Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach
Abstract
1. Introduction
1.1. Characteristics of circRNAs
1.2. Functions of circRNAs
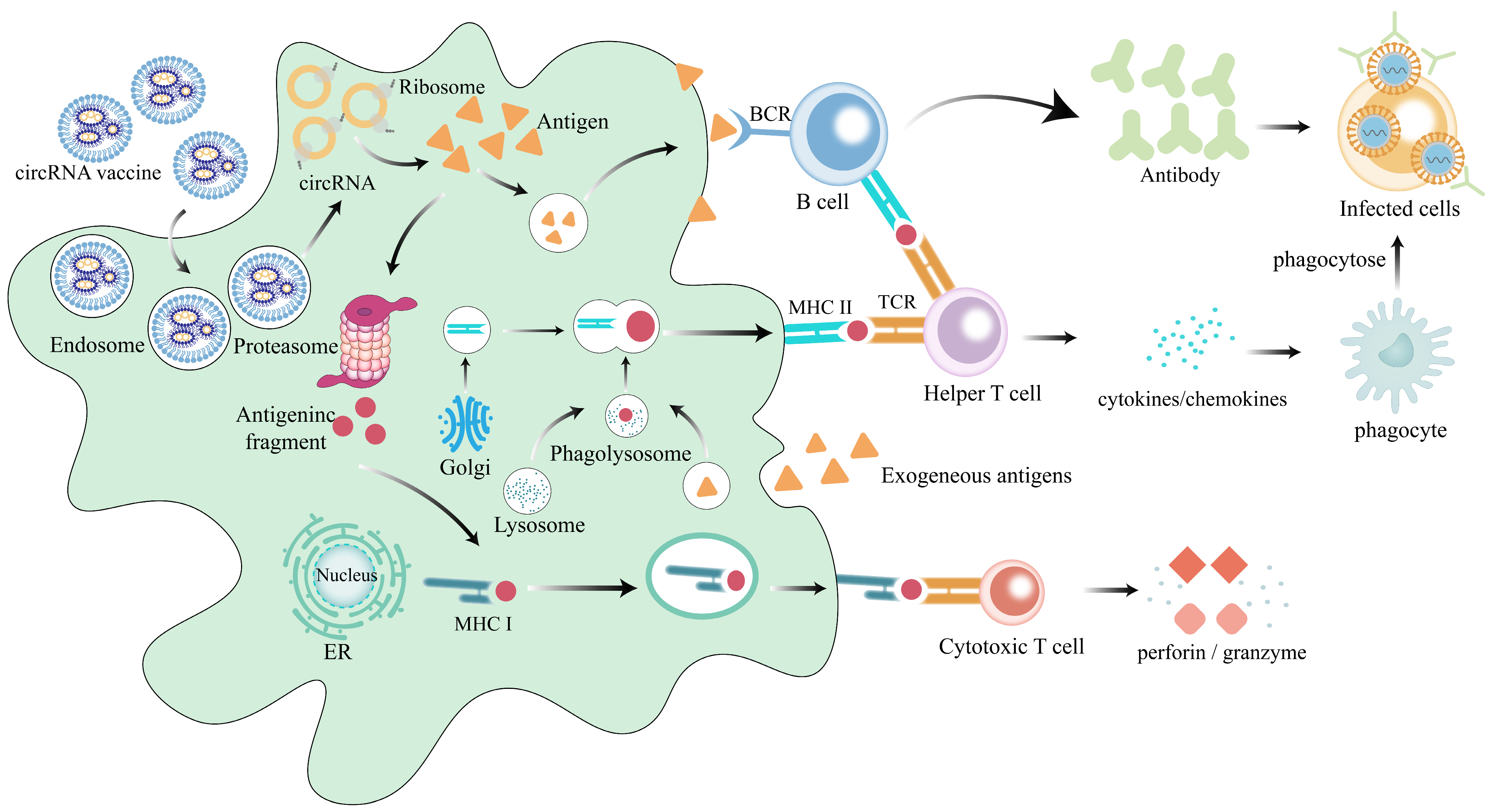
1.3. The Functional Mechanism of circRNA Vaccines
1.4. Feasibility of circRNA as a Vaccine Vector
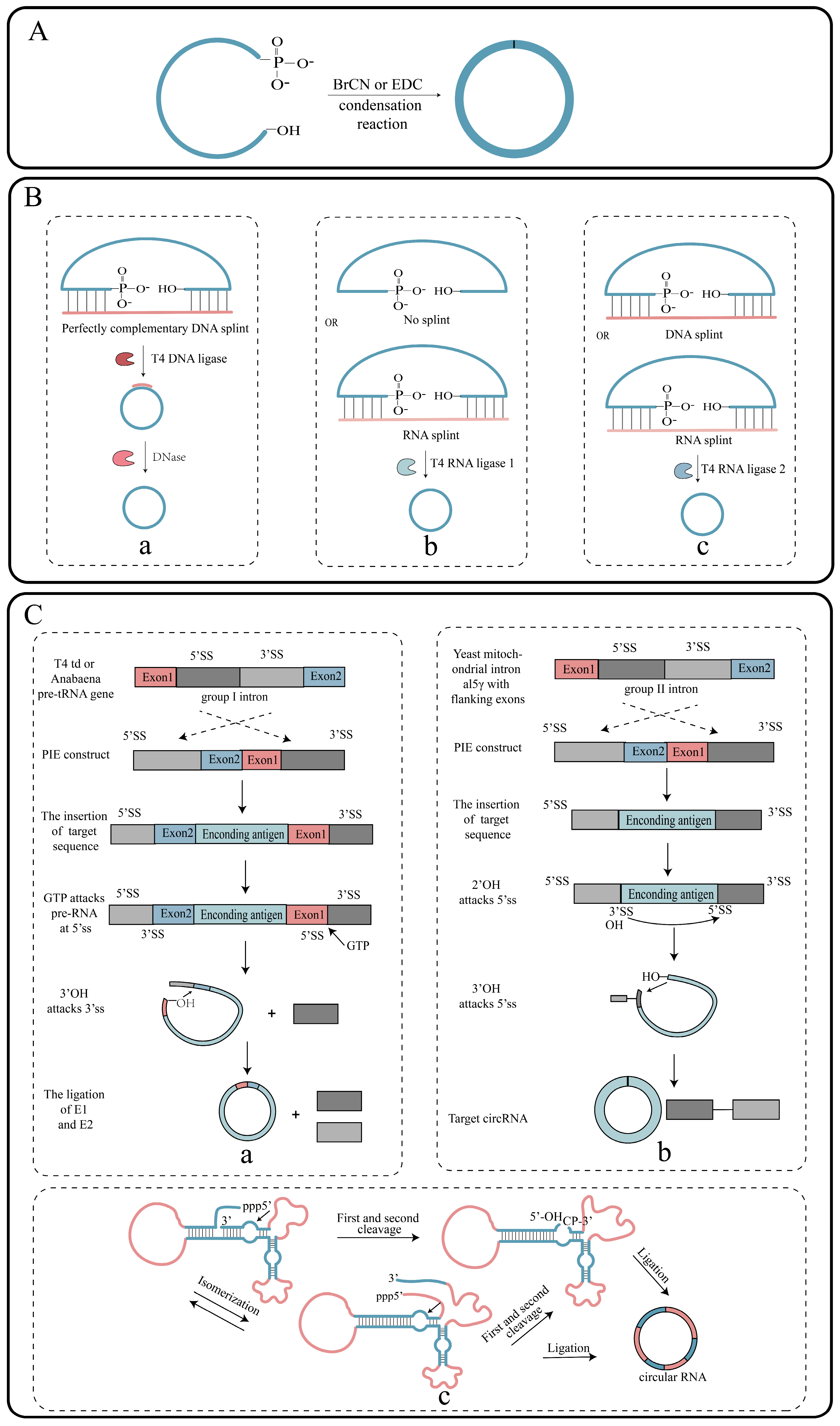
2. Synthesis of circRNAs In Vitro
2.1. Synthesis of Linear RNA Precursors
2.1.1. Chemical Strategies
2.1.2. Enzymatic Strategies
2.2. Circularization of RNA
2.2.1. Chemical Strategies
2.2.2. Enzymatic Strategies
2.2.3. Ribozyme Strategy
3. Delivery System for circRNA Vaccines
3.1. Lipid Nanoparticle-Based Delivery Systems
3.2. Other Delivery Systems
4. Advantages of circRNA Vaccines
4.1. Enhanced Stability of circRNA Vaccines
4.2. Induction of Robust Cellular Immunity
4.3. Induction of Robust Humoral Immunity
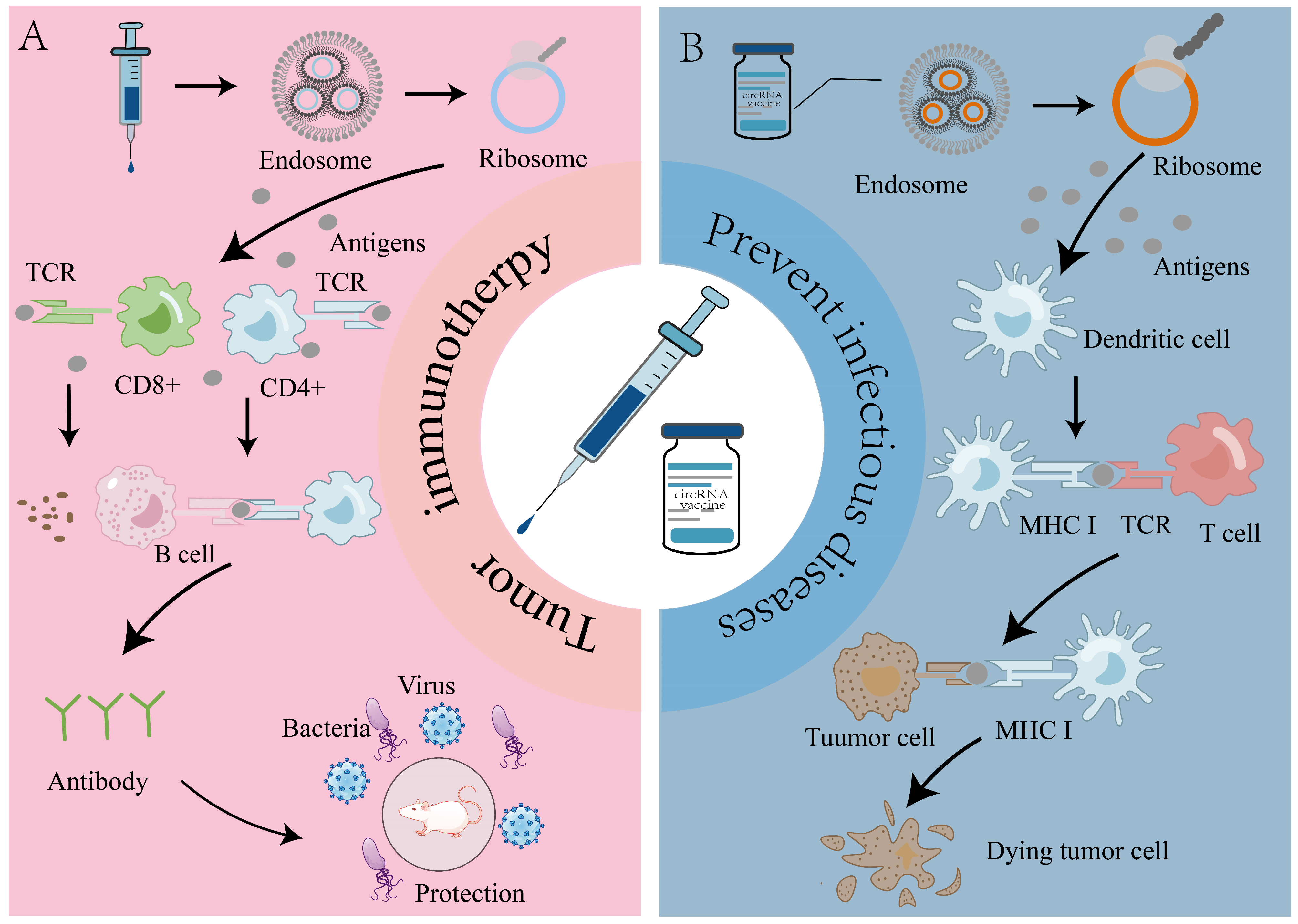
5. Application of circRNA Vaccines in Prevention of Infectious Diseases
6. Role of circRNA Vaccines in Tumor Therapy
6.1. Application of circRNA Vaccines in CAR-T and TCR-T Therapy
6.2. CircRNA Vaccine Used in Combination with PD-1 for Tumor Treatment
7. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Wu, J.; Liu, J.; Wang, Y.; Xu, T.; Zhang, M.; Zhuang, M.; Zou, L.; Sun, W.; et al. Genome-wide perturbations of A-to-I RNA editing dysregulated circular RNAs promoting the development of cervical cancer. Comput. Biol. Med. 2023, 166, 107546. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wilusz, J.E. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014, 28, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, B.; Chen, B.; Bliim, N.; Ueberham, U.; Arendt, T.; Janitz, M. The emerging role of circular RNAs in transcriptome regulation. Genomics 2017, 109, 401–407. [Google Scholar] [CrossRef]
- Yang, R.; Wang, R.C. Research Techniques Made Simple: Studying Circular RNA in Skin Diseases. J. Investig. Dermatol. 2021, 141, 2313–2319.e1. [Google Scholar] [CrossRef]
- Wilusz, J.E. A 360° view of circular RNAs: From biogenesis to functions. Wiley Interdiscip. Rev. RNA 2018, 9, e1478. [Google Scholar] [CrossRef]
- Zheng, S.; Tian, Q.; Yuan, Y.; Sun, S.; Li, T.; Xia, R.; He, R.; Luo, Y.; Lin, Q.; Fu, Z.; et al. Extracellular vesicle-packaged circBIRC6 from cancer-associated fibroblasts induce platinum resistance via SUMOylation modulation in pancreatic cancer. J. Exp. Clin. Cancer Res. 2023, 42, 324. [Google Scholar] [CrossRef]
- Dai, W.; Wu, X.; Li, J.; Tang, W.; Wang, Y.; Xu, W.; Han, D.; Xu, X.; Xu, X. Hedgehog-Gli1-derived exosomal circ-0011536 mediates peripheral neural remodeling in pancreatic cancer by modulating the miR-451a/VGF axis. J. Exp. Clin. Cancer Res. 2023, 42, 329. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Liu, M.; Zhang, Y.; Zhou, Z.; Luo, W.; Fung, K.-M.; Xu, C.; Bronze, M.S.; Houchen, C.W.; et al. Circular RNA ANAPC7 Inhibits Tumor Growth and Muscle Wasting via PHLPP2–AKT–TGF-β Signaling Axis in Pancreatic Cancer. Gastroenterology 2022, 162, 2004–2017.e2. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017, 14, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Lu, L.; Wu, C.; Pan, X.; Liu, B.; Zhang, Y.; Wang, Y.; Wu, W.; Yan, B.; Zhang, Y.; et al. circHIPK3 prevents cardiac senescence by acting as a scaffold to recruit ubiquitin ligase to degrade HuR. Theranostics 2022, 12, 7550–7566. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhong, J.; Pan, X.; Su, Z.; Xu, Y.; Zhang, M.; Chen, X.; Chen, N.; Yu, T.; Zhou, Q. A novel intronic circular RNA circFGFR1int2 up-regulates FGFR1 by recruiting transcriptional activators P65/FUS and suppressing miR-4687-5p to promote prostate cancer progression. J. Transl. Med. 2023, 21, 840. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, D.; Caponnetto, A.; Brex, D.; Mirabella, F.; Barbagallo, C.; Lauretta, G.; Morrone, A.; Certo, F.; Broggi, G.; Caltabiano, R.; et al. CircSMARCA5 Regulates VEGFA mRNA Splicing and Angiogenesis in Glioblastoma Multiforme Through the Binding of SRSF1. Cancers 2019, 11, 194. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Wang, Y.; Deng, X.; Yan, Q.; Fan, C.; Zhang, S.; Zhang, S.; Gong, Z.; Shi, L.; et al. Circular RNA circPVT1 promotes nasopharyngeal carcinoma metastasis via the β-TrCP/c-Myc/SRSF1 positive feedback loop. Mol. Cancer 2022, 21, 192. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.-L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e29. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, E.E.; Kim, J.; Yang, R.; Chamseddin, B.; Ni, C.; Gusho, E.; Xie, Y.; Chiang, C.-M.; Buszczak, M.; et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat. Commun. 2019, 10, 2300. [Google Scholar] [CrossRef]
- Li, B.; Zhu, L.; Lu, C.; Wang, C.; Wang, H.; Jin, H.; Ma, X.; Cheng, Z.; Yu, C.; Wang, S.; et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat. Commun. 2021, 12, 295. [Google Scholar] [CrossRef]
- Zheng, W.; Chu, Q.; Yang, L.; Sun, L.; Xu, T. Circular RNA circDtx1 regulates IRF3-mediated antiviral immune responses through suppression of miR-15a-5p-dependent TRIF downregulation in teleost fish. PLoS Pathog. 2021, 17, e1009438. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Sun, J.; Sun, X. Identification and Validation of an Aging-Associated circRNA-miRNA-mRNA Network in Neovascular Age-Related Macular Degeneration. Gerontology 2023, 69, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Wang, J.-N.; Cao, Q.-C.; Sun, R.-X.; Zhu, H.-J.; Zhang, Y.-R.; Ji, J.-D.; Liu, Q.-H. m6A modification of circSPECC1 suppresses RPE oxidative damage and maintains retinal homeostasis. Cell Rep. 2022, 41, 111671. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Li, X.; Nan, F.; Jiang, S.; Gao, X.; Guo, S.-K.; Xue, W.; Cui, Y.; Dong, K.; Ding, H.; et al. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell 2019, 177, 865–880.e21. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Accounts Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, C.H.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Elsen, P.J.V.D.; Gobin, S.J.P.; van Eggermond, M.C.J.A.; Peijnenburg, A. Regulation of MHC class I and II gene transcription: Differences and similarities. Immunogenetics 1998, 48, 208–221. [Google Scholar] [CrossRef]
- Heesters, B.A.; van der Poel, C.E.; Das, A.; Carroll, M.C. Antigen Presentation to B Cells. Trends Immunol. 2016, 37, 844–854. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Katikaneni, D.S.; Jin, L. B cell MHC class II signaling: A story of life and death. Hum. Immunol. 2019, 80, 37–43. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef]
- Yue, X.; Zhong, C.; Cao, R.; Liu, S.; Qin, Z.; Liu, L.; Zhai, Y.; Luo, W.; Lian, Y.; Zhang, M.; et al. CircRNA based multivalent neuraminidase vaccine induces broad protection against influenza viruses in mice. NPJ Vaccines 2024, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, K.; Yang, K.; Ma, W.; Qi, S.; Yu, X.; He, J.; Lin, X.; Yu, G. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744.e16. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Li, X.; Wu, W.; Jiang, H.; Zheng, Y.; Zhou, J.; Ye, X.; Lu, J.; Wang, W.; et al. A single-dose circular RNA vaccine prevents Zika virus infection without enhancing dengue severity in mice. Nat. Commun. 2024, 15, 8932. [Google Scholar] [CrossRef]
- Cai, Z.; Wuri, Q.; Song, Y.; Qu, X.; Hu, H.; Cao, S.; Wu, H.; Wu, J.; Wang, C.; Yu, X.; et al. CircRNA-Loaded DC Vaccine in Combination with Low-Dose Gemcitabine Induced Potent Anti-Tumor Immunity in Pancreatic Cancer Model; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Schindewolf, C.; Braun, S.; Domdey, H. In vitro Generation of a Circular Exon from a Linear Pre-mRNA Transcript. Nucleic Acids Res. 1996, 24, 1260–1266. [Google Scholar] [CrossRef]
- Obi, P.; Chen, Y.G. The design and synthesis of circular RNAs. Methods 2021, 196, 85–103. [Google Scholar] [CrossRef]
- Beckert, B.; Masquida, B. Synthesis of RNA by in vitro transcription. Methods Mol. Biol. 2011, 703, 29–41. [Google Scholar] [CrossRef]
- Dolinnaya, N.G.; Sokolova, N.I.; Ashirbekova, D.T.; Shabarova, Z.A. The use of BrCN for assembling modified DNA duplexes and DNA-RNA hybrids; comparison with water-soluble carbodiimide. Nucleic Acids Res. 1991, 19, 3067–3072. [Google Scholar] [CrossRef]
- Müller, S.; Appel, B. In vitro circularization of RNA. RNA Biol. 2016, 14, 1018–1027. [Google Scholar] [CrossRef]
- Petkovic, S.; Müller, S. RNA self-processing: Formation of cyclic species and concatemers from a small engineered RNA. FEBS Lett. 2013, 587, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, M.; Been, M. Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res. 1992, 20, 5357–5364. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, A.; Johansen, S.D. Nuclear group I introns in self-splicing and beyond. Mob. DNA 2013, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, S.; Song, J.; Han, S.R.; Kim, J.H.; Lee, S.-W. Efficient circular RNA engineering by end-to-end self-targeting and splicing reaction using Tetrahymena group I intron ribozyme. Mol. Ther. Nucleic Acids 2023, 33, 587–598. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Pyle, A.M. Group II Intron Self-Splicing. Annu. Rev. Biophys. 2016, 45, 183–205. [Google Scholar] [CrossRef]
- Mikheeva, S.; Hakim-Zargar, M.; Carlson, D.; Jarrell, K. Use of an engineered ribozyme to produce a circular human exon. Nucleic Acids Res. 1997, 25, 5085–5094. [Google Scholar] [CrossRef]
- Hieronymus, R.; Müller, S. Engineering of hairpin ribozyme variants for RNA recombination and splicing. Ann. N. Y. Acad. Sci. 2019, 1447, 135–143. [Google Scholar] [CrossRef]
- Diegelman, A.M.; Daubendiek, S.L.; Kool, E.T. Generation of RNA Ladders by Rolling Circle Transcription of Small Circular Oligodeoxyribonucleotides. BioTechniques 1998, 25, 754–758. [Google Scholar] [CrossRef]
- Strohbach, D.; Novak, N.; Müller, S. Redox-Active Riboswitching: Allosteric Regulation of Ribozyme Activity by Ligand-Shape Control. Angew. Chem. Int. Ed. 2006, 45, 2127–2129. [Google Scholar] [CrossRef]
- Jeong, M.; Lee, Y.; Park, J.; Jung, H.; Lee, H. Lipid nanoparticles (LNPs) for in vivo RNA delivery and their breakthrough technology for future applications. Adv. Drug Deliv. Rev. 2023, 200, 114990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, B.; Wu, Y.; Lee, R.J.; Lee, L.J. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer Res. 2011, 31, 1619–1626. [Google Scholar] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ye, T.; Yang, Y.; Li, E.; Zhang, K.; Wang, Y.; Chen, S.; Hu, J.; Zhang, K.; Liu, F.; et al. Circular RNA vaccines against monkeypox virus provide potent protection against vaccinia virus infection in mice. Mol. Ther. 2024, 32, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, G.; Wang, Y.; Zhuang, Q.; Cai, Z.; Li, Y.; Gao, S.; Li, F.; Zhang, C.; Zhao, B.; et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. Medcomm 2024, 5, e667. [Google Scholar] [CrossRef]
- Wan, J.; Wang, Z.; Wang, L.; Wu, L.; Zhang, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. mBio 2024, 15, e0177523. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Sun, J.; Chen, Y.; Du, Y.; Tan, Y.; Wu, L.; Zhai, M.; Wei, L.; Li, N.; et al. Intratumoral delivered novel circular mRNA encoding cytokines for immune modulation and cancer therapy. Mol. Ther. Nucleic Acids 2022, 30, 184–197. [Google Scholar] [CrossRef]
- Unti, M.J.; Jaffrey, S.R. Highly efficient cellular expression of circular mRNA enables prolonged protein expression. Cell Chem. Biol. 2024, 31, 163–176. [Google Scholar] [CrossRef]
- Yu, X.; Bai, Y.; Han, B.; Ju, M.; Tang, T.; Shen, L.; Li, M.; Yang, L.; Zhang, Z.; Hu, G.; et al. Extracellular vesicle-mediated delivery of circDYM alleviates CUS-induced depressive-like behaviours. J. Extracell. Vesicles 2022, 11, e12185. [Google Scholar] [CrossRef]
- Fan, L.; Yao, L.; Li, Z.; Wan, Z.; Sun, W.; Qiu, S.; Zhang, W.; Xiao, D.; Song, L.; Yang, G.; et al. Exosome-Based Mitochondrial Delivery of circRNA mSCAR Alleviates Sepsis by Orchestrating Macrophage Activation. Adv. Sci. 2023, 10, 2205692. [Google Scholar] [CrossRef]
- Seephetdee, C.; Bhukhai, K.; Buasri, N.; Leelukkanaveera, P.; Lerdwattanasombat, P.; Manopwisedjaroen, S.; Phueakphud, N.; Kuhaudomlarp, S.; Olmedillas, E.; Saphire, E.O.; et al. A circular mRNA vaccine prototype producing VFLIP-X spike confers a broad neutralization of SARS-CoV-2 variants by mouse sera. Antivir. Res. 2022, 204, 105370. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, X.; Qin, H.; Li, Y.; Zhu, J.; Yin, B.; Zheng, Q.; Zuo, C.; Cao, H.; Tong, Z.; et al. FGF18 encoding circular mRNA-LNP based on glycerolipid engineering of mesenchymal stem cells for efficient amelioration of osteoarthritis. Biomater. Sci. 2024, 12, 4427–4439. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, Y.; Solek, N.C.; Chen, J.; Gong, F.; Varley, A.J.; Golubovic, A.; Pan, A.; Dong, S.; Zheng, G.; et al. Tumor-Tailored Ionizable Lipid Nanoparticles Facilitate IL-12 Circular RNA Delivery for Enhanced Lung Cancer Immunotherapy. Adv. Mater. 2024, 36, e2400307. [Google Scholar] [CrossRef]
- Probst, J.; Weide, B.; Scheel, B.; Pichler, B.J.; Hoerr, I.; Rammensee, H.-G.; Pascolo, S. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007, 14, 1175–1180. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: The Fourth Century. Clin. Vaccine Immunol. 2009, 16, 1709–1719. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, Y.K. Molecular mechanisms of circular RNA translation. Exp. Mol. Med. 2024, 56, 1272–1280. [Google Scholar] [CrossRef]
- Spriggs, K.A.; Stoneley, M.; Bushell, M.; Willis, A.E. Re-programming of translation following cell stress allows IRES-mediated translation to predominate. Biol. Cell 2008, 100, 27–38. [Google Scholar] [CrossRef]
- Amaya, L.; Grigoryan, L.; Li, Z.; Lee, A.; Wender, P.A.; Pulendran, B.; Chang, H.Y. Circular RNA vaccine induces potent T cell responses. Proc. Natl. Acad. Sci. USA 2023, 120, e2302191120. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lee, E.E.; Kim, J.; Choi, J.H.; Kolitz, E.; Chen, Y.; Crewe, C.; Salisbury, N.J.H.; Scherer, P.E.; Cockerell, C.; et al. Characterization of ALTO-encoding circular RNAs expressed by Merkel cell polyomavirus and trichodysplasia spinulosa polyomavirus. PLoS Pathog. 2021, 17, e1009582. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, C.; Liu, Y. Circular RNA circ_0091579 Promotes Hepatocellular Carcinoma Proliferation, Migration, Invasion, and Glycolysis Through miR-490-5p/CASC3 Axis. Cancer Biother. Radiopharm. 2021, 36, 863–878. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, L.; Geng, S.; Yang, L.; Lv, X.; Xin, S.; Xu, T. CircMIB2 therapy can effectively treat pathogenic infection by encoding a novel protein. Cell Death Dis. 2023, 14, 578. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Peletta, A.; Lemoine, C.; Courant, T.; Collin, N.; Borchard, G. Meeting vaccine formulation challenges in an emergency setting: Towards the development of accessible vaccines. Pharmacol. Res. 2023, 189, 106699. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, S.; Wen, H.; Rao, M.; Zhang, P.; Peng, W.; Cui, Y.; Yang, H.; Tan, C.; Chen, J.; et al. Development of a novel circular mRNA vaccine of six protein combinations against Staphylococcus aureus. J. Biomol. Struct. Dyn. 2023, 41, 10525–10545. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: An open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Hu, X.; Karthigeyan, K.P.; Herbek, S.; Valencia, S.M.; Jenks, J.A.; Webster, H.; Miller, I.G.; Connors, M.; Pollara, J.; Andy, C.; et al. Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine. J. Infect. Dis. 2024, 230, 455–466. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Narayanan, E.; Liu, Q.; Tsybovsky, Y.; Boswell, K.; Ding, S.; Hu, Z.; Follmann, D.; Lin, Y.; Miao, H.; et al. A multiclade env–gag VLP mRNA vaccine elicits tier-2 HIV-1-neutralizing antibodies and reduces the risk of heterologous SHIV infection in macaques. Nat. Med. 2021, 27, 2234–2245. [Google Scholar] [CrossRef] [PubMed]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Zhong, J.; Wu, X.; Leng, Z.; Liu, M.; Wang, Y.; Wang, Y.; Yang, X.; Huang, N.; et al. Lnc-H19-derived protein shapes the immunosuppressive microenvironment of glioblastoma. Cell Rep. Med. 2024, 5, 101806. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, X.; Ye, S.; Zhang, J.; Liao, J.; Zhang, N.; Zeng, X.; Wang, J.; Yang, B.; Zhang, Y.; et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 2023, 625, 593–602. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Peng, X.; Yang, Y.; Chen, Q.; Liu, J.; She, Q.; Tan, J.; Lou, C.; Liao, Z.; et al. mRNA vaccine in cancer therapy: Current advance and future outlook. Clin. Transl. Med. 2023, 13, e1384. [Google Scholar] [CrossRef]
- Ma, L.; Dichwalkar, T.; Chang, J.Y.H.; Cossette, B.; Garafola, D.; Zhang, A.Q.; Fichter, M.; Wang, C.; Liang, S.; Silva, M.; et al. Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019, 365, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Kos, F.J.; He, T.-F.; Yin, H.H.; Li, M.; Hardwick, N.; Zurcher, K.; Schmolze, D.; Lee, P.; Pillai, R.K.; et al. Complete regression of cutaneous metastases with systemic immune response in a patient with triple negative breast cancer receiving p53MVA vaccine with pembrolizumab. OncoImmunology 2017, 6, e1363138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhao, H.; Zhou, K.; Hua, X.; Zhang, X. Scarless circular mRNA-based CAR-T cell therapy elicits superior anti-tumor efficacy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Jeremy, S.A.; Maria Lia, P.; Leo, I.G.; Matthew, A.L.; Michael, L.W.; Jon, E.A.; Amitkumar, M.; Enkhtsetseg, P.; David, G.M.; Charalambos, A.; et al. Pivotal Safety and Efficacy Results from Transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed/Refractory (R/R) Large B Cell Lymphomas. Blood 2019, 134, 241. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
- Ma, L.; Hostetler, A.; Morgan, D.M.; Maiorino, L.; Sulkaj, I.; Whittaker, C.A.; Neeser, A.; Pires, I.S.; Yousefpour, P.; Gregory, J.; et al. Vaccine-boosted CAR T crosstalk with host immunity to reject tumors with antigen heterogeneity. Cell 2023, 186, 3148–3165.e20. [Google Scholar] [CrossRef]
- Mackensen, A.; Haanen, J.B.; Koenecke, C.; Alsdorf, W.; Wagner-Drouet, E.; Borchmann, P.; Heudobler, D.; Ferstl, B.; Klobuch, S.; Bokemeyer, C.; et al. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: The phase 1 BNT211-01 trial. Nat. Med. 2023, 29, 2844–2853. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, L.; Wang, X.; Li, J.; Yin, J.; Gao, F.; Liao, X.; Zhang, C.; Yin, Q.; Zhao, C.; et al. Synergically enhanced anti-tumor immunity of in vivo CAR by circRNA vaccine boosting. bioRxiv 2024. [Google Scholar] [CrossRef]
- He, J.; Xiong, X.; Yang, H.; Li, D.; Liu, X.; Li, S.; Liao, S.; Chen, S.; Wen, X.; Yu, K.; et al. Defined tumor antigen-specific T cells potentiate personalized TCR-T cell therapy and prediction of immunotherapy response. Cell Res. 2022, 32, 530–542. [Google Scholar] [CrossRef]
- Baulu, E.; Gardet, C.; Chuvin, N.; Depil, S. TCR-engineered T cell therapy in solid tumors: State of the art and perspectives. Sci. Adv. 2023, 9, eadf3700. [Google Scholar] [CrossRef]
- Shen, L.; Yang, J.; Zuo, C.; Xu, J.; Ma, L.; He, Q.; Zhou, X.; Ding, X.; Wei, L.; Jiang, S.; et al. Circular mRNA-based TCR-T offers a safe and effective therapeutic strategy for treatment of cytomegalovirus infection. Mol. Ther. 2024, 32, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Panesso, M.; Uría, M.L.; Renedo, B.; Esperalba, J.; Benítez-Carabante, M.I.; Mendoza-Palomar, N.; Alonso, L.; Oliveras, M.; Diaz-De-Heredia, C. CMV hyperimmune globulin as salvage therapy for recurrent or refractory CMV infection in children undergoing hematopoietic stem cell transplantation. Front. Pediatr. 2023, 11, 1197828. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Zhu, Y.; Shen, Y.-Y.; Xu, Q.-Y.; Tang, H.-Y.; Cui, N.-X.; Jiang, L.; Dai, X.-M.; Chen, W.-Q.; Lin, Q.; et al. The role of PD-1 signaling in health and immune-related diseases. Front. Immunol. 2023, 14, 1163633. [Google Scholar] [CrossRef] [PubMed]
- Ajona, D.; Ortiz-Espinosa, S.; Moreno, H.; Lozano, T.; Pajares, M.J.; Agorreta, J.; Bértolo, C.; Lasarte, J.J.; Vicent, S.; Hoehlig, K.; et al. A Combined PD-1/C5a Blockade Synergistically Protects against Lung Cancer Growth and Metastasis. Cancer Discov. 2017, 7, 694–703. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Yuan, W.; Wang, H.; Chen, K.; Li, D.; Li, D. PD-1/PD-L1 interaction up-regulates MDR1/P-gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget 2017, 8, 99901–99912. [Google Scholar] [CrossRef]
- Bin Wang, H.; Yao, H.; Li, C.S.; Liang, L.X.; Zhang, Y.; Chen, Y.X.; Fang, J.; Xu, J. Rise of PD-L1 expression during metastasis of colorectal cancer: Implications for immunotherapy. J. Dig. Dis. 2017, 18, 574–581. [Google Scholar] [CrossRef]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node–targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef]
- Yang, L.; Wilusz, J.E.; Chen, L.L. Biogenesis and Regulatory Roles of Circular RNAs. Annu. Rev. Cell Dev. Biol. 2022, 38, 263–289. [Google Scholar] [CrossRef]


| System Type | System Name | Mechanisms | Advantages | References |
|---|---|---|---|---|
| Naked RNA delivery | Intratumoral injection | The naked RNA is taken up by DCs through micropinocytosis. | Avoid side effects Good targeting | [58] |
| VLPs (virus-like particles) | VLP | VLP possesses viral structural proteins but lacks a viral genome, and its mechanism of entering cells is consistent with that of natural viruses. | Targeted delivery Good safety | [59] |
| Extracellular vesicle | EV | Exosome fuses with the plasma membrane of the target cell or is endocytosed directly into the cell to release the payload. | High tissue penetration Good biocompatibility | [60,61] |
| LNPs | LNP | LNP is taken up via endocytosis. The pH of the mature endosome decreases, causing the LNP to become protonated, which leads to the fusion with the endosomal membrane and the release of circRNA. | Simple preparation High effectiveness Large load | [33,34,56,62] |
| TG6A-LNP | TG6A (an ionizable glycerolipid) undergoes rapid degradation upon entering the cytoplasm, releasing the circRNA. | Excellent degradability High protein expression | [63] | |
| H1L1A1B3 LNP | H1L1AB3 is a tumor-targeting ionizable lipid that can directly release drugs to the tumor site. | High transfection efficiency | [64] | |
| mLNP | By modifying ionizable lipids with mannose, DCs can take up nanoparticles via mannose receptor-mediated endocytosis. | Stable targeting Stable physical properties | [57] |
| Advantages | Disadvantages | |
|---|---|---|
| Stability | High stability and resistance to RNase hydrolysis | Unknown |
| Security | Contains no virus ingredients, no risk of infection | Unknown clinical safety issues |
| Immunogenicity | It can induce both humoral immunity and high proportion of neutralizing antibodies | Potential adverse effects caused by immunogenicity |
| Production | No complicated modifications required | High production cost and unstable cyclization efficiency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, T.; Yang, Z.; Zhao, J.; Gao, Y.; Li, F.; Yang, R. Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2025, 26, 379. https://doi.org/10.3390/ijms26010379
Bu T, Yang Z, Zhao J, Gao Y, Li F, Yang R. Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach. International Journal of Molecular Sciences. 2025; 26(1):379. https://doi.org/10.3390/ijms26010379
Chicago/Turabian StyleBu, Tian, Ziyu Yang, Jian Zhao, Yanmei Gao, Faxiang Li, and Rong Yang. 2025. "Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach" International Journal of Molecular Sciences 26, no. 1: 379. https://doi.org/10.3390/ijms26010379
APA StyleBu, T., Yang, Z., Zhao, J., Gao, Y., Li, F., & Yang, R. (2025). Expanding the Potential of Circular RNA (CircRNA) Vaccines: A Promising Therapeutic Approach. International Journal of Molecular Sciences, 26(1), 379. https://doi.org/10.3390/ijms26010379






