keene, C.D.; et al. Downregulation of cannabinoid receptor 1 from neuropeptide Y interneurons in the basal ganglia of patients with Huntington’s disease and mouse models. Eur. J. Neurosci. 2013, 37, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Naydenov, A.V.; Sepers, M.D.; Swinney, K.; Raymond, L.A.; Palmiter, R.D.; Stella, N. Genetic rescue of CB1 receptors on medium spiny neurons prevents loss of excitatory striatal synapses but not motor impairment in HD mice. Neurobiol. Dis. 2014, 71, 140–150. [Google Scholar] [CrossRef]
- Blazquez, C.; Chiarlone, A.; Sagredo, O.; Aguado, T.; Pazos, M.R.; Resel, E.; Palazuelos, J.; Julien, B.; Salazar, M.; Borner, C.; et al. Loss of striatal type 1 cannabinoid receptors is a key pathogenic factor in Huntington’s disease. Brain 2011, 134, 119–136. [Google Scholar] [CrossRef]
- Denovan-Wright, E.M.; Robertson, H.A. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience 2000, 98, 705–713. [Google Scholar] [CrossRef]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef]
- Gotz, M.R.; Collado, J.A.; Fernandez-Ruiz, J.; Fiebich, B.L.; Garcia-Toscano, L.; Gomez-Canas, M.; Koch, O.; Leha, A.; Munoz, E.; Navarrete, C.; et al. Structure-Effect Relationships of Novel Semi-Synthetic Cannabinoid Derivatives. Front. Pharmacol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Ruiz-Calvo, A.; Bajo-Graneras, R.; Maroto, I.B.; Zian, D.; Grabner, G.F.; Garcia-Taboada, E.; Resel, E.; Zechner, R.; Zimmermann, R.; Ortega-Gutierrez, S.; et al. Astroglial monoacylglycerol lipase controls mutant huntingtin-induced damage of striatal neurons. Neuropharmacology 2019, 150, 134–144. [Google Scholar] [CrossRef]
- Nadal, X.; Del Rio, C.; Casano, S.; Palomares, B.; Ferreiro-Vera, C.; Navarrete, C.; Sanchez-Carnerero, C.; Cantarero, I.; Bellido, M.L.; Meyer, S.; et al. Tetrahydrocannabinolic acid is a potent PPARgamma agonist with neuroprotective activity. Br. J. Pharmacol. 2017, 174, 4263–4276. [Google Scholar] [CrossRef]
- Zou, S.L.; Kumar, U. Somatostatin and cannabinoid receptors crosstalk in protection of huntingtin knock-in striatal neuronal cells in response to quinolinic acid. Neurochem. Int. 2019, 129, 104518. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Martire, A.; Petrosino, S.; Popoli, P.; Di Marzo, V. Symptom-related changes of endocannabinoid and palmitoylethanolamide levels in brain areas of R6/2 mice, a transgenic model of Huntington’s disease. Neurochem. Int. 2008, 52, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Battista, N.; Valenza, M.; Mastrangelo, N.; Malaponti, M.; Catanzaro, G.; Centonze, D.; Finazzi-Agro, A.; Cattaneo, E.; Maccarrone, M. In vitro and in vivo models of Huntington’s disease show alterations in the endocannabinoid system. FEBS J. 2013, 280, 3376–3388. [Google Scholar] [CrossRef] [PubMed]
- Samuel, D.; Legoff, L.K.; Kumar, U.; Errami, M.; Scarfone, E.; Nieoullon, A. Changes in Striatal Cholinergic, Gabaergic, Dopaminergic and Serotoninergic Biochemical Markers after Kainic Acid-Induced Thalamic Lesions in the Rat. J. Neural Transm. Park. Dis. Dement. Sect. 1990, 2, 193–203. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Boyes, B.E.; McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 1988, 38, 1285–1291. [Google Scholar] [CrossRef]
- Gerhard, A.; Pavese, N.; Hotton, G.; Turkheimer, F.; Es, M.; Hammers, A.; Eggert, K.; Oertel, W.; Banati, R.B.; Brooks, D.J. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006, 21, 404–412. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef]
- Kofalvi, A.; Rodrigues, R.J.; Ledent, C.; Mackie, K.; Vizi, E.S.; Cunha, R.A.; Sperlagh, B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: A combined immunochemical and pharmacological analysis. J. Neurosci. 2005, 25, 2874–2884. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br. J. Pharmacol. 2009, 156, 1029–1040. [Google Scholar] [CrossRef]
- Cilia, R. Molecular Imaging of the Cannabinoid System in Idiopathic Parkinson’s Disease. Int. Rev. Neurobiol. 2018, 141, 305–345. [Google Scholar]
- Rojo-Bustamante, E.; Abellanas, M.A.; Clavero, P.; Thiolat, M.L.; Li, Q.; Luquin, M.R.; Bezard, E.; Aymerich, M.S. The expression of cannabinoid type 1 receptor and 2-arachidonoyl glycerol synthesizing/degrading enzymes is altered in basal ganglia during the active phase of levodopa-induced dyskinesia. Neurobiol. Dis. 2018, 118, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, J.M. CB1 cannabinoid receptor signalling in Parkinson’s disease. Curr. Opin. Pharmacol. 2003, 3, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Mandolino, G.; Galuppo, M.; Bramanti, P.; Mazzon, E. Cannabinoids: New Promising Agents in the Treatment of Neurological Diseases. Molecules 2014, 19, 18781–18816. [Google Scholar] [CrossRef] [PubMed]
- Gubellini, P.; Picconi, B.; Bari, M.; Battista, N.; Calabresi, P.; Centonze, D.; Bernardi, G.; Finazzi-Agro, A.; Maccarrone, M. Experimental parkinsonism alters endocannabinoid degradation: Implications for striatal glutamatergic transmission. J. Neurosci. 2002, 22, 6900–6907. [Google Scholar] [CrossRef]
- Hurley, M.J.; Mash, D.C.; Jenner, P. Expression of cannabinoid CB1 receptor mRNA in basal ganglia of normal and parkinsonian human brain. J. Neural. Transm. 2003, 110, 1279–1288. [Google Scholar] [CrossRef]
- Pisani, A.; Fezza, F.; Galati, S.; Battista, N.; Napolitano, S.; Finazzi-Agro, A.; Bernardi, G.; Brusa, L.; Pierantozzi, M.; Stanzione, P.; et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005, 57, 777–779. [Google Scholar] [CrossRef]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic Changes of Anandamide in the Cerebrospinal Fluid of Parkinson’s Disease Patients. Mov. Disord 2010, 25, 920–924. [Google Scholar] [CrossRef]
- Van Laere, K.; Casteels, C.; Lunskens, S.; Goffin, K.; Grachev, I.D.; Bormans, G.; Vandenberghe, W. Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol. Aging 2012, 33, 620.e1. [Google Scholar] [CrossRef]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. Faseb J. 2000, 14, 1432–1438. [Google Scholar] [CrossRef]
- Meschler, J.P.; Howlett, A.C. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology 2001, 40, 918–926. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov. Disord. 2008, 23, S497–S508. [Google Scholar] [CrossRef] [PubMed]
- Maneuf, Y.P.; Crossman, A.R.; Brotchie, J.M. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson’s disease. Exp. Neurol. 1997, 148, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernandez-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Moreno-Martet, M.; Rodriguez-Cueto, C.; Palomo-Garo, C.; Gomez-Canas, M.; Valdeolivas, S.; Guaza, C.; Romero, J.; Guzman, M.; Mechoulam, R.; et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011, 163, 1365–1378. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Rana, A.Q.; Malik, S.H.; Rizvi, S.F.H.; Akhter, S.; Vannabouathong, C.; Sarfraz, Z.; Rana, R. Comprehensive Examination of Therapies for Pain in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 2018, 51, 190–206. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R(+)WIN55,212. J. Clin. Investig. 2003, 111, 1231–1240. [Google Scholar] [CrossRef]
- Lyman, W.D.; Sonett, J.R.; Brosnan, C.F.; Elkin, R.; Bornstein, M.B. Delta-9-Tetrahydrocannabinol—A Novel Treatment for Experimental Autoimmune Encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef]
- Sanchez, A.J.; Gonzalez-Perez, P.; Galve-Roperh, I.; Garcia-Merino, A. R-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzox azin-6-yl]-1-naphtalenylmethanone (WIN-2) ameliorates experimental autoimmune encephalomyelitis and induces encephalitogenic T cell apoptosis: Partial involvement of the CB(2) receptor. Biochem. Pharmacol. 2006, 72, 1697–1706. [Google Scholar]
- Arevalo-Martin, A.; Garcia-Ovejero, D.; Gomez, O.; Rubio-Araiz, A.; Navarro-Galve, B.; Guaza, C.; Molina-Holgado, E.; Molina-Holgado, F. CB2 cannabinoid receptors as an emerging target for demyelinating diseases: From neuroimmune interactions to cell replacement strategies. Br. J. Pharmacol. 2008, 153, 216–225. [Google Scholar] [CrossRef]
- de Lago, E.; Moreno-Martet, M.; Cabranes, A.; Ramos, J.A.; Fernandez-Ruiz, J. Cannabinoids ameliorate disease progression in a model of multiple sclerosis in mice, acting preferentially through CB1 receptor-mediated anti-inflammatory effects. Neuropharmacology 2012, 62, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzman, M.; Galve-Roperh, I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. Faseb J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Newton, C.; Widen, R.; Friedman, H.; Klein, T.W. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 2001, 423, 235–241. [Google Scholar] [CrossRef]
- Coopman, K.; Smith, L.D.; Wright, K.L.; Ward, S.G. Temporal variation in CB2R levels following T lymphocyte activation: Evidence that cannabinoids modulate CXCL12-induced chemotaxis. Int. Immunopharmacol. 2007, 7, 360–371. [Google Scholar] [CrossRef]
- Ghosh, S.; Preet, A.; Groopman, J.E.; Ganju, R.K. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol. Immunol. 2006, 43, 2169–2179. [Google Scholar] [CrossRef]
- Dittel, B.N. Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor. Br. J. Pharmacol. 2008, 153, 271–276. [Google Scholar] [CrossRef]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007, 122, 259–270. [Google Scholar] [CrossRef]
- Mohammad, M.G.; Hassanpour, M.; Tsai, V.W.; Li, H.; Ruitenberg, M.J.; Booth, D.W.; Serrats, J.; Hart, P.H.; Symonds, G.P.; Sawchenko, P.E.; et al. Dendritic cells and multiple sclerosis: Disease, tolerance and therapy. Int. J. Mol. Sci. 2012, 14, 547–562. [Google Scholar] [CrossRef]
- Greter, M.; Heppner, F.L.; Lemos, M.P.; Odermatt, B.M.; Goebels, N.; Laufer, T.; Noelle, R.J.; Becher, B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 2005, 11, 328–334. [Google Scholar] [CrossRef]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; Ogarra, A. Dendritic Cells Produce Il-12 and Direct the Development of Th1 Cells from Naive Cd4(+) T-Cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar] [CrossRef]
- Vaknin-Dembinsky, A.K.; Balashov, A.K.; Weiner, H.L. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J. Immunol. 2006, 176, 7768–7774. [Google Scholar] [CrossRef]
- Maestroni, G.J.M. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. Faseb J. 2004, 18, 1914. [Google Scholar] [CrossRef] [PubMed]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.Q.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Weiser, S.; Miu, J.; Ball, H.J.; Hunt, N.H. Interferon-gamma synergises with tumour necrosis factor and lymphotoxin-alpha to enhance the mRNA and protein expression of adhesion molecules in mouse brain endothelial cells. Cytokine 2007, 37, 84–91. [Google Scholar] [CrossRef]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Ortega-Gutierrez, S.; Molina-Holgado, E.; Arevalo-Martin, A.; Correa, F.; Viso, A.; Lopez-Rodriguez, M.L.; Di Marzo, V.; Guaza, C. Activation of the endocannabinoid system as a therapeutic approach in a murine model of multiple sclerosis. Faseb J. 2005, 19, 1338. [Google Scholar] [CrossRef]
- Docagne, F.; Muneton, V.; Clemente, D.; Ali, C.; Loria, F.; Correa, F.; Hernangomez, M.; Mestre, L.; Vivien, D.; Guaza, C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol. Cell. Neurosci. 2007, 34, 551–561. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.; Moro, M.A.; Martinez-Orgado, J. Cannabinoids in Neurodegenerative Disorders and Stroke/Brain Trauma: From Preclinical Models to Clinical Applications. Neurotherapeutics 2015, 12, 793–806. [Google Scholar] [CrossRef]
- Hardiman, O.; van den Berg, L.H.; Kiernan, M.C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 639–649. [Google Scholar] [CrossRef]
- Foran, E.; Trotti, D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid. Redox Signal. 2009, 11, 1587–1602. [Google Scholar] [CrossRef] [PubMed]
- Ferraiuolo, L.; Kirby, J.; Grierson, A.J.; Sendtner, M.; Shaw, P.J. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2011, 7, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Renton, A.E.; Majounie, E.; Waite, A.; Simon-Sanchez, J.; Rollinson, S.; Gibbs, J.R.; Schymick, J.C.; Laaksovirta, H.; van Swieten, J.C.; Myllykangas, L.; et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron 2011, 72, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, L.G.; Dick, J.R.; Pryce, G.; Petrosino, S.; Di Marzo, V.; Baker, D.; Greensmith, L. Increasing cannabinoid levels by pharmacological and genetic manipulation delay disease progression in SOD1 mice. FASEB J. 2006, 20, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Witting, A.; Weydt, P.; Hong, S.; Kliot, M.; Moller, T.; Stella, N. Endocannabinoids accumulate in spinal cord of SOD1 G93A transgenic mice. J. Neurochem. 2004, 89, 1555–1557. [Google Scholar] [CrossRef]
- Moreno-Martet, M.; Espejo-Porras, F.; Fernandez-Ruiz, J.; de Lago, E. Changes in endocannabinoid receptors and enzymes in the spinal cord of SOD1(G93A) transgenic mice and evaluation of a Sativex((R)) -like combination of phytocannabinoids: Interest for future therapies in amyotrophic lateral sclerosis. CNS Neurosci. Ther. 2014, 20, 809–815. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef]
- Espejo-Porras, F.; Piscitelli, F.; Verde, R.; Ramos, J.A.; Di Marzo, V.; de Lago, E.; Fernandez-Ruiz, J. Changes in the endocannabinoid signaling system in CNS structures of TDP-43 transgenic mice: Relevance for a neuroprotective therapy in TDP-43-related disorders. J. Neuroimmune Pharm. 2015, 10, 233–244. [Google Scholar] [CrossRef]
- Shoemaker, J.L.; Seely, K.A.; Reed, R.L.; Crow, J.P.; Prather, P.L. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J. Neurochem. 2007, 101, 87–98. [Google Scholar] [CrossRef]
- de Lago, E.; Moreno-Martet, M.; Espejo-Porras, F.; Fernández-Ruiz, J. Endocannabinoids and amyotrophic lateral sclerosis. In Cannabinoids in Neurologic and Mental Disease; Fattore, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–124. [Google Scholar]
- Di Forti, M.; Morgan, C.; Dazzan, P.; Pariante, C.; Mondelli, V.; Marques, T.R.; Handley, R.; Luzi, S.; Russo, M.; Paparelli, A.; et al. High-potency cannabis and the risk of psychosis. Br. J. Psychiatry 2009, 195, 488–491. [Google Scholar] [CrossRef]
- Di Forti, M.; Sallis, H.; Allegri, F.; Trotta, A.; Ferraro, L.; Stilo, S.A.; Marconi, A.; La Cascia, C.; Marques, T.R.; Pariante, C.; et al. Daily Use, Especially of High-Potency Cannabis, Drives the Earlier Onset of Psychosis in Cannabis Users. Schizophr. Bull. 2014, 40, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Marconi, A.; Di Forti, M.; Lewis, C.M.; Murray, R.M.; Vassos, E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr. Bull. 2016, 42, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Bassir Nia, A.; Medrano, B.; Perkel, C.; Galynker, I.; Hurd, Y.L. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. J. Psychopharmacol. 2016, 30, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Pinilla, P.; Lopez-Gil, J.; Crespo-Facorro, B. Immune system: A possible nexus between cannabinoids and psychosis. Brain Behav. Immun 2014, 40, 269–282. [Google Scholar] [CrossRef]
- Freeman, D.; Dunn, G.; Murray, R.M.; Evans, N.; Lister, R.; Antley, A.; Slater, M.; Godlewska, B.; Cornish, R.; Williams, J.; et al. How Cannabis Causes Paranoia: Using the Intravenous Administration of a dagger(9)-Tetrahydrocannabinol (THC) to Identify Key Cognitive Mechanisms Leading to Paranoia. Schizophr. Bull. 2015, 41, 391–399. [Google Scholar] [CrossRef]
- Morrison, P.D.; Zois, V.; McKeown, D.A.; Lee, T.D.; Holt, D.W.; Powell, J.F.; Kapur, S.; Murray, R.M. The acute effects of synthetic intravenous Delta(9)-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol. Med. 2009, 39, 1607–1616. [Google Scholar] [CrossRef]
- Buckley, P.F.; Miller, B.J.; Lehrer, D.S.; Castle, D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009, 35, 383–402. [Google Scholar] [CrossRef]
- Ujike, H.; Takaki, M.; Nakata, K.; Tanaka, Y.; Takeda, T.; Kodama, M.; Fujiwara, Y.; Sakai, A.; Kuroda, S. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Mol. Psychiatry 2002, 7, 515–518. [Google Scholar] [CrossRef]
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Massey, B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Papanti, D.; Schifano, F.; Botteon, G.; Bertossi, F.; Mannix, J.; Vidoni, D.; Impagnatiello, M.; Pascolo-Fabrici, E.; Bonavigo, T. “Spiceophrenia”: A systematic overview of “spice”-related psychopathological issues and a case report. Hum. Psychopharmacol. 2013, 28, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Koethe, D.; Giuffrida, A.; Schreiber, D.; Hellmich, M.; Schultze-Lutter, F.; Ruhrmann, S.; Klosterkotter, J.; Piomelli, D.; Leweke, F.M. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br. J. Psychiatry 2009, 194, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Borgan, F.; Laurikainen, H.; Veronese, M.; Marques, T.R.; Haaparanta-Solin, M.; Solin, O.; Dahoun, T.; Rogdaki, M.; Salokangas, R.K.; Karukivi, M.; et al. In Vivo Availability of Cannabinoid 1 Receptor Levels in Patients With First-Episode Psychosis. JAMA Psychiatry 2019, 76, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Sewell, R.A.; Ranganathan, M.; D’Souza, D.C. Cannabinoids and psychosis. Int. Rev. Psychiatry 2009, 21, 152–162. [Google Scholar] [CrossRef]
- Rodriguez-Munoz, M.; Sanchez-Blazquez, P.; Callado, L.F.; Meana, J.J.; Garzon-Nino, J. Schizophrenia and depression, two poles of endocannabinoid system deregulation. Transl. Psychiatry 2017, 7, 1291. [Google Scholar] [CrossRef]
- Rapp, C.; Walter, A.; Studerus, E.; Bugra, H.; Tamagni, C.; Rothlisberger, M.; Borgwardt, S.; Aston, J.; Riecher-Rossler, A. Cannabis use and brain structural alterations of the cingulate cortex in early psychosis. Psychiatry Res. Neuroimaging 2013, 214, 102–108. [Google Scholar] [CrossRef]
- Ho, B.C.; Wassink, T.H.; Ziebell, S.; Andreasen, N.C. Cannabinoid receptor 1 gene polymorphisms and marijuana misuse interactions on white matter and cognitive deficits in schizophrenia. Schizophr. Res. 2011, 128, 66–75. [Google Scholar] [CrossRef][Green Version]
- Kucerova, J.; Tabiova, K.; Drago, F.; Micale, V. Therapeutic potential of cannabinoids in schizophrenia. Recent Pat. CNS Drug Discov. 2014, 9, 13–25. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkotter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018, 235, 1923–1932. [Google Scholar] [CrossRef]
- Hallak, J.E.; Machado-de-Sousa, J.P.; Crippa, J.A.; Sanches, R.F.; Trzesniak, C.; Chaves, C.; Bernardo, S.A.; Regalo, S.C.; Zuardi, A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Braz. J. Psychiatry 2010, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ferretjans, R.; Moreira, F.A.; Teixeira, A.L.; Salgado, J.V. The endocannabinoid system and its role in schizophrenia: A systematic review of the literature. Rev. Bras. Psiquiatr. 2012, 34, S163–S193. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.L.; Carlsson, A.; Nilsson, M. Schizophrenia: From dopamine to glutamate and back. Curr. Med. Chem. 2004, 11, 267–277. [Google Scholar] [CrossRef]
- Clifford, D.B. Tetrahydrocannabinol for Tremor in Multiple-Sclerosis. Ann. Neurol. 1983, 13, 669–671. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Goldman, R.S.; Burdick, K.E.; Malhotra, A.K.; Lencz, T.; Patel, R.C.; Woerner, M.G.; Schooler, N.R.; Kane, J.M.; Robinson, D.G. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia—Is it a practice effect? Arch. Gen. Psychiatry 2007, 64, 1115–1122. [Google Scholar] [CrossRef]
- Borgan, F.; Beck, K.; Butler, E.; McCutcheon, R.; Veronese, M.; Vernon, A.; Howes, O.D. The effects of cannabinoid 1 receptor compounds on memory: A meta-analysis and systematic review across species. Psychopharmacology 2019, 236, 3257–3270. [Google Scholar] [CrossRef]
- Riegel, A.C.; Lupica, C.R. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J. Neurosci. 2004, 24, 11070–11078. [Google Scholar] [CrossRef]
- Tanda, G.; Pontieri, F.E.; DiChiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu 1 opioid receptor mechanism. Science 1997, 276, 2048–2050. [Google Scholar] [CrossRef]
- Voruganti, L.N.P.; Slomka, P.; Zabel, P.; Mattar, A.; Awad, A.G. Cannabis induced dopamine release: An in-vivo SPECT study. Psychiatry Res. Neuroimaging 2001, 107, 173–177. [Google Scholar] [CrossRef]
- Tzavara, E.T.; Li, D.L.; Moutsimilli, L.; Bisogno, T.; Di Marzo, V.; Phebus, L.A.; Nomikos, G.G.; Giros, B. Endocannabinoids activate transient receptor potential vanilloid 1 receptors to reduce hyperdopaminergia-related hyperactivity: Therapeutic implications. Biol. Psychiatry 2006, 59, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Sanchez, A.; Sanchez-Blazquez, P.; Rodriguez-Munoz, M.; Garzon, J. HINT1 protein cooperates with cannabinoid 1 receptor to negatively regulate glutamate NMDA receptor activity. Mol. Brain 2013, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Muller-Vahl, K.R.; Emrich, H.M. Cannabis and schizophrenia: Towards a cannabinoid hypothesis of schizophrenia. Expert Rev. Neurother. 2008, 8, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [CrossRef]
- Boucher, A.A.; Hunt, G.E.; Karl, T.; Micheau, J.; McGregor, I.S.; Arnold, J.C. Heterozygous neuregulin 1 mice display greater baseline and Delta(9)-tetrahydrocannabinol-induced c-Fos expression. Neuroscience 2007, 149, 861–870. [Google Scholar] [CrossRef]
- Boucher, A.A.; Arnold, J.C.; Duffy, L.; Schofield, P.R.; Micheau, J.; Karl, T. Heterozygous neuregulin 1 mice are more sensitive to the behavioural effects of Delta9-tetrahydrocannabinol. Psychopharmacology 2007, 192, 325–336. [Google Scholar] [CrossRef]
- Norton, N.; Williams, H.J.; Owen, M.J. An update on the genetics of schizophrenia. Curr. Opin. Psychiatry 2006, 19, 158–164. [Google Scholar] [CrossRef]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef]
- Harrison, P.J. The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology 2004, 174, 151–162. [Google Scholar] [CrossRef]
- Lewis, D.A.; Moghaddam, B. Cognitive dysfunction in schizophrenia: Convergence of gamma-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006, 63, 1372–1376. [Google Scholar] [CrossRef]
- Tsai, G.; Coyle, J.T. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002, 42, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B.; Marsicano, G.; Maldonado, R.; Hillard, C.J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 2015, 16, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Henquet, C.; Di Forti, M.; Morrison, P.; Kuepper, R.; Murray, R.M. Gene-environment interplay between cannabis and psychosis. Schizophr. Bull. 2008, 34, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Guimaraes, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Hill, M.N.; Patel, S.; Campolongo, P.; Tasker, J.G.; Wotjak, C.T.; Bains, J.S. Functional interactions between stress and the endocannabinoid system: From synaptic signaling to behavioral output. J. Neurosci. 2010, 30, 14980–14986. [Google Scholar] [CrossRef]
- Hill, M.N.; Hillard, C.J.; Bambico, F.R.; Patel, S.; Gorzalka, B.B.; Gobbi, G. The Therapeutic Potential of the Endocannabinoid System for the Development of a Novel Class of Antidepressants. Trends Pharmacol. Sci. 2009, 30, 484–493. [Google Scholar] [CrossRef]
- Alteba, S.; Korem, N.; Akirav, I. Cannabinoids reverse the effects of early stress on neurocognitive performance in adulthood. Learn Mem 2016, 23, 349–358. [Google Scholar] [CrossRef]
- Hyman, S.M.; Sinha, R. Stress-related factors in cannabis use and misuse: Implications for prevention and treatment. J. Subst. Use Addict. Treat. 2009, 36, 400–413. [Google Scholar] [CrossRef]
- van Laar, M.; van Dorsselaer, S.; Monshouwer, K.; de Graaf, R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction 2007, 102, 1251–1260. [Google Scholar] [CrossRef]
- Georgiades, K.; Boyle, M.H. Adolescent tobacco and cannabis use: Young adult outcomes from the Ontario Child Health Study. J. Child Psychol. Psychiatry 2007, 48, 724–731. [Google Scholar] [CrossRef]
- Degenhardt, L.; Coffey, C.; Romaniuk, H.; Swift, W.; Carlin, J.B.; Hall, W.D.; Patton, G.C. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction 2013, 108, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, C.; Spradlin, A.; McLaughlin, R.J. A naturalistic examination of the perceived effects of cannabis on negative affect. J. Affect. Disord. 2018, 235, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bahorik, A.L.; Campbell, C.I.; Sterling, S.A.; Leibowitz, A.; Travis, A.; Weisner, C.M.; Satre, D.D. Adverse impact of marijuana use on clinical outcomes among psychiatry patients with depression and alcohol use disorder. Psychiatry Res. 2018, 259, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar] [CrossRef]
- Deutschenbaur, L.; Beck, J.; Kiyhankhadiv, A.; Muhlhauser, M.; Borgwardt, S.; Walter, M.; Hasler, G.; Sollberger, D.; Lang, U.E. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 325–333. [Google Scholar] [CrossRef]
- Mathews, D.C.; Henter, I.D.; Zarate, C.A. Targeting the Glutamatergic System to Treat Major Depressive Disorder Rationale and Progress to Date. Drugs 2012, 72, 1313–1333. [Google Scholar] [CrossRef]
- Naughton, M.; Clarke, G.; O’Leary, O.F.; Cryan, J.F.; Dinan, T.G. A review of ketamine in affective disorders: Current evidence of clinical efficacy, limitations of use and pre-clinical evidence on proposed mechanisms of action. J. Affect. Disord. 2014, 156, 24–35. [Google Scholar] [CrossRef]
- Hill, M.N.; Gorzalka, B.B. Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav Pharmacol 2005, 16, 333–352. [Google Scholar] [CrossRef]
- Reich, C.G.; Taylor, M.E.; McCarthy, M.M. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav. Brain Res. 2009, 203, 264–269. [Google Scholar] [CrossRef]
- Denson, T.F.; Earleywine, M. Decreased depression in marijuana users. Addict. Behav. 2006, 31, 738–742. [Google Scholar] [CrossRef]
- Prentiss, D.; Power, R.; Balmas, G.; Tzuang, G.; Israelski, D.M. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. Jaids J. Acquir. Immune Defic. Syndr. 2004, 35, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.J.; Pope, H.J.; Brown, M.E. Do patients use marijuana as an antidepressant? Depression 1996, 4, 77–80. [Google Scholar] [CrossRef]
- Blaas, K. Treating depression with cannabinoids. Cannabinoids 2008, 3, 8–10. [Google Scholar]
- Gorzalka, B.B.; Hill, M.N. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1575–1585. [Google Scholar] [CrossRef]
- Botsford, S.L.; Yang, S.; George, T.P. Cannabis and Cannabinoids in Mood and Anxiety Disorders: Impact on Illness Onset and Course, and Assessment of Therapeutic Potential. Am. J. Addict. 2020, 29, 9–26. [Google Scholar] [CrossRef]
- Childs, E.; Lutz, J.A.; de Wit, H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. 2017, 177, 136–144. [Google Scholar] [CrossRef]
- Rubino, T.; Guidali, C.; Vigano, D.; Realini, N.; Valenti, M.; Massi, P.; Parolaro, D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 2008, 54, 151–160. [Google Scholar] [CrossRef]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simoes, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2016, 10, 294. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shohami, E. Endocannabinoids and traumatic brain injury. Mol. Neurobiol. 2007, 36, 68–74. [Google Scholar] [CrossRef]
- Panikashvili, D.; Simeonidou, C.; Ben-Shabat, S.; Hanus, L.; Breuer, A.; Mechoulam, R.; Shohami, E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001, 413, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, A.B.; Siopi, E.; Finn, D.P.; Marchand-Leroux, C.; Garcia-Segura, L.M.; Jafarian-Tehrani, M.; Viveros, M.P. CB1 and CB2 cannabinoid receptor antagonists prevent minocycline-induced neuroprotection following traumatic brain injury in mice. Cereb Cortex 2015, 25, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Georgopoulos, S.; Felekouras, E.; Patsouris, E.; Theocharis, S. The effect of endocannabinoid system in ischemia-reperfusion injury: A friend or a foe? Expert Opin. Ther. Targets 2015, 19, 1261–1275. [Google Scholar] [CrossRef]
- England, T.J.; Hind, W.H.; Rasid, N.A.; O’Sullivan, S.E. Cannabinoids in experimental stroke: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2015, 35, 348–358. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Frison, L.; Halperin, J.L.; Lane, D.A. Identifying Patients at High Risk for Stroke Despite Anticoagulation A Comparison of Contemporary Stroke Risk Stratification Schemes in an Anticoagulated Atrial Fibrillation Cohort. Stroke 2010, 41, 2731–2738. [Google Scholar] [CrossRef]
- Shohami, E.; Bass, R.; Trembovler, V.; Weidenfeld, J. The effect of the adrenocortical axis upon recovery from closed head injury. J. Neurotraum 1995, 12, 1069–1077. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, F.; Wang, M.X.; Wang, X.D.; Liu, P.; Ma, L.Z. Effect of stem cell-based therapy for ischemic stroke treatment: A meta-analysis. Clin. Neurol. Neurosurg. 2016, 146, 1–11. [Google Scholar] [CrossRef]
- Tchantchou, F.; Tucker, L.B.; Fu, A.H.; Bluett, R.J.; McCabe, J.T.; Patel, S.; Zhang, Y. The fatty acid amide hydrolase inhibitor PF-3845 promotes neuronal survival, attenuates inflammation and improves functional recovery in mice with traumatic brain injury. Neuropharmacology 2014, 85, 427–439. [Google Scholar] [CrossRef]
- Hansen, H.H.; Schmid, P.C.; Bittigau, P.; Lastres-Becker, I.; Berrendero, F.; Manzanares, J.; Ikonomidou, C.; Schmid, H.H.; Fernandez-Ruiz, J.J.; Hansen, H.S. Anandamide, but not 2-arachidonoylglycerol, accumulates during in vivo neurodegeneration. J. Neurochem. 2001, 78, 1415–1427. [Google Scholar] [CrossRef]
- Mann, A.; Smoum, R.; Trembovler, V.; Alexandrovich, A.; Breuer, A.; Mechoulam, R.; Shohami, E. Palmitoyl Serine: An Endogenous Neuroprotective Endocannabinoid-Like Entity After Traumatic Brain Injury. J. Neuroimmune Pharmacol. 2015, 10, 356–363. [Google Scholar] [CrossRef]
- Tchantchou, F.; Zhang, Y. Selective inhibition of alpha/beta-hydrolase domain 6 attenuates neurodegeneration, alleviates blood brain barrier breakdown, and improves functional recovery in a mouse model of traumatic brain injury. J. Neurotrauma 2013, 30, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Gaetz, M. The neurophysiology of brain injury. Clin. Neurophysiol. 2004, 115, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Panikashvili, D.; Mechoulam, R.; Beni, S.M.; Alexandrovich, A.; Shohami, E. CB1 cannabinoid receptors are involved in neuroprotection via NF-kappa B inhibition. J. Cereb. Blood Flow Metab. 2005, 25, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Shohami, E.; Novikov, M.; Bass, R. Long-Term Effect of Hu-211, a Novel Noncompetitive Nmda Antagonist, on Motor and Memory Functions after Closed-Head Injury in the Rat. Brain Res. 1995, 674, 55–62. [Google Scholar] [CrossRef]
- Knoller, N.; Levi, L.; Shoshan, I.; Reichenthal, E.; Razon, N.; Rappaport, Z.H.; Biegon, A. Dexanabinol (HU-211) in the treatment of severe closed head injury: A randomized, placebo-controlled, phase II clinical trial. Crit. Care Med. 2002, 30, 548–554. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Murray, G.; Henney, H.; Kassem, N.; Legrand, V.; Mangelus, M.; Muizelaar, J.P.; Stocchetti, N.; Knoller, N. Efficacy and safety of dexanabinol in severe traumatic brain injury: Results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006, 5, 38–45. [Google Scholar] [CrossRef]
- Shearer, J.A.; Coker, S.J.; Carswell, H.V.O. Detrimental effects of 2-arachidonoylglycerol on whole blood platelet aggregation and on cerebral blood flow after a focal ischemic insult in rats. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H967–H977. [Google Scholar] [CrossRef]
- Pires, P.W. Cannabinoids during ischemic strokes: Friends or foes? Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H1155–H1156. [Google Scholar] [CrossRef]
- Choi, S.H.; Mou, Y.S.; Silva, A.C. Cannabis and Cannabinoid Biology in Stroke Controversies, Risks, and Promises. Stroke 2019, 50, 2640–2645. [Google Scholar] [CrossRef]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef]
- Rumalla, K.; Reddy, A.Y.; Mittal, M.K. Recreational marijuana use and acute ischemic stroke: A population-based analysis of hospitalized patients in the United States. J. Neurol. Sci. 2016, 364, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Falkstedt, D.; Danielsson, A.K. Response by Falkstedt et al. to Letters Regarding Article, “ Cannabis, Tobacco, Alcohol Use, and the Risk of Early Stroke: A Population-Based Cohort Study Among 45 000 Swedish Men”. Stroke 2017, 48, E134. [Google Scholar] [CrossRef] [PubMed]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernandez-Tiedra, S.; Lorente, M.; Egia, A.; Vazquez, P.; Blazquez, C.; Torres, S.; Garcia, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Velasco, G.; Carracedo, A.; Blazquez, C.; Lorente, M.; Aguado, T.; Haro, A.; Sanchez, C.; Galve-Roperh, I.; Guzman, M. Cannabinoids and gliomas. Mol. Neurobiol. 2007, 36, 60–67. [Google Scholar] [CrossRef]
- Aguado, T.; Carracedo, A.; Julien, B.; Velasco, G.; Milman, G.; Mechoulam, R.; Alvarez, L.; Guzman, M.; Galve-Roperh, I. Cannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesis. J. Biol. Chem. 2007, 282, 6854–6862. [Google Scholar] [CrossRef]
- Blazquez, C.; Gonzalez-Feria, L.; Alvarez, L.; Haro, A.; Casanova, M.L.; Guzman, M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef]
- Galli, R.; Binda, E.; Orfanelli, U.; Cipelletti, B.; Gritti, A.; De Vitis, S.; Fiocco, R.; Foroni, C.; Dimeco, F.; Vescovi, A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004, 64, 7011–7021. [Google Scholar] [CrossRef]
- Galve-Roperh, I.; Sanchez, C.; Cortes, M.L.; Gomez del Pulgar, T.; Izquierdo, M.; Guzman, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- .Hemmati, H.D.; Nakano, I.; Lazareff, J.A.; Masterman-Smith, M.; Geschwind, D.H.; Bronner-Fraser, M.; Kornblum, H.I. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA 2003, 100, 15178–15183. [Google Scholar] [CrossRef]
- Ignatova, T.N.; Kukekov, V.G.; Laywell, E.D.; Suslov, O.N.; Vrionis, F.D.; Steindler, D.A. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia 2002, 39, 193–206. [Google Scholar] [CrossRef]
- Kondo, T.; Setoguchi, T.; Taga, T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc. Natl. Acad. Sci. USA 2004, 101, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Zhou, J.; Claypool, K.; Tang, D.G. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005, 65, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; de Ceballos, M.L.; Gomez del Pulgar, T.; Rueda, D.; Corbacho, C.; Velasco, G.; Galve-Roperh, I.; Huffman, J.W.; Ramon y Cajal, S.; Guzman, M. Inhibition of glioma growth in vivo by selective activation of the CB(2) cannabinoid receptor. Cancer Res. 2001, 61, 5784–5789. [Google Scholar] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Rueda, D.; Navarro, B.; Martinez-Serrano, A.; Guzman, M.; Galve-Roperh, I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002, 277, 46645–46650. [Google Scholar] [CrossRef]
- Aguado, T.; Monory, K.; Palazuelos, J.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzman, M.; Galve-Roperh, I. The endocannabinoid system drives neural progenitor proliferation. Faseb J. 2005, 19, 1704. [Google Scholar] [CrossRef]
- Blazquez, C.; Casanova, M.L.; Planas, A.; Gomez Del Pulgar, T.; Villanueva, C.; Fernandez-Acenero, M.J.; Aragones, J.; Huffman, J.W.; Jorcano, J.L.; Guzman, M. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003, 17, 529–531. [Google Scholar] [CrossRef]
- Gomez Del Pulgar, T.; De Ceballos, M.L.; Guzman, M.; Velasco, G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2002, 277, 36527–36533. [Google Scholar] [CrossRef]
- Mechoulam, R.; Spatz, M.; Shohami, E. Endocannabinoids and neuroprotection. Sci. STKE 2002, 2002, re5. [Google Scholar] [CrossRef]
- McAllister, S.D.; Chan, C.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J. Neuro-Oncol. 2005, 74, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Calatozzolo, C.; Salmaggi, A.; Pollo, B.; Sciacca, F.L.; Lorenzetti, M.; Franzini, A.; Boiardi, A.; Broggi, G.; Marras, C. Expression of cannabinoid receptors and neurotrophins in human gliomas. Neurol. Sci. 2007, 28, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Lorente, M.; Carracedo, A.; Torres, S.; Natali, F.; Egia, A.; Hernandez-Tiedra, S.; Salazar, M.; Blazquez, C.; Guzman, M.; Velasco, G. Amphiregulin Is a Factor for Resistance of Glioma Cells to Cannabinoid-Induced Apoptosis. Glia 2009, 57, 1374–1385. [Google Scholar] [CrossRef]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef]
- Velasco, G.; Galve-Roperh, I.; Sanchez, C.; Blazquez, C.; Guzman, M. Hypothesis: Cannabinoid therapy for the treatment of gliomas? Neuropharmacology 2004, 47, 315–323. [Google Scholar] [CrossRef]
- Blazquez, C.; Salazar, M.; Carracedo, A.; Lorente, M.; Egia, A.; Gonzalez-Feria, L.; Haro, A.; Velasco, G.; Guzman, M. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008, 68, 1945–1952. [Google Scholar] [CrossRef]
- Carracedo, A.; Lorente, M.; Egia, A.; Blazquez, C.; Garcia, S.; Giroux, V.; Malicet, C.; Villuendas, R.; Gironella, M.; Gonzalez-Feria, L.; et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 2006, 9, 301–312. [Google Scholar] [CrossRef]
- Lorente, M.; Torres, S.; Salazar, M.; Carracedo, A.; Hernandez-Tiedra, S.; Rodriguez-Fornes, F.; Garcia-Taboada, E.; Melendez, B.; Mollejo, M.; Campos-Martin, Y.; et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011, 18, 959–973. [Google Scholar] [CrossRef]
- Holash, J.; Maisonpierre, P.C.; Compton, D.; Boland, P.; Alexander, C.R.; Zagzag, D.; Yancopoulos, G.D.; Wiegand, S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 1999, 284, 1994–1998. [Google Scholar] [CrossRef]
- Zagzag, D.; Amirnovin, R.; Greco, M.A.; Yee, H.; Holash, J.; Wiegand, S.J.; Zabski, S.; Yancopoulos, G.D.; Grumet, M. Vascular apoptosis and involution in gliomas precede neovascularization: A novel concept for glioma growth and angiogenesis. Lab. Investig. 2000, 80, 837–849. [Google Scholar] [CrossRef]
- Vajkoczy, P.; Farhadi, M.; Gaumann, A.; Heidenreich, R.; Erber, R.; Wunder, A.; Tonn, J.C.; Menger, M.D.; Breier, G. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J. Clin. Investig. 2002, 109, 777–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldman, C.K.; Kim, J.; Wong, W.L.; King, V.; Brock, T.; Gillespie, G.Y. Epidermal Growth-Factor Stimulates Vascular Endothelial Growth-Factor Production by Human-Malignant Glioma-Cells—A Model of Glioblastoma-Multiforme Pathophysiology. Mol. Biol. Cell 1993, 4, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Pommery, N.; Wattez, N.; Bailly, C.; Henichart, J.P. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: Implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate 2003, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Galve-Roperh, I.; Sanchez, C. Ceramide: A new second messenger of cannabinoid action. Trends Pharmacol. Sci. 2001, 22, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Herrera, B.; Carracedo, A.; Diez-Zaera, M.; del Pulgar, T.G.; Guzman, M.; Velasco, G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006, 312, 2121–2131. [Google Scholar] [CrossRef]
- Hinz, B.; Ramer, R. Anti-tumour actions of cannabinoids. Br. J. Pharmacol. 2019, 176, 1384–1394. [Google Scholar] [CrossRef]
- Adamson, C.; Kanu, O.O.; Mehta, A.I.; Di, C.; Lin, N.; Mattox, A.K.; Bigner, D.D. Glioblastoma multiforme: A review of where we have been and where we are going. Expert Opin. Investig. Drugs 2009, 18, 1061–1083. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Sandalcioglu, I.E.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef]
- Blazquez, C.; Carracedo, A.; Salazar, M.; Lorente, M.; Egia, A.; Gonzalez-Feria, L.; Haro, A.; Velasco, G.; Guzman, M. Down-regulation of tissue inhibitor of metalloproteinases-1 in gliomas: A new marker of cannabinoid antitumoral activity? Neuropharmacology 2008, 54, 235–243. [Google Scholar] [CrossRef]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Parolaro, D.; Massi, P. Cannabinoids as potential new therapy for the treatment of gliomas. Expert Rev. Neurother. 2008, 8, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Duarte, M.J.; Blazquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sanchez, C.; Velasco, G.; Gonzalez-Feria, L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Ladin, D.A.; Soliman, E.; Griffin, L.; Van Dross, R. Preclinical and Clinical Assessment of Cannabinoids as Anti-Cancer Agents. Front. Pharmacol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Scott, K.A.; Dennis, J.L.; Dalgleish, A.G.; Liu, W.M. Inhibiting Heat Shock Proteins Can Potentiate the Cytotoxic Effect of Cannabidiol in Human Glioma Cells. Anticancer Res. 2015, 35, 5827–5837. [Google Scholar]
- Gilman, J.M.; Schuster, R.M.; Potter, K.W.; Schmitt, W.; Wheeler, G.; Pachas, G.N.; Hickey, S.; Cooke, M.E.; Dechert, A.; Plummer, R.; et al. Effect of Medical Marijuana Card Ownership on Pain, Insomnia, and Affective Disorder Symptoms in Adults: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e222106. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Cell. Mol. Mech. Drugs Abus. Neurotox. Cocaine GHB Substituted Amphetamines 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-Gonzalez, M.; Srivastava, R.K.; Cutando, L.; Ortega-Alvaro, A.; Ruehle, S.; Remmers, F.; Bindila, L.; Bellocchio, L.; Marsicano, G.; et al. Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 9904–9909. [Google Scholar] [CrossRef]
- van Amerongen, G.; Kanhai, K.; Baakman, A.C.; Heuberger, J.; Klaassen, E.; Beumer, T.L.; Strijers, R.L.M.; Killestein, J.; van Gerven, J.; Cohen, A.; et al. Effects on Spasticity and Neuropathic Pain of an Oral Formulation of Delta 9-tetrahydrocannabinol in Patients With Progressive Multiple Sclerosis. Clin. Ther. 2018, 40, 1467–1482. [Google Scholar] [CrossRef]
- Callen, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortes, A.; Mallol, J.; Casado, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid Receptors CB1 and CB2 Form Functional Heteromers in Brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; de Medina, V.S.; Rivas-Santisteban, R.; Callado, C.S.C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1-CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Luquin, N.; Rico, A.J.; Gomez-Bautista, V.; Roda, E.; Dopeso-Reyes, I.G.; Vazquez, A.; Martinez-Pinilla, E.; Labandeira-Garcia, J.L.; Franco, R.; et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: Changes following experimental parkinsonism. Brain Struct. Funct. 2015, 220, 2721–2738. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.L.; Somvanshi, R.K.; Kumar, U. Somatostatin Receptor 5 Is a Prominent Regulator of Signaling Pathways in Cells with Coexpression of Cannabinoid Receptors 1. Neuroscience 2017, 340, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Marsicano, G.; Lutz, B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999, 11, 4213–4225. [Google Scholar] [CrossRef]
- Katona, I.; Freund, T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14, 923–930. [Google Scholar] [CrossRef]
- Azad, S.C.; Monory, K.; Marsicano, G.; Cravatt, B.F.; Lutz, B.; Zieglgansberger, W.; Rammes, G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J. Neurosci. 2004, 24, 9953–9961. [Google Scholar] [CrossRef]
- Monory, K.; Massa, F.; Egertova, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef]
- Colizzi, M.; McGuire, P.; Pertwee, R.G.; Bhattacharyya, S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neurosci. Biobehav. Rev. 2016, 64, 359–381. [Google Scholar] [CrossRef]
- Chang, L.; Cloak, C.; Yakupov, R.; Ernst, T. Combined and Independent Effects of Chronic Marijuana Use and HIV on Brain Metabolites. J. Neuroimmune Pharm. 2006, 1, 65–76. [Google Scholar] [CrossRef]
- Muetzel, R.L.; Marjanska, M.; Collins, P.F.; Becker, M.P.; Valabregue, R.; Auerbach, E.J.; Lim, K.O.; Luciana, M. In vivo (1)H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage. Clin. 2013, 2, 581–589. [Google Scholar] [CrossRef]
- Prescot, A.P.; Locatelli, A.E.; Renshaw, P.F.; Yurgelun-Todd, D.A. Neurochemical alterations in adolescent chronic marijuana smokers: A proton MRS study. NeuroImage 2011, 57, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Prescot, A.P.; Renshaw, P.F.; Yurgelun-Todd, D.A. gamma-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013, 129, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.H.; Carey, P.D.; Stein, D.J.; Ferrett, H.L.; Spottiswoode, B.S.; Renshaw, P.F.; Yurgelun-Todd, D.A. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav. Brain Res. 2013, 246, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Hajos, N.; Freund, T.F. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology 2002, 43, 503–510. [Google Scholar] [CrossRef]
- Katona, I.; Rancz, E.A.; Acsady, L.; Ledent, C.; Mackie, K.; Hajos, N.; Freund, T.F. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 2001, 21, 9506–9518. [Google Scholar] [CrossRef]
- Wager-Miller, J.; Westenbroek, R.; Mackie, K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem. Phys. Lipids 2002, 121, 83–89. [Google Scholar] [CrossRef]
- Akirav, I.; Fattore, L. Cannabinoid CB1 and Dopamine D1 Receptors Partnership in the Modulation of Emotional Neural Processing. Front. Behav. Neurosci. 2011, 5, 67. [Google Scholar]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferre, S.; Casado, V.; Agnati, L.; Cortes, A.; Mallol, J.; Fuxe, K.; Canela, E.I.; et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar]
- Carriba, P.; Ortiz, O.; Patkar, K.; Justinova, Z.; Stroik, J.; Themann, A.; Muller, C.; Woods, A.S.; Hope, B.T.; Ciruela, F.; et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology 2007, 32, 2249–2259. [Google Scholar]
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Kanaide, M.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharmacol. Sci. 2008, 108, 308–319. [Google Scholar]
- Navarro, G.; Carriba, P.; Gandia, J.; Ciruela, F.; Casado, V.; Cortes, A.; Mallol, J.; Canela, E.I.; Lluis, C.; Franco, R. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Sci. World J. 2008, 8, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, R.; Gupta, A.; Gagnidze, K.; Lim, M.P.; Gomes, I.; Lee-Ramos, D.; Nieto, N.; Devi, L.A. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011, 30, 2350–2363. [Google Scholar] [CrossRef] [PubMed]
- Tebano, M.T.; Martire, A.; Chiodi, V.; Pepponi, R.; Ferrante, A.; Domenici, M.R.; Frank, C.; Chen, J.F.; Ledent, C.; Popoli, P. Adenosine A2A receptors enable the synaptic effects of cannabinoid CB1 receptors in the rodent striatum. J. Neurochem. 2009, 110, 1921–1930. [Google Scholar] [CrossRef]
- Ward, R.J.; Pediani, J.D.; Milligan, G. Heteromultimerization of Cannabinoid CB1 Receptor and Orexin OX1 Receptor Generates a Unique Complex in Which Both Protomers Are Regulated by Orexin A. J. Biol. Chem. 2011, 286, 37414–37428. [Google Scholar] [CrossRef]
- Schoffelmeer, A.N.M.; Hogenboom, F.; Wardeh, G.; De Vries, T.J. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 2006, 51, 773–781. [Google Scholar] [CrossRef]
- Rios, C.; Gomes, I.; Devi, L.A. mu opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006, 148, 387–395. [Google Scholar] [CrossRef]
- Milligan, G. G-protein-coupled receptor heterodimers: Pharmacology, function and relevance to drug discovery. Drug Discov. Today 2006, 11, 541–549. [Google Scholar] [CrossRef]
- Milligan, G. G-protein-coupled receptor dimerisation: Molecular basis and relevance to function. Biochim. Biophys. Acta 2007, 1768, 825–835. [Google Scholar] [CrossRef]
- Fuxe, K.; Jacobsen, K.X.; Hoistad, M.; Tinner, B.; Jansson, A.; Staines, W.A.; Agnati, L.F. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: Relationship to the dopamine innervation. Neuroscience 2003, 119, 733–746. [Google Scholar] [CrossRef]
- Kearn, C.S.; Blake-Palmer, K.; Daniel, E.; Mackie, K.; Glass, M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005, 67, 1697–1704. [Google Scholar] [CrossRef]
- Bagher, A.M.; Laprairie, R.B.; Kelly, M.E.; Denovan-Wright, E.M. Antagonism of Dopamine Receptor 2 Long Affects Cannabinoid Receptor 1 Signaling in a Cell Culture Model of Striatal Medium Spiny Projection Neurons. Mol. Pharmacol. 2016, 89, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Jarrahian, A.; Watts, V.J.; Barker, E.L. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2004, 308, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Marcellino, D.; Carriba, P.; Filip, M.; Borgkvist, A.; Frankowska, M.; Bellido, I.; Tanganelli, S.; Muller, C.E.; Fisone, G.; Lluis, C.; et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology 2008, 54, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Hudson, B.D.; Hebert, T.E.; Kelly, M.E. Physical and functional interaction between CB1 cannabinoid receptors and beta2-adrenoceptors. Br. J. Pharmacol. 2010, 160, 627–642. [Google Scholar] [CrossRef]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.H.; Ganju, R.K. Crosstalk between Chemokine Receptor CXCR4 and Cannabinoid Receptor CB2 in Modulating Breast Cancer Growth and Invasion. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef]
- Cinar, R.; Freund, T.F.; Katona, I.; Mackie, K.; Szucs, M. Reciprocal inhibition of G-protein signaling is induced by CB(1) cannabinoid and GABA(B) receptor interactions in rat hippocampal membranes. Neurochem. Int. 2008, 52, 1402–1409. [Google Scholar] [CrossRef]
- Balenga, N.A.; Martinez-Pinilla, E.; Kargl, J.; Schroder, R.; Peinhaupt, M.; Platzer, W.; Balint, Z.; Zamarbide, M.; Dopeso-Reyes, I.G.; Ricobaraza, A.; et al. Heteromerization of GPR55 and cannabinoid CB2 receptors modulates signalling. Br. J. Pharmacol. 2014, 171, 5387–5406. [Google Scholar] [CrossRef]
- Musella, A.; Fresegna, D.; Rizzo, F.R.; Gentile, A.; Bullitta, S.; De Vito, F.; Guadalupi, L.; Centonze, D.; Mandolesi, G. A novel crosstalk within the endocannabinoid system controls GABA transmission in the striatum. Sci. Rep. 2017, 7, 7363. [Google Scholar] [CrossRef]
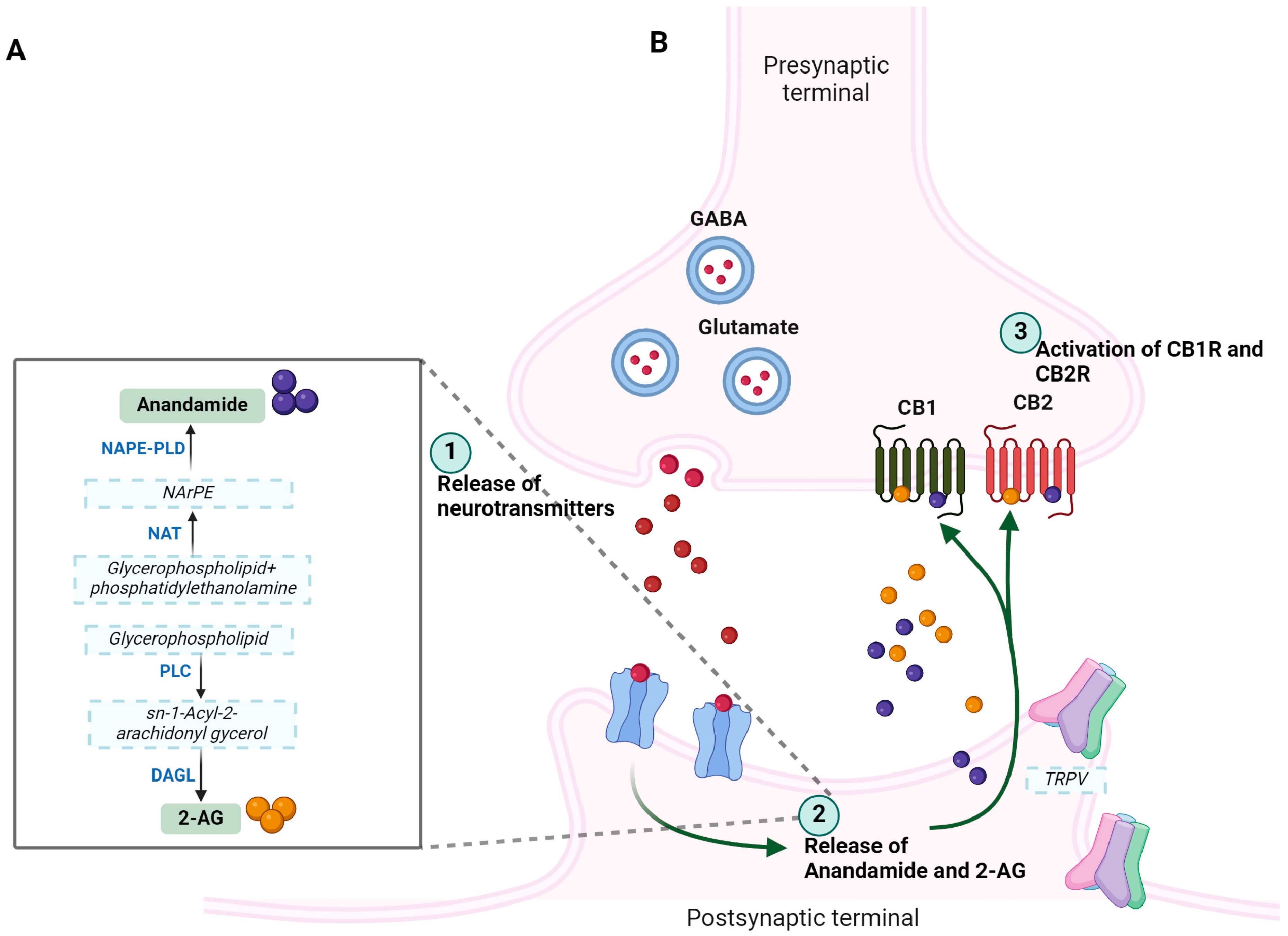
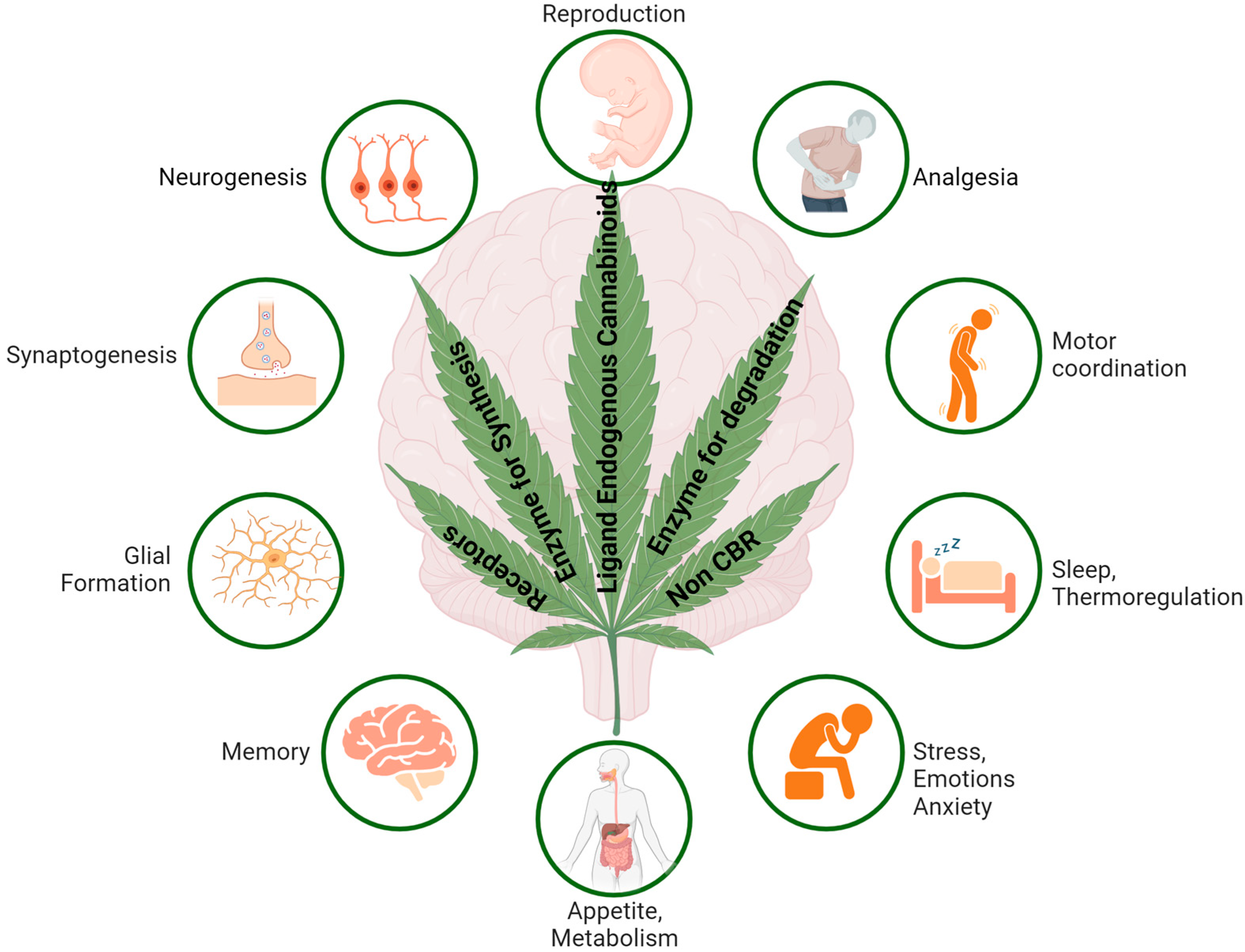
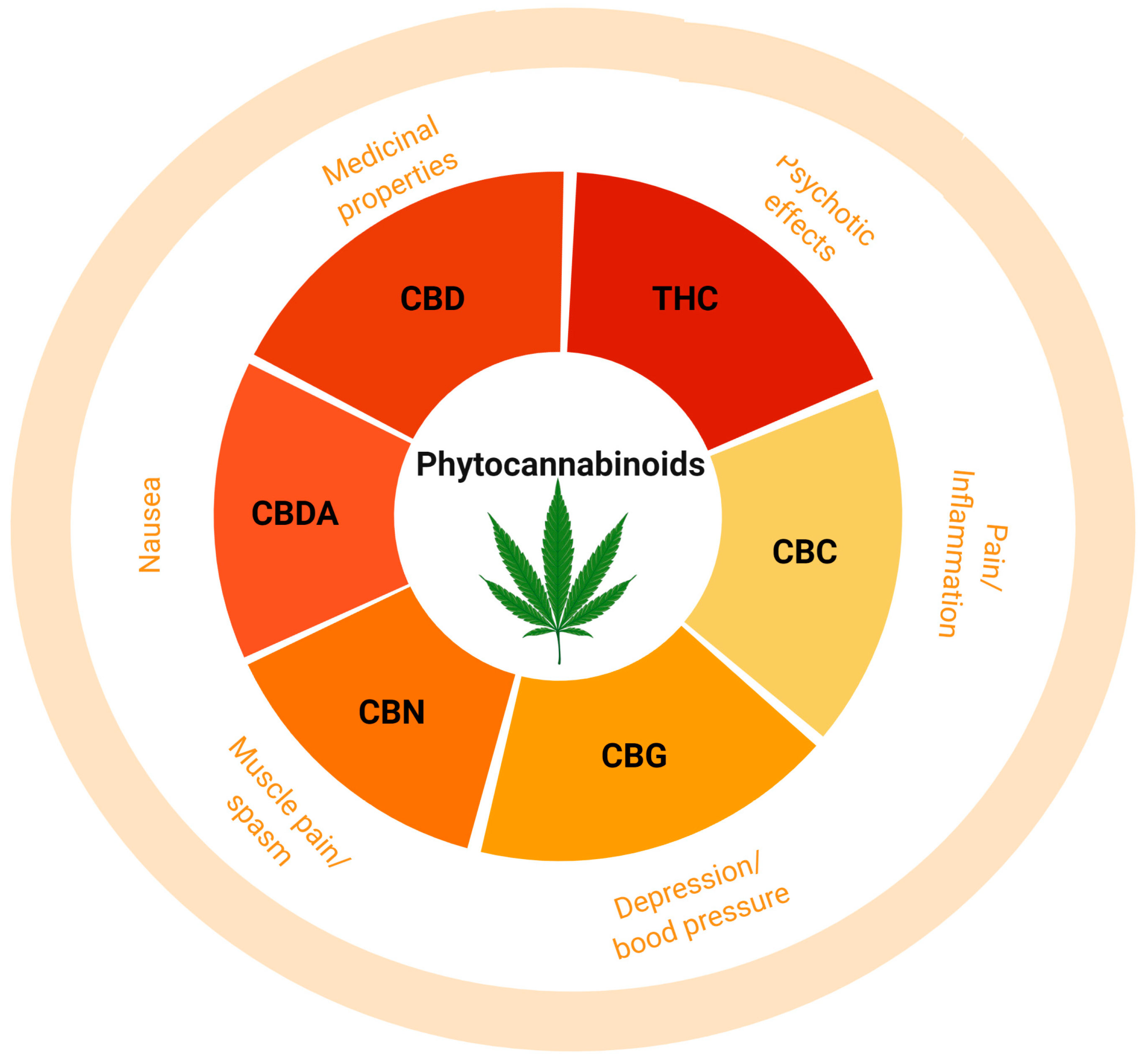
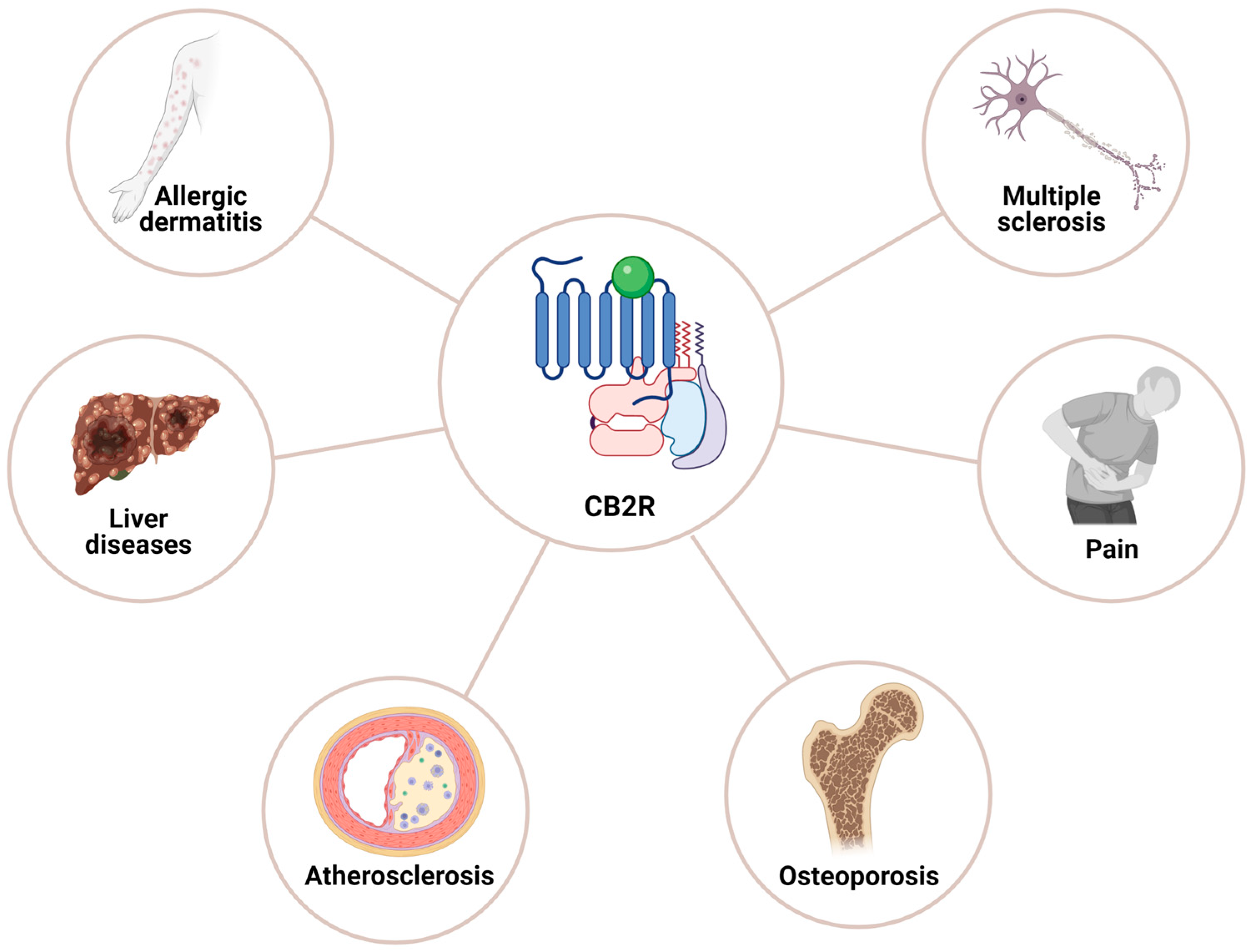

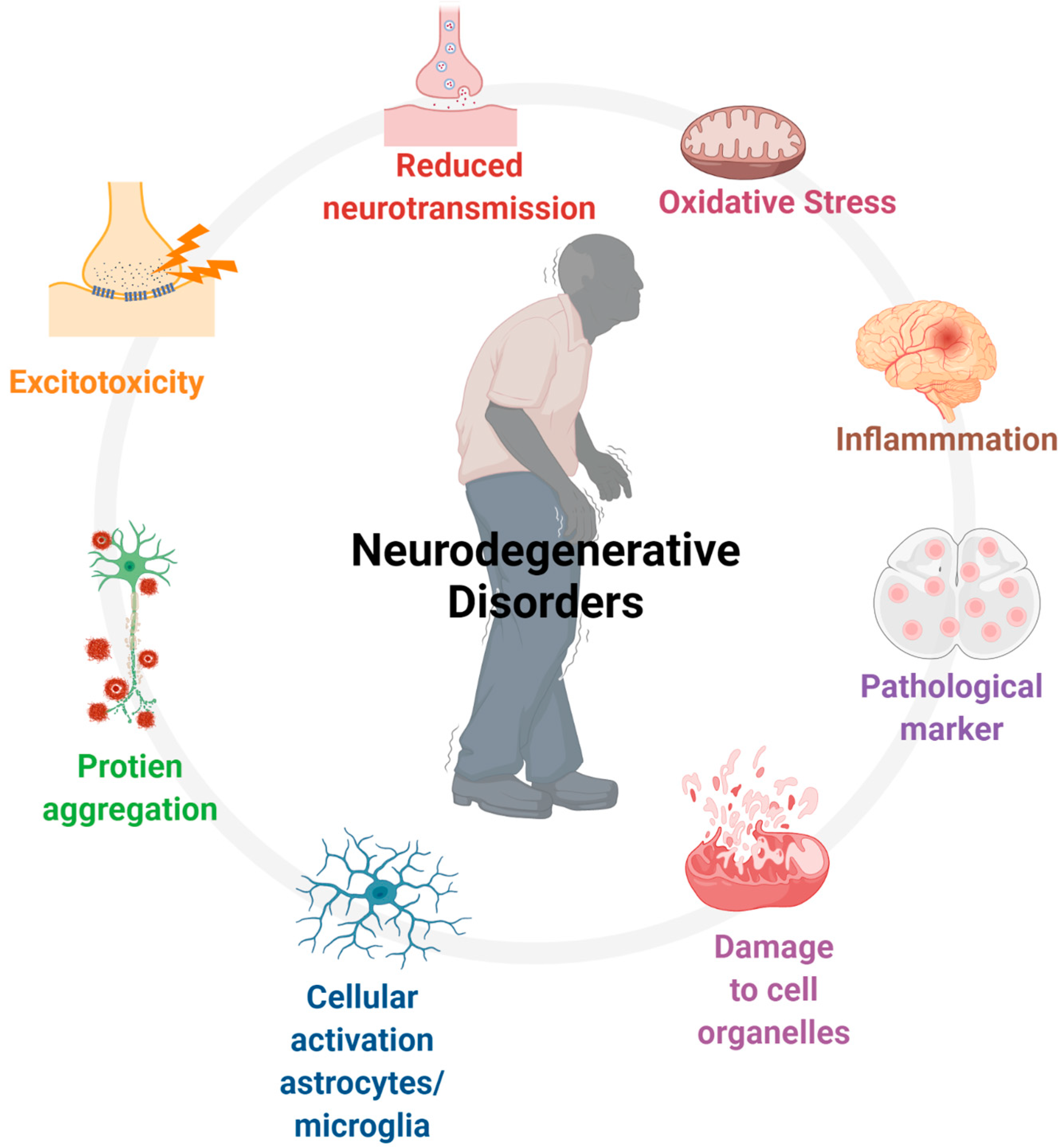
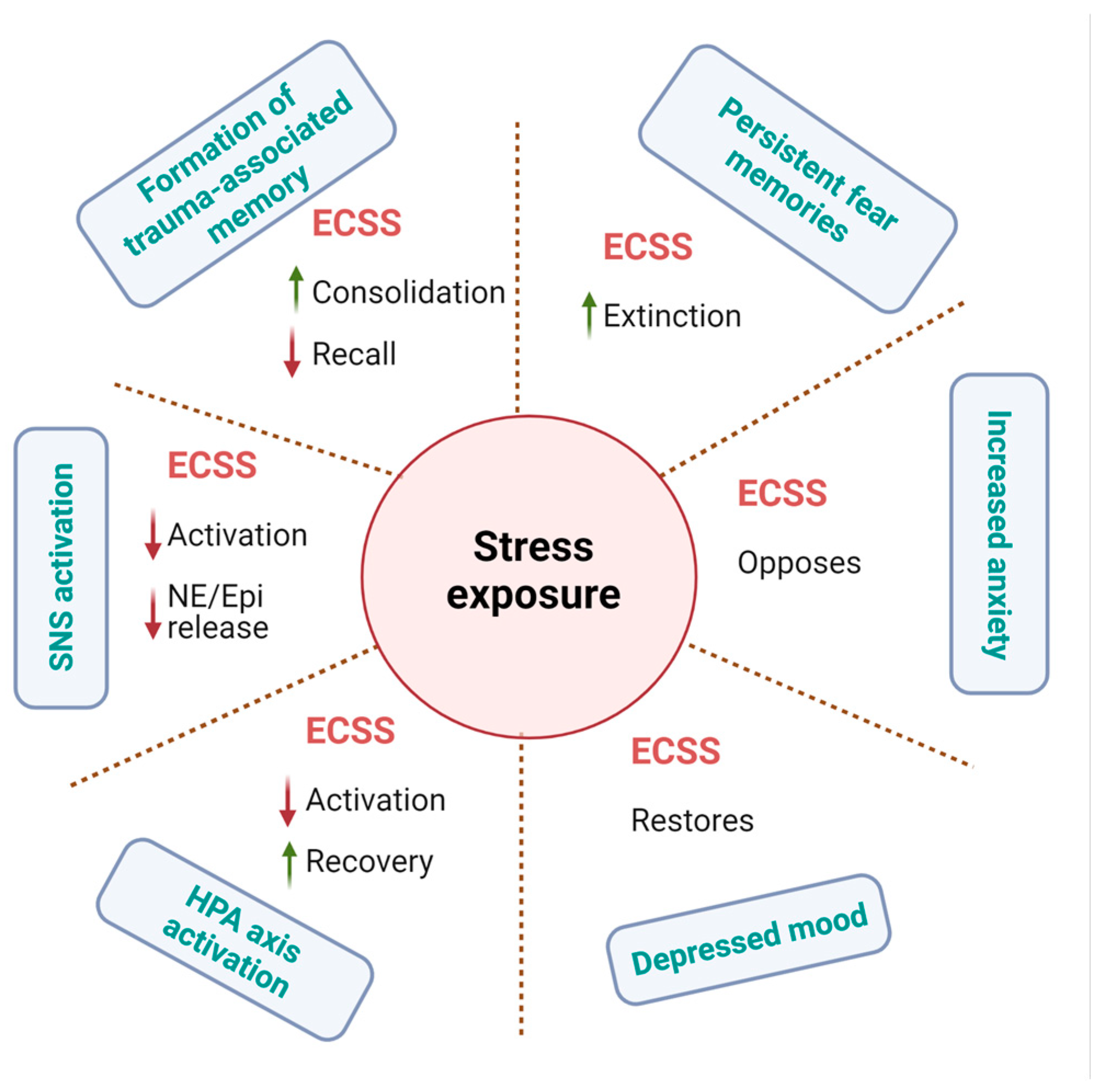
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, U. Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders. Int. J. Mol. Sci. 2025, 26, 152. https://doi.org/10.3390/ijms26010152
Kumar U. Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders. International Journal of Molecular Sciences. 2025; 26(1):152. https://doi.org/10.3390/ijms26010152
Chicago/Turabian StyleKumar, Ujendra. 2025. "Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders" International Journal of Molecular Sciences 26, no. 1: 152. https://doi.org/10.3390/ijms26010152
APA StyleKumar, U. (2025). Cannabinoids: Role in Neurological Diseases and Psychiatric Disorders. International Journal of Molecular Sciences, 26(1), 152. https://doi.org/10.3390/ijms26010152




