Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms
Abstract
:1. Introduction
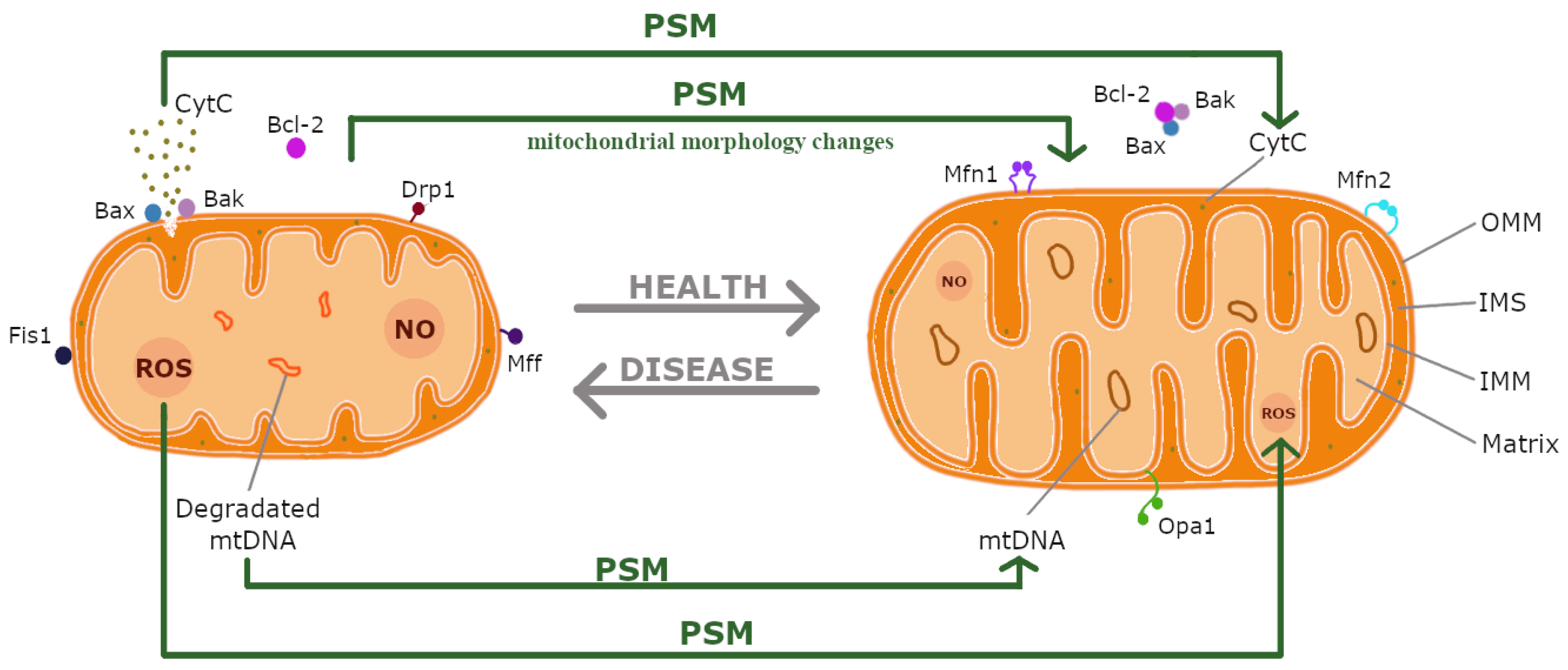
2. Mitochondria
2.1. Mitochondria: Functions and Structure
2.2. Mitochondrial Dynamics
2.3. Mitochondrial Dysfunctions and Associated Diseases
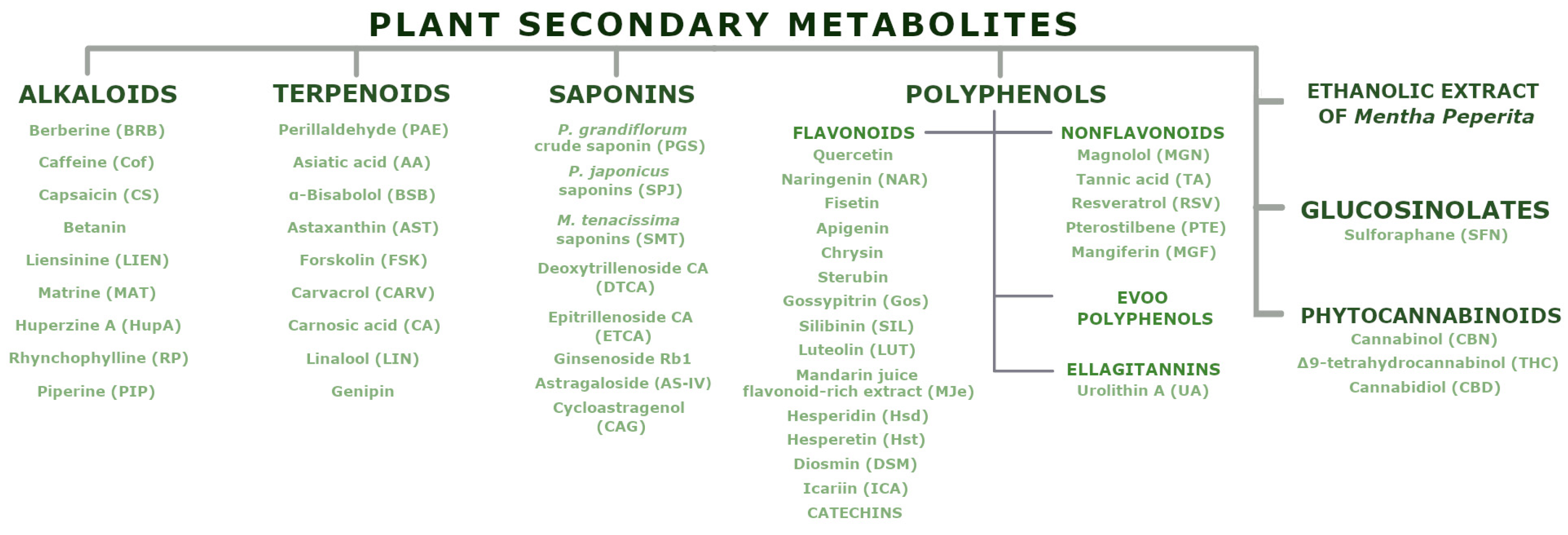
3. Plant Secondary Metabolites
3.1. Alkaloids
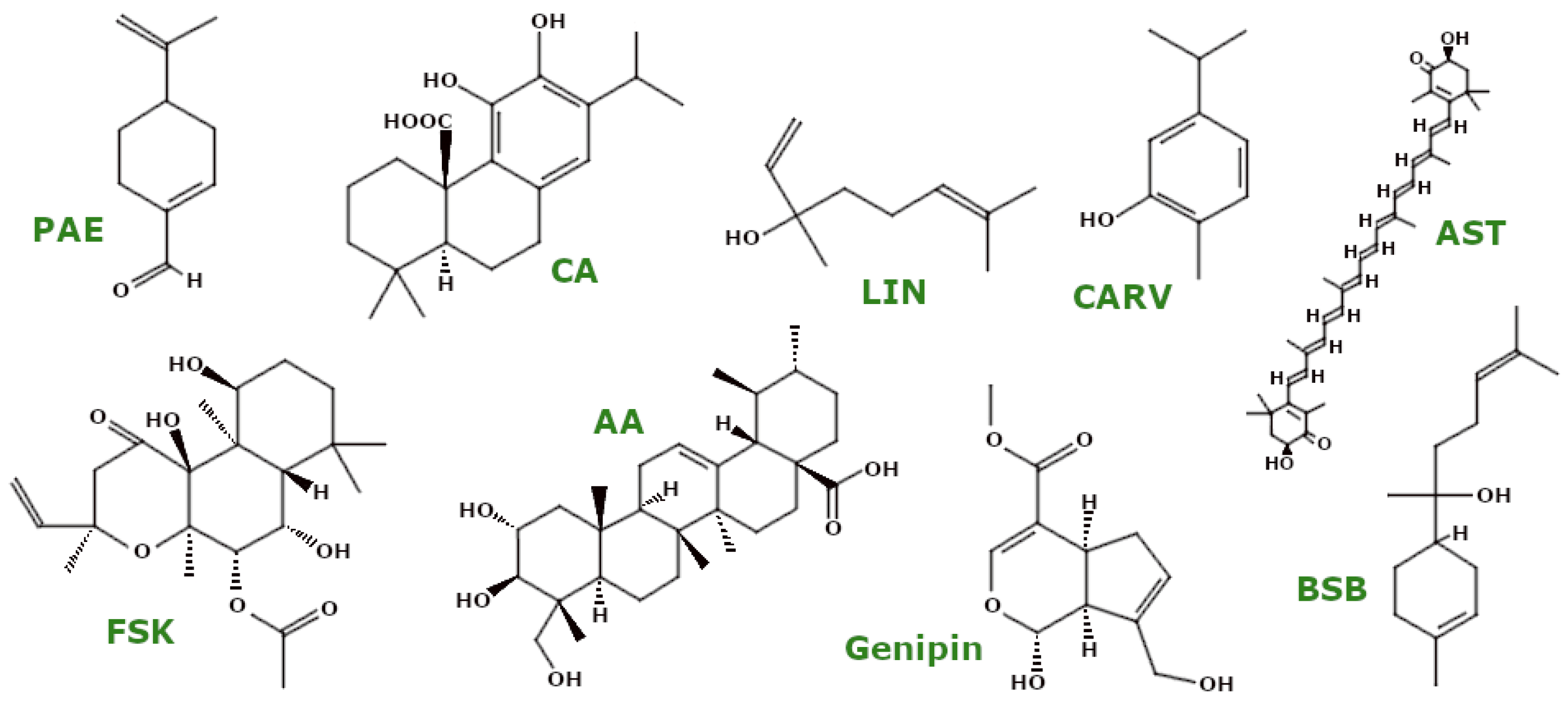
3.2. Terpenoids
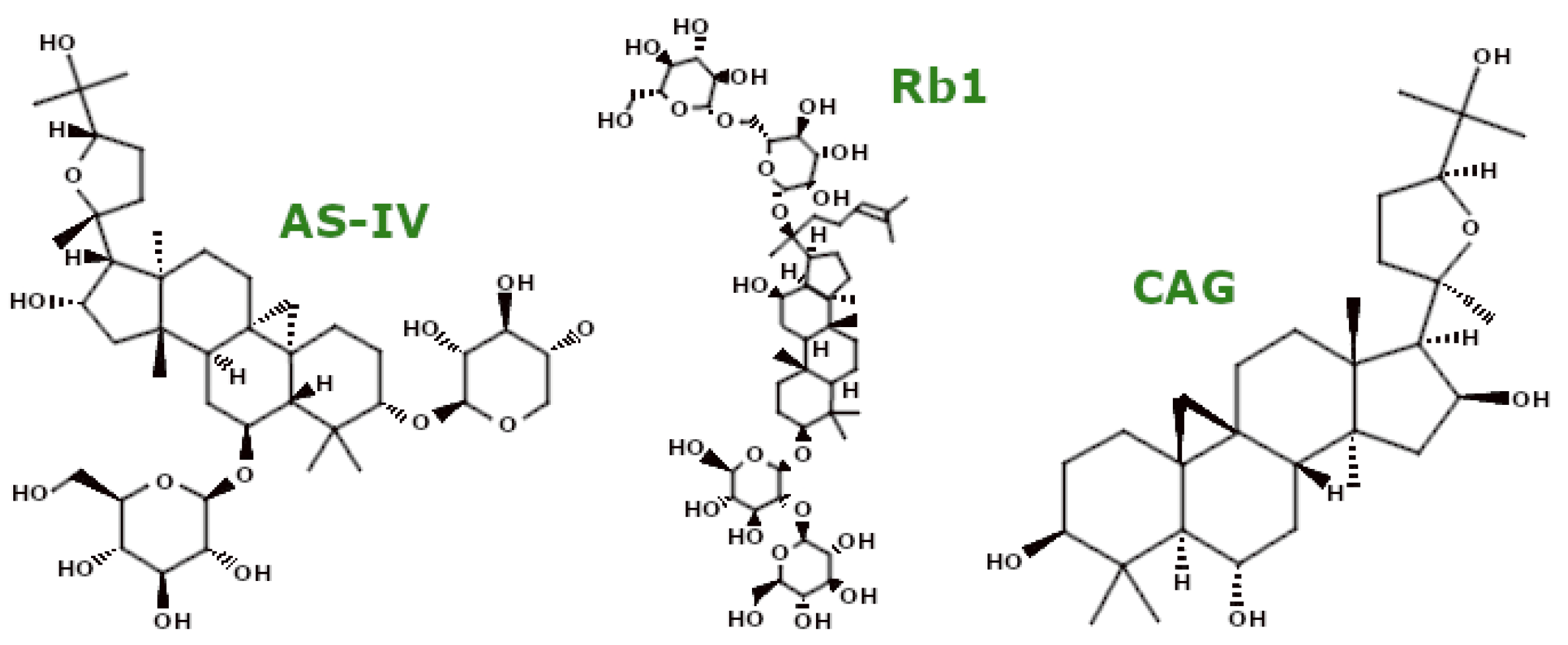
3.3. Saponins
3.4. Polyphenols
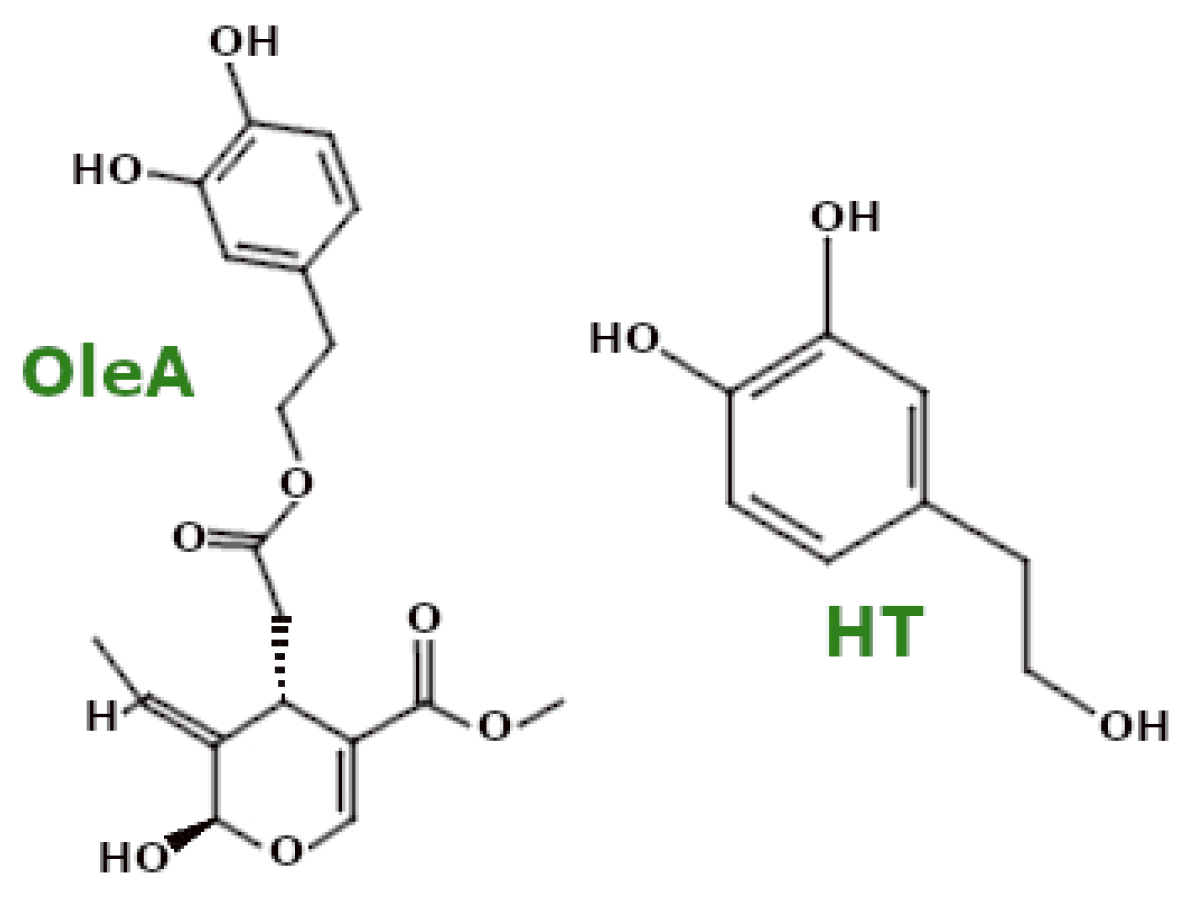
3.4.1. Polyphenols—EVOO Polyphenols
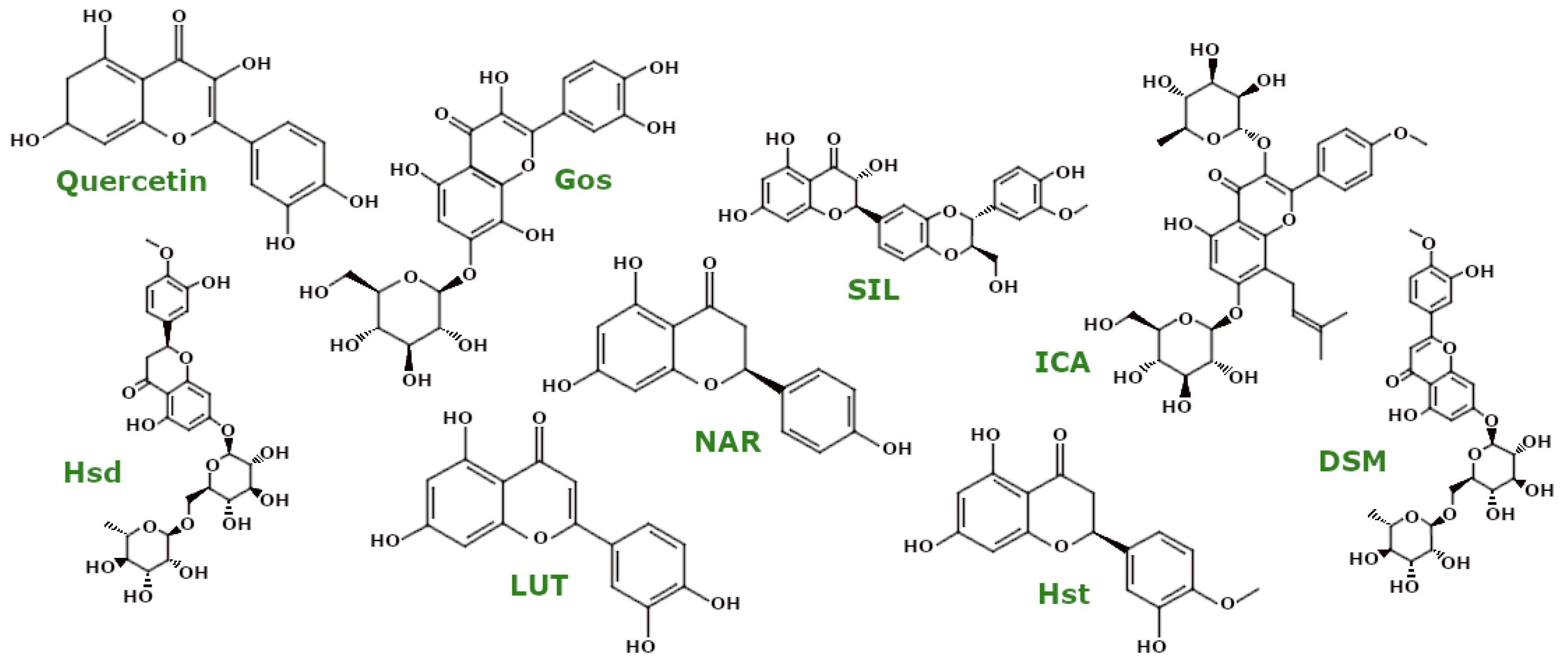
3.4.2. Polyphenols—Flavonoids
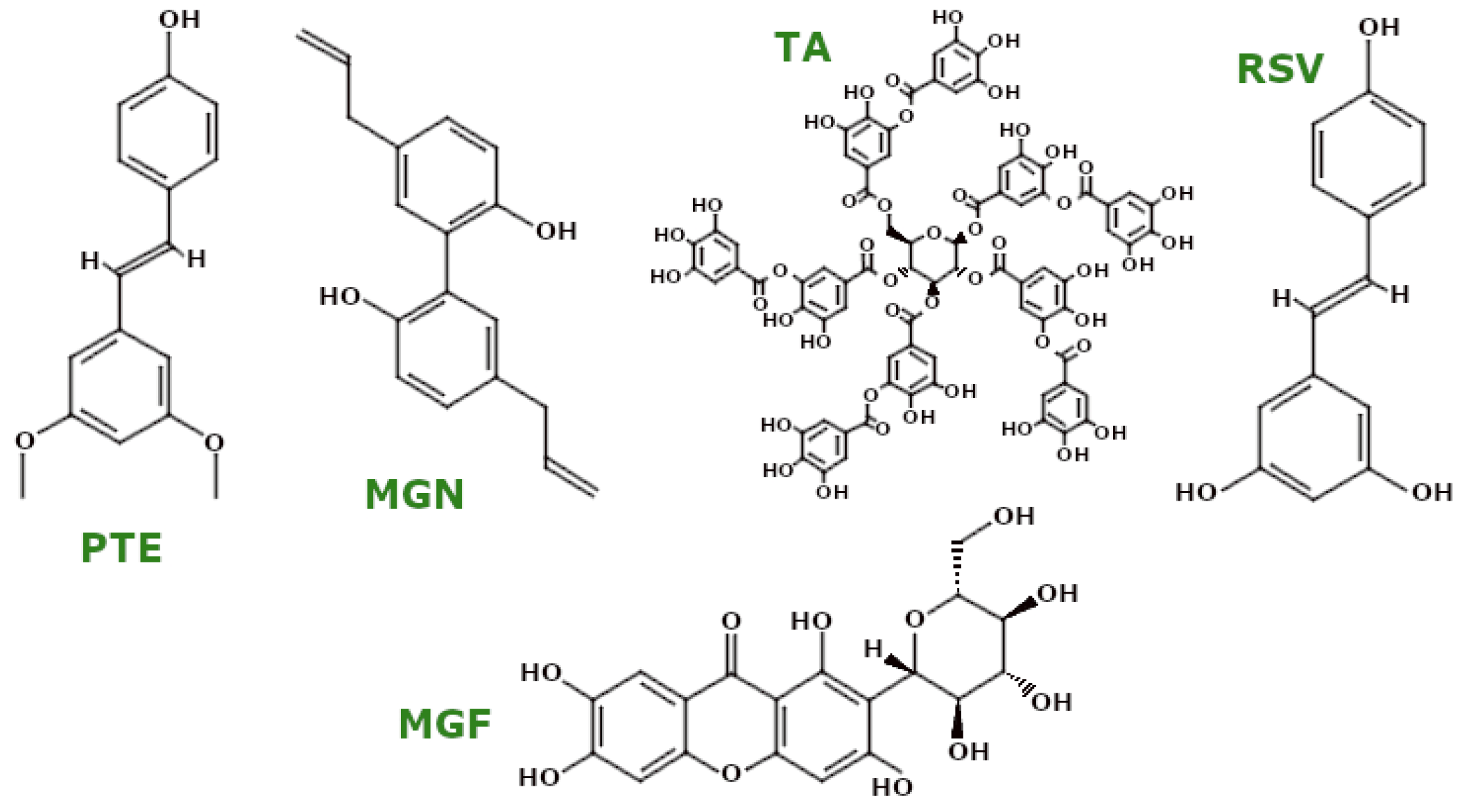
3.4.3. Polyphenols—Non-Flavonoid Polyphenols
3.4.4. Polyphenols—Ellagitannins
3.5. Glucosinolates
3.6. Ethanolic Extract of Mentha Peperita (EthMP)
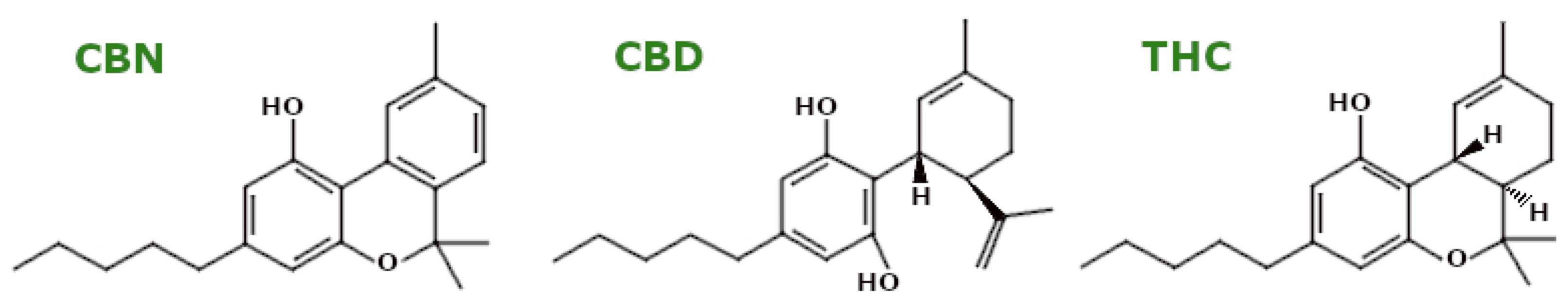
3.7. Phytocannabinoids
4. Future Perspective in Mitochondria Modulation: Synthetic, Semi-Synthetic, and PSMs in Drug Development
5. Limitations and Risks in the Utilisation of PSMs
6. Conclusions
7. Methodology
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, J.R.; Nunnari, J. Mitochondrial Form and Function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, Q.; Shi, H.; Li, F.; Duan, Y.; Guo, Q. New Insights into the Role of Mitochondrial Dynamics in Oxidative Stress-Induced Diseases. Biomed. Pharmacother. 2024, 178, 117084. [Google Scholar] [CrossRef]
- Heindel, J.J.; Lustig, R.H.; Howard, S.; Corkey, B.E. Obesogens: A Unifying Theory for the Global Rise in Obesity. Int. J. Obes. 2024, 48, 449–460. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhu, L.; Huang, Y.; Chen, J.; Tang, Q. Mitochondria: Fundamental Characteristics, Challenges, and Impact on Aging. Biogerontology 2024, 25, 923–941. [Google Scholar] [CrossRef] [PubMed]
- Sinha, J.K.; Jorwal, K.; Singh, K.K.; Han, S.S.; Bhaskar, R.; Ghosh, S. The Potential of Mitochondrial Therapeutics in the Treatment of Oxidative Stress and Inflammation in Aging. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, X.; Ma, J.; Liu, S.; Jin, X.; Liu, B. Unveiling the Longevity Potential of Natural Phytochemicals: A Comprehensive Review of Active Ingredients in Dietary Plants and Herbs. J. Agric. Food Chem. 2024, 72, 24908–24927. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xiao, Y.; Guo, J.; Lv, Z.; Chen, W. Relevance and Regulation of Alternative Splicing in Plant Secondary Metabolism: Current Understanding and Future Directions. Hortic. Res. 2024, 11, uhae173. [Google Scholar] [CrossRef]
- Mu, J.-K.; Li, Y.-Q.; Shi, T.-T.; Yu, L.-P.; Yang, Y.-Q.; Gu, W.; Li, J.-P.; Yu, J.; Yang, X.-X. Remedying the Mitochondria to Cure Human Diseases by Natural Products. Oxidative Med. Cell. Longev. 2020, 2020, 5232614. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yu, C.; Dong, X.; Yang, F.; Wang, M.; Wen, Z.; Su, M.; Li, B.; Yang, L. New Insights into Mitochondria in Health and Diseases. Int. J. Mol. Sci. 2024, 25, 9975. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R. The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family. Cold Spring Harb. Perspect. Biol. 2022, 14, a041046. [Google Scholar] [CrossRef]
- Kulikov, A.V.; Shilov, E.S.; Mufazalov, I.A.; Gogvadze, V.; Nedospasov, S.A.; Zhivotovsky, B. Cytochrome c: The Achilles’ Heel in Apoptosis. Cell. Mol. Life Sci. 2011, 69, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Yapa, N.M.B.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial Dynamics in Health and Disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Nunnari, J. Mitochondria at the Crossroads of Health and Disease. Cell 2024, 187, 2601–2627. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Grel, H.; Woznica, D.; Ratajczak, K.; Kalwarczyk, E.; Anchimowicz, J.; Switlik, W.; Olejnik, P.; Zielonka, P.; Stobiecka, M.; Jakiela, S. Mitochondrial Dynamics in Neurodegenerative Diseases: Unraveling the Role of Fusion and Fission Processes. Int. J. Mol. Sci. 2023, 24, 13033. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-H.; Xu, C.-Z.; Liu, Y.; Lu, Z.-L.; Fu, T.-L.; Li, G.-R.; Deng, Y.; Luo, G.-Q.; Ding, S.; Li, N.; et al. Mitochondrial Quality Control in Human Health and Disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular Mitophagy: Mechanism, Roles in Diseases and Small Molecule Pharmacological Regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Quinn, P.M.J.; Moreira, P.I.; Ambrósio, A.F.; Alves, C.H. PINK1/PARKIN Signalling in Neurodegeneration and Neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef]
- Diao, R.Y.; Gustafsson, Å.B. Mitochondrial Quality Surveillance: Mitophagy in Cardiovascular Health and Disease. Am. J. Physiol.-Cell Physiol. 2021, 322, C218–C230. [Google Scholar] [CrossRef] [PubMed]
- Hallacli, E.; Kayatekin, C.; Nazeen, S.; Wang, X.H.; Sheinkopf, Z.; Sathyakumar, S.; Sarkar, S.; Jiang, X.; Dong, X.; Maio, R.D.; et al. The Parkinson’s Disease Protein Alpha-Synuclein Is a Modulator of Processing-Bodies and mRNA Stability. Cell 2022, 185, 2035–2056.e33. [Google Scholar] [CrossRef] [PubMed]
- Trigo, D.; Avelar, C.; Fernandes, M.; Sá, J.; da Cruz e Silva, O. Mitochondria, Energy, and Metabolism in Neuronal Health and Disease. FEBS Lett. 2022, 596, 1095–1110. [Google Scholar] [CrossRef]
- Jakovljevic, N.K.; Pavlovic, K.; Jotic, A.; Lalic, K.; Stoiljkovic, M.; Lukic, L.; Milicic, T.; Macesic, M.; Gajovic, J.S.; Lalic, N.M. Targeting Mitochondria in Diabetes. Int. J. Mol. Sci. 2021, 22, 6642. [Google Scholar] [CrossRef]
- Alka, K.; Kumar, J.; Kowluru, R.A. Impaired Mitochondrial Dynamics and Removal of the Damaged Mitochondria in Diabetic Retinopathy. Front. Endocrinol. 2023, 14, 1160155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.N.; Zhou, M.Q.; Guo, J.; Zheng, H.J.; Tang, J.; Zhang, C.; Liu, Y.N.; Liu, W.J.; Wang, Y.X. Mitochondrial Dysfunction and Diabetic Nephropathy: Nontraditional Therapeutic Opportunities. J. Diabetes Res. 2021, 2021, 1010268. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Lee, K.-M.; Kim, Y.-Y.; Lee, D.-Y.; Kim, E.; Kim, D.-H.; Yun, J. Berberine Induces Mitophagy through Adenosine Monophosphate-Activated Protein Kinase and Ameliorates Mitochondrial Dysfunction in PINK1 Knockout Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2023, 25, 219. [Google Scholar] [CrossRef]
- Wang, C.; Zou, Q.; Pu, Y.; Cai, Z.; Tang, Y. Berberine Rescues D-Ribose-Induced Alzheimer’s Pathology via Promoting Mitophagy. Int. J. Mol. Sci. 2023, 24, 5896. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sheng, W.; Tan, Z.; Ren, Q.; Wang, R.; Stoika, R.; Liu, X.; Liu, K.; Shang, X.; Jin, M. Treatment of Parkinson’s Disease in Zebrafish Model with a Berberine Derivative Capable of Crossing Blood Brain Barrier, Targeting Mitochondria, and Convenient for Bioimaging Experiments. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 249, 109151. [Google Scholar] [CrossRef] [PubMed]
- Phogat, A.; Singh, J.; Malik, V.; Kumar, V. Neuroprotective Potential of Berberine against Acetamiprid Induced Toxicity in Rats: Implication of Oxidative Stress, Mitochondrial Alterations, and Structural Changes in Brain Regions. J. Biochem. Mol. Toxicol. 2023, 37, e23434. [Google Scholar] [CrossRef] [PubMed]
- Yadawa, A.K.; Srivastava, P.; Singh, A.; Kumar, R.; Arya, J.K.; Rizvi, S.I. Berberine Attenuates Brain Aging via Stabilizing Redox Homeostasis and Inflammation in an Accelerated Senescence Model of Wistar Rats. Metab. Brain Dis. 2024, 39, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-C.; Wang, M.-H.; Fang, C.-H.; Lin, Y.-W.; Soung, H.-S. Neuroprotective Potentials of Berberine in Rotenone-Induced Parkinson’s Disease-like Motor Symptoms in Rats. Brain Sci. 2024, 14, 596. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Youn, E.; Shim, Y.-H. Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis Elegans Model. Nutrients 2021, 13, 2517. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gan, C.; Gao, Z.; Huang, Y.; Wu, S.; Zhang, D.; Wang, X.; Sheng, J. Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria. Front. Cell Dev. Biol. 2020, 8, 822. [Google Scholar] [CrossRef]
- Han, T.-H.; Park, M.K.; Nakamura, H.; Ban, H.S. Capsaicin Inhibits HIF-1α Accumulation through Suppression of Mitochondrial Respiration in Lung Cancer Cells. Biomed. Pharmacother. 2022, 146, 112500. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, L.; Hu, T.; Yin, D.; He, H.; He, M. Capsaicin Protects Cardiomyocytes against Lipopolysaccharide-Induced Damage via 14-3-3γ-Mediated Autophagy Augmentation. Front. Pharmacol. 2021, 12, 659015. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Bahiraei, T.; Ahdeno, S.; Vatanpour, S.; Pourahmad, J. Evaluation of Cytotoxic Activity of Betanin Against U87MG Human Glioma Cells and Normal Human Lymphocytes and Its Anticancer Potential Through Mitochondrial Pathway. Nutr. Cancer 2021, 73, 450–459. [Google Scholar] [CrossRef]
- Hafez, A.; Jamali, Z.; Samiei, S.; Khezri, S.; Salimi, A. Reduction of Doxorubicin-Induced Cytotoxicity and Mitochondrial Damage by Betanin in Rat Isolated Cardiomyocytes and Mitochondria. Hum. Exp. Toxicol. 2021, 40, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, S.; Wang, L.; Ceylan, A.F.; Ren, J.; Zhang, Y. Mitophagy Inhibitor Liensinine Suppresses Doxorubicin-Induced Cardiotoxicity through Inhibition of Drp1-Mediated Maladaptive Mitochondrial Fission. Pharmacol. Res. 2020, 157, 104846. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.-J.; Huang, X.-H.; Zheng, C.-C.; Yin, X.-F.; Li, B.; He, Q.-Y. Liensinine Perchlorate Inhibits Colorectal Cancer Tumorigenesis by Inducing Mitochondrial Dysfunction and Apoptosis. Food Funct. 2018, 9, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine Alleviates Cisplatin-Induced Acute Kidney Injury by Inhibiting Mitochondrial Dysfunction and Inflammation via SIRT3/OPA1 Pathway. J. Cell. Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wei, R.; Yao, S. Matrine Has Pro-Apoptotic Effects on Liver Cancer by Triggering Mitochondrial Fission and Activating Mst1-JNK Signalling Pathways. J. Physiol. Sci. 2019, 69, 185–198. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Q.; Zhu, X.; Wang, Y. ABAD/17β-HSD10 Reduction Contributes to the Protective Mechanism of Huperzine a on the Cerebral Mitochondrial Function in APP/PS1 Mice. Neurobiol. Aging 2019, 81, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.-J.; Cui, L.-Q.; Li, P.; Wang, Y.-B.; Zhang, X.-Z.; Guo, M.-L. Rhynchophylline Ameliorates Myocardial Ischemia/Reperfusion Injury through the Modulation of Mitochondrial Mechanisms to Mediate Myocardial Apoptosis. Mol. Med. Rep. 2019, 19, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Zhu, Y.; Wang, Y.; Liu, X.; Nie, X.; Zhao, J.; Wang, W.; Cheng, J. Rhynchophylline Regulates Calcium Homeostasis by Antagonizing Ryanodine Receptor 2 Phosphorylation to Improve Diabetic Cardiomyopathy. Front. Pharmacol. 2022, 13, 882198. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, Y.; Sheng, Y.; Wang, J.; Ruan, S.; Han, C. Mechanism of Piperine in Affecting Apoptosis and Proliferation of Gastric Cancer Cells via ROS-mitochondria-associated Signalling Pathway. J. Cell. Mol. Med. 2021, 25, 9513–9522. [Google Scholar] [CrossRef]
- Kaushik, P.; Ali, M.; Salman, M.; Tabassum, H.; Parvez, S. Harnessing the Mitochondrial Integrity for Neuroprotection: Therapeutic Role of Piperine against Experimental Ischemic Stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, X.; Jiang, Y.; Jin, B.; Wang, L. Functions of Representative Terpenoids and Their Biosynthesis Mechanisms in Medicinal Plants. Biomolecules 2023, 13, 1725. [Google Scholar] [CrossRef]
- Fang, M.; Liu, Y.; Gao, X.; Yu, J.; Tu, X.; Mo, X.; Zhu, H.; Zou, Y.; Huang, C.; Fan, S. Perillaldehyde Alleviates polyQ-Induced Neurodegeneration through the Induction of Autophagy and Mitochondrial UPR in Caenorhabditis Elegans. BioFactors 2024, 51, e2089. [Google Scholar] [CrossRef] [PubMed]
- Varada, S.; Chamberlin, S.R.; Bui, L.; Brandes, M.S.; Gladen-Kolarsky, N.; Harris, C.J.; Hack, W.; Brumbach, B.H.; Quinn, J.F.; Gray, N.E. Asiatic Acid Improves Mitochondrial Function, Activates Antioxidant Response in the Mouse Brain and Improves Cognitive Function in Beta-Amyloid Overexpressing Mice. bioRxiv 2024, 2024.02.21.581270. [Google Scholar]
- Yi, C.; Song, M.; Sun, L.; Si, L.; Yu, D.; Li, B.; Lu, P.; Wang, W.; Wang, X. Asiatic Acid Alleviates Myocardial Ischemia-Reperfusion Injury by Inhibiting the ROS-Mediated Mitochondria-Dependent Apoptosis Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 3267450. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Lin, T.-Y.; Pan, T.-L.; Wang, P.-W.; Chiu, K.-M.; Lee, M.-Y.; Wang, S.-J. Asiatic Acid Prevents Cognitive Deficits by Inhibiting Calpain Activation and Preserving Synaptic and Mitochondrial Function in Rats with Kainic Acid-Induced Seizure. Biomedicines 2021, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Meeran, M.F.N.; Azimullah, S.; Bader Eddin, L.; Dwivedi, V.D.; Jha, N.K.; Ojha, S. α-Bisabolol, a Dietary Bioactive Phytochemical Attenuates Dopaminergic Neurodegeneration through Modulation of Oxidative Stress, Neuroinflammation and Apoptosis in Rotenone-Induced Rat Model of Parkinson’s Disease. Biomolecules 2020, 10, 1421. [Google Scholar] [CrossRef]
- Brasil, F.B.; Bertolini Gobbo, R.C.; Souza De Almeida, F.J.; Luckachaki, M.D.; Dall’Oglio, E.L.; De Oliveira, M.R. The Signaling Pathway PI3K/Akt/Nrf2/HO-1 Plays a Role in the Mitochondrial Protection Promoted by Astaxanthin in the SH-SY5Y Cells Exposed to Hydrogen Peroxide. Neurochem. Int. 2021, 146, 105024. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Ding, F.; Zhang, Y.; Wang, Y.; Wang, Y.; Zhang, Y.; Zhu, F.; Zhang, G.; Zheng, X.; Jia, G.; et al. Astaxanthin Inhibits H2O2-Induced Excessive Mitophagy and Apoptosis in SH-SY5Y Cells by Regulation of Akt/mTOR Activation. Mar. Drugs 2024, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Zhang, C.; Lu, W. Progress in Heterologous Biosynthesis of Forskolin. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab009. [Google Scholar] [CrossRef]
- Alharbi, M.; Alshammari, A.; Kaur, G.; Kalra, S.; Mehan, S.; Suri, M.; Chhabra, S.; Kumar, N.; Alanazi, W.A.; Alshanwani, A.R.; et al. Effect of Natural Adenylcyclase/cAMP/CREB Signalling Activator Forskolin against Intra-Striatal 6-OHDA-Lesioned Parkinson’s Rats: Preventing Mitochondrial, Motor and Histopathological Defects. Molecules 2022, 27, 7951. [Google Scholar] [CrossRef]
- Zare Mehrjerdi, F.; Niknazar, S.; Yadegari, M.; Akbari, F.A.; Pirmoradi, Z.; Khaksari, M. Carvacrol Reduces Hippocampal Cell Death and Improves Learning and Memory Deficits Following Lead-Induced Neurotoxicity via Antioxidant Activity. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Nazıroğlu, M. A Novel Antagonist of TRPM2 and TRPV4 Channels: Carvacrol. Metab. Brain Dis. 2022, 37, 711–728. [Google Scholar] [CrossRef] [PubMed]
- AlKahtane, A.A.; Ghanem, E.; Bungau, S.G.; Alarifi, S.; Ali, D.; AlBasher, G.; Alkahtani, S.; Aleya, L.; Abdel-Daim, M.M. Carnosic Acid Alleviates Chlorpyrifos-Induced Oxidative Stress and Inflammation in Mice Cerebral and Ocular Tissues. Environ. Sci. Pollut. Res. 2020, 27, 11663–11670. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Chen, W.-J.; Fu, R.-H.; Tsai, C.-W. Upregulation of OPA1 by Carnosic Acid Is Mediated through Induction of IKKγ Ubiquitination by Parkin and Protects against Neurotoxicity. Food Chem. Toxicol. 2020, 136, 110942. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Huang, Y.-N.; Fu, R.-H.; Liao, Y.-H.; Kuo, T.-Y.; Tsai, C.-W. Promotion of Mitochondrial Biogenesis via the Regulation of PARIS and PGC-1α by Parkin as a Mechanism of Neuroprotection by Carnosic Acid. Phytomedicine 2021, 80, 153369. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Khodaparast, F.; Bohlooli, S.; Hashemidanesh, N.; Baghal, E.; Rezagholizadeh, L. Linalool Reverses Benzene-Induced Cytotoxicity, Oxidative Stress and Lysosomal/Mitochondrial Damages in Human Lymphocytes. Drug Chem. Toxicol. 2022, 45, 2454–2462. [Google Scholar] [CrossRef]
- Rosado-Ramos, R.; Poças, G.M.; Marques, D.; Foito, A.; Sevillano, D.M.; Lopes-da-Silva, M.; Gonçalves, L.G.; Menezes, R.; Ottens, M.; Stewart, D.; et al. Genipin Prevents Alpha-Synuclein Aggregation and Toxicity by Affecting Endocytosis, Metabolism and Lipid Storage. Nat. Commun. 2023, 14, 1918. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.-X.; Chen, Y.-Y.; Li, Z.; Zheng, S.-J.; Wan, W.-J.; Ji, Y.; Hu, K. Genipin Relieves Diabetic Retinopathy by Down-Regulation of Advanced Glycation End Products via the Mitochondrial Metabolism Related Signaling Pathway. World J. Diabetes 2023, 14, 1349–1368. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Dias, M.M.; Barreiro, M.F.; Pinho, S.P. Saponins as Natural Emulsifiers for Nanoemulsions. J. Agric. Food Chem. 2022, 70, 6573–6590. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.-J.; Kim, S.; Kim, J.-J.; Jang, G.Y.; Moon, M.; Kim, H.D. Crude Saponin from Platycodon Grandiflorum Attenuates Aβ-Induced Neurotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Signaling Pathways. Antioxidants 2021, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wang, J.-X.; Xiong, Z.-E.; Hu, S.-S.; Zhou, A.-J.; Yuan, D.; Zhang, C.-C.; Zhou, Z.-Y.; Wang, T. Saponins from Panax Japonicus Improve Neuronal Mitochondrial Injury of Aging Rats. Pharm. Biol. 2023, 61, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jin, S.; Shao, W.; Zhu, L.; Yan, S.; Lu, J. Saponins of Marsdenia Tenacissima Promotes Apoptosis of Hepatocellular Carcinoma Cells through Damaging Mitochondria Then Activating Cytochrome C/Caspase-9/Caspase-3 Pathway. J. Cancer 2022, 13, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.-G.; Yong, Y.-Y.; He, C.-L.; Li, Y.-P.; Zhou, X.-Y.; Yu, L.; Chen, Q.; Lan, C.; Liu, J.; Yu, C.-L.; et al. Novel 18-Norspirostane Steroidal Saponins: Extending Lifespan and Mitigating Neurodegeneration through Promotion of Mitophagy and Mitochondrial Biogenesis in Caenorhabditis elegans. Mech. Ageing Dev. 2024, 218, 111901. [Google Scholar] [CrossRef]
- Ni, X.-C.; Wang, H.-F.; Cai, Y.-Y.; Yang, D.; Alolga, R.N.; Liu, B.; Li, J.; Huang, F.-Q. Ginsenoside Rb1 Inhibits Astrocyte Activation and Promotes Transfer of Astrocytic Mitochondria to Neurons against Ischemic Stroke. Redox Biol. 2022, 54, 102363. [Google Scholar] [CrossRef]
- Jiang, L.; Yin, X.; Chen, Y.-H.; Chen, Y.; Jiang, W.; Zheng, H.; Huang, F.-Q.; Liu, B.; Zhou, W.; Qi, L.-W.; et al. Proteomic Analysis Reveals Ginsenoside Rb1 Attenuates Myocardial Ischemia/Reperfusion Injury through Inhibiting ROS Production from Mitochondrial Complex I. Theranostics 2021, 11, 1703–1720. [Google Scholar] [CrossRef]
- Xia, M.-L.; Xie, X.-H.; Ding, J.-H.; Du, R.-H.; Hu, G. Astragaloside IV Inhibits Astrocyte Senescence: Implication in Parkinson’s Disease. J. Neuroinflamm. 2020, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, J.; Lan, J.; Li, Q.; Ke, L.; Jiang, Q.; Li, Y.; Zhang, H.; Zhong, H.; Yang, P.; et al. Astragaloside IV Protects against Autoimmune Myasthenia Gravis in Rats via Regulation of Mitophagy and Apoptosis. Mol. Med. Rep. 2024, 30, 129. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Zhou, M.; Li, H.; Zhou, T.; Liu, X.; Huang, Q.; Yang, S.; Xiang, Q.; Yu, R. Phenylsulfate-Induced Oxidative Stress and Mitochondrial Dysfunction in Podocytes Are Ameliorated by Astragaloside IV Activation of the SIRT1/PGC1α/Nrf1 Signaling Pathway. Biomed. Pharmacother. 2024, 177, 117008. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Y.; Xue, M.; Xia, F.; Zhu, L.; Li, Y.; Jia, D.; Chen, S.; Xu, G.; Lei, Y. Astragaloside IV Ameliorates Fat Metabolism in the Liver of Ageing Mice through Targeting Mitochondrial Activity. J. Cell. Mol. Med. 2021, 25, 8863–8876. [Google Scholar] [CrossRef]
- Ben, Y.; Hao, J.; Zhang, Z.; Xiong, Y.; Zhang, C.; Chang, Y.; Yang, F.; Li, H.; Zhang, T.; Wang, X.; et al. Astragaloside IV Inhibits Mitochondrial-Dependent Apoptosis of the Dorsal Root Ganglion in Diabetic Peripheral Neuropathy Rats Through Modulation of the SIRT1/P53 Signaling Pathway. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Dou, B.; Zou, Y.; Han, H.; Liu, D.; Ke, Z.; Wang, Z. Cycloastragenol Upregulates SIRT1 Expression, Attenuates Apoptosis and Suppresses Neuroinflammation after Brain Ischemia. Acta Pharmacol. Sin. 2020, 41, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Gorantla, V.R.; Mahalakshmi, A.M.; Ghanekar, R.K.; Yang, J.; Sakharkar, M.K.; Chidambaram, S.B. Polyphenols, Autophagy and Neurodegenerative Diseases: A Review. Biomolecules 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Bertolini, A.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Relieve Synergistically Autophagy Dysregulation in a Cellular Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7225. [Google Scholar] [CrossRef] [PubMed]
- Madiha, S.; Batool, Z.; Tabassum, S.; Liaquat, L.; Sadir, S.; Shahzad, S.; Naqvi, F.; Saleem, S.; Yousuf, S.; Nawaz, A.; et al. Quercetin Exhibits Potent Antioxidant Activity, Restores Motor and Non-Motor Deficits Induced by Rotenone Toxicity. PLoS ONE 2021, 16, e0258928. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-W.; Han, R.; He, H.-J.; Li, J.; Chen, S.-Y.; Gu, Y.; Xie, C. Administration of Quercetin Improves Mitochondria Quality Control and Protects the Neurons in 6-OHDA-Lesioned Parkinson’s Disease Models. Aging 2021, 13, 11738–11751. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Dai, S.; Liu, Y.; Zhang, F.; Peng, C.; Li, Y. Quercetin Protects Ethanol-Induced Hepatocyte Pyroptosis via Scavenging Mitochondrial ROS and Promoting PGC-1α-Regulated Mitochondrial Homeostasis in L02 Cells. Oxidative Med. Cell. Longev. 2022, 2022, 4591134. [Google Scholar] [CrossRef]
- Kesh, S.; Kannan, R.R.; Balakrishnan, A. Naringenin Alleviates 6-Hydroxydopamine Induced Parkinsonism in SHSY5Y Cells and Zebrafish Model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108893. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.H.; Fatima, M.; Ali, M.; Rizvi, M.A.; Mondal, A.C. Naringenin Alleviates Paraquat-Induced Dopaminergic Neuronal Loss in SH-SY5Y Cells and a Rat Model of Parkinson’s Disease. Neuropharmacology 2021, 201, 108831. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zeng, L.; Yan, F.; Liu, J.; Qin, M.; Wang, F.; Zhang, X. Long-Term Oral Administration of Naringenin Counteracts Aging-Related Retinal Degeneration via Regulation of Mitochondrial Dynamics and Autophagy. Front. Pharmacol. 2022, 13, 919905. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yu, C.R.; Lien, H.; Sheu, G.; Cherng, S. Cytotoxicity of Naringenin Induces Bax-mediated Mitochondrial Apoptosis in Human Lung Adenocarcinoma A549 Cells. Environ. Toxicol. 2020, 35, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Song, H.; Cho, J.H. Flavonoids Mitigate Neurodegeneration in Aged Caenorhabditis Elegans by Mitochondrial Uncoupling. Food Sci. Nutr. 2020, 8, 6633–6642. [Google Scholar] [CrossRef] [PubMed]
- Goujon, M.; Liang, Z.; Soriano-Castell, D.; Currais, A.; Maher, P. The Neuroprotective Flavonoids Sterubin and Fisetin Maintain Mitochondrial Health under Oxytotic/Ferroptotic Stress and Improve Bioenergetic Efficiency in HT22 Neuronal Cells. Antioxidants 2024, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Bécquer-Viart, M.Á.; Armentero-López, A.; Alvarez-Almiñaque, D.; Fernández-Acosta, R.; Matos-Peralta, Y.; D’Vries, R.F.; Marín-Prida, J.; Pardo-Andreu, G.L. Gossypitrin, a Naturally Occurring Flavonoid, Attenuates Iron-Induced Neuronal and Mitochondrial Damage. Molecules 2021, 26, 3364. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Wang, C.; Chen, Y.; Liu, P.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Silibinin Attenuates Motor Dysfunction in a Mouse Model of Parkinson’s Disease by Suppression of Oxidative Stress and Neuroinflammation along with Promotion of Mitophagy. Physiol. Behav. 2021, 239, 113510. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Liu, W.; Song, S.; Fu, J.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Oral Administration of Silibinin Ameliorates Cognitive Deficits of Parkinson’s Disease Mouse Model by Restoring Mitochondrial Disorders in Hippocampus. Neurochem. Res. 2021, 46, 2317–2332. [Google Scholar] [CrossRef]
- Tie, F.; Fu, Y.; Hu, N.; Wang, H. Silibinin Protects against H2O2-Induced Oxidative Damage in SH-SY5Y Cells by Improving Mitochondrial Function. Antioxidants 2022, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Esselun, C.; Bruns, B.; Hagl, S.; Grewal, R.; Eckert, G.P. Impact of Silibinin A on Bioenergetics in PC12APPsw Cells and Mitochondrial Membrane Properties in Murine Brain Mitochondria. Antioxidants 2021, 10, 1520. [Google Scholar] [CrossRef]
- Iyengar, R.M.; Devaraj, E. Silibinin Triggers the Mitochondrial Pathway of Apoptosis in Human Oral Squamous Carcinoma Cells. Asian Pac. J. Cancer Prev. 2020, 21, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Naia, L.; Pinho, C.M.; Dentoni, G.; Liu, J.; Leal, N.S.; Ferreira, D.M.S.; Schreiner, B.; Filadi, R.; Fão, L.; Connolly, N.M.C.; et al. Neuronal Cell-Based High-Throughput Screen for Enhancers of Mitochondrial Function Reveals Luteolin as a Modulator of Mitochondria-Endoplasmic Reticulum Coupling. BMC Biol. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, W.; Sun, S.; Li, C.; Zhang, Y.; Ren, J. Luteolin Attenuates Doxorubicin-Induced Cardiotoxicity Through Promoting Mitochondrial Autophagy. Front. Physiol. 2020, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pan, Z.; Du, H.; Chen, X.; Zhu, X.; Hao, W.; Zheng, Q.; Tang, X. Luteolin Induces Apoptosis by Impairing Mitochondrial Function and Targeting the Intrinsic Apoptosis Pathway in Gastric Cancer Cells. Oncol. Lett. 2023, 26, 327. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Shen, F.; Liu, J.; Tang, H.; Zhang, K.; Teng, X.; Yang, F. The Protective Effect of Luteolin on Chicken Spleen Lymphocytes from Ammonia Poisoning through Mitochondria and Balancing Energy Metabolism Disorders. Poult. Sci. 2023, 102, 103093. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Lombardo, G.E.; Russo, C.; Musumeci, L.; Gangemi, S.; Calapai, G.; Barreca, D.; Navarra, M. A Flavonoid-Rich Extract of Mandarin Juice Counteracts 6-OHDA-Induced Oxidative Stress in SH-SY5Y Cells and Modulates Parkinson-Related Genes. Antioxidants 2021, 10, 539. [Google Scholar] [CrossRef]
- Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.B.L.; Moreira, A.L.; Boeira, S.P.; Cattelan Souza, L. Hesperidin Protects against Behavioral Alterations and Loss of Dopaminergic Neurons in 6-OHDA-Lesioned Mice: The Role of Mitochondrial Dysfunction and Apoptosis. Metab. Brain Dis. 2021, 36, 153–167. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Z.; Deng, L.; Xia, S.; Mu, Q.; Xiao, B.; Xiu, Y.; Liu, Z. Hesperetin Promotes Bladder Cancer Cells Death via the PI3K/AKT Pathway by Network Pharmacology and Molecular Docking. Sci. Rep. 2024, 14, 1009. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cai, Z.; Schindler, F.; Afjehi-Sadat, L.; Montsch, B.; Heffeter, P.; Heiss, E.H.; Weckwerth, W. Elevated PINK1/Parkin-Dependent Mitophagy and Boosted Mitochondrial Function Mediate Protection of HepG2 Cells from Excess Palmitic Acid by Hesperetin. J. Agric. Food Chem. 2024, 72, 13039–13053. [Google Scholar] [CrossRef]
- Huang, M.; Singh, N.; Kainth, R.; Khalid, M.; Kushwah, A.S.; Kumar, M. Mechanistic Insight into Diosmin-Induced Neuroprotection and Memory Improvement in Intracerebroventricular-Quinolinic Acid Rat Model: Resurrection of Mitochondrial Functions and Antioxidants. Evid.-Based Complement. Altern. Med. 2022, 2022, 8584558. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Ni, L.; Hu, F.; Yue, B.; Zheng, Y.; Wang, T.; Kumar, A.; Wang, Y.; Wang, J.; et al. Icariin Ameliorates D-Galactose-Induced Cell Injury in Neuron-like PC12 Cells by Inhibiting MPTP Opening. Curr. Med. Sci. 2024, 44, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Li, S.; Luo, R.; Wu, X.; Zhang, Y.; Liao, Z.; Song, Y.; Wang, K.; Zhao, K.; Yang, S.; et al. Icariin Protects Human Nucleus Pulposus Cells from Hydrogen Peroxide-Induced Mitochondria-Mediated Apoptosis by Activating Nuclear Factor Erythroid 2-Related Factor 2. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2020, 1866, 165575. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced Inhibition of SIRT6/NF-κB Triggers Redox Mediated Apoptosis and Enhances Anti-tumor Immunity in Triple-negative Breast Cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, H.; Pan, Q.; Kang, J.; Liang, Z.; Zhang, R. The Combination of β-Asarone and Icariin Inhibits Amyloid-β and Reverses Cognitive Deficits by Promoting Mitophagy in Models of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 7158444. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Park, J.Y.; Kang, K.S.; Hwang, G.S. Neuroprotective Effect of Gallocatechin Gallate on Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2021, 26, 1387. [Google Scholar] [CrossRef]
- Yu, J.; Gao, X.; Zhang, L.; Shi, H.; Yan, Y.; Han, Y.; Wu, C.; Liu, Y.; Fang, M.; Huang, C.; et al. Magnolol Extends Lifespan and Improves Age-Related Neurodegeneration in Caenorhabditis Elegans via Increase of Stress Resistance. Sci. Rep. 2024, 14, 3158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, H.; Wang, H.; Wei, L.; Niu, L. Magnolol Alleviates IL-1β-Induced Dysfunction of Chondrocytes Through Repression of SIRT1/AMPK/PGC-1α Signaling Pathway. J. Interferon Cytokine Res. 2020, 40, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Hardy, M.; Zielonka, J.; Weh, K.; Zielonka, M.; Boyle, K.A.; Abu Eid, M.; McAllister, D.; Bennett, B.; Kresty, L.A.; et al. Mitochondria-Targeted Magnolol Inhibits OXPHOS, Proliferation, and Tumor Growth via Modulation of Energetics and Autophagy in Melanoma Cells. Cancer Treat. Res. Commun. 2020, 25, 100210. [Google Scholar] [CrossRef]
- Tao, C.; Chen, J.; Huang, X.; Chen, Z.; Li, X.; Li, Y.; Xu, Y.; Ma, M.; Wu, Z. CT1-3, a Novel Magnolol-Sulforaphane Hybrid Suppresses Tumorigenesis through Inducing Mitochondria-Mediated Apoptosis and Inhibiting Epithelial Mesenchymal Transition. Eur. J. Med. Chem. 2020, 199, 112441. [Google Scholar] [CrossRef]
- Azimullah, S.; Meeran, M.F.N.; Ayoob, K.; Arunachalam, S.; Ojha, S.; Beiram, R. Tannic Acid Mitigates Rotenone-Induced Dopaminergic Neurodegeneration by Inhibiting Inflammation, Oxidative Stress, Apoptosis, and Glutamate Toxicity in Rats. Int. J. Mol. Sci. 2023, 24, 9876. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Tabassum, H.; Parvez, S. Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1α/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2020, 57, 2870–2885. [Google Scholar] [CrossRef]
- Li, C.-C.; Tsai, B.C.-K.; Annseles Rajula, S.; Hsu, C.-H.; Chen, M.-C.; Kuo, C.-H.; Yeh, C.-M.; Hsieh, D.J.-Y.; Kuo, W.-W.; Huang, C.-Y. Tannic Acid Impedes the Proliferation of Bladder Cancer Cells by Elevating Mitochondrial Pathways of Apoptosis. Cell Biochem. Biophys. 2024, 82, 1325–1333. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, G.; Lin, L.; Zhang, Y.; Luo, X.; Ma, X.; Aiyisake, A.; Cheng, Q. Resveratrol Attenuates Sepsis-Induced Cardiomyopathy in Rats through Anti-Ferroptosis via the Sirt1/Nrf2 Pathway. J. Investig. Surg. Off. J. Acad. Surg. Res. 2023, 36, 2157521. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Q.; Dong, B.; Geng, H.; Wang, Y.; Han, D.; Zhu, X.; Liu, H.; Zhang, Z.; Yang, Y.; et al. Resveratrol Alleviates Lipopolysaccharide-Induced Liver Injury by Inducing SIRT1/P62-Mediated Mitophagy in Gibel Carp (Carassius gibelio). Front. Immunol. 2023, 14, 1177140. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, Z.; Zhou, G.; Niu, Q.; Chen, J.; Li, P.; Dong, L.; Xia, T.; Zhang, S.; Wang, A. SIRT1-Dependent Mitochondrial Biogenesis Supports Therapeutic Effects of Resveratrol against Neurodevelopment Damage by Fluoride. Theranostics 2020, 10, 4822–4838. [Google Scholar] [CrossRef] [PubMed]
- Adedara, A.O.; Babalola, A.D.; Stephano, F.; Awogbindin, I.O.; Olopade, J.O.; Rocha, J.B.T.; Whitworth, A.J.; Abolaji, A.O. An Assessment of the Rescue Action of Resveratrol in Parkin Loss of Function-Induced Oxidative Stress in Drosophila Melanogaster. Sci. Rep. 2022, 12, 3922. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Ye, Y.; Zhu, G.; Xu, Y.; Sun, J.; Wu, H.; Feng, F.; Wen, Z.; Jiang, S.; Li, Y.; et al. Resveratrol Induces Human Colorectal Cancer Cell Apoptosis by Activating the Mitochondrial Pathway via Increasing Reactive Oxygen Species. Mol. Med. Rep. 2021, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ren, D.; Feng, X.; Huang, J.; Wang, D.; Li, T.; Zhang, D. Neuroprotective and Anti-Inflammatory Effect of Pterostilbene Against Cerebral Ischemia/Reperfusion Injury via Suppression of COX-2. Front. Pharmacol. 2021, 12, 770329. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Q.; Wang, X.; Cheng, H.; Yu, J.; Li, Y.; Luo, J.; Zhang, Q.; Wu, J.; Zhang, G. Pterostilbene Attenuates Microglial Inflammation and Brain Injury after Intracerebral Hemorrhage in an OPA1-Dependent Manner. Front. Immunol. 2023, 14, 1172334. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene Promotes Mitochondrial Apoptosis and Inhibits Proliferation in Glioma Cells. Sci. Rep. 2021, 11, 6381. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lai, C.; Luo, J.; Ding, Y.; Chen, Q.; Guan, Z. Mangiferin Prevents the Impairment of Mitochondrial Dynamics and an Increase in Oxidative Stress Caused by Excessive Fluoride in SH-SY5Y Cells. J. Biochem. Mol. Toxicol. 2021, 35, e22705. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.-J.; Hwangbo, H.; Bang, E.; Kim, H.-S.; Yun, S.J.; Moon, S.-K.; Kim, W.-J.; Kim, G.-Y.; Lee, S.-O.; et al. Activation of Heme Oxygenase-1 by Mangiferin in Human Retinal Pigment Epithelial Cells Contributes to Blocking Oxidative Damage. Biomol. Ther. 2024, 32, 329–340. [Google Scholar] [CrossRef]
- Wang, X.-L.; Feng, S.-T.; Wang, Y.-T.; Zhang, N.-N.; Guo, Z.-Y.; Yan, X.; Yuan, Y.-H.; Wang, Z.-Z.; Chen, N.-H.; Zhang, Y. Mangiferin, a Natural Glucoxilxanthone, Inhibits Mitochondrial Dynamin-Related Protein 1 and Relieves Aberrant Mitophagic Proteins in Mice Model of Parkinson’s Disease. Phytomedicine 2022, 104, 154281. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hou, G.; Cao, J.; Yin, Y.; Zhao, Y.; Cheng, L. Mangiferin Alleviates Mitochondrial ROS in Nucleus Pulposus Cells and Protects against Intervertebral Disc Degeneration via Suppression of NF-κB Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6632786. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; Jo, E.-R.; Song, H. Urolithin A Attenuates Auditory Cell Senescence by Activating Mitophagy. Sci. Rep. 2022, 12, 7704. [Google Scholar] [CrossRef]
- D’Amico, D.; Olmer, M.; Fouassier, A.M.; Valdés, P.; Andreux, P.A.; Rinsch, C.; Lotz, M. Urolithin A Improves Mitochondrial Health, Reduces Cartilage Degeneration, and Alleviates Pain in Osteoarthritis. Aging Cell 2022, 21, e13662. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A Improves Muscle Strength, Exercise Performance, and Biomarkers of Mitochondrial Health in a Randomized Trial in Middle-Aged Adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.; Alvir, R.V.; Pradeepkiran, J.A.; Hindle, A.; Vijayan, M.; Ramasubramaniam, B.; Kumar, S.; Reddy, A.P.; Reddy, P.H. A Combination Therapy of Urolithin A+EGCG Has Stronger Protective Effects than Single Drug Urolithin A in a Humanized Amyloid Beta Knockin Mice for Late-Onset Alzheimer’s Disease. Cells 2022, 11, 2660. [Google Scholar] [CrossRef]
- Maycotte, P.; Illanes, M.; Moreno, D.A. Glucosinolates, Isothiocyanates, and Their Role in the Regulation of Autophagy and Cellular Function. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Brasil, F.B.; de Almeida, F.J.S.; Luckachaki, M.D.; Dall’Oglio, E.L.; de Oliveira, M.R. The Isothiocyanate Sulforaphane Prevents Mitochondrial Impairment and Neuroinflammation in the Human Dopaminergic SH-SY5Y and in the Mouse Microglial BV2 Cells: Role for Heme Oxygenase-1. Metab. Brain Dis. 2023, 38, 419–435. [Google Scholar] [CrossRef]
- Folbergrová, J.; Ješina, P.; Otáhal, J. Protective Effect of Sulforaphane on Oxidative Stress and Mitochondrial Dysfunction Associated with Status Epilepticus in Immature Rats. Mol. Neurobiol. 2023, 60, 2024–2035. [Google Scholar] [CrossRef]
- Napoli, E.; Flores, A.; Mansuri, Y.; Hagerman, R.J.; Giulivi, C. Sulforaphane Improves Mitochondrial Metabolism in Fibroblasts from Patients with Fragile X-Associated Tremor and Ataxia Syndrome. Neurobiol. Dis. 2021, 157, 105427. [Google Scholar] [CrossRef]
- Anjum, R.; Raza, C.; Faheem, M.; Ullah, A.; Chaudhry, M. Neuroprotective Potential of Mentha Piperita Extract Prevents Motor Dysfunctions in Mouse Model of Parkinson’s Disease through Anti-Oxidant Capacities. PLoS ONE 2024, 19, e0302102. [Google Scholar] [CrossRef]
- Liang, Z.; Soriano-Castell, D.; Kepchia, D.; Duggan, B.M.; Currais, A.; Schubert, D.; Maher, P. Cannabinol Inhibits Oxytosis/Ferroptosis by Directly Targeting Mitochondria Independently of Cannabinoid Receptors. Free Radic. Biol. Med. 2022, 180, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Drummond-Main, C.D.; Ahn, Y.; Kesler, M.; Gavrilovici, C.; Kim, D.Y.; Kiroski, I.; Baglot, S.L.; Chen, A.; Sharkey, K.A.; Hill, M.N.; et al. Cannabidiol Impairs Brain Mitochondrial Metabolism and Neuronal Integrity. Cannabis Cannabinoid Res. 2023, 8, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Noskova, E.; Fernández, R.; García, J.; Ochoa, E.; Domínguez-Fernández, C.; Adell, A.; Laso, A.; Andrés, M.F.; González-Coloma, A.; Astigarraga, E.; et al. Screening System of Cannabis Sativa Extracts Based on Their Mitochondrial Safety Profile Using Cytochrome c Oxidase Activity as a Biomarker. Int. J. Mol. Sci. 2023, 24, 1315. [Google Scholar] [CrossRef]
- Yang, S.; Hu, B.; Wang, Z.; Zhang, C.; Jiao, H.; Mao, Z.; Wei, L.; Jia, J.; Zhao, J. Cannabinoid CB1 Receptor Agonist ACEA Alleviates Brain Ischemia/Reperfusion Injury via CB1–Drp1 Pathway. Cell Death Discov. 2020, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodríguez De Fonseca, F.; et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016, 7, 476. [Google Scholar] [CrossRef]
- Pinky, P.D.; Majrashi, M.; Fujihashi, A.; Bloemer, J.; Govindarajulu, M.; Ramesh, S.; Reed, M.N.; Moore, T.; Suppiramaniam, V.; Dhanasekaran, M. Effects of Prenatal Synthetic Cannabinoid Exposure on the Cerebellum of Adolescent Rat Offspring. Heliyon 2021, 7, e06730. [Google Scholar] [CrossRef]
- Ma, L.; Jia, J.; Niu, W.; Jiang, T.; Zhai, Q.; Yang, L.; Bai, F.; Wang, Q.; Xiong, L. Mitochondrial CB1 Receptor Is Involved in ACEA-Induced Protective Effects on Neurons and Mitochondrial Functions. Sci. Rep. 2015, 5, 12440. [Google Scholar] [CrossRef]
- Rupprecht, A.; Theisen, U.; Wendt, F.; Frank, M.; Hinz, B. The Combination of Δ9-Tetrahydrocannabinol and Cannabidiol Suppresses Mitochondrial Respiration of Human Glioblastoma Cells via Downregulation of Specific Respiratory Chain Proteins. Cancers 2022, 14, 3129. [Google Scholar] [CrossRef]
- Delerue, T.; Tribouillard-Tanvier, D.; Daloyau, M.; Khosrobakhsh, F.; Emorine, L.J.; Friocourt, G.; Belenguer, P.; Blondel, M.; Arnauné-Pelloquin, L. A Yeast-Based Screening Assay Identifies Repurposed Drugs That Suppress Mitochondrial Fusion and mtDNA Maintenance Defects. Dis. Model. Mech. 2019, 12, dmm036558. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.-L.; Huang, H.; Zeng, X.; Duan, C.-Y. Targeting Mitochondrial Quality Control: New Therapeutic Strategies for Major Diseases. Mil. Med. Res. 2024, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, E.O. Drug Repurposing for Mitochondrial Diseases Using a Pharmacological Model of Complex I Deficiency in the Yeast Yarrowia lipolytica. bioRxiv 2020, 2020.01.08.899666. [Google Scholar]
- Tcherniuk, S.O.; Chesnokova, O.; Oleinikov, I.V.; Potopalsky, A.I.; Oleinikov, A.V. Anti-Malarial Effect of Semi-Synthetic Drug Amitozyn. Malar. J. 2015, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.; Kumar, J. Biosynthesis of Anticancer Phytochemical Compounds and Their Chemistry. Front. Pharmacol. 2023, 14, 1136779. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.K.; Jbara, M.; Brik, A. Chemical and Semisynthesis of Modified Histones. J. Pept. Sci. 2016, 22, 252–259. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hsieh, Y.-H.; Powers, Z.M.; Kao, C.-Y. Defeating Antibiotic-Resistant Bacteria: Exploring Alternative Therapies for a Post-Antibiotic Era. Int. J. Mol. Sci. 2020, 21, 1061. [Google Scholar] [CrossRef]
- Somuncu, B.; Ekmekcioglu, A.; Antmen, F.M.; Ertuzun, T.; Deniz, E.; Keskin, N.; Park, J.; Yazici, I.E.; Simsek, B.; Erman, B.; et al. Targeting Mitochondrial DNA Polymerase Gamma for Selective Inhibition of MLH1 Deficient Colon Cancer Growth. PLoS ONE 2022, 17, e0268391. [Google Scholar] [CrossRef]
- Datta, S.; Mahdi, F.; Ali, Z.; Jekabsons, M.B.; Khan, I.A.; Nagle, D.G.; Zhou, Y.-D. Toxins in Botanical Dietary Supplements: Blue Cohosh Components Disrupt Cellular Respiration and Mitochondrial Membrane Potential. J. Nat. Prod. 2014, 77, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bhakti, M.; Thankamani, M. Benefits of Morinda citrifolia L. Fruit Extract on Methotrexate Induced Mitochondrial Toxicity—An In Vivo Study in Rat Liver Mitochondria: Life Sciences-Biotechnology for Prospective Medical Science. Int. J. Life Sci. Pharma Res. 2021, 11, L45–L53. [Google Scholar] [CrossRef]
- Gray, N.E.; Sampath, H.; Zweig, J.A.; Quinn, J.F.; Soumyanath, A. Centella Asiatica Attenuates Amyloid-β-Induced Oxidative Stress and Mitochondrial Dysfunction. J. Alzheimer’s Dis. 2015, 45, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Stansley, B.J.; Yamamoto, B.K. L-Dopa-Induced Dopamine Synthesis and Oxidative Stress in Serotonergic Cells. Neuropharmacology 2013, 67, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, J.; Schiefen, T.; Güber, T.; Henkel, J.; Uter, J.; Steinhardt, J.; Wilms, B.; Brüggemann, N. Levodopa Impairs the Energy Metabolism of the Basal Ganglia In Vivo. Ann. Neurol. 2024, 95, 849–857. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Song, P.; Mazzulli, J.R.; Zampese, E.; Wong, Y.C.; Jeon, S.; Santos, D.P.; Blanz, J.; Obermaier, C.D.; Strojny, C.; et al. Dopamine Oxidation Mediates Mitochondrial and Lysosomal Dysfunction in Parkinson’s Disease. Science 2017, 357, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Li, Z.; Kang, X.; Li, T.; Isaev, N.K.; Smirnova, E.A.; Shen, H.; Liu, L.; Yu, Y. L-DOPA Ameliorates Hippocampus-Based Mitochondria Respiratory Dysfunction Caused by GCI/R Injury. Biomed. Pharmacother. 2024, 175, 116664. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S. Antibacterial and Phytochemical Studies on Twelve Species of Indian Medicinal Plants. Afr. J. Biomed. Res. 2007, 10, 175–181. [Google Scholar] [CrossRef]
- Husaini, I.P.A.; Maulany, R.; Oka, N.P. Factors Affecting the Intensity of the Use of Medicinal Plants in Minasatene Resort, Bantimurung-Bulusaraung National Park, South Sulawesi: A Case Study in Indonesia. Int. J. Sci. Manag. Stud. 2022, 5, 105–110. [Google Scholar] [CrossRef]
- Hassen, G.; Belete, G.; Carrera, K.G.; Iriowen, R.O.; Araya, H.; Alemu, T.; Solomon, N.; Bam, D.S.; Nicola, S.M.; Araya, M.E.; et al. Clinical Implications of Herbal Supplements in Conventional Medical Practice: A US Perspective. Cureus 2022, 14, e26893. [Google Scholar] [CrossRef] [PubMed]
- Assadzadeh, R.; Shamaei, S.; Manouchehri, A. Drug Interaction of Hypericum Perforatum with Routine Chemical Drugs. Indian J. Forensic Med. Toxicol. 2021, 15, 3450–3454. [Google Scholar] [CrossRef]
- Anekwe, I.I.; Chikwendu, C.I.; Amadi, E.S.; Nwogwugwu, N.U.; Ihenetu, F.C. Gas Chromatography-Mass Spectrometry Analysis of Bioactive Compounds of Curcuma Longa Leaves Extract. Int. J. Biol. Chem. Sci. 2023, 17, 1199–1207. [Google Scholar] [CrossRef]
- Funk, J.L.; Schneider, C. Perspective on Improving the Relevance, Rigor, and Reproducibility of Botanical Clinical Trials: Lessons Learned from Turmeric Trials. Front. Nutr. 2021, 8, 782912. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef] [PubMed]
- Berberine (Chloride) (CAS 633-65-8). Available online: https://www.caymanchem.com/product/10006427 (accessed on 19 November 2024).
- Machmudah, S.; Kitada, K.; Sasaki, M.; Goto, M.; Munemasa, J.; Yamagata, M. Simultaneous Extraction and Separation Process for Coffee Beans with Supercritical CO2 and Water. Ind. Eng. Chem. Res. 2011, 50, 2227–2235. [Google Scholar] [CrossRef]
- Molajafari, F.; Li, T.; Abbasichaleshtori, M.; D., M.H.Z.; Cozzolino, A.F.; Fandrick, D.R.; Howe, J.D. Computational Screening for Prediction of Co-Crystals: Method Comparison and Experimental Validation. CrystEngComm 2024, 26, 1620–1636. [Google Scholar] [CrossRef]
- Capsaicin. Available online: https://www.selleckchem.com/datasheet/capsaicin-S199002-DataSheet.html (accessed on 15 November 2024).
- Milenković, A.N.; Stanojević, L.P. Black Pepper: Chemical Composition and Biological Activities. Adv. Technol. 2021, 10, 40–50. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Peng, Y.; Tan, C.; Wang, J.; Xue, H.; Xu, P.; Tao, F. Rapid Production of L-DOPA by Vibrio Natriegens, an Emerging next-Generation Whole-Cell Catalysis Chassis. Microb. Biotechnol. 2022, 15, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Sha, L.; Zuo, Q.; Wei, R.; Sun, C.; Wei, J.; Wang, M. Room-Temperature Stable Perillaldehyde—Natural Cyclodextrin Inclusion Complexes: Preparation, Characterization, Thermal Stability, Water Solubility, Antioxidant Activity and Slow–Release Performance. J. Mol. Struct. 2024, 1312, 138483. [Google Scholar] [CrossRef]
- Soumyanath, A.; Zhong, Y.-P.; Henson, E.; Wadsworth, T.; Bishop, J.; Gold, B.G.; Quinn, J.F. Centella Asiatica Extract Improves Behavioral Deficits in a Mouse Model of Alzheimer′s Disease: Investigation of a Possible Mechanism of Action. Int. J. Alzheimer’s Dis. 2012, 2012, 381974. [Google Scholar] [CrossRef]
- Tai, Y.; Wang, H.; Yao, P.; Sun, J.; Guo, C.; Jin, Y.; Yang, L.; Chen, Y.; Shi, F.; Yu, L.; et al. Biosynthesis of α-Bisabolol by Farnesyl Diphosphate Synthase and α-Bisabolol Synthase and Their Related Transcription Factors in Matricaria recutita L. Int. J. Mol. Sci. 2023, 24, 1730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, L.; Guo, Y.; Zhang, T.; Qiao, X.; Wang, J.; Xu, J.; Xue, C. Hydrophilic Astaxanthin: PEGylated Astaxanthin Fights Diabetes by Enhancing the Solubility and Oral Absorbability. J. Agric. Food Chem. 2020, 68, 3649–3655. [Google Scholar] [CrossRef]
- Singh, P.; Suryanarayana1, M.A. Effect of Solvents and Extraction Methods on Forskolin Content from Coleus Forskholii Roots. Indian J. Pharm. Sci. 2020, 81, 1136–1140. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical Properties and Antimicrobial Efficacy of Carvacrol Nanoemulsions Formed by Spontaneous Emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef] [PubMed]
- Cerisuelo, J.P.; Gavara, R.; Hernández-Muñoz, P. Antimicrobial-Releasing Films and Coatings for Food Packaging Based on Carvacrol and Ethylene Copolymers. Polym. Int. 2015, 64, 1747–1753. [Google Scholar] [CrossRef]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus Proximus Serg. Essential Oil and Its Major Constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef]
- Vaka, S.R.K.; Shivakumar, H.N.; Repka, M.A.; Murthy, S.N. Formulation and Evaluation of Carnosic Acid Nanoparticulate System for Upregulation of Neurotrophins in the Brain upon Intranasal Administration. J. Drug Target. 2013, 21, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Cháfer, A.; Fornari, T.; Berna, A.; Ibañez, E.; Reglero, G. Solubility of Solid Carnosic Acid in Supercritical CO2 with Ethanol as a Co-Solvent. J. Supercrit. Fluids 2005, 34, 323–329. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM Fragrance Ingredient Safety Assessment, Linalool, CAS Registry Number 78-70-6. Food Chem. Toxicol. 2015, 82, S29–S38. [Google Scholar] [CrossRef]
- Carpentieri, S.; Režek Jambrak, A.; Ferrari, G.; Pataro, G. Pulsed Electric Field-Assisted Extraction of Aroma and Bioactive Compounds from Aromatic Plants and Food By-Products. Front. Nutr. 2022, 8, 792203. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, C.I.S.; de Sousa, M.N.A.; Filho, G.G.A.; Freitas, F.O.R.; Uchoa, D.P.L.; Nobre, M.S.C.; Bezerra, A.L.D.; Rolim, L.A.D.M.M.; Morais, A.M.B.; Nogueira, T.B.S.S.; et al. Antifungal Activity of Linalool against Fluconazole-Resistant Clinical Strains of Vulvovaginal Candida albicans and Its Predictive Mechanism of Action. Braz. J. Med. Biol. Res. 2022, 55, e11831. [Google Scholar] [CrossRef]
- Augustine, E.; Deng, P.; Mou, C.; Okamura, M.; Woolley, B.; Horowitz, M.; Bettinger, C.J. Control Release and Diffusion-Reaction Kinetics of Genipin-Eluting Fibers Using an in Vitro Aneurysm Flow Model. ACS Biomater. Sci. Eng. 2021, 7, 5144–5153. [Google Scholar] [CrossRef]
- Zhao, X.; Song, K.; Wang, S.; Zu, Y.; Li, N.; Yu, X. Micronization of the Pharmaceutically Active Agent Genipin by an Antisolvent Precipitation Process. Chem. Eng. Technol. 2013, 36, 33–42. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshamsan, A.; Alkholief, M.; Alsarra, I.A.; Ali, R.; Haq, N.; Anwer, M.K.; Shakeel, F. Solubility Measurement and Various Solubility Parameters of Glipizide in Different Neat Solvents. ACS Omega 2020, 5, 1708–1716. [Google Scholar] [CrossRef]
- Jiang, L.; Niu, H.; Chen, Y.; Li, X.; Zhao, Y.; Zhang, C.; Li, M. Quality Control of Platycodon Grandiflorum (Jacq.) A. DC. Based on Value Chains and Food Chain Analysis. Sci. Rep. 2023, 13, 14048. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Chen, J.; Li, X.; Gu, W.; Zhang, F.; Cao, G.; Yu, J. Optimization of the Ultrasonic-Assisted Extraction Technology of Steroidal Saponins from Polygonatum kingianum Collett & Hemsl and Evaluating Its Quality Planted in Different Areas. Molecules 2022, 27, 1463. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, W.; Desikan Seshadri, V.D.; Cao, G. Synthesis and Characterization of Gold Nanoparticles from Marsdenia tenacissima and Its Anticancer Activity of Liver Cancer HepG2 Cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3029–3036. [Google Scholar] [CrossRef]
- Ye, B.; Li, J.; Li, Z.; Yang, J.; Niu, T.; Wang, S. Anti-Tumor Activity and Relative Mechanism of Ethanolic Extract of Marsdenia tenacissima (Asclepiadaceae) against Human Hematologic Neoplasm in vitro and in vivo. J. Ethnopharmacol. 2014, 153, 258–267. [Google Scholar] [CrossRef]
- Qiu, W.-Q.; Yu, L.; He, C.-L.; Wu, J.-M.; Law, B.Y.-K.; Yu, C.-L.; Qin, D.-L.; Zhou, X.-G.; Wu, A.-G. Two 18-Norspirostane Steroidal Saponins as Novel Mitophagy Enhancers Improve Alzheimer’s Disease. Clin. Transl. Med. 2023, 13, e1390. [Google Scholar] [CrossRef]
- Wang, L.-H.; Song, Y.-T.; Chen, Y.; Cheng, Y.-Y. Solubility of Artemisinin in Ethanol + Water from (278.2 to 343.2) K. J. Chem. Eng. Data 2007, 52, 757–758. [Google Scholar] [CrossRef]
- Vo-An, Q.; Nguyen, T.C.; Nguyen, Q.T.; Vu, Q.T.; Truong, C.D.; Nguyen, T.L.; Ly, T.N.L.; Bach, L.G.; Thai, H. Novel Nanoparticle Biomaterial of Alginate/Chitosan Loading Simultaneously Lovastatin and Ginsenoside RB1: Characteristics, Morphology, and Drug Release Study. Int. J. Polym. Sci. 2021, 2021, 5214510. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, G.; Pan, G.; Fawcett, J.P.; A., J.; Sun, J. Transport and Bioavailability Studies of Astragaloside IV, an Active Ingredient in Radix Astragali. Basic Clin. Pharmacol. Toxicol. 2004, 95, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wei, K.; Jiang, J.; Zhang, L. Extraction Optimization of Astragaloside IV by Response Surface Methodology and Evaluation of Its Stability during Sterilization and Storage. Molecules 2021, 26, 2400. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, Y.; Chen, S.; Chen, L.; Sun, L.; Cao, M.; Li, C.; Zhou, X. Astragaloside IV Alleviates Ammonia-Induced Apoptosis and Oxidative Stress in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 600. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Qin, Y.; Geng, X.; Wang, R.; Tan, W.; Mou, S. The Construction of Oligonucleotide-Cycloastragenol and the Renoprotective Effect Study. Front. Bioeng. Biotechnol. 2022, 10, 1027517. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Yang, Y.; Liu, L.; Zhou, P.; Zhu, Y.; Chai, Y.; Chen, K.; Tang, W.; Huang, Q.; Zhang, C. Research Progress on the Mechanism of Astragaloside IV in the Treatment of Asthma. Heliyon 2023, 9, e22149. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A. Olive Oil Microemulsions as a Biomimetic Medium for Enzymatic Studies: Oxidation of Oleuropein. J. Am. Oil Chem. Soc. 2005, 82, 335–340. [Google Scholar] [CrossRef]
- Tasioula-margari, M.; Okogeri, O. Isolation and Characterization of Virgin Olive Oil Phenolic Compounds by HPLC/UV and GC-MS. J. Food Sci. 2001, 66, 530–534. [Google Scholar] [CrossRef]
- Masrijal, C.D.P.; Harmita, H.; Iskandarsyah, I. Improving transdermal drug delivery system for medroxyprogesterone acetate by olive oil and dimethylsulfoxide (dmso) as penetration enhancers: In vitro penetration study. Int. J. Pharm. Pharm. Sci. 2020, 12, 12–15. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; de Leeuw, T.C.; te Poele, E.M.; Pijning, T.; Dijkhuizen, L.; Liu, W. Enzymatic Glucosylation of Polyphenols Using Glucansucrases and Branching Sucrases of Glycoside Hydrolase Family 70. Crit. Rev. Food Sci. Nutr. 2023, 63, 5247–5267. [Google Scholar] [CrossRef]
- Wisudyaningsih, B.; Setyawan, D. Co-Crystallization of Quercetin and Isonicotinamide Using Solvent Evaporation Method. Trop. J. Pharm. Res. 2019, 18, 697–702. [Google Scholar] [CrossRef]
- Liu, F.; Peng, B.; Li, M.; Ma, J.; Deng, G.; Zhang, S.; Sheu, W.C.; Zou, P.; Wu, H.; Liu, J.; et al. Targeted Disruption of Tumor Vasculature via Polyphenol Nanoparticles to Improve Brain Cancer Treatment. Cell Rep. Phys. Sci. 2022, 3, 100691. [Google Scholar] [CrossRef]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical Properties and Biological Activities of Hesperetin and Naringenin in Complex with Methylated β-Cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, J.; Fan, Y.; Wang, X.; Zheng, S.; Feng, J.; Li, J.; Fan, Z.; Li, G.; Ye, Q. Naringenin: A Promising Therapeutic Agent against Organ Fibrosis. Oxidative Med. Cell. Longev. 2021, 2021, 1210675. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Cuéllar, A.; Nossin, E.; Monan, M. Iron Chelating Activity of Gossypitrin Isolated from the Petals of Talipariti elatum Sw. (Fryxell) Malvaceae. J. Agric. Stud. 2017, 5, 1. [Google Scholar] [CrossRef]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef] [PubMed]
- Pielorz, S.; Węglińska, M.; Mazurek, S.; Szostak, R. Quantitative Determination of Diosmin in Tablets by Infrared and Raman Spectroscopy. Molecules 2022, 27, 8276. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Melzer, J.; Reichling, J.; Brignoli, R.; Meier, R. An Updated Systematic Review of the Pharmacology of Silymarin. Forsch. Komplementärmed. Res. Complement. Med. 2007, 14, 70–80. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Panayiotou, C.; Vardas, M.; Balaskas, N.; Kostomitsopoulos, N.G.; Tsaroucha, A.K.; Valsami, G. A Comprehensive Review of the Cardiovascular Protective Properties of Silibinin/Silymarin: A New Kid on the Block. Pharmaceuticals 2022, 15, 538. [Google Scholar] [CrossRef]
- Sato, A.; Shinozaki, N.; Tamura, H. Secoiridoid Type of Antiallergic Substances in Olive Waste Materials of Three Japanese Varieties of Olea Europaea. J. Agric. Food Chem. 2014, 62, 7787–7795. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and Quantification of Polyphenols from Kinnow (Citrus reticulate L.) Peel Using Ultrasound and Maceration Techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Majumdar, S.; Srirangam, R. Solubility, Stability, Physicochemical Characteristics and In Vitro Ocular Tissue Permeability of Hesperidin: A Natural Bioflavonoid. Pharm. Res. 2009, 26, 1217–1225. [Google Scholar] [CrossRef]
- Bisen, A.C.; Rawat, P.; Sharma, G.; Sanap, S.N.; Agrawal, S.; Kumar, S.; Kumar, A.; Choudhury, A.D.; Kamboj, S.; Narender, T.; et al. Hesperidin: Enrichment, Forced Degradation, and Structural Elucidation of Potential Degradation Products Using Spectral Techniques. Rapid Commun. Mass Spectrom. 2023, 37, e9615. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J. Solubility of Hesperetin in Various Solvents from (288.2 to 323.2) K. J. Chem. Eng. Data 2008, 53, 1649–1650. [Google Scholar] [CrossRef]
- Sharma, S.K.; Bhatt, P. Controlled Release of Bi-Layered EGCG Tablets Using 3D Printing Techniques. J. Pharm. Res. Int. 2020, 32, 5–13. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Chang, Q.; Zhang, L.; Wang, G.; Chen, W.; Miao, X.; Zheng, Y. A Strategy for the Improvement of the Bioavailability and Antiosteoporosis Activity of BCS IV Flavonoid Glycosides through the Formulation of Their Lipophilic Aglycone into Nanocrystals. Mol. Pharm. 2013, 10, 2534–2542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Lin, C.-W.; Lin, L.-C.; Chiu, A.W.; Chen, K.-K.; Tsai, T.-H. Analysis of Biliary Excretion of Icariin in Rats. J. Agric. Food Chem. 2010, 58, 9905–9911. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, H.; Li, L.; Li, Y.; Zhang, R. β-Asarone Inhibits Amyloid-β by Promoting Autophagy in a Cell Model of Alzheimer’s Disease. Front. Pharmacol. 2020, 10, 1529. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, P.; Ganesan, P.; Prabakaran, D.S.; Gupta, P.K.; Jonnalagadda, S.; Govindarajan, K.; Vishnu, R.; Sivalingam, K.; Sodha, S.; Choi, D.-K.; et al. Lipid Nanoparticles Improve the Uptake of α-Asarone into the Brain Parenchyma: Formulation, Characterization, In Vivo Pharmacokinetics, and Brain Delivery. AAPS PharmSciTech 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.; Melzig, M.F. Comparative Study of the Cytotoxicity and Genotoxicity of Alpha- and Beta-Asarone. Sci. Pharm. 2012, 80, 663–668. [Google Scholar] [CrossRef]
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.-E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics 2021, 13, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Zhu, B.-W.; Chen, T.; Chen, L.-H.; Wu, D.; Hu, J.-N. Construction of Magnolol Nanoparticles for Alleviation of Ethanol-Induced Acute Gastric Injury. J. Agric. Food Chem. 2024, 72, 7933–7942. [Google Scholar] [CrossRef]
- Jin, Y.C.; Kim, K.J.; Kim, Y.M.; Ha, Y.M.; Kim, H.J.; Yun, U.J.; Bae, K.H.; Kim, Y.S.; Kang, S.S.; Seo, H.G.; et al. Anti-Apoptotic Effect of Magnolol in Myocardial Ischemia and Reperfusion Injury Requires Extracellular Signal-Regulated Kinase1/2 Pathways in Rat In Vivo. Exp. Biol. Med. 2008, 233, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef] [PubMed]
- Kruthika, N.L.; Raju, G.B.; Prabhakar, S. Degradation of Tannic Acid Powered by TiO2 Nanoparticles. Mater. Sci. Forum 2013, 734, 117–126. [Google Scholar] [CrossRef]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazué, F.; Ghiringhelli, F.; Latruffe, N. Transport, Stability, and Biological Activity of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Atanasova, V.; Tardif, C.; Richard-Forget, F. Stilbenoids as Promising Natural Product-Based Solutions in a Race against Mycotoxigenic Fungi: A Comprehensive Review. J. Agric. Food Chem. 2023, 71, 5075–5092. [Google Scholar] [CrossRef] [PubMed]
- Gerber, G.; Fox, L.; Gerber, M.; Preez, J.; van Zyl, S.; Boneschans, B.; Plessis, J. Stability, Clinical Efficacy, and Antioxidant Properties of Honeybush Extracts in Semi-Solid Formulations. Pharmacogn. Mag. 2015, 11, s337–s351. [Google Scholar] [CrossRef]
- Purnomo, Y.; Soeatmadji, D.W.; Sumitro, S.B.; Widodo, M.A. Anti-Diabetic Potential of Urena lobata Leaf Extract through Inhibition of Dipeptidyl Peptidase IV Activity. Asian Pac. J. Trop. Biomed. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Tian, J.; Zhao, B.; Wei, X.-F.; Su, Y.-L.; Li, C.-Y.; Cao, S.-G.; Ji, T.-F.; Wang, L. The Effect of Ultrasound on Lipase-Catalyzed Regioselective Acylation of Mangiferin in Non-Aqueous Solvents: Original Article. J. Asian Nat. Prod. Res. 2010, 12, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lessard-Lord, J.; Plante, P.-L.; Desjardins, Y. Purified Recombinant Enzymes Efficiently Hydrolyze Conjugated Urinary (Poly)Phenol Metabolites. Food Funct. 2022, 13, 10895–10911. [Google Scholar] [CrossRef]
- Sim, H.W.; Lee, W.-Y.; Lee, R.; Yang, S.Y.; Ham, Y.-K.; Lim, S.D.; Park, H.-J. The Anti-Inflammatory Effects of Broccoli (Brassica oleracea L. Var. Italica) Sprout Extract in RAW 264.7 Macrophages and a Lipopolysaccharide-Induced Liver Injury Model. Curr. Issues Mol. Biol. 2023, 45, 9117–9131. [Google Scholar] [CrossRef]
- Singh, M.; Jha, A.; Kumar, A.; Hettiarachchy, N.; Rai, A.K.; Sharma, D. Influence of the Solvents on the Extraction of Major Phenolic Compounds (Punicalagin, Ellagic Acid and Gallic Acid) and Their Antioxidant Activities in Pomegranate Aril. J. Food Sci. Technol. 2014, 51, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Tavčar, E.; Vidak, M. Experimental Investigation and Thermodynamic Modelling of Cannabidiol and Curcumin in Different Solvents. J. Mol. Liq. 2024, 410, 125511. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anchimowicz, J.; Zielonka, P.; Jakiela, S. Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms. Int. J. Mol. Sci. 2025, 26, 380. https://doi.org/10.3390/ijms26010380
Anchimowicz J, Zielonka P, Jakiela S. Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms. International Journal of Molecular Sciences. 2025; 26(1):380. https://doi.org/10.3390/ijms26010380
Chicago/Turabian StyleAnchimowicz, Julia, Piotr Zielonka, and Slawomir Jakiela. 2025. "Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms" International Journal of Molecular Sciences 26, no. 1: 380. https://doi.org/10.3390/ijms26010380
APA StyleAnchimowicz, J., Zielonka, P., & Jakiela, S. (2025). Plant Secondary Metabolites as Modulators of Mitochondrial Health: An Overview of Their Anti-Oxidant, Anti-Apoptotic, and Mitophagic Mechanisms. International Journal of Molecular Sciences, 26(1), 380. https://doi.org/10.3390/ijms26010380





