Cyclic Imines and Their Salts as Universal Precursors in the Synthesis of Nitrogen-Containing Alkaloids
Abstract
1. Introduction
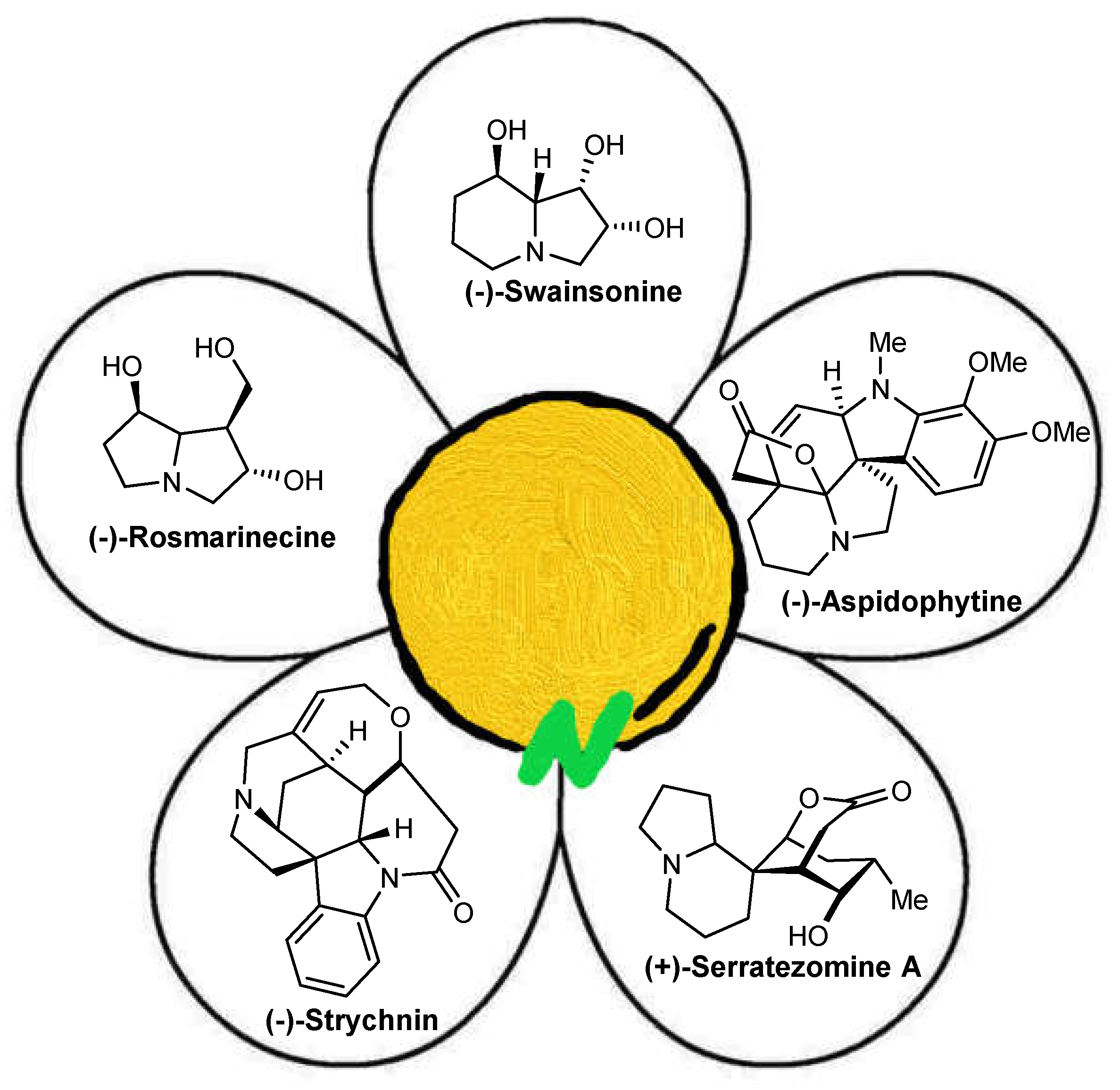
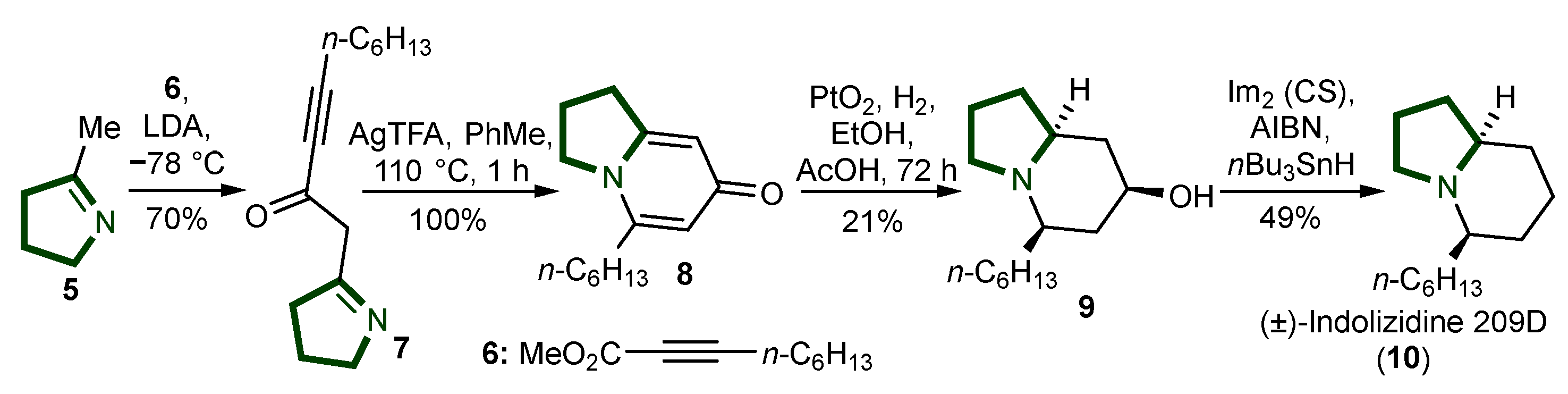
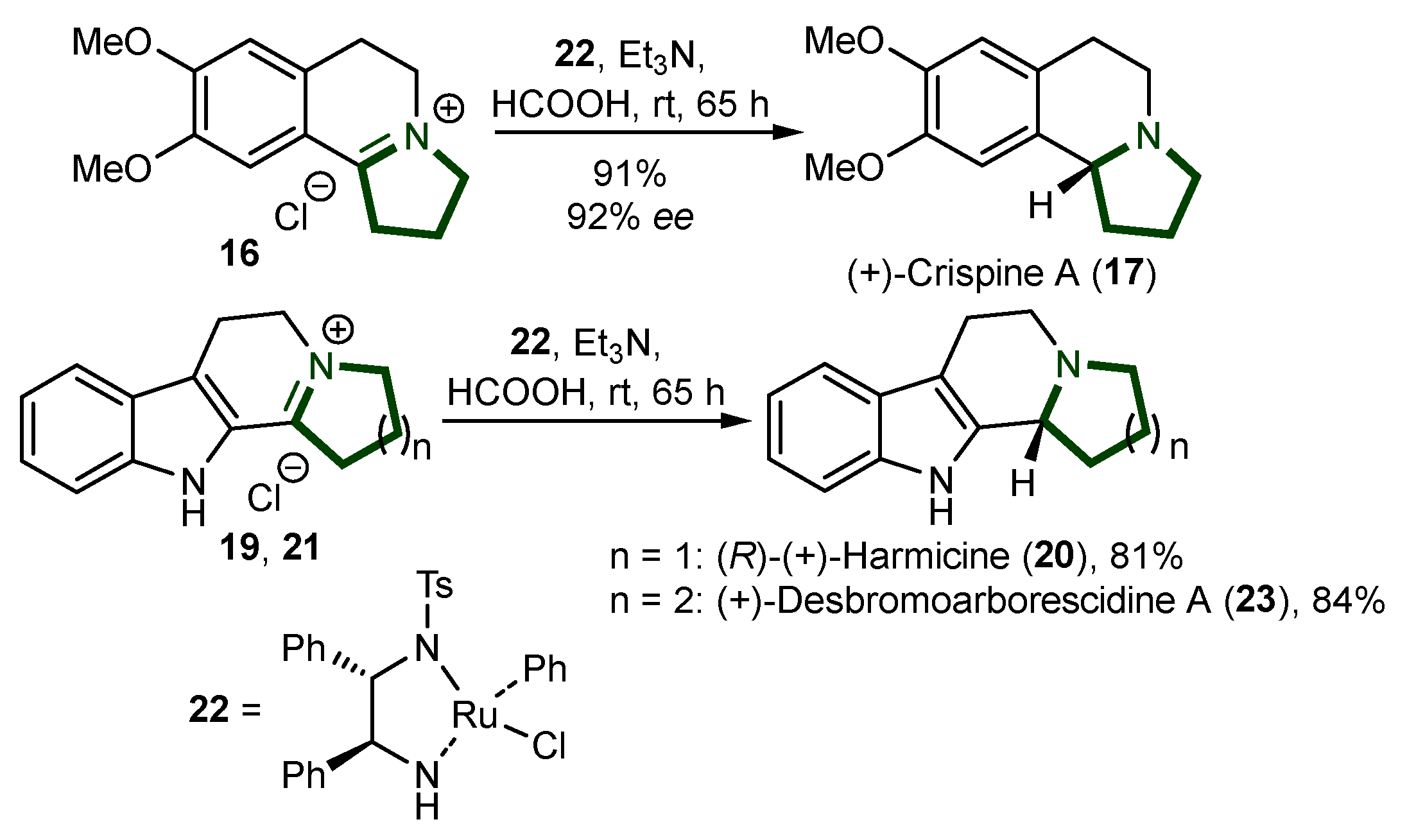
2. Synthesis of Indolizidine Alkaloids
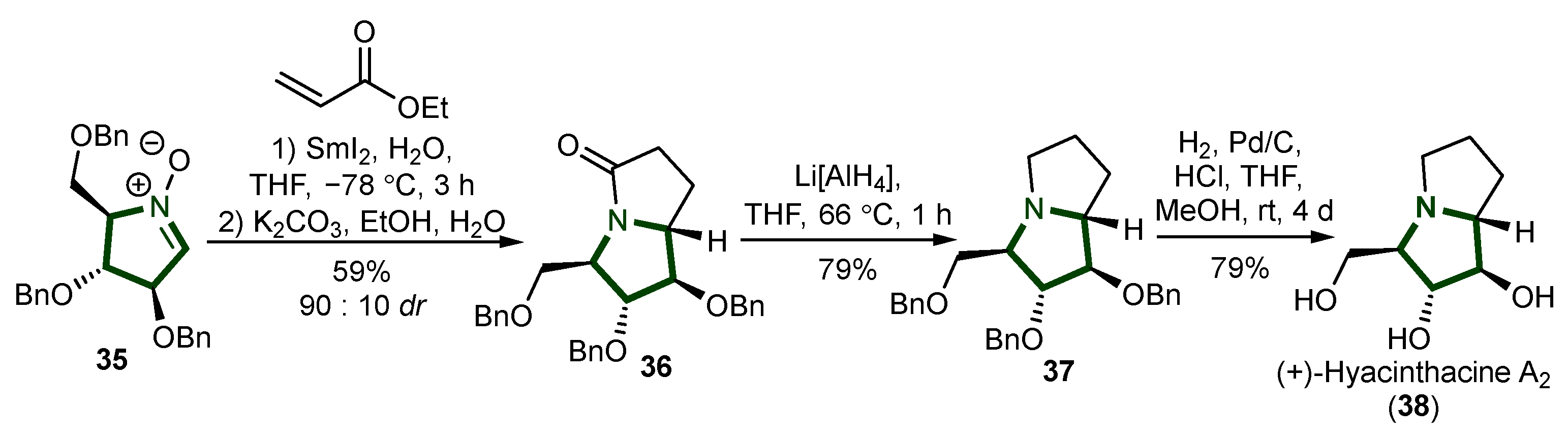
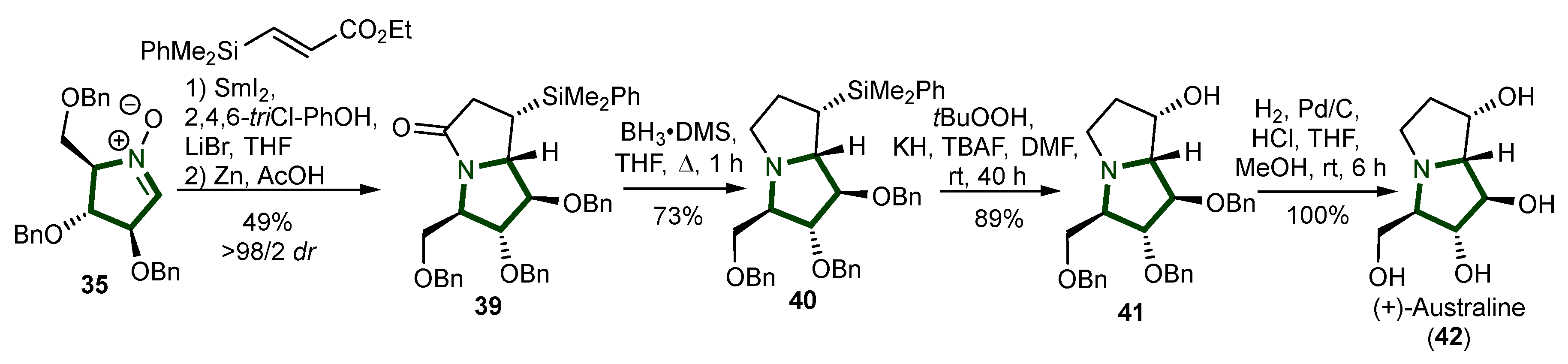
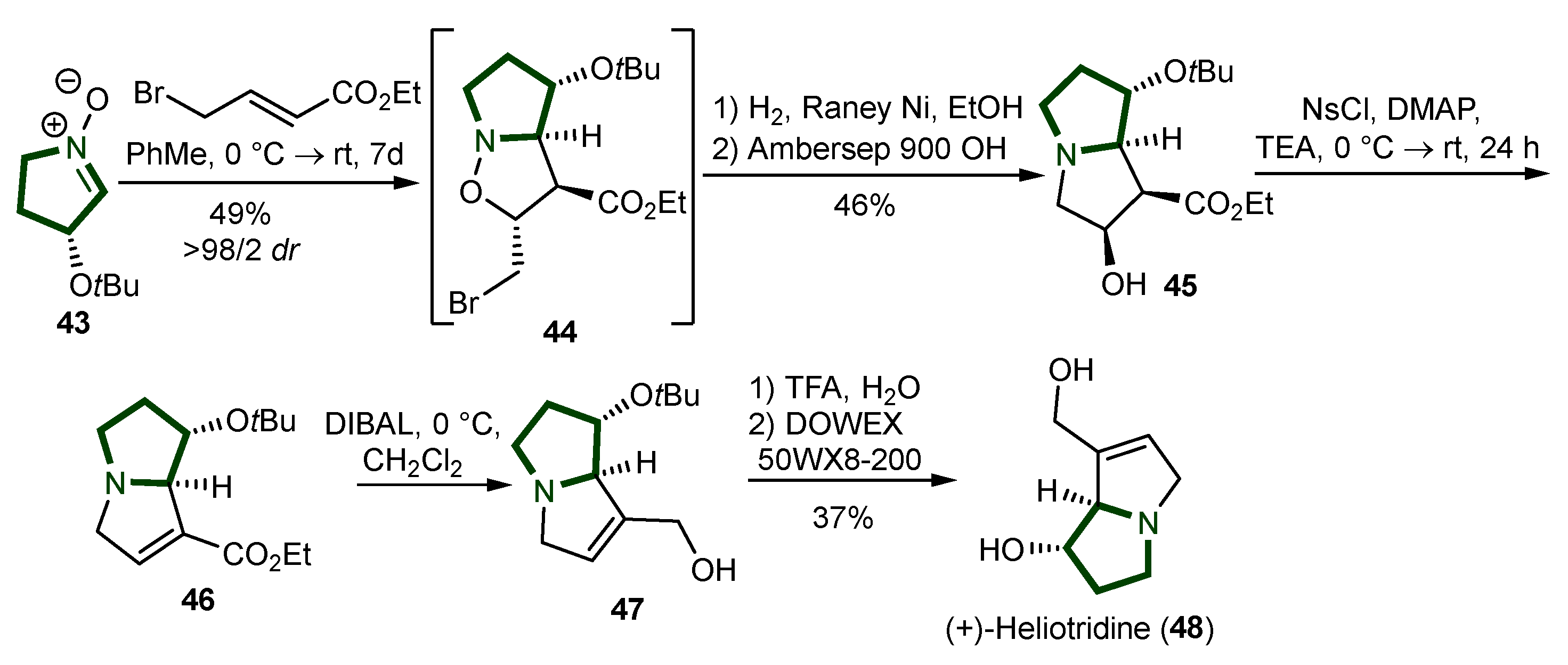
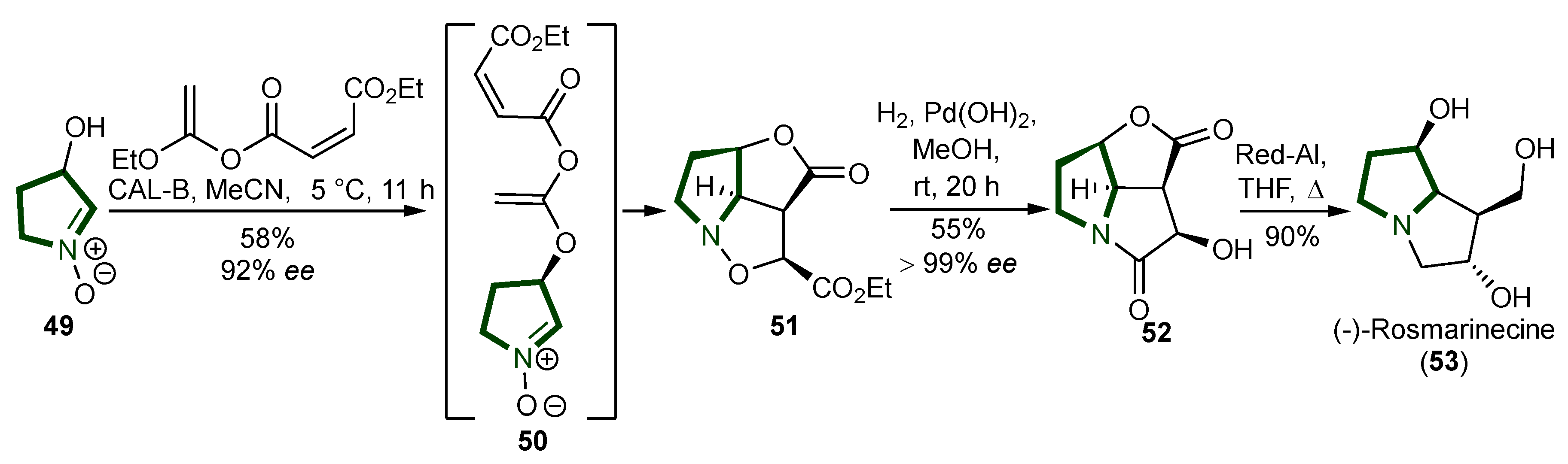
3. Synthesis of Pyrrolizidine Alkaloids
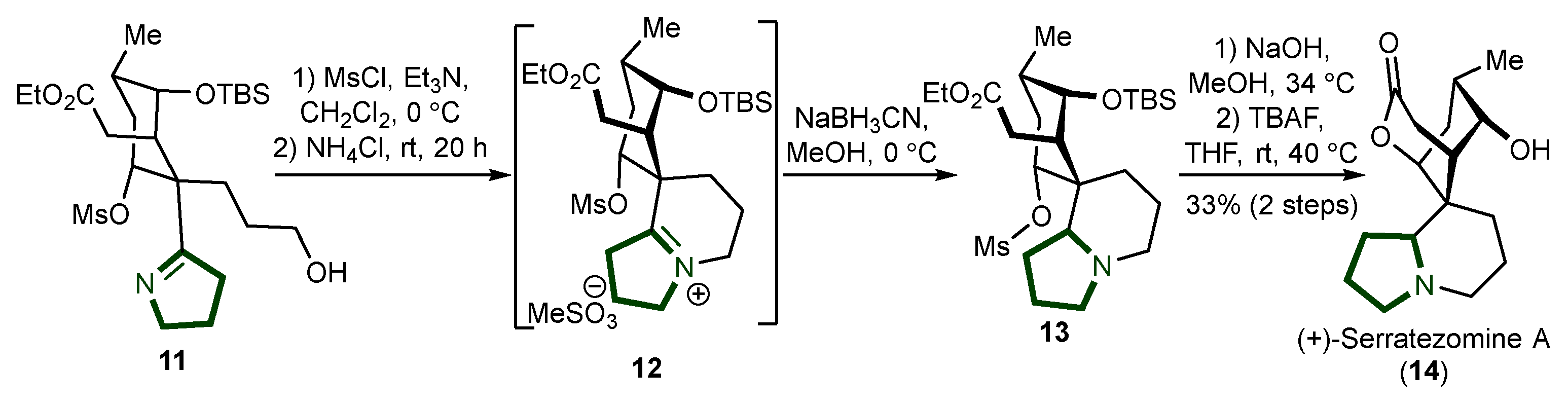
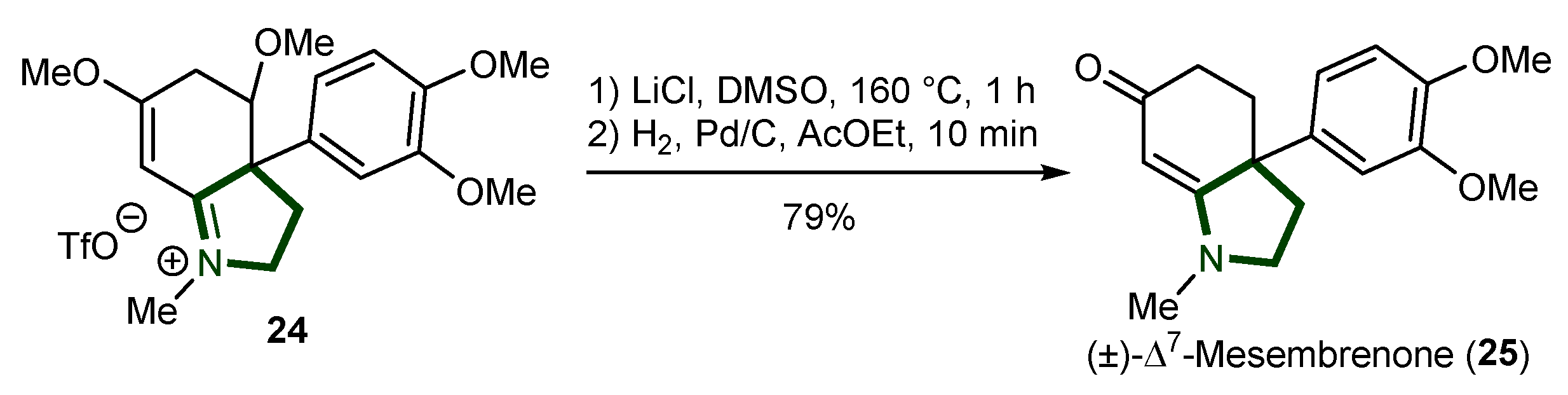
4. Synthesis Quinolizidine Alkaloids
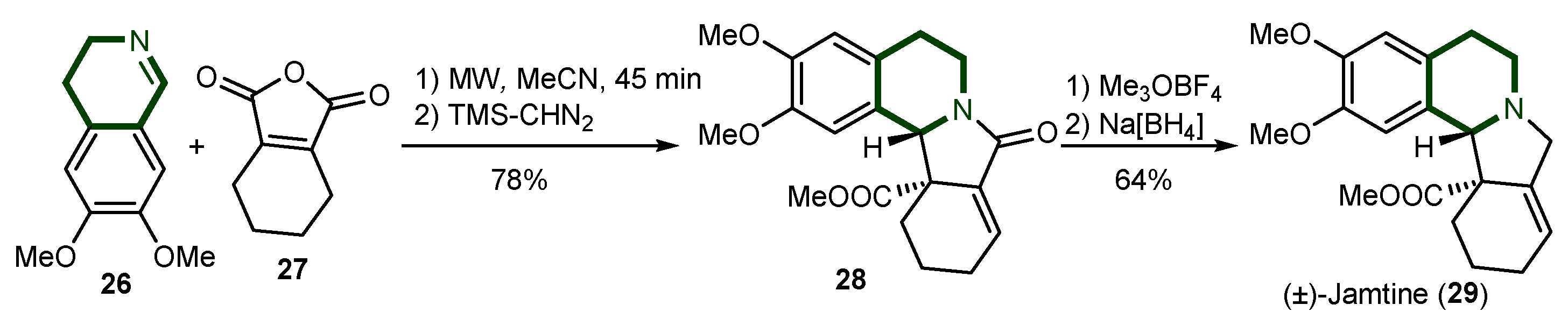
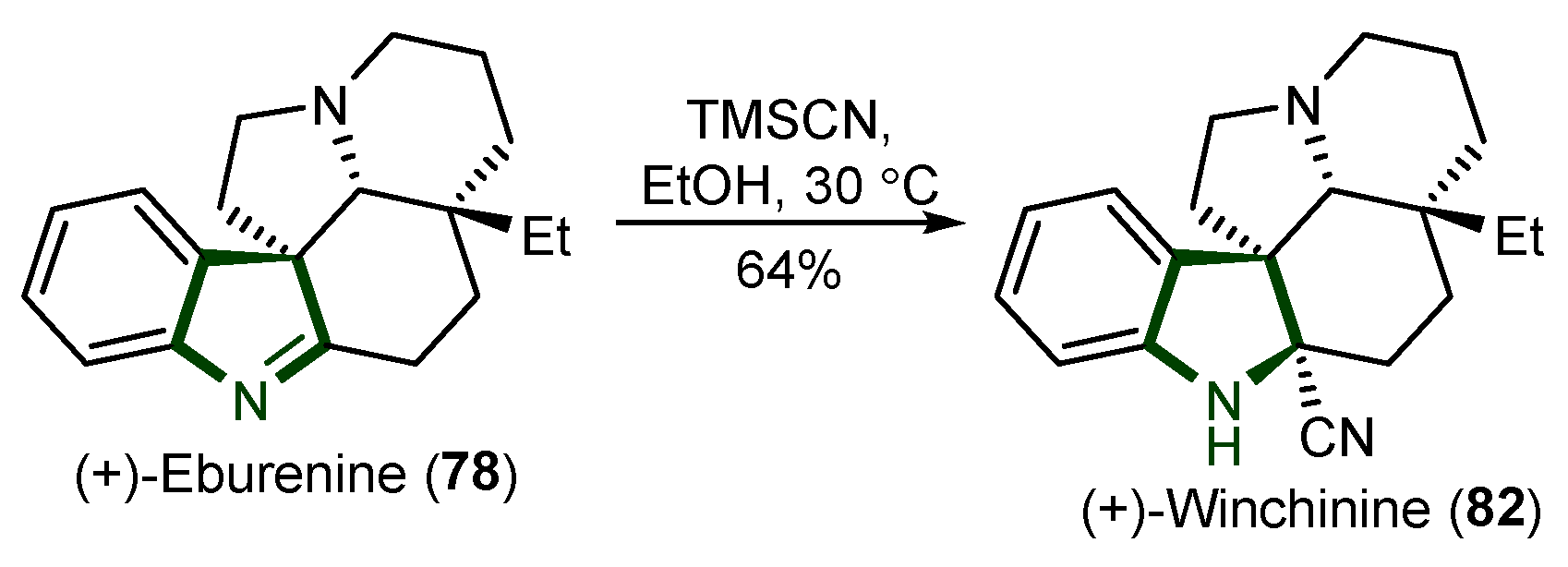
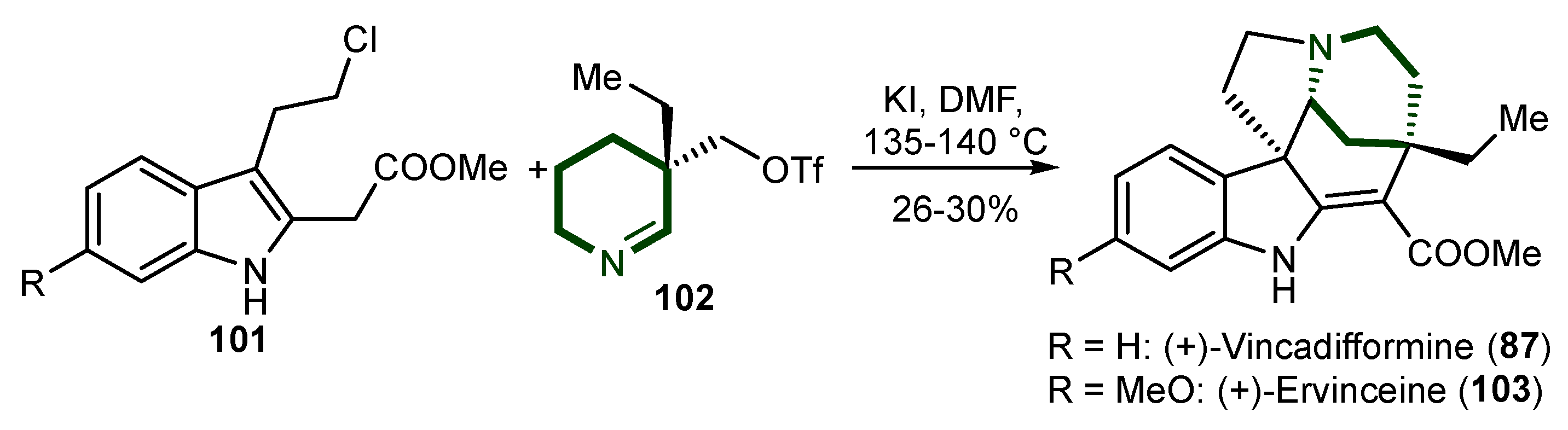
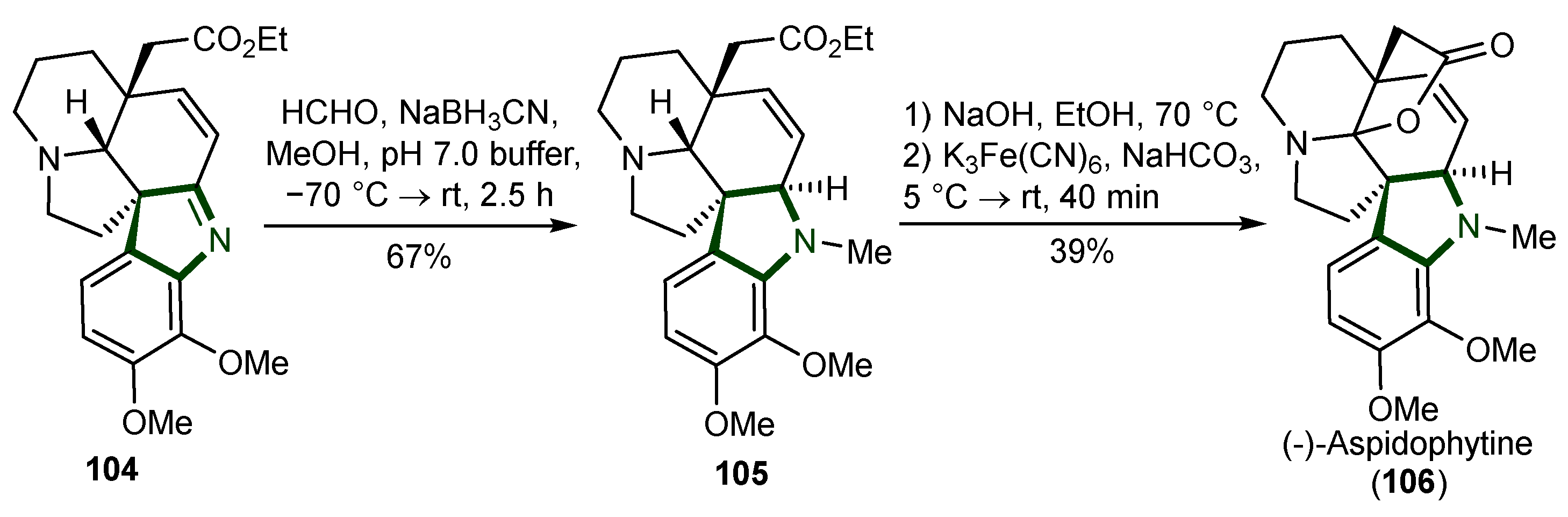
5. Synthesis of Aspidosperma Alkaloids
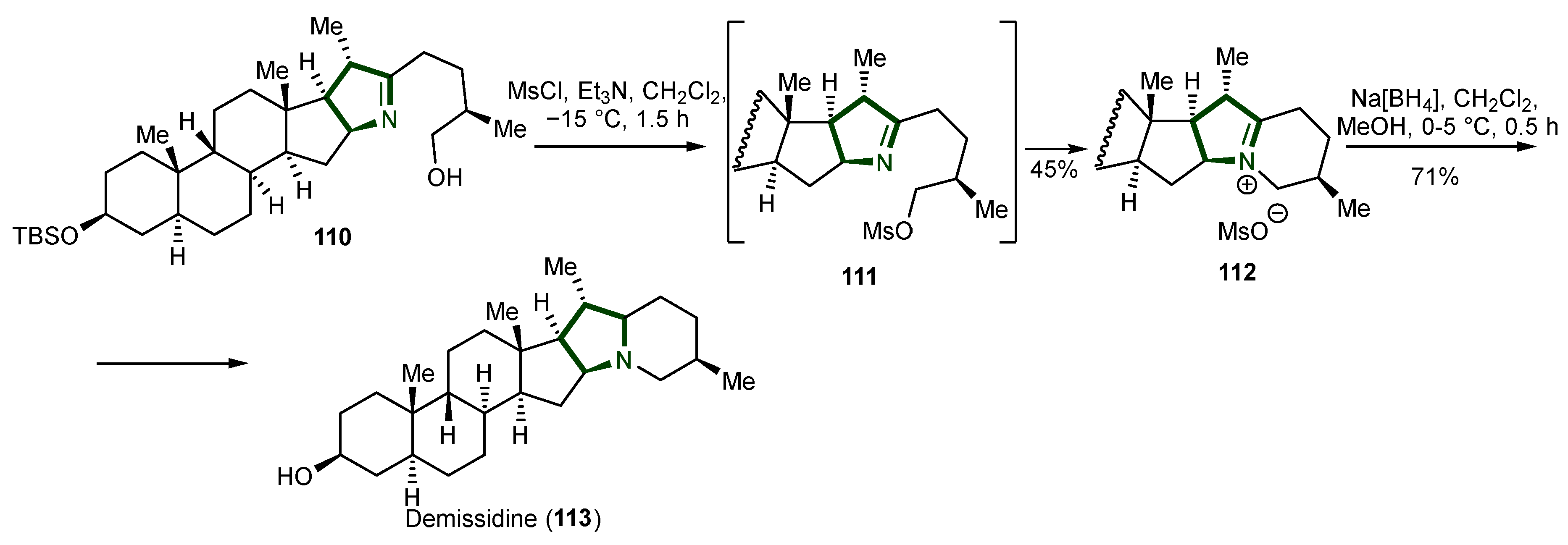
6. Synthesis of Steroidal Alkaloids
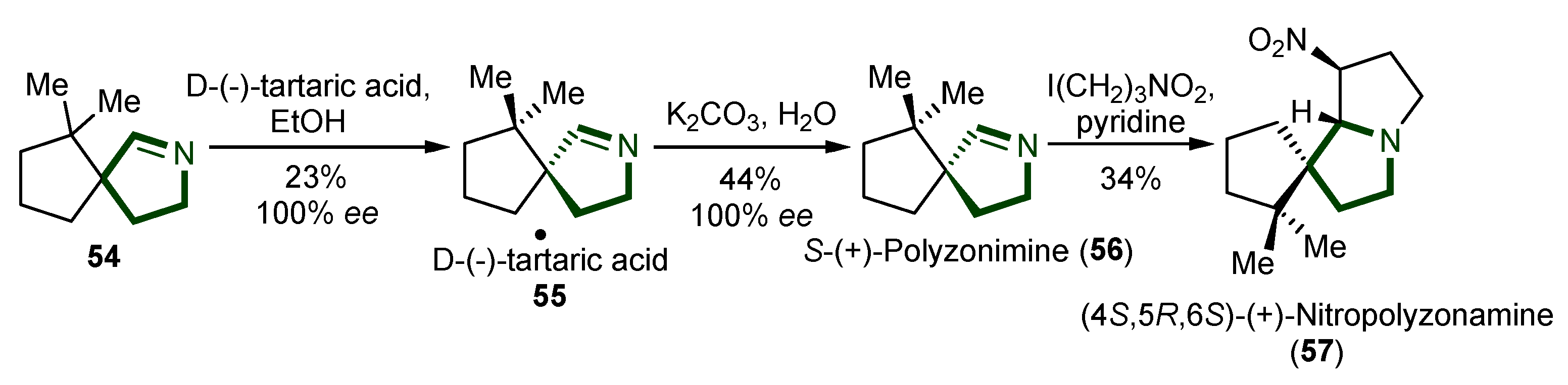
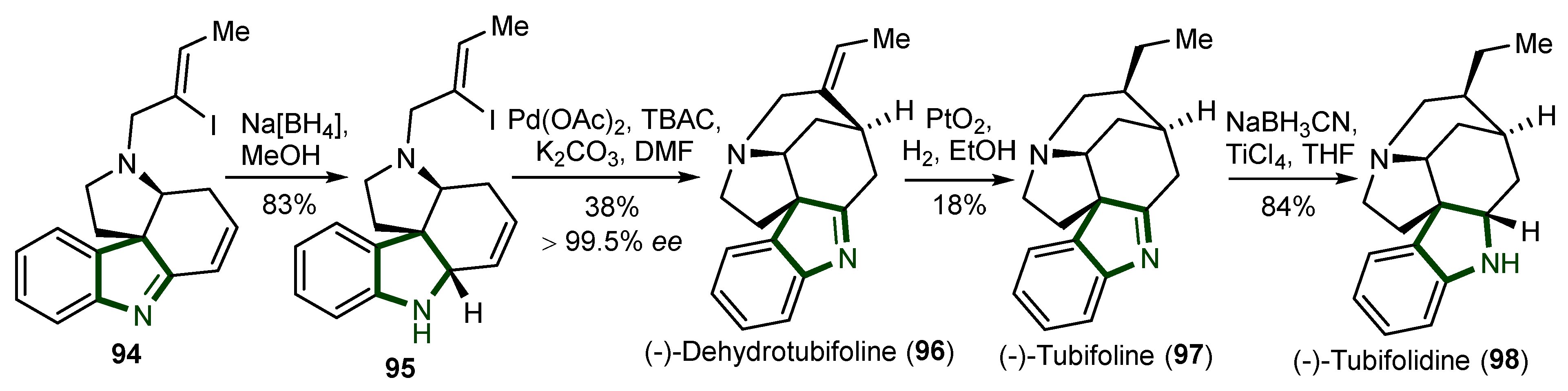
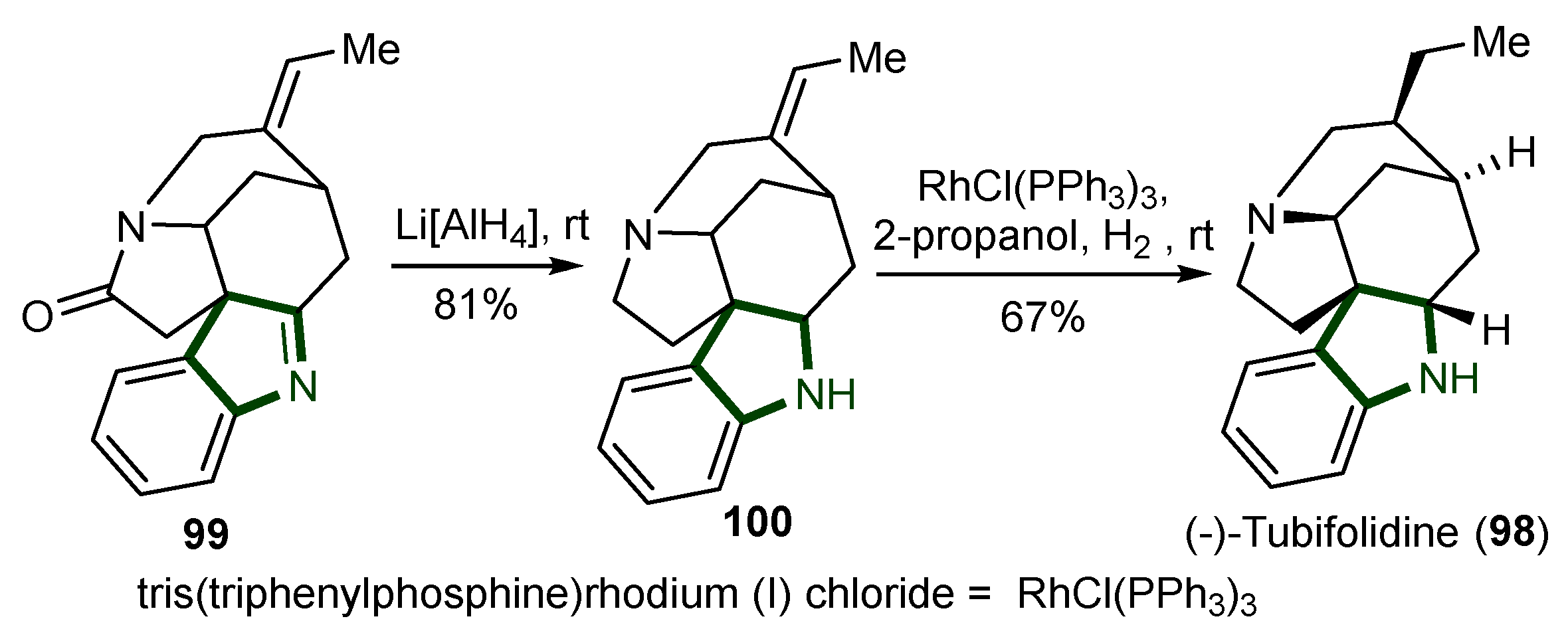
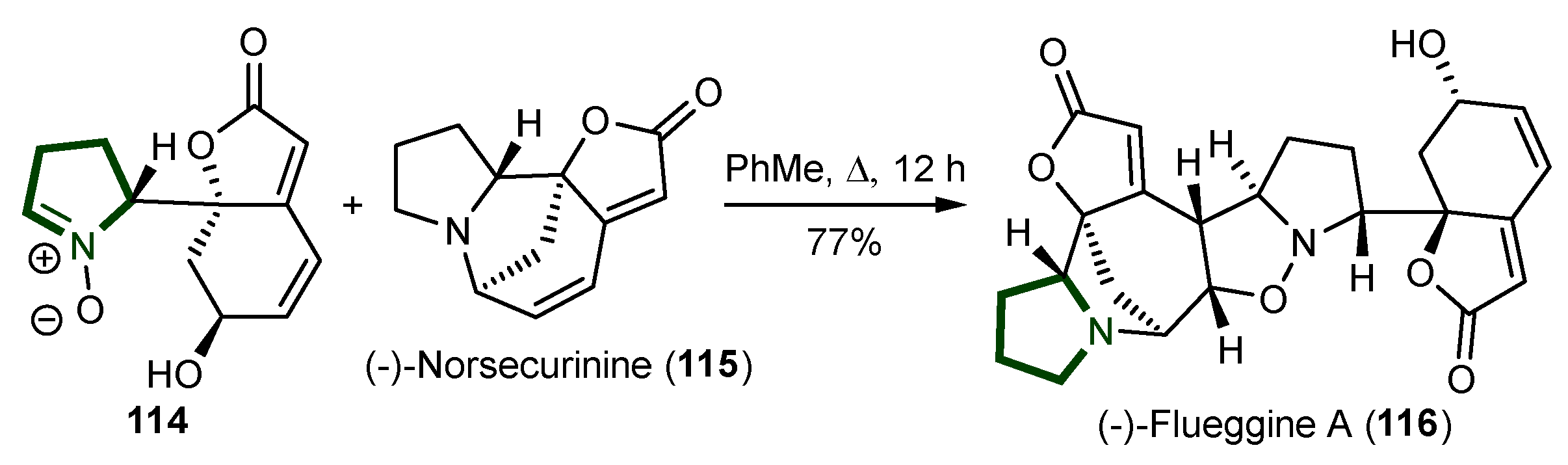
7. Synthesis of Other Alkaloids
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wink, M. A Short History of Alkaloids. In Alkaloids; Springer: Boston, MA, USA, 1998; pp. 11–44. [Google Scholar]
- Ding, Y.-Y.; Zhou, H.; Deng, P.; Zhang, B.-Q.; Zhang, Z.-J.; Wang, G.-H.; Zhang, S.-Y.; Wu, Z.-R.; Wang, Y.-R.; Liu, Y.-Q. Antimicrobial Activity of Natural and Semi-Synthetic Carbazole Alkaloids. Eur. J. Med. Chem. 2023, 259, 115627. [Google Scholar] [CrossRef]
- Uzor, P.F. Alkaloids from Plants with Antimalarial Activity: A Review of Recent Studies. Evid.-Based Complement. Altern. Med. 2020, 2020, 8749083. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.X.; Curtis, M.A.; Sperry, J. Pyridine Alkaloids with Activity in the Central Nervous System. Bioorganic Med. Chem. 2020, 28, 115820. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological Activities and Toxicities of Alkaloids on Human Health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Silva, G.L.; Lee, I.-S.; Kinghorn, A.D. Special Problems with the Extraction of Plants. In Natural Products Isolation; Humana: Totowa, NJ, USA, 1998; pp. 343–363. [Google Scholar]
- Dash, A.K. The Dark Side of Paclitaxel. Oncol. Rev. 2010, 4, 71–72. [Google Scholar] [CrossRef]
- Varga, S.; Angyal, P.; Martin, G.; Egyed, O.; Holczbauer, T.; Soós, T. Total Syntheses of (−)-Minovincine and (−)-Aspidofractinine through a Sequence of Cascade Reactions. Angew. Chem. Int. Ed. 2020, 59, 13547–13551. [Google Scholar] [CrossRef]
- Kataja, A.O.; Masson, G. Imine and Iminium Precursors as Versatile Intermediates in Enantioselective Organocatalysis. Tetrahedron 2014, 70, 8783–8815. [Google Scholar] [CrossRef]
- Cheng, D.; Shao, Y. Organocatalytic Asymmetric Transformations Involving the Cyclic Imine Moiety in Indole and Isoindole Related Heterocycles. Adv. Synth. Catal. 2018, 360, 3614–3642. [Google Scholar] [CrossRef]
- Iwanejko, J.; Wojaczyńska, E. Cyclic Imines—Preparation and Application in Synthesis. Org. Biomol. Chem. 2018, 16, 7296–7314. [Google Scholar] [CrossRef]
- Kuang, Z.; Ding, X.-B.; Furkert, D.P.; Brimble, M.A. Synthesis and α-Functionalisation of Cyclic Imines. Synlett 2024, 35, 1813–1816. [Google Scholar] [CrossRef]
- Xu, Z.; Kovács, E. Beyond Traditional Synthesis: Electrochemical Approaches to Amine Oxidation for Nitriles and Imines. ACS Org. Inorg. Au 2024, 4, 471–484. [Google Scholar] [CrossRef]
- Zhang, J.; Morris-Natschke, S.L.; Ma, D.; Shang, X.; Yang, C.; Liu, Y.; Lee, K. Biologically Active Indolizidine Alkaloids. Med. Res. Rev. 2021, 41, 928–960. [Google Scholar] [CrossRef]
- Chakraborty, I.; Jana, S. Synthetic Developments on the Indolizidine Alkaloid, Harmicine. Synthesis 2013, 45, 3325–3331. [Google Scholar] [CrossRef]
- Dorling, P.R.; Huxtable, C.R.; Colegate, S.M. Inhibition of Lysosomal α-Mannosidase by Swainsonine, an Indolizidine Alkaloid Isolated from Swainsona Canescens. Biochem. J. 1980, 191, 649–651. [Google Scholar] [CrossRef] [PubMed]
- El Nemr, A. Synthetic Methods for the Stereoisomers of Swainsonine and Its Analogues. Tetrahedron 2000, 56, 8579–8629. [Google Scholar] [CrossRef]
- Tan, X.; Chen, A.J.; Wu, B.; Zhang, G.-S.; Ding, G. Advance of Swainsonine Biosynthesis. Chin. Chem. Lett. 2018, 29, 417–422. [Google Scholar] [CrossRef]
- Pyne, S. Recent Developments on the Synthesis of (-)-Swainsonine and Analogues. Curr. Org. Synth. 2005, 2, 39–57. [Google Scholar] [CrossRef]
- Drogalin, A. Advances in the Chemistry of (−)-D-Swainsonine. Chem. Sel. 2022, 7, e202201905. [Google Scholar] [CrossRef]
- Olden, K.; Breton, P.; Grzegorzewski, K.; Yasuda, Y.; Gause, B.L.; Oredipe, O.A.; Newton, S.A.; White, S.L. The Potential Importance of Swainsonine in Therapy for Cancers and Immunology. Pharmacol. Ther. 1991, 50, 285–290. [Google Scholar] [CrossRef]
- Elbein, A.D.; Solf, R.; Dorling, P.R.; Vosbeck, K. Swainsonine: An Inhibitor of Glycoprotein Processing. Proc. Natl. Acad. Sci. USA 1981, 78, 7393–7397. [Google Scholar] [CrossRef]
- Li, B.; Zhang, C.; Zhu, Y.; Sun, P.; Fan, S.; Wang, W.; Tian, Y.; Lu, H. Development of Novel Formulation for Sustained Release of Drug to Prevent Swainsonine-Containing Plants Poisoning in Livestock. Animals 2023, 13, 2646. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Irie, R.; Katsuki, T. Short Step Enantioselective Synthesis of (-)-Swainsonine. Chirality 2000, 12, 464–468. [Google Scholar] [CrossRef]
- Ho, H.E.; James, M.J.; O’Brien, P.; Taylor, R.J.K.; Unsworth, W.P. Ag(I)-Catalyzed Synthesis of Azabicyclic Alkaloid Frameworks from Ketimine-Tethered Ynones: Total Synthesis of Indolizidine 209D. Org. Lett. 2018, 20, 1439–1443. [Google Scholar] [CrossRef]
- Daly, J.W.; Myers, C.W.; Whittaker, N. Further Classification of Skin Alkaloids from Neotropical Poison Frogs (Dendrobatidae), with a General Survey of Toxic/Noxious Substances in the Amphibia. Toxicon 1987, 25, 1023–1095. [Google Scholar] [CrossRef]
- Takahata, H.; Kubota, M.; Ihara, K.; Okamoto, N.; Momose, T.; Azer, N.; Eldefrawi, A.T.; Eldefrawi, M.E. New Synthesis of All the Four Stereoisomers of Indolizidine 209D and Their Affinity for Nicotinic Acetylcholine Receptor. Tetrahedron Asymmetry 1998, 9, 3289–3301. [Google Scholar] [CrossRef]
- Morita, H.; Arisaka, M.; Yoshida, N.; Kobayashi, J. Serratezomines A−C, New Alkaloids from Lycopodium s Erratum Var. s Erratum. J. Org. Chem. 2000, 65, 6241–6245. [Google Scholar] [CrossRef]
- Chandra, A.; Pigza, J.A.; Han, J.-S.; Mutnick, D.; Johnston, J.N. Total Synthesis of the Lycopodium Alkaloid (+)-Serratezomine A. J. Am. Chem. Soc. 2009, 131, 3470–3471. [Google Scholar] [CrossRef][Green Version]
- Mohan, C.; Krishna, R.B.; Sivanandan, S.T.; Ibnusaud, I. Synthesis of Pyrrolo[2,1-a]Isoquinoline Class of Natural Product Crispine A. Eur. J. Org. Chem. 2021, 2021, 4911–4926. [Google Scholar] [CrossRef]
- Pässler, U.; Knölker, H.-J. The Pyrrolo[2,1-a]Isoquinoline Alkaloids. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2011; pp. 79–151. [Google Scholar]
- Zhang, L.; Li, D.; Yu, S. Pharmacological Effects of Harmine and Its Derivatives: A Review. Arch. Pharmacal Res. 2020, 43, 1259–1275. [Google Scholar] [CrossRef]
- Spindola, H.M.; Vendramini-Costa, D.B.; Rodrigues, M.T.; Foglio, M.A.; Pilli, R.A.; Carvalho, J.E. The Antinociceptive Activity of Harmicine on Chemical-Induced Neurogenic and Inflammatory Pain Models in Mice. Pharmacol. Biochem. Behav. 2012, 102, 133–138. [Google Scholar] [CrossRef]
- Saha, S.; Venkata Ramana Reddy, C.; Patro, B. Facile Two-Step Synthesis of Crispine A and Harmicine by Cyclopropylimine Rearrangement. Tetrahedron Lett. 2011, 52, 4014–4016. [Google Scholar] [CrossRef]
- Szawkało, J.; Czarnocki, S.J.; Zawadzka, A.; Wojtasiewicz, K.; Leniewski, A.; Maurin, J.K.; Czarnocki, Z.; Drabowicz, J. Enantioselective Synthesis of Some Tetrahydroisoquinoline and Tetrahydro-β-Carboline Alkaloids. Tetrahedron Asymmetry 2007, 18, 406–413. [Google Scholar] [CrossRef]
- Szawkało, J.; Zawadzka, A.; Wojtasiewicz, K.; Leniewski, A.; Drabowicz, J.; Czarnocki, Z. First Enantioselective Synthesis of the Antitumour Alkaloid (+)-Crispine A and Determination of Its Enantiomeric Purity by 1H NMR. Tetrahedron Asymmetry 2005, 16, 3619–3621. [Google Scholar] [CrossRef]
- Gallagher-Duval, S.; Lapointe, V.; Bélanger, G. Functionalized Polyhydroquinolines from Amino Acids Using a Key One-Pot Cyclization Cascade and Application to the Synthesis of (±)-Δ 7-Mesembrenone. Org. Lett. 2021, 23, 8606–8611. [Google Scholar] [CrossRef]
- Krstenansky, J.L. Mesembrine Alkaloids: Review of Their Occurrence, Chemistry, and Pharmacology. J. Ethnopharmacol. 2017, 195, 10–19. [Google Scholar] [CrossRef]
- Sidjimova, B.; Denev, R.; Nikolova, M.; Bastida, J.; Berkov, S. Dynamics of Alkaloid Accumulation in Narcissus Cv. Hawera: A Source of Sceletium-Type Alkaloids. Z. Für Naturforschung C 2024, 79, 73–79. [Google Scholar] [CrossRef]
- Meyer, G.M.J.; Wink, C.S.D.; Zapp, J.; Maurer, H.H. GC-MS, LC-MSn, LC-High Resolution-MSn, and NMR Studies on the Metabolism and Toxicological Detection of Mesembrine and Mesembrenone, the Main Alkaloids of the Legal High “Kanna” Isolated from Sceletium Tortuosum. Anal. Bioanal. Chem. 2015, 407, 761–778. [Google Scholar] [CrossRef]
- Gericke, J.; Harvey, B.H.; Pretorius, L.; Ollewagen, T.; Benecke, R.M.; Smith, C. Sceletium Tortuosum-Derived Mesembrine Significantly Contributes to the Anxiolytic Effect of Zembrin®, but Its Anti-Depressant Effect May Require Synergy of Multiple Plant Constituents. J. Ethnopharmacol. 2024, 319, 117113. [Google Scholar] [CrossRef] [PubMed]
- Pérard-Viret, J.; Souquet, F.; Manisse, M.-L.; Royer, J. An Expeditious Total Synthesis of (±)-Jamtine Using Condensation between Imine and Acid Anhydride. Tetrahedron Lett. 2010, 51, 96–98. [Google Scholar] [CrossRef]
- Logesh, R.; Das, N.; Adhikari-Devkota, A.; Devkota, H.P. Cocculus Hirsutus (L.) W.Theob. (Menispermaceae): A Review on Traditional Uses, Phytochemistry and Pharmacological Activities. Medicines 2020, 7, 69. [Google Scholar] [CrossRef]
- Thavamani, B.S.; Mathew, M.; Dhanabal, S. Cocculus Hirsutus: Molecular Docking to Identify Suitable Targets for Hepatocellular Carcinoma by in Silico Technique. Pharmacogn. Mag. 2016, 12, 350. [Google Scholar] [CrossRef][Green Version]
- Ameena, S.; Rajesh, N.; Anjum, S.M.; Khadri, H.; Riazunnisa, K.; Mohammed, A.; Kari, Z.A. Antioxidant, Antibacterial, and Anti-Diabetic Activity of Green Synthesized Copper Nanoparticles of Cocculus Hirsutus (Menispermaceae). Appl. Biochem. Biotechnol. 2022, 194, 4424–4438. [Google Scholar] [CrossRef] [PubMed]
- Salve, N.R.; Mishra, D.N. Select Phytocompounds from Cocculus Hirsutus (L.) W. Theob. Extract Having Anti-Cancer Potential as Identified by LC-MS and in Silico Studies. Adv. Zool. Bot. 2024, 12, 47–62. [Google Scholar] [CrossRef]
- Renner, U.; Kernweisz, P. Alkaloide AusSchizozygia Caffaeoides (Boj.) Baill. Experientia 1963, 19, 244–246. [Google Scholar] [CrossRef]
- Kariba, R.M.; Houghton, P.J.; Yenesew, A. Antimicrobial Activities of a New Schizozygane Indoline Alkaloid from Schizozygia Coffaeoides and the Revised Structure of Isoschizogaline. J. Nat. Prod. 2002, 65, 566–569. [Google Scholar] [CrossRef]
- Xu, Z.; Bao, X.; Wang, Q.; Zhu, J. An Enantioselective Total Synthesis of (−)-Isoschizogamine. Angew. Chem. 2015, 127, 15150–15153. [Google Scholar] [CrossRef]
- Macel, M. Attract and Deter: A Dual Role for Pyrrolizidine Alkaloids in Plant–Insect Interactions. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef]
- Coulombe, R.A. Pyrrolizidine Alkaloids in Foods. Adv. Food Nutr. Res. 2003, 45, 61–99. [Google Scholar]
- Moreira, R.; Pereira, D.; Valentão, P.; Andrade, P. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ruan, W.; Vrieling, K. Current Knowledge and Perspectives of Pyrrolizidine Alkaloids in Pharmacological Applications: A Mini-Review. Molecules 2021, 26, 1970. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Desvergnes, S.; Py, S.; Vallée, Y. Total Synthesis of (+)-Hyacinthacine A 2 Based on SmI 2 -Induced Nitrone Umpolung. J. Org. Chem. 2005, 70, 1459–1462. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Schwikkard, S.L.; Crouch, N.R. The Chemistry and Biological Activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R. Pyrrolizidine Alkaloids (July 1998 to June 1999). Nat. Prod. Rep. 2000, 17, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Gilles, P.; Py, S. SmI 2 -Mediated Cross-Coupling of Nitrones with β-Silyl Acrylates: Synthesis of (+)-Australine. Org. Lett. 2012, 14, 1042–1045. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Benson, M.; Wong, R.Y.; Tropea, J.E.; Elbein, A.D. Australine, a Novel Pyrrolizidine Alkaloid Glucosidase Inhibitor from Castanospermum Australe. J. Nat. Prod. 1988, 51, 1198–1206. [Google Scholar] [CrossRef]
- Tropea, J.E.; Molyneux, R.J.; Kaushal, G.P.; Pan, Y.T.; Mitchell, M.; Elbein, A.D. Australine, a Pyrrolizidine Alkaloid That Inhibits Amyloglucosidase and Glycoprotein Processing. Biochemistry 1989, 28, 2027–2034. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Jamasbi, N.; Mohajer, F. The Synthesis of Australine and Its Stereoisomers as Naturally Pyrrolizidine Alkaloids. Mini-Rev. Org. Chem. 2024, 21, 40–57. [Google Scholar] [CrossRef]
- Fayed, M.A.A. Heliotropium; a Genus Rich in Pyrrolizidine Alkaloids: A Systematic Review Following Its Phytochemistry and Pharmacology. Phytomedicine Plus 2021, 1, 100036. [Google Scholar] [CrossRef]
- Culvenor, C. The Alkaloids of Heliotropium Europaeum L.. II. Isolation and Structures of the Third Major Alkaloid and Two Monor Alkaloids, and Isolation of the Principal n-Oxides. Aust. J. Chem. 1954, 7, 287. [Google Scholar] [CrossRef]
- Pisaneschi, F.; Cordero, F.M.; Brandi, A. A Concise Total Synthesis of (+)-Heliotridine. Eur. J. Org. Chem. 2003, 2003, 4373–4375. [Google Scholar] [CrossRef]
- DE WAAL, H.L. South African Senecio Alkaloids. Nature 1940, 146, 777–778. [Google Scholar] [CrossRef]
- Nemoto, H.; Tanimoto, K.; Kanao, Y.; Omura, S.; Kita, Y.; Akai, S. Protecting-Group-Free Catalytic Asymmetric Total Synthesis of (−)-Rosmarinecine. Tetrahedron 2012, 68, 7295–7301. [Google Scholar] [CrossRef]
- Meinwald, J.; Smolanoff, J.; McPhail, A.T.; Miller, R.W.; Eisner, T.; Hicks, K. Nitropolyzonamine: A Spirocyclic Nitro Compound from the Defensive Glands of a Milliped (Polyzonium rosalbum). Tetrahedron Lett. 1975, 16, 2367–2370. [Google Scholar] [CrossRef]
- Kunert, O.; Pferschy-Wenzig, E.M.; Orthaber, A.; Raspotnig, G.; Bodner, M. Alkaloids from Millipedes: A Re-Evaluation of Defensive Exudates from Polyzonium Germanicum. Front. Ecol. Evol. 2023, 11, 1212452. [Google Scholar] [CrossRef]
- Mori, K.; Takagi, Y. Enantioselective Synthesis of Polyzonimine and Nitropolyzonamine, Spirocyclic Compounds from the Defensive Glands of a Millipede, Polyzonium Rosalbum. Tetrahedron Lett. 2000, 41, 6623–6625. [Google Scholar] [CrossRef]
- Michael, J.P. Simple Indolizidine and Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 1–498. [Google Scholar] [CrossRef]
- Michael, J.P. Simple Indolizidine and Quinolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2001; pp. 91–258. [Google Scholar] [CrossRef]
- Cely-Veloza, W.; Kato, M.J.; Coy-Barrera, E. Quinolizidine-Type Alkaloids: Chemodiversity, Occurrence, and Bioactivity. ACS Omega 2023, 8, 27862–27893. [Google Scholar] [CrossRef]
- Snider, B.B.; Grabowski, J.F. Total Synthesis of (−)-Senepodine G and (−)-Cermizine C. J. Org. Chem. 2007, 72, 1039–1042. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morita, H.; Hirasawa, Y.; Shinzato, T.; Kobayashi, J. New Phlegmarane-Type, Cernuane-Type, and Quinolizidine Alkaloids from Two Species of Lycopodium. Tetrahedron 2004, 60, 7015–7023. [Google Scholar] [CrossRef]
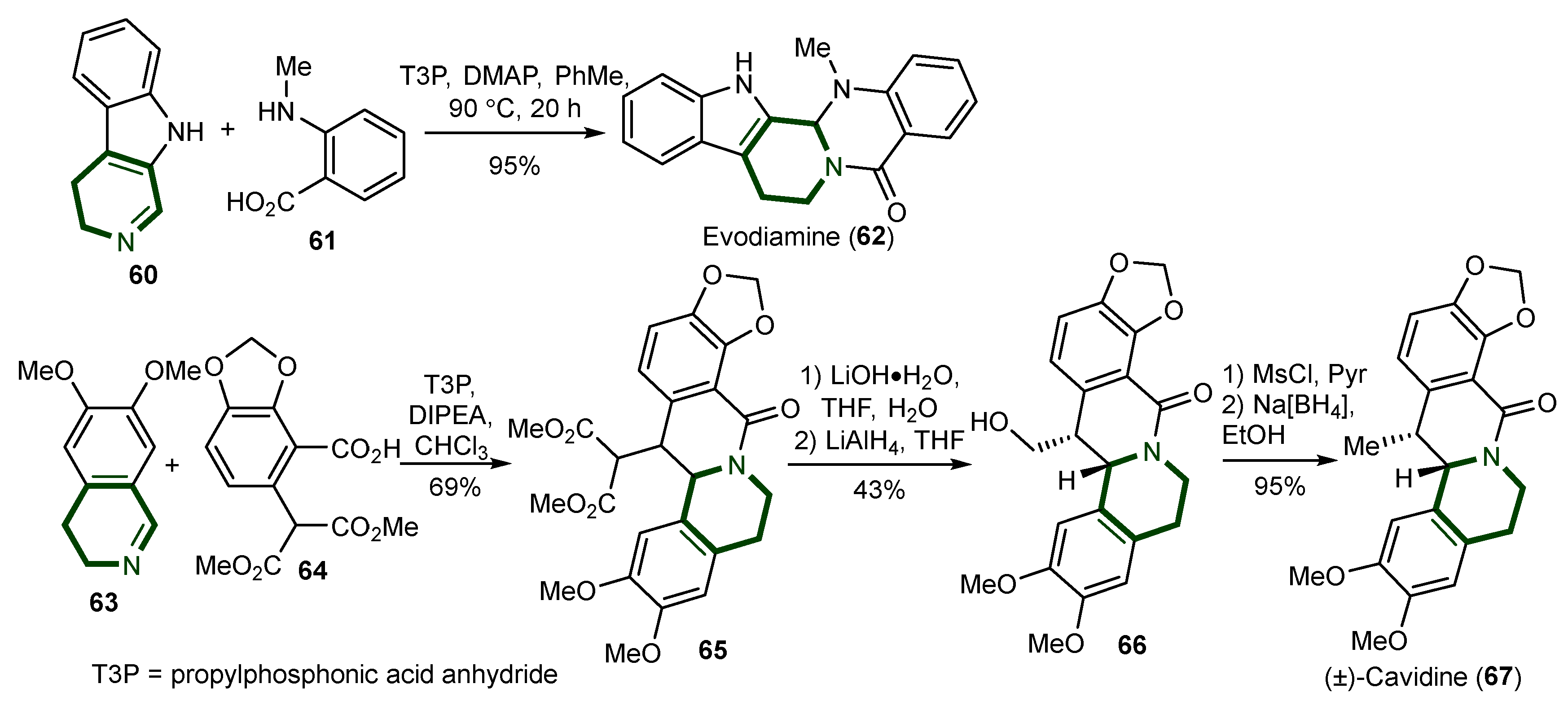
- Kitsiou, C.; Unsworth, W.P.; Coulthard, G.; Taylor, R.J.K. Substrate Scope in the Direct Imine Acylation of Ortho-Substituted Benzoic Acid Derivatives: The Total Synthesis (±)-Cavidine. Tetrahedron 2014, 70, 7172–7180. [Google Scholar] [CrossRef]
- Yu, H.; Jin, H.; Gong, W.; Wang, Z.; Liang, H. Pharmacological Actions of Multi-Target-Directed Evodiamine. Molecules 2013, 18, 1826–1843. [Google Scholar] [CrossRef]
- Sun, Q.; Xie, L.; Song, J.; Li, X. Evodiamine: A Review of Its Pharmacology, Toxicity, Pharmacokinetics and Preparation Researches. J. Ethnopharmacol. 2020, 262, 113164. [Google Scholar] [CrossRef]
- Panda, M.; Tripathi, S.K.; Zengin, G.; Biswal, B.K. Evodiamine as an Anticancer Agent: A Comprehensive Review on Its Therapeutic Application, Pharmacokinetic, Toxicity, and Metabolism in Various Cancers. Cell Biol. Toxicol. 2023, 39, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiong, Y.; Peng, Y.; Zhang, X.; Li, S.; Peng, Y.; Peng, X.; Zhuo, L.; Jiang, W. Natural Product Evodiamine-Inspired Medicinal Chemistry: Anticancer Activity, Structural Optimization and Structure-Activity Relationship. Eur. J. Med. Chem. 2023, 247, 115031. [Google Scholar] [CrossRef]
- Solanki, R.; Patel, S. Evodiamine and Its Nano-based Approaches for Enhanced Cancer Therapy: Recent Advances and Challenges. J. Sci. Food Agric. 2024, 104, 8430–8444. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, X.; Zhang, H.; He, Z.; Zhi, W.; Liu, F.; Wang, Y.; Niu, X. Anti-Ulcerogenic Effect of Cavidine against Ethanol-Induced Acute Gastric Ulcer in Mice and Possible Underlying Mechanism. Int. Immunopharmacol. 2016, 38, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, H.; Li, W.; Mu, Q.; Yao, H.; Wang, Y. Anti-Inflammatory Effects of Cavidine In Vitro and In Vivo, a Selective COX-2 Inhibitor in LPS-Induced Peritoneal Macrophages of Mouse. Inflammation 2015, 38, 923–933. [Google Scholar] [CrossRef]
- Niu, X.; Liu, F.; Li, W.; Zhi, W.; Zhang, H.; Wang, X.; He, Z. Cavidine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via NF-ΚB Signaling Pathway in Vivo and in Vitro. Inflammation 2017, 40, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Wang, Z.; Mei, R.; Zhang, W.; Ma, D.; Xu, D.; Xie, X.; She, X. Short and Scalable Total Synthesis of Myrioneuron Alkaloids (±)-α,β-Myrifabral A and B. Org. Lett. 2016, 18, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.C.; Jossang, A.; Sévenet, T.; Nguyen, V.H.; Bodo, B. Absolute Configuration of Myrobotinol, New Fused-Hexacyclic Alkaloid Skeleton from Myrioneuron Nutans. J. Org. Chem. 2007, 72, 9826–9829. [Google Scholar] [CrossRef]
- Pham, V.C.; Jossang, A.; Sévenet, T.; Nguyen, V.H.; Bodo, B. Novel Alkaloids from Myrioneuron Nutans. Eur. J. Org. Chem. 2009, 2009, 1412–1416. [Google Scholar] [CrossRef]
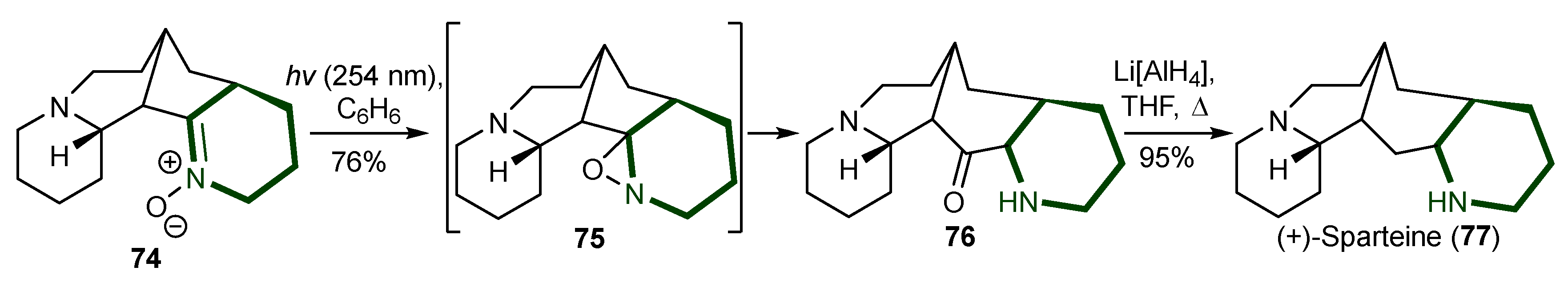
- Smith, B.T.; Wendt, J.A.; Aubé, J. First Asymmetric Total Synthesis of (+)-Sparteine. Org. Lett. 2002, 4, 2577–2579. [Google Scholar] [CrossRef] [PubMed]
- Chuzel, O.; Riant, O. Sparteine as a Chiral Ligand for Asymmetric Catalysis. In Chiral Diazaligands for Asymmetric Synthesis; Springer: Berlin/Heidelberg, 2005; pp. 59–92. [Google Scholar]
- Dearden, M.J.; McGrath, M.J.; O’Brien, P. Evaluation of (+)-Sparteine-like Diamines for Asymmetric Synthesis. J. Org. Chem. 2004, 69, 5789–5792. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sirasani, G.; Andrade, R.B. Aspidosperma and Strychnos Alkaloids: Chemistry and Biology. In The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 1–143. [Google Scholar]
- de Almeida, V.L.; Silva, C.G.; Silva, A.F.; Campana, P.R.V.; Foubert, K.; Lopes, J.C.D.; Pieters, L. Aspidosperma Species: A Review of Their Chemistry and Biological Activities. J. Ethnopharmacol. 2019, 231, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lopchuk, J.M. Recent Advances in the Synthesis of Aspidosperma-Type Alkaloids. In Progress in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–25. [Google Scholar]
- Wang, N.; Xiao, X.; Liu, C.; Yao, H.; Huang, N.; Zou, K. Recent Advances in the Total Synthesis of Aspidosperma and Kopsia Alkaloids Using Tetracyclic Pyridocarbazoles as Versatile Building Blocks. Adv. Synth. Catal. 2022, 364, 2479–2501. [Google Scholar] [CrossRef]
- Wang, N.; Jiang, X. Synthetic Approaches to Tricyclic Aminoketones in the Total Synthesis of Aspidosperma and Kopsia Alkaloids. Chem. Rec. 2021, 21, 295–314. [Google Scholar] [CrossRef]
- Goel, B.; Jain, S.K. Semisynthesis: Bridging Natural Products and Novel Anticancer Therapies. Eur. J. Med. Chem. Rep. 2024, 12, 100218. [Google Scholar] [CrossRef]
- Florencia, M.; Brunella, B. Commentary on the Obtention of Semi-Synthetic Derivatives from Natural Products for Medicinal Applications: Advances, Challenges, and Perspectives. Curr. Med. Chem. 2024, 32, 1–7. [Google Scholar] [CrossRef]
- Shemet, A.; Carreira, E.M. Total Synthesis of (−)-Rhazinilam and Formal Synthesis of (+)-Eburenine and (+)-Aspidospermidine: Asymmetric Cu-Catalyzed Propargylic Substitution. Org. Lett. 2017, 19, 5529–5532. [Google Scholar] [CrossRef]
- Kozmin, S.A.; Iwama, T.; Huang, Y.; Rawal, V.H. An Efficient Approach to Aspidosperma Alkaloids via [4 + 2] Cycloadditions of Aminosiloxydienes: Stereocontrolled Total Synthesis of (±)-Tabersonine. Gram-Scale Catalytic Asymmetric Syntheses of (+)-Tabersonine and (+)-16-Methoxytabersonine. Asymmetric Sy. J. Am. Chem. Soc. 2002, 124, 4628–4641. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Du, S.; Li, D.; Jiang, X. Divergent Asymmetric Total Synthesis of (+)-Vincadifformine, (−)-Quebrachamine, (+)-Aspidospermidine, (−)-Aspidospermine, (−)-Pyrifolidine, and Related Natural Products. Org. Lett. 2017, 19, 3167–3170. [Google Scholar] [CrossRef]
- Banwell, M.G.; Lupton, D.W.; Willis, A.C. Application of the Palladium(0)-Catalyzed Ullmann Cross-Coupling Reaction in a Total Synthesis of (±)-Aspidospermidine and Thus Representing an Approach to the Lower Hemisphere of the Binary Indole—Indoline Alkaloid Vinblastine. Aust. J. Chem. 2005, 58, 722. [Google Scholar] [CrossRef]
- Gnecco, D.; Vázquez, E.; Galindo, A.; Terán, J.L.; Orea, L.; Bernès, S.; Enríquez, R.G. Synthesis of an Aspidosperma Alkaloid Precursor: Synthesis of (+)-Aspidospermidine. Arkivoc 2003, 2003, 185–192. [Google Scholar] [CrossRef]
- Toczko, M.A.; Heathcock, C.H. Total Synthesis of (±)-Aspidospermidine. J. Org. Chem. 2000, 65, 2642–2645. [Google Scholar] [CrossRef]
- Serdaroglu, G.; Uludag, N. Concise Total Synthesis of (±)-Aspidospermidine and Computational Study: FT-IR, NMR, NBO, NLO, FMO, MEP Diagrams. J. Mol. Struct. 2018, 1166, 286–303. [Google Scholar] [CrossRef]
- Antropow, A.H.; Garcia, N.R.; White, K.L.; Movassaghi, M. Enantioselective Synthesis of (−)-Vallesine: Late-Stage C17-Oxidation via Complex Indole Boronation. Org. Lett. 2018, 20, 3647–3650. [Google Scholar] [CrossRef]
- Akhgari, A.; Laakso, I.; Seppänen-Laakso, T.; Yrjönen, T.; Vuorela, H.; Oksman-Caldentey, K.; Rischer, H. Determination of Terpenoid Indole Alkaloids in Hairy Roots of Rhazya Stricta (Apocynaceae) by GC-MS. Phytochem. Anal. 2015, 26, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Madni, A.; Iqbal, M.A.; Batool, R.; Mahmood, A.; Riaz, H.; Hasnain, M.; Akram, M.A.; Zahid, A. Evaluation of Chemical Composition of Ethanolic Extract of Rhazya Stricta and Its Total Phenolic and Flavonoid Contents. Chem. Afr. 2022, 5, 533–542. [Google Scholar] [CrossRef]
- Fukuda, Y.; Shindo, M.; Shishido, K. Total Synthesis of (−)-Aspidospermine via Diastereoselective Ring-Closing Olefin Metathesis. Org. Lett. 2003, 5, 749–751. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, H.F.; Evenson, M.A.; Drescher, P.; Sparwasser, C.; Madsen, P.O. Isolation and Biological Activity of Aspidospermine and Quebrachamine from an Aspidosperma Tree Source. J. Pharm. Biomed. Anal. 1994, 12, 1283–1287. [Google Scholar] [CrossRef]
- Mitaine-Offer, A.-C.; Sauvain, M.; Valentin, A.; Callapa, J.; Mallié, M.; Zèches-Hanrot, M. Antiplasmodial Activity of Aspidosperma Indole Alkaloids. Phytomedicine 2002, 9, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Amiri, Z.; Kafshdarzadeh, K.; Zadsirjan, V. Synthesis of Indole Derivatives as Prevalent Moieties Present in Selected Alkaloids. RSC Adv. 2021, 11, 33540–33612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ju, X.; Ma, S.; Du, C.; Zhang, W.; Li, H.; Wang, X.; Xie, X.; She, X. Asymmetric Total Synthesis of (+)-Winchinine B. J. Org. Chem. 2019, 84, 14994–15000. [Google Scholar] [CrossRef]
- Li, Y.-T.; Gao, X.-D.; Zhang, W.; Huang, X.-J.; Zhang, D.-M.; Jiang, R.-W.; Wang, L.; Zhang, X.-Q.; Ye, W.-C. Winchinines A and B, Two Unusual Monoterpene Indole Alkaloids with a Third Nitrogen Atom from Winchia Calophylla. RSC Adv. 2016, 6, 59657–59660. [Google Scholar] [CrossRef]
- Lee, K.; Boger, D.L. Total Syntheses of (−)-Kopsifoline D and (−)-Deoxoapodine: Divergent Total Synthesis via Late-Stage Key Strategic Bond Formation. J. Am. Chem. Soc. 2014, 136, 3312–3317. [Google Scholar] [CrossRef]
- Zhao, S.; Andrade, R.B. Development and Scope of the Arene-Fused Domino Michael/Mannich Reaction: Application to the Total Syntheses of Aspidosperma Alkaloids (−)-Aspidospermidine, (−)-Tabersonine, and (−)-Vincadifformine. J. Org. Chem. 2017, 82, 521–531. [Google Scholar] [CrossRef]
- Zhao, S.; Andrade, R.B. Domino Michael/Mannich/ N -Alkylation Route to the Tetrahydrocarbazole Framework of Aspidosperma Alkaloids: Concise Total Syntheses of (−)-Aspidospermidine, (−)-Tabersonine, and (−)-Vincadifformine. J. Am. Chem. Soc. 2013, 135, 13334–13337. [Google Scholar] [CrossRef]
- Wagnières, O.; Xu, Z.; Wang, Q.; Zhu, J. Unified Strategy to Monoterpene Indole Alkaloids: Total Syntheses of (±)-Goniomitine, (±)-1,2-Dehydroaspidospermidine, (±)-Aspidospermidine, (±)-Vincadifformine, and (±)-Kopsihainanine A. J. Am. Chem. Soc. 2014, 136, 15102–15108. [Google Scholar] [CrossRef] [PubMed]
- Kai, T.; Zhang, L.; Wang, X.; Jing, A.; Zhao, B.; Yu, X.; Zheng, J.; Zhou, F. Tabersonine Inhibits Amyloid Fibril Formation and Cytotoxicity of Aβ(1–42). ACS Chem. Neurosci. 2015, 6, 879–888. [Google Scholar] [CrossRef]
- Qian, C.; Wang, J.; Lin, W.; Chen, Y.; Yang, J.; Liu, M.; Sun, X.; Wu, H.; Zhang, M.; Wang, Y.; et al. Tabersonine Attenuates Obesity-induced Renal Injury via Inhibiting NF-κB-mediated Inflammation. Phytother. Res. 2023, 37, 2353–2363. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Chen, L.; Deng, Y.; Zheng, Z.; Ming, Y. Tabersonine Induces the Apoptosis of Human Hepatocellular Carcinoma In Vitro and In Vivo. Anti-Cancer Agents Med. Chem. 2024, 24, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, Y.; Liu, Y.; Yi, Q.; Xu, Z. Tabersonine Enhances Cisplatin Sensitivity by Modulating Aurora Kinase A and Suppressing Epithelial–Mesenchymal Transition in Triple-Negative Breast Cancer. Pharm. Biol. 2024, 62, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, W.; Hong, S.; Shao, J.; Chen, J.; Chattipakorn, N.; Wu, D.; Luo, W.; Liang, G. Tabersonine, a Natural NLRP3 Inhibitor, Suppresses Inflammasome Activation in Macrophages and Attenuate NLRP3-Driven Diseases in Mice. Acta Pharmacol. Sin. 2023, 44, 1252–1261. [Google Scholar] [CrossRef]
- Shen, X.-L.; Zhao, R.-R.; Mo, M.-J.; Peng, F.-Z.; Zhang, H.-B.; Shao, Z.-H. Catalytic Enantioselective and Divergent Total Synthesis of (+)-10-Oxocylindrocarpidine, (+)-Cylindrocarpidine, (−)- N -Acetylcylindrocarpinol, and (+)-Aspidospermine. J. Org. Chem. 2014, 79, 2473–2480. [Google Scholar] [CrossRef]
- He, W.; Hu, J.; Wang, P.; Chen, L.; Ji, K.; Yang, S.; Li, Y.; Xie, Z.; Xie, W. Highly Enantioselective Tandem Michael Addition of Tryptamine-Derived Oxindoles to Alkynones: Concise Synthesis of Strychnos Alkaloids. Angew. Chem. 2018, 130, 3868–3871. [Google Scholar] [CrossRef]
- Liu, X.; Lou, M.; Bai, S.; Sun, G.; Qi, X. Asymmetric Total Syntheses of Strychnos Alkaloids via Selective Fischer Indolization. J. Org. Chem. 2022, 87, 5199–5212. [Google Scholar] [CrossRef]
- Uludag, N. An Effective Approach to the Strychnos Alkaloids: Total Synthesis of Tubifolidine. Chem. Nat. Compd. 2021, 57, 491–496. [Google Scholar] [CrossRef]
- Pandey, G.; Khamrai, J.; Mishra, A.; Maity, P.; Chikkade, P.K. Iminium Ion-Enamine Cascade Reaction Enables the Asymmetric Total Syntheses of Aspidosperma Alkaloids Vincadifformine and Ervinceine. Tetrahedron 2018, 74, 6317–6327. [Google Scholar] [CrossRef]
- Pandey, G.; Prasanna, K.C. Iminium Ion Cascade Reaction in the Total Synthesis of (+)-Vincadifformine. Org. Lett. 2011, 13, 4672–4675. [Google Scholar] [CrossRef]
- Sumi, S.; Matsumoto, K.; Tokuyama, H.; Fukuyama, T. Enantioselective Total Synthesis of Aspidophytine. Org. Lett. 2003, 5, 1891–1893. [Google Scholar] [CrossRef]
- Satoh, H.; Ueda, H.; Tokuyama, H. Divergent Total Synthesis of (−)-Aspidophytine and Its Congeners via Fischer Indole Synthesis. Tetrahedron 2013, 69, 89–95. [Google Scholar] [CrossRef]
- Banwell, M.G.; White, L.V.; Ye, S.Y. Formal Total Syntheses of (+)- and (−)-Aspidophytine from a Common, Homochiral Precursor. J. Org. Chem. 2022, 87, 14407–14421. [Google Scholar] [CrossRef] [PubMed]
- Mroue, M.A.; Euler, K.L.; Ghuman, M.A.; Alam, M. Indole Alkaloids of Haplophyton Crooksii. J. Nat. Prod. 1996, 59, 890–893. [Google Scholar] [CrossRef]
- Dey, P.; Kundu, A.; Chakraborty, H.J.; Kar, B.; Choi, W.S.; Lee, B.M.; Bhakta, T.; Atanasov, A.G.; Kim, H.S. Therapeutic Value of Steroidal Alkaloids in Cancer: Current Trends and Future Perspectives. Int. J. Cancer 2019, 145, 1731–1744. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, M.; Cheng, K.; Yu, P.; Wei, X.; Shi, Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 2016, 36, 119–143. [Google Scholar] [CrossRef]
- Huang, Y.; Li, G.; Hong, C.; Zheng, X.; Yu, H.; Zhang, Y. Potential of Steroidal Alkaloids in Cancer: Perspective Insight Into Structure–Activity Relationships. Front. Oncol. 2021, 11, 733369. [Google Scholar] [CrossRef] [PubMed]
- Troost, B.; Mulder, L.M.; Diosa-Toro, M.; van de Pol, D.; Rodenhuis-Zybert, I.A.; Smit, J.M. Tomatidine, a Natural Steroidal Alkaloid Shows Antiviral Activity towards Chikungunya Virus in Vitro. Sci. Rep. 2020, 10, 6364. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Xu, P.; Zheng, J.; Deng, X.; Ye, Q.; Huang, Z.; Wang, N. Effects and Mechanisms of Natural Alkaloids for Prevention and Treatment of Osteoporosis. Front. Pharmacol. 2022, 13, 1014173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-D.; Shi, Y.; Wu, J.-J.; Lin, J.-R.; Tian, W.-S. Synthesis of Demissidine and Solanidine. Org. Lett. 2016, 18, 3038–3040. [Google Scholar] [CrossRef] [PubMed]
- Vronen, P.J.E.; Koval, N.; Groot, A. de The Synthesis of 16-Dehydropregnenolone Acetate (DPA) from Potato Glycoalkaloids. Arkivoc 2003, 2004, 24–50. [Google Scholar] [CrossRef]
- Wollmann, B.M.; Størset, E.; Kringen, M.K.; Molden, E.; Smith, R.L. Prediction of CYP2D6 Poor Metabolizers by Measurements of Solanidine and Metabolites—A Study in 839 Patients with Known CYP2D6 Genotype. Eur. J. Clin. Pharmacol. 2023, 79, 523–531. [Google Scholar] [CrossRef]
- Medwid, S.; Schwarz, U.I.; Choi, Y.; Keller, D.; Ross, C.; Kim, R.B. Solanidine Metabolites as Diet-Derived Biomarkers of CYP2D6-Mediated Tamoxifen Metabolism in Breast Cancer Patients. Clin. Pharmacol. Ther. 2024, 116, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Wojtkielewicz, A.; Kiełczewska, U.; Baj, A.; Morzycki, J.W. Synthesis of Demissidine Analogues from Tigogenin via Imine Intermediates. Int. J. Mol. Sci. 2021, 22, 10879. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.-X.; Wang, Y.; Zhang, D.-M.; Jiang, R.-W.; Wang, G.-C.; Shi, J.-M.; Huang, X.-J.; Chen, W.-M.; Che, C.-T.; Ye, W.-C. Flueggines A and B, Two New Dimeric Indolizidine Alkaloids from Flueggea Virosa. Org. Lett. 2011, 13, 3888–3891. [Google Scholar] [CrossRef]
- Bailly, C. Traditional Uses, Pharmacology and Phytochemistry of the Medicinal Plant Flueggea Virosa (Roxb. Ex Willd.) Royle. Future Pharmacol. 2024, 4, 77–102. [Google Scholar] [CrossRef]
- Wei, H.; Qiao, C.; Liu, G.; Yang, Z.; Li, C. Stereoselective Total Syntheses of (−)-Flueggine A and (+)-Virosaine B. Angew. Chem. 2013, 125, 648–652. [Google Scholar] [CrossRef]
- Ma, N.; Yao, Y.; Zhao, B.-X.; Wang, Y.; Ye, W.-C.; Jiang, S. Total Synthesis of Securinega Alkaloids (−)-Norsecurinine, (−)-Niruroidine and (−)-Flueggine A. Chem. Commun. 2014, 50, 9284–9287. [Google Scholar] [CrossRef] [PubMed]
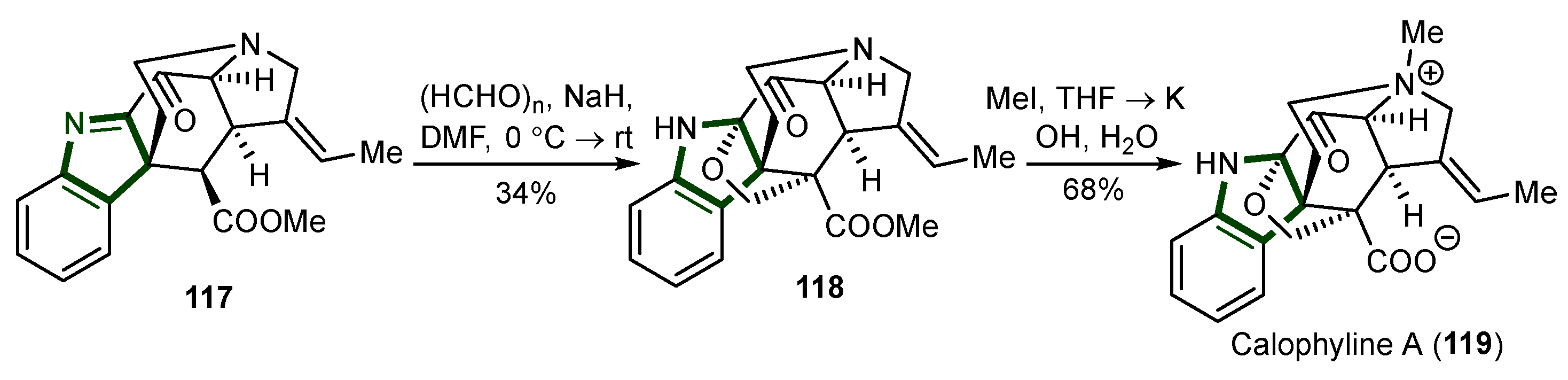
- Li, L.-M.; Yang, T.; Liu, Y.; Liu, J.; Li, M.-H.; Wang, Y.-T.; Yang, S.-X.; Zou, Q.; Li, G.-Y. Calophyline A, a New Rearranged Monoterpenoid Indole Alkaloid from Winchia Calophylla. Org. Lett. 2012, 14, 3450–3453. [Google Scholar] [CrossRef]
- Li, G.; Xie, X.; Zu, L. Total Synthesis of Calophyline A. Angew. Chem. 2016, 128, 10639–10642. [Google Scholar] [CrossRef]
- Xie, X.; Wei, B.; Li, G.; Zu, L. Unified Total Syntheses of Structurally Diverse Akuammiline Alkaloids. Org. Lett. 2017, 19, 5430–5433. [Google Scholar] [CrossRef] [PubMed]
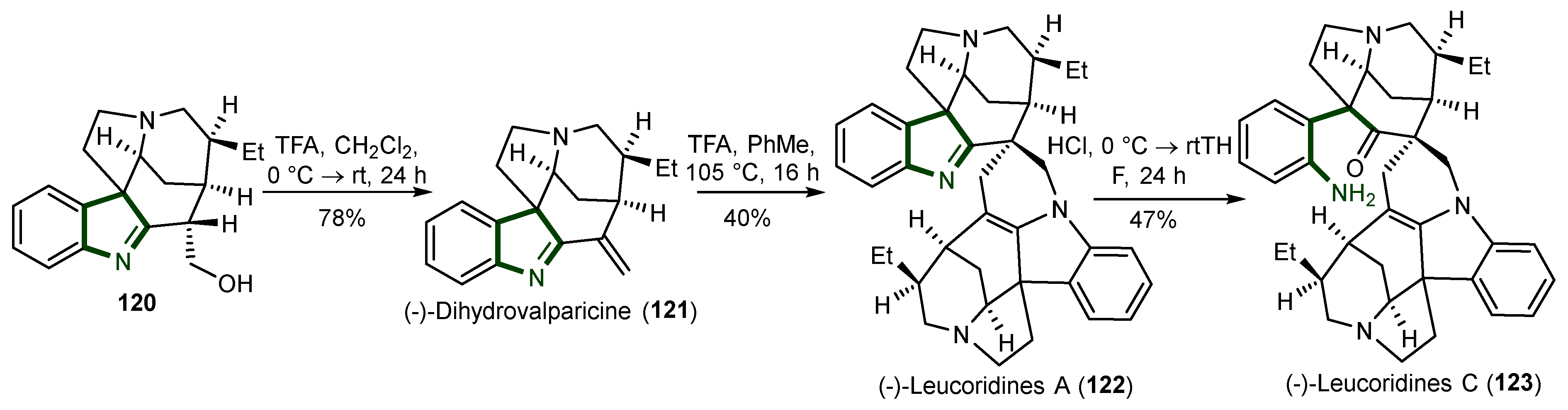
- Gan, C.-Y.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.-S. Leucoridines A−D, Cytotoxic Strychnos − Strychnos Bisindole Alkaloids from Leuconotis. J. Nat. Prod. 2010, 73, 1107–1111. [Google Scholar] [CrossRef]
- Kokkonda, P.; Brown, K.R.; Seguin, T.J.; Wheeler, S.E.; Vaddypally, S.; Zdilla, M.J.; Andrade, R.B. Biomimetic Total Syntheses of (−)-Leucoridines A and C through the Dimerization of (−)-Dihydrovalparicine. Angew. Chem. 2015, 127, 12823–12826. [Google Scholar] [CrossRef]
- Benayad, S.; Beniddir, M.A.; Evanno, L.; Poupon, E. Biomimetic Assembly of Leucoridine A. Eur. J. Org. Chem. 2015, 2015, 1894–1898. [Google Scholar] [CrossRef]
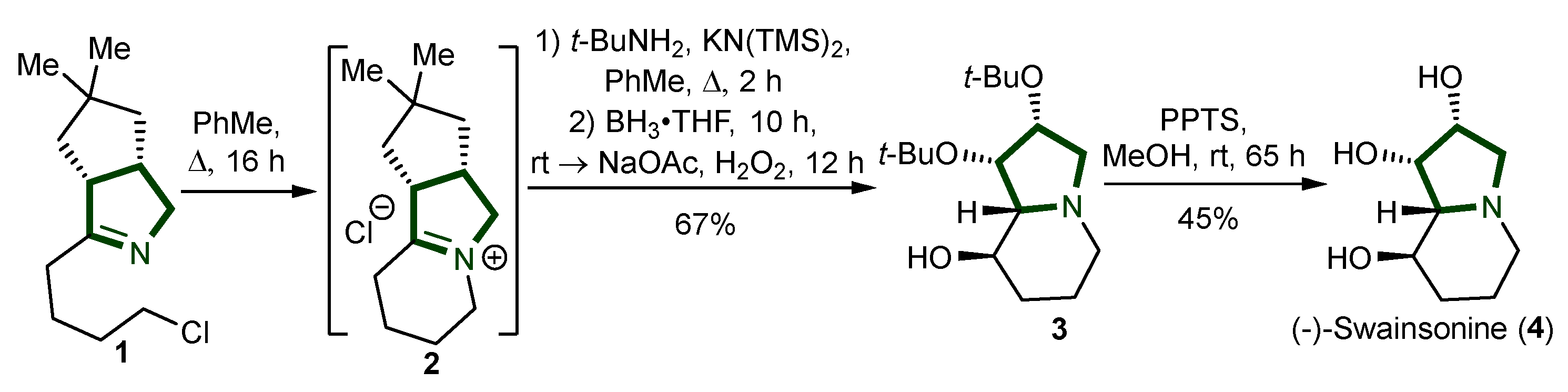


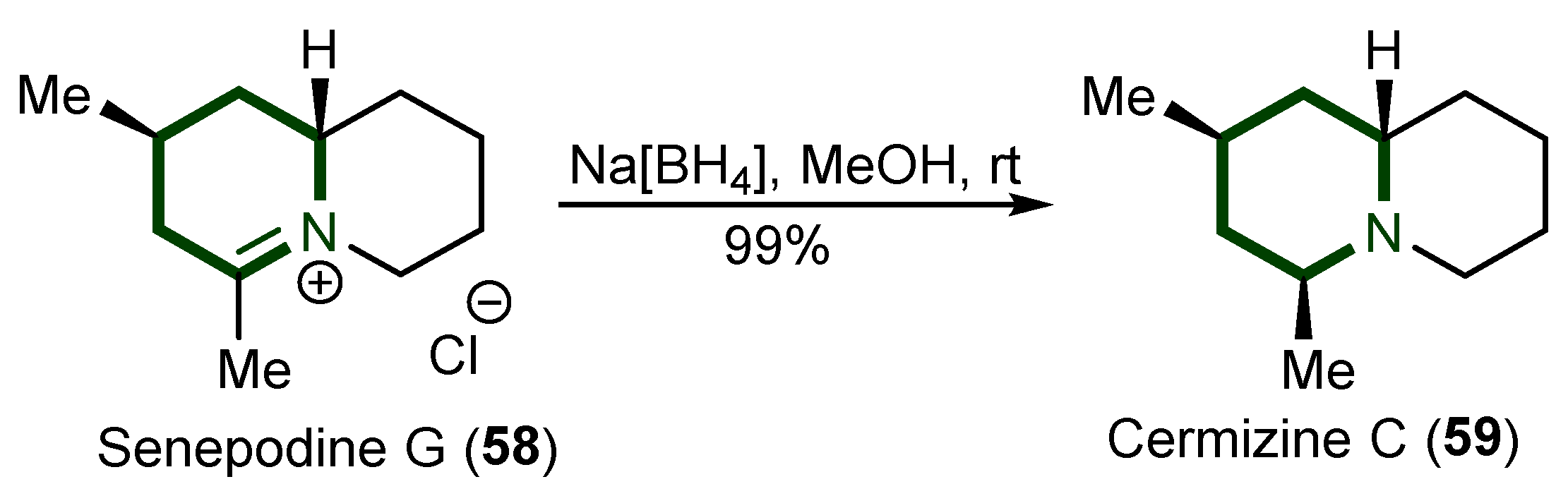

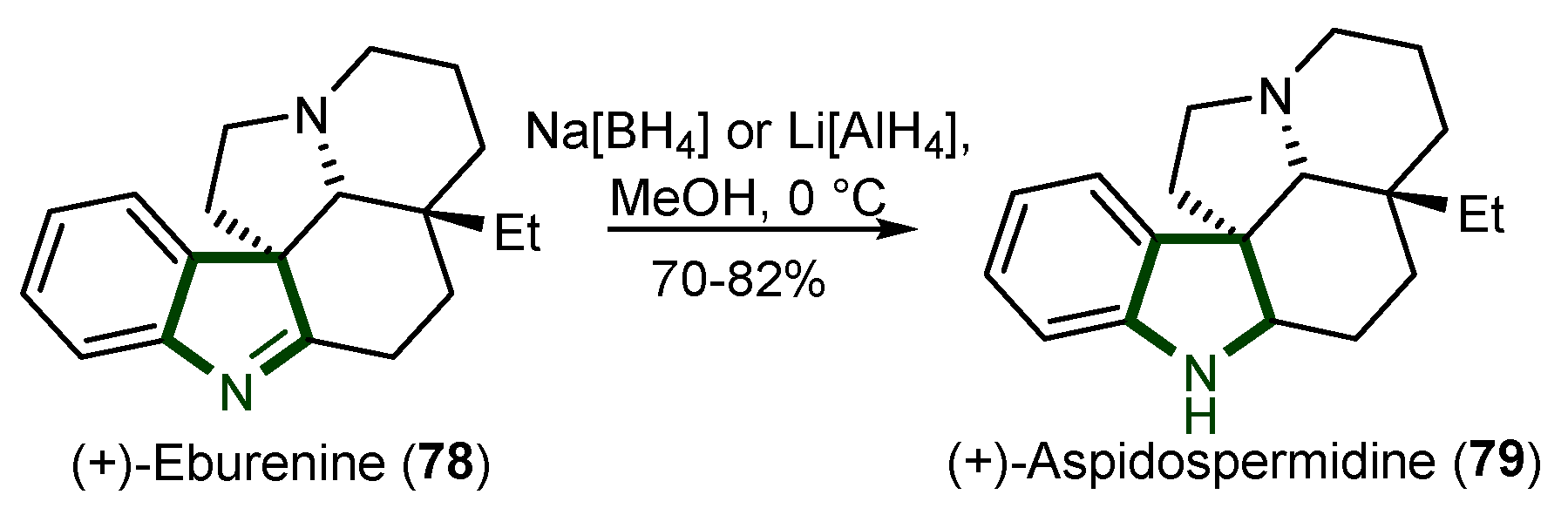
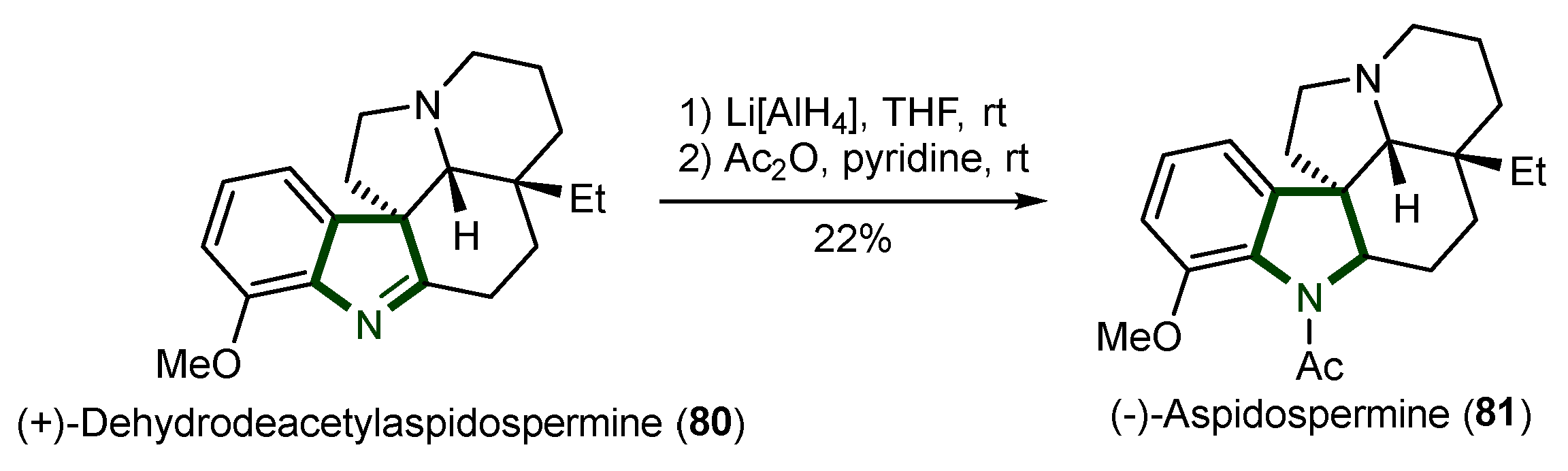




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolobochkin, A.; Gazizov, A.; Sidlyaruk, N.; Akylbekov, N.; Zhapparbergenov, R.; Burilov, A. Cyclic Imines and Their Salts as Universal Precursors in the Synthesis of Nitrogen-Containing Alkaloids. Int. J. Mol. Sci. 2025, 26, 288. https://doi.org/10.3390/ijms26010288
Smolobochkin A, Gazizov A, Sidlyaruk N, Akylbekov N, Zhapparbergenov R, Burilov A. Cyclic Imines and Their Salts as Universal Precursors in the Synthesis of Nitrogen-Containing Alkaloids. International Journal of Molecular Sciences. 2025; 26(1):288. https://doi.org/10.3390/ijms26010288
Chicago/Turabian StyleSmolobochkin, Andrey, Almir Gazizov, Nikita Sidlyaruk, Nurgali Akylbekov, Rakhmetulla Zhapparbergenov, and Alexander Burilov. 2025. "Cyclic Imines and Their Salts as Universal Precursors in the Synthesis of Nitrogen-Containing Alkaloids" International Journal of Molecular Sciences 26, no. 1: 288. https://doi.org/10.3390/ijms26010288
APA StyleSmolobochkin, A., Gazizov, A., Sidlyaruk, N., Akylbekov, N., Zhapparbergenov, R., & Burilov, A. (2025). Cyclic Imines and Their Salts as Universal Precursors in the Synthesis of Nitrogen-Containing Alkaloids. International Journal of Molecular Sciences, 26(1), 288. https://doi.org/10.3390/ijms26010288






